Abstract
Rice straw was sequentially pre-treated with sulphuric acid (3% v/v) and sodium hydroxide (4% w/v) which resulted in the removal of ~ 90% hemicelluloses and ~ 55% lignin, respectively. The pre-treated rice straw was saccharified with cellulases to produce 787 mg/g reducing sugars, which were further fermented with Saccharomyces cerevisae HAU. The acid hydrolysate produced after acid treatment was also subjected to fermentation by Pichia stipitis NCIM 3499. The hexose and pentose fermentation leads to production of 26.9 g/L and 9.4 g/L of ethanol (79% and 93% fermentation efficiency, respectively). High purity (98%) lignin (146 mg/g dry substrate) was precipitated by direct acidification with HCl from the spent liquor obtained after alkali treatment. Extracted lignin was characterized by FTIR and TGA. Overall, the study aimed at effective utilization of all major polymers of rice straw into valuable products making the bioethanol production process more sustainable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The interest in development of alternative fuels has been revitalized due to rapid decline in fossil fuel reserves. The world’s present scenario of growth in population and consumption of energy is not far short of reaching the limit of earth’s capacity. Hence, it is high time to explore new and sustainable ways of generating chemicals, energy as well as food. Biofuel production is one such promising solution but production of first-generation biofuels has evoked competition between food security and energy requirements. In order to strike a balance between food and energy, second- and third-generation biofuels are possible solutions. Bioethanol has emerged as a dominant biofuel in the twentieth century and its global production from biomass is expected to reach 125 × 109 L by 2020 as many countries seek to reduce oil imports [1]. The production of ethanol from lignocellulosic biomass i.e. second-generation bioethanol, is currently being studied worldwide [2]. Second-generation bioethanol production from lignocellulose, considering all its individual components viz. cellulose, hemicellulose and lignin, opens up a whole pandora of promising applications in bio-refinery. Cellulose and hemicellulose are the major sources of hexose and pentose sugars, respectively, which can be fermented to produce a variety of biofuels like bioethanol, biobutanol or can be processed into polymeric matrix nanocomposites [3]. To convert the polymeric constituents of lignocellulosic biomass into simple sugars, it is necessary to deconstruct the structure, via pre-treatment [4]. Among physical and chemical pre-treatments necessary to remove the barriers and make cellulose more accessible to enzymes, dilute acid and alkali treatment are the most practiced strategies [2, 5,6,7]. Lignin on the other hand is a complex 3D polyaromatic matrix that prevents enzymes from accessing regions of cellulose [8]. Lignin has emerged as a major by-product of bioethanol production and is usually dumped in water bodies along with other waste products of the process. Various lignin-derived products in this waste are toxic, hence this practice is hazardous to the environment. However, this problem can be solved if the lignin from this waste could be harnessed. Moreover, lignin is a potential source of various valuable products such as fuel, phenolic resins, substitute for phenol formaldehyde, emulsifier, dispersant or chelating agents and as fat mimetics in emulsion-based foods [9,10,11,12].
Rice straw is one of the most abundantly available agricultural waste on earth and is rich in cellulose (28–36%), hemicellulose (23–28%) and lignin (15–23%) [13, 14]. Recovery of all these biopolymers in a single process will make the second-generation biofuel industry commercially viable and sustainable. Till date, all pre-treatment processes employed for second-generation biofuel production leaves at least one of the components wasted and untapped for its potential.
Dilute acid pre-treatment is known to extract hemicellulose fraction in the form of pentoses along with small amount lignin as well [4, 15] resulting in biomass rich in cellulose and lignin. The liquid fraction obtained after acid treatment is pentose-rich which can be fermented using pentose fermenting organisms like Pichia stipitis and Pichia pastoris, etc. [16, 17]. Of course, the furans, furfurals and phenolics produced during acid treatment have to be removed from the fraction before fermentation as they act as fermentation inhibitors. Various methods of detoxifications are documented in the literature [18, 19]. Delignification of the biomass left after acid treatment presents a logical next step. Alkali pre-treatment is the most widely used methods to enhance the digestibility of the lignocellulosic biomass because dilute alkali treatment leads to swelling of biomass and thus increases the internal surface area, decrease in crystallinity and disruption of lignin structure [7]. Compared to acid/oxidative reagents, alkali treatment works best to break the ester bonds between lignin, hemicellulose and cellulose [20]. Sodium hydroxide has been most widely used as the delignifying agent [7]. Delignification results in a biomass, which contains cellulose as a major component. The black liquor obtained after alkaline pre-treatment is similar to kraft lignin and can be selectively precipitated to obtain pure fractions of lignin. Lignin has found great number of applications such as a base for material application in the field of bioplastics, nano-composites and nano-particles, as adsorbents in solutions, protective UV-absorbents and dispersants [21,22,23]. The biomass left after alkali treatment is rich in cellulose which can be hydrolysed using cellulases to simpler sugars i.e. glucose, which can further be fermented to ethanol by hexose-fermenting organisms like Saccharomyces and Zymomonas mobilis etc. [24, 25].
