Abstract
Agricultural residues could become feedstocks for biobased products as they are renewable, carbon neutral, and do not compete with food. In India, > 130 MT rice straw is available annually for alternate uses. Exploiting this abundant biomass for biochemical production will pave way for bio-based economy. Rice straw is highly recalcitrant due to lignin-carbohydrate complex and high ash. For production of value added products, the cellulose fraction is very important and also lignin can be used. However, for overall economic efficiency, it is imperative to separate and recover these fractions maximally from biomass and convert them into high value products at high titers and efficiency. Biomass has to be deconstructed to access these fractions. An improvised pretreatment with sodium hydroxide (NaOH) coupled with acidified water wash enabled high retrieval of cellulose and lignin. More than 80% of cellulose present in raw rice straw was recovered in pretreated solids and lignin (> 65%) recovered from acidification of alkali prehydrolysates/wash waters. Enzymatic hydrolysis of solids with commercial cellulases resulted in 80–100% glucan conversion at 6% and 3% loading respectively yielding ~ 5.5% and 3.3% sugar syrups which can be fermented to value added chemicals. Saccharomyces cerevisiae LN fermented hydrolysates with 77–97% efficiency producing 0.508 gg−1 and 0.403 gg−1ethanol within 24 h consuming all glucose while xylose was unutilized. Material calculations showed that this process converted 63% of cellulose present in rice straw to ethanol potentially yielding 135 L ethanol and ~ 100 Kg lignin per ton of rice straw with limited water use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Use of biomass-derived energy/biochemicals can mitigate climate change by lowering CO2 emission, reduce petroleum dependence, and offer socio-economic benefits. Rice straw is a major agricultural waste biomass generated in South and Southeast Asia where rice is a staple food and is widely grown. In 2013–2014, rice production was around 741 million tons worldwide with China and India being the largest producers. Based on the rice grain-to-straw ratio of 1–1.5, huge amount of rice straw is produced [1, 2].
The rice straw is commonly burnt in-situ in the field to facilitate quick planting of the next crop, resulting in the low air quality and waste of a valuable resource which could be used for energy or manure/fertilizer [3]. The development of alternate strategies for utilization of straw would therefore provide benefits to the growth of rural economy, employment generation, and reduction of local air pollution from burning crop residues.
It has been shown that rice straw can be effectively utilized for bioenergy production in the form of solid biofuels as pellets or briquettes, biogas through anaerobic digestion, and bioethanol production which can yield energy equivalent of 8.0 GJ/ton and 5.6 GJ/ton, respectively. The life cycle analyses (LCA) further revealed that such application of rice straw as biomethanation of paddy straw reduces net global warming potential by 2750 CO2 kg emissions/ton, while bioethanol production showed net global warming potential reduction of 2549 CO2 kg emissions/ton. The pelletization of paddy straw for improved cookstove showed net global warming potential reduction of 2459 CO2 kg emissions/ton [4]. The results of LCA showed that per ton of dry rice straw, the bioethanol pathway resulted in the highest environmental sustainability with regard to reductions in global warming and resource depletion potentials [2]. Thus, it makes a lot of sense to further study these approaches for alternate uses of rice straw and improve the process efficiencies to facilitate their deployment on commercial scale.
The most desirable traits of a successful, sustainable cellulosic bioenergy enterprise are greenhouse gas (GHG) mitigation, integration with agriculture system, and techno-economic feasibility [5]. Therefore, bioethanol production from rice straw is an attractive option as it helps in lowering GHG emissions, is available in plenty as agri-residue, and also provides opportunity for saving on use of petroleum as it can be easily blended with petrol and used for transport fuel.
Rice straw, a lignocellulosic biomass, is composed of cellulose, hemicellulose, lignin, and ash. Theoretically, like any other lignocellulosic biomass, it could be used as a feedstock material in bioethanol production. In lignocellulosic biomass, cellulose and lignin fractions are most important for production of bioenergy, biofuels, and biochemicals. However, its application potential for bioethanol production depends on its cellulose content, physical structure (e.g. crystallinity), chemical composition, and availability. Percent content of cellulose in biomass and its structural crystallinity are the most important factors for bioethanol production from plant biomass [6]. To access this cellulose for production of value added products, the lignocellulosic biomass has to be first pretreated and then enzymatically hydrolyzed to yield fermentable sugars. A number of physical/chemical/biological pretreatments and their combinations have been applied till date [7, 8]. Each processing step has a profound effect on structure of biomass components, release of fermentable and other sugars, lignin, and degradation products [9]. However, pretreatment of choice should be the one with less severity, less use of harsh chemicals/conditions, and maximum preservation of cellulose as it is the primary substrate that is converted to bioethanol [10].
