Abstract
Hericium erinaceus has been widely used in food and medicine because of its various promising bioactivities. This study investigated the effects of Tween series surfactant and agar addition on mycelial growth and β-glucan in liquid submerged culture of the medicinal mushroom H. erinaceus. The addition of Tween 80 in liquid medium resulted in higher dry cell weight (DCW) and β-glucan production, with values of 6.06 and 1.21 g/L for 3.0% (w/v) and 7.27 and 1.22 g/L for 4.0% (w/v), respectively. This corresponded to a 1.82 relative ratio of DCW to control and a 1.86 relative ratio of β-glucan to control for 3.0% (w/v) and a 2.18 relative ratio of DCW to control and a 1.87 relative ratio of β-glucan to control for 4.0% (w/v), respectively. Further, the mycelial pellets were altered by the addition of agar in liquid medium, forming small pellets. The pellet diameter decreased with increasing medium viscosity, and the smallest pellet diameter, 1.5 mm, was attained with the addition of 0.3% (w/v) agar. The medium viscosity was 48.7 mPa s. Moreover, β-glucan production increased with a decreasing pellet diameter, and the highest β-glucan production, 0.86 g/L, was observed with 0.3% (w/v) agar added to the medium. The influence of flask type on DCW and β-glucan production was investigated which were almost 1.2-fold higher in baffled Erlenmeyer flasks than in normal Erlenmeyer flasks. Finally, cultivation with agar addition in a baffled Erlenmeyer flask was determined to be most effective for DCW and β-glucan production, yielding the highest values of 7.74 and 1.43 g/L, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hericium erinaceus, known as Yamabushitake in Japan, is a medicinal mushroom [1]. Its polysaccharides can prevent, alleviate, or treat major diseases including cancer, gastric ulcer, diabetes, hyperlipidemia, hepatic injury, and neurodegenerative diseases [2,3,4,5]. In particular, β-glucan is a major bioactive compound known to have biological activities including anti-cancer, anti-inflammatory, and immune-modulating properties. Due to the specific physical properties of β-glucan, such as water solubility, viscosity, and gelation, it has been increasingly used by the food and other industries [6]. Therefore, it is required to be produced on a large scale and should be available throughout the year. Polysaccharides can be isolated from the fruiting bodies of mushrooms, cultured mycelia, and cultured broth [7]. Fruiting body cultivation takes 2–3 months for harvesting based on solid cultures using substrates such as wood, grain, or sawdust [8], Moreover, its growth is seasonal. To overcome these problems, mycelial cultivation using submerged culture techniques can be used as an alternative method of polysaccharide production at an industrial scale with a shorter cultivation period (8–10 days) [9]. Therefore, in our previous study, the optimal carbon source required to produce H. erinaceus mycelia in submerged cultivation was investigated. Furthermore, yogurt whey as an alternative nitrogen source was used, which was determined to be favorable for mycelial growth and β-glucan production [10]. However, there are only a few studies on mycelial growth and β-glucan production by H. erinaceus with submerged cultivation.
Tween 80 is one of the most favorable surfactants for mycelial growth and exopolysaccharide production with medicinal fungi, such as Grifola frondosa [11], Schizophyllum commune [12], and Pleurotus tuber-regium [13]. Some researchers have indicated that it significantly increases cell membrane permeability. This increased cell membrane permeability might be attributed to changes in the composition and content of unsaturated fatty acids in the cell membrane, which could facilitate the uptake of nutrients, as well as the secretion of metabolites such as β-glucan [14,15,16].
In this study, we evaluated the effects of Tween series surfactants and agar on H. erinaceus mycelial growth and β-glucan production in submerged culture. To prevent the aggregation of mycelia and to miniaturize the pellets, agar was added into the medium. Next, the effect of a culture reactor, specifically a baffled Erlenmeyer shake flask use, on mycelial growth and β-glucan production was investigated. Finally, cultivation with additives in a baffled Erlenmeyer flask was investigated.
2 Materials and methods
2.1 Microorganism
Fungal mycelia of H. erinaceus were kindly provided by Oze Trading Co., Japan, in this study and cultured on potato dextrose agar (Wako Pure Chemical Industries, Ltd.) plates at 25°C.
