Abstract
Method
Twenty inactive males (BMI 27.67 ± 0.88 kg/m2, age 49.15 ± 2.58 years) participated in an eight-week were randomly assigned to one of three intervention groups (combined (CT), resistance (RT), and aerobic (AT)) exercise modalities to assess within-subject and between group changes in glycolipid profile. Data were analyzed using repeated measures ANCOVA.
Result
Pre-post mean values of body fat percentage (%BF), area under the curve (AUC), low density lipoprotein (LDL), high density lipoprotein (HDL) and total cholesterol (TC) decreased in all three groups. The main effect of exercise modality on the AUC (F (2, 26) = 10.577, P = 0.001, η2 = 0.569) was significant. Post-hoc analyses revealed that the RT group (-30.653 ± 6.766, p = 0.001) with 11.53% and the CT group (M = -0.896, SE = 3.347, P = 0.015) with 3.79% exhibited significantly greater reductions in AUC compared to the AT group. LDL levels showed significant different between groups (F (2, 26) = 6.33, p = 0.009, η2 = 0.442), specially significantly 3.7% lowered in AT (MD = 4.783, SE = 1.563, P = 0.002) and 3.79% lower in CT (MD = 4.57, SE = 1.284, P = 0.008) groups compared to the RT group. AT significantly reduced TC by 17.716 ± 5.705 mg/dL (p = 0.02) compared to RT, representing a 7.97% decrease.
Conclusion
Exercise type significantly influences lipid profiles and glycemic control. Notably, both aerobic and combined training demonstrated a superior ability to modulate the lipid profile, and resistance training and combined training were more effective in reducing the AUC.
Trial registration
May, 31st 2024. Registration no: PACTR202405463745521 “Retrospectively registered”.
Similar content being viewed by others
Introduction
Obesity is the condition of having an abnormal or excessive accumulation of fat that poses a health risk. A body mass index (BMI) of over 30 is considered to be indicative of obesity [1]. The prevalence of obesity increased with age in this study, with the highest prevalence (44.3%) among middle-aged adults (40–59 years old) [2]. While women and older adults are generally more likely to experience obesity, these data suggest a trend across all age groups [3]. The incidence of obesity has been on the rise in recent decades and has been linked to a variety of health and socioeconomic issues [4]. It is important to prioritize strategies that can help reduce the healthcare costs associated with obesity.
The combined estimates of the prevalence of obesity and overweight in Middle Eastern countries were 21.17% and 33.14%, respectively [5]. The combined crude prevalence rate of obesity in Nigeria was 14.3% [6]. Like in many other countries, overweight and obesity are becoming increasingly prevalent public health issues in Ethiopia [7].
Obesity is a significant risk factor for and contributor to increased morbidity and mortality, especially as individuals age, most importantly from cardiovascular disease (CVD) and diabetes but also from cancer and chronic diseases, including osteoarthritis, liver and kidney disease, sleep apnea, and depression [8, 9].
We can therefore strongly suggest that interventions improving both blood sugar control and cholesterol levels would be highly effective in preventing cardiovascular diseases [10]. Optimizing exercise for health goes beyond just choosing an activity. The global fitness business is moving in the direction of exercise for better health results [11].
A personalized approach that considers the type of exercise is required [12]. Exercise has been shown to enhance glycemic control and improve blood lipid profiles in individuals both with and without type 2 diabetes, as supported by different studies [13, 14]. Therefore, enhancing cardio-metabolic risk factors with sustained exercise modalities can lead to better health outcomes [15, 16]. Regular physical exercise enhances IR and glycolipid metabolism, which lowers the problems linked to obesity [17]. The current guidelines for managing adult obesity state that improving cardiometabolic health need regular multimodal exercise [18].
Extensive research has been conducted on the effects of aerobic training modalities of exercise on various health outcomes [13, 19, 20]. For clinically successful weight reduction that aims to enhance many cardiometabolic health indices, regular endurance training is essential [21, 22]. During aerobic exercise workouts, the body burns fat for most of its energy and becomes better at breaking down fat stores (lipolysis) [23, 24]. National guidelines on physical activity and recommendations for public health were released by the American College of Sports Medicine and the Centers for Disease Control and Prevention. All healthy individuals between the ages of 18 and 65 must engage in moderate-intensity aerobic (endurance) physical activity for at least 150 min per week or vigorous-intensity aerobic physical activity for at least 75 min per week in order to promote and maintain their health [25].
