Abstract
Rice straw, an abundant agro-residue, is available for energy production. In many parts of Asian countries, it is burnt on fields causing harm to the environment. Rice straw contains lignin, cellulose, hemicelluloses, and silicates making it recalcitrant. Pretreatment processes disintegrate lignin-carbohydrate matrix for efficient bioconversion of polysaccharides to fermentable sugars. A good number of physical, biological and chemical processes have been tried but degradation of polysaccharides and subsequent fermentation is still a challenge. Alkaline pretreatment causes effective delignification and swelling of biomass. The present study was performed on alkaline pretreatment of rice straw with 1% NaOH by autoclaving for 30 min at 121 °C at 10% solid loading. It was extracted with water to remove lignins, solids separated by filtrations and washed again to neutralize the pH. Water washing also led to removal of phenolic inhibitors. High (63%) glucan enrichment was obtained with concomitant lignin loss. Dry matter loss was around 50%. Enzymatic saccharification of the pretreated solids at 5 and 10% with Accellerase® 1500 for 24 h at 50 °C gave saccharification efficiency 76 and ~ 50% respectively. Hydrolysates containing 18 and 23 gL−1 sugars, supplemented with minimal salts, yeast extract, fermented by S. cerevisiae LN for 24 h yielded ~ 2 and 4 gL−1 ethanol with fermentation efficiency 55–66%. Thus, NaOH pretreatment is a cost effective option for ethanol production from rice straw. Lignin removed in prehydrolysates can be recovered by acidification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Rice straw is an abundant feedstock available for alternate uses and energy production, but difficult to process. Its conversion to ethanol presents challenges like developing cost effective process and selecting an appropriate pretreatment process. Alkaline pretreatments with NaOH are effective in delignification. This study aimed at achieving a more basic understanding of pretreatment and enzymatic hydrolysis at solids and glucan loadings, identify points for improving the efficiency and effectiveness of the lignocellulose conversion. NaOH pretreatment is a relatively simple, cost effective pretreatment method for ethanol production from rice straw hydrolysates. We also demonstrated presence of degraded lignins in prehydrolysates which can be removed by simple water extraction and can be recovered by lowering the pH of extracts for value added applications.
Introduction
Biofuels, offer the means for slowing down and mitigating climate change by lowering CO2 emissions, reduce dependence on fast depleting petroleum reserves and also socio-economic benefits for rural communities. They unarguably, are the most promising renewable energy sources. Liquid biofuels, including bioethanol and biodiesel, can be blended with petrol and diesel and cater to transport sector which consumes more than 50% of petro based energy. Bioethanol can be blended with petrol at (5–85%) thus can potentially enable global society to supplement/trim demand for petroleum. Most of the countries worldwide are working towards overcoming the dependence and usage of petroleum and have delineated timeline targets in this direction. In consonance with rest of the world, India also launched its National Biofuel Mission in 2003 with a view to switch to cleaner energy and established blending targets making 5% ethanol blending mandatory by 2011, then gradually increase to 10% by 2016–2017 and to 20% thereafter [1]. As is evident from current scenario, it is far from its targets. For meeting 10% blending target, about 29 billion L ethanol is required which will increase to 44 billion L by 2022. The main source of bioethanol in India is molasses and is not clearly sustainable because of sugarcane being highly water intensive crop, high sugar demand by population and diversion of ethanol to beverage and chemical industry where they get higher price [2]. Currently, only 6.7 billion L of molasses based bioethanol is available and there is a deficit of 22.3 billion L [3]. Therefore, there is urgent need to explore alternative feedstock for bioethanol including cereal grains, sugar and lignocellulosic biomass. On the other hand, diverse wastes from agriculture, forestry and food processing wastes have been used for production of biobased value added products [4].
Second generation bioethanol from lignocellulosic biomass is more desirable as biomass is the most abundant, avoids food and fuel controversy, and is C neutral. Globally, 1.4 billion t agricultural wastes are produced annually [5]. This abundantly present biomass, is a tremendous source of energy and can be utilized in modern industry for large-scale production of ethanol and other value added products, which in turn enhance the bio-based economy of the world as well as resolve the issue of its disposal.
