Abstract
This study identified phenotypic traits appropriate for biotechnological applications of 118 yeasts isolated from cachaça distilleries. Different properties were verified: capacity to use alternative carbon sources; ability to tolerate high concentrations of sucrose, ethanol, methanol, aluminum and zinc as well as different pH values and foam production. Pichia guilliermondii and Pichia anomala strains were identified as the most promising ones for application in the second-generation biofuel industry, showing ability to grow on high glycerol concentrations. Other isolates, identified as Saccharomyces cerevisiae, produced bioethanol comparable to the industrial strains, and were therefore ideal for use in the first-generation ethanol industry. Some of these strains also showed high resistance to aluminum, as observed in sugarcane juice, and to inter-cycle washings with diluted sulphuric acid, as performed in the industrial bioethanol production process. In summary, yeast isolates from cachaça distilleries displayed robustness and phenotypic plasticity, which makes them interesting for biotechnological applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing demand for energy is an irreversible global phenomenon. Intensive use of fossil fuels, the main component of the energy matrix of most societies, is observed for decades as unsustainable in the long term. Thus, search for cleaner, renewable energy sources has increased in the past few decades. Among these, biofuels have been proposed as a short-term solution, fitting a transition stage before better options are available [2, 16]. This is mostly due to their relative chemical similarity to fossil fuels, which reduces the need for large modifications in current combustion engines and fuel distribution logistics. Some countries such as Brazil already have biofuels on their energy matrix. Since the late 1970s, ethanol produced by a few Saccharomyces cerevisiae industrial strains has been added to gasoline or directly used in motor vehicles.
Brazilian bioethanol production is commonly performed in very-high-gravity (VHG) fermentations using highly concentrated substrates such as sugarcane juice and molasses and producing high ethanol yields [6], thus reducing the costs of ethanol distillation [13]. In addition, the Brazilian bioethanol production process recycle yeast cells. After fermentation, they are collected by centrifugation and incubated in dilute sulphuric acid (pH 2.0–2.5) for 1–3 h. This treatment reduces bacterial contamination but also lowers the vitality of yeast cells, which causes a considerable delay in the start-up of the subsequent fermentation cycle [8]. Moreover, in certain areas in Brazil, the soil is rich in aluminum and/or zinc. When sugarcane is grown on such soils, the aluminum and/or zinc content in the sugarcane juice can be high enough to inhibit yeast fermentation [33]. An additional problem in the alcohol industry is related to the production of foam that impairs the fermentation vats to operate at full capacity. This requires continuous utilization of antifoaming products, thereby increasing ethanol production costs [13]. Altogether, despite the fact that bioethanol processes are industrially well established and operative for 4 decades, these problems configure the need for technological upgrade and performance amelioration.
Another well-developed biofuel is biodiesel, which is produced by the transesterification of triacylglycerols. At present, chemical methods drive most biodiesel production process; however, strong efforts are being made to develop biorefineries for the biological production of esters. On the other hand, the biodiesel production process yields crude glycerol waste [approximately 10 % (v/v) of the whole reaction product]. In view of extremely large amounts of crude glycerol produced worldwide, this is being considered for conversion into valuable goods. Microbial processing of these wastes into valuable goods, namely succinic, citric, or propionic acids, hydrogen, and ethanol, is a promising alternative as some microorganisms are not affected by the chemical contaminants present in crude glycerol [24, 31, 35].
In a totally separate economy sector, the production of cachaça (the Brazilian spirit) also starts by fermenting sugarcane juice and molasses. Unlike the case of bioethanol, in this process, yeasts are not washed after each fermentation cycle. In addition, the initial inoculum is not made of selected yeast starters, and fermentations are conducted by a spontaneous community of species and strains. S. cerevisiae, the predominant yeast species, has to cope with: (1) competition from many other types of yeast as well as bacteria; (2) high environmental temperature fluctuations owing to the absence of temperature control in industrial premises; (3) low a w , generated by sugarcane juice at 20 % sucrose (18–20° Brix); and (4) increasing amounts of ethanol during each fermentation cycle. These combined extreme conditions contribute to the natural selection of robust yeast strains with unusual physiological characteristics, which are presumably interesting for diverse biotechnological applications, namely addressing the problems from the above-mentioned bioethanol and biodiesel industrial sectors.
In this study, 118 yeast strains obtained from different cachaça distilleries covering a large geographical area of Brazil, including the states of Bahia, Espírito Santo, Minas Gerais and Rio de Janeiro were used. Yeast cells were initially selected according to (1) tolerance to high concentrations of ethanol; sugar and high temperatures; (2) fermentative capacity and (3) ability to flocculate [41]. These cachaça yeast isolates were characterized bearing in mind the putative future utilization in the production of bioethanol (first generation) and/or in sustainable management of biofuel wastes, i.e., utilization of residues from bioethanol and biodiesel production to generate yeast-based added value products. Yeast isolates showed unusual high phenotypic plasticity and robustness. Two of them, identified as strains of Pichia guilliermondii and P. anomala, presented interesting characteristics for biodiesel waste utilization. Another isolate, an S. cerevisiae strain, presented remarkably promising characteristics for producing ethanol in the presence of aluminum and was further resistant to continuous acid washing. The present results emphasize that a considerable degree of biotechnological innovation and improvement can be derived from the exploitation of the microbial biodiversity of spontaneous fermentations without the need for cost- and time-consuming laborious genetic manipulation.
