Abstract
Sparassis crispa contains various bioactive substances, such as β-glucan, which exhibits antitumor activity. In this study, we investigated the effect of the mycelial shape of S. crispa and the combination of flask type and agitation method on β-glucan production. With the combination of the Erlenmeyer flask and shaken culture, the mycelia grew in the shape of pellets, whereas with the combination of the baffled Erlenmeyer flask and stirred culture, the mycelia grew in the shape of filaments. The dried cell weight (DCW) and β-glucan production of the filamentous mycelia were 5.91 g/L and 1.71 g/L, respectively, 1.34-fold and 1.73-fold higher, respectively, than that of the pelleted mycelia (4.42 g/L and 0.99 g/L, respectively). The production was further increased using the homogenization process; the DCW was 1.03-fold (7.23 g/L) higher and β-glucan production 1.34-fold (3.50 g/L) higher, respectively, than that without the treatment (7.01 g/L and 2.61 g/L, respectively). In the filamentous mycelia, β-glucan production increased with suppressed ethanol production, and a negative correlation was observed between β-glucan production and ethanol production. In the cultivation of S. crispa mycelia, filamentous mycelia have been suggested to be more suitable for β-glucan production than pelleted mycelia.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mushrooms have long been considered a food source. Recently, they have attracted considerable attention owing to their therapeutic potential and have become a new subject for research. Nutritionally, they are rich in essential proteins, indigestible carbohydrates, unsaturated fats, minerals, and various vitamins, which have led to the increased consumption and development of various processed products [1]. Many studies have been conducted on the antitumor activity of mushrooms. The therapeutic effects of mushrooms include inhibition of cancer cell proliferation, dysregulation of the proportion of cells in cell-cycle phases, induction of autophagy and phagocytosis, improvement of the immune system response, and induction of apoptosis via upregulation of pro-apoptotic factors and downregulation of anti-apoptotic genes [2]. Furthermore, recent studies have shown that the intake of mushrooms can reduce the probability of depression [3].
Sparassis crispa, known as Hanabiratake in Japan, has been described as a mushroom with potential therapeutic applications. The medicinal properties of this mushroom are mainly attributed to its rich content of six-branched 1,3-β-glucan (SCG). Although the degree of branching is relatively lower than that of SCG, the mycelia of S. crispa have been reported to contain similar glucans [4, 5]. The medical effects of SCG have been reported to enhance hematopoietic response [5], activate human leukocytes and the immune system [6], inhibit tumor-induced angiogenesis, suppress tumor growth and metastasis in the lungs [7], promote the expression of inducible NO synthase (iNOS) in macrophage-like RAW264.7 cells [8], prevent stroke and hypertension [9], and increase the synthesis of type I collagen [10]. Three new phthalides (Hanabiratakelide A (1), B (2), and C (3)) were isolated from the fruiting bodies of S. crispa, and these phthalides showed stronger superoxide dismutase-like activity than vitamin C and exhibited antioxidant, anti-inflammatory, and antitumor activities [11].
As observed in the cultivation of Ganoderma lucidum, the solid culture of mushroom germplasm on wood, grain, and sawdust takes 2 to 3 months for harvesting. [12]. According to a survey of several strains of S. crispa, the cultivation of S. crispa fruit bodies on solid culture required 2–3 months for mycelium growth and another 1–2 months for harvesting [13]. To solve these problems, several mycelial submerged cultures have been studied for obtaining functional substances such as polysaccharides [14, 17,18,19]. Polysaccharides, the main bioactive substance in mushrooms, can be isolated not only from mushroom fruiting bodies but also from cultured mycelia and culture broth [15]. The challenge in submerged mushroom culture is to achieve the same production of bioactive substances as that in the cultivation of mushroom fruiting bodies. Considering the advantages of shorter culture time and reproducibility, submerged culture is the best technology for mushroom cultivation at the industrial level [16].
