Abstract
Biotechnology is constantly explored to develop methods for wastewater treatment to have a clean environment. Discharge of untreated wastewaters in the soil and water environment is the major cause of environmental pollution in soil and surface and groundwater bodies. Current wastewater treatment technologies are not sufficient to meet the ever-growing demands due to rapid industrialization and population growth, and they are also energy-consuming and cost-intensive. Therefore, it is necessary to develop an energy-efficient treatment method. The rise in global energy demand is projected to increase by 37% by 2040. As the conventional energy sources are also depleting at a fast rate, it is necessary to adopt renewable energy to meet the demands of future energy scenario. Microbial fuel cell (MFC) is a technology in which electricity is generated from wastewater using microorganism as a biocatalyst, simultaneously achieving wastewater treatment. In this system, microorganism mediates the direct conversion of chemical energy stored in biodegradable organic matter of wastewater into electrical energy. Many researchers have developed a variety of MFC-centered hybrid wastewater treatment plants at a pilot scale, for the treatment of wastewater from different industries such as dye, brewery, and dairy industries, domestic wastewater, and landfill leachate. Irrespective of the potential of MFC as a renewable energy source and its wide applicability for wastewater treatment, the technique is not yet established successfully for field applications. Low-energy performance and excessive internal resistance are the limiting factors in its practical application. Researchers have performed multiple strategic attempts to minimize these factors by developing efficient reactor designs for minimizing internal resistance, stacking multiple reactors into one enlarge system (modularization). Modularization is one of the common strategies followed by researchers for pilot-scale MFC setup. This prevents the unnecessary distance between anode and cathode and enhances COD removal rate. Also, using various forms of electrodes such as carbon brushes, nickel foam, etc. to provide large surface area and modification of electrodes using nanomaterials have shown power density enhancement. This chapter discusses the potential applications and practical limitations of MFC for its effectiveness in wastewater treatment and contaminant removal and energy generation at bench and pilot scale.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Clean water and electricity are one of the basic human requirements, but unfortunately these are denied to large swathes of world population. The rise in energy demand of the world is projected to increase by about 37% by 2040 (International Energy Agency). The current sufficiency in the supply of different energy resources available for the short term may paint an image of the future with an easy and clear path to sustainable energy development and also hide different challenges involved in doing so. The rise in the usage of different energy resources varies based on the risk of disruption involved in supply and the harm their usage can cause to the environment. For example, the growth in liquefied natural gas has been highest of those of all the fossil fuels at about more than half while the usage of coal is seen to decline. Along with the energy resource crisis, the problem of waste generation makes the situation even worse, especially wastewater. There is an increasing demand for freshwater across the globe. The usage of freshwater has tripled over the past 50 years. It is increasing by 64 trillion liters every year as per data by UN, UNESCO, and FAO. As per a report published by Central Pollution Control Board (CPCB) of India, in March 2015, the sewage generation from urban areas in the country is estimated at 61,948 million liters per day (mld), against which a sewage treatment capacity of 23,277 mld is available with us. The remaining untreated sewage either flows into rivers and other water bodies or percolates into the ground. Wastewater treatment is an energy-intensive process and requires lots of energy each year for treatment. According to an estimate, 0.349 kWh of electrical energy per cubic meter of wastewater is required for activated sludge processes in the USA (Oh et al. 2010). There is a strong nexus between water and energy. For example, water and wastewater utilities consume 3% of the electricity in the USA, which accounts for 35% of the total municipal energy budgets. Activated sludge (aerobic digestion) is one of the well-established processes and has been used in most wastewater treatment systems. Although it is an efficient process, it is chemical- and energy-intensive with high capital and significant operational cost (Lu et al. 2017).
To overcome these problems, microbial fuel cells (MFCs) offer a potential solution as they can concurrently be used for wastewater treatment and electricity generation. MFCs have long been considered as an attractive technology for converting various organic wastes directly into electricity using electrogenic bacterial cells in the anode compartment. Most MFCs have been operated using anaerobic or facultative aerobic bacteria which oxidize various organic components including glucose, sewage sludge, brewery waste, dairy waste, and petroleum hydrocarbon. The performances of the MFCs vary with its configuration, electrode configuration, bacterial community, and specific substrate concentration (Lu et al. 2017). Apart from producing electricity, MFC can also be used as efficient wastewater treatment technology and water quality monitoring. In wastewater treatment technology, MFCs have advantage over other technologies in that it can also treat high soluble BOD content wastewater (Waller and Trabold 2013) and produce less sludge. So, various researches are focused to commercialize MFC technology due to its advantage over other technologies of treating wastewater.
