Abstract
Bioethanol production from agro-food industry waste is one of the suitable alternatives to fossil fuels. In this study, six agro-food wastes were evaluated to select those with a high concentration of starch or fermentable sugars for bioethanol processing. Yeast’s ability to produce biofuels by fermentation is affected by temperature. In this study, thermotolerant yeasts, obtained from natural sources in Algeria (soil and agro-food waste), were selected for their capacity to produce and tolerate bioethanol. Three wastes were selected, two of which had significant starch content. In order to get fermentable sugars, these wastes underwent chemical and enzymatic hydrolysis. The selected yeasts were identified by morphological, physiological, biochemical, and molecular characterization. Enzymatic and acid hydrolysis of whole potato and durum wheat bran released (190 g/L and 130 g/L) and (138.40 g/L and 90.03 g/L) reducing sugars, respectively. Among the isolated strains, three were found to be able to produce bioethanol, namely Candida tropicalis, Candida glabrata, and Saccharomyces cerevisiae. These strains were identical to those stored in the data bank with 99%, 100%, and 100%, respectively. In addition, C. glabrata and C. tropicalis exhibited an ethanol tolerance of up to 14%, while S. cerevisiae tolerates up to 15%. Interestingly, enzymatic hydrolysis–treated potatoes produced a considerable amount of bioethanol after 48 h of fermentation by S. cerevisiae (7.525% (v/v)), C. glabrata (6.80% (v/v)), and C. tropicalis (4.50% (v/v)). Taken together, our findings suggest that S. cerevisiae and whole potato waste could be considered good candidates for industrial bioethanol production at high temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
World demand for energy is continuing to rise significantly as a result of the rapid growth in the human population, the expansion of agriculture and industry, and the growth of the transportation industry. Thus, the increment in the consumption of fuels derived from petrochemicals is becoming a serious problem, particularly in terms of air pollution, which has an impact on both human health and global warming. Therefore, it is deemed necessary to find alternatives for these fuels. In this context, producing energy from agricultural and agro-industrial activity wastes could be a potential solution. Indeed, these wastes are rich in renewable organic matter (biomass), and they make it possible to reduce dependence on oil and thus the environment pollution [1]. In light of all this, particular attention must be given to the better management of organic waste and particularly the by-products from agricultural residues. Hence, their use as a raw material for bioenergy production could make a good alternative to fossil fuels. Bioethanol can be produced by renewable natural resources such as plants containing sucrose (beets, sugar cane, etc.) or starch (wheat, corn, potatoes, etc.); accordingly, it represents a promising source of energy. The production of biofuel is thought to be influenced by temperature, one of the factors that greatly affect yeast fermentation ability. The temperature in Algeria rises considerably and tends to increase with global warming, especially during the summer when average temperatures vary between 32 and 44 °C [2]. Therefore, the selection of thermotolerant yeast strains is crucial to deal with climate change. Importantly, high-temperature fermentation has several benefits, such as lowering the cost of tank cooling, obtaining higher saccharification yields, and minimizing bacterial contamination [3]. Heretofore, plant crops were devoted to produce ethanol, which has drawn criticism because it competes with its use for human and animal food in countries such as the USA and Brazil [4]. The production of bioethanol from sources other than those used for human or animal consumption has attracted considerable interest in scientific studies [3, 5, 6]. According to Tse et al. [7], agricultural and agro-food industry wastes present an opportunity to produce ethanol due to the abundant and the available supplies, biodegradability, high carbon and nutrient content, and their utility in managing the economic and the environmental issues of the wastes from industry [5]. In Algeria, food industries have generated a huge amount of starchy wastes such as durum wheat bran, potato, rinse water, cutting water, quenching water, and process water. These wastes are produced annually at 60 million tons, 10 million tons, 3.8 million m3, 3.3 million m3, 1.5 million m3, and 58,000 m3, respectively. In this study, we sought to isolate and identify locally thermotolerant yeast strains from the soil of Bechar region as well as agro-food wastes. Moreover, we aimed to examine their ethanolic fermentation on some selected wastes at high temperature, in order to contribute to the recovery of agro-food wastes.
2 Materials and methods
2.1 Agro-food waste selection
There are several raw materials used for the production of bioethanol. In this study, six wastes were chosen based on their abundance and availability (Table 1). Except for whole potatoes provided by Chipsou factory in Rouiba, Algeria, all other waste products were used directly. The potatoes were cleaned using tap water and sliced into small pieces measuring 2 cm in length, 2 cm in width, and 4 mm in thickness using an electric slicer. The slices were dried in an oven for 9–10 h at a temperature of 60 °C. The resulting powder was obtained through grinding and sieving with a 500-μm sieve. Finally, the powder was packaged in polyethylene bags and stored in a refrigerator at 4 °C until it was analyzed.
2.2 Physicochemical and biochemical analysis of agro-food wastes
The physicochemical properties of agro-food wastes were analyzed to assess their potential for bioethanol production and biotransformation. AFNOR methods were used to measure the pH, total solids, ash, and fat contents of all the collected agro-food wastes [8]. The electrical conductivity at 20 °C was measured using a Jenway 4520 conductivity meter, as stated by Rodier [9], while total nitrogen and protein amounts were determined using the Kjeldahl method [8]. Also, the estimation of reducing sugars was conducted employing the 3,5-dinitrosalycilic acid (DNS) method, which was introduced by Miller [10]. In addition, glucose was measured enzymatically through the glucose oxidase–peroxidase GODPOD method developed by Trinder [11]. The starch content was carried out using a colorimetric method described by Uarrota et al. [12] with some modifications.
Three milliliters of the starch solution was placed in a test tube, and 1 mL of an I/KI solution was added. The resulting mixture was then assessed for the intensity of its blue color using a spectrophotometer set at 600 nm against a reagent blank; the amount of starch present in the sample was calculated according to a standard curve prepared in the range of 10–50 mg of soluble starch per milliliter (mL), treated in the same manner as the test sample. Additionally, to investigate the chemical oxygen demand (COD) of both liquid and solid samples, the protocols recommended by Rodier [9] and Noguerol–Arias et al. [13] were followed, respectively. Biological oxygen demand after 5 days (BOD5) of both liquid and solid samples was performed using methods described by Rodier [9] and Kolář et al. [14], respectively. Mineral salts such as Na+, Ca2+, K+, Zn2+, and Mg2+ were quantified using atomic absorption spectrophotometry [8]. The phosphorus was measured by the colorimetric method [15], and chlorides were evaluated by titrimetry [8].
2.3 Isolation, selection, and identification of thermotolerant yeast strains producing bioethanol
2.3.1 Isolation of the thermotolerant yeasts
Various yeast strains were obtained from diverse sources, including soil and agro-food waste. Soil samples were gathered from two distinct regions, namely Bechar and Jijel, while agro-food waste samples were obtained from two local factories, which were TAFNA starch plant (Maghnia, Algeria) and EURL MARAVILLA (Chipsou) (Rouiba, Algeria). Approximately 100 g of soil was placed in a sterile plastic bag, while liquid waste was put into plastic bottles. The solid sample mainly potato was crushed and homogenized in a sterile mortar. One gram of substrate was dissolved in a sterile sodium citrate solution (2% w/v) previously heated to 45 °C in order to soften it, dissolve its constituents, and release the microbial cells [16]. The yeasts were isolated on yeast peptone glucose (YPG) agar medium containing 10 g/L of yeast extract, 5 g/L peptone, 20 g/L glucose, 20 g/L agar, and 0.05 g/L chloramphenicol. The different cultures were isolated by conventional methods of decimal dilutions [17]. The inoculation was done on the surface by spreading 0.1 mL of each dilution in transverse streaks. The dishes were then incubated at 37 °C for 72 h, and pure cultures were stored at 4 °C.
