Abstract
Daily large quantities of kitchen wastes rich in starchy components are discarded into the environment. The current study documents the use of starchy kitchen wastes (SKW) as a low-cost effective substrate for bacterial cellulose (BC) production and loaded with carbonaceous compounds to remove cationic dyes from wastewater. Box-Behnken design (BBD) was applied to optimize the maximum production of reducing sugars by enzymatic hydrolysis of SKW and the hydrolysate at different concentrations used as precursors for BC biosynthesis using Komagataeibacter hansenii AS.5. SEM, FT-IR, and mechanical properties were performed for BC membranes characterization. The synthesized BC membranes were loaded with either charcoal or graphite and evaluated for removal of methylene blue (MB) as a representative cationic dye. The BBD showed that 148.0 g/l of reducing sugars (54.8% saccharification) can be produced using 24.3% of SKW (84.3 g/l/dry) treated with 2-ml amylase (313 U/ml) at 50 °C for 5 h and 40 min. The optimum concentrations of SKW hydrolysate produced 2.11 g/l of BC membranes (0.3 g/l/day). The original and loaded membranes showed absorption capacities of 53, 98.7, and 100% removal of MB using BC, graphite-BC, and charcoal-BC membranes, respectively. Therefore, SKW can be used as alternative medium for BC production and applied successfully for dye removal.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cellulose, the most abundant polymer on earth, can be produced from many sources including plants and microorganisms. Among microbial celluloses, BC is effective in terms of purity (free from lignin and hemicellulose) and homogeneity [1, 2]. An extracellular product with the same form of cellulose in nature and widely produced by acetic acid bacteria such as Komagataeibacter hansenii and Komagataeibacter xylinus [3], BC has unique structural advantages over PC (plant cellulose), such as high degree of polymerization, crystallinity and surface area, and nano-fibrillary network [4]. A major drawback in the widespread commercial application of BC is its relatively expensive production process. This could be attributed to the low productivity of known applied strains and the costly cultivation media requirements, (approximately 30% of the total BC production cost) [5], Therefore, medium cost reduction represents a key step towards extensive application of BC. To achieve this, a wide range of biomass wastes was surveyed including tobacco waste extract [6], orange peel [7], citrus juices [8], beverage industrial waste [9], cotton-based waste textiles [10], and black tea [11]. Food wastes, originating from processing industries, restaurants, canteens, households, and markets, are important sources of biomass wastes. According to the Food and Agriculture Organization of the United Nations (FAO), 1.3 billion tons of food wastes are produced, which is around one-third of food produced for human consumption on a global scale [12]. In many countries, food waste is usually incinerated, composted or directly land filled with other wastes after simple recycling [13]. With growing environmental awareness, several studies have reported the application of food kitchen wastes towards production of bioelectricity [14], biogas [15, 16], volatile fatty acids and H2 [17], enzymes [18], biofuels [19, 20], and pullulan [21]. Starch kitchen wastes (SKW) could be a potential candidate for BC production upon enzymatic hydrolysis. Amylase enzyme converts SKW to free glucose and reducing sugars which in turn can be fermented into BC. Optimization of the SKW hydrolysis process is crucial and directly affects the final product yield. Statistical designing of this optimization process reduces both time and effort compared to one variable at time method (OVAT) [22]. In addition, all studied variables are optimized at the same time where interaction between such variables could also be evaluated [23, 24]. Among statistical designs, BBD represents an efficient tool with a small manageable set of data and requirements for process parameter optimization of kitchen waste [25]. Based on the unique physical and chemical properties, BC is used in wound healing [26], oil separation [27], skin and bone tissue engineering [28], bioelectrode [29], cosmetics [30], heavy metal removal [31, 32], and dye removal [33]. MB, commonly used for dye removal in several works, and removal of MB itself from textile, dyeing, printing, and coating industries, has become one of the major environmental issues [34]. Due to the low efficiency of BC for dye removal, many attempts were dedicated to manipulating its structure including BC/polydopamine/TiO2 [35], BC/attapulgite magnetic structure [32], and BC/polydopamine/reduced graphene oxide [33]. Charcoal (Ch) and graphite (Gr) are worldwide naturally occurring biomaterials characterized by their high porosity and efficient absorption for a long time. Ch has been used for water treatment, harmful gas elimination, antibacterial efficacy, blood purification, absorption, and electromagnetic shielding [36, 37]. Furthermore, Gr is a naturally occurring mineral, used for battery application, graphene production, and removal of dyes [38, 39]. In the present investigation, the SKW matrix from different sources was collected and submitted to BBD optimization in order to maximize the production of reducing sugars. The final optimized hydrolysate with high reducing sugars at different concentrations was used as a carbon source for the production of BC. The obtained BC membranes were characterized by scanning electron microscope (SEM), Fourier-transform infrared spectroscopy (FT-IR), and other mechanical features. Finally, the BC gel-like matrix was applied for the removal of MB compared to Ch and Gr-loaded BC matrices.
2 Materials and methods
2.1 Collection and analysis of SKW
SKW is a mixture of house kitchen wastes including bread, rice, pasta, and noodles. The collected wet wastes were milled through a laboratory blender to obtain a well-homogenized mash with small particle size. The prepared mash was packaged and stored at 4 °C till use, to prevent acidification during the storage time. The SKW was analyzed for moisture, solid, and reducing sugar contents.
2.2 Amylase source and activity determination
Amylase used throughout this work was produced by the locally isolated Bacillus methylotrophicus SCJ4 [40]. The enzyme was prepared in a stock solution (0.1 mg/ml), where its units (313 U/ml) were assayed according to the iodine colorimetric method [41] with slight modification. The dried form of the enzyme was prepared through freeze drying using the culture supernatant as source of the enzyme.