Materials and methods
Strains and culture conditions
Yeast strains used in this study originated from sugarcane fermentation vats in cachaça distilleries from diverse geographical localizations in Brazil. Yeast colonies were isolated as described previously [41]. The commercial bioethanol strains PE2, CAT1, and Ethanol Red (Fermentec, Brazil) and the laboratory strain S. cerevisiae BY4741 were used as controls. For short-term maintenance, yeasts were cultured in YPD [1 % (w/v) yeast extract, 2 % (w/v) peptone, with 2 % (w/v) dextrose] supplemented with 2 % (w/v) agar and kept at 4 °C. Long-term maintenance was guaranteed by the storage of samples from YPD-grown cultures at −80 °C with 30 % (v/v) glycerol. All strains are available in the culture collection of the Federal University of Ouro Preto (propp@ufop.br). Growth assays were performed in 96-microwell plates containing 200 µL YP [1 % (w/v) yeast extract, 2 % (w/v) peptone] that was differently supplemented. Plates were incubated at 30 °C without shaking. Batch cultures were performed at 30 °C, with 1:5 air-to-liquid ratio and 200 rpm orbital shaking (New Brunswick Model G200). Inoculation was standardized to initial OD600nm = 0.1 using an overnight culture. Growth was monitored following OD600nm in a spectrophotometer BioMate3 (Thermo Scientific). The specific growth rate (μ) was estimated from OD600nm measurements. Drop tests were performed using 10× serial dilutions of YPD-grown OD600nm ≈ 1.0 batch cultures on sterile deionized water. Plates were incubated at 30 °C.
Growth in different carbon sources and under stressful conditions
The ability to use different carbon sources was tested in YP batch cultures with 2 % (w/v) xylose, ferulic acid, p-coumaric acid. Additionally, the ability to tolerate the following stress agents was tested in YPD: acetic acid (2.0–6.0 g/L), formic acid (0.5–3.0 g/L), furfural (10–60 mmol/L, hydroxymethyl-2-furaldehyde (HMF) (10–60 mmol/L) [1]. Also, growth on increasing concentrations of glycerol [2, 5, 10, 12, 15, 20, 25 % (w/v)] was tested. Tolerance to increasing concentrations of ethanol or methanol [2, 5, 10, 12, 15, 20 % (v/v)] as well as a wide range of pH values (from 2.5 to 10.5 NaOH or HCl adjusted) was screened in YPD microwell plates. Results from microwell assays of chosen strains were confirmed by drop tests in solid media using tenfold dilutions of an initial inoculum of 107 cells/mL. Tolerance to zinc and aluminum was tested by drop tests in solid YPD supplemented with 10 mmol/L AlCl3, 5 mmol/L Al2(SO4)3 or 5 mmol/L ZnSO4.
Small-scale fermentations
Small-scale industrial-like batch fermentations were performed on fermentation tubes (5.5 × 14 cm) (handmade at Katholieke Universiteit Leuven, Belgium) (Fig. 1). These were kept still at 30 °C, except for an initial 2-h period with orbital shaking (350 rpm). Fermentations were started at initial OD600nm = 5.0 (approximately 5 × 107 yeast cells) and were noninvasively followed by weighing the flasks. Ethanol production was estimated by weight loss. Foaming was monitored by measuring the thickness of the foam layer on top of the fermentation medium. Starter cultures were obtained from pre-cultures of YP with 10 % (w/v) glucose or sucrose (150 mL) and incubated for 48 h at 30 °C and 200 rpm. These were centrifuged (3,000 rpm, 5 min, 4 °C), and the pellets were re-suspended in 10 mL YP with 20 % sucrose, 33 % glucose, or 2 % xylose and used to inoculate 90 mL of identical fermentation media, total 100 mL of medium. To test for fermentation in the presence of 10 mmol/L Al2(SO4)3, cells were pregrown in 3 mL of YPD for 24 h (200 rpm, 30 °C). This pre-inoculum was used to inoculate 100 mL of 10 % sucrose, grown for 2 days (200 rpm, 30 °C) until the stationary phase. Cells were then harvested by centrifugation and used to inoculate 20 % sucrose supplemented with 10 mmol/L Al2(SO4)3. Small-scale fermentations with 2 % xylose in the presence of inhibitors acetic acid (2.0 g/L), formic acid (0.5 g/L), furfural (10 mmol/L) and HMF (10 mmol/L) were performed as described.
Acid washing between fermentation cycles
Small-scale fermentation cycles of 24 h in 20 % sucrose were intercalated with an acid washing treatment of yeast cells. At the end of each cycle, approximately 6 × 108 yeast cells were harvested by centrifugation and resuspended for 1 h in sulphuric acid diluted in water to pH 2.0, following which they were harvested again by centrifugation and reused in a subsequent fermentation. This procedure was repeated up to six cycles. The viability of yeasts in vats was quantified by counting the viable cells in a Neubauer chamber after staining with methylene blue.