Resistance training has been demonstrated to increase the body's consumption of glucose by inducing a hypertrophy response and a type shift in the muscular fibers of exercised muscles [26]. It can help obese people manage their weight [27] and become more insulin-sensitive, manage lipid profile and glycemic control in obese adults [28, 29]. High-intensity resistance training burns mostly carbohydrates for immediate energy [24]. It also triggers the release of hormones such as growth hormone and testosterone [30]. By influencing the body's chemistry, hormones promote muscle growth and make it easier to access the body's ability to burn glucose derived from fat stores (lipolysis) [31]. Increased overall muscle mass, an increase in the number of insulin receptors in muscle cells, and an increase in the number of glucose transporter (GLUT) proteins are some probable underlying mechanisms for the beneficial effects of resistance (strength) training [26]. The combined exercise program had a positive impact on body mass and composition as well as enhanced glucose and lipid metabolism [21, 32], which includes both aerobic and resistance exercise within a single session. Research has confirmed the benefits of combined training in improving glycemic management and lipid parameter health in obese adults [21, 33, 34]. Current ADA guidelines advocate a combination of aerobic and resistance training, which may be the most beneficial exercise modality for regulating lipid profile and glucose levels [35].
Regardless of the type or intensity of exercise, hormonal changes affecting blood lipid levels are not significantly impacted when individuals burn the same amount of calories [36]. To accurately assess the impact of exercise programs on health, participants need to follow a strict dietary monitoring protocol [37]. This involved carefully tracking the participants' food intake, often through a food frequency questionnaire, to ensure that any observed changes were primarily due to the exercise intervention itself and not influenced by significant shifts in their dietary habits [38].
There is a lack of research evaluating the effects of resistance and aerobic exercise on the risk factors associated with glycolipids profile, and even fewer have studied the two types of training separately or in combination. Further studies are required to better understand how various exercise modalities affect these risk variables while controlling dietary practices, given the rising burden of chronic disease. Hence, the objective of this study was to assess and compare the efficacy of various exercise modes (AT, RT, and CT exercise) and changes over time in enhancing lipid profile changes and glucose tolerance among adults who are obese.
Methods and materials
This study is reported following the CONSORT guidelines.
Research setting and design
This research combined pretest posttest with a repeated measures design component to capture within-subject changes over time with a randomized parallel group experimental design to assess between-group differences.
The study involved physically inactive male persons between the ages of 45 and 60 years who had a BMI greater than 24.9 kg/m2. The participants were selected from a volunteer pool of inactive residents of Debre Markos town, Ethiopia and were informed through local radio and notice board postings.
The inclusion criteria were as follows: (a) had a BMI > 24.9 kg/m2, (b) were aged between 45 and 60 years, (c) volunteered to participate, (d) were physically inactive (not achieving 30–60 min per day or 150 min per week of moderate intensity exercise or 20–60 min per day (75 min per week) of vigorous intensity [39] and cleared a medical history form the physical activity readiness questionnaire, and (d) were able to perform the necessary exercises. The exclusion criteria were as follows: (a) any cardiovascular, respiratory, or muscle-skeletal disorders precluding physical exercise; (b) uncontrolled hyperglycemia (≥ 126 mg/d) or hypertension (a resting blood pressure ≥ 140/100 mm Hg); and (c) active infection, (d) acute myocardial infarction, stroke, trauma, surgery or severe liver dysfunction.
The ideal sample size for our investigation was determined by using the G*Power program. We determined a sample size of 21 people, as described by Sousa et al., taking into account zero correlation among each measure TGC, Total-c, LDL-c, and HDL-c in the AT [40], a significance level (α) of 0.05, and a power of 0.8. An overall sample size of 24 was needed, accounting for a 10% nonresponse rate. Our study's intended statistical power and significance level were achieved by successfully detecting the given effect size with this sample size.
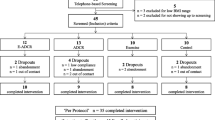
The current investigation recruited and registered a total of 36 male overweight and obese individuals. After applying the inclusion criteria, 32 out of the 36 volunteers who we had initially registered as physically inactive remained. The researchers were created a final study group consisting of 24 participants via a simple random selection procedure. After that, these people were randomized at random to one of three exercise groups (each with eight participants): combined training, resistance training, or aerobic training. The study was conducted in Debere Markos, Ethiopia, using homogeneous samples and balanced randomization (1:1:1) (Fig. 1). The data collectors were blinded to one another during the study.
Before obtaining their informed consent, participants were completely told about all procedures, hazards and protocols to ensure ethical conduct. This satisfied the requirements set forth by the American College of Sports Medicine [39]. Additionally, the Sport Academy research ethics committee at Debre Markos University reviewed and provided input on all procedures involving human subjects. Lastly, the study was carried out in accordance with the ethical standards provided in the 2000 revision of the Declaration of Helsinki.