2.3 Statistical optimization for enzymatic hydrolysis of SKW
For SKW hydrolysis optimization, BBD was applied using three variables, namely SKW concentration, enzyme concentration, and hydrolysis time. As indicated in Table 1, each variable was studied at three different levels coded − 1, 0, and 1 representing the low, middle, and high values of each variable. The BBD matrix implies 12 different combinations (trials) and 3 central trials to detect handling errors (Table 1), and each row and column represent an experiment and a variable used, respectively. All trials were incubated at 50 °C, pH 6, according to the optimum conditions for enzyme activity [42]. The amount of released reducing sugars was measured as the response (Y) and culture conditions were taken as independent variables (X). Each trial was conducted in triplicate and mean values were represented as responses. The experimental results were fitted by regression to a predictive quadratic polynomial model that gave reducing sugars production (Y) as a function of culture conditions (X):
where Y is the expected response; B0 is the model constant; X1, X2, and, X3 are the variables under study; B1, B2, and B3 are the linear coefficients; B12, B13, and B23 are the cross-interacted coefficient; and B11, B22, and B33 are the quadratic coefficients. The results of BBD were represented in the form of surface plots, where each plot was developed by two independent variables on X and Y axes with the dependent variable (response) on the Z axis, while the third variable was set at its middle value.
2.4 Assay of SKW hydrolysate
SKW hydrolysis progress was evaluated by measuring the reducing sugars released during the course of hydrolysis. The released reducing sugars were determined with the dinitrosalicylic acid (DNS) method [43], wherein brief, 1 ml of sample was mixed with 1.5 ml of freshly prepared DNS solution and boiled for 10 min. The resulting color was measured at 575 nm with a standard curve of glucose. Percentage of saccharification was calculated according to [44] as = amount of reducing sugar × 0.9 × 100/initial amount of SKW, where the 0.9 is the molar mass conversion of starch to sugars.
2.5 Model accuracy and verification
The accuracy of BBD results was verified by comparing the expected yield at model level with the real measured yield, upon applying the model conditions in the laboratory.
2.6 Data analysis and 3D graphs representation
Regression analysis and analysis of variance (ANOVA test) were conducted through the statistical analyses on Microsoft Excel 2010, where the 3D graphs representing the effect of each two factors on waste hydrolysis were created through the STATISTICA 7 program.
2.7 Microorganism and preinoculum
In the present investigation, Komagataeibacter hansenii AS.5 (K. hansenii AS.5) was used as the BC-producing strain, as described in an earlier study [45]. Preinoculum was prepared by using Hestrin and Schramm (HS) medium, with the following composition (g/l): 20 D-glucose, 5 peptone, 5 yeast extract, 2.7 Na2HPO4, and 1.15 citric acid [46]. The medium was inoculated with a single colony of K. hansenii AS.5 and incubated at 30 °C, 200 rpm for 48 h, to activate the producer strain. The bacterial strain was maintained on HS agar slants at 4 °C for further use.
2.8 Production of BC using SKW hydrolysate
The enzymatic hydrolysis condition of SKW with the highest reducing sugar yield was used for BC production. Under optimum conditions for SKW enzymatic hydrolysis, the hydrolysate was centrifuged at 5000 × for 15 min to remove sediments and the supernatant was collected. Glucose-free HS media were used as a basal medium for BC production from SKW. As an alternative carbon source, the glucose-free HS medium was supplemented with 10% SKW hydrolysate supernatant. Medium pH was adjusted to 6.0, inoculated with 10% (v/v) of K. hansenii AS.5 preinoculum, and incubated for 7 days at 30 °C in static conditions. By the end of the incubation time, the BC pellicle (gel) at the air–liquid interface was observed. The flasks containing media were sterilized by autoclave at 121 °C for 15 min.
2.9 Effect of SKW hydrolysate concentrations on BC production
Different concentrations of SKW hydrolysate (20, 40, 60, 80, and 100%) were added to glucose-free HS media as carbon sources. Erlenmeyer flasks (250 ml) were inoculated with 10% (v/v) preinoculum and cultivated statically at 30 °C for 7 days. Each culture was performed in triplicate and the mean values for BC are given as g/l.
2.10 BC purification and quantification
By the end of the fermentation course, the observed BC at the air–liquid interface was collected, transferred to another container, and purified in several steps. First, BC was washed three times with distilled water (DW) to remove the remaining components of the culture medium. Subsequently, the BC was treated 2–3 times with 0.5% NaOH solution at 90 °C for 30 min to remove the remaining bacteria and other impurities adsorbed on the BC, and then washed 3–4 times with DW until a neutral pH bright-white BC was obtained. Finally, the purified BC was dried at 70 °C overnight to get a constant weight. BC production has been reported as gram dry weight of cellulose per liter of the medium (g/l) [47].
2.11 Characterization of BC membrane
SEM (JEOL JSM 6360 LA, Japan) was used to investigate the surface structure of the BC samples. The SEM experiment was performed at a magnification of 5000–15,000 × . The functional groups and chemical bonds of BC samples were performed using FT-IR (JASCO FTIR-4100 E, Japan), operated in absorption mode with the wavenumber range from 4000 to 500 cm−1, using a resolution of 1 cm−1. A universal tensile testing machine (Universal Testing Machine, model: AG-I/50 N-10 KN, Japan) was used to determine the mechanical properties of dried BC membranes. The BC membrane was cut into rectangular strips (10 cm long and 1 cm wide) for measurement, with a gauge length of 20 mm. BC membrane thickness was determined by an electronic digital micrometer before the examination. Each test was performed using 3 specimens and the average of the results was recorded.