Preliminary reports concerning the use of MFC for wastewater treatment were mostly focused on MFC with low volumetric capacity (less than 1 L) and using synthetic wastewater. However, for commercialization purposes, it requires scaling-up, and studies are needed on pilot scale using real wastewater or operated in treatment plants. Recently, various studies have focused on scaling-up MFCs. For example, a 90L MFC was built by stacking anode and cathode modules into a reactor vessel which was reported to produce 0.056 kWh/m3 energy and 171±8.4 mW/m2 power densities from each module and reached 87.6% COD removal efficiency while treating brewery wastewater (Dong et al. 2015). Zhang et al. (2013a, b) designed two 4 liter tubular MFCs to treat primary effluent wastewater and reached 65–70% COD removal efficiency in the continuous mode of operation at a HRT of 11 h, and the maximum energy production was 0.0255 kWh/m3.
It is a common approach to stack multiple small reactors to form a bigger system, as this greatly reduces the internal resistance, and the total volume capacity of the reactor can be increased by just increasing the number of each single reactor. This also offers the advantage as each reactor can function and be maintained as an independent unit. Zhuang et al. (2012a, b) reported the energy output and COD removal of 6 W/m3and 87/1%, respectively, by stacking 40 tubular units in MFCs with 10 L volumetric capacity while treating brewery wastewater. Ge et al. (2013) stacked 96 tubular MFC modules to build an MFC system with 200 L liquid volume to treat primary effluent wastewater and obtained maximum power density of 6.4 W/m3. These studies show that the stacking of small segmented reactors might be measured as a suitable option for scale-up. This approach would be better for maximizing the power production of systems; however, minimizing internal resistances and energy losses due to fluid flow and reducing dead space are difficult to achieve.
Electrode configuration and positioning is another great challenge in making MFC a scalable technology. Frequently used electrodes can be categorized according to their configurations, the plane electrode and the three-dimensional electrode. Carbon cloth and carbon paper come under plane electrode, whereas carbon brushes/felt comes under three- dimensional electrodes. A good configuration and properties for anodes include a large surface area for bacterial adhesion and efficient current collection.
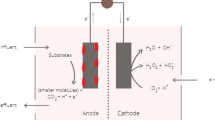
This chapter focuses on design and operation of MFCs, with emphasis on its application in wastewater treatment (Fig. 10.1).
10.2 MFC: Design and Operation
Many MFC designs have been proposed and operated in laboratory with different reactor configurations. Various designs have different positioning of basic components of MFC such as anode, cathode, and separator. Below is the short introduction about the components of MFC.
10.2.1 Components of MFC
The main components of MFC include the electrodes, i.e., cathode and anode, and the separator which is a semipermeable membrane which allows the exchange of protons.
10.2.2 Electrodes
Electrode is the significant component in determining the performance and cost of MFC. Electrode design is one of the challenging problems in making MFCs a cost-effective and scalable technology. A variety of carbonaceous material are used as electrodes.
10.2.3 Anodes
Anode materials are responsible for the electron transfer efficiency which is an important factor for MFC performance. A large variety of carbon materials are used as anode in MFC due to their conductivity, chemical stability, cost-effectiveness, and biocompatibility.
-
(a)
Granular carbon/graphite – It is generally used as an anode in MFC for its high surface area and porosity. Graphite granules of diameter between 1.5 and 5 mm provide an estimated surface area between 817 and 2720 m2 m-3 of the reactor volume, which is much higher than flat electrodes (Rabaey et al. 2005). This type of anode configuration is achieved by filling the anodic chamber with carbon/graphite granules. Tubular reactor is the best suited configuration for using granule anode. Although granular anodes facilitate good energy output, filling the reactor with granules decreases the volume of the anodic chamber. Moreover, clogging of pores in the electrode may be due to suspended solids in wastewater which may hinder the long-term continuous use of reactor.
-
(b)
Carbon cloth – It is the most common material for plain electrodes and can be used in most of the single-chamber MFC configuration due to its flexible nature. In tubular MFC configuration, carbon cloth wrapped around the tube acts as cathode, which is only possible due to its flexible nature. Kim et al. (2010) used carbon cloth as anode in tubular MFC by wrapping around a perspex cylinder and fitted centrally in the tube reactor.