The first data were collected prior to the intervention, while the second data were collected at the end of the 8-week intervention. The data were collected at the Debre Markos referral hospital. We measured lipid profiles (TC, HDL and LDL) and glucose tolerance as primary outcomes and body fat percentage as a secondary outcome. The periods of follow-up (intervention) were from April 20/ 2022 to June 2022.
Measurement of study variables
Oral Glucose Tolerance Test (OGTT)
The oral glucose tolerance test (OGTT) measures how well the body can breakdown and use sugar (glucose) as well as clear it from the blood stream [41]. The glucose AUC (area under the curve) is a measure used in glucose tolerance tests to quantify the body's response to glucose over a specified period. It represents the total exposure to glucose following an intervention, like consuming a glucose load. AUC is calculated by plotting blood glucose levels over time and measuring the area under this curve [42]. After an overnight fast, blood samples for determination of glucose concentrations were taken at 0, 30, 60, and 120 min after a standard 75 g of oral glucose dissolved in 300 ml of water was given orally, and the blood samples were allowed to drink within 5 min [43]. Blood samples were taken 48 h after the previous training session and after a 12-h fast. After being drawn, blood samples were centrifuged and kept at -80 °C for 30 min. Glucose was analyzed by the hexokinase method (COBAS, Roche), and its intracoefficient of variation ranged between 1.58% (µ = 64.7 mg/dl) and 1.38% (µ = 369 mg/dl). The area under the curve of glucose was calculated using the trapezoidal rule and compared. Plasma glucose (PG-AUCs) were calculated by trapezoidal approximation of PG levels [44]. PG levels at x min were defined as PG(x), and the PG-AUC was calculated as follows:
Lipid profile
After a 12-h fasting period, 5 ml of blood was collected from the left median cubital vein of a seated individual at 8:00 AM. Following immediate centrifugation and refrigeration, the samples were analyzed within 24 h. An enzymatic method utilizing an Alpha X autoanalyzer with E2HL-100 kits and a sensitivity of 0.1 mmol/dL (Hitachi, Tokyo, Japan) was employed for lipid measurement.
Body fat percentage
Abdominal, thigh, suprailiac and triceps skinfolds were measured on the right side of the body to the nearest 0.5 mm with a Lange caliper (Cambridge Scientific Instruments, Cambridge, MD, USA). All skinfolds were measured by the same technician. A reliability criterion of 2 mm was established for triplicate measurements, and the mean of these measurements was used for analytical purposes. To determine body fat percentage from skinfold measurements, we initially computed body density values using an equation specifically designed for older adults [45].
Body density = (0.29288 × sum of all the skinfolds) – (0.0005 × sum of all of the skinfolds squared) + (0.15845 × age) – 5.76377.
The body density equations were converted into fat percentage by using the Siri equation: %BF = ((4.95/D) – 4.50) × 100 for the purpose of the analysis [46].
Average daily energy intake
We utilized a 24-h interactive questionnaire with several passes that was developed and validated for use in developing countries. Instruments for measuring food frequency were adapted from Regassa IF, et al.,(2021) [47] The three 24-h sessions were held on Monday, Wednesday and Saturday to capture variation in intakes throughout different days of the week. We applied the Ethiopian food composition table to estimate nutrient and energy levels from dietary data. The names of foods and drinks, their descriptions, cooking methods, and amounts from both 24-h periods were coded and submitted to the NutriSurvey200 [48]. After determining the frequency of consumption per day, we used the product sum approach to determine daily food intake. Daily food intake = ∑ (food item's stated consumption frequency, translated to times per day) * (portion size ingested of that food). The daily average energy intake was also determined as follows: ADEi = ∑daily food intake/number of data collected days.
Exercise intervention protocol
The individuals in the training groups were assigned to aerobic training (AT), resistance training (RT), or combined training (CT). The exercise regimens involved three weekly sessions over a period of eight weeks, with each session lasting 60 min. During each exercise session, participants engaged in a 5 to 10 min warm-up, followed by 30 to 40 min of main training. Finally, they concluded with 5 to 10 min of cool-down. The resistance training (RT) program consisted of six exercises per session, specifically targeting the major muscle groups of the body. The exercises were performed standing plantar flexion, squatting, machine leg press, neutral reowing, bicep curl, triceps pulley, dumbbell curl, and vertical bench press. The exercise routine involved three sets per day, with 8 to 12 repetitions at an intensity of 50% to 75% of their one-repetition maximum (1RM). The remaining intervals between each set and between training sessions were approximately 1 to 1.5 min and 48 to 72 h, respectively [49]. The aerobic exercise involved using a treadmill at an intensity level ranging from 50 to 75% of the maximum heart rate (HR max). The goal was to burn approximately 500 cal per session [50]. The combined exercise group (CT) followed a training program that included the same amount of exercises as the aerobic group (AT) and the resistance group (RT), which performed three exercises per session.