Therefore, tracking the flow of cellulose and its maximum recovery in the process of ethanol production and applying these calculations for the evaluation and optimization of lignocellulosic biomass conversion processes providing information on overall material recovery, recovery of individual sugars, and solubilization of biomass components is fundamentally important. Based on material balance calculations, overall ethanol yields for biochemical conversion of biomass could be calculated [11]. The evaluation of biomass conversion processes through material balance templates is crucial for its commercialization as it gives an idea about the efficiency of the total process, brings about comparison between different available processes, and thereby guides in policy making [5, 9].
In this context, material flow of cellulose in rice straw treated with improvised NaOH pretreatment method leading to high cellulose conversion to ethanol was traced, and lignin was recovered. The material flow calculations were performed to depict cellulose recovery and ethanol productivity for economic viability.
2 Materials and methods
2.1 Biomass feedstock
The biomass substrate, rice straw var. Pusa Basmati 1121, was obtained from farms of the Indian Agricultural Research Institute, New Delhi. The straw was air-dried, chopped and milled using mini Mill (Zexter, India), and sieved through 40-μm mesh. The sieved samples were stored at room temperature until used further.
2.2 Microorganism
The fermentation of hydrolysate was carried out using yeast Saccharomyces cerevisiae LN ITCC 8246, obtained from Division of Microbiology, IARI, New Delhi. The culture was maintained and grown on MGYP (Malt extract, 3 g L−1; glucose, 10 g L−1; yeast extract, 3 g L−1; peptone, 5 g L−1) at 30 °C for 72 h. Culture was stored at 4 °C on MGYP slants and periodically subcultured.
2.3 Enzymes
Commercially available cellulases, Accellerase®1500 (Genencor) and Cellulase from Trichoderma reesei ATCC 26921* (Sigma-C2730), were used for saccharification experiments. The total reducing sugar was estimated by dinitrosalicylic acid (DNSA) reagent method [12].
2.4 Pretreatment of rice straw with NaOH
The alkali pretreatment of raw rice straw was carried out using 1% NaOH (w/v). Initially, 10 g dried and sieved biomass having moisture content 5% was taken in 5 different Erlenmeyer flasks of 500-mL volume. About 100 mL of 1% NaOH was added in each flask (1:10 loading), mixed well, and autoclaved at 121 °C and 15 psi for 30 min. After autoclaving, 100-mL distilled water was added in each flask and mixed well. The contents of all 5 flasks were pooled and filtered to separate the liquid fraction (liquor) from the solid biomass. The volume of collected liquor was measured and stored at 4 °C until further use for sugar estimation and lignin recovery by acid precipitation.
The solid fraction of treated biomass was washed twice with 500-mL distilled water to remove the residual NaOH from treated biomass and finally washed with 500-mL acidified water to neutralize the pH and reduce water consumption in neutralizing the pH [13]. After washing and filtration, wet pretreated biomass was collected. The composition of the pretreated biomass was analyzed for moisture content using RADWAG MA 50.R (Bracka, Poland) moisture analyzer and cellulose and lignin content. The biomass was stored at 4 °C in a zip lock bag until used further for enzymatic saccharification to prevent the moisture loss. Volume of all the washing fractions was also measured, and lignin content was estimated by reading absorbance at 205 nm, and sugar content was estimated by DNSA method [12]. The fractions were pooled and used for recovering the acid precipitable lignin by acidification using H2SO4.
2.5 Lignin recovery from wash waters
Acid insoluble lignin (AIL) was recovered from all the washing fractions by acidification, and the pH of washing fractions was measured. Initial 3 washing/liquid fractions were strongly alkaline, so their pH was reduced to 1–2 using concentrated H2SO4 and left overnight for the precipitation of AIL present in washing/liquid fractions. The AIL precipitates were recovered by centrifugation at 10,000 rpm for 15 min. The AIL pellet was washed twice using 50-mL distilled water. The washed pellet was oven dried at 60 °C and weighed.