In submerged cultures of mushroom mycelia, the cells receive and metabolize nutrients, creating spherical mycelial pellets of different sizes [17]. Several studies have been conducted for controlling the pellet size formed by the mycelia. The pellet size can be minimized by adding agar or carboxymethyl cellulose, which are high-molecular-weight compounds, to the culture medium [18, 19]. When a pellet becomes larger than a certain size, self-digestion of the center of the pellet occurs because of lack of oxygen inside the pellet, and the center of the pellet is absent [18]. As autolysis has an important effect on cell metabolism and product synthesis [20, 21], control of pellet size is necessary to efficiently increase the mycelia in the pellet state. The culture methods used for forming these pellets include shaking and airlift bioreactor cultures. Shaking cultures have been predominantly used in several previous studies [22, 23]. However, common bioreactors and industrial-scale culture tanks are generally stirred with agitator blades. In submerged cultures, in addition to the composition of the medium, the intensity of agitation has a significant effect on the formation and structure of the pellets. This is another major factor that affects the formation and structure of the pellets. Especially in bioreactors aimed at industrialization, the effects of agitation speed and shearing action of agitator blades on the morphology and productivity of filamentous fungi could be factors to be considered. An inverse relationship exists between the agitation strength and pellet size. The pellets become smaller and more compact with stronger agitation, which is considered to prevent the formation of pellets. Such observations have been documented for several filamentous fungi [18, 24, 25]. The shearing effect of agitator blades causes an obvious decrease in mycelial yield and polysaccharide production [26]; however, some studies have shown effective results in mycelial cultivation in agitated or homogenized cultures [26, 27]. Therefore, it is necessary to investigate the effects of agitator blades and water flow on mycelium growth for achieving the mass cultivation of mycelia. In this study, we used an agitator as a model for agitator blades and compared it with a shaken culture to determine the amount of DCW, β-glucan, and ethanol.
Baffled Erlenmeyer flasks show superior mass transfer capacity and shear formation compared with flasks without baffles [28]. An increase in agitation rate generates shear stress, which is usually detrimental to cell integrity [29] and reduces the size of the mycelial aggregates. In submerged cultures of Ganoderma lucidum, the initial volumetric oxygen transfer coefficient affected the growth rate of cells and production of ganoderic acid [30]. Therefore, baffled Erlenmeyer flasks are expected to be suitable for efficient production of mycelia.
In this study, we examined the efficient production of β-glucan by adjusting pellet size using physical methods without additives in the cultivation of S. crispa mycelia. In addition to using a baffled Erlenmeyer flask instead of a normal Erlenmeyer flask, we examined stirred cultures using an agitator instead of shaking and investigated combinations of miniaturization to identify the most efficient conditions for β-glucan production through S. crispa mycelium culture.
2 Materials and methods
2.1 Microorganism
Sparassis crispa (Oze Trading Co., Japan) was used in this study. The mycelia were cultured on potato dextrose agar (FUJIFILM Wako Pure Chemical Industries Co.) plates at 25 °C.
2.2 Medium and culture method
Pre-culture was performed by dispensing 50 mL of basic medium (Basal medium; glucose 30 g/L, polypeptone 6.3 g/L, yeast extract 0.2 g/L, Kirk salt 10 mL/L) adjusted to pH 5.0 with 0.4 M phthalate buffer into 200-mL Erlenmeyer flasks [31]. A 5-mm-square piece of S. crispa mycelium was inoculated into an Erlenmeyer flask filled with basal medium. The compositions of the Kirk salt and Kirk trace element solutions are shown in Tables 1 and 2, respectively. All medium components were purchased from Wako Pure Chemicals. The inoculated flasks were incubated in a rotary shaker (TAITEC, BR-300LF) at 25 °C and 100 rpm for 7 days. For the main culture, 60 mL of the basic medium was dispensed into 300-mL Erlenmeyer flasks. The preculture medium was homogenized (15,000 rpm for 20 s) using a polytron homogenizer (Model PT-MR 2100, Kinematica AG, Switzerland) and inoculated into the main culture flask at 2% (v/v). After inoculation, flasks were incubated at 25 °C. In the main culture, the glucose concentration was measured at least every 2 days, and the incubation time was defined as the number of days taken for the residual glucose concentration to reach 0 g/L.
2.3 Cultivation
2.3.1 Comparison of flask shape and agitation method
We investigated the effect of a normal 300-mL Erlenmeyer flask and a 300-mL baffled Erlenmeyer flask on mycelial growth. The baffled Erlenmeyer flask has three protrusions inside, which can generate a complex water flow in the liquid inside the flask. In addition to shaking the culture with a rotary shaker, stirred culture with an agitator and a magnetic stirrer (as shown in Fig. 1) was examined to investigate the effect of the combination of flask shape and agitation method on mycelial growth.