-
(c)
Carbon brushes – High surface area and efficient electron collection properties make carbon brush an ideal electrode material for MFC (Wei et al. 2011). It was first testified by Logan et al. (2007). The brushes were made of carbon fibers looped into a twisted core of two conductive titanium wires. The diameter and mass of fibers affect the performance of MFC. Liu et al. (2013) investigated that the smaller diameter brush facilitates more energy output, but the effect of fiber loading on energy output was not clear. However, studies do indicate that dense fiber loading leads to clumping and causes hindrance in bacterial access to fiber surface as well as substrate access into the fiber interior. Zhang et al. (2013a, b) used carbon brushes (100 cm long) as anode in tubular MFC deployed in the primary effluent of municipal wastewater treatment plant.
10.2.4 Cathode
The electrons produced in the anodic chamber are transferred to the cathode via external circuit where reductions of electrons occur. In dual-chamber MFC, ferricyanide is used as electron acceptor and converted to ferrocyanide. In single-chamber MFCs, water is formed by reduction reaction in the presence of atmospheric oxygen. Oxygen is most commonly used as electron acceptor due to its high affinity to acquire electron and easy availability, and its end product is only water. Graphite, carbon, and steel are commonly used as a cathode electrode (Fan et al. 2007). Reduction potential of graphite and carbon is very low, and it usually leads to high over-potential. So, platinum is used as catalyst for oxygen reduction reaction at the cathode, but due to its high cost, it is limited to lab scale only. Many researchers focused on using alternative to platinum catalyst to low-cost metal such as Fe (III), cobalt complexes (Cheng et al. 2006), and manganese oxide (Rhoads et al. 2005).
10.2.5 Separator
Separator is employed in MFC to physically separate the anodic and cathodic chambers or cathode for a single-chamber MFC and migration of proton from anodic to cathodic chambers. Various types of separator have been extensively studied for MFC including cation-exchange membrane (CEM) (Kim et al. 2010; Zhang et al. 2013a, b), anion-exchange membrane (AEM), microfiltration membrane (MFM), ultrafiltration membrane (UFM), and salt bridge. Separator has large contribution in the cost of MFC. In the study conducted by Ge et al. (2016), separator (CEM) contributed to 60% of the total cost (Table 10.1).
Two of the basic designs of MFC are single-chamber MFC and dual-chamber MFC.
10.2.6 Single-Chamber Microbial Fuel Cells (SC-MFCs)
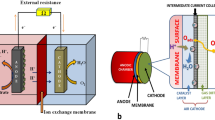
SC-MFC offers simpler and cost-saving designs which only have an anodic chamber and cathode directly facing the air. This is the simpler type of MFC in which cathode is manifested directly to air and is now widely used. Nowadays researchers have shown that oxygen can act as terminal electron acceptor that eliminates the necessities of catholyte solution. Liu and Logan (2004) designed the first SC-MFC of cylindrical shape having anode placed inside it and cathode wrapped outside. A single-compartment MFC was designed by Park et al. (2018) consisting of a rectangular chamber having anode inside it and cathode which is directly exposed to the air. Cheng and Logan (2011) designed a SC-MFC consisting of a cylindrical structure with anode placed into it, and a cathode made of carbon cloth material coated with platinum was enclosed around the reactor. In single-chamber MFC, stacking MFCs is more easily achieved and offers higher voltage/current (Fig. 10.2).
10.2.7 Dual-Chamber Microbial Fuel Cells (DC-MFCs)
The traditional two-compartment MFC has “H” shape, in which a proton-exchange membrane (PEM) is used to separate the anodic chamber and the cathodic chamber. Sometimes salt bridge is also used that allows the protons to cross the membrane to cathode. This kind of MFC is used for the study of the basic parameter of microbial fuel cells such as electrodes, biofilms, proton-exchange membrane, and substrates. Dual-chamber MFCs are generally larger in size due to the presence of extra chamber; the distance between two electrodes (anode and cathode) is more, thus causing high internal resistance. Also the chemicals used in cathodic side should be environment-friendly. The use of membranes in DC-MFCs prevents the diffusion of oxygen from cathode to anode. Zhang et al. (2010) constructed a plate-shaped DC-MFC in which cation-exchange membrane (CEM) is used to separate the anode and cathode, and potassium ferricyanide was used as the electron acceptor to complete the circuit (Table 10.2).
Based on the above criteria and components, many MFC reactor designs have been proposed and operated in the laboratory; despite the great efforts and research, they are still challenging for MFC to be used in practical application. Many MFC systems have been developed at milliliter scale, but in order for practical applications to be comprehended, it is required that it should be capable of treating large quantities of wastewater. System scaling-up is one of the major obstacles for MFC to be used for practical application. It has been observed that there is a logarithmic relationship between power output and reactor volume. The declining power output in large scale is due to the fact that a proper mixing and large distance between anode and cathode hamper the electricity generation. For scaling-up MFC, research approaches the modularization strategies in which multiple reactors are stacked into one system.