2.6 Compositional analysis of treated and untreated rice straw
The cell wall components, mainly cellulose and lignin, and moisture were quantified in the treated and untreated paddy straw. The moisture content of the biomass was estimated using moisture analyzer RADWAG MA 50.R (Bracka, Poland). The Updegraff method was used for estimating cellulose content [14]. The cellulose enrichment was calculated as
The lignin was estimated following the National Renewable Energy Laboratory (NREL), Laboratory Analysis Protocol-003 standard protocol [15]. The delignification percentage was calculated with respect to total lignin present in raw rice straw.
2.7 Enzymatic saccharification of pretreated rice straw
Rice straw hydrolysates were prepared by subjecting pretreated solids to enzymatic hydrolysis with Accellerase®1500 and Cellulase (Sigma) with slight modifications as done in [3]. The alkali pretreated rice straw biomass having cellulose 52.75% ± 0.78 and 70% moisture was used for enzymatic hydrolysis. Saccharification was carried out using 1.923 g and 3.846 g (equivalent to 3.0 and 6.0% glucan loading respectively) mixed with 150 μL and 300 μL enzyme respectively in the 0.05 M sodium citrate buffer pH 4.8. Total volume of reaction mixture was 10 mL in 30-mL bottles. The reaction mixture was incubated at 50 °C and 150 rpm in shaker water bath for 96 h. The 0.5-mL samples were withdrawn at 24 h intervals; reaction stopped by heating the mixture at boiling temperature for 5 min. The liquid fraction was collected and the total reducing sugars were estimated by DNS assay [12] and glucose and xylose by HPLC. The saccharification efficiency was calculated as described by Saritha et al. [16]:
Liquid fraction was collected as sugar rich hydrolysate and its volume was measured. It was used for fermentation by Saccharomyces cerevisiae LN ITCC 8246 for ethanol production.
2.8 Fermentation of Hydrolysate using Saccharomyces cerevisiae LN ITCC 8246
The sugar rich hydrolysates (10 mL) in 15-mL screw capped flat bottom vials, supplemented with mineral salts and 0.1% YE [17], were used to check the growth, sugar utilization, and ethanol production by S. cerevisiae LN. Controls consisted of 3.5 and 5.0% glucose in minimal medium. S. cerevisiae LN, grown overnight in MGYP broth, with optical density of 0.8 at 660 nm was used as inoculum at 10% rate and incubated at 30 °C for 120 h under static conditions. Aliquots were withdrawn at 24 h intervals, and optical density was checked at 660 nm to measure growth. Samples were then centrifuged at 5000 rpm for 5 min, and the supernatant was used for estimation of sugar consumption and ethanol production by HPLC. The fermentation efficiency was calculated as referred in Sharma et al. [18]:
2.9 Material flow calculation
Mass/material flow analysis was done after each step of experiment including alkali pretreatment, enzymatic hydrolysis, and fermentation.
A total of 50 g of raw rice straw biomass having 5% moisture, constituting total 47.50 g of dry weight, was used for alkali pretreatment. After pretreatment and washing, four different liquor fractions obtained in this process were collected, measured, and analyzed for sugar concentration and lignin content in them. The volumes of four liquor fractions having pH 10.0, 9.7, 9.4, and 1.8 were measured and total volume recovered was determined. The total reducing sugars were estimated by DNS method in washing fractions, and acid insoluble lignin (AIL) was extracted by acidification using concentrated H2SO4 and weighed after drying.
The weight of total solid pretreated biomass recovered after alkali treatment (wt. of wet biomass), moisture content, and total dry weight was measured.
2.10 Analytical methods
2.10.1 High performance liquid chromatography for sugars and ethanol quantification
The culture samples were centrifuged at 8000 rpm for 10 min and filtered through 0.2-μm Nylon-66 Syringe Filters. Samples were run on Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA) at 65 °C using 5-mM H2SO4 as a mobile phase at 0.5 mL−1 and measured with a Shodex RI-101 Refractive Index detector (Shoko Scientific Co. Ltd., Yokohama, Japan) as described by Sharma et al. [19].
2.11 Statistical analysis
All the experimental data was statistically analyzed using SPSS (Version 21.0. Armonk, NY: IBM Corp).