2.3.2 Examination of stirring speed in stirred culture and medium volume
The effect of agitation speed on mycelial growth was investigated in a stirred culture. The optimal agitation speed for β-glucan production was investigated at 250, 500, and 750 rpm in a 300-mL baffled Erlenmeyer flask with 60 mL of culture medium.
We examined the effect of the culture medium volume on mycelial growth. Change in the medium volume is expected to alter the contact frequency between the agitator and mycelium and affect the shape of the mycelium. We investigated the effect of four different culture volumes, 20, 40, 60, and 100 mL, on mycelium shape; the cultures were placed in a 300-mL baffled Erlenmeyer flask and stirred at 500 rpm.
2.3.3 Examination of homogenization
A previous study on a culture of Lentinus edodes showed that the mycelial fragments in the culture medium increased with homogenization treatment and the number of mycelial fragments increased with homogenization time [27]. Mycelial fragments are considered to facilitate access to nutrient sources and dissolved oxygen. Therefore, the homogenization process would enable efficient production of mycelia. The effect of homogenization on the incubation period, DCW, and β-glucan content was examined for shaken and stirred cultures in 300-mL flasks containing 40 mL of medium. The homogenization was performed using a homogenizer (Model PT-MR 2100, Kinematica AG, Switzerland) at 15,000 rpm for 10 s every 2 days of the incubation period.
2.4 Analysis method
After incubation, the mycelia were filtered from the culture medium using a filter paper (Advantec no. 131) and washed with distilled water five times the volume of culture medium. The washed mycelia were dried in a constant-temperature dryer at 37 °C for 2 days, cooled to room temperature in a desiccator under reduced pressure, and subsequently weighed. The dried cell weight (DCW) was measured using the method described above. The glucose concentration was determined using the mutarotase GOD method (FUJIFILM Wako Pure Chemical Industries, Co.). Ethanol content was determined by analyzing the supernatant obtained by filtration using HPLC (Shimadzu RID-10A). The column used for HPLC was an Aminex HPX-87X (300 mm × 7.8 mm), and the column temperature was 65 °C. β-Glucan in DCW was measured using a mushroom and yeast β-glucan assay kit (Megazyme International Ireland Ltd., Bray Business Park Co.). All experiments were performed in triplicate, and the average values of the three tests are reported.
3 Results and discussion
3.1 Comparison of shaken and stirred cultures in normal and baffled Erlenmeyer flasks
Table 3 presents the incubation days, DCW, amount of β-glucan, and amount of ethanol produced for S. crispa mycelia under the combination of normal/baffled Erlenmeyer flasks and shaken/stirred culture conditions. The incubation days were less for the shaken culture than for the stirred culture, and the DCW and β-glucan content tended to be higher with the baffled flask than with the normal flask. The shortest incubation period was 8 days, for the baffled Erlenmeyer flask/shaken culture condition, and the condition with the maximum DCW was 5.91 ± 0.32 g/L for the baffled Erlenmeyer flask/stirred culture. Using the same agitation method, mycelial growth was enhanced in the baffled Erlenmeyer flask compared to that in the normal Erlenmeyer flask, indicating that the baffled Erlenmeyer flask was superior in promoting mycelial growth. One reason for this superiority is assumed to be that the baffles in the Erlenmeyer flask increased the oxygen supply because of sufficient agitation. Furthermore, the amount of ethanol was higher in the shaken culture than in the stirred culture, and the amount of ethanol was higher in the normal flask than in the baffled flask, which is in contrast to the trend for the DCW and β-glucan amounts.
Figure 2 shows the mycelium shape of S. crispa at the end of cultivation under each condition. Typically, pellets of various sizes are formed in a normal Erlenmeyer flask and shaken. However, even in the same culture, the use of a baffled Erlenmeyer flask minimized pellet size. This is considered to be the result of the complex water flow created by the three baffled protrusions inside the Erlenmeyer flask, which prevented the mycelia from connecting with each other and led to miniaturization of the pellets. Studies on Aspergillus niger have shown that hyphal cells usually form twisted pellets in Erlenmeyer flasks, whereas they mainly form loose clumps in baffled Erlenmeyer flasks [28]. Meanwhile, in the stirred culture, S. crispa mycelia grew as filaments rather than pellets in both normal and baffled Erlenmeyer flasks. This may be because of the physical shearing of the mycelia by the agitator. Furthermore, more filamentous mycelia appeared to form in the baffled Erlenmeyer flasks than in the normal Erlenmeyer flasks. The synergistic effect of the combination of the stirrer and the baffles possibly render the mycelia even finer, and consequently, the mycelium was dispersed so that it covered the entire culture medium. Therefore, the amounts of DCW and β-glucan increased significantly in the baffled Erlenmeyer flask culture because sufficient oxygen supply prevented autolysis from occurring inside the pellet and allowed efficient growth through aerobic respiration.