2.12 Preparation of BC gels loaded with Ch or Gr
BC gels were obtained from K. hansenii AS.5 using SKW hydrolysate as a carbon source. BC gel (~ 4 g wet, 5 × 5 cm diameter, and 0.5 cm thickness) was loaded by Ch and Gr through the swelling diffusion method [48]. About 0.5 g of Ch or Gr was stirred separately in 100-ml DW for 1 h, and homogenized through ultrasonication (750 wt., 20 KH, pulse 45, Amp 1) for 15 min to obtain a homogenous dispersion. Once a homogenous aqueous dispersion of Ch or Gr was obtained, the original BC gels were immersed in an Erlenmeyer flask containing Ch or Gr dispersion for 24 h, 200 rpm at room temperature, to ensure complete absorption of BC gels with Ch or Gr. In post-incubation, the BC gels were removed and then immersed briefly in DW for 30 s and wiped lightly once with tissue to remove non-absorbed Ch or Gr from BC gel surface. Water-soaked BC gels were taken as control. Finally, the original BC and BC-loaded gels were placed in a Petri dish and kept at 4 °C in the refrigerator until use [48].
2.13 Application of BC-Ch- and BC-Gr-amended gels for MB dye removal
The three prepared gels (original BC, BC-Ch, and BC-Gr) were tested individually for their ability to remove 10 mg/l of MB using a pressure-free system. The system consists of two chambers connected by a medium junction that contains one of the tested membranes (Fig. 1). Fifty milliliters of 10 mg/l dye solution was added to the upper chamber and allowed to pass through the membrane to the lower chamber. Optical densities (OD) of the upper and lower liquids were measured at 655 nm, and the percentage of dye removal by each membrane was calculated. The reusability of BC-Ch- and BC-Gr-amended gels was also evaluated to determine the full capacity and durability of the two membranes in successive dye removal processes. For this, the used gels in the previous dye removal cycle were washed in 50-ml PBS buffer (pH 7.0) and the released dye was determined at different time intervals at 655 nm.
2.13.1 Dye retention capacity
To evaluate the dye retention capacity for original BC, BC-Ch, and BC-Gr gels, the three gels used in the previous dye removal experiment were washed in 50-ml PBS buffer (pH 7.0) for 2 h and the released dye was quantitated over different time intervals at 655 nm compared to 10 mg/l MB dye solution as control (100%).
3 Results and discussion
3.1 BBD for enzymatic hydrolysis of SKW
The high starch content in the kitchen wastes qualified them for several biotechnological applications [49]. In this study, the used SKW was a mixture of collected house kitchen wastes with estimated moisture to solid contents of 65.3% and 34.7%, respectively, with an initial reducing sugar concentration of 25.8 g/l. The SKW was hydrolyzed by Bacillus methylotrophicus SCJ4 amylase, where the released fermentable sugars were used as the main substrate for BC production. During enzymatic hydrolysis of the SKW, BBD was applied to evaluate the optimum conditions for maximum production of the reducing sugars. BBD was also widely applied for various optimization processes including enzyme production [50], waste degradation process [51, 52], and waste composting [25]. In the present investigation, three variables were evaluated for their effects on SKW hydrolysis at three levels. These variables include SKW concentration, enzyme volume, and hydrolysis time. The matrix design was built from 12 different trial combinations, with three central trials, to evade the handling errors. The data (Table 1) indicated variation in the amount of reduced sugars resulting in different trials ranging from 34.38 to 135.1 g/l with 15.5 and 60.8% scarification, respectively. The variation thus had a direct impact of the optimization process on the hydrolysis results. The significance F value represents the statistical significance of the used variables on the experiment’s response (reducing sugars), where values below 0.05 indicate significance while that below 0.01 indicates high significance [53]. In this experiment, the value of significance F (0.001958) indicated a highly significant effect of the applied variables upon the estimated response. BBD depends upon a second-order polynomial model in predicting the optimum experimental conditions for the desired response (maximum reducing sugars). From the regression analysis, the applied polynomial model (Eq. 1) can be written as:
where X1, X2, and X3 are variables under study.
The regression analysis results (R square = 0.97) showed high accuracy of the applied model, where the closer the R value to 1 indicates a strong correlation between measured and predicted values at the model level [22]. The result of correction factor adjusted R square (0.926) also revealed a strong correlation between estimated and predicted response values, where only less than 7.4% of the response could not be fitted within the applied model. The ANOVA summary of the BBD model represents the degrees of freedom (df), sum-of-squares (SS), mean square (MS), F-ratio (F), and significance F values as presented in Table 2. The interaction consequences of the independent variables on the measured response were visualized with surface plots (Fig. 2). The plot indicated that waste concentration implied positive interaction with enzyme volume and hydrolysis time in contrary to enzyme concentration that interacted negatively with hydrolysis time. The Solver function of MS Excel was applied to determine the exact parameter values at the used model. The results implied that using SKW concentration of 24.3 g/100 ml (84.3 g/l/dry) with enzyme volume of 2 ml (0.1 mg/ml), for 5 h and 40 min hydrolysis time, resulted in maximum production of simple sugars of 151 g/l (56% saccharification).
3.1.1 Model verification
To evaluate the applicability and accuracy of the quadratic polynomial model, a verification experiment was conducted under the predicted optimal conditions as following: SKW concentration of 24.3 g/100 ml, with enzyme volume of 2 ml for hydrolysis time of 5 h and 40 min at 50 °C and 200 rpm. The estimated reducing sugar was 148.0 g/l (54.8% saccharification), while the expected was 151 g/l (56% saccharification). Thus, a high degree of accuracy (98.0%) is evidence of model validation. Kim and his colleagues applied response surface methodology (RSM) based on the central composite design for optimization of food waste hydrolysis to obtain the predicted and estimated reducing sugar concentrations as 117.0 and 120.1 g/l, respectively [54]. Another study reported maximum reducing sugar as 100 g/l upon enzymatic hydrolysis for food wastes using a cellulolytic enzyme [55]. The hydrolysis time of 5 h and 40 min in the current study is in agreement with Bhatt and his colleagues who reported an optimum time of 6 h for kitchen wastes hydrolysis by amylase [18].