Considering the far-fetched scenario of lignocellulosic bio-refinery’s self-sustaining status, the present study focuses on usage of rice straw as a raw material for bioethanol production and lignin recovery. In the present study, attempts were made to recover each fraction of lignocellulose by exploiting dilute acid (H2SO4) and dilute alkali (NaOH) pre-treatments sequentially. The recovery of all the biopolymers of lignocellulose in a single process will definitely make it possible to obtain full value of feedstock and help produce more than a single product in a bio-refinery–based approach and hence it is anticipated to be more sustainable.
Material and Methods
Raw Material
Rice straw was collected from local fruit vendors. It was milled and sieved to obtain particles of mesh size between 40 and 60. The sieved rice straw was washed thoroughly with distilled water and dried overnight at 60 °C before use. The chemical composition of rice straw (cellulose, hemicellulose, klason-lignin, ash and moisture) was estimated using standard TAPPI protocols (α-Cellulose–TAPPI Method T203 om–83; Klason lignin–TAPPI Method T222 om–83; Pentosans–TAPPI Method T223 hm–84; Moisture–TAPPI Method T208 om–84 and Ash–TAPPI method 211om–93).
Microorganisms
Pichia stipitis NCIM 3499 and Saccharomyces cerevisae HAU, were obtained from the culture collection of Lignocellulose Biotechnology Laboratory, University of Delhi South Campus, New Delhi. The yeasts were grown on plates containing (g/L): yeast extract, 3; malt extract, 3; peptone, 5; glucose/xylose, 20 and agar, 20, as described by Gupta and coworkers [4]. The cultures were sub-cultured every 15 days.
Pre-treatment
Dilute Acid Treatment
Dilute acid pre-treatment of ground rice straw was performed by adding sulphuric acid (1–6% v/v) to feedstock slurry at variable solid loadings (10–20% w/v). The slurry was then subjected to 121 °C/15 psi for varying time periods (15–60 min) [4]. After pre-treatment, liquid fraction was separated from the solid biomass using a double layered muslin cloth. The biomass was washed till neutral pH and the acid hydrolysate obtained after treatment was analysed for total reducing sugar, phenolics and furans. The acid hydrolysate was further detoxified and used as a medium for pentose fermentation.
Alkaline Treatment
The acid treated lignocellulosic biomass was subsequently treated with dilute NaOH solution in anticipation to remove the lignin fraction from the interwoven mesh. Mixture of acid-treated rice straw was further treated with aqueous NaOH (2–6% w/v) at 121 °C/15 psi for varying time periods (15–60 min) at different substrate consistencies (8–14% w/v) [26]. After the hydrolysis time, the biomass was washed with water till neutral pH and sieved through double layered muslin cloth. The sieved biomass was evaluated for holocellulose (cellulose and hemicellulose) and lignin content. Sequentially, pre-treated biomass was further used for enzymatic saccharification studies. The black liquor obtained as a by-product of alkali treatment was used to recover lignin.
X-ray Diffraction (XRD) Analysis
The cellulose crystallinity index (CI) of raw, acid-treated and alkali-treated rice straw was measured with XRD using Ultima IV X-ray Diffraction system (Rigaku Corporation, Japan). The dried samples were scanned in a 2θ range from 7 to 40° using step size of 0.02° and Cu/Kα radiation generated at 35 kV and 35 mA. The CI of the samples were calculated from the XRD spectra readings using Segal peak method with the formula:
Where I002 corresponds to peak height at 22.2–22.5° (2θ) and IAm corresponds to peak height of the amorphous cellulose at 18.4–18.8° (2θ).
Lignin Precipitation
Black spent liquor obtained after optimum dilute NaOH (4%) treatment (1 L) was taken in a 2-L glass beaker and conc. HCl was slowly added (under constant stirring) to bring its pH to 2 [27]. The precipitated lignin was separated by centrifugation at 8500 rpm for 20 min (Sigma table top Centrifuge 6 K15). The precipitates were washed thrice with hot water (60 °C) to remove salts. Finally, the precipitates were dried at 60 °C till constant weight.
Fourier Transform Infrared (FTIR) of Lignin Fractions
The Fourier Transform Infrared Spectroscopy (FTIR) of the lignin fractions was recorded with FTIR spectrometer (Perkin Elmer Spectrum I FTIR) by making potassium bromide pellets. The region between 4000 and 400 cm−1 with resolution of 4 cm−1 was recorded.
Thermogravimetric Analysis
The thermogravimetric analysis (LabSys EVO, SETARAM Instrumentation) was carried out under N2 atmosphere with a purge gas flow of 55 cm 3 min−1 from 30 to 500 °C with the heating rate of 10 °C min−1. 10-mg sample was used for the analysis of the thermogravimetric behaviour of the precipitated lignin.