When producing a target substance in a reactor, an agitated culture is used, and, sometimes, the shearing force of the agitator blades becomes a problem. In this case, the agitator blades rotate at a high speed, and the shearing force at the tip of the blades is large, which can easily damage filamentous fungi such as molds and actinomycetes, adversely affecting the production of useful substances such as itaconic acid from Aspergillus niger [32, 33]. However, the amount of mycelia obtained using the stirred culture was higher than that obtained using the shaking culture (Table 3). S. crispa mycelia have been suggested as being tolerant to physical stress, and comparable productivity can be possibly achieved in reactors or vessels using agitator blades.
Meanwhile, the consumption rate of glucose decreased, and the incubation time increased for the stirred culture compared to that for the shaking culture. The reason for this effect is considered to be the increase in the viscosity of the culture medium as the mycelia became filamentous due to stirring. The high viscosity of the medium restricted the rate of mass transfer of nutrients [34], and the reduced rate of glucose consumption extended the cultivation period. Ethanol production after the end of the culture period was higher in the shaken culture than in the stirred culture, and higher β-glucan was obtained in the stirred culture than in the shaken culture. The same tendency was observed in the comparison between the normal and baffled flasks. In shaken culture and/or normal Erlenmeyer flasks, mycelia grow as pellets; thus, glucose is probably consumed anaerobically, except on the pellet surface. Ethanol is mainly produced via glycolysis. Therefore, more glycolysis occurred in the shaken culture. Meanwhile, in stirred culture and/or baffled flasks, oxygen supply to the filamentous mycelia was sufficient, and aerobic respiration was more dominant, which probably led to an increased production of β-glucan under efficient glucose metabolism.
3.2 Effect of stirring speed in stirred culture
Table 4 presents the results for S. crispa mycelium cultivation under 250, 500, and 750 rpm stirring conditions, using the shaken culture as the control. At a stirring speed of 250 rpm, the DCW was almost the same as that of the control shaken culture (5.56 ± 0.58 g/L). However, the incubation time of the stirred culture was more than twice that of the shaking culture (18 days). At 250 rpm, the agitation was insufficient to supply oxygen, and the incubation time probably increased significantly. In the stirred culture, the rate of glucose consumption increased with stirring speed, and the incubation time was shortened from 18 days (250 rpm) to 13 days (500 and 750 rpm). In a culture of Trichoderma harzianum, the yield of 6-pentyl-α-pyrone was reported to decrease with increasing stirring rate [35]. Hydrodynamic stress above a certain level has been suggested to exert a negative effect on mycelial growth.
Figure 3 shows the shape of the S. crispa mycelia at the end of incubation at each stirring speed. The mycelial pellets became finer as the agitation speed was increased. A higher agitation speed is considered to increase the shearing force in the culture medium and prevent pellet formation. Consistent with the results presented in Table 3, the amount of β-glucan increased and the amount of ethanol decreased as filamentation progressed.
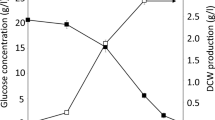
From the above results, the culture conditions (shape of the Erlenmeyer flask and agitation method) appear to affect the shape of the mycelia; consequently, DCW, β-glucan production, and ethanol production varied significantly. Figure 4 presents the correlation of these three factors with the culture and agitation conditions. A positive correlation was observed between β-glucan content and DCW. As β-glucan is a component of the mycelial cell wall, an increase in DCW leads to an increase in β-glucan. In contrast, a negative correlation was observed between the amounts of β-glucan and ethanol. Upon comparing the correlation diagram with the shape of the mycelia at the end of cultivation, we observed that the amount of ethanol produced was higher and the amount of β-glucan was lower when the mycelia grew as pellets, whereas the amount of ethanol production was lower and the amount of β-glucan was higher when the mycelia grew as filaments. This is probably because when the mycelia form pellets, oxygen deficiency occurs inside the pellets, and glucose is consumed by anaerobic respiration (ethanol fermentation) instead of aerobic respiration, which prevents sufficient growth of the mycelia. Furthermore, autolysis was reported to occur at the center of all pellets larger than 2 mm, among the pellets obtained from a culture of Phanerochaete chrysosporium, which is a basidiomycete, as is S. crispa [24].