Among the aforementioned agro-residue, rice straw is plentiful agro-residue available for alternate use in India, many Asian and other countries. Rice straw is an ideal feedstock as it is available at very low price and can be converted to sugar rich slurries which can be fermented to ethanol or any other very high value added chemical. Due to cropping system pattern practiced in North India, it has to be removed from the fields at the earliest and farmers resort to burning it on fields despite deleterious effects on environment and soil ecology. This low value fiber is generally consumed by brick kilns, paper and packaging industry. Rice straw, as most lignocelluloses, consists of linear chains of highly crystalline and non-porus cellulose, a homopolymer of β (1–4) linked glucose whereas, hemicelluloses are relatively loose, less crystalline and branched heteropolysaccharides of mannan, xylan, glucan, galactan and arabinan [6]. Lignin is highly heterogenous, amorphous three dimensional network of phenylpropane units and aromatic alcohols. It is inherently resistant to microbial attack because of phenolic monomers such as coniferyl alcohol, sinapyl alcohol, p-coumaryl alcohol [7]. In addition, rice straw contains silicates which make it indigestible. Intrinsic structural and compositional properties of biomass substrate and the pretreatment conditions employed have profound effect on efficacy of enzymatic hydrolysis and subsequent fermentation of sugars released [8]. Apparently lignin more drastically affects hydrolysis by cellulase enzymes by unproductive binding to enzymes whereas effect of hemicelluloses is less obvious [9].
Pretreatment technologies have been developed to disintegrate lignin carbohydrate matrix and enhance accessible surface area of carbohydrate, so that low dosage of hydrolytic enzymes is utilized with minimum bioconversion time and minimum generation of inhibitory products. Various factors considered for pretreatment to be efficient, include rapid decrystallization of cellulose, depolymerization of hemicelluloses, low energy input, recovery of value added products (such as lignins) and limited formation of inhibitors. Choicest methodologies should involve minimal degradation of polymeric sugars like hemicelluloses and cellulose while maximizing the hydrolysis [10]. Therefore, diverse combinations of physical, biological and chemical processes, compromises and tradeoffs, have been tried for biomass deconstruction. The most widely used methods are grinding/milling, steam explosion [11], autohydrolysis [12], acid treatment [13], alkali treatment [14] and many other methods. Amongst the pretreatment methods, alkaline pretreatment is more advantageous as it utilizes non-polluting and less corrosive chemicals. Alkaline treatment directly affects the cell wall, thus resulting in more effective delignification as well as swelling of the biomass. Overall process for ethanol production from NaOH pretreated paddy straw was laid [15]. Alkaline pretreatment using sodium hydroxide causes the lignocellulose to swell, thereby increasing the surface area while reducing the degree of polymerization (DP) and crystallinity of the material and it is not as energy-intensive as some of the other pretreatment options because it can be performed at ambient temperatures and pressures, although longer reaction times may be needed to obtain the same level of digestibility offered by other forms of pretreatments. During alkaline pretreatment, very little of the saccharide fractions are solubilized, meaning that nearly maximum saccharides can be recovered during subsequent processing steps, which is desirable to obtain higher productivity. Many reports, describing NaOH pretreatment under different conditions, including soaking in alkaline solution at low temperature to retain the hemicellulose in the solids, for ethanol production from rice straw and other biomass sources have been summarized in Table 1.
Enzymatic hydrolysis of pretreated biomass involves cleaving of cellulose and hemicellulose by cellulases and xylanases resulting in production of glucose from cellulose and hexoses and pentoses released from hemicellulose [28]. For efficient hydrolysis generally indigenous/commercially available enzymes with high activities are used e.g. Accellerase from Genencor [29,30,31], a combination of two enzymes—Speczyme CP (Genencor, Denmark) and Novozyme 188 (Novozymes A/S, Denmark) [32], Accellerase®1500 supplemented with xylanase [33].
Saccharomyces cerevisiae, the brewer’s yeast is the established cell factory for commercial ethanol production. S. cerevisiae ferments hexose sugars with high efficiency due to its high ethanol tolerance and fermentation rates thus yielding ethanol close to the theoretical maximum (0.51 g g−1 hexose sugar consumed). It has also been employed to ferment biomass hydrolysates to ethanol [29, 34, 35].