3.2 Evaluation of the effect of SKW hydrolysate upon BC production
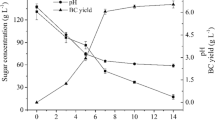
BC production cost is one of the main obstacles towards its wide commercial applications. Most of this high cost could be attributed to carbon sources and hence, the reduction in this cost is widely desirable [49]. Using SKW as an alternative source for BC production represents a dual solution for both environmental and economical point of view. Initially, 10% of optimized hydrolysate was supplemented to glucose-free HS media to achieve 0.32 g/l BC after 7 days. In order to investigate the effect of SKW hydrolysate concentration on BC production, SKW hydrolysate was added exogenously to the glucose-free HS media at varying concentrations from 20 to 100% and the BC productions were compared after 7 days of static cultivation. The results revealed that the SKW hydrolysate had a positive effect on BC production at 20, 40, 60, 80, and 100% concentrations to produce 0.82, 1.26, 1.48, 1.64, and 2.11 of BC g/l respectively. This suggests that the gradual increase in BC production was proportional to the increase in the concentration of the SKW hydrolysate supplemented to the medium with a maximum production yield at 100%. BC production at 100% (2.11 g/l) (0.3 g/l/day) represented a 2.5-fold increase in the BC production compared to that at 20% SKW concentration (0.82 g/l) (0.1 g/l/day). These results are consistent with other studies that reported maximum BC production between 2.0 and 2.25 g/l using SKW hydrolysate by Acetobacter xylinum [56, 57]. The addition of different substrate concentrations to production media may enhance the production process as reported by [6], in which the maximum BC production was 2.2-fold the initial production. The yield of BC from food wastes differs based on factors such as pretreatment of food waste [58], type of food waste applied [59], and production conditions [60]. Collectively, the results indicated that extra concentration of SKW may be a useful strategy to improve BC production as a culture medium.
3.3 Purification and characterization of BC membrane
The raw BC membranes collected from the liquid–air interface of the production media were purified and cleaned from remaining medium components and bacterial debris with 0.5% Sodium hydroxide (Fig. 3). The impact of different concentrations of SKW hydrolysate (20–100%) on the properties and characteristics of the purified BC membranes was investigated with different analytical methods, including surface structure by SEM and functional and chemical ponds with FT-IR spectroscopy along with their mechanical properties.
3.3.1 SEM analysis
The SEM results clearly indicated that the purified BC membranes obtained from different concentrations of SKW hydrolysate are porous and homogeneous 3-dimensional network-like structures of randomly arranged cellulosic fibers (Fig. 3). The scanning results of all membranes (20–100%) also indicated nearly similar morphological structures with no significant difference. These results are concurrent with data from other studies that reported the BC production with nearly identical membrane morphological structure, using different media and/or varied substrate concentrations [60, 61].
3.3.2 FT-IR spectroscopy
FT-IR spectroscopy was used to determine the chemical structure of the BC membranes including the functional groups and molecular bonding properties using the bond vibration frequencies [62]. The FT-IR spectra of all BC membranes exhibited several clear absorption peaks from approximately 3336.2 to 869.7 cm−1 with all typical bands of the BC in the five samples as shown in Fig. 4. From the data obtained (Table 3), we can conclude that all the BC samples share no obvious difference in FTIR spectra, indicating that the obtained BC membranes in all concentrations of SKW hydrolysate have the same chemical composition with no influence on their functional groups. The similarity in the FT-IR for BC membrane from SKW in the current study and other studies [61, 63] indicated that the SKW is a good candidate for BC production and implied no contamination or by-products in the formed membranes. Moreover, FT-IR data analysis indicated the presence of crystalline regions and confirming the purity of all produced BC membranes [64]. Our results are similar to those in earlier studies, where the similarity in FT-IR results of produced BC membranes at different concentrations of the same media as well as at different media compositions had been reported [6, 65,66,67].
3.3.3 Mechanical properties
Different mechanical properties of the produced BC membranes from SKW hydrolysate at 20, 40, 60, 80, and 100% concentrations were performed to include: maximum load (0.49, 0.98, 1.80, 2.35, and 2.53 N, respectively), tensile strength (74.3 78.1, 93.3, 129.6, and 162.5 MPa, respectively), elongation at peak (0.48, 0.51, 0.55, 0.84, and 1.25%, respectively), Young’s modulus (74.3, 80.4, 131, 195, and 216 MPa, respectively), and thickness (0.10, 0.15, 0.22, 0.27, and 0.31 mm, respectively) of the dried BC membranes. The results indicated that an increase in the membrane thickness was proportional to the increase in SKW hydrolysate concentrations, which is in agreement with other reports [68]. This increase directly improved the mechanical properties with maximum values at 100% concentration (0.31 mm). Several studies reported the optimization of various parameters that enhance the mechanical properties of produced BC membranes including production time [67], sodium fluoride concentrations [66], static conditions [75], and BC-producing strain [63].