3.3 Effect of medium volume in stirred culture
Table 5 presents the results of S. crispa mycelium cultivation in 20, 40, 60, and 100 mL of medium. DCW was significantly lower when the volume of culture medium was 20 or 100 mL than when it was 40 or 60 mL. The maximum DCW, 6.79 g/L, was obtained at 40 mL of culture volume. The maximum amount of β-glucan, 2.88 g/L, was also obtained at 40 mL. In contrast, the amount of ethanol was maximum, 11.27 g/L, when the volume of the culture solution was 20 mL.
The shape of the mycelia at the end of the incubation period is shown in Fig. 5. At a volume of 20 mL, it is in the form of a pellet; however, at a volume of 40 mL or more, filaments are formed. One reason for this effect could be that the liquid level was lower than that of the agitator owing to the lower volume of the culture liquid. Because the agitator was not in the liquid, the mixing of the culture broth was insufficient, causing the mycelia to stagnate. This may have caused the mycelia to agglomerate and form large pellets. The DCW and β-glucan contents were the lowest among the four examined species (4.73 g/L and 1.22 g/L, respectively), probably because of the internal oxygen deficiency caused by pellet formation. In contrast, when the medium volume was 100 mL, S. crispa mycelia did not grow as filamentous as when the volume was 40 or 60 mL. This may be because under the same stirring conditions (500 rpm) with a larger amount of culture medium, the movement of the medium became slower, which may have caused aggregation and pelleting of the mycelia. The amount of dissolved oxygen was reported to decrease as with increase in medium volume at the same agitation speed [36]. At 100 mL, agitation was insufficient, and the supply of nutrients and oxygen became inefficient, which is believed to have extended the incubation period. Although not as large as 20 mL, a decrease in β-glucan production and an increase in ethanol production were observed; these are characteristics of mycelia that have grown into pellets.
3.4 Effect of homogenization on mycelium culture
As pellets were formed, β-glucan production decreased, and ethanol production increased. This is attributed to the lack of oxygen inside the pellets. However, it is assumed that the production of β-glucan can be increased if the pellet size can be intentionally reduced. Therefore, the physical miniaturization of mycelium pellets by homogenization was expected to exhibit a very similar trend to the growth of filamentous mycelia in the shaken culture.
Table 6 presents the incubation days, amount of DCW, β-glucan, and ethanol production of S. crispa mycelia cultivated with homogenization treatment. As expected, the amounts of DCW and β-glucan were higher, and ethanol production was lower in the homogenized culture for both the shaken and stirred cultures. Interestingly, the effect of homogenization differed between the shaken and stirred cultures. In the shaken cultures, homogenization increased the DCW and β-glucan content by up to 1.33- and 1.39-fold, respectively. The correlation between the amounts of β-glucan, DCW, and ethanol production is shown in Fig. 4. The amount of β-glucan increased as DCW increased. The amount of DCW and β-glucan increased in the shaking culture because the homogenization prevented an anaerobic condition in the pellet and mycelia, which would normally undergo autolysis. In contrast, homogenization in the stirred culture resulted in 1.03-fold higher DCW and 1.34-fold higher β-glucan production. The increase in β-glucan was approximately 30% greater than the increase in DCW. The pellet mycelia grew after withstanding the shearing force of the homogenizer; however, the filamentous mycelia were damaged by the strong shearing force of the homogenizer. As lactic acid bacteria secrete extracellular polysaccharides to protect themselves from environmental stress [37,38,39], basidiomycetes might secrete extracellular polysaccharides to prevent damage. Secreted extracellular polysaccharides may interact with the filamentous mycelia and grow into larger filamentous mycelia, forming a biofilm. The DCWs obtained in this manner contain a large amount of extracellular polysaccharides, which may have resulted in an increase in β-glucan content. Figure 6 shows the shape of the S. crispa mycelia at the end of the culture. Homogeneous pellets and filamentous mycelia were formed by miniaturization in both shaken and stirred cultures. In the shaken culture, the homogenized mycelia re-agglomerated and formed pellets because of the shaking action, while in the stirred culture, the finely divided mycelia grew into filaments. Because the presence of pellets affected ethanol production, the ethanol production in the shaken culture was higher than that in the stirred culture.
