Abstract
Bioactive polysaccharides extracted from Ganoderma lucidum (G. lucidum) have been widely applied in food and medicine for their multiple functions. In this study, G. lucidum exopolysaccharide (EPS) production in submerged fermentation was stimulated by Tween 80. The addition of 0.25% Tween 80 on day 3 gave a maximum production of mycelial biomass and EPS, with an increase of 19.76 and 137.50%, respectively. Analysis of fermentation kinetics showed that glucose was consumed faster after adding Tween 80, while the expression of EPS biosynthesis-related genes and ATP generation were greatly improved. Moreover, Tween 80 resulted in the significant accumulation of reactive oxygen species and increased cell membrane and cell wall permeability. The EPS from Tween 80-containing medium had higher contents of carbohydrate and uronic acid, lower molecular weight, and higher antioxidant activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals than those of EPS produced in the absence of Tween 80. This study provides further evidence to clarify the stimulatory effects of Tween 80 in fermentation and provides a guide for the production of bioactive G. lucidum EPS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ganoderma lucidum (G. lucidum), commonly known as Lingzhi in China, is a traditional medicinal mushroom that has been widely used for treating and preventing various diseases in Asia [1]. Polysaccharides, the major bioactive components of G. lucidum, have been applied in the food and pharmaceutical industries because of their antioxidant, anti-inflammatory, antimicrobial and antitumor capabilities [2]. Polysaccharides can be isolated from fruiting bodies, cultured mycelium and cultured broth [3]. Normally, it takes months to harvest fruiting bodies by solid cultures using substrates such as wood, grain or sawdust [4]. The quality of the fruiting body is also seriously affected by cultivation management [5]. In contrast, submerged fermentation of mycelium is an effective alternative approach that can obtain more bioactive components than the harvesting of fruiting bodies with a shorter culture period, a consistent product and seasonal independence [6, 7].
Many efforts have been made to improve polysaccharide production by submerged fermentation to satisfy growing consumer demand. Controlling the pH, temperature and dissolved oxygen content or the optimization of the medium composition have been reported [4]. Recently, the addition of chemical agents has become a new strategy to improve fermentation performance. Surfactants, vegetable oil and fatty acids are low-cost materials and can be removed from fermentation mixtures by simple evaporation. Many of these materials have been used as effective stimulants to enhance the production efficiency of useful metabolites (e.g., polysaccharides [8], pigments [9], and enzymes [10]) in microorganisms.
Tween 80, namely polyoxyethylene glycol sorbitan monooleate, is one of the most important nonionic surfactants that can decrease surface and interfacial tensions as well as increase solubility and bioavailability [11]. Although Tween 80 has been widely applied to increase the production of xanthan, pullulan and curdlan [12,13,14], its usage to increase exopolysaccharide (EPS) production of mushrooms in submerged fermentation is not common. In particular, the effect of Tween 80 on EPS production in G. lucidum has not yet been studied. The mechanism underlying the increased EPS production in the presence of Tween 80 was related to the fatty acid composition of the cell membrane and the activity of polysaccharide biosynthesis enzymes [14, 15]. More information is still needed to obtain an in-depth understanding.
In this study, Tween 80 was added to the liquid medium of G. lucidum. The Tween 80 concentration and addition time were optimized to improve EPS yield. The mechanism by which Tween 80 enhanced G. lucidum EPS production was investigated. Finally, the antioxidant activity and the structural characteristics of the EPS were also evaluated primarily.