Molecular identification of yeast strains
Yeast genomic DNA was extracted [26] and subjected to polymerase chain reaction (PCR) amplification of the 18S rRNA internal transcribed spacer (ITS) regions (ITS1/5, 8S/ITS2) using the primers ITS1 + ITS4 [43]. DNA electrophoresis was performed using standard procedures. MW markers were a 1-kb DNA ladder from Promega and 100-bp DNA ladder from Axygen. The amplicon (880 pb) was analyzed by restriction fragment length polymorphism (RFLP) using HhaI, HaeIII, and HinfI and compared with a control correspondent sequence originating from S. cerevisiae BY4741 [15]. Different ITS PCR products were purified using standard procedures and sequenced by capillary electrophoresis (Sanger method) using the ABI3130 platform Life Technologies (Myleus Biotechnology, Belo Horizonte, Minas Gerais, Brazil). Sequences were analyzed using the National Centre for Biotechnology Information (NCBI) database.
Reproducibility
All experiments were conducted in triplicate and repeated twice.
Results
Physiological properties of 118 Brazilian cachaça yeast strains
Fermentation vats from cachaça distilleries from several states of Brazil were used to isolate 118 yeast strains [41]. These were subjected to a physiological survey, including (1) the ability to grow on biofuel industry wastes; (2) elevated tolerance to high alcohol concentrations and (3) tolerance to other stressful conditions: low pH, the presence of aluminum, zinc or VHG fermentation conditions (Supplementary Material, S1).
Cellular growth in different carbon sources
The chemical composition of crude biodiesel waste varies according to several production constraints, namely the type of fat or oil used in the process [17]. Nonetheless, glycerol is the most relevant compound present in biodiesel wastes [30]. Therefore, the ability of each of the 118 yeast isolates to grow on glycerol (reagent grade) as unique sole carbon and energy source was tested in concentrations spanning from 2 to 25 % (w/v). All strains grew on 2 % (w/v) glycerol, although only a few could grow on glycerol concentrations above 10 % (Supplementary Material, S1). Strains LBCM15 and LBCM105 grew well on 20 and 25 % (w/v) glycerol, respectively, compared with 15 % (w/v) for CAT1, PE2, and Ethanol Red industrial strains used as controls and 5 % (w/v) for the laboratory strain BY4741 (Fig. 2; Table 1).
Viability phenotype of candidate strains (LBCM15 and LBCM105) on a glycerol and b lignocellulosic component-based media. Drop tests were performed from cellular suspensions containing approximately 107 cells/mL in solid medium: YPD, YP + 25 % glycerol, YP + 2 % xylose, YP + 2 % ferulic acid and YP + 2 % coumaric acid (w/v)
The bioethanol industry produces very high amounts of a sugarcane fibrous dry waste rich in lignin and hemicellulose, bagasse. This is hardly degraded by microbes, namely yeasts. Cachaça yeast isolates were tested for the ability to grow on the most prominent components of sugarcane bagasse, xylose, ferulic acid or coumaric acid, as sole carbon and energy sources. Eight strains (LBCM08, LBCM14, LBCM15, LBCM19, LBCM46, LBCM99, LBCM105 and LBCM106) could grow on xylose, two of which (LBCM15 and LBCM105) could also grow on ferulic acid and one of which (LBCM105) could also grow on coumaric acid (Fig. 2). This last strain was further challenged with compounds that are produced during the pretreatment of lignocellulosic biomass: furfural, acetic acid, formic acid and 5-hydroxymethyl-2-furfuraldehyde (HMF) in the presence of which it could still grow on glucose (Table 2).
Tolerance to alcohol
Biodiesel wastes contain high amounts of methanol and less frequently contains ethanol [30]. In addition, regardless of the type of industrial fermentation, yeasts have to cope with very high amounts of alcohol accumulated from each fermentation cycle to the next. Therefore, the tolerance of yeasts to ethanol and methanol up to 20 % (v/v) in glucose-based medium was tested. None of the strains could grow in presence of 20 % (v/v) alcohol. Twelve strains could grow in presence of 15 % (v/v) ethanol (Supplementary Material, S1). Again, strains LBCM15 and LBCM105 stood out for growing in the presence of 12 and 15 % (v/v) methanol and ethanol, respectively (Table 1). In addition, when these strains were grown in YP 20 % of sucrose for 24 h, the industrial strain PE2 produced 106.77 g/L of ethanol, while LBCM15 and LBCM105 produced 61.05 and 64.26 g/L of ethanol, respectively.
Other stress conditions
The bioethanol industry uses cleaning treatments with dilute sulphuric acid (pH 2.0–2.5) to eliminate bacteria contaminations between fermentation cycles [3]. Although that does not apply to the cachaça production process, in view of future applications, 118 yeast isolates were characterized with regard to their resistance to low pH in glucose-based medium (Supplementary Material, S1). Half of the strains grew well at pH values as low as 2.4, including the above-mentioned strains LBCM15 and LBCM105 (Table 3). In addition, high pH was tested. No growth was observed at pH above 9.5. The broader pH range allowing growth was 2.4–9.5 observed with strains LBCM15, LBCM37, LBCM47, LBCM97, and LBCM105. Moreover, the soils in which sugarcane is produced often contain elevated amounts of metal ions that pass on to sugarcane juice. This is therefore rich in zinc and aluminum, both of which are highly toxic to yeasts [3]. Aluminum in the form of Al2(SO4)3 and AlCl3 as well as ZnSO4 was used to supplement fermentation media (
Supplementary Material, S1). From the 118 strains tested, eight could tolerate 10 mmol/L AlCl3 and 5 mmol/L ZnSO4 (LBCM03, LBCM05, LBCM47, LBCM54, LBCM69, LBCM78, LBCM79, and LBCM105).