3.4 Removal of MB dye by the prepared gels
The increased application of synthetic dyes in several industries implies a huge amount of the discharged dyes in wastewater samples, representing a direct environmental and human health threat [76]. MB, a cationic dye, is widely applied in many industries and has been reported to be a good model for dye removal in multiple studies [77, 78]. Due to the environmental aspects and high cost of the most reported dye removal techniques [77], an ecofriendly economic method for the removal of dyes from wastewater is highly demanded. BC gels obtained from 100% SKW hydrolysate loaded with Ch (BC-Ch) or Gr (BC-Gr) (Fig. 5) were tested for their ability to remove MB dye (10 mg/l) compared with plain BC gel. As shown in Fig. 6, the three gels revealed a varied capacity to remove the MB dye. However, this dye removal capacity was dramatically reduced with repeated application. The plain BC gel was able to absorb 53% of the dye in the first application round. The dye removal capacity dropped to 36.3 and 30.3% in the second and third cycles, respectively. In this study, BC-Ch-amended gel revealed the maximum dye removal activity (100%) at its first application round (Fig. 7), with 96 and 94% dye removal capacity after the second and third cycles of application, respectively. Carbon-based materials such as activated carbon have been previously used for the efficient removal of dyes from aqueous solutions at moderate temperature and neutral pH [79]. Some researchers preferred use of activated charcoal loaded by titanate nanotubes for the removal of different dyes such as MB, cationic rhodamine B, and anionic methyl orange [80]. Other materials such as clays [81, 82], zeolites [83], and siliceous materials [84] have been also been used for the adsorption of dyes from solutions. On the other hand, the BC-Gr-amended gel reported a moderate remediation ability of 98.7% dye removal in the first round, with 91.6 and 89% of the dye removal capacity after three successive cycles of remediation, respectively. The results are supported by other studies that reported the application of graphene in dye and heavy metal remediation [39, 85]. Our results indicated that BC has the potential capacity for trapping MB dye (53%), and this bio-sorption capacity could be improved through manipulation of the plain BC with Ch (100% dye trapping) or Gr (98.7%). The reusability results also indicated the superiority of Ch-amended BC gel with only 6% loss in dye bio-sorption capacity, compared to BC-Gr and BC that lost about 11% and 69.6% of their dye bio-sorption activity after the third round of reusing. The mean flow rate of the dye-containing water through the three gels was 1.19, 0.49, and 0.63 ml/min for BC, BC-Ch, and BC-Gr gels, respectively.
Statistical analysis of the obtained results was performed using SPSS (version 23) program. As shown in Table 4 part A, the mean difference among all cycles of the membranes BC, BC-Ch, and BC-Gr was greater than 0.05, indicating insignificance. On the other hand, as shown in Table 4 part B, the mean differences among the membranes BC and BC-Ch, BC and BC-Gr, BC-Ch and BC, and BC-Gr and BC-Ch were all ≤ 0.05 indicating significance.
3.4.1 Dye retention capacity
Compared to plain BC membranes, the ability of the prepared gels (BC-Ch, and BC-Gr) to retain the trapped dye was evaluated. For this, the used gels in the previous dye removal cycle were washed with 50-ml PBS buffer (pH 7.0) for 2 h, and the released dye % was determined at different time intervals at 655 nm. As shown in Table 5, the three gels exhibited a distinct affinity for the trapped dye. The maximum released dye was 45.7% from plain BC after 1 h. BC-Ch gel had the highest affinity for trapping dye where the maximum released dye was 4.5% after 1 h upon three cycles of dye removal. However, BC-Gr gel showed a maximum release of 11.7% MB dye after 2 h for cycle 3. The limited dye released amounts from BC-Ch- and BC-Gr-amended gels, compared to plain BC gel, over time indicated a strong adsorption status between the dye and Ch- and Gr-amended membranes, and reflecting the effect of Ch in enhancing the BC activity towards synthetic dye removal. This limited absorption of cationic dyes to BC could be attributed to the weak anion nature of most natural cellulosic fibers [86], and hence modification of its abundant (OH) surface groups enhanced their dye removal activity [87].
4 Conclusion
The enzymatic hydrolysis optimization of SKW resulted in 148.0 g/l of reducing sugars (54.8% saccharification) through applying BBD optimum conditions as following 243 g/l of wet SKW (84.3 g/l/dry) treated by 2 ml amylase (313 U/ml) at 50 °C for 5.66 h. The SKW hydrolysate has been used as a raw material for BC production after the optimization of its enzymatic hydrolysis using BBD. The maximum BC weight of 2.11 g/l (0.3 g/l/day) was obtained after the microbial fermentation of 100% of the optimum SKW hydrolysate. Both SEM and FTIR analyses showed the formation of a porous and homogeneous 3-dimensional network of the cellulose fibers and the representative functional groups, respectively. The mechanical properties of BC membranes were also affected by the concentration of SKW hydrolysate. Loading the biosynthesized BC membrane with graphite or charcoal showed maximum percentages of MB removal of 98.7 and 100%, respectively. Our study thus suggests that charcoal-loaded BC originating from starchy kitchen waste can be effectively used for the treatment of dye-contaminated wastewater.