Materials and methods
Strains and culture conditions
G. lucidum YW01 was obtained from the Guangdong Institute of Microbiology (Guangzhou, China) and cultivated on potato dextrose agar (PDA; Huankai Microbial, China) plates at 28 ℃. To prepare the seed culture, fresh mycelia from PDA plates were transferred to 250 mL conical flasks containing 100 mL of modified Martin broth (20 g/L glucose, 5 g/L tryptone, 2 g/L yeast extract, 0.5 g/L MgSO4 and 1 g/L KH2PO4), and cultured at 28 °C and 150 rpm in a shaker for 7 days. Then, the seed culture was inoculated at 10% (v/v) into 100 mL of modified Martin broth and cultured at 28 °C and 150 rpm. Tween 80 (Macklin, China) at concentrations of 0.06, 0.12, 0.25, 0.5, 0.75, 1 and 1.25% (w/v) was added to the medium on day 0 of the seven-day fermentation. Subsequently, the optimal concentration of Tween 80 was added to the medium on days 0, 1, 3, or 5 of the seven-day fermentation to optimize the addition time.
Determination of biomass, polysaccharide production and residual sugar
Mycelia were separated from the fermentation broth by vacuum filtration. The mycelia were washed with distilled water and dried at 50 ℃ to a constant weight for biomass determination. The dry mycelia were then extracted with boiling water for 2 h. The extract and mycelium-free fermentation broth were added with four volumes of absolute ethanol, respectively, and allowed to stand overnight at 4 ℃. The precipitated intracellular polysaccharide (IPS) from mycelia and EPS from the fermentation broth were collected by centrifugation. The contents were assayed by the phenol sulfuric acid method [16]. The amount of residual sugar in the fermentation broth was measured by the 3,5-dinitrosalicylic acid method [17].
Compositional analysis of EPS
The carbohydrate content of the EPS was measured by the phenol sulfuric acid method. The protein content was measured by Bradford’s method [18]. The uronic acid content was estimated by the meta-hydroxydiphenyl method [19]. The residual Tween 80 content was determined by the cobalt thiocyanate ammonium color-developing method [20].
The molecular weight (Mw) of the EPS was determined using a high-performance gel permeation chromatography system (Shimadzu, Japan) equipped with a TSK-G5000PWXL column (7.5 × 300 mm) and a refractive index detector. The mobile phase was ultrapure water with a flow rate of 1 mL/min, and the column temperature was maintained at 30 ℃. The Mw of the EPS was calculated using the calibration curve of dextran standards.
Real-time quantitative PCR
Fresh mycelia were collected from the fermentation broth and ground into a powder with liquid nitrogen. Total RNA was extracted from 100 mg of mycelium powder using a Plant RNA Extraction Kit (Tiangen Biotech, China) according to the manufacturer's instructions. The PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Japan) was used for cDNA synthesis, and TB Green Premix Ex Taq (Takara) was used for real-time quantitative PCR. PCR was performed on an Applied Biosystems QuantStudio 6 (Thermo Scientific, USA). The 18S rRNA gene was used as an internal gene for normalization, and the relative gene expression was analyzed according to the \({2}^{{ - \Delta \Delta C_{{\text{T}}} }}\) method [21]. The primers used are shown in Supplemental Table S1.
ATP detection assay
Fresh mycelia were ground into a powder with liquid nitrogen. Then, 20 mg of mycelium powder was resuspended in the lysis reagent of an ATP Assay Kit (Beyotime, China) and centrifuged at 4 ℃ and 12,000 rpm for 5 min. The supernatant was used for ATP detection according to the protocol of the ATP Assay Kit, which is based on the luciferase-luciferin reaction.
Assessment of cell membrane permeability
Fresh 5-day-fermented mycelia (300 mg) were treated with 1 mL of 1.5% (w/v) lywallzyme (Guangdong Institute of Microbiology) containing 0.6 M mannitol at 30 ℃ for 2 h. The cells were washed twice and stained with propidium iodide (PI; Sigma, USA) for 30 min at room temperature in the dark. The fluorescence intensity was measured using a flow cytometer (BD Bioscience, USA).
Quantification of β-1,3-glucan and chitin
Five-day-fermented mycelia were ground into a fine powder with liquid nitrogen. The aniline blue assay was used to determine the content of β-1,3-glucan [22]. Briefly, 20 mg mycelium powder was extracted in 1 mL of 1 M NaOH at 52 ℃ for 30 min. Then, 50 μL of the extract was mixed with 185 μL of aniline blue solution (0.067% aniline blue, 0.35 N HCl and 0.98 M glycine–NaOH; pH 9.5). The mixture was incubated at 52 ℃ for 30 min and then cooled to room temperature. The absorbance was measured using a Cytation 5 Cell Imaging Multi-Mode Reader (BioTek, USA) with excitation and emission wavelengths of 405 nm and 460 nm, respectively.
