Abstract
An abundant agricultural residue, rice straw (RS) was pretreated using ammonia fiber expansion (AFEX) process with less than 3% sugar loss. Along with commercial cellulase (Spezyme® CP) at 15 filter paper unit/g of glucan, the addition of Multifect® Xylanase at 2.67 mg protein/g glucan and Multifect® Pectinase at 3.65 mg protein/g glucan was optimized to greatly increase sugar conversion of AFEX-treated RS. During enzymatic hydrolysis even at 6% glucan loading (equivalent to 17.8% solid loading), about 80.6% of glucan and 89.6% of xylan conversions (including monomeric and oligomeric sugars) were achieved. However, oligomeric glucose and xylose accounted for 12.3% of the total glucose and 37.0% of the total xylose, respectively. Comparison among the three ethanologenic strains revealed Saccharomyces cerevisiae 424A(LNH-ST) to be a promising candidate for RS hydrolysate with maximum ethanol metabolic yield of 95.3% and ethanol volumetric productivity of 0.26 g/L/h. The final concentration of ethanol at 37.0 g/L was obtained by S. cerevisiae 424A(LNH-ST) even with low cell density inoculum. A biorefinery combining AFEX pretreatment with S. cerevisiae 424A(LNH-ST) in separate hydrolysis and fermentation could achieve 175.6 g EtOH/kg untreated rice straw at low initial cell density (0.28 g dw/L) without washing pretreated biomass, detoxification, or nutrient supplementation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, ethanol is widely considered to be one of the most important alternatives to petroleum. Lignocellulosic feedstocks, due to their abundance and low cost, have become attractive raw materials for ethanol production compared to starch- and sucrose-based materials. Fuels derived from lignocellulosic biomass also hold the potential for clean and renewable transportation energy (Bothast and Schlicher 2005; Farrell et al. 2006; Lin and Tanaka 2006).
Ammonia fiber expansion (AFEX) offers an effective pretreatment technology for recalcitrant lignocellulosic materials (Belkacemi et al. 1998; Teymouri et al. 2006). This physiochemical method has been shown to improve substrate digestibility several fold through the decrystallization of cellulose, the partial depolymerization of hemicellulose, and the cleavage of lignin carbohydrate complex linkages (Balan et al. 2008). Furthermore, the AFEX-treated biomass was shown to produce less inhibitors than dilute acid pretreatment (manuscript in preparation). Enzymatic hydrolysate from AFEX-treated corn stover exhibited high fermentability without any costly processes like washing the pretreated biomass, detoxification, and nutrient supplementation (Lau et al. 2008; Lau and Dale 2009).
From an economic feasibility standpoint, a high solid loading with satisfactory conversion yields (to fermentable sugars) is required to reduce the cost of distillation during production. High solid loading (above 30%) has already been widely used in the starch-based ethanol industry (Bayrock and Ingledew 2001). The Department of Energy in the USA has launched several cellulosic ethanol projects, suggesting that a final ethanol concentration above 40 g/L is economically desirable (Fan et al. 2003; Wingren et al. 2003). Economic evaluation revealed that the energy expenditure was reduced by about two-thirds if the initial ethanol concentration was increased from 1% to 5% in a single distillation unit for a final concentration of 94.5% (w/w; Zacchi and Axelsson 1989). However, due to enzyme inhibition by sugars and degradation products (generated during pretreatment), conversion of biomass to fermentable sugars usually decreases with an increase in solid loading (Byung-Hwan and Hanly 2008; Lübbert and Jøgensen 2001). It has been reported that the cellulose conversion is reduced to approximately 33.3% at 40% (w/w) solid loading when compared to 3% (w/w) solid loading. Both cellulose and hemicellulose conversion decreased approximately linearly with the increase in solid loading (Jørgensen et al. 2007). Furthermore, fermentation of hydrolysate at high solid loading may also be hampered by high viscosity and accumulation of inhibitors like furfural, acetic acid, phenolic lignin degradation products, etc. (Lu et al. 2008; Jørgensen et al. 2007).
Traditionally, rice straw (RS) was incinerated to produce fertilizer minerals in agricultural fields after harvest resulting in a large amount of environmental pollution. To overcome this problem, some states (such as California, USA) have implemented state laws to restrict the burning of RS (Forrest et al. 1997). With nearly 800 million dry tons produced worldwide annually, RS holds great potential for the cellulosic ethanol market.