In each session, participants performed endurance exercises before moving on to strength exercises. Details of the general training intervention approach are outlined in Table 1. This specific exercise order was selected to explore the impact of aerobic training preceding strength training [51]. To minimize potential confounding factors, participants were explicitly advised not to engage in any additional resistance-type or aerobic training throughout the duration of the study.
Statistical analysis
The data were analyzed using SPSS version 26 (SPSS Inc., Chicago, IL). A paired t test was used to examine the differences in the baseline and follow-up variables within the group because within-subject information in the RM-ANCOVA output was inaccurate [52]. The researchers ensured reliable results by applying a Bonferroni correction for multiple comparisons in their RM-ANCOVA, with average daily energy intake as the covariate, which was performed for HDL, LDL, TC, AUC and %BF. Changes over time were compared among participants, while exercise types were compared among groups. Interactions between these factors were also investigated. All the statistical tests were two-tailed, and a p value of 0.05 or less was considered to indicate statistical significance.
Results
Table 2 summarizes the descriptive data characteristics at baseline and the adjusted absolute changes in %BF, AUC, HDL, LDL and TC levels during the study period. Four participants were dropped from the experiment due to exercise-induced injuries, parting a total of 20 participants who finished the study and were included in the analysis, 7 in the AT, 6 in the RT and 7 in the CT.
The average ages of the participants in the respective groups were AT = 49.00 ± 2.08, RT = 49.83 ± 3.06 and CT = 48.71 ± 2.87. The average body mass index of participant in AT, RT and CT group were 27.84 ± 1.10, 27.45 ± 0.74 and 27.70 ± 0.85 respectively. The results revealed no significant differences in any of the variables among the three groups in the pretest, suggesting successful randomization of the study participants.
However, there were significant differences in the %BF, AUC, HDL, LDL and TC between the pretest and posttest after 8 weeks of intervention in all three groups. The results indicated that after they received the aerobic training, body fat percentage in the aerobic training group was t (6) = 9.306, p < 0.01; in the RT group, t (5) = 11.158, p < 0.01; and in the CT group, t (6) = 8.294, p < 0.01. The area under the curve for the older adults’ means for the pretest and posttest results differed significantly between the aerobic training groups: t (6) = 7.054, p < 0.001; RT: t (5) = 11.904, p < 0.001; and CT: t (6) = 9.56, p < 0.001. We also found that high-density lipoprotein levels significantly improved between the pretest and posttest scores in the aerobic training group. This improvement was observed for AT (P < 0.012), RT (P < 0.013) and CT (P < 0.001). The study additionally demonstrated a statistically significant improvement in AT (t (6) = 33.806, p < 0.001), RT (t (6) = 12.504, p < 0.001) and CT (t (6) = 10.405, p < 0.001) in low-density lipoprotein (LDL) cholesterol levels between the pretest and posttest within the aerobic training group. In all three groups, the participants' total cholesterol levels decreased significantly after the training compared to before they started, as shown in Table 3.
Table 4 shows that the main effect of exercise modality on the AUC was significant, F (2, 16) = 10.577, P = 0.001, η2 = 0.569. Post hoc analyses using the Bonferroni post hoc criterion for significance indicated that the area under the curve from the OGTT was significantly lower for RT (-30.653 ± 6.766, p = 0.001) with 11.53% and CT (M = -0.896, SE = 3.347, P = 0.015) with 3.79% exhibited greater significant reduction compared to AT.
Low-density lipoprotein F (2, 26) = 6.33, p = 0.009, η2 = 0.442 parameters were significantly lower in the AT by 3.7% and CT by 3.79% groups (MD = 4.783, SE = 1.563, P = 0.002 and M = 4.57, SE = 1.284, P = 0.008) respectively than in the RT group. There was a significant difference in total cholesterol between the training modalities, F (2, 16) = 4.849, P = 0.023, η2 = 0.377. Interns with independent variables AT showed a significant reduction in RT (MD = -17.716, SE = 5.705, P = 0.02) representing 7.97% reduction. Despite observing a significant difference between the pretest and posttest results for high-density lipoprotein (HDL) and percentage of body fat (%BF), there was no significant difference attributable to the independent variables affecting these variables.
There was a significant group × time interaction for the area under the curve from the OGTT test results (F (2, 16) = 9.002, p = 0.002), η2 = 0.530, high-density lipoprotein: F (2, 16) = 4.064, P = 0.037, η2 = 0.337, TC: F (2, 16) = 15.075, P < 0.001, η2 = 0.653), and no significant interaction for body fat percentage and high-density lipoprotein was detected. The results suggest that time had a different effect on the AUC, LDL and TC depending on the group, while the effect of time on %BF and HDL was the same across all groups.