A chitin detection assay was performed as follows [22]: first, 5 mg of mycelium powder was treated with 3 mL of saturated KOH at 130 ℃ for 1 h. After cooling to room temperature, the sample was mixed with 8 mL of ice-cold 75% (v/v) ethanol and placed in an ice bath for 15 min. The mixture was combined with 300 μL of 13.3% (w/v) Celtite 545 and centrifuged at 4 ℃ and 5,000 rpm for 5 min. The precipitate was washed with ice-cold 40% (v/v) ethanol and ice-cold water and resuspended in 0.5 mL of distilled water. The resuspended solution, standard solution (10 μg/mL glucosamine) and blank control (distilled water) were combined with 0.5 mL of 5% (w/v) NaNO2 and 0.5 mL of 5% (w/v) KHSO4 and centrifuged at 4 ℃ at 10,000 rpm for 5 min. Then, 150 μL of supernatant was mixed with 450 μL of water and 200 μL of 12.5% (w/v) NH4 sulfamate. The mixture was combined with 0.5 mL of 5 mg/mL 3-methylbenzthiazolinone-2-hydrazone and boiled for 3 min. After cooling to room temperature, the solution was incubated with 200 μL of 0.83% (w/v) FeCl3 for 30 min. The absorbance at 650 nm was read on a visible light spectrophotometer (Thermo Scientific). The concentration of glucosamine decomposed by chitin was calculated as follows: [(A650 unknown − A650 blank) × 10 μg/mL] / (A650 standard − A650 blank).
Measurement of reactive oxygen species (ROS)
The level of intracellular ROS was detected by the 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma) assay. Five-day-fermented mycelia were stained with 10 μM DCFH-DA in the dark for 20 min and washed twice with phosphate-buffered saline (PBS; Sangon, China). The fluorescence was detected using a Cytation 5 Cell Imaging Multi-Mode Reader with excitation and emission wavelengths of 488 and 525 nm, respectively.
Antioxidant activity assay
The antioxidant activity of the EPS was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH; Sigma) radical scavenging method [23]. Briefly, 1 mL of EPS solution at a series of concentrations was mixed with 1 mL of 0.1 mM DPPH. Each mixture was incubated in the dark at room temperature for 30 min, and then their absorbances were measured at 517 nm.
The DPPH radical scavenging activity of each sample was calculated according to the following equation:
where A0 is the absorbance of the control group without EPS, Ax is the absorbance of the EPS sample, and Ai is the absorbance for the background without DPPH.
Statistical analysis
Unless otherwise stated, all data are presented as the mean ± standard deviation of three replicates. Statistical analysis was performed using the Student's t test. A p value of < 0.05 was considered statistically significant.
Results and discussion
Mycelial growth and EPS production under different Tween 80 concentrations and addition times
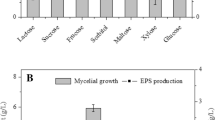
The G. lucidum mycelial biomass and EPS yield achieved by supplementing 0.06–1.25% Tween 80 on day 0 are shown in Fig. 1a. Tween 80 at concentrations of 0.06–0.25% had no significant effect on mycelial biomass accumulation, while at a concentration above 0.25%, Tween 80 inhibited mycelial growth. EPS production reached a maximum yield of 1.02 g/L when the added Tween 80 concentration increased to 0.25% but then decreased to 0.71 g/L when the Tween 80 concentration increased to 1.25%. The optimal concentration of Tween 80 for mycelial growth and EPS production of G. lucidum was found to be 0.25%. A high concentration of Tween 80 resulted in the formation of excessive foam, which caused an adverse effect not only on the sterile environment but also on mass and heat transfer during submerged fermentation [8]. This might explain the reduction in G. lucidum biomass and EPS yield when the Tween 80 concentration was higher than 0.25%. Previous studies reported that the optimal concentrations of Tween 80 used for EPS production of Aureobasidium pullulans and Agrobacterium sp. were 0.5 and 1.6%, respectively [12, 14]. This discrepancy indicated that different species responded differently to this additive.