References
Lima H, Nascimento E, Andrade F et al (2017) Bacterial cellulose production by Komagataeibacter hansenii ATCC 23769 Using Sisal Juice-An Agroindustry Waste. Braz J Chem Eng 34(3):671–680
Wu C (2010) Production and characterization of optically transparent nanocomposite film. Faculty of forestry
Noree S, Tongdang C, Sujarit K et al (2021) Application of raw starch degrading enzyme from Laceyella sacchari LP175 for development of bacterial cellulose fermentation using colored rice as substrate. 3 Biotech 11(3):1–11
Sai H, Fu R, Xing L et al (2015) Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl Mater Interfaces 7(13):7373–7381
Jozala AF, de Lencastre-Novaes LC, Lopes AM et al (2016) Bacterial nanocellulose production and application: a 10-year overview. Appl Microbiol Biotechnol 100(5):2063–2072
Ye J, Zheng S, Zhang Z et al (2019) Bacterial cellulose production by Acetobacter xylinum ATCC 23767 using tobacco waste extract as culture medium. Bioresour Technol 274:518–524
Kuo C-H, Huang C-Y, Shieh C-J et al (2019) Hydrolysis of orange peel with cellulase and pectinase to produce bacterial cellulose using Gluconacetobacter xylinus. Waste Biomass Valorization 10(1):85–93
Andritsou V, De Melo EM, Tsouko E et al (2018) Synthesis and characterization of bacterial cellulose from citrus-based sustainable resources. ACS Omega 3(8):10365–10373
Fan X, Gao Y, He W et al (2016) Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydr Polym 151:1068–1072
Hong F, Guo X, Zhang S et al (2012) Bacterial cellulose production from cotton-based waste textiles: enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour Technol 104:503–508
Sharma C, Bhardwaj NK, Pathak P (2021) Static intermittent fed-batch production of bacterial nanocellulose from black tea and its modification using chitosan to develop antibacterial green packaging material. J Clean Prod 279:123608
Mansoorian HJ, Mahvi AH, Jafari AJ et al (2013) Bioelectricity generation using two chamber microbial fuel cell treating wastewater from food processing. Enzyme Microb Technol 52(6–7):352–357
Wen Z, Wang Y, De Clercq D (2016) What is the true value of food waste? A case study of technology integration in urban food waste treatment in Suzhou City, China. J Clean Prod 118:88–96
Mohamed SN, Hiraman PA, Muthukumar K et al (2020) Bioelectricity production from kitchen wastewater using microbial fuel cell with photosynthetic algal cathode. Bioresour Technol 295:122226
Neto JG, Ozorio LV, de Abreu TCC et al (2021) Modeling of biogas production from food, fruits and vegetables wastes using artificial neural network (ANN). Fuel 285:119081
Xue S, Wang Y, Lyu X et al (2020) Interactive effects of carbohydrate, lipid, protein composition and carbon/nitrogen ratio on biogas production of different food wastes. Bioresour Technol 312:123566
Slezak R, Grzelak J, Krzystek L et al (2017) The effect of initial organic load of the kitchen waste on the production of VFA and H2 in dark fermentation. Waste Manage 68:610–617
Bhatt B, Prajapati V, Patel K et al (2020) Kitchen waste for economical amylase production using Bacillus amyloliquefaciens KCP2. Biocatal Agric Biotechnol 26:101654
Battista F, Frison N, Pavan P et al (2020) Food wastes and sewage sludge as feedstock for an urban biorefinery producing biofuels and added-value bioproducts. J Chem Technol Biotechnol 95(2):328–338
Taha TH, Abu-Saied M, Elnaggar EM et al (2019) Using starchy waste as a promising raw material for bioethanol production with consequence purification using chitosan/sodium alginate polymeric membrane. J Biosci Appl Res 5(2):154–166
Rishi V, Sandhu AK, Kaur A et al (2020) Utilization of kitchen waste for production of pullulan to develop biodegradable plastic. Appl Microbiol Biotechnol 104(3):1307–1317
Tayeb AM, Tony MA, Mansour SA (2018) Application of Box-Behnken factorial design for parameters optimization of basic dye removal using nano-hematite photo-Fenton tool. Appl Water Sci 8(5):1–9
Mannan S, Fakhru’l-Razi A, Alam MZ (2007) Optimization of process parameters for the bioconversion of activated sludge by Penicillium corylophilum, using response surface methodology. J Environ Sci 19(1):23–28
Sharma S, Malik A, Satya S (2009) Application of response surface methodology (RSM) for optimization of nutrient supplementation for Cr (VI) removal by Aspergillus lentulus AML05. J Hazard Mater 164(2–3):1198–1204
Iqbal M, Nadeem A, Sherazi F et al (2015) Optimization of process parameters for kitchen waste composting by response surface methodology. Int J Environ Sci Technol 12(5):1759–1768
Ahmed J, Gultekinoglu M, Edirisinghe M (2020) Bacterial cellulose micro-nano fibres for wound healing applications. Biotechnol Adv 107549
Wang F-p, Zhao X-j, Wahid F et al (2021) Sustainable, superhydrophobic membranes based on bacterial cellulose for gravity-driven oil/water separation. Carbohydr Polym 253:117220
Pang M, Huang Y, Meng F et al (2020) Application of bacterial cellulose in skin and bone tissue engineering. Eur Polym J 122:109365
Xie Y, Zheng Y, Fan J et al (2018) Novel electronic–ionic hybrid conductive composites for multifunctional flexible bioelectrode based on in situ synthesis of poly (dopamine) on bacterial cellulose. ACS Appl Mater Interfaces 10(26):22692–22702
Pacheco G, de Mello CV, Chiari-Andréo BG et al (2018) Bacterial cellulose skin masks—Properties and sensory tests. J Cosmet Dermatol 17(5):840–847
Bhattacharya A, Sadaf A, Dubey S et al (2020) Production and characterization of Komagataeibacter xylinus SGP8 nanocellulose and its calcite based composite for removal of Cd ions. Environ Sci Pollut Res 1–8