Discussion
Over an intervention time of eight weeks, exercise emerged as a potent metabolic health intervention for obese older adult men. This program resulted in a remarkable decrease in body fat percentage and the levels of both “bad” cholesterol (LDL and TC). Simultaneously, it enhances the body’s capacity to regulate blood sugar (glucose tolerance). Intriguingly, throughout the program, “good” cholesterol (HDL) levels improved while average daily energy intake was controlled. These findings align with studies involving longer exercise durations, such as the 32-week program described [40].
These findings have established a physiological foundation supporting the hypothesis that resistance training may elicit favorable alterations in glucose tolerance. Similarly, other investigators have reported that strength training also reduces basal and glucose-stimulated insulin levels [53, 54]. In contrast, both the aerobic training (AT) and resistance training (RT) groups demonstrated improvements in glucose tolerance (Smutok et al., 1994).
Our analysis indicated that compared with RE, AE and concurrent training are the most effective exercise modalities for reducing LDL and TC in older adults. The present study implemented an 8-month aerobic exercise intervention involving 111 randomly selected participants. The results indicated that the exercise group experienced a significant reduction in total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels among centrally obese older adults [55]. A similar study involving sedentary older adults who participated in a 16-week exercise intervention was performed. Similarly, studies on the effects of different exercise modalities on lipid metabolism in individuals with concurrent type 2 diabetes and obesity According to a narrative review, regular exercise helps to raise HDL-C levels [56] while lowering TG, TC, VLDL, and LDL-C at the same time [57]. The findings of the other comprehensive review revealed that there were no noteworthy variations in low-density lipoprotein cholesterol levels between the AEDT and ST groups [58]. A separate investigation revealed that exercise modality had no effect on blood lipids. Over time, the lipid levels were decreased in all groups. There was a considerable decrease in triglycerides, low- and high-density lipoprotein, and total cholesterol. The decline in all groups could [59]. This discrepancy could be attributed to inadequate energy expenditure during resistance exercise compared to aerobic exercise with low to moderate intensity [60].
The results of this study highlight that there were no significant differences in the effect on high-density lipoprotein (HDL) or percentage of body fat (%BF) levels among AT, RT and CT. These results align with the work of Moraleda et al., who similarly demonstrated that HDL cholesterol remained significantly unchanged across groups [61] and % BF [62]. In contrast, in a previous study, resistance exercise exhibited a more robust association with HDL levels than did aerobic exercise (β = 2.56210, p < 0.0001 vs. 1.33748, p < 0.0001) [63]. In contrast to our results, fat mass was reduced more in the AT and CT groups than in the RT group [62].
One limitation of this study is the absence of a non-intervention control group, which restricts the ability to fully attribute observed changes solely to the intervention. While the study was designed with random assignment and control of key confounding factors, the lack of a traditional control group limits the ability to isolate the effects of the intervention from other potential influences. Future research should consider incorporating a non-exercising control group to strengthen the validity of causal inferences and provide a more comprehensive understanding of the intervention’s impact. However, the observed differences in the effectiveness of each modality suggest that individualized exercise prescriptions could optimize outcomes, especially for older adults. Future studies should explore the long-term effects of these interventions and evaluate whether combining different modalities yields synergistic benefits. Additionally, more diverse participant groups and the inclusion of non-exercising control groups will be essential in validating these results and refining clinical guidelines.
Conclusion
Aerobic training (AT) and combined training (CT) were more effective at reducing low-density lipoprotein (LDL) and total cholesterol (TC) levels. Moreover, resistance training (RT) and combined training (CT) demonstrated greater efficacy than aerobic training (AT) in reducing glucose intolerance among previously inactive obese older adults. Interestingly, there was a significant change in the pretest and posttest levels of high-density lipoprotein (HDL) and percentage of body fat (%BF). However, when comparing aerobic training (AT), resistance training (RT), or a combination of both programs, there was no significant difference. Interestingly, the effect of time on AUC, LDL, and TC varied depending on the group, while the effect of time on %BF and HDL remained consistent across all groups.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to data security before publication but are available from the corresponding author on reasonable request.
References
Hordern MD, Dunstan DW, Prins JB, Baker MK, Singh MA, Coombes JS. Exercise prescription for patients with type 2 diabetes and pre-diabetes: a position statement from Exercise and Sport Science Australia. J Sci Med Sport. 2012;15(1):25–31.
Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133: 155217.
Lin X, Li H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front Endocrinol (Lausanne). 2021;12: 706978.
Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–98.