The optimal concentration of Tween 80 (0.25%) was then added on days 0, 1, 3, and 5 within the fermentation period. These time points represented the beginning, early exponential phase, middle exponential phase and late exponential phase of fermentation, respectively, based on the strain culturing features. The results revealed that the maximal mycelial biomass (7.24 g/L) and EPS yield (1.33 g/L) were achieved by adding Tween 80 at the middle exponential phase (on day 3) and were significantly increased by 19.76 and 137.50% compared to the control, respectively (Fig. 1b). A previous study found that Tween 80 as an oxygen vector increased oxygen transfer efficiency and promoted the production of metabolites [24]. The oxygen demand was remarkably increased as cell growth and metabolic activity increased, leading to the removal of dissolved oxygen in the middle and late exponential phase [25]. The addition of Tween 80 at these phases might efficiently alleviate oxygen limitation, resulting in improved mycelial growth and EPS production. It is noteworthy that the addition of Tween 80 at the late exponential phase caused lower mycelial biomass and EPS yield than those achieved with the addition of Tween 80 at the middle exponential phase. This was possibly due to the decreased cell viability in the late exponential phase. Thus, 0.25% Tween 80 added at day 3 was selected for subsequent studies.
Effects of Tween 80 on fermentation kinetics of G. lucidum
Comparison of the fermentation performance of G. lucidum with and without 0.25% Tween 80 is shown in Fig. 2. The results revealed that the mycelial growth rate of the Tween 80 group was faster than that of the control, and the biomass increased to 7.39 g/L on day 8 versus 6.08 g/L in the control (Fig. 2a). IPS production was also significantly promoted by Tween 80 (Fig. 2b). The IPS yield of the Tween 80 group reached the maximum level of 0.51 g/L on day 8 and then decreased until day 10. The EPS yield of the Tween 80 group increased faster than that of the control and reached 1.45 g/L on day 10 (Fig. 2c). Tween 80 increased EPS production primarily by stimulating mycelial growth and IPS production. Furthermore, the glucose in the Tween 80-containing medium was consumed more rapidly than that in the absence of Tween 80 (Fig. 2d). At the end of the fermentation, the glucose content in the Tween 80-containing medium (2.76 g/L) was lower than that in the control group (4.61 g/L). These results implied that the nutrient absorption efficiency was enhanced in Tween 80-treated G. lucidum, and it subsequently increased the mycelial biomass and EPS yield.
Culture pH is one of the important environmental factors affecting the cell growth and polysaccharide production of G. lucidum [26]. Time-course changes in pH value with and without the presence of 0.25% Tween 80 are shown in Fig. S1. During the first 6 days of fermentation, the consumption of substrate and dissolved oxygen could cause a decrease in the pH value [26]. The minimum pH value of the Tween 80 group was 3.61 on day 6 compared to 3.70 in the control, which was in accordance with the higher glucose consumption rate of the Tween 80 group. Thereafter, the pH of the control group marginally increased to 3.83, while that in the Tween 80 group remained relatively constant between 3.61 and 3.67. It was reported that the intracellular components would be released due to the lysis of cells at the end of fermentation, thereby increasing the pH value of the culture broth [27]. The lower final pH value of the Tween 80-containing medium implied that Tween 80 could prevent disintegration due to the shearing forces, improving EPS yield [15].