In depth study was carried out into NaOH pretreatment as an effective method for deconstruction and enzymatic saccharification of rice straw of locally grown variety into sugars and fermentation of hydrolysate for ethanol production.
Materials and Methods
Raw Material
The substrate, rice straw var. Pusa Basmati 1121 was obtained from farms of the IARI, New Delhi. The straw was air dried to lower moisture content, chopped and milled using Mini Mill (Zexter, India) and sieved through mesh size 10 to get particle size 2 mm.
Microorganism
The fermentation of hydrolysate was carried out using yeast Saccharomyces cerevisiae LN ITCC 8246, obtained from Division of Microbiology, IARI, New Delhi. The culture was maintained and grown on MGYP (malt extract: 3 g L−1, glucose: 10 g L−1, yeast extract: 3 g L−1, peptone: 5 g L−1) at 30 °C for 72 h. Culture was stored at 4 °C on MGYP slants and subcultured periodically.
Enzymes
Commercially available cellulase, Accellerase®1500 (Genencor, Denmark) was used for saccharification experiments. The cellulolytic activity of enzyme was assayed to be 29 FPU (Filter Paper Unit) and endoglucanase activity 1746 CMCase U g−1 (Carboxy methyl cellulase) [36, 37]. The reducing sugars released were assayed by DNS method [38].
Alkaline Pretreatment of Rice Straw
For pretreatment, 10 g ground rice straw was taken in a 500 mL Erlenmeyer flask and moistened with 100 mL of 1% NaOH in 1:10 loading. The treatment was carried out in autoclave at 121 °C, 15 psi pressure, for 30 min. After cooking, 100 mL distilled water was added to the flask and stirred for 30 min on magnetic stirrer and filtered through muslin cloth and filtrate was collected. The treated biomass was further washed with distilled water to remove residual NaOH and neutralize pH. The absorbance of alkaline extract was read at 205 nm to determine presence of lignin. Sugars in extracts were assayed using DNS. The solids were analyzed for moisture content, cellulose and lignin content. The biomass was air-dried overnight to lower moisture content to < 10% and stored at 4 °C for further use in saccharification.
Compositional Analysis of Treated and Untreated Rice Straw
The cell wall components mainly, cellulose, lignin and moisture were studied in the treated and untreated rice straw. The moisture content of the biomass was estimated using moisture analyzer RADWAG MA 50.R (Bracka, Poland). Updegraff method was used for estimating cellulose content [39]. The cellulose enrichment was calculated as:
The lignin was estimated following NREL LAP-003 standard protocol [40]. The delignification percentage was calculated with respect to total lignin present in raw rice straw.
Saccharification of Pretreated Rice Straw
Pretreated solids containing 47.27% cellulose and ~ 9% moisture were subjected to enzymatic hydrolysis. Saccharification was carried out using 5 and 10% solids loading (0.5 and 1.0 g of pretreated rice straw in 10 mL reaction mixture corresponding to 2.1 and 4.2% glucan/cellulose loading respectively). The rice straw was hydrolyzed with commercially available Accellerase®1500 (Enzyme loading rate was 0.5 mL enzyme per g of glucan as prescribed by manufacturer), at 150 rpm, 50 °C for 24 h. The final volume was kept at 10 mL using citrate buffer (50 mM) pH 4.8 in both the cases. The samples (0.5 mL) were withdrawn after 24 h of incubation, reaction stopped and the total reducing sugars were estimated by DNS assay. The saccharification efficiency was calculated as described by Saritha et al. [41].