From all the above-mentioned results, two strains, LBCM15 and LBCM105, emerge as being able to tolerate the highest alcohol concentrations, the lowest pH, and the presence of aluminum and zinc (Table 3) as well as being able to grow on 20–25 % glycerol (Fig. 2). This makes them promising candidates for further exploitation in innovative processes of biodiesel waste consumption. These same characteristics also make strain LBCM105, together with strain LBCM47 (able to tolerate low pH and the presence of Al/Zn), good candidates for first-generation ethanol production. Furthermore, the strains LBCM08, LBCM14, LBCM15, LBCM19, LBCM46, and LBCM99, but mainly strain LBCM105, could be considered good candidates for the development of second-generation bioethanol production processes owing to their ability to convert hemicellulose and lignin constituents into biomass (Supplementary Material, S1).
Sugarcane juice-like sucrose fermentations
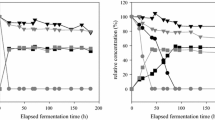
These results prompted us to assay small-scale ethanol fermentations using sugarcane juice as a substrate for testing the 12 strains that displayed a higher tolerance to ethanol: LBCM07, LBCM10, LBCM13, LBCM17, LBCM23, LBCM25, LBCM28, LBCM44, LBCM47, LBCM65, LBCM67, LBCM105 and LBCM107. Fermentations were performed on YP with 20 % sucrose (average sucrose concentration in sugarcane juice) (Fig. 3a; Supplementary Material, S2A). Strains LBCM10, LBCM13, LBCM25 and LBCM47 could achieve these ethanol yields in 24 h similar to the control industrial strain PE2 (Fig. 3a), while the strains LBCM07, LBCM17, LBCM28, LBCM44, LBCM65, LBCM67, LBCM105, LBCM107 showed slower ethanol production (Supplementary Material, S2A). In addition, the production of foam was monitored (Supplementary Material, S3A, C and D). Strains showed three distinct patterns of foam production throughout fermentation: (1) strains LBCM10, LBCM28 and LBCM44 produced foam during the entire fermentation; (2) strains LBCM13, LBCM17, LBCM23, LBCM65, LBCM67, LBCM105 and LBCM107 produced foam mainly during the first 24 h and gradually decreased production without ceasing; and (3) strains LBCM07, LBCM25 and LBCM47, similarly to the industrial stain PE2, did not produce foam after 72 h.
Profile of ethanol production in small-scale fermentation of different yeast strains in 20 % sucrose (a) and 33 % glucose—VHG fermentation (b). The strains LBCM13 (Ο), LBCM10 (×), LBCM25 (∆), and LBCM47 (-) could achieve ethanol yields close to the maximum in 24 h, as the industrial strain PE2 (●) (a). The strains LBCM17 (▼), LBCM25 (∆), LBCM47 (-), and LBCM67 (□) showed higher ethanol production capacity (higher than 90 %) similar the industrial strain PE2 (●) (b)
VHG fermentations
These strains were also subsequently used to mimic VHG fermentations using YP with 33 % glucose. Ethanol production was monitored (Fig. 3b; Supplementary Material, S2B). None of the strains reached the same production levels as the industrial strain PE2. The production of foam was globally much less intense than that in the 20 % sucrose fermentation (Supplementary Material S3B, D and F). Again, three distinct patterns could be distinguished: (1) strain LBCM28 presented a constant although very low foam production; (2) strain LBCM10 gradually decreased production, without ceasing it; and (3) strains LBCM07, LBCM13, LBCM17, LBCM23, LBCM25, LBCM44, LBCM47, LBCM65, LBCM67 and LBCM105 as well as the control industrial strain PE2 did not produce foam at all.
Sulphuric acid washings
Bearing in mind the acid cleaning treatments used by the bioethanol industry between fermentation cycles, ethanol production was further measured in the presence of dilute sulphuric acid (pH 2.0). High initial cell densities (approximately 6 × 108 yeast cells) were used to cope with the predicted loss of cell viability. The ethanol produced was quantified (Fig. 4a), and the viability of the culture (Fig. 4b) after the sixth 24-h cycle was monitored. The best results were obtained with strains LBCM37 and LBCM47, for which the total ethanol production was approximately 85 g/L, which was 20 % more than that by the industrial strain PE2 in YP with 20 % sucrose. Cellular viability closely followed the variations in ethanol production after each 24-h cycle. Notably, strain LBCM37 lost significantly more viability than all others, which is quite surprising considering its higher ability to produce ethanol.
Effect of low pH treatment on ethanol production during recycling of different yeast strains in small-scale fermentations. The fermentations were performed at 30 °C in 100 mL of YP + 20 % sucrose. Cycles of 24 h were intercalated with washings in diluted sulphuric acid six consecutive times. The different bars represent each cycle of fermentation. Final ethanol production (a). Viable cell number after each fermentation cycles (24 h). The viable cell number was assessed based on cell counting in the end of the each fermentation cycle (b). Cycles: first cycle (black bar), second cycle (gray bar), third cycle (white bar with horizontal lines), fourth cycle (white bar with oblique lines), fifth cycle (grid bar) and sixth cycle (white bar)
Aluminum toxicity
The addition of 10 mmol/L Al2(SO4)3 to 20 % sucrose fermentations (not shown) highlighted the isolates able to keep producing ethanol despite the presence of this contaminant. Strains LBCM05 and LBCM47 were the best performers, producing 1.5-fold (69 g/L) more ethanol than the industrial strain PE2 after 133 h of fermentation (not shown), which makes them excellent candidates for first-generation bioethanol production.