3 Results and discussion
The key to stopping in-situ straw burning and motivate farmers to reap environmental benefits of rice straw utilization can come only by suggesting a feasible alternative and profitable strategy. Rice straw utilization for fuels and fertilizer for enhancing environmental sustainability is recommended. The LCA studies have shown that conversion of rice straw to bioethanol is the most environmentally sustainable pathway with regard to global warming and resource depletion potentials. For successful commercial deployment of a rice straw to ethanol bioprocess, it is important to separate and maximally recover the cellulose and lignin components, maximally utilize cellulose, and convert it to desired product/ethanol with highest efficiency. In this work, material flow of cellulose present in rice straw to ethanol using an improvised cost-effective NaOH treatment was analyzed and process efficiency calculated.
3.1 Pretreatment and compositional analysis of rice straw
The cellulose and lignin content present in the untreated rice straw were 32.28 and 15.89%, respectively. In the present study, after the pretreatment of the rice straw with 1% NaOH cellulose content was enriched to 52.75% and lignin content was reduced to 9.93%, pretreatment resulted in increased cellulose content by 61.19% and lignin loss by 37.51% (Table 1). Lignin removal is higher in this improved method than the previous work, where only 17.4% lignin was removed during alkali pretreatment [3]. Total 87.17 g wet pretreated solids were obtained. This alkali pretreatment was associated with ~ 44% dry matter loss in side streams, which is in accordance with the previous study (57.23%) [3] (Table 1). The level of cellulose enrichment in rice straw variety used in this improvised procedure was higher as compared from previous pretreatments [3, 16, 17, 20, 21]. Xue et al. [13] reported 20–28% wt. loss and 67–85% lignin loss and improvement in cellulose content 17–36% in Jerusalem artichoke stalks by treatment with different concentrations of NaOH and H2O2. Loss of materials in every stage of operation is inevitable, and this material loss should be accounted to draw a conclusive economic analysis [9].
It is highly desirable that lignin is removed and more sugars be preserved in solids during pretreatment to enable higher glucan loadings and solubilization during enzymatic hydrolysis leading to high ethanol titers [10, 22]. The solids were extracted and washed twice with distilled water and final wash with acidified water to neutralize pH. Also, washing removes degradation products, which may adversely affect efficiency of enzymatic hydrolysis of solids [23].
The alkali extracts showed absorbance ranging from 0.797 to 0.029 at 205 nm in the five washing fractions indicating presence of solubilized lignins. The absorbance values of wash waters decreased with subsequent washings (Table 2). The total sugars released during pretreatment into washings were 186.34 mg L−1 (423 mg in 2270 mL). Total 4.938 g AIL was recovered from total liquor recovered (2270 mL) during alkali pretreatment. Thus, minimal amounts of sugars and high amount of lignins were removed from solids by rinsing them with deionized water and acidified water. Alkaline pretreatment and washing with acidified water is an effective strategy for improving enzymatic hydrolysis and sugar yields. Xue et al. [13] were able to limit waste water generation from solid washing by using water washing/HCL neutralization. Washings removed any soluble sugars, degradation products, and acid/alkali/solvents from solids, presence of which can potentially inhibit sugar release from solids by enzymes [24]. High levels of lignins present in these fractions prevent their fermentation to ethanol without prior detoxification and pH adjustment. These lignins can be easily recovered by acidification of extracts as they precipitate at low pH [3, 25].
The extent of lignin and xylan removal varies in pretreated solids as a result of type of pretreatment applied. Dilute acid pretreatment leads to xylan removal, while dilute alkali pretreatments are advantageous causing effective delignification at relatively low temperature conditions and showed enhanced sugar release and delignification as in Co-solvent Enhanced Lignocellulosic Fractionation (CELF). Lignin removal had a more positive effect on biomass digestion than xylan removal by other hydrothermal and acid pretreatment [24]. The most commonly used alkali reagents are sodium hydroxide, potassium hydroxide, calcium hydroxides, and ammonium salts. Among these, sodium hydroxide is the most extensively used [26]. Treating lignocellulose with alkali reagents degrade the side chains of esters and glycosides causing structural modification of lignin, cellulose swelling, cellulose decrystallization, and hemicellulose solvation [27, 28].
3.2 Saccharification of pretreated rice straw by commercial cellulases and production of sugar rich hydrolysates
The pretreated rice straw was further subjected to hydrolysis by commercially available cellulase (Accellerase®1500 and Sigma cellulase) enzymes. With enriched cellulose in pretreated solids, higher glucan loadings of 3% and 6% could be made corresponding to ~ 20% and ~ 40% wet solids. The corresponding loading rate was ~ 6% and ~ 12% solids on dry weight basis (Table 3).