10.2.8 Multimodule MFC Reactors
This is a promising configuration for scale-up, as optimal cross-sectional dimensions could be maintained while increasing the volume by extending the tube in the orthogonal dimension. This configuration geometric feature also helps in mass transfer with minimum pressure losses. Carbon brush anode is mostly used in tubular MFC either at the lab scale or pilot scale (Zhang et al. 2013a, b). Carbon cloth anode is used in tubular MFC by wrapping it on a thin pipe and fitted centrally on the tubular MFC. To increase the volume in tubular MFC, researchers generally vary the length of the tube, and the diameter of the tube is generally maintained at 3–6 cm. The size of the tube length has large impact on COD removal rate. In municipal wastewater treatment plant, 53% COD removal was observed by 100-cm-long tubular MFC (Zhang et al. 2013a, b), while in the laboratory, 49% COD removal is observed by 23-cm-long tubular MFC (Kim and Logan 2013). When raw sludge from primary sedimentation tank was used as substrate, about 69% COD removal was observed with 70 cm tube length.
10.3 Strategies for Improving Power Output
High MFC power output is due to the efficient harvesting of electrons from the anode and transferring them to the cathode, where it is finally accepted by oxygen within a certain limit of time. Wei et al. (2011) reported that to improve the performance of MFCs in terms of power generation, it is necessary to modify electrodes to increase their electron capturing capacity. Zhuang et al. (2012a, b) reported that to enhance the power generation and pollutant removal from wastewater, MFC units could be connected either in parallel or in series. Alatraktchi et al. (2014) reported a power density of 461.6 mW/m2 using electrodes modified by coating them with gold nanoparticles, which was 1.88 times higher than the unmodified electrode (carbon paper). Wu et al. (2012) achieved a maximum power density of 320 mW/m3 using white-rot fungus, which was significantly higher as compared to the abiotic control, i.e., 50 mW/m3. Ren et al. (2013) reported that the charge and discharge frequency significantly influences the current output, COD removal efficiency, and charge recovery efficiency.
Below are some of the given factors which should be considered while scaling-up MFC to get improved power output.
-
(a)
Internal Resistance
It is one of the main factors that affects the power density in liter-scale MFC, and as already discussed, increasing the volume of reactor leads to increased internal resistance. The same will also occur in the multiple cell systems stacked up by combining small MFC modules in series or parallel connection (Zhuang et al. 2012a, b). Therefore, it is necessary to quantify the internal resistance of each component of MFC reactor to identify the limiting factors of the system (Fan et al. 2012). Many studies have been conducted to minimize the internal resistance by improving the reactor configuration (Dong et al. 2015; Feng et al. 2014), increasing conductivity of substrate (Zuo et al. 2014), adding ion-exchange resins to chamber (Zuo et al. 2014), and improving the electrode configuration. Feng et al. (2014) compared the internal resistance distribution between a lab-scale MFC module and a pilot MFC module with similar structure to identify the limiting factors during enlargement for further enhancing the performance in the application-oriented pilot MFC system and derived the following equation to identify the increase internal resistance during enlargement:
where Pp.max is the maximum power output from a pilot-scale MFC could be, E is the potential difference, K is the enlarge ratio of reactor cross-sectional area, and Rp,real and R cubic refer to the internal resistance in pilot and cubic reactors.
Zuo et al. (2014) mixed salt solution with influent to increase the conductivity of anolyte which decreased the internal resistance. The internal structure of MFC also greatly influences the internal resistance, thus indicating that abiotic factors such as catalysts, electrodes, electrolytes, and reactor types, along with biotic factors, should also be considered, if one wishes to optimize power generation.
-
(b)
Biofouling
Bacterial consortia endeavor to grow on every surface in its surrounding area. Membrane is also exposed to the wastewater in the anode chamber, so biofilm formation on its surface is quite obvious, which leads to biodegradation of the membrane, i.e., biofouling. Biofouling of both membrane and cathode electrode over time decreases the current. Biofouling leads to a decrease in the surface area required for migration of ions to cathode and thus a decrease of the efficiency of MFC (Sonawane et al. 2013). It is one of the major problems in of MFC in the long run, especially when operated in wastewater treatment plant. Biofouling of cathode affects the power generation as it reduces the surface for oxygen uptake. Zhang et al. (2013a, b) observed that by simply washing the cathode with water, it quickly restored the current generation, but biofilm formation occurs quickly again once the MFC is installed back. Biofouling is one of the serious problems that needs to be improved by developing new membranes with lower internal resistance or less subject to biofouling to achieve long-term performance of MFC.