Expression of EPS biosynthesis-related genes with Tween 80 addition
The polysaccharide biosynthetic pathway of G. lucidum has been partially elucidated. Phosphoglucomutase (PGM) and uridine diphosphate glucose pyrophosphorylase (UGP) are key enzymes for the production of precursors of polysaccharides [28]. The expression levels of the genes pgm and ugp in Tween 80-treated G. lucidum are presented in Fig. 3. The results showed that the expression levels of pgm and ugp were greatly stimulated by the presence of Tween 80. The maximum transcription levels of these two genes were achieved on day 8, and they were 3.14- and 2.98-fold greater than those observed in the control, respectively. These findings corresponded to the EPS production pattern under Tween 80 treatment (Fig. 2c). Tween 80 also induced transcription of the related polysaccharide biosynthesis genes in Aureobasidium pullulans and Agrobacterium sp. for pullulan and curdlan production, respectively [12, 24]. Therefore, it can be proposed that Tween 80 regulated EPS biosynthesis by affecting the expression level of related genes in G. lucidum.
Intracellular ATP levels under Tween 80 conditions
Since ATP directly provides energy for various cellular processes, such as precursor generation and EPS secretion in the biosynthesis of EPS [28], the ATP levels were also measured during fermentation. A large amount of energy was needed to support the rapid growth of mycelia and EPS synthesis during the first 6 days of fermentation, which led to a fast increase in ATP generation (Fig. 4). Thereafter, ATP generation gradually decreased as cell viability decreased. It is noteworthy that the ATP levels of the Tween 80 group were greater than those of the control during the whole fermentation process. A previous study also showed that Tween 80-treated Agrobacterium sp. produced more ATP and curdlan than the untreated strain [12]. ATP generation mainly depends on the oxidative phosphorylation of the respiratory chain [29]. The increase in oxygen transfer efficiency induced by Tween 80 could be conducive to oxidative phosphorylation, which provided more ATP for EPS synthesis and secretion [12, 24].
Changes in cell membrane permeability and cell wall components by Tween 80
PI is a red-fluorescent nuclear dye and is normally blocked by the cell membrane. This dye will only bind to nucleic acids of cells where the plasma membrane has been permeabilized [30]. Therefore, the effect of Tween 80 on cell membrane permeability was studied by measuring the fluorescence intensity of PI-stained cells. As seen in Fig. 5, the fluorescence intensity of Tween 80-treated G. lucidum cells was 1.79-fold higher than that of control cells, indicating that Tween 80 significantly increased the cell membrane permeability. The increased cell membrane permeability might be attributed to changes in the composition and contents of unsaturated fatty acids in the cell membrane, which could facilitate the uptake of nutrients as well as secretion of macromolecule EPS [14, 15].
The contents of β-1,3-glucan and chitin in the cell wall of G. lucidum were also measured. As shown in Fig. 6a, the contents of β-1,3-glucan and chitin of the cell wall in the Tween 80 treatment group were significantly decreased by 17.66 and 23.19% compared with those in the control, respectively. Moreover, the transcription levels of putative glucan synthase genes (GL20535, GL24465 and GL24554) and chitin synthase genes (GL15273, GL18134, GL25613, GL27969, GL28060, GL30737, GL30799 and GL31550) [31] were determined. The transcription levels of these genes were significantly downregulated in Tween 80-treated G. lucidum (Fig. 6b, c). As the main components of the fungal cell wall, β-1,3-glucan and chitin play an important role in cell wall structure [32]. The decrease of β-1,3-glucan and chitin had a positive impact on cell wall permeability, which was beneficial for EPS secretion [22].
The cell wall components were altered in Tween 80-treated G. lucidum. a The contents of β-1,3-glucan and chitin. b The expression levels of putative glucan synthase genes. c The expression levels of putative chitin synthase genes. Values are the mean ± standard deviation of three replicates. **P < 0.01
Previous studies confirmed that ROS accumulation resulted in the increased cell membrane and cell wall permeability [33, 34]. The increased ROS level in Tween 80-treated G. lucidum (Fig. S2) here might have also contributed to the elevation of the cell membrane and cell wall permeability. The new evidence we provided is the first to show that Tween 80 modulates fungal cell membrane and cell wall permeability. A high concentration of Tween 80 inhibited mycelial growth and EPS production (Fig. 1a) because it severely affected the cell membrane and cell wall integrity, which might be harmful to the cells. The addition of Tween 80 at the early exponential phase might cause irreversible injury to the immature cell membrane and cell wall, leading to lower biomass and EPS yield outcomes (Fig. 1b).