2.2 Culture media and cultivation
To preculture the fungus, fresh mycelia from potato dextrose agar plates were inoculated into 200-mL Erlenmeyer flasks containing 50 mL of medium consisting of the following ingredients buffered with 0.4 M phthalic acid (pH 5.0): glucose 30 g/L, polypeptone 6.3 g/L, yeast extract 0.2 g/L, KH2PO4 1.0 g/L MgSO4·7H2O 0.5 g/L (basal medium). All nutrients were purchased from Wako Pure Chemicals. The flasks were incubated in a rotary shaker (TAITEC, Bio-Shaker BR-300LF) at 25°C with 100 rpm for 7 days.
The main culture was carried out in 300-mL Erlenmeyer flasks containing 60 mL of basal medium. The pre-culture was homogenized with a polytron homogenizer (Model PT-MR 2100, Kinematica AG, Switzerland) for 20 s at 1500 rpm. Two percent (v/v) homogenized pre-cultures were inoculated into the main culture media. The flasks were then incubated at 25°C with 100 rpm. The incubation periods (end of culture) were defined as the day at which the glucose concentration reached 0 g/L. The mycelial morphological characteristics were observed with the naked eye, and the average pellet diameter was measured with specimen samples taken from each flask. For each sample, at least 20 pellets were measured using a ruler to attain the average pellet diameter. The cultivation was performed in duplicate, and the mean results are shown.
2.3 Cultivation with additives
The effects of surfactant, specifically the Tween series (Tween 20, Tween 40, Tween 60, and Tween 80), or agar addition on H. erinaceus culture were studied. The concentrations of Tween series and agar used were 0.5, 1.0, 2.0, 3.0, and 4.0% (w/v) (Tween series) and 0.05, 0.1, 0.2, and 0.3% (w/v) (agar). These additives were supplemented in liquid media and the sample was cultivated at 25°C on a rotary shaker, as described previously herein, with shaking at 100 rpm.
2.4 Shake flasks
A comparison of cultivation conditions by type of flask used was also performed. Shake flasks used for cultivation were typical (normal) Erlenmeyer flasks with or without baffles. Each 300-mL baffled Erlenmeyer flask consisted of a conventional flask (SIBATA, Erlenmeyer flask, narrow neck, Borosilicate Glass, SIBATA SCIENTIFIC TECHNOLOGY, Japan) with three indentations, which were 7.0 cm high, 1.0 cm wide, and 0.8 cm deep.
2.5 Analytical methods
After cultivation, mycelia were filtered from the culture medium using ADVANTEC no.131 filter paper [10]. The filtered mycelial pellets were washed with 300 mL distilled water and air-dried for 2 days at 35°C. Subsequently, the sample was stored in a vacuum desiccator with silica gel at room temperature. The dry cell weight (DCW) was then determined. Glucose concentrations were determined by the mutarotase GOD method (Glucose C-Test; Wako Pure Chemicals, Osaka, Japan). To determine the β-glucan content in the DCW, generated glucose via lichenase treatment was determined by mutarotase GOD method. The glucose from β-glucan in the DCW was assayed using the Mushroom and Yeast β-glucan Assay Kit (Megazyme International Ireland Ltd., Bray Business Park, Bray, Co. Wicklow, Ireland). All experiments were carried out in triplicate, and individual assays were performed at least twice.
3 Results and discussion
3.1 Effects of Tween surfactants on cell growth and β-glucan production
The DCW and β-glucan are two of the most desired products resulting from the cultivation of H. erinaceus. To accelerate the production of these components, we previously reported the effects of various carbon sources and the effects of yogurt whey addition as an alternative nitrogen source on mycelia (DCW) and β-glucan production [10]. In this study, the effects of surfactant Tween series addition on H. erinaceus in submerged cultivation were investigated. Liu et al. reported the effects of surfactant Tween 80 addition on mycelial growth and exopolysaccharide production in Cordyceps sinensis [17]; specifically, the DCW and exopolysaccharide yields were increased, and the highest exopolysaccharide yield was attained with the addition of 1.5% Tween 80, which was 2-fold higher than that in control conditions (without Tween 80).