-
(c)
Modularization
Another tactic for improving power output in MFC is to stack multiple small reactors together to form a bigger system rather than having a singular large unit. This strategy is followed by most researchers to improve the power output at a large scale. Apart from electricity generation, COD removal efficiency also depends on the number of modules and reactor configuration. Dong et al. (2015) reported COD removal of 87.6%, at an HRT of 72 h, while Zhuang et al. (2012a, b) achieved 87.1% COD removal with an HRT of 48 h only, both using brewery wastewater. The volumetric capacity of the above two studies was 90 L and 10 L, respectively, but the reactor configuration was different. In the study by Dong et al. (2015), anode and cathode modules were placed in a single vessel, and in the report by Zhuang et al. (2012a), 40 units of tubular MFC were stacked to form a serpentine-type MFC. It should be noted that when 96 tubular MFC modules of total volumetric capacity of 200 L were used to treat primary effluent, 76.8% COD removal efficiency was achieved at an HRT of 18 h only (Ge et al. 2016). These efforts have proved that it is more appropriate to stack multiple MFC modules, since it allows maintaining minimum distance between anode and cathode, and each single reactor can function and be maintained independently. Although Haeger et al. (2014) constructed a single reactor instead of having modules in which anode and cathode are rolled up around a central manifold in a spiral fashion, the electrolytes flow through it producing power density of 170 W/m3. These studies indicate that efficiency of MFC largely depends on its configuration, and more studies are required to achieve a module configuration in which maximum COD removal efficiency is achieved with a minimum HRT and maximum power production.
-
(d)
Use of Modified Electrodes
Approaches to modify the surface of electrodes to enhance surface area and improve conductivity have recently gained popularity. Electrode configuration relates to flat electrode, three-dimensional electrode (carbon felt), and brush electrode. The surface properties of electrode material are one of the deciding factors that affect the bacterial attachment to anode and electron reduction at cathode. One of the approaches to enhance power production in MFCs is using modified electrode to facilitate electron transfer to the anode and improve bacterial adhesion capability (Wei et al. 2011). Strategies used to modified anode include (i) use of metal oxide/graphene as electroactive coatings for electrode, (ii) surface treatment with chemical or physical methods (iii), use of conductive polymer to enhance conductivity of electrode, (iv) and increase surface area by enhancing configuration such as carbon brush, carbon mesh, stainless steel mesh, etc. For cathode, generally flat electrode is used with platinum on carbon coating to enhance its activity.
-
(e)
Hydraulic Retention Time (HRT)
HRT is one of the important parameters in the wastewater treatment process; it also greatly affects the operational cost and power generation in MFC (Akman et al. 2013). Excessively long HRTs in MFC reduce the organic loading rate, thereby causing fast depletion of substrate and reducing the cell performance, as the microbes attached to the anodic chamber could not receive sufficient electron sources to produce sufficient amounts of electrons, whereas shorter HRTs favor the development of non-exoelectrogenic microorganism, which causes reduction in the electrochemical performance, thus leading to lower columbic efficiency and less efficient COD removal. Also, when an excessively low HRT is applied to MFCs during the continuous wastewater inflow, it prevents the attachment of microbes to the anode surfaces, thus preventing the formation of confluent biofilm on anodic surface. In this way, HRT readily affects the power generation and effluent quality. In a continuous wastewater flow, MFC having HRT between 0.5 and 2 days, when HRT was decreased, enabled the organic load of the anode chamber to facilitate the microbes to generate more electrons by degrading organic matter, thus releasing more electrons and enhancing the power generation efficiency. Akman et al. (2013) and Hiegemann et al. (2016) reported the power density of 80, 82, and 73 mW/m2 with HRT of 12, 22, and 44 h, respectively.
The effect of temperature on MFC performance is also reported. MFCs operated higher temperatures were reported to give better performance in terms of power generation than those which operated under ambient temperature conditions (Ahn and Logan 2010).