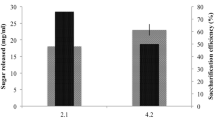
The amount of sugars released and saccharification efficiency achieved with both the enzymes are presented in Table 4 and Fig. 1. One hundred percent of glucan solubilization was achieved (sugar released 33 g L−1) in case of 3% glucan with both the enzymes within 48 h. Accellerase®1500 released 47.34 sugars, while Sigma cellulase released 53.50 g L−1 at 72 and 48 h, respectively, with 6% glucan loading. The highest saccharification efficiency achieved in case of 6% glucan loading was 71.01% and 80.25% with Accellerase and cellulase (Sigma) respectively (Table 4). With improvisation, better glucan loadings and solubilization could be achieved than reported earlier [3]. Solids loading at higher rate (more than 15% dry matter) may not be beneficial as cellulose conversion efficiency gets reduced due to factors like high viscosity and mass transfer [29]. The glucan loading, solids loading, and glucan conversion achieved in this study were at par with that reported by Zhong et al. [30] who achieved increased sugar conversion (80.6% glucan and 89.6% xylan) upon hydrolysis by mixture of enzymes which included cellulase Spezyme®, Multifect® xylanase, and Multifect® pectinase with 6% glucan loading in case of AFEX treated rice straw corresponding to 17.8% solids loading.
The results were statistically analyzed using Univariate Analysis of Variance by SPSS 16.0 software. Significant difference was obtained due to treatments, i.e., 3% and 6% glucan loading at 1% significance level on the amount of sugar released. However, effect due to the enzymes was at par.
3.3 Fermentation of sugars by S. cerevisiae LN and ethanol production
The fermentation of sugar rich hydrolysates obtained from hydrolysis of treated rice straw and control containing glucose was carried out using S. cerevisiae LN. The maximum fermentation efficiency of 97% and 76% (Fig. 2) was observed in hydrolysates obtained from 3.0 and 6.0% glucan respectively with complete utilization of glucose within 24 h, and the highest ethanol produced was 10.34 and 15.13 g L−1 respectively (Fig. 3). S. cerevisiae LN produced 0.508 gg−1 and 0.403 gg−1 ethanol from glucose present in hydrolysates obtained from 3% and 6% glucan loading respectively. It showed ethanol productivity of 0.63 g L−1 h−1. In synthetic controls containing 3.5 and 5% glucose, the highest ethanol yields were 19.53 and 26.7 g L−1 in 24 h and 48 h of fermentation (Fig. 4). In hydrolysates obtained from 3% glucan loading and synthetic medium controls with only glucose as C source, the fermentation efficiency was 100%, while it was about 80% in case of hydrolysates from 6% glucan loading. Thus, S. cerevisiae LN completely consumed glucose present in NaOH treated rice straw hydrolysates and produced ethanol with high efficiency, while xylose was largely unutilized. Xylose utilization/ fermentation is affected by concentration of nutrients, cell density, and degradation products from pretreatment [23].
The results were statistically analyzed using Univariate Analysis of Variance by SPSS 16.0 software. Significant difference was obtained due to treatments, i.e., 3% and 6% glucan loading, on both the amount of xylose utilized and ethanol produced. However, for glucose, statistical analysis could not be performed due to majority of its values corresponding to zero.
3.4 Material flow of cellulose/glucan and lignin recovery
Flow of lignin and translation of glucan from raw rice straw to ethanol is shown in Fig. 5.
This improvised cost effective NaOH pretreatment method led to recovery of 80% of cellulose present in original raw rice straw in pretreated solids. Following the scenario of 3% glucan loading, whole of glucan loaded was converted to soluble sugars with 100% efficiency within 48 h using any of the commercial cellulase used. In the subsequent fermentation of hydrolysates with Saccharomyces cerevisiae LN ITCC 8246, whole of glucose present is fermented to ethanol with 100% efficiency. Thus, overall with this process 5.39 g ethanol could be produced from 50 g of rice straw. This method can potentially produce 136 L ethanol/ton rice straw along with ~ 98 kg lignin and 14.85 g yeast biomass. Sixty-three percent of glucose present in rice straw was recovered as ethanol by this process.