Okati-Aliabad H, Ansari-Moghaddam A, Kargar S, Jabbari N, Prevalence of obesity and overweight among adults in the middle east countries from,. to 2020: a systematic review and meta-analysis. Journal of Obesity. 2000;2022:2022.
Adeloye D, Ige-Elegbede JO, Ezejimofor M, Owolabi EO, Ezeigwe N, Omoyele C, et al. Estimating the prevalence of overweight and obesity in Nigeria in 2020: a systematic review and meta-analysis. Ann Med. 2021;53(1):495–507.
Kassie AM, Abate BB, Kassaw MW. Prevalence of overweight/obesity among the adult population in Ethiopia: a systematic review and meta-analysis. BMJ Open. 2020 Aug 6;10(8):e039200. doi: 10.1136/bmjopen-2020-039200. PMID: 32764091; PMCID: PMC7412611.
Ghodeshwar GK, Dube A, Khobragade D. Impact of Lifestyle Modifications on Cardiovascular Health: A Narrative Review. Cureus. 2023;15(7): e42616.
Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121(6):21–33.
De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, et al. European guidelines on cardiovascular disease prevention in clinical practice; third joint task force of European and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of eight societies and by invited experts). Eur J Prev Cardiol. 2003;10(4):S1–10.
Newsome ANM, Reed R, Sansone J, Batrakoulis A, Mcavoy C, Parrott MW. 2024 ACSM Worldwide Fitness Trends Future Directions of the Health and Fitness Industry. ACSM’s Health Fitness J. 2024;28(1):14–26.
Ekkekakis P. Let them roam free? Physiological and psychological evidence for the potential of self-selected exercise intensity in public health. Sports Med. 2009;39:857–88.
Kim K-B, Kim K, Kim C, Kang S-J, Kim HJ, Yoon S, et al. Effects of exercise on the body composition and lipid profile of individuals with obesity: a systematic review and meta-analysis. Journal of obesity & metabolic syndrome. 2019;28(4):278.
Solomon TP. Sources of inter-individual variability in the therapeutic response of blood glucose control to exercise in type 2 diabetes: going beyond exercise dose. Front Physiol. 2018;9:896.
Wewege MA, Thom JM, Rye K-A, Parmenter BJ. Aerobic, resistance or combined training: A systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis. 2018;274:162–71.
Lee D-c, Brellenthin AG, Lanningham-Foster LM, Kohut ML, Li Y. Aerobic, resistance, or combined exercise training and cardiovascular risk profile in overweight or obese adults: the CardioRACE trial. Eur Heart J. 2024;45(13):1127–42.
Liu Y, Liu SX, Cai Y, Xie KL, Zhang WL, Zheng F. Effects of combined aerobic and resistance training on the glycolipid metabolism and inflammation levels in type 2 diabetes mellitus. J Phys Ther Sci. 2015;27(7):2365–71.
Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J Nutr Health Aging. 2021;25(7):824–53.
Doewes RI, Gharibian G, Zaman BA, Akhavan-Sigari R. An updated systematic review on the effects of aerobic exercise on human blood lipid profile. Curr Probl Cardiol. 2023;48(5): 101108.
Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44:211–21.
Batrakoulis A, Jamurtas AZ, Metsios GS, Perivoliotis K, Liguori G, Feito Y, et al. Comparative Efficacy of 5 Exercise Types on Cardiometabolic Health in Overweight and Obese Adults: A Systematic Review and Network Meta-Analysis of 81 Randomized Controlled Trials. Circ Cardiovasc Qual Outcomes. 2022;15(6):e008243.
Guo J, Lou Y, Zhang X, Song Y. Effect of aerobic exercise training on cardiometabolic risk factors among professional athletes in the heaviest-weight class. Diabetol Metab Syndr. 2015;7(1):78.
Noland RC. Exercise and regulation of lipid metabolism. Prog Mol Biol Transl Sci. 2015;135:39–74.
Brown RC. Nutrition for optimal performance during exercise: carbohydrate and fat. Curr Sports Med Rep. 2002;1(4):222–9.
Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–34.
Schoenfeld BJ. Potential Mechanisms for a Role of Metabolic Stress in Hypertrophic Adaptations to Resistance Training. Sports Med. 2013;43(3):179–94.
Sword, David O. PT, DPT, CSCS. Exercise as a Management Strategy for the Overweight and Obese: Where Does Resistance Exercise Fit in?. Strength Cond J. 2012;34(5):47–55. https://doi.org/10.1519/SSC.0b013e31826d9403.
Ismail AD, Alkhayl FFA, Wilson J, Johnston L, Gill JMR, Gray SR. The effect of short-duration resistance training on insulin sensitivity and muscle adaptations in overweight men. Exp Physiol. 2019;104(4):540–5.