Saccharification efficiency was calculated as follows:
Fermentation Using S. cerevisiae LN
The sugar rich hydrolysates (10 mL) in 15 mL screw capped flat bottom vials, supplemented with mineral salts and 0.1% YE [29] were used to check the growth, sugar utilization and ethanol production by S. cerevisiae LN. Controls consisted of 2% glucose in defined medium. A 10% inoculum of S. cerevisiae LN, grown on MGYP broth, with optical density of 0.8 at 660 nm, was used and incubated at 30 °C for 24 h under static conditions. Aliquots were withdrawn periodically and optical density was measured at 660 nm to measure growth. Samples were then centrifuged at 8000×g rpm for 10 min and the supernatant was used for estimation of sugar consumption and ethanol production. Fermentation efficiency (%) was calculated by using following equation:
Gas Chromatographic Quantification of Alcohol Produced after Fermentation
The culture samples were centrifuged at 8000×g rpm for 10 min and the supernatants were diluted appropriately, filtered through 0.2 µm Nylon-66 syringe filters. 1 µL sample was injected and analyzed by a dual column gas chromatography, Shimadzu GC-2014 (Kyoto, Japan) fitted with a flame ionization detector (220 °C). Porapak N Column (Chromatopak Analytical Instrumentation, Mumbai, India) of 2 m length and 3 mm inner diameter was used at 170 °C, isothermally. Flow rate was 30 mL min−1 with a pressure of 340 KPa [42].
Statistical Analysis
All the experiments were carried out in triplicates using Completely Randomised Design and statistically analyzed using one way ANOVA (SPSS Version 21.0. Armonk, NY: IBM Corp). Student’s T test was done to determine the effect of glucan loading on cellulose saccharification.
Results and Discussion
Rice straw var. Pusa Basmati 1121 used in this study had 29% cellulose. One of the main goals of pretreatment step is to enrich cellulose content in substrate so that more concentrated sugar syrups are obtained after hydrolysis. The pretreatment is applied to remove the lignin and to decrease the DP and crystallinity of cellulose, which are the major hurdles for hydrolysis of cellulose. Alkaline pretreatment have been reported to be much effective for delignification of grass species and softwoods as they have higher content of syringyl units in lignin. Syringyl lignins are more easily delignified at higher pH. Pretreatment with NaOH alone is technically appropriate as it selectively delignifies the biomass and conserves more holocellulose than acidic or neutral pretreatments [10, 43]. NaOH pretreatment was selected in this study as alkali efficiently dissolves lignin and thus enriches cellulose content in pretreated biomass. It has emerged as a frontrunner amongst chemical pretreatments because of obvious advantages such as low cost, less corrosive and energy.
Pretreatment and Compositional Analysis of Rice Straw
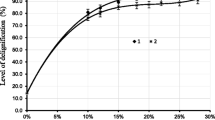
In the present study, the pretreated rice straw contained 47.3% cellulose on dry wt. basis as compared to 29% in raw rice straw. The pretreatment of the rice straw with 1% NaOH resulted in 63% cellulose enrichment and about 17.4% lignin loss. Although alkaline pretreatments involving Ammonia give very high delignification, Kim et al. stated that a high percentage of lignin removal was not essential for effective conversion of lignocellulosic biomass to fermentable sugars [10]. The level of cellulose enrichment in rice straw variety used in this procedure was fairly high as compared from other pretreatments [29, 33, 41, 44]. This alkali pretreatment was associated with 57.23% dry matter loss. Various pretreatment methods have been adopted across the world under diverse conditions and varying degree of saccharification efficiency have been achieved (Table 1). Improved tensile strength and elasticity of wheat straw treated with NaOH was achieved compared to other oxalic acid and hydrothermal treatments [45]. Alkaline hydrolysis, degrades the lignin and decreases the polymerization and crystallinity of the cellulose making it more susceptible to hydrolysis by enzymes, has been found as the most viable and advantageous process [10]. The pretreatment of Parthenium sp. with 1% NaOH increased the cellulose content by 30.5% with decrease in lignin content by 16.6% [16]. Dai et al. [46] observed reduction in lignin content to 5.1–11.8% from 12.7% and increased cellulose content to 56.8–69.2% from 51.7% in NaOH/Urea treated rice straw.