Enzymatic Hydrolysis
The enzymatic hydrolysis of the pre-treated biomass was carried out using in-house SSF produced cellulase enzyme from Trichoderma sp. (FPU, 9 IU/mL; CMCase, 60 IU/mL; BGL, 20 IU/mL) [28]. Sequentially, pre-treated rice straw (2.0 g each) at 10% (w/v) substrate consistency in 50 mM citrate phosphate buffer (pH 5.0) was taken in 50-mL flasks and were pre-incubated at respective temperatures on a rotatory incubator shaker (New Brunswick Scientific, Germany) for 2 h [26]. The enzyme dose (10–35 FPU/g), hydrolysis time (0–24 h), temperature (30–60 °C), pH (4–7) and addition of surfactants (Tween 20, tween 40, tween 80, PEG) were varied one at a time to obtain maximum sugar release. The saccharification was continued till 24 h and the hydrolysate was analysed for total reducing sugar content. The methods of estimation of total reducing sugar estimation has been described in “Analytical Methods”. Saccharification yield was calculated according to the formula:
Where S is concentration of sugar in the hydrolysate (mg/mL), V is the total volume of hydrolysate obtained after reaction (mL) and W is the substrate weight (g).
Statistical Optimization of Enzymatic Saccharification by Response Surface Methodology (RSM)
The enzymatic hydrolysis conditions for pre-treated rice straw were further optimized using RSM of CCD following the OFAT. The levels of five independent variables FPase dosage, temperature, substrate consistency, incubation time and tween 80 concentration were optimized by the statistical software Design expert 9.0 (Stat-ease, USA). Each factor was studied at five different levels (Table 1). A set of 50 experiments was carried out. The variables were taken at a central coded value considered as zero. Upon completion of experiment, the average of reducing sugar concentration was taken as dependent variable response.
Ethanol Fermentation
The acid hydrolysate obtained after acid treatment of rice straw was detoxified with 2% w/v activated charcoal by stirring for 30 min. The pH of the hydrolysate was brought to 6.0 by adding NaOH solution (50% w/v) and supplemented with yeast extract (0.3% w/v), malt extract (0.2% w/v), peptone (0.2% w/v) and soybean meal (0.4% w/v) before its use of fermentation medium. The medium (50 mL) was suspended in 250-mL Erlenmeyer flask and inoculated with 10% v/v (OD600nm ˜ 0.5) secondary inoculum of P. stipitis NCIM 3499 (primary inoculum of 24 h) and were incubated at 30 °C/175 rpm for 72 h in a rotary shaker (Innova-40, New Brunswick Scientific, Germany) [4]. Samples were withdrawn after every 4 h and were analysed for ethanol and biomass content. The saccharified hydrolysate rich in hexoses was supplemented with yeast extract (0.3% w/v), malt extract (0.3% w/v), peptone (0.5% w/v) and soybean meal (0.4% w/v) and pH calibrated to five; 50 mL broth suspended in 250 -L Erlenmeyer flasks were inoculated with 10% v/v (OD600nm ˜ 0.5) secondary inoculum of S. cerevisae (primary inoculum of 24 h) and were incubated at 30 °C/150 rpm for 48 h in a rotary shaker (Innova-40, New Brunswick Scientific, Germany). Samples were withdrawn after every 4 h and assessed for ethanol and biomass content. The methods of estimation have been described in “Analytical Methods”. Ethanol yield was calculated by using the following formula:
Where E is the ethanol concentration obtained (g/L) and ME is the maximum theoretical ethanol concentration that can be produced (g/L).
Analytical Methods
The raw material was extracted with alcohol-benzene (1:2 v/v) and the chemical composition (cellulose, klason lignin, hemicellulose, moisture and ash content) of rice straw was determined following standard TAPPI protocols(α-Cellulose–TAPPI Method T203 om–83; Klason lignin–TAPPI Method T222 om–83; Pentosans–TAPPI Method T223 hm–84;Moisture–TAPPI Method T208 om–84 and Ash–TAPPI method 211om–93). The ethanol content in the fermented broth was determined by Gas Chromatography (Perkin–Elmer, Clarus 500) with an elite-wax (cross bond-polyethylene glycol) column (30.0 m × 0.25 mm) at an oven temperature of 90 °C and flame ionization detector (FID) at 200 °C. The ethanol standards were prepared using commercial grade ethanol (Merck, Darmstadt, Germany). Nitrogen with a flow rate of 0.5 mL/min was used as the carrier gas. Total reducing sugars were estimated by the DNS method of Miller (1959) [29]. Moreover, the total phenolics released were determined by the Folin–Ciocalteu reagent method [30] using vanillin as a standard. Total furans were estimated by the method of Martinez (2001) [31]. The OD600nm of culture filtrate was measured with a double beam spectrophotometer (Specord 200, Analytic Jena, Germany) and the dry biomass of yeast cells was measured after drying the pellets at 70 °C until constant weight. All the experiments were performed in triplicates and results mentioned are the mean values of the observations.