4 Conclusion
In the culture of S. crispa mycelia, filamentous mycelial growth suppressed ethanol production and promoted β-glucan production, leading to a negative correlation between ethanol production and β-glucan production. The optimal conditions were as follows: stirred culture in a baffled flask, 40 mL of culture medium, and homogenization during culture. Compared to the corresponding values for the conditions before the study (normal Erlenmeyer flask, shaken culture, 60 mL of culture medium, no homogenization), the DCW was 1.11-fold higher (7.23 g/L); β-glucan content, 1.77-fold higher (3.50 g/L); and ethanol content, 0.26-fold lower (1.48 g/L). However, since filamentous mycelia tend to require a longer incubation period, further research is needed regarding shortening the incubation period for producing β-glucan more efficiently.
Data availability
Not applicable.
Code availability
Not applicable.
References
Yadav D, Negi PS (2021) Bioactive components of mushrooms: processing effects and health benefits. Food Res Int 148:110599. https://doi.org/10.1016/j.foodres.2021.110599
Nowakowski P, Markiewicz-Żukowska R, Bielecka J, Mielcarek K, Grabia M, Socha K (2021) Treasures from the forest: evaluation of mushroom extracts as anti-cancer agents. Biomed Pharmacother 143:112106. https://doi.org/10.1016/j.biopha.2021.112106
Ba DM, Gao X, Al-Shaar L, Muscat JE, Chinchilli VM, Beelman RB, Richie JP (2021) Mushroom intake and depression: a population-based study using data from the US National Health and Nutrition Examination Survey (NHANES), 2005–2016. J Affect Disord 294:686–692. https://doi.org/10.1016/j.jad.2021.07.080
Kimura T (2013) Natural products and biological activity of the pharmacologically active cauliflower mushroom Sparassis crispa. Biomed Res Int 2013:982317. https://doi.org/10.1155/2013/982317
Ohno N, Miura NN, Nakajima M, Yadomae T (2000) Antitumor 1,3-beta-glucan from cultured fruit body of Sparassis crispa. Biol Pharm Bull 23:866–872. https://doi.org/10.1248/bpb.23.866
Ohno N, Nameda S, Harada T, Miura NN, Adachi Y, Nakajima M, Yoshida K, Yoshida H, Yadomae T (2003) Immunomodulating activity of a β-glucan preparation, SCG, extracted from a culinary–medicinal mushroom, Sparassis crispa Wulf.:Fr. (Aphyllophoromycetideae), and application to cancer patients. Int J Med Mushrooms 5:359–368. https://doi.org/10.1615/InterJMedicMush.v5.i4.30
Yamamoto K, Kimura T, Sugitachi A, Matsuura N (2009) Anti-angiogenic and anti-metastatic effects of β-1,3-D-glucan purified from Hanabiratake, Sparassis crispa. Biol Pharm Bull 32:259–263. https://doi.org/10.1248/bpb.32.259
Lee SY, Lee YG, Byeon SE, Han S, Choi SS, Kim AR, Lee J, Lee SJ, Hong S, Cho JY (2010) Mitogen activated protein kinases are prime signalling enzymes in nitric oxide production induced by soluble β-glucan from Sparassis crispa. Arch Pharm Res 33:1753–1760. https://doi.org/10.1007/s12272-010-1107-3
Yoshitomi H, Iwaoka E, Kubo M, Shibata M, Gao M (2011) Beneficial effect of Sparassis crispa on stroke through activation of Akt/eNOS pathway in brain of SHRSP. J Nat Med 65:135–141. https://doi.org/10.1007/s11418-010-0475-9
Kwon AH, Qiu Z, Hashimoto M, Yamamoto K, Kimura T (2009) Effects of medicinal mushroom (Sparassis crispa) on wound healing in streptozotocin-induced diabetic rats. Am J Surg 197:503–509. https://doi.org/10.1016/j.amjsurg.2007.11.021
Yoshikawa K, Kokudo N, Hashimoto T, Yamamoto K, Inose T, Kimura T (2010) Novel phthalide compounds from Sparassis crispa (Hanabiratake), Hanabiratakelide A-C, exhibiting anti-cancer related activity. Biol Pharm Bull 33:1355–1359. https://doi.org/10.1248/bpb.33.1355