During the pretreatment biomass loses with every step. For economic efficient process, the loss should be accounted, minimized, and utilized less water. It was observed that out of the 47.50 g initial dry weight of biomass, 26.17 g biomass was recovered after pretreatment with enriched cellulose content of about 52.75% from 32.28% in raw rice straw. There was dry matter loss in side stream (washings); 55.1% rice straw (dry wt. basis) was recovered after the pretreatment process. Loss of materials in every stage of operation is inevitable, and this material loss should be accounted to draw a conclusive economic analysis [9].
4 Conclusions
The evaluation of this improvised NaOH pretreatment of rice straw coupled with acidic- water washing, tracking the flow of cellulose to ethanol, and recovery of lignin, showed that this process converted 63% cellulose present in raw rice straw to ethanol. Using rice straw feedstock treated with this improvised method and fermenting hydrolysates with Saccharomyces cerevisiae LN ITCC 8246 could yield ~ 135 L of ethanol/ton of dry rice straw with minimal generation of wash waters and about 100 kg of lignin recovery which could be chemically/biologically converted to very high value products. This study provides a scalable platform and hence can be employed on industry standard. Further advancements can be made by improving enzyme hydrolysis and using xylose utilizing strain.
References
Gadde B, Menke C, Wassmann R (2009) Rice straw as a renewable energy source in India, Thailand, and the Philippines: overall potential and limitations for energy contribution and greenhouse gas mitigation. Biomass Bioenergy 33(11):1532–1546
Gheewala SH, Silalertruksa T, Nilsalab P, Mungkung R, Perret SR, Chaiyawannakarn N (2013) Implications of the biofuels policy mandate in Thailand on water: the case of bioethanol. Bioresour Technol 150:457–465
Sharma S, Nandal P, Arora A (2019) Ethanol production from NaOH pretreated rice straw: a cost effective option to manage rice crop residue. Waste Biomass Valor 10(11):3427–3434
Trivedi A, Verma AR, Kaur S, Jha B, Vijay V, Chandra R, Vijay VK, Subbarao PM, Tiwari R, Hariprasad P, Prasad R (2017) Sustainable bio-energy production models for eradicating open field burning of paddy straw in Punjab, India. Energy 127:310–317
Robertson GP, Hamilton SK, Barham BL, Dale BE, Izaurralde RC, Jackson RD, Landis DA, Swinton SM, Thelen KD, Tiedje JM (2017) Cellulosic biofuel contributions to a sustainable energy future: choices and outcomes. Science 356:6345
Dinh Tuan P, Dinh Minh Hiep N (2009) Analysis of material balance in the application of different sources of biomass for bioethanol production. J Sci Technol 72:6–11
Hong YY, Wang YT, Zhu SM, Luo XC, Li S, Zhuo M, Zhou T, Zhu MJ (2019) Improved enzymatic hydrolysis and ethanol production by combined alkaline peroxide and ionic liquid-water mixtures pretreatment of rice straw. J Chem Technol 94(5):1451–1459
Wei HL, Wang YT, Hong YY, Zhu MJ (2020) Pretreatment of rice straw with recycled ionic liquids by phase-separation process for low-cost biorefinery. Biotechnol Appl Biochem
Akanksha K, Sukumaran RK, Pandey A, Rao SS, Binod P (2016) Material balance studies for the conversion of sorghum Stover to bioethanol. Biomass Bioenergy 85:48–52
Tsegaye B, Balomajumder C, Roy P (2019) Optimization of microwave and NaOH pretreatments of wheat straw for enhancing biofuel yield. Energy Convers Manag 186:82–92
Hatzis C, Riley C, Philippidis G (1996) Detailed material balance and ethanol yield calculations for the biomass-to-ethanol conversion process. In seventeenth symposium on biotechnology for fuels and chemicals (pp. 443-459). Humana press, Totowa, NJ
Miller GL (1959) Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Xue C, Zhang X, Wang J, Xiao M, Chen L, Bai F (2017) The advanced strategy for enhancing biobutanol production and high-efficient product recovery with reduced wastewater generation. Biotechnol biofuels 10(1):148
Updegraff DM (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32(3):420–424
Templeton D, Ehrman T (1995) Chemical analysis and testing task: LAP-003 (determination of acid-insoluble lignin in biomass). National Renewable Energy Laboratory, Golden