The alkali extracts showed absorbance 0.433 at 205 nm indicating presence of the degraded soluble lignin. The absorbance values of wash waters decreased for subsequent washings (Table 2) showing that lignins have been removed from solids after alkaline pretreatment and washing. The liquid fractions of wash wasters also had sugars. Thus washings were important in facilitating the subsequent enzymatic hydrolysis by making pH favorable and removing inhibition of enzymes by phenolic byproducts from lignins. High levels of lignins present in the hydrolysate fractions prevent their fermentation to ethanol without prior detoxification and pH adjustment. The lignins can be easily recovered from alkaline prehydrolysates and wash waters by acidification of extracts as they precipitate at low pH [44, 47]. The alkaline pretreatment yielded pretreated biomass with low lignin and S free lignins. The lignins can find high value applications in chemical industry as adhesives, extenders and antioxidants [48, 49].
Saccharification of Pretreated Rice Straw by Cellulase (Accellerase®1500)
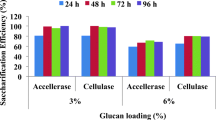
The pretreated rice straw was further subjected to enzymatic hydrolysis by commercially available cellulase (Accellerase®1500) enzyme. Higher sugar release (23 gL−1) was obtained in case of 10% solid loading corresponding to 4.2% glucan loading while 18 gL−1 sugars were present in treatment with 5% solids loading corresponding to 2.1% glucan loading (Supplementary table 1). The cellulose conversion (saccharification efficiency) was 50 and 76% respectively (Fig. 1). The levels of sugars obtained in enzymatic hydrolysates obtained from different glucan loadings were significantly different as shown by t-test. Thus, at higher solids and glucan loadings, higher concentrations of sugars in hydrolysates were obtained but cellulose conversion efficiency was lower. The higher substrate loadings result in higher sugar concentrations in hydrolysate but cellulose conversion to sugars is lesser. Higher solids loadings and sugar concentrations are desirable for getting higher ethanol titres and thus efficient and cost effective production of ethanol from lignocellulosics. However, operations at higher solids presents challenges like higher viscosity resulting in lower efficiency in mass and heat transfer and higher energy consumption for mixing. Also, high substrate content gives higher concentrations of inhibitors causing the enzyme inhibition [42]. In the pretreated rice straw, 0.567 g g−1 biomass sugar recovered as compared to untreated rice straw 0.270 g g−1 biomass in 48 h of hydrolysis by cellulase and xylanase enzymes [50]. At 2% solids loading of acid pretreated rice straw, 71.8% conversion of sugar polymers was obtained while at higher solid loadings yields decreased [51]. Saccharification efficiency 76% was obtained in this study from 2.1% glucan loading of alkali pretreated rice straw while it decreased to 50% for 4.2% glucan loading.
Fermentation of Sugars by S. cerevisiae LN and Ethanol Production
The fermentation process of sugar rich hydrolysate obtained from hydrolysis of treated rice straw and control containing synthetic sugars was carried out using S. cerevisiae LN. The highest fermentation efficiency of 80.98% was found in control containing synthetic sugars (Table 3). The maximum fermentation efficiency of 66.38% was observed in hydrolysate containing 4.2% sugars. The ethanol produced was 0.34 g g−1 (ethanol 4 mg mL−1 with sugar consumption of 11.89 mg mL−1). While, hydrolysate obtained from 2.1% sugar produced ethanol 1.94 mg mL−1 with 6.93 mg mL−1 sugar consumption. The fermentation efficiency 54.73% was obtained. Table 4 summarizes some reports on ethanol production using NaOH pretreatment and fermentation efficiency obtained in this study were comparable. Thus, NaOH treatment is a suitable, simple and cost effective method for rice straw pretreatment. The main consideration is copious amount of water required for washing and pH neutralization, this also results in dry matter loss. However, a strategy like using acidified water for washing can help in limiting water usage.
Xu et al. also stated that alkali treatment could give higher sugar yields than other pretreatments but large amount of water required for NaOH removal and neutralization is a drawback [53]. In North India, rice crop is grown over a vast acreage and about 16.8 million tonnes of low value rice straw is available in Punjab and Haryana alone according to estimates in 2012–2013 [54]. Alkali pretreatment using low cost NaOH can be a promising strategy to utilize rice straw to produce ethanol thus offering alternative use and cleaner option for energy.