Results and Discussion
Dilute Acid Pre-treatment of Rice Straw
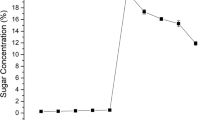
Chemical analysis of rice straw revealed that it consisted of 35.63% cellulose, 18.06% hemicellulose, 31.97% lignin, 10.24% ash and 4.40% moisture. Generally, acid treatment of lignocellulose results in almost complete solubilization of hemicellulose to its constituent monomeric sugars and partial digestion of cellulose and lignin [32]. Therefore, the total amount of sugar released can be related to effectiveness of acid pre-treatment. The acid pre-treatment was optimized to recover maximum sugars in the acid hydrolysate. Sugars, phenolics and furans were assessed and quantified in the acid hydrolysate. The optimum concentration of H2SO4 was determined by carrying out the treatment with 1, 2, 3, 4, 5 and 6% (v/v) H2SO4 at 121 °C for 30 min using 10% (w/v) solid loading (Fig. 1). The reducing sugar yield increased with increasing acid concentration from 1 to 3% with maximum sugar release (160 mg/g) at 3% acid concentration (Fig. 1). Further increase in the acid concentration lead to decline in sugar recovery and increase in furan concentration indicating conversion of sugars to furans and furfurals at high acid concentration. Pre-treatment time was optimized by performing the process for different time periods keeping the temperature, acid concentration and solid loading fixed at 121 °C, 3% v/v and 10% w/v, respectively. The results showed that maximum sugar (156 mg/g) could be recovered when the treatment was operated for 30 min. Further increase in the time period resulted in formation of more phenolics and furans (11.1 mg/g and 150 mg/L, respectively). In an attempt to check whether the treatment gives better results at high solid loadings, different consistencies of the biomass were also tested. It was found that increased solid loading did increase the concentration of sugar in the hydrolysate but total sugar yield kept decreasing. Thus, 10% (w/v) solid loading based on corresponding maximum sugar yield was considered optimum for further studies (Fig. 1). The process was able to separate out about 90% hemicellulose in the form of pentoses in the acid hydrolysate. Kim and Kim [33] were able to remove 87.9% hemicellulose content from empty palm fruit bunches with acid treatment, which is in agreement with results of this study. Hsu and coworkers [15] have reported the maximum sugar release of 144 mg/g of the rice straw and 2.1 g/L combined furfurals and HMFs at the severity factor of 1.7–2.0 [15]. Lee and co-workers [34] have reported 121.3 mg/g total sugar release from rice straw by treating it at 120 °C with 1% acid, which is lower than that obtained in the present studies. Jung and co-workers [32] treated oil palm empty fruit bunches (EMB) at 190 °C with 1% (w/v) sulphuric acid which resulted in sugar yield of 14.7 mg/g EFB.
Alkali Treatment of the Acid-Hydrolysed Rice Straw
The acid-treated rice straw was freed of lignin by treating it with dilute NaOH. Alkaline hydrolysis causes saponification of ester bonds cross-linking hemicelluloses and celluloses to lignin [8]. Different concentrations (2–6% w/v) of NaOH were tested to obtain maximum delignification from the biomass out of which 4% NaOH removed 45.5% lignin, leaving 79.2% holocellulose in the biomass after 15 min of treatment at 121 °C (Table 2). Since higher alkali concentration did not cause any improvement in the removal of lignin, 4% NaOH was considered optimum for further studies. Pre-treatment time of 30 min was observed as the optimum period for removal of 54.6% lignin (Table 3). Further, alkaline treatment at different biomass consistencies revealed that 8% solid loading resulted in maximum delignification (54.9%). Even though 8% substrate load had a slight better delignification, 10% was chosen optimum for further studies as increased solid load led to removal of equivalent amount of lignin (54.6%). Increasing the biomass load beyond 10% led to decrease in delignification (Table 4). Thus, treatment of biomass (10%) with 4% (w/v) NaOH for 30 min at 121 °C resulted in maximum delignification of acid-treated rice straw; NaOH treatment has been known to enhance enzymatic hydrolysis as it results in swelling of the substrate, thereby increasing the internal surface area [7, 35]. In present study, the final pre-treated biomass consisted of 83% holocellulose which was available for hydrolysis with cellulases. Lee and team [34] applied sequential acid and alkali treatment on corn stover and observed that there was increase in the glucan levels of up to 82% and lignin levels as low as 5.5%. They also reported better saccharification results when two pre-treatment methods were applied sequentially. Han et al. [8] reported 42% lignin removal from the alkali-treated wheat straw, coherently increasing the cellulose content by 45%, which is in agreement with our present findings. Kim and Kim [33] used very high concentration of NaOH (10 M) to treat empty palm fruit bunch fibre at room temperature for 4 h leading to 70% lignin removal. Findings of present study are at par with previous studies, with total 55% delignification at lower NaOH concentration (4% w/v) within 30 min.
XRD Analysis
Cellulose CI of raw, acid-treated and sequential alkali-treated rice straw was calculated from the XRD spectra (Fig.2). XRD spectra shows three sharp peaks corresponding to crystalline cellulose present in raw rice straw. The CI for raw rice straw was calculated to be 42% and was observed to be increased to 56% after acid-treatment. This observation is in accordance with the fact that acid-treatment removes amorphous hemicellulosic and available amorphous cellulosic regions which leads to increase in CI of the substrate. Further treatment with alkali lead to decrease in the CI to 40% supporting the fact that the alkali-treatment leads to decrease in CI of cellulose by removing the recalcitrant lignin and subsequently open up the structure of the substrate.