Figure 1 shows the basal glucose consumption time course and growth curve of H. erinaceus in submerged culture. The initial glucose concentration of 20.7 g/L decreased to zero during 8-day cultivation period, and 3.33 g/L of DCW was achieved. The influence of Tween series (Tween 20, 40, 60, and 80) and concentrations (0.5, 1.0, 2.0, 3.0, and 4.0% (w/v)) on the DCW, β-glucan, and relative ratios of DCW and β-glucan to control values of H. erinaceus in the culture media are summarized in Table 1. The DCW of H. erinaceus was found to increase at all concentrations of Tween 40, 60, and 80, and the relative ratios of the DCW to control values were 1.16–2.18 (the highest, at 4.0% (w/v) Tween 80). In contrast, the remarkable increase in β-glucan was observed only with Tween 80 at 3.0% (w/v) and 4.0% (w/v), representing high concentrations; the relative ratios of β-glucan to control values were 1.86 at 3.0% (w/v) and 1.87 at 4.0% (w/v). From this result, it was concluded that Tween 40 and 60 were only accumulated in the fungus cell bodies; therefore, the addition of these contributed little to metabolite production, such as β-glucan production. Yang et al. also reported the effect of Tween 80 on metabolite production (exopolysaccharide production) in submerged mycelial cultures of Ganoderma lucidum [15]. The addition of 0.25% (w/v) Tween 80 on day 3 resulted in the maximum production of DCW and exopolysaccharide, with an increase of 19.8 and 137.5%, respectively. Moreover, it was found that Tween 80 significantly increased cell membrane permeability. The increased cell membrane permeability could facilitate the uptake of nutrients, biosynthetic activity for exopolysaccharide production, and membrane permeability for exopolysaccharide secretion [14, 16]. Tween 80 is composed of fatty acid esters of polyoxyethylene sorbitan. The fatty acid composition is primarily oleic acid, but other fatty acids, such as palmitic or linoleic acid, may be included [18]. Zhan et al. reported that Tween 80 is one of the stimulatory agents for the submerged culture of mushroom (P. tuber-regium) mycelia [16]. With the addition of Tween 80 into the medium, the amount of total fatty acids in the mycelial lipids of P. tuber-regium was significantly enhanced; especially, the amount of oleic acid was significantly increased. Therefore, they suggested that the oleic acid contained in Tween 80 could be incorporated into the mycelial cell membrane of P. tuber-regium, increasing its fatty acid (oleic acid) composition and the cell membrane permeability. Xu et al. also reported that oleic acid and Tween 80 had favorable effects on mycelia and exopolysaccharide production in submerged culture of Inonotus obliquus. Tween 80 could be hydrolyzed by microbial enzymes, such as lipase, to release oleic acid [19]. The effect of Tween 80 might be partially attributed to its convert to oleic acid. Oleic acid was proved to increase the amount of mycelia and exopolysaccharides [20]. However, with the addition of 4% (w/v) of Tween 80 into the medium, the glucan content in the DCW decreased. This result indicated that more than 4% (w/v) of Tween 80 addition could increase the DCW, but might have no effect on β-glucan production. While, no effect of shortening the cultivation period (at the end of glucose consumption) was observed over the entire Tween series. With Tween 20 at concentrations of 2, 3, and 4% (w/v), marked inhibition of cell growth and no consumption of glucose were observed after 4 days of cultivation. Therefore, the cells were recovered from the medium in the middle of cultivation, and no β-glucan was observed in the mycelia. Similar phenomena were observed in submerged cultures of Grifola frondosa when Tween 20 was added to the medium (0.1–1.0% (w/v)) [11]. It was speculated that lower carbon chain lengths (12C side chain) of surfactants with high concentrations might damage the cell membrane or interact with other bio-compounds in the cell, resulting in low cell growth.