Conclusions
The pretreatment of the rice straw with 1% NaOH is suitable to release lignin and leads to better cellulose enrichment, obtaining sugar rich hydrolysate. The ethanol production by S. cerevisiae LN was better with fermentation efficiency of 66.38%. The constraints of excess water usage for washing and neutralizing pH can be overcome by further experimentation by washing with mildly acidified water and also manipulating pretreatment parameters to get higher delignification and higher cellulose enrichment. This process is cost effective and can be adapted on larger scale for ethanol production from rice straw.
References
National Policy on Biofuels Ministry of New and Renewable Energy (MNRE): Government of India (2009)
Shinoj, P., Raju, S.S., Joshi, P.K.: India’s biofuels production programme; need for prioritizing alternative options. Indian J. Agric. Sci. 81(5), 391–397 (2011)
Annual Report. Ministry of Petroleum and Natural Gas (MPNG), New Delhi, India (2016–2017). http://www.petroleum.nic.in
Pleissner, D., Demichelis, F., Mariano, S., Fiore, S., Guitierrez, I.M.N., Schneider, R., Venus, J.: Direct production of lactic acid based on simultaneous saccharification and fermentation of mixed restaurant food waste. J. Cleaner Prod. 143, 615–623 (2017)
Saini, J.K., Saini, R., Tewari, L.: Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. Biotech 5(4), 337–353 (2015)
Chandrakant, P., Bisaria, V.S.: Simultaneous bioconversion of cellulose and hemicellulose to ethanol. Crit. Rev. Biotechnol. 18, 295–331 (1998)
Grabber, J.H.: How do lignin composition, structure, and cross-linking affect degradability? A review of cell wall model studies. Crop Sci. 45, 820–831 (2005)
Chandra, R.P., Bura, R., Mabee, W.E., Berlin, A., Pan, X., Saddler, J.N.: Substrate pretreatment: The key to effective enzymatic hydrolysis of lignocellulosics? Adv. Biochem. Eng. Biotechnol. 108, 67–93 (2007)
Wyman, C.E.: Handbook on Bioethanol: Production and Utilization. Taylor and Francis, Washington D.C. (1996)
Kim, S.J., Lee, Y.Y., Kim, T.H.: A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Biores. Technol. 199, 42–48 (2016)
Liu, Z.H., Chen, H.Z.: Xylose production from corn stover biomass by steam explosion combined with enzymatic digestibility. Biores. Technol. 193, 345–356 (2015)
Timug, B., Mohan, M., Chilukoti, B., Sasmal, S., Banerjee, T., Goud, V.V.: Optimization of dilute acid and hot water pretreatment of different lignocellulosic biomass: a comparative study. Biomass Bioenerg. 81, 9–18 (2015)
Kim, J.S., Lee, Y.Y., Torget, R.W.: Cellulosic hydrolysis under extremely low sulfuric acid and high temperature conditions. Appl. Biochem. Biotechnol. 91–93, 331–340 (2001)
Yoo, C.G., Nghiem, N.P., Hicks, K.B., Kim, T.H.: Pretreatment of corn stover using low-moisture anhydrous ammonia (LMAA) process. Biores. Technol. 102, 10028–10034 (2011)
Wati, L., Kumari, S., Kundu, B.S.: Paddy straw as substrate for ethanol production. Indian J. Microbiol. 47, 26–29 (2007)
Pandiyan, K., Tiwari, R., Singh, S., Nain, P.K., Rana, S., Arora, A., Singh, S.B., Nain, L.: Optimization of enzymatic saccharification of alkali pretreated Parthenium sp. using response surface methodology. Enzyme Res. (2014). https://doi.org/10.1155/2014/764898
McIntosh, S., Vancov, T.: Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass Bioenerg. 35, 3094–3103 (2011)
Jeya, M., Zhang, Y.W., Kim, I.W., Lee, J.K.: Enhanced saccharification of alkali-treated rice straw by cellulase from Trametes hirusta and statistical optimization of hydrolysis conditions by RSM. Biores. Technol. 100(21), 5155–5161 (2009)