Yang H, Min W, Bi P, Zhou H, Huang F (2013) Stimulatory effects of Coix lacryma-jobi oil on the mycelial growth and metabolites biosynthesis by the submerged culture of Ganoderma lucidum. Biochem Eng J 76:77–82. https://doi.org/10.1016/j.bej.2013.04.012
Ryu SR, Ka KH, Park H, Bak WC, Lee BH (2009) Cultivation characteristics of Sparassis crispa strains using sawdust medium of Larix kaempferi. Kor J Mycol 37:49–54. https://doi.org/10.4489/KJM.2009.37.1.049
Singh U, Gautam A, Singha TK, Tiwari A, Tiwari P, Sahai V, Sharma S (2020) Mass production of Pleurotus eryngii mycelia under submerged culture conditions with improved minerals and vitamin D2. LWT 131:109665. https://doi.org/10.1016/j.lwt.2020.109665
Wasser SP (2011) Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl Microbiol Biotechnol 89:1323–1332. https://doi.org/10.1007/s00253-010-3067-4
Bakratsas G, Polydera A, Katapodis P, Stamatis H (2021) Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods 4:100086. https://doi.org/10.1016/j.fufo.2021.100086
Petre M, Petre P (2016) Chapter 1 - Biotechnology of mushroom growth through submerged cultivation. In: Petre M (ed) Mushroom Biotechnology. Academic Press, Cambridge, pp 1–18
Yang FC, Yang MJ, Cheng SH (2009) A novel method to enhance the mycelia production of Ganoderma lucidum in submerged cultures by polymer additives and agitation strategies. J Taiwan Inst Chem Eng 40:148–154. https://doi.org/10.1016/j.jtice.2008.09.003
Wen Y, Erika AH, Aiqi F, Arnold LD (2003) Effects of carboxymethylcellulose and carboxypolymethylene on morphology of Aspergillus fumigatus NRRL 2346 and fumagillin production. Curr Microbiol 46:24–27. https://doi.org/10.1007/s00284-002-3711-z
Perez-Leblic MI, Reyes F, Martinez MJ, Lahoz R (1982) Cell wall degradation in the autolysis of filamentous fungi. Mycopathologia 80:147–155. https://doi.org/10.1007/BF00437577
Xu N, Liu Y, Hu Y, Zhou M, Wang C, Dongsheng L (2016) Autolysis of Aspergillus oryzae mycelium and effect on volatile flavor compounds of soy sauce. J Food Sci 81:1883–1890. https://doi.org/10.1111/1750-3841.13396
Asada C, Okumura R, Sasaki C, Nakamura Y (2012) Acceleration of Hericium erinaceum mycelial growth in submerged culture using yogurt whey as an alternative N source. Adv Biosci Biotechnol 3:828–832. https://doi.org/10.4236/abb.2012.37103
Okumura R, Nakamura Y, Sasaki C, Asada C (2021) Effects of Tween series and agar additives on mycelia biomass and β-glucan production by Hericium erinaceus in submerged culture. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01342-y
Gibbs PA, Seviour RJ, Schmid F (2000) Growth of filamentous fungi in submerged culture: Problems and possible solutions. Crit Rev Biotechnol 20:17–48. https://doi.org/10.1080/07388550091144177
Papagianni M (2004) Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv 22:189–259. https://doi.org/10.1016/j.biotechadv.2003.09.005
Lee BC, Bae JT, Pyo HB, Choe TB, Kim ST, Hwang HJ, Yun JW (2004) Submerged culture conditions for the production of mycelial biomass and exopolysaccharides by the edible basidiomycete Grifola frondose. Enzyme Microb Technol 35:369–376. https://doi.org/10.1016/j.enzmictec.2003.12.015
Tan YH, David M (1992) Convenient and effective methods for in vitro cultivation of mycelium and fruiting bodies of Lentinus edodes. Mycol Res 96:1077–1084. https://doi.org/10.1016/S0953-7562(09)80119-6
Li C, Xia JY, Chu J, Wang YH, Zhuang YP, Zhang SL (2013) CFD analysis of the turbulent flow in baffled shake flasks. Biochem Eng J 70:140–150. https://doi.org/10.1016/j.bej.2012.10.012