10.4 Application in Wastewater Treatment
The MFC technology utilizes the potential of exoelectrogenic bacteria by releasing the energy containing wastewater while simultaneously treating it. Many researchers have demonstrated power generation using wastewater as substrate and reported COD and pollutant removal simultaneously (Hiegemann et al. 2016; Akman et al. 2013). The microorganisms growing on the anode oxidize the organic matter content of wastewater and transfer the electrons generated to the anode. As the electrons flow from the anode to the cathode through an external circuit, a current is produced which is sometimes stored in capacitors. Therefore, MFCs can be one of the most promising technologies for getting renewable energy and treating wastewater with great eco-friendly benefits. Previously researchers mainly focused on optimizing the power generation and neglecting wastewater treatment; however, lately, attention is being given to both aspects.
Application of MFC in wastewater treatment was first demonstrated by Habermann and Pommerin (1991). Logan (2008) reported a maximum power generation of 330 kW/day using an MFC installed at the wastewater treatment plant of a food processing plant.
Heilmann and Logan (2006) demonstrated electricity generation from protein-rich municipal wastewaters. They used three different protein-rich waste streams, i.e., bovine serum albumin, peptone, and slaughterhouse wastewater with three different BODs of 1100 mg/L, 500 mg/L, and 1420 mg/L, respectively, and reported power densities of 354, 269, and 80 mW/m2, respectively, with BOD removal efficiencies of 90%, 86%, and 93%, respectively. They concluded that less complex protein generates more power than the complex protein. It may thus be concluded that the higher the complexity of wastewater, the lower will be the columbic efficiency. Zhuang et al. (2012a, b) constructed an MFC system of 10 L volume that consists of 40 individual tubular MFCs to treat synthetic wastewater and reported a maximum power density of 4.1 W/m3.
Jiang et al. (2011) constructed a 16 L MFC module to treat municipal wastewater and achieved 80% of COD removal but reported low-energy generation due to excessive internal resistance. Zhang et al. (2013a, b) designed two tubular microbial fuel cells (MFCs) of volume 4 L to treat municipal wastewater and achieved similar COD removal efficiency of 65–70% with both types of MFCs. They used activated carbon powder as catalyst in one MFC while platinum along with carbon powder on other MFC. Both the MFCs removed 65–70% chemical oxygen demand at a hydraulic retention time of 11 h and reduced 50% suspended solids (Table 10.3).
10.5 Conclusion
MFC reactor design and configuration are the greatest challenges in making the MFC effective for power generation and COD removal efficiency. In wastewater treatment plant, COD removal is one of the important parameters, and it will be beneficial that the energy required for its treatment is obtained from the wastewater itself. Scaling-up MFC is still facing lots of challenges such as increasing internal resistance, dead space, biofouling, and long-term usability. Further studies on more optimization of reactor configurations are expected to address these challenges and enhance the potential of large-scale MFCs with conventional wastewater treatment systems.
References
Ahn Y, Logan BE (2010) Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour Technol 101:469–475. https://doi.org/10.1016/j.biortech.2009.07.039
Akman D, Cirik K, Ozdemir S, Ozkaya B, Cinar O (2013) Bioelectricity generation in continuously-fed microbial fuel cell: effects of anode electrode material and hydraulic retention time. Bioresour Technol 149:459–464. https://doi.org/10.1016/J.BIORTECH.2013.09.102
Alatraktchi FA, Zhang Y, Angelidaki I (2014) Nanomodification of the electrodes in microbial fuel cell: impact of nanoparticle density on electricity production and microbial community. Appl Energy 116:216–222. https://doi.org/10.1016/j.apenergy.2013.11.058
Cheng S, Logan BE (2011) Increasing power generation for scaling up single-chamber air cathode microbial fuel cells. Bioresour Technol 102(6):4468–4473. https://doi.org/10.1016/j.biortech.2010.12.104
Cheng S, Liu H, Logan BE (2006) Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (Nafion and PTFE) in single chamber microbial fuel cells. Environ Sci Technol 40:364–369. https://doi.org/10.1021/es0512071