Gupta, R., Khasa, Y.P., Kuhad, R.C.: Evaluation of pretreatments methods in improving the enzymatic saccharification of cellulosic materials. Carbohyd. Polym. 84, 1103–1109 (2011)
Cheng, Y.S., Zheng, Y., Yu, C.W., Dooley, T.M., Jenkins, B.M., VanderGheynst, J.S.: Evaluation of high solids alkaline pretreatment of rice straw. Appl. Biochem. Biotechnol. 162, 1768–1784 (2010)
Park, Y.C., Kim, J.S.: Comparison of various alkaline pretreatment methods of Lignocellulosic biomass. Energy. 47, 31–35 (2012)
Wang, Z., Keshwani, D.R., Redding, A.P., Cheng, J.J.: Sodium hydroxide pretreatment and enzymatic hydrolysis of coastal Bermuda grass. Biores. Technol. 101, 3583–3585 (2010)
Kataria, R., Ghosh, S.: NaOH pretreatment and enzymatic hydrolysis of Saccharum spontaneum for reducing sugars production. Energy Sources A 36, 1028–1035 (2014)
Li, M., Si, S., Hao, B., Zha, Y., Wan, C., Hong, S., Kang, Y., Jia, J., Zhang, J., Li, M., Zhao, C., Tu, Y., Zhou, S., Peng, L.: Mild alkali-pretreatment effectively extracts guaiacyl-rich lignin for high lignocellulose digestibility coupled with largely diminishing yeast fermentation inhibitors in Miscanthus. Biores. Technol. 169, 447–454 (2014)
Jaisamut, K., Paulova, L., Patakova, P., Rychtera, M., Melzoch, K.: Optimization of alkali pretreatment of wheat straw to be used as substrate for biofuels production. Plant Soil Environ. 59, 537–542 (2013)
Naseeruddin, S., Yadav, K.S., Sateesh, L., Manikyam, A., Desai, S., Venkateswar, R.L.: Selection of the best chemical pretreatment of lignocellulosic substrates Prosopis juliflora. Biores. Technol. 136, 542–549 (2013)
Lee, J.W., Kim, J.Y., Jang, H.M., Lee, M.W., Park, J.M.: Sequence dilute acid and alkali pretreatment of corn stover: Sugar recovery efficiency and structural characterization. Biores. Technol. 182, 296–301 (2015)
Taherzadeh, M.J., Niklasson, C.: Ethanol from lignocellulosic materials: pretreatment, acid and enzymatic hydrolyses, and fermentation. In ACS symposium series, Oxford University Press, Oxford (2004)
Arora, A., Priya, T., Sharma, P., Sharma, S., Nain, L.: Evaluating biological pretreatment as a feasible methodology for ethanol production from paddy straw. Biocatal. Agric. Biotechnol. 8, 66–72 (2016)
Jung, Y.H., Park, H.M., Kim, D.H., Park, Y.C., Seo, J.H., Kim, K.H.: Combination of high solids loading pretreatment and ethanol fermentation of whole slurry of pretreated rice straw to obtain high ethanol titers and yields. Biores. Technol. 198, 861–866 (2015)
Saritha, M., Rajan, K., Carrier, D.J., Nain, L., Arora, A.: Insights into biological delignification of rice straw by Trametes hirsuta and Myrothecium roridum and comparison of saccharification yields with dilute acid pretreatment. Biomass Bioenerg. 76, 54–60 (2015)
Agudelo, R.A., García-Aparicio, M.P., Görgens, J.F.: Steam explosion pretreatment of triticale (× Triticosecale wittmack) straw for sugar production. New Biotechnol. 33(1), 153–163 (2016)
Choudhary, J., Saritha, M., Nain, L., Arora, A.: Enhanced saccharification of steam-pretreated rice straw by commercial cellulases supplemented with xylanase. J. Bioprocess Biotech. 4(7), 188–194 (2014)
Sindhu, R., Kuttiraja, M., Prabisha, T.P., Binod, P., Sukumaran, R.K., Pandey, A.: Development of a combined pretreatment and hydrolysis strategy of rice straw for the production of bioethanol and biopolymer. Biores. Technol. 215, 110–116 (2016)
Hashem, M., Ali, E.H., Abdel-Basset, R.: Recycling rice straw into biofuel. J. Agric. Sci. Technol. 15(4), 709–721 (2013)
Ghosh, P., Ghose, T.K.: Bioethanol in India: Recent past and emerging future. Adv. Biochem. Eng. Biotechnol. 85, 1–27 (2003)