2.3.2 The selection of thermotolerant yeasts
The isolated yeast strains were purified and selected based on their ability to produce and tolerate high concentrations of bioethanol. A colony from a Petri dish was transferred to glass tubes containing 10 mL of YPG broth and incubated at 37 °C for 24 h. Five milliliters of the obtained culture was transferred into a 100-mL Erlenmeyer flask containing 20 mL of YPG broth and was further incubated at 37 °C for 18 h with stirring at 150 rpm [18]. The resulting active cells were centrifuged at 8000 rpm for 10 min, rinsed twice with sterile distilled water, and served as inoculum. The alcoholic fermentation was carried out in 250-mL Erlenmeyer flasks containing 100 mL of sterile synthetic YPG broth at pH 5 and was incubated at 37 °C for 48 h with the agitation of 150 rpm. The effects of temperature (37 °C, 40 °C, and 45 °C), pH (4, 5, and 6), and substrate concentration (glucose) (10%, 15%, 20%, 25%, and 30% w/v) were studied on bioethanol production. The ethanol content was determined at the end of each fermentation.
2.3.3 Ethanol tolerance test
Yeast suspensions were transferred into glass tubes containing 10 mL of YGP broth mixed with a variable volume of absolute ethanol in order to obtain different concentrations (0%, 5%, 10%, 11%, 12%, 13%, 14%, 15%, 16%, and 20% (v/v)) [6, 19, 20]. The tubes were then incubated at 37 °C for 48 h. The cell concentration in each tube was measured at the beginning and the end of fermentation by determining the optical density at 600 nm, which is proportional to the number of cells [21].
2.3.4 Identification of strains producing bioethanol
The yeast strains were identified through conventional techniques outlined by Kurtzman and Fell [22] according to their morphological, physiological, and biochemical characteristics. The cell morphology is examined using an optical microscope (MOTIC B3-220-PL). After incubation of yeasts for 48 h on YPG agar medium at 37 °C, a single colony of yeast was mixed in a droplet of sterile distilled water on glass slide and smeared until the smear dry off. The smear was then stained using diluted methylene blue dye, air dried, and observed under light microscope at magnification × 100. Additionally, White et al.’s [23] molecular approach was employed for further identification. The QIA fast R genomic extraction kit was firstly used to extract DNA from the isolated yeast strains. Then, polymerase chain reaction (PCR) was performed for the internal transcribed spacer (ITS) region of 26S rDNA using the following primers: ITS1 5′-TCCGTAGGTGAACCTGCGG-3′ and ITS4 5′-TCCTCCGCTTATTGATATGC-3′. The D1/D2 domain of the 26S rDNA was also amplified by PCR using the following primers: LROR 5′ACCCGCTGAACTTAAGC3′ and LR6 5′CGCCAGTTCTGCTTACC3′. The amplification of DNA and sequencing were done in a final volume of 50 μL reaction volume (10 mM Tris HCl, 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTPs, 1.25 IU Taq polymerase, 0.2 mM each primer, and 0.2 μL of DNA). The PCR was conducted in a DNA thermal cycler (Biometra, Germany). The reaction underwent 35 cycles, and the resulting products, along with their restriction fragments, were detected by electrophoresis on a 1.5% agarose gel. Furthermore, the obtained sequences were matched with those available in the National Center for Biotechnology and Information’s (NCBI) GenBank using the Basic Local Alignment Search Tool (BLAST). The highest alignment score was then used to determine the species. Additionally, the neighbor-joining method was used to construct a phylogenetic tree, utilizing Mega 6 software, as described by Altschul et al. [24].
2.4 Production of bioethanol on selected agro-food wastes
Three agro-food wastes were selected for bioethanol production based on their sugar content. Except quenching water, the potato powder and the durum wheat bran require a pre-treatment by hydrolysis of starch to fermentable sugar mainly glucose which will be fermented into ethanol by alcoholic fermentation.
2.4.1 Starch hydrolysis in selected agro-food wastes
The hydrolysis of agro-food waste was done by acid and enzymatic hydrolysis. The waste acid hydrolysis was performed as described by Taibi et al. [25] with some adjustments. Fifty grams of both potato powder and durum wheat bran was suspended at 35% (w/v) of hydrochloric acid solution (1 M HCl) which was heated at 100 °C for 2 h. After cooling in the open air, the substrate was recovered by filtration. pH was adjusted using a solution of 5 M NaOH until it was equal to 5 in order to eliminate the fermentation inhibitors [26]. The enzymatic hydrolysis process consisted of two stages: liquefaction and saccharification. According to Taibi et al. [26], liquefaction involved mixing 50 g of substrate with a phosphate buffer solution (Weight ratio 1:3) and 5 mL of 0.15% (w/v) alpha-amylase (30 U/mg) and heating the mixture at 100 °C for 10 min, followed by a 2-h liquefaction step at 90 °C and pH 6. The resulting solution was centrifuged, and the supernatant was analyzed for reducing sugars according to the method of Miller [10]. After liquefaction, the mixture pH was adjusted to 4.8 with 1 M HCl and cooled to 60 °C. Twenty milliliters of Aspergillus niger amyloglucosidase (120 U/mg) was added, and saccharification was carried out for 4 h at 60 °C. The enzyme was then inactivated by increasing the temperature to 100 °C, and samples were analyzed for reducing sugars as described above [26].
2.4.2 Fermentation conditions
Each pure isolated yeast was transferred from an agar culture colony to a glass tube containing 10 mL of YPG broth and incubated at 37 °C for 24 h. Then, 5 mL of this culture was added to 10 mL of sterilized YPG medium and stirred at 150 rpm for 18 h at 37 °C [18]. Active cells were centrifuged at 8000 rpm for 10 min, washed twice with sterile distilled water, and used as an inoculum. The fermentations were carried out in Erlenmeyer flasks containing 100 mL of sterile fermentation medium with 10% (v/v) inoculum size, incubated at 37 °C for 48 h under agitation speed of 150 rpm. All experiments were conducted in triplicate.
2.4.3 Analytical methods
Cell growth was determined by measuring optical density at 600 nm with a UV–Visible Spectrophotometer Varian 50 Tablet, and cell numeration was carried out using a Thoma hemocytometer [27]. A standard absorbance curve was established as a function of the cell population number. The ethanol concentration expressed in alcoholic degree % (v/v) was measured using an ebulliometer of the Dujardin– Salleron type (Paris, France) based on the variances in boiling points between water and alcohol, as indicated by a reference table [28]. Moreover, the concentration of ethanol (g/L) was measured using headspace GC–FID to establish a calibration curve between the ethanol concentration and the alcoholic degree.
Ethanol productivity (Qp, g/L/h) was calculated using the following equation:
where P is the ethanol concentration (g/L) and t is the fermentation time (h) giving the highest ethanol concentration.
Ethanol yield (Yp/s, g/g) was calculated as the actual produced ethanol and expressed as g ethanol per g of utilized sugar.
The ethanol fermentation efficiency (Ey, %) was calculated by the following equation:
Ey = (Yp/s/0.511) × 100
where Yp/s is the ethanol yield (g/g) and 0.511 is the theoretical maximum ethanol yield per unit of glucose from glycolytic fermentation (g/g).
2.5 Statistical analysis
All analyses were performed in triplicate, and results were expressed as average values ± standard deviation. The data were analyzed for statistical significance using one-way and two-way analysis of variance (ANOVA), followed by Duncan’s multiple range test (p < 0.05). The statistical software package XLSTAT v. 14 was used in the analysis of the experimental data.