Clauwaert P, Mulenga S, Aelterman P, Verstraete W (2009) Litre-scale microbial fuel cells operated in a complete loop. Appl Microbiol Biotechnol 83:241–247. https://doi.org/10.1007/s00253-009-1876-0
Dong Y, Qu Y, He W et al (2015) A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode. Bioresour Technol 195:66–72. https://doi.org/10.1016/j.biortech.2015.06.026
Fan YZ, Hu HQ, Liu H (2007) Enhanced Coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. J Power Sources 171:348–354. https://doi.org/10.1016/j.jpowsour.2007.06.220
Fan Y, Han S-K, Liu H (2012) Improved performance of CEA microbial fuel cells with increased reactor size. Energy Environ Sci 5:8273–8280. https://doi.org/10.1039/c2ee21964f
Feng Y, He W, Liu J, Wang X, Qu YRN (2014) A horizontal plug flow and stackable pilot microbial fuel cell for municipal wastewater treatment. Bioresour Technol 156:132–138. https://doi.org/10.1016/j.biortech.2013.12.104
Ge Z, Zhang F, Grimaud J, Hurst J, He Z (2013) Long-term investigation of microbial fuel cells treating primary sludge or digested sludge. Bioresour Technol 136:509–514. https://doi.org/10.1016/j.biortech.2013.03.016
Ge Z, He Z, Ren ZJ, Zeeman G, Saakes M, Sleutels TH, Buisman CJ (2016) Long-term performance of a 200 liter modularized microbial fuel cell system treating municipal wastewater: treatment, energy, and cost. Environ Sci Water Res Technol 2(2):274–281. https://doi.org/10.1039/C6EW00020G
Ghadge AN, Jadhav DA, Ghangrekar MM (2016) Wastewater treatment in pilot-scale microbial fuel cell using multielectrode assembly with ceramic separator suitable for field application. Environ Prog Sustain Energy 35:1809–1817. https://doi.org/10.1002/ep.12403
Habermann W, Pommer EH (1991) Biological fuel cells with sulphide storage capacity. Appl Microbiol Biotechnol 35:128–133. https://doi.org/10.1007/BF00180650
Haeger A, Forrestal C, Xu P, Ren ZJ (2014) High performance spiral wound microbial fuel cell with hydraulic characterization. Bioresour Technol 174:287–293. https://doi.org/10.1016/j.biortech.2014.09.153
Heidrich ES, Edwards SR, Dolfing J, Cotterill SE, Curtis TP (2014) Performance of a pilot scale microbial electrolysis cell fed on domestic wastewater at ambient temperatures for a 12 month period. Bioresour Technol 173:87–95. https://doi.org/10.1016/j.biortech.2014.09.083
Hiegemann H, Herzer D, Nettmann E, Lubken M, Schulte P, Schmelz G, Hoffmann SG, Wichern M (2016) An integrated 45 L pilot microbial fuel cell system at a full scale wastewater treatment plant. Bioresour Technol 218:115–122. https://doi.org/10.1016/j.biortech.2016.06.052
Heilmann J, Logan BE (2006) Production of electricity from proteins using a microbial fuel cell. Water Environ Res 78:531–537. https://doi.org/10.2175/106143005X73046
Jiang D, Curtis M, Troop E, Scheible K, McGrath J, Hu B, Suib S, Raymond D, Li B (2011) A pilot-scale study on utilizing multi-anode/cathode microbial fuel cells (MAC MFCs) to enhance the power production in wastewater treatment. Int J Hydrogen Energy 36:876–884. https://doi.org/10.1016/J.IJHYDENE.2010.08.074
Kim Y, Logan BE (2013) Microbial desalination cells for energy production and desalination. Desalination 308:122–130. https://doi.org/10.1016/j.desal.2012.07.022
Kim JR, Premier GC, Hawkes FR et al (2010) Modular tubular microbial fuel cells for energy recovery during sucrose wastewater treatment at low organic loading rate. Bioresour Technol 101:1190–1198. https://doi.org/10.1016/j.biortech.2009.09.023
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38(14):4040–4046. https://doi.org/10.1021/es0499344
Liu Y, Liu H, Wang C, Hou SX, Yang N (2013) Sustainable energy recovery in wastewater treatment by microbial fuel cells: stable power generation with nitrogen doped graphene cathode. Environ Sci Technol 47(23):13889–13895. https://doi.org/10.1021/es4032216
Logan BE (2008) Microbial fuel cells. John Wiley and Sons, Hoboken
Logan BE, Cheng S, Watson V, Estadt G (2007) Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ Sci Technol 41(9):3341–3346. https://doi.org/10.1021/es062644y
Lu M, Chen S, Babanova S et al (2017) Long-term performance of a 20-L continuous flow microbial fuel cell for treatment of brewery wastewater. J Power Sources 356:274–287. https://doi.org/10.1016/j.jpowsour.2017.03.132