Pentose fermentation
Finally, strain LBCM105 was challenged with a small-scale fermentation using YP supplemented with 2 % xylose. This strain fermented xylose and produced 2 g/L ethanol after 120 h of fermentation, while the industrial strain PE2 produced around 1 g/L (Fig. 5). These amounts were 5- and 10-fold smaller, respectively, than the ethanol these strains produce from identical amounts of glucose (approximately 10 g/L). However, this strain (LBCM105) showed a reduction of approximately 30 % in the ethanol production in the presence of the inhibitors furfural, acetic acid, formic acid and HMF (not shown).
Molecular identification of the selected yeast strains
In view of their promising characteristics, yeast strains LBCM15, LBCM25, LBCM47 and LBCM105 were submitted to taxonomic identification by molecular techniques on the basis of 5.8 S-ITS rRNA region amplification and restriction analysis. The restriction profiles of the PCR products of yeast strains LBCM25 and LBCM47 were similar to those of S. cerevisiae BY4741 (control), while those of LBCM15 and LBCM105 were different (not shown). The PCR products of strains LBCM15 and LBCM105 were sequenced and revealed 98 and 88 % identity, respectively, to the corresponding regions from the type strains of P. guilliermondii (GenBank: FJ527876.1) and P. anomala (GenBank: FJ713067.1). The degree of similarity allows classifying LBCM15 and LBCM105 strains as P. guilliermondii and P. anomala, while strains LBCM25 and LBCM47 are most probably S. cerevisiae.
Discussion
The sugarcane fermentation vats from cachaça production processes yielded 118 yeast isolates that were used to survey physiological traits bearing in mind specific biotechnological applications. These were as follows: (1) the utilization of bioethanol and biofuel industry wastes and (2) the improvement of ethanol yields from sugarcane juice, namely in VHG fermentation conditions. This demanded the verification of the ability of these yeasts to use carbon sources other than sucrose or glucose and the corresponding ethanol production.
Contamination of fermentations with bacteria, mainly species of Lactobacillus, is a well-known significant industrial problem causing production losses up to 22 % [3, 9]. These bacteria can produce lactic and acetic acid, diacetyl, reuterin and proteinaceous compounds affecting yeast growth and ethanol production capacity [9]. Several techniques, such as antibiotics, ammonia and acid treatment are currently being employed in an attempt to inhibit unwanted microbial growth [3, 9]. However, importantly, these yeasts were also tested for their resistance to the high amounts of ethanol accumulated during consecutive cycles, together with the low pH from washes with diluted sulphuric acid performed between fermentation cycles.
Finally, it was also important to test the resistance of these strains and their fermentative ability to the presence of inhibitory metals present in sugarcane juice, mainly aluminum and zinc.
The reasoning underlying the choice of sugarcane fermentation vats from cachaça distilleries for this purpose is related to these fermentations occurring in environments of high osmotic pressure and high temperatures (a physiological trait not explored in this survey) and relying on spontaneous microbial fauna. Strong competition between different microorganisms, together with continuous exposition to considerable levels of alcohol and reutilization of “foot-of-vat” without treatment between fermentative cycles, generates a constant natural selective pressure imposed by environmental conditions [29]. Yeast strains spontaneously occurring in sugarcane fermentation vats must be natural ethanol producers under stressful conditions because the industrial environment favors confined evolution and selection for industrially favorable characteristics. Fermentation vats are thus an excellent source of yeast biodiversity already adapted to harsh industrial environments. Further spontaneous genetic hybridization events between different yeast strains [4] may also contribute to strain amelioration. Results obtained with this physiological survey reflect this industrial natural selection effect and highlight the high plasticity of these yeasts, showing these strains as natural candidates for several possible industrial applications.
Glycerol is the major waste product from the biodiesel industry, presently produced in excessive amounts [30]. Interesting high added value solutions for its use are not available. This happens because crude glycerol from biodiesel contains a significant number of contaminants that preclude its direct utilization for microbial feed [11, 22, 32, 44]. Nevertheless, glycerol and alcohol (more frequently methanol than ethanol) are the most relevant chemicals present in biodiesel wastes [30]. Therefore, yeasts were surveyed for their ability to grow in crescent concentrations of glycerol up to 25 % and in the presence of high concentrations of methanol and ethanol up to 15 %. Two strains, LBCM15 and LBCM105, identified as P. guilliermondii and P. anomala, respectively, could use glycerol up to 25 % (w/v). Both P. guilliermondii and P. anomala are good glycerol consumers [21, 34, 38], used in many biotechnological applications [42]. Furthermore, LBCM15 and LBCM105 could tolerate methanol or ethanol up to 15 % (v/v). These strains also presented an impressively resilient ethanol production ability in the presence of aluminum and low pH on either 20 % sucrose or 33 % glucose. In particular, P. anomala is recognizably robust because it is able to grow in the presence of high ethanol concentrations [23, 39], at low pH, under high osmotic pressure, and under low oxygen tension [28]. Moreover, in contrast to S. cerevisiae, P. anomala is tolerant to Zn2+ (up to 500 mg/L), Cd2+ [40], Hg2+, Ni2+, Cr6+, Pb2+, Cu2+, and Co2+ [5, 7, 12]. Isolation of two Pichia species from cachaça fermentation vats is not a surprising fact. These species are easily found in water, soil, plants, or crops suitable for ensilage. Furthermore, many authors reported their presence, particularly during early stages of cachaça fermentation [36]. Their robustness (to tolerate biotic and abiotic stresses), together with their plasticity (using a diverse set of carbon and energy sources) should explain their almost ubiquitous nature in such harsh conditions as those found in cachaça production vats.