Saritha M, Arora A, Nain L (2012) Pretreatment of paddy straw with Trametes hirsuta for improved enzymatic saccharification. Bioresour Technol 104:459–465
Arora A, Priya S, Sharma P, Sharma S, Nain L (2016) Evaluating biological pretreatment as a feasible methodology for ethanol production from paddy straw. Biocatal Agric Biotechnol 8:66–72
Sharma S, Arora A, Sharma P, Singh S, Nain L, Paul D (2018) Notable mixed substrate fermentation by native Kodamaea ohmeri strains isolated from Lagenaria siceraria flowers and ethanol production on paddy straw hydrolysates. Chem Cent J 12(1):8
Sharma S, Varghese E, Arora A, Singh KN, Singh S, Nain L, Paul D (2018) Augmenting pentose utilization and ethanol production of native Saccharomyces cerevisiae LN using medium engineering and response surface methodology. Front Bioeng Biotechnol 6:132
Choudhary JM, Nain L, Arora A (2014) Enhanced saccharification of steam-pretreated rice straw by commercial cellulases supplemented with xylanase. J Bioprocess Biotech 4(7):1
Saritha M, Arora A, Singh S, Nain L (2013) Streptomyces griseorubens mediated delignification of paddy straw for improved enzymatic saccharification yields. Bioresour Technol 135:12–17
Stoklosa RJ, Hodge DB (2015) Fractionation and improved enzymatic deconstruction of hardwoods with alkaline delignification. Bioenerg Research 8(3):1224–1234
Lau MW, Dale BE (2009) Cellulosic ethanol production from AFEX-treated corn Stover using Saccharomyces cerevisiae 424A (LNH-ST). Proc Natl Acad Sci 106(5):1368–1373
Kothari N, Holwerda EK, Cai CM, Kumar R, Wyman CE (2018) Biomass augmentation through thermochemical pretreatments greatly enhances digestion of switch grass by Clostridium thermocellum. Biotechnol biofuels 11(1):219
Arora A, Nain L, Gupta JK (2005) Solid-state fermentation of wood residues by Streptomyces griseus B1, a soil isolate, and solubilization of lignins. World J Microbiol Biotechnol 21(3):303–308
Kumar R, Mago G, Balan V, Wyman CE (2009) Physical and chemical characterizations of corn Stover and poplar solids resulting from leading pretreatment technologies. Bioresour Technol 100(17):3948–3962
Cheng YS, Zheng Y, Yu CW, Dooley TM, Jenkins BM, VanderGheynst JS (2010) Evaluation of high solids alkaline pretreatment of rice straw. Appl Biochem Biotechnol 162(6):1768–1784
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4(1):7
Wi SG, Choi IS, Kim KH, Kim HM, Bae HJ (2013) Bioethanol production from rice straw by popping pretreatment. Biotechnol Biofuels 6(1):166
Zhong C, Lau MW, Balan V, Dale BE, Yuan YJ (2009) Optimization of enzymatic hydrolysis and ethanol fermentation from AFEX-treated rice straw. Appl Microbiol Biotechnol 84(4):667–676
Acknowledgments
Authors are thankful to the Director of Indian Agricultural Research Institute (IARI), Pusa, New Delhi, for providing infrastructure facilities for carrying out this research. Authors acknowledge support by Ms. Jyoti Singh for performing HPLC analysis. Authors are also thankful to Dr. K N Singh and Dr. Mrinmoy Ray from the ICAR-Indian Agricultural Statistics Research Institute (IASRI) for aiding in statistical analysis of data.
Funding
WS and AS received fellowship from ICAR-AMAAS (Application of Microorganisms in Agriculture and Allied Sectors) India vide grant No. 12-124.
Author information
Authors and Affiliations
Contributions
WS carried out the experiments, prepared and edited the manuscript. AA received funding from ICAR-AMAAS (Application of Microorganisms in Agriculture and Allied Sectors) India vide grant No. 12-124, conceptualized the study, designed the experiments prepared and finalized the manuscript. AS carried out the experiments. SS and PN did sugar analyses and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 516 kb)
Rights and permissions
About this article
Cite this article
Samar, W., Arora, A., Sharma, A. et al. Material flow of cellulose in rice straw to ethanol and lignin recovery by NaOH pretreatment coupled with acid washing. Biomass Conv. Bioref. 13, 2233–2242 (2023). https://doi.org/10.1007/s13399-021-01278-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01278-3