Generally, cellulosic ethanol production requires the following three major processes: pretreatment, saccharification, and fermentation. RS was more recalcitrant than other agricultural residues such as corn stover (Balan et al. 2008). Therefore, a mushroom spent straw (MSS)/AFEX technology integrating a biological pretreatment by white rot fungi followed by AFEX pretreatment was used in that work. Treating RS with a white rot fungus (Pleurotus ostreeatus) benefits enzymatic hydrolysis by selectively degrading recalcitrant lignin, while the fungus utilizes free sugars and cohydrolyzed oligosaccharides for growth of their fruiting bodies (Balan et al. 2008; Cohen et al. 2002). However, 23.2% of glucan and 20.1% of xylan were lost during MSS pretreatment. As shown in that work (Balan et al. 2008), they investigated the effects of various AFEX pretreatment conditions on the conversion of RS by glucan and xylan. Through the optimization of AFEX conditions, glucan conversion was increased to 92% after MSS/AFEX at 15 FPU (filter paper unit)/g glucan loading. However, only 55% of xylan conversion was observed. In this paper, we have investigated to improve the xylan conversion for AFEX-treated RS, by appropriate addition of Multifect® xylanase and Multifect® pectinase in addition to Spezyme® CP cellulase and Novozyme™ 188 during hydrolysis. The production of monomeric and oligomeric sugars at different solid loadings during enzymatic hydrolysate is discussed thoroughly. Comparison of glucose and xylose co-fermentation was conducted using three different yeast strains, including Saccharomyces cerevisiae 424A(LNH-ST) and Pichia stipitis (FPL-061 and DX-26). A biorefinery involving AFEX pretreatment and saccharification and fermentation at optimized conditions was integrated to analyze feedstock-to-ethanol by mass balance.
Materials and methods
Materials
Rice straw was obtained from California, USA and air-dried to approximately 7.92% moisture content (based on total dry biomass). Pre-milled RS, passed through a 5-mm screen, was stored in the refrigerator (4 °C) until further use.
AFEX pretreatment
AFEX pretreatment was performed in a bench top Parr reactor under optimized conditions previously described (Balan et al. 2008). The optimal conditions in this experiment were as follows: reaction temperature, 140 °C; moisture content, 80% (on dry weight basis); residence time, 30 min; and ratio of ammonia to biomass, 1:1 g ammonia/g dry biomass. The excess ammonia present in the pretreated RS was removed by flushing with nitrogen for 20 min and then left under the fume hood for 3–4 h.
Composition analysis
Composition was performed on untreated RS and AFEX-treated RS to the laboratory analysis protocol (LAP) from the National Renewable Energy Laboratory (NREL 2004).
Enzymatic hydrolysis
Enzymatic hydrolysis was performed according to the LAP-009 protocol from the National Renewable Energy Laboratory (Golden, CO, USA; NREL 2004). Spezyme® CP, Multifect® Xylanase, and Multifect® Pectinase were obtained from Genecor Inc., and Novozyme™ 188 was purchased from Sigma-Aldrich Co. Spezyme® CP and Novozyme™ 188 were loaded at 15 FPU/g cellulose and 64 pNPGU/g cellulose, respectively. The AFEX-treated RS was enzymatically hydrolyzed without any prior washing or detoxification. Tetracycline was added at a concentration of 40 mg/L as an antibiotic during the enzymatic hydrolysis.
The reaction was conducted at pH 4.8, 50 °C, and 150 rpm for 1% glucan loading. For 3% or 6% loading, the shaker speed was 250 rpm in order to achieve good mixing performance. The hydrolysis samples were heat-treated at 100 °C on a block heater (Eppendorf, Westbury, NY, USA) for 20 min to denature the enzyme mixture and then filtered through a 0.22-μm Whatman membrane filter at predetermined time periods (72 and 168 h) as described in the previous report (Balan et al. 2008).