Production of bioethanol from sugarcane leads to the accumulation of huge amounts of the sugarcane bagasse, a material composed of hemicellulose and lignin. Its polysaccharide fraction is composed of cellulose and xylose-rich hemicellulose, and its polyphenolic fraction is composed of several aromatic monomers, derived from hydroxycinnamic acids such as ferulic or coumaric acids [10, 27]. Pichia species were described as capable of using multiple substrates [19] and are among the few organisms that can metabolize ferulic acid [14, 18, 20, 37]. Accordingly, P. anomala, strain LBCM105, could grow on ferulic and coumaric acids as well as ferment xylose. The ethanol produced from xylose by strain LBCM105 was less than in sucrose- or glucose-based fermentations. Still, this strain can be considered for the future improvement of fermentation for second-generation bioethanol production from sugarcane bagasse.
S. cerevisiae is the most robust ethanol-producing microorganism [25, 45]. In this survey, S. cerevisiae strain LBCM47 stood out as a good candidate for the improvement of first-generation ethanol production, beyond the already excellent yeasts in the bioethanol industry, CAT1, PE2, and Ethanol Red. Apart from the capacity to produce higher concentrations of ethanol in 20 % sucrose or 33 % glucose (VHG), its capacity to actively survive very low pH (around 2.4) as well as aluminum toxicity suggests that it may ally a better ethanol performance with a high resilience to industrial harsh conditions. In addition, the foam production pattern observed is compatible with its industrial utilization with less expenditure in anti-foam agents.
Well-known processes used for decades (like bioethanol), or for centuries (like cachaça), are often not standardized or quality controlled as well as are poorly studied. Studies such as this are mandatory to generate molecular tools for monitoring industrial fermentations. However, more importantly, studies such as this demonstrate the importance of exploring the biodiversity associated with industrial environments, of c achaça production processes or others, to find the tools required to solve problems, to improve performance, or to generate novel processes required to ensure sustainability in the long run.
References
Almeida JRM, Modig T, Petersson A, Hähn-Hägerdal B, Lidén G, Gorwa-Grauslund MF (2007) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol 82:340–349
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pre-treatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851-486
Amorim HV, Lopes ML, de Castro Oliveira JV, Buckeridge MS, Goldman GH (2011) Scientific challenges of bioethanol production in Brazil. Appl Microbiol Biotechnol 91:1267–1275
Badotti F, Vilaça ST, Arias A, Rosa CA, Barrio E (2014) Two interbreeding populations of Saccharomyces cerevisiae strains coexist in cachaça fermentations from Brazil. FEMS Yeast Res 14(2):289–301
Bahafid W, Sayel H, Joutet N, Ghachtouli N (2011) Removal mechanism of hexavalent chromium by a novel strain Pichia anomala isolated from industrial effluents of Fez (Marocco). J Environ Eng Sci 5:980–991
Balat M, Balat H (2009) Recent trends in global production and utilization of bio-ethanol fuel. Appl Energ 86:2273–2282
Basso LC, Basso TO, Rocha S (2011) Ethanol production in Brazil: the industrial process and its impact on yeast fermentation. In: Bernardes MAS (ed) Biofuel production: recent developments and prospects. INTECH e-book, São Paulo
Basso LC, de Amorim HV, de Oliveira AJ, Lopes ML (2008) Yeast selection for fuel ethanol production in Brazil. FEMS Yeast Res 8:1155–1163
Beckner M, Ivey ML, Phister TG (2011) Microbial contamination of fuel ethanol fermentations. Lett Appl Microbiol 53:387–394
Betancur G, Pereira N (2010) Sugarcane bagasse as feedstock for second generation ethanol production, part I: diluted acid pretreatment optimization. Electron J Biotechnol 13(3):10
Choi WJ (2008) Glycerol-based biorefinery for fuels and chemicals. Recent Pat Biotechnol 2:173–180
de Souza Oliveira RP, Rivas Torres B, Zilli M, de Araújo Viana Marques D, Basso LC, Converti A (2009) Use of sugar cane vinasse to mitigate aluminum toxicity to Saccharomyces cerevisiae. Arch Environ Contam Toxicol 57:488–494
Demeke MM, Dumortier F, Li Y, Braeckx T, Foulquié-Moreno MR, Thevelein JM (2013) Combining inhibitory tolerance and D-Xylose fermentation in industrial Saccharomyces cerevisiae for efficient lignocellulose based bioethanol production. Biotechnol Biofuels 6:1–17