Oh ST, Kim JR, Premier GC, Lee TH, Kim C, Sloan WT (2010) Sustainable waste water treatment: how might MFC contribute? Biotech Adv 28:871–881. https://doi.org/10.1016/j.biotechadv.2010.07.008
Park Y, Nguyen VK, Park S, Yu J, Lee T (2018) Effects of anode spacing and flow rate on energy recovery of flat-panel air-cathode microbial fuel cells using domestic wastewater. Bioresour Technol 258:57–63. https://doi.org/10.1016/j.biortech.2018.02.097
Rabaey K, Clauwaert P, Aelterman P, Verstraete W (2005) Tubular microbial fuel cells for efficient electricity generation. Environ Sci Technol 39:8077–8082. https://doi.org/10.1021/es050986i
Ren S, Xia X, Yuan L, Liang P, Huang X (2013) Enhancing charge harvest from microbial fuel cells by controlling the charging and discharging frequency of capacitors. Bioresour Technol 146:812–815. https://doi.org/10.1016/j.biortech.2013.08.055
Rhoads A, Beyenal H, Lewandowski Z (2005) Microbial fuel cell using anaerobic respiration as an anodic reaction and biomineralized manganese as a cathodic reactant. Environ Sci Technol 39:4666–4671. https://doi.org/10.1021/es048386r
Sonawane JM, Gupta A, Ghosh PC (2013) Multi-electrode microbial fuel cell (MEMFC): a close analysis towards large scale system architecture. Int J Hydrog Energy 38:5106–5114. https://doi.org/10.1016/j.ijhydene.2013.02.030
Waller MG, Trabold TA (2013) Review of microbial fuel cells for wastewater treatment: large-scale applications, future needs and current research gaps. ASME. International Conference on Fuel Cell Science, Engineering and Technology, ASME 2013 11th International Conference on Fuel Cell Science, Eng. and Technol: V001T01A011. doi:10.1115/FuelCell2013-18185.
Wei J, Liang P, Huang X (2011) Recent progress in electrodes for microbial fuel cells. Bioresour Technol 102(20):9335–9344. https://doi.org/10.1016/j.biortech.2011.07.019
Wu C, Liu XW, Li WW, Sheng GP, Zang GL, Cheng YY (2012) A white-rot fungus is used as a biocathode to improve electricity production of a microbial fuel cell. Appl Energy 98:594–596. https://doi.org/10.1016/j.apenergy.2012.02.058
Wu S, Li H, Zhou X et al (2016) A novel pilot-scale stacked microbial fuel cell for efficient electricity generation and wastewater treatment. Water Res 98:396–403. https://doi.org/10.1016/j.watres.2016.04.043
Yazdi H, Gaviria LA, Ren ZJ (2015) Pluggable microbial fuel cell stacks for septic wastewater treatment and electricity production. Bioresour Technol 180:258–263. https://doi.org/10.1016/j.biortech.2014.12.100
Zhang F, Jacobson KS, Torres P et al (2010) Effects of anolyte recirculation rates and catholytes on electricity generation in a litre-scale upflow microbial fuel cell. Energy Environ Sci 3:1347. https://doi.org/10.1039/c001201g
Zhang F, Ge Z, Grimaud J, Hurst J, He Z (2013a) Long-term performance of liter-scale microbial fuel cells treating primary effluent installed in a municipal wastewater treatment facility. Environ Sci Technol 47(9):4941–4948. https://doi.org/10.1021/es400631r
Zhang F, Ge Z, Grimaud J et al (2013b) In situ investigation of tubular microbial fuel cells deployed in an aeration tank at a municipal wastewater treatment plant. Bioresour Technol 136:316–321. https://doi.org/10.1016/j.biortech.2013.02.107
Zhuang L, Yuan Y, Wang Y, Zhou S (2012a) Long-term evaluation of a 10-liter serpentine-type microbial fuel cell stack treating brewery wastewater. Bioresour Technol 123:406–412. https://doi.org/10.1016/j.biortech.2012.07.038
Zhuang L, Zheng Y, Zhou S et al (2012b) Scalable microbial fuel cell (MFC) stack for continuous real wastewater treatment. Bioresour Technol 106:82–88. https://doi.org/10.1016/j.biortech.2011.11.019
Zuo K, Cai J, Liang S et al (2014) A ten liter stacked microbial desalination cell packed with mixed ion-exchange resins for secondary effluent desalination. Environ Sci Technol 48:9917–9924. https://doi.org/10.1021/es502075r
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Geetanjali, Agrahari, R., Kumar, S., Rani, R. (2020). Microbial Fuel Cell-Based Process for Wastewater Treatment and Power Generation. In: Gothandam, K., Ranjan, S., Dasgupta, N., Lichtfouse, E. (eds) Environmental Biotechnology Vol. 1. Environmental Chemistry for a Sustainable World, vol 44. Springer, Cham. https://doi.org/10.1007/978-3-030-38192-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-38192-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-38191-2
Online ISBN: 978-3-030-38192-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)