3 Results and discussion
3.1 Physicochemical and biochemical characterization of agro-food wastes
Based on the data presented in Table 2, various nutrient compounds in all the tested agro-food wastes showed diversity and richness. The variation in nutrient content may be linked to the origin and initial composition of the used material during the production process, as stated by Slopiecka et al. [29]. Bioethanol is generated by the fermentation of simple sugars present in biomass as well as sugars resulting from a prior treatment by enzymatic or chemical hydrolysis of agro-food wastes containing high starch content [30]. In the present study, only three wastes among the tested agro-food wastes were selected as potential raw materials for bioethanol production regarding their important starch and sugar contents, namely quenching water with 50 g/L of sugar, whole potatoes with 60% of starch, and durum wheat bran with 41.50% of starch. These high amounts of starch and sugar in these three wastes are generally linked to the initial composition of starchy raw materials (potatoes, wheat, and corn) used during the production process and its operating conditions [31, 32]. The high initial sugar concentration is an important influencing parameter which has a direct effect on yeast cell growth and alcoholic fermentation rate [6, 7]. Furthermore, selected agro-food wastes have a slightly acidic pH and a total nitrogen concentration greater than 0.50% (w/w), corresponding to a protein concentration of more than 4% (w/w). The availability of nitrogen and proteins in the culture medium has a direct effect on the yeast growth. Insufficient amounts of these nutrients can impede or reduce the speed of alcoholic fermentation, resulting in the formation of undesirable sensory compounds, such as sulfur derivatives [33]. The agro-food waste contains low fat content which varies from 0.4 to 3.65%. This variation depends on the type of agro-food waste and its constituents [29].
The presence of fatty acids in the fermentation medium increases the tolerance of yeasts to ethanol, which leads to a high production of ethanol [34]. Additionally, our findings reported that biological oxygen demand concentrations over 5 days (BOD5) vary from 903.80 to 7522 mg/L, while chemical oxygen demand concentrations (COD) present values between 907 and 14225 mg/L. The COD/BOD5 ratio ranges from 1.53 to 2.48, demonstrating that COD values are higher than BOD5 values in all tested samples. In general, these two parameters vary considerably depending on the type and characteristics of the waste [35]. The high values of COD and BOD5 in our agro-food waste constitute a crucial environmental pollution factor which affects on the one hand the physical and chemical structure of the soil, and on the other hand, it reduces the aquatic life by increasing the concentration of dissolved oxygen [36]. Furthermore, a high electrical conductivity was observed, varying from 0.74 to 11.70 mS/cm depending on the presence of ions, their total concentration, their mobility, and their valence, as well as the temperature of the solution; these data demonstrate the abundance and richness of these discharges in various mineral salts [37]. These results were confirmed by atomic absorption spectrometry, and a diversity of mineral elements was revealed in the samples, including Na, Ca, K, Zn, Mg, and P, which vary in the range of 0.01 (Zn) to 2.12 (K) g/100g. Indeed, these micronutrients are crucial for microbial development and the synthesis of bioethanol [38]. They play an important role in the fermentation process and affect the ethanol yield [39]. Several studies have been carried out to highlight the importance of each element. Jones and Greenfield [40] cited that sodium (Na) improves the sugar assimilation by contributing to a good alcoholic fermentation, while magnesium (Mg) increases yeast tolerance to ethanol [41]. In addition, it has been demonstrated that magnesium in the fermentation medium affects cell growth, glucose consumption, and ethanol production; on the other hand, lack of magnesium triggers stress responses and reduces the expression of genes involved in energy metabolism [42]. In the presence of zinc (Zn), Ismail et al. [43] have also noted a considerable improvement in ethanol tolerance. According to Alminderej et al. [44], calcium (Ca) enhances bioethanol production and yeast tolerance to high ethanol concentrations. Also, phosphorus can be provided to yeasts in the form of phosphate salts, which are required for the creation of adenosine triphosphate (ATP), phospholipids, and nucleic acids [45]. According to Manuel Fernando et al [46], the addition of phosphate salt increased the ATP, which improved the efficiency of the fermentation process. Thus, the greatest improvement in ethanol production derived specifically from the increase in K+ concentration and the reduction in acidity of the fermentation medium [47]. In the same line, our findings have proven that these wastes constitute attractive and nutrient-rich raw materials for yeast growth and biofuel production.
3.2 Isolation of yeast strains producing bioethanol
A total of thirty thermotolerant yeast strains were isolated from different natural sources (Table 3). Six of which were able to produce bioethanol on YPG broth at 37 °C. Strains Q1 and Q2 were isolated from quenching water, C1 from cutting water, P1 and P2 from whole potato, and B2 from the soil of Bechar. Our results are in line with previous studies, in which thermotolerant yeasts were able to grow and produce ethanol from 37 °C to 45 °C [48,49,50] and even up to 50 °C [51]. Consequently, the six isolates that produced ethanol at 37 °C were chosen to select the best-performing yeast strains in ethanol production and tolerance.
3.3 Selection of thermotolerant yeast strains producing bioethanol
The effects of temperature, pH, substrate concentration, and ethanol tolerance were studied in order to select the most efficient yeast strains that are capable of producing and tolerating large amounts of ethanol in the YPG broth.
3.3.1 Effect of the substrate concentration
Figure 1 demonstrates that all isolated yeast strains tolerate a sugar concentration up to 25% (w/v), at which point strains Q1, B1, P1, C1, P2, and Q2 produce ethanol the most, with maximum values of 11.45%, 9.15%, 8.35%, 7.05%, 5.45%, and 3.80% (v/v), respectively. Moreover, the data suggests that as the substrate concentration increases, the ethanol production yield also increases until it reaches a maximum yield at a concentration of 25% (w/v). Our results are in concordance with those reported by Lin et al. [27]; their data showed that the ethanol yield produced by S. cerevisiae starts to decline above a substrate concentration of 30%. Similarly, Osho [21] and Triwahyuni et al. [52] have conducted studies showing that high sugar concentrations (25% and 30% (w/v)) in the fermentation environment inhibit cell growth and ethanol production. At a concentration of 40% (w/v), cell growth is completely halted as water infiltrates through the cytoplasmic membrane of the cells to balance intra- and extracellular concentrations. This decrease in production yield decrement could be attributed to the high concentration of sugars which creates osmotic stress in the yeasts [53].
3.3.2 Effect of temperature
According to thermodynamic laws, temperature has an impact on biological processes. Indeed, yeasts can only work in a narrow range of “optimal” temperatures, and they cannot survive in a critical temperature, like all other living organisms [54]. Figure 2 shows that the optimum temperature for ethanol production seems to be 37 °C for all the tested yeast strains with maximum ethanol contents of 11.45%, 9.15%, 8.35%, 7.10%, 5.45%, and 3.80% (v/v) corresponding to strains Q1, B1, P1, C1, P2, and Q2, respectively. In addition, based on the aforementioned graph, we noted that any deviation from the optimum temperature, either increment or decrement, results in a decline in ethanol production yield for all the tested isolates. Eventually, at a temperature of 45 °C, ethanol production stopped entirely for all strains except for strain B1, which managed to produce a minimal amount of bioethanol at a concentration of 0.5% (v/v). This decrement in production can be justified by the denaturation of the tertiary structure and inactivation of enzymes that regulate microbial activity and the fermentation process [55]. Our findings are in agreement with those demonstrated by Nuanpeng et al. [56] with a temperature of 37 °C as optimal for bioethanol production using S. cerevisiae DBKKUY–5. A number of publications have proven that temperature increment enhances the inhibiting effect of ethanol on cellular activities by lowering yield cell and ethanol production [57, 58]. However, a very high temperature causes many changes in the cell that ultimately affect protein structures and function, accumulate denatured and aggregated biomacromolecules, and lead to growth inhibition or cell death [59]. Zhang et al. [60] showed that high temperature can not only trigger high DNA damage of S. cerevisiae but also trigger other biological abnormalities including loss of cell wall integrity. Moreover, Duhan et al. [61] have reported a temperature of 35 °C as optimal for bioethanol production on potatoes using S. cerevisiae MTCC–170, whereas Ünal et al. [62] have noted higher ethanol yield at 30 °C using S. cerevisiae. Generally, ethanol production depends on fermentation temperature which profoundly affects yeast growth and fermentation performance [63].