Ghosh S, Sachan A, Sen SK, Mitra A (2007) Microbial transformation of ferulic acid to vanillic acid by Streptomyces sannanensis MTCC 6637. J Ind Microbiol Biotechnol 34:131–138
Guillamón JM, Sabaté J, Barrio E, Cano J, Querol A (1998) Rapid identification of wine yeast species based on RFLP analysis of the ribosomal internal transcribed spacer (ITS) region. Arch Microbiol 169:387–392
Hamelinck C, Hooidjonk G, Faaij A (2005) Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenerg 28:384–410
Hansen C, Hernandez A, Mullan B, Moore K, Trezona-Murray M, King R, Pluske J (2009) A chemical analysis of samples of crude glycerol to growing-finishing pigs on performance, plasma metabolites and meat quality at slaughter. Anim Prod Sci 49:154–161
Huang H, Chen L, Tokashiki M, Ozawa T, Taira T, Ito S (2012) An endogenous factor enhances ferulic acid decarboxylation catalyzed by phenolic acid decarboxylase from Candida guilhermondii. AMB Express 2:4–14
Jeffries TW, Grigoriev IV, Grimwood J, Laplaza JM, Aerts A, Salamov A, Schmutz J, Lindquist E, Dehal P, Shapiro H, Jin YS, Passoth V, Richardson PM (2007) Genome sequence of the lignocellulose-bioconverting and xylose-fermenting yeast Pichia stipitis. Nat Biotechnol 25:319–326
Karmakar B, Vohra RM, Nandanwar H, Sharma P, Gupta KG, Sobti RC (2000) Rapid degradation of ferulic acid via 4-vinylguaiacol and vanillin by a newly isolated strain of Bacillus coagulans. J Biotechnol 80:195–202
Lages F, Silva-Graça M, Lucas C (1999) Active glycerol uptake is a mechanism underlying halotolerance in yeasts: a study of 42 species. Microbiol 145(Pt 9):2577–2585
Lee SJ, Kim SB, Kang SW, Han SO, Park C, Kim SW (2012) Effect of crude glycerol-derived inhibitors on ethanol production by Enterobacter aerogenes. Bioprocess Biosyst Eng 35:85–92
Lee YJ, Choi YR, Lee SY, Park JT, Shim JH, Park KH, Kim JW (2011) Screening wild yeast strains for alcohol fermentation from various fruits. Mycobiology 39:33–39
Levinson W, Kurantz C, Kuo T (2007) Characterization of Yarrowia lipolytica and related species for citric acid production from glycerol. Enz Microb Technol 41:292–295
Liu ZL, Moon J, Andersh BJ, Slininger PJ, Weber S (2008) Multiple gene-mediated NAD(P)H-dependent aldehyde reduction is a mechanism of in situ detoxification of furfural and 5-hydroxymethylfurfural by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 81:743–753
Looke M, Kristjuhan K, Kristjuhan A (2011) Extraction of genomic DNA from yeasts for PCR-based applications. Biotechniques 50:325–328
Masarin F, Gurpilhares DB, Baffa DC, Barbosa MH, Carvalho W, Ferraz A, Milagres AM (2011) Chemical composition and enzymatic digestibility of sugarcane clones selected for varied lignin content. Biotechnol Biofuels 4:55
Passoth V, Olstorpe M, Schnürer J (2011) Past, present and future research directions with Pichia anomala. Anton Van Leeuw 99:121–125
Pereira FB, Guimarães PMR, Teixeira JA, Domingues L (2011) Robust industrial Saccharomyces cerevisiae strains for very high gravity bio-ethanol fermentations. J Biosci Bioeng 112:130–131
Petersen PD, Lau J, Ebert B, Yang F, Verhertbruggen Y, Kim JS, Varanasi P, Suttangkakul A, Auer M, Loquél D, Scheller HV (2012) Engineering of plants with improved properties as biofuels feedstocks by vessel-specific complementation of xylan biosynthesis mutants. Biotechnol Biofuels 5:84
Posada JA, Rincón LE, Cardona CA (2012) Design and analysis of biorefineries based on raw glycerol: addressing the glycerol problem. Bioresour Technol 111:282–293
Pott RW, Howe CJ, Dennis JS (2013) Photofermentation of crude glycerol from biodiesel using Rhodopseudomonas palustris: comparison with organic acids and the identification of inhibitory compounds. Bioresour Technol 130:725–730
Purcena LLA, Di Medeiros MCB, Leandro WM, Fernandes KF (2014) Effects of organic and conventional management of sugar cane crop on soil physicochemical characteristics and phosphomonoesterase activity. J Agric Food Chem 62:1456–1463
Rivaldi JD, Sarrouh BF, Branco RF, de Mancilha IM, da Silva SS (2012) Biotechnological utilization of biodiesel-derived glycerol for the production of ribonucleotides and microbial biomass. Appl Biochem Biotechnol 167:2054–2067
Sabourin-Provost G, Hallenbeck PC (2009) High yield conversion of a crude glycerol fraction from biodiesel production to hydrogen by photofermentation. Bioresour Technol 100:3513–3517
Schwan RF, Mendonça AT, da Silva JJ Jr, Rodrigues V, Wheals AE (2001) Microbiology and physiology of Cachaça (Aguardente) fermentations. Anton Van Leeuw 79:89–96