Effects of Tween 80 on antioxidant activity and structural composition of EPS
DPPH is a stable radical used to assess the free radical scavenging ability of antioxidants in vitro. Multiple hydroxyls in polysaccharides contribute to their antioxidant activity, as they act as donors of hydrogen atoms or electrons in the transformation of DPPH radicals into their reduced form [35]. EPS of the control group (EPS-control) and Tween 80 group (EPS-Tween 80) scavenged DPPH radicals in a dose-dependent manner (Fig. 7). The DPPH radical scavenging activity of EPS-Tween 80 was significantly higher than that of EPS-control, and a maximum of 56.13% at 2 mg/mL was observed. The enhanced production of EPS-Tween 80 with higher antioxidant activity might respond to the ROS accumulation stimulated by Tween 80 (Fig. S2). When ROS accumulation exceeded a certain extent, it could induce oxidative stress and cell damage [34]. In this case, the fungus produced more EPS with higher antioxidant activity to protect cells under stress by maintaining cell morphology and eliminating ROS [34, 36, 37].
The bioactivity of polysaccharides is strongly affected by their structural characteristics, and the chemical composition and molecular weight of EPS were investigated. As shown in Table 1, there was no Tween 80 left in EPS-Tween 80 and it could be confirmed that there was no disturbance in the antioxidant activity assay. The carbohydrate and uronic acid contents of EPS-Tween 80 were significantly higher than those of EPS-control. Moreover, there was no significant difference in protein content between the two groups (Table 1). It was found that the polysaccharides in some plants and fungi with relatively high carbohydrate and uronic acid contents exhibited strong antioxidant activity due to the enhanced electron- or hydrogen atom-donating capacity [18, 38, 39]. We assumed that the higher antioxidant activity of EPS-Tween 80 than that of EPS-control was related to its higher carbohydrate and uronic acid contents. Furthermore, polysaccharides with relatively low Mw seemed to have more reductive hydroxyl groups for the elimination of free radicals than those with high Mw [38]. Thus, the lower Mw of EPS-Tween 80 than that of EPS-control might contribute to a higher antioxidant activity (Table 1). Overall, Tween 80 improved the antioxidant activity of EPS by altering its chemical composition and molecular weight. It should be noted that the antioxidant activity of EPS-Tween 80 might be affected by other structural characteristics, such as monosaccharide composition and the glycosidic bond content, which need to be further investigated.
Conclusions
In summary, the addition of Tween 80 at the middle exponential phase of G. lucidum under submerged fermentation could significantly increase mycelial growth and EPS production. ROS accumulation stimulated by Tween 80 might result in elevation of the cell membrane and cell wall permeability, thereby facilitating nutrient absorption and EPS biosynthesis. The expression of EPS biosynthesis-related genes and ATP generation were also stimulated by Tween 80. Moreover, Tween 80 increased the antioxidant activity of EPS by altering its structural composition. Due to the low cost of Tween 80, this is an economically feasible application to enhance bioactive G. lucidum EPS production.