3.3.3 Effect of pH
pH plays an essential role in the production of bioethanol. Figure 3 illustrates that the difference in pH affects the amount of the produced bioethanol. pH 5 gave the highest ethanol content for all six isolated yeast strains where they have maximum ethanol contents of 11.35%, 9.05%, 8.40%, 7.00%, 5.45%, and 3.80% (v/v) corresponding to strains Q1, B1, P1, C1, P2, and Q2, respectively. According to Darvishi and Abolhasan [64], an increase in the rate of ethanol production at pH 5 was noted. These results are in agreement with our finding as well as those obtained by Narendranath and Power [65], and they stated that yeast is an acidophilic organism and grows in an acidic environment. Because acidic conditions promote greater yeast development and inhibit the growth of harmful bacteria, alcohol production in acidic environments is important [27]. A pH between 4 and 6 is considered as optimum pH where the yeast can grow better, depending to several parameters such as the temperature, the amount of oxygen, and the yeast strain. Moreover, it has been noted that an increase or a decrease in the pH on either side of the optimum value for all the yeast isolates leads automatically to a decrease in the ethanol production yield. In addition, this inappropriate pH also has a negative effect on yeast cells because it can affect the structure of the cell wall and modify the conformation of proteins protruding from the plasma membrane; at the same time, it also impacts the organization and function of cell membrane lipids and disrupts the function of proteins embedded in membranes [66]. Narendranath and Power [65] have reported that the extracellular pH changes from the optimum pH may be too difficult for the cell to maintain a constant intracellular pH and the enzymes may not function normally. Also, if the enzymes are disabled, the yeast cell will not be able to grow and produce ethanol efficiently. Additionally, when the pH is lower than 4 or higher than 6, a more extended incubation period is required, and ethanol concentration decreases considerably [67]. It has been also signaled that more acidic and basic media retard yeast metabolic pathways and inhibit cell growth, resulting in a lower ethanol yield [68].
3.4 Ethanol tolerance
Figure 4 illustrates the tolerance of several yeast strains to different ethanol concentrations. A heterogeneity of ethanol resistance levels was reported in the different species. Strain Q2 seems to be less resistant compared to the other tested strains. Indeed, the Q2 strain has the capacity to withstand ethanol concentrations up to 11% (v/v). Above this value, only the other strains can survive. The strains C1 and P2 tolerate a concentration up to 12% (v/v) compared to 14% (v/v) for the strains P1 and B1, while the strain Q1 has the highest ethanol resistance compared to other strains (15%). This variability in ethanol tolerance could be explained by the variation of the strain’s biochemical composition. Numerous studies demonstrated the alteration of cellular lipid composition in response to ethanol exposure [69, 70]. Furthermore, You et al. [69] and Archana et al. [70] have reported that unsaturated fatty acids such as palmitoleic and oleic acids play an important role in overcoming the toxic effects of ethanol in S. cerevisiae cells, and when it is exposed to ethanol stress, the proportion of monounsaturated fatty acids increases with a concomitant decrease in saturated fatty acids. This unsaturated fatty acid composition could be a useful criterion to assess the potential ethanol tolerance of other microorganisms. Similarly, Boudjema et al. [71] have advocated a range of ethanol tolerance between 11 and 14% (v/v) for their isolated yeasts. In the same line, Nguyen et al. [72] have mentioned a total cell growth inhibition when the ethanol concentration is between 11.8 and 13% (w/v) for four different strains (C. tropicalis OP010088, Meyerozyma guilliermondii OP010064, C. tropicalis OP010090, and M. guilliermondii OP010065). On the other hand, yeasts generally cannot tolerate an ethanol concentration superior than 16%. Yet, several studies by Hawaz et al. [6], Urano et al. [19], and Moneke et al. [20] have succeeded to isolate yeast strains supporting up to 20% ethanol. This variation in tolerance could be attributed to the difference in genetic makeup of the yeast isolates and their ecological origin [6]. The ethanol resistance mechanism involves many interactions between signal transduction pathways and regulatory networks, as well as a complex network at the genomic level. Transcription dynamics and profiling results of important gene sets, such as heat shock proteins, provided an understanding of the tolerance mechanism [73]. According to our results, all selected yeast strains can produce bioethanol at significantly variable levels. Based on the one-way ANOVA statistical analysis of the data presented in Fig. 4, the tolerance to ethanol is significantly different according to the yeast strains tested. The Q1, P1, and B1 strains are significantly distinguished from the other strains by their tolerance to high amounts of ethanol. Table 4 shows the efficiency of bioethanol production by the six isolates at the different tested parameters (temperature, pH, and substrate concentration). It has been shown that the concentrations of bioethanol produced at all parameters are significantly different (p < 0.05) depending on the tested yeast strains. However, among these tested isolates, Q1, B1, and P1 strains produce significantly higher concentrations of bioethanol than the other isolates at all tested parameters. Thus, these three strains could be good candidates for the bioethanol production from agro-food wastes and are selected for further study.
3.5 Identification of yeast isolates
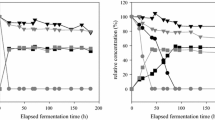
The isolated strains were confirmed molecularly by comparing the obtained PCR-amplified sequences with those stored in GenBank of the National Center for Biotechnology and Information (NCBI) using the BLAST algorithm. Phylogenetic analysis of the isolated strains revealed that they belong to two different genera: Saccharomyces and Candida. Strain Q1 is closely related to S. cerevisiae CBS 2888 (KY109257.1) with similarity of 100% (Fig. 6), whereas strains P1 and B1 seem close to C. tropicalis L2 (MK752673.1) and C. glabrata CBS 5278 (KY106475.1), respectively, with similarities of 99% and 100% (Fig. 7). Finally, these identified yeast strains are named S. cerevisiae Z0730–8635I (Q1), C. tropicalis Z0730–8636I (P1), and C. glabrata Z0730–8637VS (B1), and they are integrated in GenBank with the following accession numbers: OP876823, OP876824, and OP876825, respectively. The primary identification of yeast isolates is based on the morphological characteristics of colonies grown on solid medium. Table 5 reveals the presence of white, rounded, creamy, smooth, domed, and buttery texture colonies, showing pseudomycelium development only in P1 strain. Furthermore, microscopic examination demonstrated that all strain isolates have ovoid cell shapes with budding asexual reproduction (Fig. 5).