Rossi MJ, Nascimento FX, Giachini AJ, Oliveira VL, Furigo A Jr (2017) Transfer and consumption of oxygen during the cultivation of the ectomycorrhizal fungus Rhizopogon nigrescens in an airlift bioreactor. Appl Microbiol Biotechnol 101:1013–1024. https://doi.org/10.1007/s00253-016-7854-4
Tang YJ, Zhong JJ (2003) Role of oxygen supply in submerged fermentation of Ganoderma lucidum for production of Ganoderma polysaccharide and ganoderic acid. Enzyme Microb 32:478–484. https://doi.org/10.1016/S0141-0229(02)00338-1
Kurosumi A, Kobayasi F, Mtui G, Nakamura Y (2006) Development of optimal culture method of Sparassis crispa mycelia and a new extraction method of antineoplastic constituent. Biochem Eng J 30:109–113. https://doi.org/10.1016/j.bej.2006.02.004
Okabe M, Lies D, Kanamasa S, Park EY (2009) Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl Microbiol Biotechnol 84:606–697. https://doi.org/10.1007/s00253-009-2132-3
Amanullah A, Christensen LH, Hansen K, Nienow AW, Thomas CR (2002) Dependence of morphology on agitation intensity in fed-batch cultures of Aspergillus oryzae and its implications for recombinant protein production. Biotechnol Bioeng 30:815–826. https://doi.org/10.1002/bit.10181
Kawagoe M, Hyakumura K, Suye S, Miki K, Naoe K (1997) Application of bubble column fermentors to submerged culture of Schizophyllum commune for production of L-malic acid. J Ferment Bioeng 84:333–336. https://doi.org/10.1016/S0922-338X(97)89254-9
Galindo E, Flores C, Larralde-Corona P, Corkidi-Blanco G, Rocha-Valadez JA, Serrano-Carreón L (2004) Production of 6-pentyl-α-pyrone by Trichoderma harzianum cultured in unbaffled and baffled shake flasks. Biochem Eng J 18:1–8. https://doi.org/10.1016/S1369-703X(03)00115-3
McDaniel LE, Bailey EG, Zimmerli A (1965) Effect of oxygen supply rates on growth of Escherichia coli: I. Studies in unbaffled and baffled shake flasks. Appl Microbiol 13:109–114. https://doi.org/10.1128/am.13.1.109-114.1965
Pittet V, Morrow K, Ziola B (2011) Ethanol tolerance of lactic acid bacteria, including relevance of the exopolysaccharide gene Gtf. J Am Soc Brew Chem 69:57–61. https://doi.org/10.1094/ASBCJ-2011-0124-01
Suzuki S, Kimoto-Nira H, Suganuma H, Suzuki C, Saito T, Yajima N (2014) Cellular fatty acid composition and exopolysaccharide contribute to bile tolerance in Lactobacillus brevis strains isolated from fermented Japanese pickles. Can J Microbiol 60:183–191. https://doi.org/10.1139/cjm-2014-0043
Sabir F, Beyatli Y, Cokmus C, Onal-Darilmaz D (2010) Assessment of potential probiotic properties of Lactobacillus spp., Lactococcus spp., and Pediococcus spp. strains isolated from kefir. J Food Sci 75:568–573. https://doi.org/10.1111/j.1750-3841.2010.01855.x
Funding
This study was financed in part by the by the Grant-in-Aid for Young Scientists (A) (Grant No. 17H04717) and the Grant-in-Aid for Scientific Research (A) (Grant No. 20H00664) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Contributions
Ryosuke Okumura: conceptualization, methodology, writing-original. Yoshitoshi Nakamura: investigation, data curation and analysis, writing-review and editing. Chikako Asada: methodology, validation, funding acquisition, supervision.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okumura, R., Nakamura, Y. & Asada, C. Efficiency of β-glucan production by Sparassis crispa depends on mycelium shape. Biomass Conv. Bioref. 14, 1939–1947 (2024). https://doi.org/10.1007/s13399-022-02555-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02555-5