References
Lin Z (2019) Ganoderma (Lingzhi) in traditional Chinese medicine and Chinese culture. Adv Exp Med Biol 1181:1–13
Cor D, Knez Z, Knez Hrncic M (2018) Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: a review. Molecules 23(3):649
Wasser SP (2011) Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl Microbiol Biotechnol 89(5):1323–1332
Yang H, Min W, Bi P, Zhou H, Huang F (2013) Stimulatory effects of Coix lacryma-jobi oil on the mycelial growth and metabolites biosynthesis by the submerged culture of Ganoderma lucidum. Biochem Eng J 76:77–82
Hu G, Zhai M, Niu R, Xu X, Liu Q, Jia J (2018) Optimization of culture condition for ganoderic acid production in Ganoderma lucidum liquid static culture and design of a suitable bioreactor. Molecules 23(10):2563
Tao TL, Cui FJ, Chen XX, Sun WJ, Huang DM, Zhang J, Yang Y, Wu D, Liu WM (2018) Improved mycelia and polysaccharide production of Grifola frondosa by controlling morphology with microparticle Talc. Microb Cell Fact 17(1):1
Yang FC, Liau CB (1998) Effects of cultivating conditions on the mycelial growth of Ganoderma lucidum in submerged flask cultures. Bioprocess Eng 19(3):233–236
Zhang BB, Cheung PC (2011) Use of stimulatory agents to enhance the production of bioactive exopolysaccharide from Pleurotus tuber-regium by submerged fermentation. J Agric Food Chem 59(4):1210–1216
Yang X, Dong Y, Liu G, Zhang C, Cao Y, Wang C (2019) Effects of nonionic surfactants on pigment excretion and cell morphology in extractive fermentation of Monascus sp. NJ1. J Sci Food Agric 99(3):1233–1239
Li PJ, Xia JL, Shan Y, Nie ZY, Wang FR (2015) Effects of surfactants and microwave-assisted pretreatment of orange peel on extracellular enzymes production by Aspergillus japonicus PJ01. Appl Biochem Biotechnol 176(3):758–771
Singh A, Van Hamme JD, Ward OP (2007) Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnol Adv 25(1):99–121
Liang Y, Zhu L, Gao M, Zheng Z, Wu J, Zhan X (2018) Influence of Tween-80 on the production and structure of water-insoluble curdlan from Agrobacterium sp. Int J Biol Macromol 106:611–619
Ghashghaei T, Soudi MR, Hoseinkhani S, Shiri M (2018) Effects of nonionic surfactants on xanthan gum production: a survey on cellular interactions. Iran J Biotechnol 16(1):e1483
Sheng L, Zhu G, Tong Q (2013) Mechanism study of Tween 80 enhancing the pullulan production by Aureobasidium pullulans. Carbohydr Polym 97(1):121–123
Zhang BB, Cheung PC (2011) A mechanistic study of the enhancing effect of Tween 80 on the mycelial growth and exopolysaccharide production by Pleurotus tuber-regium. Bioresour Technol 102(17):8323–8326
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Miller LG (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Biochem 31(3):426–428
Xu X, Wu P, Wang T, Yan L, Lin M, Chen C (2019) Synergistic effects of surfactant-assisted biodegradation of wheat straw and production of polysaccharides by Inonotus obliquus under submerged fermentation. Bioresour Technol 278:43–50
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54(2):484–489
Wei-Juan YAN, Jian-Nong W, Li-Jun S (2009) Qualitative and quantitative determination of tween 80 in 42 traditional Chinese medicine injections. J Fourth Mil Med Univ 30(21):2366–2369
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3(6):1101–1108
Ma Z, Xu M, Wang Q, Wang F, Zheng H, Gu Z, Li Y, Shi G, Ding Z (2019) Development of an efficient strategy to improve extracellular polysaccharide production of Ganoderma lucidum using l-phenylalanine as an enhancer. Front Microbiol 10:2306
Zhu S, Du C, Yu T, Cong X, Liu Y, Chen S, Li Y (2019) Antioxidant activity of selenium-enriched peptides from the protein hydrolysate of Cardamine violifolia. J Food Sci 84(12):3504–3511