The morphological identification of the strains was followed by physiological and biochemical characterization (Table 6). These results indicate a variation in sugars fermentation and assimilation from one strain to another. Interestingly, all our isolates were able to use glucose during their growth. Moreover, trehalose was also efficiently assimilated by all strains, whereas it was fermented only by P1 and B1 strains. On the other hand, all the isolates present a negative test for lactose and starch. Furthermore, only the P1 isolate showed an ability to assimilate xylose and adonitol which are not assimilable by the others, while unlike the other strains, the Q1 isolate was able to use raffinose. The selected yeast isolates seem to belong to two different genera of yeast such as Saccharomyces and Candida, based on comparisons between all of our findings and the characteristics listed by Kurtzman and Fell [22], Boudjema et al. [71], Silva et al. [74], and Zhang et al. [75]. Indeed, isolate B1 was found to have the characteristics of C. glabrata. Interestingly, as stated by Risan [76], C. glabrata does not produce pseudohyphae at temperatures above 37 °C and has smooth, oval, cream-colored colonies. Risan [76] and Devadas [77] have mentioned that C. glabrata only ferments and assimilates glucose and trehalose. In fact, the Q1 isolate was found to have the characteristics of S. cerevisiae. Kavanagh [78] stated that colonies of S. cerevisiae are typically creamy with an oval shape. Based on the findings of Asyikeen et al. [79], Boudjema [80], and Moussan et al. [81], isolate Q1 can be identified as S. cerevisiae due to its ability to ferment sucrose, maltose, fructose, glucose, and raffinose but not lactose. This idea is confirmed by El Nemr [82] who has reported that S. cerevisiae cells are unable to ferment lactose due to a lack of lactase or β-galactosidase. Table 5 reveals that the P1 isolate is highly similar to C. tropicalis, which is consistent with the findings of other researchers. For instance, Ebabhi et al. [83] and Maikan et al. [84] successfully isolated C. tropicalis strains from local food samples, which exhibited creamy, flat, and smooth colony characteristics. Similarly, Boudjema et al. [71] and Shariq and Sohail [85] identified a yeast strain with ovoid cells, mycelium formation, and fermentation capabilities for glucose, galactose, sucrose, xylose, maltose, and trehalose as C. tropicalis.
3.6 Production of bioethanol from selected agro-food wastes
Table 7 summarizes the results of bioethanol production from three selected agro-food wastes using the different three isolated yeast strains. On the basis of the statistical analysis of the data presented in Table 7, it has been shown that the bioethanol production is significantly varied according to the tested yeast strains and the selected agro-food wastes. This variation could be related to different factors, namely the substrate, the hydrolysis process, and the strain type used in the fermentation process [67]. Bioethanol concentrations and their productivity values vary significantly from 10.81 to 62.46 g/L and from 0.19 to 1.30 g/L/h, respectively. Additionally, from Table 7 and Fig. 8, the highest ethanol concentrations are produced by S. cerevisiae and C. glabrata, at 7.85% and 6.80% (v/v), respectively; these results are in agreement with those cited by Techaparin et al. [58] which showed that the thermotolerant S. cerevisiae KKU–VN8 strain could produce the maximum amount of ethanol at a temperature of 37 °C from a nutritional substrate containing 15% (w/v) of glucose.
Unlike S. cerevisiae, several Candida strains are recognized for their inability to grow sufficiently and produce ethanol under anaerobic conditions [86]. Importantly, our results indicate that C. glabrata was able to produce ethanol more efficiently and grow under anaerobic conditions and that its fermentation efficiency was not significantly different from that of S. cerevisiae (p < 0.05).
With regard to S. cerevisiae, the obtained results have demonstrated that this strain presents a significantly high yield (p < 0.05) compared to C. tropicalis and a significantly better ethanol productivity (p < 0.05) than the other tested strains (Table 7, Fig. 8). These results support the widespread use of S. cerevisiae as the most effective starter in the industrial production of bioethanol [57] because it tolerates high ethanol concentrations and a wide pH range which makes the process less susceptible to infections [27, 67, 87]. On the other hand, the C. tropicalis strain has demonstrated its ability to produce ethanol from agro-food waste, but at a lower concentration comparing to the other two strains. These results are in concordance with those cited by Mussato et al. [88], who have obtained an ethanol yield of 5.45% (v/v) by C. tropicalis and 14.8 (v/v) by S. cerevisiae. Whole potatoes and durum wheat bran were used as raw materials, and they underwent acid and enzymatic hydrolysis to release fermentable sugars into the medium and avoid slow progress of fermentation due to insufficient intake of fermentable carbohydrates for yeast metabolism [7]. The amounts of bioethanol produced from the two agro-food wastes after acid and enzymatic hydrolysis ranged from 22.24 to 52.60 g/L and from 28.55 to 62.46 g/L, respectively. The initial concentration of substrate seems to have an impact on the production capacity, which explains the high yield of bioethanol production from potatoes in both processes. In addition, the statistical analysis shows that the fermentation of the enzymatic hydrolysates produces a higher bioethanol yield than that of the two tested residual acid hydrolysates. This difference could be attributed to the high amount of fermentable sugars that are released during the enzymatic hydrolysis comparing to acid hydrolysis which is characterized by releasing other inhibitor substances such as organic acids and furaldehydes (furfural and 5-hydroxymethyl furfural) [89]. Despite their low concentrations, these substances are able to alter the metabolism, lengthen the lag phase, damage cell membranes, and acidify the cytoplasm, which results in the reduction of ethanol production and productivity [90]. On the other hand, enzymatic hydrolysis already has high yields of simple sugars (85–95%), generates few phenolic compounds and effluents to be treated, and does not cause corrosion problems [91]. Regarding our results, the additional amylases were crucial for the alcoholic fermentation of the three tested yeast strains. This was demonstrated in the previous study of Vincent et al. [92] by the fact that C. glabrata did not produce ethanol from starch in the absence of these enzymes. The fermentation of the quenching water has been carried out. This contained 51.31 g/L of reducing sugars, and the production of bioethanol using the three tested yeast strains was between 10.81 and 13.97 g/L. Although this waste did not require any treatment contrary to other tested wastes, the rate of bioethanol production remains low. This could be explained by the low concentration of fermentable sugars that are released in this waste, which leads to low bioethanol production [93]. Several studies have been conducted on the production of bioethanol from different substrates including sugarcane, sugar beet, and sweet sorghum. The most common starches are corn and wheat [3, 44, 62]. The variations of ethanol yields change considerably from one substrate to another, mainly due to differences in the conversion efficiency of the different raw materials (acid hydrolysis and enzymatic hydrolysis), the composition of the culture medium, the fermentation process, and the used yeast strain whether commercially or isolated locally.
4 Conclusion
In the study, thirty strains of yeast were isolated from two Algerian natural sources (soil and agro-food waste). Among them, three thermotolerant yeast strains were successfully selected, specifically S. cerevisiae Q1, C. tropicalis P1, and C. glabrata B1. The strains, isolated from three agro-food wastes, were chosen for their ability to produce and to tolerate ethanol from three agro-food wastes. Interestingly, tolerance levels have been observed at high temperatures up to 40 °C and at ethanol concentrations up to 16% (v/v). The ethanol production capacities of these yeast strains were maximal at 37 °C. Moreover, whole potato wastes and durum wheat bran underwent acid and enzymatic hydrolysis of starch in order to release fermentable sugars. According to the results, the enzymatic hydrolysis made it possible to have a better yield of sugars, in particular from potato (190 g/L). The highest ethanol concentrations are obtained after 48 h of fermentation as follows: 7.525% (v/v), 6.80% (v/v), and 4.50% (v/v) on potatoes treated by hydrolysis enzymatic by S. cerevisiae, C. glabrata, and C. tropicalis, respectively. Taken together, our results provide important information regarding S. cerevisiae and whole potato waste for bioethanol production and represent a promising starting point for improving high-temperature industrial ethanol production processes in the near future.
Data availability
Not applicable.