Chen X, Cui J, Xu X et al (2020) Bacterial cellulose/attapulgite magnetic composites as an efficient adsorbent for heavy metal ions and dye treatment. Carbohydr Polym 229:115512
Hu Y, Yue M, Yuan F et al (2021) Bio-inspired fabrication of highly permeable and anti-fouling ultrafiltration membranes based on bacterial cellulose for efficient removal of soluble dyes and insoluble oils. J Membr Sci 621:118982
Mohammed M, Shitu A, Ibrahim A (2014) Removal of methylene blue using low cost adsorbent: a review. Rese J Chem ISSN 2231:606X
Yang L, Chen C, Hu Y et al (2020) Three-dimensional bacterial cellulose/polydopamine/TiO2 nanocomposite membrane with enhanced adsorption and photocatalytic degradation for dyes under ultraviolet-visible irradiation. J Colloid Interface Sci 562:21–28
Yang F-C, Wu K-H, Huang J-W et al (2012) Preparation and characterization of functional fabrics from bamboo charcoal/silver and titanium dioxide/silver composite powders and evaluation of their antibacterial efficacy. Mater Sci Eng C 32(5):1062–1067
Zhang L, Li H, Song Q et al (2014) Surface characterisation and in vitro behaviour of carbon/carbon composites with various surface modifications. Mater Technol 29(3):134–138
Jara AD, Betemariam A, Woldetinsae G et al (2019) Purification, application and current market trend of natural graphite: A review. Int J Min Sci Technol 29(5):671–689
Yusuf M, Elfghi F, Zaidi SA et al (2015) Applications of graphene and its derivatives as an adsorbent for heavy metal and dye removal: a systematic and comprehensive overview. RSC Adv 5(62):50392–50420
El-Gendi H, Azab MS, El-Fakharany EM et al (2016) Purification and Characterization of Contemporaneously Produced Alkaline Protease and α-amylase Enzymes from Locally Isolated Bacillus methylotrophicus SCJ4. PSM Biol Res 1(2):88–95
Fuwa H (1954) A new method for microdetermination CF amylase activity by the use of amylose as the substrate. J Biochem 41(5):583–603
El-Gendi H, Azab MS, Soliman NA et al (2016) Application of Plackett-Burman Design for Optimization of Alkaline Protease and alpha-amylase Production by the Marine Bacterium Bacillus methylotrophicus SCJ4. Res J Pharm Biol Chem 7(4):899–909
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Lomthong T, Netprasom P, Kancharu N et al (2021) Very high Gravity (VHG) Bioethanol Production Using Modified Simultaneous Saccharification and Fermentation of Raw Cassava Chips with Molasses by Kluyveromyces marxianus DMKU-KS07. Waste Biomass Valorization 12(7):3683–3693
Saleh AK, Farrag A, Soliman N et al (2019) Evaluation of culture requirments for cellulose production by Egyptian local isolate alongside reference strain Gluconacetobacter hansenii ATCC 23769. Pak J Biotechnol 16(2):69–80
Hestrin S, Schramm M (1954) Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J 58(2):345
Hsieh J-T, Wang M-J, Lai J-T et al (2016) A novel static cultivation of bacterial cellulose production by intermittent feeding strategy. J Taiwan Inst Chem Eng 63:46–51
Kiziltas EE, Kiziltas A, Rhodes K et al (2016) Electrically conductive nano graphite-filled bacterial cellulose composites. Carbohydr Polym 136:1144–1151
Talan A, Tiwari B, Yadav B et al (2020) Food Waste Valorization: Energy Production Using Novel Integrated Systems. Bioresour Technol 124538
Özmen I (2020) Optimization for coproduction of protease and cellulase from Bacillus subtilis M-11 by the Box-Behnken design and their detergent compatibility. Braz J Chem Eng 37(1):49–59
Radhathirumalaiarasu S, Mahalakshmi GK (2020) Optimized parameters using Box-Behnken design methodology facilitate enhanced phenol degradation of Bacillus cereus PB1 by immobilization and adsorption. Environ Qual Manage 30(2):13–20
Solomon D, Kiflie Z, Van Hulle S (2020) Using Box–Behnken experimental design to optimize the degradation of Basic Blue 41 dye by Fenton reaction. Int J Ind Chem 1–11
Rastogi A, Banerjee R (2020) Statistical optimization of bacterial cellulose production by Leifsonia soli and its physico-chemical characterization. Process Biochem 91:297–302
Kim JK, Oh BR, Shin H-J et al (2008) Statistical optimization of enzymatic saccharification and ethanol fermentation using food waste. Process Biochem 43(11):1308–1312
Song H-J, Li H, Seo J-H et al (2009) Pilot-scale production of bacterial cellulose by a spherical type bubble column bioreactor using saccharified food wastes. Korean J Chem Eng 26(1):141–146
Wang Q, Tian D, Hu J et al (2020) Harvesting bacterial cellulose from kitchen waste to prepare superhydrophobic aerogel for recovering waste cooking oil toward a closed-loop biorefinery. ACS Sustain Chem Eng 8(35):13400–13407
Li WL, Hua J et al (2015) Production of nano bacterial cellulose from waste water of candied jujube-processing industry using Acetobacter xylinum. Carbohydr Polym 120:115–119
Kaur A, Kuhad RC (2019) Valorization of Rice Straw for Ethanol Production and Lignin Recovery Using Combined Acid-Alkali Pre-treatment. BioEnergy Res 1–13
Revin V, Liyaskina E, Nazarkina M et al (2018) Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz J Microbiol 49:151–159
Moon S-H, Park J-M, Chun H-Y et al (2006) Comparisons of physical properties of bacterial celluloses produced in different culture conditions using saccharified food wastes. Biotechnol Bioproc Eng 11(1):26
Machado RT, Meneguin AB, Sábio RM et al (2018) Komagataeibacter rhaeticus grown in sugarcane molasses-supplemented culture medium as a strategy for enhancing bacterial cellulose production. Ind Crops Prod 122:637–646