3.2 Effects of agar on cell growth and the production of β-glucan
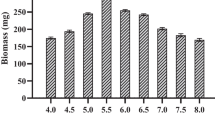
Pellet morphology and size play a major role in metabolite production [21]. The pelletization of mycelia depends on culture conditions and is thought to be strain-specific [12]. Generally, autolysis of cells occurs in large pellets due to limitations oxygen and nutrients [22, 23]. Zmak et al. also found that autolysis occurs in the pellet center in all pellet sizes (6.1 mm, 11 mm, and 16 mm) of Phanerochaete chrysosporium after 150 h of cultivation, except for the smallest pellets (2.0 mm) formed in Erlenmeyer flasks [24]. Therefore, pellet miniaturization of less than 2 mm in size was investigated by adding agar to the medium to increase the viscosity of the medium. Yang et al. [25] investigated the effect of various polymer additives in the culture of G. lucidum on mycelial growth. With agar (0.4% (w/v)) addition, the viscosity of the medium increased and the pellets were minimized by reducing mycelium aggregation. In this study, 0.05, 0.1, 0.2, and 0.3% (w/v) agar concentrations were studied. Figure 2 shows the mycelial micromorphology of H. erinaceus in the medium with and without agar at the end of culture. An increase in agar concentrations resulted in a significant decrease in the pellet size. The influences of agar concentration on the DCW, β-glucan, and pellet size (diameter) of H. erinaceus in the cultures are summarized in Table 2. The pellet diameters varied from 4.3 mm without agar to 1.5 mm with 0.3% (w/v) of agar. DCW and β-glucan production with 0.3% (w/v) agar were almost 1.3-fold higher than those of the control (without agar). Generally, as the size of the pellet increases, the diffusion of nutrients, particularly oxygen, into the center of the pellets becomes limited [25]. Pellet morphology and pellet diameter affect the amounts of secondary metabolites, and Supramani et al. reported that the morphology of Ganoderma pfeifferi mycelium was affected by medium pH and medium agitation speed. Pellets with larger diameter were associated with a higher DCW, whereas small dispersed pellets were associated with high exopolysaccharide, for example, β-glucan production [21]. Based on microscopic observations, it can be seen that there were many crystal particles (agar) in the medium with agar (data not shown), and the mycelium tips adhered to the crystal particles. The presence of crystal particles might also prevent the formation of mycelium aggregates [25]. In the study, as 1.5 mm of pellet size (less than 2 mm) was attained at 0.3% (w/v) of agar concentration, higher concentrations than 0.3% (w/v) were not investigated.
3.3 Comparison of culture efficiency using Erlenmeyer flasks with and without baffles
To investigate the influence of incubators on cell growth and metabolite production, we evaluated the submerged culture of H. erinaceus in normal and baffled Erlenmeyer flasks. DCW production, β-glucan production, and pellet size (diameter) of H. erinaceus in the cultures using normal and baffled Erlenmeyer flasks are summarized in Table 3. DCW production and β-glucan production in baffled Erlenmeyer flasks were almost 1.2-fold higher than those in normal Erlenmeyer flasks. Moreover, the pellet size in a baffled Erlenmeyer flask (1.6 mm) was smaller than that in a normal Erlenmeyer flask (4.5 mm). Bermek et al. reported the morphology and ligninolytic enzyme production in the wood-degrading fungus Trichophyton rubrum in submerged cultures using normal and baffled Erlenmeyer flasks. In baffled Erlenmeyer flasks, fungal morphologies were notably different, the pellet size diameter was small (approximately 2–4 mm), and free mycelia were observed. In contrast, large ball formation was observed in normal Erlenmeyer flasks. It was clear that limited oxygen conditions were responsible for the formation of large pellets. Baffled Erlenmeyer flasks have a unique design, creating strong convection stirring, and thus allowing better mixing and air uptake into the medium [26]. Recently, Ángeles-Argáiz et al. also reported that mycelia of the ectomycorrhizal mushroom Laccaria trichodermophora in submerged culture in a baffled Erlenmeyer flask were fragmented and the pellets were compact with growing hyphae throughout their surface. In a normal Erlenmeyer flask, the mycelia formed larger and fewer filamentary pellets [27]. Larger pellets have deficiencies in oxygen and nutrient diffusion, which leads to low growth [28].
3.4 Effects of agar or Tween 80 on cell growth and the production of β-glucan in baffled Erlenmeyer flask
Figure 3 shows H. erinaceus DCW and β-glucan production in a baffled Erlenmeyer flask with and without 3.0% (w/v) of Tween 80 or 0.3% (w/v) agar. The highest β-glucan concentration, 1.89 g/L, was attained when 0.3% (w/v) agar was added to the medium with a shorter cultivation period (5 days). However, no effect was observed when 3.0% (w/v) of Tween 80 was added to the medium. This is because the viscosity remained low (3.0 mPa s), which is similar to that without agar (3.3 mPa s), and there might have been limitations in oxygen uptake into the pellets [29].