References
Bisht A, Kamboj N, Bisht A, Kamboj V, Bharti M (2022) An intensive approach to the renewable energy recovery from agro waste–a review. In: Bahukhandi KD, Kamboj N, Kamboj V (eds) Environmental pollution and natural resource management. Springer Proceedings in Earth and Environmental Sciences. Springer, Cham, pp 19–38. https://doi.org/10.1007/978-3-031-05335-1_2
Sahabi–Abed S (2022) Assessment of future climate projections in Algeria using statistical downscaling model. International Journal of Big Data Mining for Global Warming 4(1):2130001. https://doi.org/10.1142/S2630534821300013
Phong HX, Klanrit P, Dung NTP, Thanonkeo S, Yamada M, Thanonkeo P (2022) High–temperature ethanol fermentation from pineapple waste hydrolysate and gene expression analysis of thermotolerant yeast Saccharomyces cerevisiae. Sci. Rep 12(1):1–16. https://doi.org/10.1038/s41598-022-18212-w
Arifin Y, Tanudjaja E, Dimyati A, Pinontoan R (2014) A second generation biofuel from cellulosic agricultural by–product fermentation using clostridium species for electricity generation. Energy Procedia 47:310–315. https://doi.org/10.1016/j.egypro.2014.01.230
Kumar D, Singh V (2020) Bioconversion of processing waste from agro–food industries to bioethanol: creating a sustainable and circular economy. In: Lin C, Kaur G, Li C, Yang X (eds) Waste valorisation: waste streams in a circular economy. Wiley, New York, pp 161–181. https://doi.org/10.1002/9781119502753.ch7
Hawaz E, Tafesse M, Tesfaye A, Beyene D, Kiros S, Kebede G, Muleta D (2022) Isolation and characterization of bioethanol producing wild yeasts from bio–wastes and co–products of sugar factories. Ann. Microbiol 72(1):1–16. https://doi.org/10.1186/s13213-022-01695-3
Tse TJ, Wiens DJ, Reaney MJ (2021) Production of bioethanol–a review of factors affecting ethanol yield. Ferment 7(4):268. https://doi.org/10.3390/fermentation7040268
AFNOR (1986) Contrôle microbiologique en agro–alimentaire, 3ème édition. Recueil de normalisation française, Paris
Rodier J (2009) L’analyse de l’eau : eaux naturelles, eaux résiduaires et eaux de mer, 9ème édition. Dunod, Paris
Miller GL (1959) Use of DNS reagent for determination of reducing sugars. Anal. Chem 31(3):426–428. https://doi.org/10.1021/ac60147a030
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem 6(1):24–27. https://doi.org/10.1177/000456326900600108
Uarrota VG, Moresco R, Coelho B, Nunes E, Peruch L, Neubert E, Rocha M, Maraschin M (2014) Metabolomics combined with chemometric tools (PCA, HCA, PLS–DA and SVM) for screening cassava (Manihot esculenta Crantz) roots during postharvest physiological deterioration. Food Chem 161:67–78. https://doi.org/10.1016/j.foodchem.2014.03.110
Noguerol-Arias J, Rodríguez-Abalde A, Romero-Merino E, Flotats X (2012) Determination of chemical oxygen demand in heterogeneous solid or semisolid samples using a novel method combining solid dilutions as a preparation step followed by optimized closed reflux and colorimetric measurement. Anal Chem 84(13):5548–5555. https://doi.org/10.1021/ac3003566
Kolář L, Gergel J, Šindelářová M, Kužel S (2002) Impact of farming intensity reduction in the Šumava foothills region on changes in soil organic matters and surface water quality. ROST VYROBA 48(9):377–381. https://doi.org/10.17221/4383-PSE
Colombié V (2005) Description de la production de spiramycine par Streptomyces ambofaciens. Modélisation métabolique, simulation et capteur logiciel. Dissertation,. Institut National des Sciences Appliquées de Toulouse
Ducastelle A, Lenoir J (1965) Contribution a l’étude de la flore microbienne du fromage de type Saint–Paulin. 1. Son évolution au cours de la maturation. Le Lait 45(447):371–378
Clark HE, Geldrich EF, Kabler PW, Huff CB (1958) Applied microbiology. International Book Company, New York
Ratnam BVV, Bandaru SR, Somalanka SR, Mendu DR, Imandi SB, Benjawada SR, Medicherla NR, Devarajan T, Karothi J, Chityala A (2007) Optimization of fermentation parameters to enhance the production of ethanol from Palmyra jaggery using Saccharomyces cerevisiae in a batch fermentor. Appl. Biochem. Biotechnol 143:224–235. https://doi.org/10.1007/s12010-007-0048-x
Urano N, Ishida M, Naito Y, Endo R, Takei T, Takashio M, Okai M (2021) Ethanol fermentation by high stress–tolerance aquatic yeasts and their mutants. Adv. microbiol 11(11):616–629. https://doi.org/10.4236/aim.2021.1111045
Moneke AN, Okolo BN, Nweke AI, Ezeogu LI, Ire FS (2008) Selection and characterisation of high ethanol tolerant Saccharomyces yeasts from orchard soil. AJB 7(24):4567–4575
Osho A (2005) Ethanol and sugar tolerance of wine yeasts isolated from fermenting cashew apple juice. AJB 4(7):660–662. https://doi.org/10.5897/AJB2005.000-3119
Kurtzman CP, Fell W (1998) The yeasts–a taxonomic study, 4th edn. Elsevier, Amsterdam
White TJ, Bruns T, Lee J, Taylor J (1990) Taylor amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Gelfand MAIDH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J. Mol. Biol 215(3):403–410. https://doi.org/10.1016/s0022-2836(05)80360-2
Taibi H, Boudries N, Abdelhai M, Lounici H (2021) White sorghum landrace and corn starches in glucose syrups production using enzymatic and acid amylolysis. J. Food Process. Preserv 45(2):1–9. https://doi.org/10.1111/jfpp.15131
Tasić MB, Konstantinović BV, Lazić ML, Veljković VB (2009) The acid hydrolysis of potato tuber mash in bioethanol production. Biochem. Eng. J 43(2):208–211. https://doi.org/10.1016/j.bej.2008.09.019
Lin Y, Zhang W, Li C, Sakakibara K, Tanaka S, Kong H (2012) Factors affecting ethanol fermentation using Saccharomyces cerevisiae BY4742. Biomass Bioenergy 47:395–401. https://doi.org/10.1016/j.biombioe.2012.09.019
Jacobson JL (2006) Introduction to wine laboratory practices and procedures. https://doi.org/10.1007/b106815
Slopiecka K, Liberti F, Massoli S, Bartocci P, Fantozzi F (2022) Chemical and physical characterization of food waste to improve its use in anaerobic digestion plants. Energy Nexus 5:100049. https://doi.org/10.1016/j.nexus.2022.100049
Robak K, Balcerek M (2018) Review of second generation bioethanol production from residual biomass. Food Technol. Biotechnol 56(2):174–187. https://doi.org/10.17113/ftb.56.02.18.5428
Teigiserova DA, Bourgine J, Thomsen M (2021) Closing the loop of cereal waste and residues with sustainable technologies: an overview of enzyme production via fungal solid–state fermentation. Sustain. Prod. Consum 27:845–857. https://doi.org/10.1016/j.spc.2021.02.010
Horstmann SW, Lynch KM, Arendt EK (2017) Starch characteristics linked to gluten–free products. Foods 6(4):29. https://doi.org/10.3390/foods6040029
Roca-Mesa H, Sendra S, Mas A, Beltran G, Torija MJ (2020) Nitrogen Preferences during Alcoholic Fermentation of Different Non-Saccharomyces Yeasts of Oenological Interest. Microorganisms 8(2):157. https://doi.org/10.3390/microorganisms8020157
Andreasen AA, Stier TJ (1954) Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J. Cell. Physiol 43:271–281. https://doi.org/10.1002/jcp.1030430303
Lim KY, Zakaria NA, Foo KY (2020) Water quality assessment of urban catchment after the large–scale flood event: the worst natural tragedy at Pahang River, Malaysia. Desalination Water Treat 175:32–42. https://doi.org/10.5004/dwt.2020.24790
Dey T, Bhattacharjee T, Nag P, Ritika GA, Kuila A (2021) Valorization of agro–waste into value added products for sustainable development. Bioresour. Technol. Rep 16:100834. https://doi.org/10.1016/j.biteb.2021.100834
Yu IT, Su JJ, Wu JF, Lee SL, Ju CC, Yen HT (2005) Dietary Modification for Reducing Electrical Conductivity of Piggery Wastewater. Asian-australas. J. Anim. Sci 18(9):1343–1347. https://doi.org/10.5713/ajas.2005.1343