Tomeleri CM, Ribeiro AS, Souza MF, Schiavoni D, Schoenfeld BJ, Venturini D, et al. Resistance training improves inflammatory level, lipid and glycemic profiles in obese older women: A randomized controlled trial. Exp Gerontol. 2016;84:80–7.
Kraemer WJ, Ratamess NA, Hymer WC, Nindl BC, Fragala MS. Growth hormone (s), testosterone, insulin-like growth factors, and cortisol: roles and integration for cellular development and growth with exercise. Front Endocrinol. 2020;11: 513110.
Loucks AB, Caiozzo MJ. The endocrine system: integrated influences on metabolism, growth, and reproduction. Acsm’s advanced exercise physiology. 2nd ed. Philadelphia (PA): Wolters Kluwer Lippincott Williams & Wilkins; 2012, p 466–506.
Śliwicka E, Popierz-Rydlewska N, Straburzyńska-Lupa A, Nikolov J, Pilaczyńska-Szcześniak Ł, Gogojewicz A. Prevention Is Better than Cure-Body Composition and Glycolipid Metabolism after a 24-Week Physical Activity Program without Nutritional Intervention in Healthy Sedentary Women. Nutrients. (2024);16(15):2536. https://doi.org/10.3390/nu16152536.
Azarbayjani MA, Abedi B, Peeri M, Rasaee MJ, Stannard SR. Effects of combined aerobic and resistant training on lipid profile and glycemic control in sedentary men. Int Med J. 2014;21(2):132–6.
Amaro-Gahete FJ, Ponce-González JG, Corral-Pérez J, Velázquez-Díaz D, Lavie CJ, Jiménez-Pavón D. Effect of a 12-Week Concurrent Training Intervention on Cardiometabolic Health in Obese Men: A Pilot Study. Front Physiol. 2021;12: 630831.
Vanhees L, Geladas N, Hansen D, Kouidi E, Niebauer J, Reiner Ž, et al. Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular risk factors: recommendations from the EACPR (Part II). Eur J Prev Cardiol. 2011;19(5):1005–33.
Crouse SF, O’Brien BC, Grandjean PW, Lowe RC, Rohack JJ, Green JS, et al. Training intensity, blood lipids, and apolipoproteins in men with high cholesterol. J Appl Physiol. 1997;82(1):270–7.
Matthews CE, Hagströmer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68.
Beck KL, Thomson JS, Swift RJ, von Hurst PR. Role of nutrition in performance enhancement and postexercise recovery. Open Access J Sports Med. 2015;6:259–67.
Ferguson B. ACSM’s Guidelines for Exercise Testing and Prescription 9th Ed. 2014. J Can Chiropr Assoc. 2014;58(3):328.
Sousa N, Mendes R, Abrantes C, Sampaio J, Oliveira J. A randomized study on lipids response to different exercise programs in overweight older men. Int J Sports Med. 2014;28:1106–11.
Bano G. Glucose homeostasis, obesity and diabetes. Best Pract Res Clin Obstet Gynaecol. 2013;27(5):715–26.
Sakaguchi K, Takeda K, Maeda M, Ogawa W, Sato T, Okada S, et al. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol Int. 2016;7(1):53–8.
Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults—a randomized clinical trial. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2009;64(1):90–5.
Sakaguchi K, Takeda K, Maeda M, Ogawa W, Sato T, Okada S, et al. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol Int. 2016;7(1):53–8.
Nevill AM, Metsios GS, Jackson AS, Wang J, Thornton J, Gallagher D. Can we use the Jackson and Pollock equations to predict body density/fat of obese individuals in the 21st century? Int J Body Compos Res. 2008;6(3):114–21.
Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition. 1993;9(5):480–91 discussion , 92.
Regassa IF, Endris BS, Habtemariam E, Hassen HY, Ghebreyesus SH. Development and validation of food frequency questionnaire for food and nutrient intakes of adults in Butajira. Southern Ethiopia Journal of Nutritional Science. 2021;10: e98.
Regassa IF, Endris BS, Habtemariam E, Hassen HY, Ghebreyesus SH. Development and validation of food frequency questionnaire for food and nutrient intakes of adults in Butajira, Southern Ethiopia. J Nutr Sci. 2021;10:e98. https://doi.org/10.1017/jns.2021.94.
Soori R, Ravasi A, Ranjbar K. The comparison of between endurance and resistance training on vaspin and adiponectin in obese middle-age men. Sport Physiology. 2014;5(20):97–114.
Asad M. Effect of 8 weeks aerobic, resistance and concurrent training on cholestrol, LDL, HDL and cardiovascular fitness in obesity male. Applied Research in Sport Management. 2013;1(3):57–64.