Ulman A (2013) An Introduction to Ultrathin Organic Films: From Langmuir--Blodgett to Self--Assembly, Academic press
He X, Meng H, Song H et al (2020) Novel bacterial cellulose membrane biosynthesized by a new and highly efficient producer Komagataeibacter rhaeticus TJPU03. Carbohyd Res 493:108030
Castro C, Zuluaga R, Álvarez C et al (2012) Bacterial cellulose produced by a new acid-resistant strain of Gluconacetobacter genus. Carbohydr Polym 89(4):1033–1037
Rozenberga L, Skute M, Belkova L et al (2016) Characterisation of films and nanopaper obtained from cellulose synthesised by acetic acid bacteria. Carbohydr Polym 144:33–40
Sun B, Zhang L, Wei F et al (2020) In situ structural modification of bacterial cellulose by sodium fluoride. Carbohydr Polym 231:115765
Zhang Y, Chen Y, Cao G et al (2020) Bacterial cellulose production from terylene ammonia hydrolysate by Taonella mepensis WT-6. Int J Bio Macromol 166:251–258
Gao G, Cao Y, Zhang Y et al (2020) In situ production of bacterial cellulose/xanthan gum nanocomposites with enhanced productivity and properties using Enterobacter sp. FY-07. Carbohydr Polym 248:116788
Qiao N, Fan X, Zhang X et al (2019) Soybean oil refinery effluent treatment and its utilization for bacterial cellulose production by Gluconacetobacter xylinus. Food Hydrocoll 97:105185
Güzel M, Akpınar Ö (2019) Production and characterization of bacterial cellulose from citrus peels. Waste Biomass Valorization 10(8):2165–2175
Chang W-S, Chen H-H (2016) Physical properties of bacterial cellulose composites for wound dressings. Food Hydrocoll 53:75–83
Vasconcelos NF, Feitosa JPA, da Gama FMP et al (2017) Bacterial cellulose nanocrystals produced under different hydrolysis conditions: properties and morphological features. Carbohydr Polym 155:425–431
Saleh AK, Soliman NA, Farrag AA et al (2020) Statistical optimization and characterization of a biocellulose produced by local Egyptian isolate Komagataeibacter hansenii AS. 5. Int J Bio Macromol 144:198–207
Fernandes M, Gama M, Dourado F et al (2019) Development of novel bacterial cellulose composites for the textile and shoe industry. Microb Biotechnol 12(4):650–661
Wang J, Tavakoli J, Tang Y (2019) Bacterial cellulose production, properties and applications with different culture methods–a review. Carbohydr Polym 219:63–76
Cheng J, Zhan C, Wu J et al (2020) Highly efficient removal of methylene blue dye from an aqueous solution using cellulose acetate nanofibrous membranes modified by polydopamine. ACS Omega 5(10):5389–5400
Bayomie OS, Kandeel H, Shoeib T et al (2020) Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Sci Rep 10(1):1–10
Zhijiang C, Ping X, Cong Z et al (2018) Preparation and characterization of a bi-layered nano-filtration membrane from a chitosan hydrogel and bacterial cellulose nanofiber for dye removal. Cellulose 25(9):5123–5137
Elhadiri N, Benchanaa M, Chikri R (2020) Activated carbon for dyes removal: modeling and understanding the adsorption process. J Chem 2020
Lin Y, Ma J, Liu W et al (2019) Efficient removal of dyes from dyeing wastewater by powder activated charcoal/titanate nanotube nanocomposites: adsorption and photoregeneration. Environ Sci Pollut Res 26(10):10263–10273
Crini G, Lichtfouse E, Wilson LD et al (2019) Conventional and non-conventional adsorbents for wastewater treatment. Environ Chem Lett 17(1):195–213
Kausar A, Iqbal M, Javed A et al (2018) Dyes adsorption using clay and modified clay: a review. J Mol Liq 256:395–407
Alpat SK, Özbayrak Ö, Alpat Ş et al (2008) The adsorption kinetics and removal of cationic dye, Toluidine Blue O, from aqueous solution with Turkish zeolite. J Hazard Mater 151(1):213–220
Sun B, Chakraborty A (2015) Thermodynamic frameworks of adsorption kinetics modeling: Dynamic water uptakes on silica gel for adsorption cooling applications. Energy 84:296–302
Leng Y, Guo W, Su S et al (2012) Removal of antimony (III) from aqueous solution by graphene as an adsorbent. Chem Eng J 211:406–411
Fardim P, Moreno T, Holmbom B (2005) Anionic groups on cellulosic fiber surfaces investigated by XPS, FTIR-ATR, and different sorption methods. J Colloid Interface Sci 290(2):383–391
dos Santos SL, de Oliveira CJ, de Sousa Bezerra RD et al (2018) Potential of cellulose functionalized with carboxylic acid as biosorbent for the removal of cationic dyes in aqueous solution. Molecules 23(4):743
Acknowledgements
The authors are grateful to Prof. Dr. Mohamed A. Abu-Saied, Polymer Materials Research Department, City of Scientific Research and Technological Applications, for supporting this work through providing the design of the dye removal system. Thanks to Dr. Khlood S. Abdel Zaher, Green Chemistry Department, National Research Centre, for FT-IR characterization of BC membranes.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Ahmed K. Saleh: methodology, investigation, formal analysis, experimental details, writing original draft. Hamada El-Gendi: methodology, investigation, formal analysis, writing original draft. Tarek H. Taha: conceptualization, investigation, writing (review and editing). Julie Basu Ray: conceptualization, final review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saleh, A.K., El-Gendi, H., Ray, J.B. et al. A low-cost effective media from starch kitchen waste for bacterial cellulose production and its application as simultaneous absorbance for methylene blue dye removal. Biomass Conv. Bioref. 13, 12437–12449 (2023). https://doi.org/10.1007/s13399-021-01973-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01973-1