4 Conclusion
In summary, the addition of Tween 80 or agar into the medium for H. erinaceus submerged cultivation could significantly increase cell growth and β-glucan production. Additionally, baffled Erlenmeyer flasks were found to be efficient for β-glucan production. In the future, a scale-up experiment is required. It will also be necessary to carry out a submerged cultivation experiment using tank batch culture and fed-batch continuous culture.
References
He X, Wang X, Fang J, Chang Y, Ning N, Guo H, Huang L, Huang X, Zhao Z (2017) Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review. Int J Biol Macromol 97:228–237. https://doi.org/10.1016/j.ijbiomac.2017.01
Cheng JH, Tsai CL, Lien YY, Lee MS, Sheu SC (2016) High molecular weight of polysaccharides from Hericium erinaceus against amyloid beta-induced neurotoxicity. BMC Complement Altern Med 16:1–9. https://doi.org/10.1186/s12906-016-1154-5
Lee JS, Hong EK (2010) Hericium erinaceus enhances doxorubicin-induced apoptosis in human hepatocellular carcinoma cells. Cancer Lett 297:144–154. https://doi.org/10.1016/j.canlet.2010.05.006
Shang HM, Song H, Xing YL, Niu SL, Ding GD, Jiang YY, Liang F (2016) Effects of dietary fermentation concentrate of Hericium caput-medusae (Bull.:Fr.) Pers. On growth performance, digestibility, and intestinal microbiology and morphology in broiler chickens. J Sci Food Agric 96:215–222. https://doi.org/10.1002/jsfa.7084
Zhang Z, Pan GLH, Pandey A, He W, Fan L (2012) Antioxidant and hepatoprotective potential of endo-polysaccharides from Hericium erinaceus grown on tofu whey. Int J Biol Macromol 51:1140–1146. https://doi.org/10.1016/j.ijbiomac.2012.09.002
Zhu F, Du B, Xu B (2016) A critical review on production and industrial applications of beta-glucans. Food Hydrocoll 52:275–288. https://doi.org/10.1016/j.foodhyd.2015.07.003
Wasser SP (2011) Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl Microbiol Biotechnol 89:1323–1332. https://doi.org/10.1007/s00253-010-3067-4
Yang H, Min W, Bi P, Zhou H, Huang F (2013) Stimulatory effects of Coix lacryma-jobi oil on the mycelial growth and metabolites biosynthesis by the submerged culture of Ganoderma lucidum. Biochem Eng J 76:77–82. https://doi.org/10.1016/j.bej.2013.04.012
Singh U, Gautam A, Singha TK, Tiwari A, Tiwari P, Sahai V, Sharma S (2020) Mass production of Pleurotus eryngii mycelia under submerged culture conditions with improved minerals and vitamin D2. Food Sci Technol-LEB 131:109665. https://doi.org/10.1016/j.lwt.2020.109665
Asada C, Okumura R, Sasaki C, Nakamura Y (2012) Acceleration of Hericium erinaceum mycelial growth in submerged culture using yogurt whey as an alternative nitrogen source. Adv Biosci Biotechnol 3:828–832. https://doi.org/10.4236/abb.2012.37103
Hsieh C, Wang HL, Chen CC, Hsu TH, Tseng MH (2008) Effect of plant oil and surfactant on the production of mycelial biomass and polysaccharides in submerged culture of Grifola frondosa. Biochem Eng J 38:198–205. https://doi.org/10.1016/j.bej.2007.07.001
Hao LM, Xing XH, Li Z, Zhang JC, Sun JX, Jia SR, Qiao CS, Wu TY (2010) Optimization of effect factors for mycelial growth and exopolysaccharide production by Schizophyllum commune. Appl Biochem Biotechnol 160:621–631. https://doi.org/10.1007/s12010-008-8507-6
Zhan BB, Chen L, Cheung PCK (2012) Proteomic insights into the stimulatory effect of Tween 80 on mycelial growth and exopolysaccharide production of an edible mushroom Pleurotus tuber-regium. Biotechnol Lett 34:1863–1867. https://doi.org/10.1007/s10529-012-0975-7
Sheng L, Zhu G, Tong Q (2013) Mechanism study of Tween 80 enhancing the pullulan production by Aureobasidium pullulans. Carbohydr Polym 97:121–123. https://doi.org/10.1016/j.carbpol.2013.04.058