Ghose, T.K.: Measurements of cellulase activities. Pure Appl. Chem. 59, 257–268 (1987)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Updegraff, D.M.: Semimicro determination of cellulose in biological materials. Annal Biochem. 32, 420–424 (1969)
Templeton, D., Ehrman, T.: Determination of acid-insoluble lignin in biomass. Laboratory Analytical Procedure. NREL 3 (1995)
Saritha, M., Arora, A., Nain, L.: Pretreatment of paddy straw with Trametes hirsuta for improved enzymatic saccharification. Biores. Technol. 104, 459–465 (2012)
Wang, R., Unrean, P., Franzen, C.J.: Model-based optimization and scale-up of multi-feed simultaneous saccharification and co-fermentation of steam pre-treated lignocellulose enables high gravity ethanol production. Biotechnol. Biofuels 9, 88–100 (2016)
Sun, Y., Cheng, J.: Hydrolysis of lignocellulosic materials for ethanol production: a review. Biores. Technol. 83, 1–11 (2002)
Saritha, M., Arora, A., Singh, S., Nain, L.: Streptomyces griseorubens mediated delignification of paddy straw for improved enzymatic saccharification yields. Biores. Technol. 135, 12–17 (2013)
Yang, X.X., He, C., Liu, J., Diao, H.: Influence on the physical properties of wheat straw via hydrothermal and chemical treatments. BioRes 11(3), 7345–7354 (2016)
Dai, Y., Si, M., Chen, Y., Zhang, N., Zhou, M., Liao, Q., Shi, D., Liu, Y.: Combination of biological pretreatment with NaOH/Urea pretreatment at cold temperature to enhance enzymatic hydrolysis of rice straw. Biores. Technol. 198, 725–731 (2015)
Arora, A., Nain, L., Gupta, J.: Solid state fermentation of wood residues by Streptomyces griseus B1, a soil isolate and solubilization of lignins. World J. Microbiol. Biotechnol. 23, 303–308 (2005)
Kurakake, M., Hirotsu, S., Shibata, M., Kubota, A., Makino, A.: Lignin antioxidants extracted from lignocellulosic biomasses by treatment with ammonia water. Ind. Crops Prod. 77, 1028–1032 (2015)
Holladay, J.E., Bozell, J.J., White, J.F., Johnson, D.: Top value-added chemicals from biomass Volume II- Results of screening for potential candidates from biorefinery lignin II. PNNL report no. PNNL-16983 (DOE contract no. DE-AC05-76RL01830). Pacific Northwest National Laboratory, Richland, WA (2007)
Wi, S.G., Choi, I.S., Kim, K.H., Kim, H.M., Bae, H.J.: Bioethanol production from rice straw by popping pretreatment. Biotechnol. Biofuels 6(1), 166–172 (2013)
Abedinifar, S., Karimi, K., Khanahmadi, M., Taherzadeh, M.J.: Ethanol production by Mucor indicus and Rhizopus oryzae from rice straw by separate hydrolysis and fermentation. Biomass Bioenerg. 33, 828–833 (2009)
Belal, E.B.: Bioethanol production from rice straw residues. Braz. J. Microbiol. 44(1), 225–234 (2013)
Xue, C., Zhang, X., Wang, J., Xiao, M., Chen, L., Bai, F.: The advanced strategy for enhancing biobutanol production and high-efficient product recovery with reduced wastewater generation. Biotechnol. Biofuels 10, 148 (2017)
Kannan, E.: Straws in the wind. The Hindu, 10 Nov 2016
Acknowledgements
This work was supported by the Application of Microorganisms in Agriculture and Allied Sectors (AMAAS), India, (Grant No. 12-124). Authors are thankful to Director, IARI for providing infrastructural facilities for carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, S., Nandal, P. & Arora, A. Ethanol Production from NaOH Pretreated Rice Straw: a Cost Effective Option to Manage Rice Crop Residue. Waste Biomass Valor 10, 3427–3434 (2019). https://doi.org/10.1007/s12649-018-0360-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0360-4