Monomeric and oligomeric sugar analysis
Enzymatic hydrolysate was centrifuged at 7,500 rpm to separate the pellets from the supernatant. The collected pellets were washed twice at a ratio of 1 g wet pellet to 10 mL of water. Samples were taken from the washed streams after each washing. Monomeric sugars (glucose and xylose) in the hydrolysate and washed streams were diluted tenfold and analyzed using Aminex HPX-87P column (Bio-Rad, Sunnyvale, CA, USA).
The washed solids were air-dried and weighed. Hydrolysate was diluted to about 5.0 g/L glucose and 2.5 g/L xylose. Oligomeric sugars in the hydrolysate and washed solids stream were acid-hydrolyzed to analyze the oligomeric sugars, following LAP-014 from NREL (NREL 2004). Standards were prepared to calculate the percent recovery of monosaccharides. Enzymatic and acid hydrolysis was conducted in duplicate.
Microorganisms and inoculum culture
S. cerevisiae 424A(LNH-ST), a derivative of ATCC 4124, is an engineered strain from Dr. Nancy Ho (Purdue University) with integrated xylose reductase, xylitol dehydrogenase, and xylulokinase genes in yeast chromosomes (Ho and Chen 1997; Sedlak and Ho 2004). P. stipitis FPL-061 (NRRL Y-21301) and P. stipitis DX-26 are from Dr. Thomas W. Jeffries (University of Wisconsin, Madison). P. stipitis FPL-061 is a mutant strain from P. stipitis CBS-6054 (ATCC 58785), maintained by selective growth on l-xylose in the presence of respiratory inhibitors antimycin A and salicyl hydroxamic acid (Sreenath and Jeffries 1997). P. stipitis DX-26 (NRRL 21304) was derived from P. stipitis FPL-061 by mutagenesis with ethyl methanesulfonate and selection for growth on d-xylose in the presence of 1.0 g/L 2′-deoxyglucose (Lu et al. 1998).
Solid cultures of S. cerevisiae and P. stipitis were prepared in YEPD medium which contained 10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose, and 20 g/L agar. Inoculum cultures of S. cerevisiae and P. stipitis were prepared in 250-mL Erlenmeyer flasks overnight. Cells grown on a YEPD agar plate were inoculated into 250 mL flasks containing 100 mL inoculum culture medium, covered with an aerobic stopper. Inoculum culture was incubated at 30 °C for 18–24 h at a rotary speed of 150 rpm. Tetracycline was added at a concentration of 40 mg/L as antibiotics in solid and liquid inoculum culture.
Fermentation and analysis
Ethanol fermentation was done using hydrolysate under semisterile conditions without any nutrient supplement. The initial pH was adjusted to 5.5 for yeast fermentation. Predetermined inoculum culture was centrifuged at 4,000 rpm and cell pellets were re-suspended into the fermentation culture. Fermentation was conducted at 30 °C with a rotary speed of 150 rpm.
Cell density was measured using a Beckman Coulter UV–Vis spectrometer at 600 nm (1-cm light path). Optical density (OD) was corrected between 0.1 and 0.8 with the dilution factors as necessary. Samples were taken and centrifuged at 13,000 rpm for 5 min. Supernatant was collected and filtered through a 0.22-μm Whatman syringe filter with a diameter of 13 mm. Ethanol, xylitol, and residual sugars were determined by a Waters HPLC. The HPLC system was composed of a Waters 410 differential refractometer (Milford, MA, USA), a UV detector, a HPLC pump (Waters 515), an external heater module (Waters), and a Waters 717 plus auto sampler. The HPLC was run on an HPX-87H column (Bio-Rad) at 0.6 mL/min flow rate at 50 °C, with 5 mM H2SO4 as the mobile phase.
Results
AFEX pretreatments and optimization of enzyme addition
Based on appearance, AFEX-treated RS retained its structure almost completely intact, but appeared slightly darker than untreated RS (Balan et al. 2008). Composition analysis revealed that untreated RS had 34.7% glucan, 15.1% xylan, 2.2% arabinan, 19.1% lignin, 16.0% ash, etc. (Table 1).