Sutherland JB, Crawford DL, Pometto AL (1983) Metabolism of cinnamic, p-coumaric and ferulic acids by Streptomyces setonii. Can J Microbiol 29:1253–1257
Taccari M, Canonico L, Comitini F, Mannazzu I, Ciani M (2012) Screening of yeasts for growth on crude glycerol and optimization of biomass production. Bioresour Technol 110:488–495
Tao N, Gao Y, Liu Y (2011) Isolation and characterization of a Pichia anomala strain: a promising candidate for bioethanol production. Braz J Microbiol 42:668–675
Vadkertiová R, Sláviková E (2006) Metal tolerance of yeasts isolated from water, soil and plant environments. J Basic Microbiol 46:145–152
Vicente MA, Fietto LG, Castro IM, dos Santos AN, Coutrim MX, Brandão RL (2006) Isolation of Saccharomyces cerevisiae strains producing higher levels of flavoring compounds for production of “cachaça” the Brazilian sugarcane spirit. Int J Food Microbiol 108:51–59
Walker GM (2011) Pichia anomala: cell physiology and biotechnology relative to other yeasts. Anton Van Leeuw 99:25–34
White T, Bruns T, Lee S, Taylor T (1990) Amplification and direct sequencing of fungal RNA genes for phylogenetic. Academic Press, New York
Yang F, Hanna MA, Sun R (2012) Value-added uses for crude glycerol: a byproduct of biodiesel production. Biotechnol Biofuels 5:13
Zaldivar J, Nielsen J, Olsson L (2001) Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol 56:17–34
Acknowledgments
This work was supported by grants from Fundação de Capacitação de Pessoal de Nível Superior from the Ministry of Education, CAPES/Brazil (PNPD 2755/2011; PCF-PVE 021/2012) and from Universidade Federal de Ouro Preto, Fundação de Amparo à Pesquisa do Estado de Minas Gerais, FAPEMIG (Process APQ-00263-10) and a research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq (Brazil) Process 304815/2012. C. Lucas is supported by FCT/MEC through Portuguese funds (PIDDAC), PEst-OE/BIA/UI4050/2014 and by a grant of Visiting Professor from the programme “Ciência sem Fronteiras”, CAPES, Brazil, Process 2021/2012.
Conflict of interest
Authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10295_2014_1528_MOESM1_ESM.tif
S1: Screening of wild yeast collection for multiple factors associated to biodiesel and bioethanol waste products. All 118 strains were tested for their ability to use glycerol (2–25%, w/v), xylose, ferulic acid, coumaric acid or maltose (2%, w/v) as carbon sources. Yeasts were also tested for their tolerance to methanol (2–20%, v/v), ethanol (2–20%, v/v), aluminum (5 mmol/L Al2(SO4)3 and 10 mmol/L AlCl3) and zinc (5 mmol/L ZnSO4). Xyl: xylose, Fer: ferulic acid, Cou: p-coumaric acid (TIFF 1851 kb)
10295_2014_1528_MOESM2_ESM.tif
S2: Profile of ethanol production in small-scale fermentation of different yeast strains, in 20% sucrose (A), and 33% glucose—VHG fermentation (B). The production of ethanol of the strains LBCM07 (⋆), LBCM17 (▼), LBCM28 (◆), LBCM44 (......), LBCM65 (■), LBCM67 (□), LBCM105 (×), LBCM107 (◊) compared with PE2 (●) (A). Production of ethanol by strains LBCM07 (⋆), LBCM10 (×), LBCM17 (▼), LBCM23 (+), LBCM28 (◆), LBCM44 (......), LBCM65 (■), LBCM105 (×), LBCM107 (◊) compared with PE2 (●) (B) (TIFF 1631 kb)
10295_2014_1528_MOESM3_ESM.tif
S3: Profile of foaming in small-scale fermentation of different yeast strains: PE2 (industrial strain) (●), LBCM07 (⋆), LBCM10 (×), LBCM13 (◯), LBCM17 (▼), LBCM23 (+), LBCM25 (△), LBCM28 (◆), LBCM44 (......), LBCM47 (-), LBCM65 (■), LBCM67 (□), LBCM105 (×), and LBCM107 (◊). Fermentation of 20% sucrose (A, C and E) and 33% glucose (VHG) fermentation (B, D and F). The strains LBCM10, LBCM28, and LBCM44 showed high foam production during the entire fermentation (A). The strains LBCM13, LBCM17, LBCM23, LBCM65, LBCM67, LBCM105, and LBCM107 presented foaming with a gradual decrease, but without ceasing it (C). The strains PE2, LBCM07, LBCM25, and LBCM47 did not show foaming after 72 h (E). The strain LBCM10 presented constant foam production during the entire fermentation (B). The strain LBCM28 presented less intense but persistent foaming during the fermentation (D). In strains LBCM07, LBCM13, LBCM17, LBCM23, LBCM25, LBCM44, LBCM47, LBCM65, LBCM67, LBCM105, LBCM107, and PE2, the production of foam not occurred during the entire fermentation (F) (TIFF 1752 kb)
Rights and permissions
About this article
Cite this article
da Conceição, L.E.F.R., Saraiva, M.A.F., Diniz, R.H.S. et al. Biotechnological potential of yeast isolates from cachaça: the Brazilian spirit. J Ind Microbiol Biotechnol 42, 237–246 (2015). https://doi.org/10.1007/s10295-014-1528-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1528-y