Tu G, Wang Y, Ji Y, Zou X (2015) The effect of Tween 80 on the polymalic acid and pullulan production by Aureobasidium pullulans CCTCC M2012223. World J Microbiol Biotechnol 31(1):219–226
Dou Y, Xiao J-H, Xia X-X, Zhong J-J (2013) Effect of oxygen supply on biomass and helvolic acid production in submerged fermentation of Cordyceps taii. Biochem Eng J 81:73–79
Hyun Mi K, Moon Ki P, Jong Won Y (2006) Culture pH affects exopolysaccharide production in submerged mycelial culture of Ganoderma lucidum. Appl Biochem Biotechnol 134(3):249–262
Li Q, Lei Y, Hu G, Lei Y, Dan D (2018) Effects of Tween 80 on the liquid fermentation of Lentinus edodes. Food Sci Biotechnol 27(4):1103–1109
Ma Z, Ye C, Deng W, Xu M, Wang Q, Liu G, Wang F, Liu L, Xu Z, Shi G, Ding Z (2018) Reconstruction and analysis of a genome-scale metabolic model of Ganoderma lucidum for improved extracellular polysaccharide production. Front Microbiol 9:3076
Neupane P, Bhuju S, Thapa N, Bhattarai HK (2019) ATP synthase: structure, function and inhibition. Biomol Concepts 10(1):1–10
Zhang N, Fan Y, Li C, Wang Q, Leksawasdi N, Li F, Wang S (2018) Cell permeability and nuclear DNA staining by propidium iodide in basidiomycetous yeasts. Appl Microbiol Biotechnol 102(9):4183–4191
Chen S, Xu J, Liu C, Zhu Y, Nelson DR, Zhou S, Li C, Wang L, Guo X, Sun Y, Luo H, Li Y, Song J, Henrissat B, Levasseur A, Qian J, Li J, Luo X, Shi L, He L, Xiang L, Xu X, Niu Y, Li Q, Han MV, Yan H, Zhang J, Chen H, Lv A, Wang Z, Liu M, Schwartz DC, Sun C (2012) Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat Commun 3:913
Bowman SM, Free SJ (2006) The structure and synthesis of the fungal cell wall. BioEssays 28(8):799–808
Liang D (2018) A salutary role of reactive oxygen species in intercellular tunnel-mediated communication. Front Cell Dev Biol 6:2
Wang L, Sha Y, Wu D, Wei Q, Chen D, Yang S, Jia F, Yuan Q, Han X, Wang J (2020) Surfactant induces ROS-mediated cell membrane permeabilization for the enhancement of mannatide production. Process Biochem 91:172–180
Zhao Y, Hu W, Zhang H, Ding C, Huang Y, Liao J, Zhang Z, Yuan S, Chen Y, Yuan M (2019) Antioxidant and immunomodulatory activities of polysaccharides from the rhizome of Dryopteris crassirhizoma Nakai. Int J Biol Macromol 130:238–244
Liao B, Huang H (2019) Structural characterization of a novel polysaccharide from Hericium erinaceus and its protective effects against H2O2-induced injury in human gastric epithelium cells. J Funct Foods 56:265–275
Liu S, Li B, Chen X, Qin Y, Li P (2019) Effect of polysaccharide from Enteromorpha prolifera on maize seedlings under NaCl stress. J Oceanol Limnol 37(4):1372–1381
Liang X, Gao Y, Fei W, Zou Y, He M, Yin L, Yuan Z, Yin Z, Zhang W (2018) Chemical characterization and antioxidant activities of polysaccharides isolated from the stems of Parthenocissus tricuspidata. Int J Biol Macromol 119:70–78
Wang C, Yu Y-B, Chen T-T, Wang Z-W, Yan J-K (2020) Innovative preparation, physicochemical characteristics and functional properties of bioactive polysaccharides from fresh okra (Abelmoschus esculentus (L.) Moench). Food Chem 320:126647
Acknowledgements
This work was supported by the Key-Area Research and Development Program of Guangdong Province (No. 2018B020205001), the Guangdong Province Innovation Team Construction Program on Modern Agriculture Industrial Technology System (The Edible Fungus) (No. 2019KJ103), the Guangdong Province Science and Technology Project (No. 2019A050520003), and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA01020304).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, X., Yang, Y., Zhang, Y. et al. Enhanced exopolysaccharide production in submerged fermentation of Ganoderma lucidum by Tween 80 supplementation. Bioprocess Biosyst Eng 44, 47–56 (2021). https://doi.org/10.1007/s00449-020-02418-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02418-1