Lignin Precipitation from the Alkali Spent Liquor
The spent liquor obtained after NaOH pre-treatment (4% w/v) of the acid treated biomass was used to recover lignin by acid precipitation. The gradual acidification with concentrated HCl resulted in the precipitation of the lignin fraction. Maximum amount of lignin that could be precipitated by this method was 14.6 g (dry weight) which corresponds to 146 mg/g dry substrate. Alkali treatment leads to the removal of 168 mg/g lignin which deduces that the method was able to precipitate 84.5% lignin from spent black liquor. The purity of precipitated lignin was assessed by Klason lignin–TAPPI Method T222 om–83 and was found to be 97.87% w/w of precipitated fraction of lignin. The acidification to pH 2 also resulted in precipitation of salts which could be removed by washing the lignin fraction with hot water (60 °C). The colour of the spent liquor was drastically reduced from dark black to pale yellow during the acidification step. The dark colour of the liquor is derived from the chromophoric functional groups, including quinones, carbonyl group containing compounds, carboxylic acids, hydroperoxy radicals and the phenolic hydroxyl groups generated during the lignin degradation that are soluble in alkaline medium. Lowering the pH also removes the lignin-derived compounds that confer dark colour to the spent liquor [27]. There have been some studies on the precipitation of lignin by acidification from the spent liquor of kraft pulping and breweries. Mussatto and coworkers [27] were able to precipitate out 81.43% of lignin solubilized in the brewer’s spent grain black liquor by acidification to pH 2.15, which articulate well with observations of this study. A similar observation was made by Santos and Curvelo [36], who also observed that decreasing the pH of black liquor of Eucalyptus urograndis wood from 13.6 to 3.0 resulted in precipitation of 87.8% of the lignin content.
FTIR Analysis of Extracted Lignin
Fourier transform infrared spectroscopy is a very sensitive and accurate technique to study physicochemical and conformational properties of lignin because various functional groups and structural fragments can be characterized by this method. The spectra (Fig.3) exhibited the fingerprint regions of true lignin i.e. peaks at 1462 and 1422 cm−1 corresponding to vibrations of aromatic methylene and methyl groups [37]; at 1360 cm−1 reflecting methyl groups owing to C-H stretching and at 1330 cm−1 corresponding to syringyl ring breathing with C-O stretching. The fingerprint region confirmed the purity of the extracted lignin. Peaks around 3432, 2934 and 2840 cm−1 present in extracted lignin reflect hydroxyl, methylene, methyl and aromatic methoxy groups in the structure. A major characteristic of lignin is the presence of a strong band at 1125 cm−1 for syringyl structures, whereas aromatic C-H in plane deformation in guiacyl type is observed at 1033 cm−1 [38]. Also, the peaks at 1030 and 1125 cm−1 correspond to C-H plane deformation in guiacyl and syringyl units, respectively.
Thermogravimetric Analysis (TGA) of Extracted Lignin
Figure 4 shows the TGA curve of the extracted lignin. Thermal degradation of the lignin sample proceeded over a range of 250–500 °C. Only 1% mass loss occurred when the temperature rose from 30 to 100 °C. After 100 °C, the degradation process is slower between 100 and 240 °C, which is depicted by appearance of a plateau on the curve. The main degradation process for the lignin sample started after 240 °C and sample mass decreased to 77% at 500 °C, proving the lignin sample to be highly thermostable. Our study is in well accordance with previous reports of thermal behaviours of the lignins showing 65–70% residual mass after 500 °C (Poletto 2017).
Saccharification of the Sequentially Pre-treated Rice Straw
Figure 5 shows the sugar yields during enzymatic hydrolysis of sequentially pre-treated rice straw. Various factors like temperature, time, pH and addition of surfactants were studied to maximize the sugar yields after hydrolysis. Different doses of cellulase (10–35 FPU/g substrate) were added to the biomass slurry and incubated for varying time periods to obtain the optimum enzyme dosage and the hydrolysis time. Rice straw hydrolysis resulted in sugar yield of 640 mg/g dry substrate with 30 FPU/g (Fig. 5) enzyme dosage after 20 h of incubation. The optimum temperature and pH for achieving maximum saccharification were found to be 50 °C and 5, respectively (Fig. 6). The effect of different surfactants was also studied on the hydrolysis of the pre-treated rice straw. It was observed that addition of 0.2% (v/v) tween 80 increased the hydrolysis yield from 637 mg/g to 672 mg/g dry substrate. Addition of non-ionic surfactants have been known to increase the saccharification efficiency of the cellulases as they prevent the non-specific binding of cellulase to exposed lignin surfaces on the substrate [39, 40]. Optimization of saccharification resulted in 81% hydrolysis of the total holocellulose (by weight) of the pre-treated rice straw. Kim and co-workers [41] observed almost complete hydrolysis of acid alkali treated empty palm fruit bunch fibres, however, the total reducing sugar yield was only 35 g/L. Kim and Kim [33] worked on empty palm fruit bunch fibres, which after being sequentially treated with acid and alkali resulted in 84% digestibility with enzyme loading of 50 FPU/g dry biomass in 48 h. Jung et al. [32] worked upon empty fruit bunches resulting in 88% total saccharification. Lee et al. [34] reported better hydrolysis of corn stover when treated sequentially with acid and alkali in comparison to only acid treatment. In another work, similarly, only alkali treatment of the substrate has been reported to yield lower sugar release. Han and coworkers [8] could only recover 61% of the total sugars from the NaOH treated wheat straw. Results of these previous studies indicate that the dual phase pre-treatment produces better results as has been observed during the present investigation.