Lepers R, Millet GY, Maffiuletti NA. Effect of cycling cadence on contractile and neural properties of knee extensors. Med Sci Sports Exerc. 2001;33(11):1882–8.
Schneider BA, Avivi-Reich M, Mozuraitis M. A cautionary note on the use of the Analysis of Covariance (ANCOVA) in classification designs with and without within-subject factors. Front Psychol. 2015;6: 135022.
Craig B, Everhart J, Brown R. The influence of high-resistance training on glucose tolerance in young and elderly subjects. Mech Ageing Dev. 1989;49(2):147–57.
Miller W, Sherman W, Ivy J. Effect of strength training on glucose tolerance and post-glucose insulin response. Med Sci Sports Exerc. 1984;16(6):539–43.
Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(19):1483–92.
Badri Al-mhanna S, Leão C, Wan Ghazali WS, Mohamed M, Batrakoulis A, Afolabi H, Abubakar B, Aldhahi M, Gülü M, Yagin FH, Nikolaidis P. Impact of Exercise on High-Density Lipoprotein Cholesterol in Adults with Overweight and Obesity: A Narrative Review. Ann Appl Sport Sci. 2024;12:e1300. https://doi.org/10.61186/aassjournal.1300.
Badri Al-mhanna S, Syaheedah W, Ghazali W, Batrakoulis A, Alkhamees N, Drenowatz C, Mohamed M, Gülü M, Afolabi H, Badicu G. (2024). Impact of Various Types of Exercise on Lipid Metabolism in Patients with Type 2 Diabetes and Concurrent Overweight/Obesity: A Narrative Review. Ann Appl Sport Sci. 2024;12:e1324. https://doi.org/10.61186/aassjournal.1324.
Al-Mhanna SB, Rocha-Rodriguesc S, Mohamed M, Batrakoulis A, Aldhahi MI, Afolabi HA, et al. Effects of combined aerobic exercise and diet on cardiometabolic health in patients with obesity and type 2 diabetes: a systematic review and meta-analysis. BMC Sports Sci Med Rehabil. 2023;15(1):165.
Boardley D, Fahlman M, Topp R, Morgan AL, McNevin N. The Impact of Exercise Training on Blood Lipids in Older Adults. Am J Geriatr Cardiol. 2007;16(1):30–5.
Reis VM, Garrido ND, Vianna J, Sousa AC, Alves JV, Marques MC. Energy cost of isolated resistance exercises across low-to high-intensities. PLoS ONE. 2017;12(7): e0181311.
Moraleda BR, Morencos E, Peinado AB, Bermejo L, Candela CG, Benito PJ, et al. Can the exercise mode determine lipid profile improvements in obese patients? Nutr Hosp. 2013;28(3):607–17.
Willis LH, Slentz CA, Bateman LA, Shields AT, Piner LW, Bales CW, et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol. 2012;113(12):1831–7.
Hsu C-S, Chang S-T, Nfor ON, Lee K-J, Lee S-S, Liaw Y-P. Effects of regular aerobic exercise and resistance training on high-density lipoprotein cholesterol levels in Taiwanese adults. Int J Environ Res Public Health. 2019;16(11):2003.
Acknowledgements
The authors would like to express their gratitude to the Debere Markos University for its fund for our research and gym manager and Servicemen for their permission, Debre Markos Referral Hospital as well as to the participants, without whose cooperation this study could not have been completed.
Adverse effect report
No adverse effects were recorded in participants during and after the intervention.
Funding
The Debre Markos University Annual Research Funding provided assistance for this work. The financial funding from Debre Markos University has been crucial in supporting the research efforts for the project. The funding body did not participate in the study's design, data collection, analysis, or interpretation, or manuscript writing.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to this work. Friew Amare conceived and designed the study methodology. Yehualaw Alemu, solomon Adamu and mollalign Enchalew involved in intervention performed the investigation. Friew and Yalemsew conducted the formal analysis. Friew and Yehualaw drafted the original manuscript. All authors participated in reviewing and editing the manuscript. All authors oversaw the project and managed project administration. All authors meet the criteria for authorship by substantially contributing to the research, manuscript preparation, and approval for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trial was approved by the Ethics Review Board of Debre Markos University of Sport Sciences Academy (Reference number: SPSC 05/22). All the participants were informed about the intervention and possible adverse events before the commencement of the trial and signed an informed consent form.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Amare, F., Alemu, Y., Enichalew, M. et al. Effects of aerobic, resistance, and combined exercise training on body fat and glucolipid metabolism in inactive middle-aged adults with overweight or obesity: a randomized trial. BMC Sports Sci Med Rehabil 16, 189 (2024). https://doi.org/10.1186/s13102-024-00982-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13102-024-00982-7