Tadmourt W, Khiari K, Boulal A, Tarabet L (2020) Waste paper valorization for bioethanol production: retreatment and acid hydrolysis optimization. Energy Sources A: Recovery Util. Environ. Eff 1–20. https://doi.org/10.1080/15567036.2020.1815908
Hadri K, Cheikh N, Ammar M, Messaoudi FZ, Boulal A (2022) Valorization of Balanites aegyptiaca fruits by production of bioethanol: study and optimization. Current Trends in Natural Sci 11(21):290–303. https://doi.org/10.47068/ctns.2022.v11i21.032
Jones RP, Greenfield PF (1984) A review of yeast ionic nutrition. Part I: Growth and fermentation requirements. Process Biochem 19:48–60
Pejin JD, Mojović LV, Pejin DJ, Kocić-Tanackov SD, Savić DS, Nikolić SB, Djukić-Vuković AP (2015) Bioethanol production from triticale by simultaneous saccharification and fermentation with magnesium or calcium ions addition. Fuel 142:58–64. https://doi.org/10.1016/j.fuel.2014.10.077
Li R, Jin M, Du J, Li M, Chen S, Yang S (2020) The magnesium concentration in yeast extracts is a major determinant affecting ethanol fermentation performance of Zymomonas mobilis. Front. bioeng. biotechnol 8:957. https://doi.org/10.3389/fbioe.2020.00957
Ismail KS, Sakamoto T, Hasunuma T, Zhao XQ, Kondo A (2014) Zinc, magnesium and calcium ion supplementation confers tolerance to acetic acid stress in industrial Saccharomyces cerevisiae utilizing xylose. Biotechnol J 9(12):1519–1525. https://doi.org/10.1002/biot.201300553
Alminderej FM, Hamden Z, El–Ghoul Y, Hammami B, Saleh SM, Majdoub H (2022) Impact of calcium and nitrogen addition on bioethanol production by S. cerevisiae fermentation from date by–products: physicochemical characterization and technical design. Ferment 8(11):583. https://doi.org/10.3390/fermentation8110583
Walker GM, Stewart GG (2016) Saccharomyces cerevisiae in the production of fermented beverages. Bev 2(4):30. https://doi.org/10.3390/beverages2040030
Rubio–Arroyo MF, Vivanco–Loyo P, Juárez M, Poisot M, Ramírez–Galicia G (2011) Bio–ethanol obtained by fermentation process with continuous feeding of yeast. Journal of the Mexican Chemical Society 55(4):242–245. https://doi.org/10.29356/JMCS.V55I4.807
Goldberg AL (2003) Protein degradation and protection against misfolded or damaged proteins. Nature 426(6968):895–899. https://doi.org/10.1038/nature02263
Pongcharoen P (2022) The ability of Pichia kudriavzevii to tolerate multiple stresses makes it promising for developing improved bioethanol production processes. Lett. Appl. Microbiol 75(1):36–44. https://doi.org/10.1111/lam.13703
Kaewkrajay C, Dethoup T, Limtong S (2014) Ethanol production from cassava using a newly isolated thermotolerant yeast strain. Sci 40(4):268–277 https://doi.org/10.2306/scienceasia1513-1874.2014.40.268
Auesukaree C, Koedrith P, Saenpayavai P, Asvarak T, Benjaphokee S, Sugiyama M, Kaneko Y, Harashima S, Boonchird C (2012) Characterization and gene expression profiles of thermotolerant Saccharomyces cerevisiae isolates from Thai fruits. J. Biosci. Bioeng 114(2):144–149. https://doi.org/10.1016/j.jbiosc.2012.03.012
Talukder AA, Easmin F, Mahmud SA, Yamada M (2016) Thermotolerant yeasts capable of producing bioethanol: isolation from natural fermented sources, identification and characterization. Biotechnol. Biotechnol. Equip 30(6):1106–1114. https://doi.org/10.1080/13102818.2016.1228477
Triwahyuni E, Sudiyani Y, Abimanyu H (2015) The effect of substrate loading on simultaneous saccharification and fermentation process for bioethanol production from oil palm empty fruit bunches. Energy Procedia 68:138–146. https://doi.org/10.1016/j.egypro.2015.03.242
Liu X, Xu W, Zhang C, Yan P, Jia S, Xu Z, Zhang ZC (2014) Vitalized yeast with high ethanol productivity. RSC Adv 4(94):52299–52306. https://doi.org/10.1039/C4RA08809C
Ho DHN, Powel C (2014) The effect temperature on the growth characteristics of ethanol producing yeast strains. Int. J. Energy Environ. Eng 2(1):1–6
Phisalaphong M, Srirattana N, Tanthapanichakoon W (2005) Mathematical modeling to investigate temperature effect on kinetic parameters of ethanol fermentation. Biochem. Eng 28(1):36–43. https://doi.org/10.1016/j.bej.2005.08.039
Nuanpeng S, Thanonkeo S, Klanrit P, Yamada M, Thanonkeo P (2023) Optimization conditions for ethanol production from sweet sorghum juice by thermotolerant yeast Saccharomyces cerevisiae: using a statistical experimental design. Fermentation 9(5):450. https://doi.org/10.3390/fermentation9050450
Huynh XP, Pornthap T, Mamoru Y, Nguyen TCG, Sukanya N, Ngo TPD (2016) Ethanol production from molasses at high temperature by thermotolerant yeasts isolated from cocoa. CTUJS 03:32–37. https://doi.org/10.22144/ctu.jen.2016.021
Techaparin A, Thanonkeo P, Klanrit P (2017) High–temperature ethanol production using thermotolerant yeast newly isolated from Greater Mekong Subregion. Braz. J. Microbiol 48(3):461–475. https://doi.org/10.1016/j.bjm.2017.01.006
Lam FH, Ghaderi A, Fink GR, Stephanopoulos G (2014) Engineering alcohol tolerance in yeast. Sci 346(6205):71–75. https://doi.org/10.1126/science.1257859
Zhang M, Shi J, Jiang L (2015) Modulation of mitochondrial membrane integrity and ROS formation by high temperature in Saccharomyces cerevisiae. Electron. J. Biotechnol 18(3):202–209. https://doi.org/10.1016/j.ejbt.2015.03.008
Duhan JS, Kumar A, Tanwar SK (2013) Bioethanol production from starchy part of tuberous plant (potato) using Saccharomyces cerevisiae MTCC–170. Afr. J. Microbiol. Res 7(46):5253–5260. https://doi.org/10.5897/AJMR2013.6122
Ünal MÜ, Chowdhury G, Şener A (2020) Effect of temperature and nitrogen supplementation on bioethanol production from waste bread, watermelon and muskmelon by Saccharomyces cerevisiae. Biofuels 13(4):395–399. https://doi.org/10.1080/17597269.2020.1724440
Zabed H, Faruq G, Sahu JN, Azirun MS, Hashim R (2014) Boyce NA (2014) Bioethanol production from fermentable sugar juice. Sci. World J 95710:1–11. https://doi.org/10.1155/2014/957102
Darvishi F, Abolhasan MN (2019) Optimization of an industrial medium from molasses for bioethanol production using the Taguchi statistical experimental–design method. Ferment 5(1):14. https://doi.org/10.3390/fermentation5010014
Narendranath NV, Power R (2005) Relationship between pH and medium dissolved solids in terms of growth and metabolism of lactobacilli and Saccharomyces cerevisiae during ethanol production. AEM 71(5):2239–2243. https://doi.org/10.1128/AEM.71.5.2239-2243.2005
Liu X, Jia B, Sun X, Ai J, Wang L, Wang C, Zhao F, Zhan J, Huang W (2015) Effect of initial pH on growth characteristics and fermentation properties of Saccharomyces cerevisiae. J. Food Sci 80:800–808. https://doi.org/10.1111/1750-3841.12813
Azhar SHM, Abdulla R, Jambo SA, Marbawi H, Gansau JA, Faik AAM, Rodrigues KF (2017) Yeasts in sustainable bioethanol production: a review. Biochem. Biophys. Rep 10:52–61. https://doi.org/10.1016/j.bbrep.2017.03.003
Hapsari MA, Pramashinta A (2013) Manufacture of bioethanol from rubber sengkong (Manihot glaziovii), for household stove fuel for efforts to accelerate kerosene conversion to biofuels. Jurnal Teknol 2(2):240–245
You KM, Rosenfield CL, Knipple DC (2003) Ethanol tolerance in the yeast Saccharomyces cerevisiae is dependent on cellular oleic acid content. AEM 69(3):1499–1503. https://doi.org/10.1128/AEM.69.3.1499-1503.2003
Archana KM, Ravi R, Anu–Appaiah KA (2015) Correlation between ethanol stress and cellular fatty acid composition of alcohol producing non–Saccharomyces in comparison with Saccharomyces cerevisiae by multivariate techniques. JFST 52(10):6770–6776https://doi.org/10.1007/s13197-015-1762-y .