Optimization of Saccharification by Response Surface Methodology (RSM)
The design of experiments (DOE) for the central composite design (CCD) of RSM along with the mean observed responses is presented in Table 5. The regression equation obtained after the ANOVA gave the level of sugar yield (mg/g) as a function of the initial values of FPase dosage (A), temperature (B), substrate consistency (C), incubation time (D) and Tween 80 concentration (E). The remaining conditions remained the same as found optimum during the optimization using OFAT approach. The response equation that represented a suitable model for sugar released from pre-treated rice straw during enzymatic hydrolysis is as follows:
where, A is FPase dosage, B is temperature, C is solid loading, D is incubation time and E is Tween 80 concentration.
Quadratic model was found to be the best fit model. The regression equation obtained after ANOVA indicated the coefficient of determination (R2) value of 0.9269 (closer the value of R2 to 1.0, the better the model is assumed) for reducing sugar yield and thus the model could explain 92.69% of the variability in the responses. The adjusted R-squared was in reasonable agreement with the predicted R-squared. The computed F-value of 21.23, which is the ratio of mean square due to error and indicates the influence of each controlled factor on the tested model, was significant at high confidence level (Table 6). The probability p value (< 0.0001) was low indicating that model terms are significant. The high adequate precision value 16.684 suggests that the model to be fit as the value more than four corresponds to fitness of the model. The three-dimensional response surfaces were plotted to study the interaction among the various factors selected. Interaction of substrate consistency with temperature revealed that at 55 °C, maximum hydrolysis occurred with 8% consistency (Fig. 7A). Tween 80-temperature interaction (Fig. 7B) and incubation time-substrate consistency interaction (Fig. 7C) inferred the same optimal conditions for hydrolysis. The conditions predicted by the model were validated and the optimum conditions obtained for the enzymatic hydrolysis of pre-treated substrate was 35 IU/g FPase, 55 °C, 8% consistency at 0.5% tween 80 after 24 h of incubation which brought out maximum sugar yield of 787 mg/g.
Fermentation of the Acid Hydrolysate and Saccharified Hydrolysate via SHF
Several studies have shown that separate hexose and pentose fermentation works better than the co-culture method (4). The detoxified acid hydrolysate containing 20 g/L sugars (mainly xylose and arabinose) was studied for the production of ethanol with P. stipitis NCIM 3499. Maximum ethanol produced was 9.36 g/L with a yield of 0.468 g/g (Fig. 8). After 56 h of fermentation, cell biomass was 5.09 g/L. Increasing the incubation time beyond 56 h leads to decrease in biomass production as the sugar was exhausted and cells began to die off. Ethanol yield in the present study is better than the studies of Gupta et al. [42] who worked on the fermentation of corn cob acid hydrolysate with P. stipitis NCIM 3499 leading to production of 11.84 g/L ethanol corresponding to 0.31 g/g yield. Alrikkson and coworkers [43] fermented the dithionite detoxified acid hydrolysates of sugarcane bagasse and obtained 18 g/L ethanol. Da Silva et al. [44] fermented hemicellulosic sugarcane bagasse hydrolysate with Scheffersomyces stipitis and achieved maximum of 15 g/L ethanol corresponding to 0.37 g/g ethanol yield which is also lower than that obtained in the present study.
Hexose sugar rich slurry (68 g/L) produced after OFAT optimized saccharification of the pre-treated rice straw was fermented by S. cerevisae HAU for ethanol production. Fermentation produced 26.87 g/L of ethanol with a yield of 0.40 g/g and 79% fermentation efficiency (Fig. 9). Biomass production after 36 h of incubation was found to be 8.67 g/L. After 36 h of incubation, biomass production stopped and decreased further. Lee and co-workers [34] studied simultaneous saccharification and fermentation of dilute acid pre-treated rice straw and reported the formation of 9 g/L ethanol after 5 days of fermentation with a recombinant strain of E. coli K011 as compared to only 6 g/L with S. cerevisae D5A which is quite lower than that obtained in the present studies. Results of this study are in agreement with Raghavi and coworkers [45] who applied the sequential acid/alkali pre-treatment of sugarcane trash and were able to obtain high sugar levels and 32 g/L ethanol with 79% fermentation efficiency. Devendra and Pandey [46] worked with rice straw hydrolysate but were able to achieve only 73.5% fermentation efficiency. Koradiya et al. [47] worked on cofermentation of pentoses and hexoses from Sorghum pioneer with a co-culture of S. cerevisae and laboratory culture N achieving a maximum ethanol yield of 0.39 g/g sugar. These findings support the assumption that separate hexose and pentose fermentation present better results as obtained by us in the present study.