Yang X, Yang Y, Zhang Y, He J, Xie Y (2020) Enhanced exopolysaccharide production in submerged fermentation of Ganoderma lucidum by Tween 80 supplementation. Bioprocess Biosyst Eng 44:47–56. https://doi.org/10.1007/s00449-020-02418-1
Zhang BB, Cjeung PC (2011) A mechanistic study of the enhancing effect of Tween 80 on the mycelial growth and exopolysaccharide production by Pleurotus tuber-regium. Bioresour Technol 102:8323–8326. https://doi.org/10.1016/j.biortech.2011.06.021
Liu YS, Wu JY (2012) Effects of Tween 80 and pH on mycelial pellets and exopolysaccharide production in liquid culture of a medicinal fungus. J Ind Microbiol Biotechnol 39:623–628. https://doi.org/10.1007/s10295-011-1066-9
Schwartzberg LS, Navari RM (2018) Safety of polysorbate 80 in the oncology setting. Adv Ther 35:754–767. https://doi.org/10.1007/s12325-018-0707-9z
Breuil C, Schindler DB, Sijger JS, Kushner DJ (1978) Stimulation of lipase production during bacterial growth on alkanes. J Bacteriol 133:601–606. https://doi.org/10.1128/JB.133.2.601-606.1978
Xu X, Quan L, Shen M (2015) Effect of chemicals on production, composition and antioxidant activity of polysaccharides of Inonotus obliquus. Int J Biol Macromol 77:143–150. https://doi.org/10.1016/j.ijbiomac.2015.03.013
Supramani S, Jailani N, Ramarao K, Zain NAM, Klaus A, Ahmad R, Wan-Mohtar WAAQI (2019) Pellet diameter and morphology of European Ganoderma pfeifferi in a repeated-batch fermentation for exopolysaccharide production. Biocatal Agric Biotechnol 19:101118. https://doi.org/10.1016/j.bcab.2019.101118
Grimm HL, Kelly S, Krull R, Hempel DC (2005) Morphology and productivity of filamentous fungi. Appl Microbiol Biotechnol 69:375–384. https://doi.org/10.1007/s00253-005-0213-5
White S, McIntyre M, Berry DR, McNeil B (2002) The autolysis of industrial filamentous fungi. Crit Rev Biotechnol 22:1–14. https://doi.org/10.1080/07388550290789432
Zmak PM, Podgornik A, Podogornik H, Koloini T (2006) Impact of pellet size on growth and lignin peroxidase activity of Phanerochaete chrysosporium. World J Microbiol Biotechnol 22:1243–1249. https://doi.org/10.1007/s11274-006-9168-7
Yang FC, Yang MJ, Cheng SH (2009) A novel method to enhance the mycelia production of Ganoderma lucidum in submerged cultures by polymer additives and agitation strategies. J Taiwan Inst Chem E 40:148–154. https://doi.org/10.1016/j.jtice.2008.09.003
Bermek H, Gülseren I, Li K, Jung H, Tamerler C (2004) The effect of fungal morphology on ligninolytic enzyme production by a recently isolated wood-degrading fungus Trichophyton rubrum LSK-27. World J Microbiol Biotechnol 20:345–349. https://doi.org/10.1023/B:WIBI.0000033055.52660.03
Ángeles-Argáiz RE, Carmona-Reyes IA, Quintero-Corrales CA, Medina-Macias FJ, Blancas-Cabrera A, Valdez-Cruz NA, Ulloa M, Trujillo-Roldán MA (2020) From field sampling to pneumatic bioreactor mycelia production of the ectomycorrhizal mushroom Laccaria trichodermophora. Fungal Biol 124:205–218. https://doi.org/10.1016/j.funbio.2020.02.003
Garcia-Ochoa F, Gomez E (2009) Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnol Adv 27:153–176. https://doi.org/10.1016/j.biotechadv.2008.10.006
Schugerl KRW, Lorenz T (1983) The use of molds in pellet form. Trends Biotechnol 1:120–123. https://doi.org/10.1016/0167-7799(83)90035-5
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okumura, R., Nakamura, Y., Sasaki, C. et al. Effects of Tween series and agar additives on mycelia biomass and β-glucan production by Hericium erinaceus in submerged culture. Biomass Conv. Bioref. 13, 3135–3141 (2023). https://doi.org/10.1007/s13399-021-01342-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01342-y