Boudjema K, Fazouane–Naimi F, Hellal A (2016) Isolation, identification of yeast strains producing bioethanol and improvement of bioethanol production on cheese whey. Turk. J. Biochem 41(3):157–166. https://doi.org/10.1515/tjb-2016-0026
Nguyen PV, Nguyen KHV, Nguyen NL, Ho XTT, Truong PH, Thi Nguyen KC (2022) Lychee–derived, thermotolerant yeasts for second–generation bioethanol production. Ferment 8(10):515. https://doi.org/10.3390/fermentation8100515
Ma M, Liu ZL (2010) Mechanisms of ethanol tolerance in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol 87:829–845. https://doi.org/10.1007/s00253-010-2594-3
Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J (2012) Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev 36(2):288–305. https://doi.org/10.1111/j.1574-6976.2011.00278.x
Zhang S, Zhao Q, Xue W, Li Y, Guo Y, Wu X, Huo S, Li Y, Li C (2021) The isolation and identification of Candida glabrata from avian species and a study of the antibacterial activities of Chinese herbal medicine in vitro. Poult Sci 100(4):101003. https://doi.org/10.1016/j.psj.2021.01.026
Risan MH (2016) Molecular identification of yeast Candida glabrata from candidemia patients in Iraq. Iraqi J Sci 57(2):808–813
Devadas SM, Ballal M, Prakash PY, Hande MH, Bhat GV, Mohandas V (2017) Auxanographic carbohydrate assimilation method for large scale yeast identification. Journal of Clinical and Diagnostic Research 11(4):DC01–DC03. https://doi.org/10.7860/JCDR/2017/25967.9653
Kavanagh K (2005) Fungi: biology and applications. John Wiley and Sons Ltd, England
Asyikeen ZN, Maaruf AG, Sahilah AM, Khan AM, Aida WW (2013) A new source of Saccharomyces cerevisiae as a leavening agent in bread making. Int. Food Res. J 20(2):967–973
Boudjema K (2016) Valorisation du lactosérum pour production de biocarburant (Bioéthanol). Doctoral dissertation, University of Boumerdes, Algeria
Moussan DFA, Tiemele LSA, Constant KA, Tanoh ALA, Théodore NDD (2019) Aptitude of three yeast strains isolated from palm wine for citrus wine production. International J. agric. environ 6(4):172–179. https://doi.org/10.14445/23942568/IJAES-V6I4P123
El Nemr TM (2001) Immobilization of recombinant strains of Saccharomyces cerevisiae for the hydrolysis of lactose in salted Domiati cheese whey. Eur. Food Res. Technol 212(2):225–227. https://doi.org/10.1007/s002170000204
Ebabhi AM, Adekunle AA, Okunowo WO, Osuntoki AA (2013) Isolation and characterization of yeast strains from local food crops. J Yeast and Fungal Res 4(4):38–43. https://doi.org/10.5897/JYFR2013.0112
Maikan HK, Jabbar S, Al–Haishawi H (2022) Isolation and identification of Candida tropicalis as a cause of cutaneous candidiasis in Kalar District, Arch. Razi Inst77(4):1367–1372. https://doi.org/10.22092/ARI.2022.357613.2066
Shariq M, Sohail M (2019) Application of Candida tropicalis MK–160 for the production of xylanase and ethanol. Journal of King Saud University–Science 31(4):1189–1194. https://doi.org/10.1016/j.jksus.2018.04.009
Moller K, Olsson L, Piskur J (2001) Ability for anaerobic growth is not sufficient for development of the petite phenotype in Saccharomyces kluyveri. Journal of Bacteriol 183(8):2485–2489. https://doi.org/10.1128/JB.183.8.2485-2489.2001
Surendhiran D, Sirajunnisa AR (2019) Role of genetic engineering in bioethanol production from algae. In: Ray RC, Ramachandran S (eds) Bioethanol production from food crops. Academic Press, United States, pp 361–381. https://doi.org/10.1016/B978-0-12-813766-6.00018-7
Mussato SI, Machado EMS, Carneiro LM, Teixeira JA (2012) Sugar metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl. Energy 92:763–768. https://doi.org/10.1016/j.apenergy.2011.08.020
Ramprakash B, Muthukumar K (2014) Comparative study on the production of biohydrogen from rice mill wastewater. International Journal of Hydrogen Energy 39(27):14613–14621 https://doi.org/10.1016/j.ijhydene.2014.06.029
Moysés DN, Reis VC, Almeida JR, Moraes LM, Torres FA (2016) Xylose fermentation by Saccharomyces cerevisiae: challenges and prospects. Int. J. Mol. Sci 17(3):207. https://doi.org/10.3390/ijms17030207
Eloutassi N, Louaste B, Boudine L, Remmal A (2014) Valorization of lignocellulosic biomass for the production of second generation bioethanol. J. Renew. Energy 17(4):600–609
Vincent M, Johnny Q, Adeni DSA, Suhaili N (2021) Potential of Candida glabrata from ragi as a bioethanol producer using selected carbohydrate substrates. Nusantara. Bioscience 13(1). https://doi.org/10.13057/nusbiosci/n130101
Wei P, Cheng LH, Zhang L, Xu XH, Chen HL, Gao CJ (2014) A review of membrane technology for bioethanol production. Renew. Sust. Energ. Rev 30:388–400. https://doi.org/10.1016/j.rser.2013.10.017
Author information
Authors and Affiliations
Contributions
Younes Gherbi and Khaled Boudjema designed the study. Younes Gherbi performed the data collection and analysis. Younes Gherbi and Khaled Boudjema analyzed the data and wrote the manuscript. All the authors commented on previous versions of the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This declaration is not applicable to this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gherbi, Y., Boudjema, K., Djeziri, M. et al. Isolation and identification of thermotolerant yeast strains producing bioethanol from agro-food wastes. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04621-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04621-y