Conclusion
An attempt to develop a bioprocess which exploits all the biopolymers of lignocellulosic rice straw has been made. Lignocellulosic biomass was first pre-treated with acid and then with alkali to specifically target the removal of hemicellulose and lignin, respectively. Acid treatment leads to removal of 90% hemicellulose from the biomass and the obtained acid hydrolysate has monomeric sugar concentration of 20 g/L. Alkali treatment leads to removal of 55% lignin and the resultant biomass had holocellulose content of 830 mg/g. The saccharification of pretreated biomass using cellulases produced 787 mg/g reducing sugars. The hexose and pentose fermentation corresponded to yield of 0.40 and 0.47 g/g, respectively. The alkali treatment followed by acid pre-treatment leads to removal of 146 mg/g of high-purity (98%) lignin corresponding to 85% lignin recovery from the black-spent liquor. The present study focused on the potential of rice straw as a source of multiple valuable products. By making use of all the components of rice straw in a bio-refinery based approach, thereby, making the process sustainable and environment friendly.
References
Pejin JD, Mojovic LV, Pejin DJ, Kocic-Tanackov SDC, Nikolic SB, Djukic-Vukovic AP (2015) Bioethanol production from Triticale by simultaneous saccharification and fermentation with magnesium or calcium ions addition. Fuel 142:58–64
Panagiotopoulos IA, Bakker RR, Vrije TD, Claassen PAM, Koukios EG (2012) Integration of first and second generation biofuels: fermentative hydrogen production from wheat grain and straw. Bioresour Technol 128:345–350
Cotana F, Cavalaglio G, Nicolini A, Gelosia M, Coccia V, Petrozzi A, Brinchi L (2014) Lignin as co-product of second generation bioethanol production from ligno-cellulosic biomass. Energy Procedia 45:52–60
Gupta R, Sharma KK, Kuhad RC (2009) Separate hydrolysis and fermentation of Prosopisjuliflora, a woody substrate, for production of cellulosic ethanol by Saccharomyces cerevisae and Pichiastipitis-NCIM 3498. Bioresour Technol 100:1214–1220
Sambusiti C, Ficara E, Malpei F, Steyer JP, Carrere H (2013) Effect of sodium hydroxide pretreatment on physical, chemical characteristics and methane production of five varieties of sorghum. Energy 55:449–456
Xin D, Yang Z, Liu F, Xu X, Zhang J (2015) Comparison of aqueous ammonia and dilute acid pretreatment of bamboo fractions: structure properties and enzymatic hydrolysis. Bioresour Technol 175:529–536
Singh R, Srivastava M, Shukla A (2015) Environmental sustainability of bioethanol production from rice straw in India: a review. Renew Sust Energ Rev 54:202–216
Han KJ, Pitman WD, Kim M, Day DF, Alison MW, McCormick ME, Aita G (2012) Ethanol production potential of sweet sorghum assessed using forage fiber analysis procedures. GCB Bioenergy 5:358–366
Boeriu CG, Bravo D, Gosselink RJA, van Dam JEG (2004) Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind Crop Prod 20(2):205–218
Park Y, Doherty WOS, Halley PJ (2008) Developing lignin based resin coatings and composites. Ind Crop Prod 27(2):163–167
Toledano A, Serrano L, Labidi J (2012) Process of olive tree pruning lignin revalorization. Chem Eng J 193(3):396–403
Stewart H, Golding M, Merino LM, Archer R, Davies C (2014) Manufacture of lignin microparticles by anti-solvent precipitation: effect of preparation temperature and presence of sodium dodecyl sulfate. Food Res Int 66:93–99
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6:4497–4559
Wiselogel A, Tyson S and Johnson D (1996) Biomass feedstock resources and composition. In: Wyman CE (ed) Handbook on bioethanol: production and utilization (applied energy technology series), pp 105–118
Hsu TC, Guo GL, Chen WH, Hwang WS (2010) Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour Technol 101:4907–4913
Silva JPA, Mussatto SI, Roberto IC, Teixeira JA (2011) Ethanol production from xylose by Pichiastipitis NRRL Y-7124 in a stirred tank bioreactor. Braz J Chem Eng 28(1):151–156
Yucel HG, Aksu Z (2015) Ethanol fermentation characteristics of Pichiastipitis yeast from sugar beet pulp hydrolysate: use of new detoxification methods. Fuel 158:793–799
Mateo S, Roberto IC, Sanchez S, Moya AJ (2013) Detoxification of hemicellulose hydrolysate from olive tree pruning residue. Ind Crop Prod 49:196–203
Soudham VP, Brandberg T, Mikkola JP, Larsson C (2014) Detoxification of acid pretreated spruce hydrolysates with ferrous sulfate and hydrogen peroxide improves enzymatic hydrolysis and fermentation. Bioresour Technol 166:559–565
Binod P, Sindhu R, Singhania RR, Vikram S, Devi L, Nagalakshmi S, Kurien N, Sukumaran RK, Pandey A (2010) Bioethanol production from rice straw: an overview. Bioresour Technol 101:4767–4774
Chung BY, Lee JT, Bai HW, Kim UJ, Bae HJ, Wi SG, Cho JY (2013) Enhanced enzymatic hydrolysis of poplar bark by combined use of gamma ray and dilute acid for bioethanol production. Radiat Phys Chem 81(8):1003–1007
Xue BL, Wen JL, Sun RC (2014) Lignin based rigid polyurethane foam reinforced with pulp fiber: synthesis and characterization. ACS Sustain Cheml Eng 1:1474–1480
Tortora M, Cavalieri F, Mosesso P, Ciaffardini F, Melone F, Crestini C (2014) Ultrasound driven assembly of lignin into microparticles for storage and delivery of hydrophobic molecules. Biomacromolecules 15:1634–1643
Lau MW, Gunawan C, Balan V, Dale BE (2010) Comparing the fermentation performance of Escherichia coli K011, Saccharomyces cerevisae 424A (LNH-ST) and Zymomonasmobilis AX101 for cellulosic ethanol production. Biotechnol Biofuels 3:11
Tan IS, Lee KT (2014) Enzymatic hydrolysis and fermentation of seaweed solid wastes for bioethanol production: an optimization study. Energy 78:53–62
Mkhize T, Mthembu LD, Gupta R, Kaur A, Kuhad RC, Reddy P, Deenadayalu N (2016) Enzymatic saccharification of aci/alkali pre-treated, mill-run and depithed sugarcane bagasse. Bioresources 11(3):6267–6285
Mussatto SI, Fernandes M, Roberto IC (2007) Lignin recovery from brewer’s spent grain black liquor. Carbohydr Polym 70:218–223
Chakraborty S, Gupta R, Jain KK, Kuhad RC (2016) Cost-effective production of cellulose hydrolysing enzymes from Trichodermasp RCK65 under SSF and its evaluation in saccharification of cellulosic substrates. Bioprocess Biosyst Eng 39(11):1659–1670
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178
Martinez A, Rodriguez ME, York SW, Preston JF, Ingram LO (2001) Use of UV absorbance to monitor furans in dilute acid hydrolysates of biomass. Biotechnol Prog 16:637–641
Jung YH, Kim IJ, Kim HK, Kim KH (2013) Dilute acid pretreatment of lignocellulose for whole slurry ethanol fermentation. Bioresour Technol 132:109–114
Kim S, Kim CH (2013) Bioethanol production using sequential acid−/alkali pretreated empty palm fruit bunch fiber. Renew Energy 54:150–155
Lee JW, Kim JY, Jang HM, Lee MW, Park JM (2015) Sequential dilute acid and alkali pretreatment of corn stover: sugar recovery efficiency and structural characterization. Bioresour Technol 182:296–301
Ibrahim MM, El-Zawawy WK, Abdel-Fattah YR, Soliman NA, Agblevor FA (2011) Comparison of alkaline pulping with steam explosion for glucose production from rice straw. Carbohydr Polym 83:720–726
Santos F and Curvelo AAS (2001) Recovery of lignins from kraft liquors. In: Proceedings of the 6th Brazilian symposium on the chemistry of lignins and other wood components, pp 310–314
Sun R, Tomkinson J (2002) Comparative study of lignins isolated by alkali and ultrasound-assisted alkali extractions from wheat straw. Ultrason Sonochem 9(2):85–93
Jahan MS, Chowdhury DAN, Islam MK, Moeiz SMI (2007) Characterization of lignin isolated from some nonwood available in Bangladesh. Bioresour Technol 98(2):465–469
Eriksson T, Borjesson J, Tjerneld F (2002) Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzym Microb Technol 31:353–364
Tu M, Saddler JN (2010) Potential enzyme cost reduction with the addition of surfactant during the hydrolysis of pretreated softwood. Appl Biochem Biotechnol 161:274–287
Kim S, Park JM, Seo JW, Kim CH (2012) Sequential acid-/alkali pretreatment of empty palm fruit bunch fiber. Bioresour Technol 109:229–233
Gupta R, Mehta G, Kuhad RC (2012) Fermentation of pentose and hexose sugars from corncob, a low cost feedstock into ethanol. Biomass Bioenergy 47:334–341
Alrikkson B, Cavka A, Jonsson LJ (2011) Improving the fermentability of enzymatic hydrolysates of lignocellulose through chemical in-situ detoxification with reducing agents. Bioresour Technol 102:1254–1263
DaSilva ASA, Inoue H, Endo T, Yano S, Bon EPS (2010) Milling pretreatment of sugarcane bagasse and straw for enzymatic hydrolysis and ethanol fermentation. Bioresour Technol 101(19):7402–7409
Raghavi S, Sindhu R, Binod P, Gnansounou E, Pandey A (2016) Development of novel sequential pretreatment strategy for the production of bioethanol from sugarcane trash. Bioresour Technol 199:202–210
DevendraLP and Pandey A (2016) Hydrotropic pretreatment on rice straw for bioethanol production. Renew Energy 98:2–8
Koradiya M, Duggirala S, Tipre D, Dave S (2016) Pretreatment optimization of Sorghum pioneer biomass for bioethanol production and its scale up. Bioresour Technol 199:142–147
Funding
This work was supported by Ministry of new and renewable energy (MNRE), India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, A., Kuhad, R.C. Valorization of Rice Straw for Ethanol Production and Lignin Recovery Using Combined Acid-Alkali Pre-treatment. Bioenerg. Res. 12, 570–582 (2019). https://doi.org/10.1007/s12155-019-09988-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-019-09988-3