AFEX-treated RS was solubilized to a free flowing liquid within 6 h of enzymatic hydrolysis, while untreated RS was still intact after 72 h and little free liquid was observed in the flasks. For the untreated RS, only 30.9% glucan conversion and 20.5% xylan conversion at 168 h were obtained (data not shown). As shown in Fig. 1, the glucan and xylan conversions of AFEX-treated RS at 72 h were 66.0% and 63.4%, respectively. Conversion at 168 h was 10% greater than that at 72 h. Previous studies have indicated that the initial hydrolysis rate is much higher than the subsequent rate due to selective initial hydrolysis of amorphous cellulose or subsequent insufficiency of new catalytic sites (Mansfield et al. 1999; Eriksson et al. 2002). The effect of Multifect® xylanase addition on enzymatic hydrolysis was conducted at 1% glucan loading. Addition of Multifect® xylanase with 2.67 mg protein/g glucan improved the glucan and xylan conversion at 72 h by nearly 6.9% and 7.8%. At 168 h, the glucan and xylan conversions were 75.8% and 74.6%, respectively. Increasing Multifect® xylanase by fourfold to 10.68 mg protein/g glucan achieves 78.9% glucan conversion and 81.6% xylan conversion.
Effect of Multifect® xylanase supplementation on saccharification for AFEX-treated rice straw. Enzyme complex: Spezyme® CP (15 FPU/g glucan), Novozyme™ 188 (64 pNPGU/g glucan), Multifect® xylanase. Addition of Multifect® xylanase was based on a loading at gram protein per gram glucan. Values are means of duplicate experiments. Enzyme loading, 1% glucan loading; temperature, 50 °C; shaking speed, 150 rpm. Values are means of duplicate experiments
A previous study has shown that Multifect® Pectinase contains Xylanase activity of 8.7 U/mg protein (Berlin et al. 2007). In order to determine the effect of Multifect® Pectinase on enzymatic hydrolysis, the Multifect® Xylanase level was fixed at 2.67 mg protein/g glucan. At 1% glucan loading, the addition of Multifect® Pectinase did not show a significant increase of glucan and xylan conversions (data not shown). Therefore, the enzymatic hydrolysis was conducted at 3% glucan loading. As shown in Fig. 2, the glucan and xylan conversions were only 70.5% and 55.0% without any addition of Multifect® Xylanase and Multifect® Pectinase. With the increase of the ratio of Multifect® Pectinase to Multifect® Xylanase, the change in glucan conversion was insignificant. However, the xylan conversion increased to 75.8% when the Multifect® Pectinase level was increased to 3.65 mg protein/g glucan. Further addition of Multifect® Pectinase did not bring more improvement in conversion to sugars.
Effect of Multifect® pectinase supplementation on saccharification for AFEX-treated rice straw. CK: without any addition of xylanase and pectinase. Enzyme complex: Spezyme® CP (15 FPU/g glucan), Novozyme™ 188 (64 pNPGU/g glucan), Multifect® xylanase (2.67 g protein/g glucan), Multifect® pectinase. Addition of Multifect® pectinase was based on a loading at gram protein per gram glucan. Enzyme loading, 3% glucan loading; temperature, 50 °C; shaking speed, 250 rpm. Values are means of duplicate experiments
Oligomeric and monomeric sugar analysis at different glucan loadings
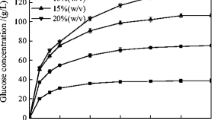
Enzymatic hydrolysis was performed at 1%, 3%, and 6% glucan loading, corresponding to 3.0%, 8.9%, and 17.6% solid loading, respectively. Sugar concentrations and glucan/xylan conversion to monomeric/oligomeric sugars are shown in Fig. 3. At 1% glucan loading, the concentrations of glucose and xylose at 168 h were 9.0 and 4.0 g/L, respectively, corresponding to 84.8% of glucan and 84.3% of xylan conversion to monomeric sugars, respectively. Glucan and xylan conversions to monomeric sugars were both dependent on glucan loadings. The increase in glucan loading from 1% to 3% led to a decrease of 3.1% in glucan conversion and a decrease of 8.5% in xylan conversion to monomeric sugars. With higher glucan loading, we see a decrease in the conversions of monomeric sugars. The glucan and xylan conversions to monomeric sugars at 6% glucan loading decreased to 70.7% and 56.4%, respectively. Previous studies have shown that high concentrations of the hydrolysis product (e.g., glucose) greatly inhibit enzyme performance (Xiao et al. 2004), which could be a possible explanation for higher decrease in the conversions to monomeric sugars at higher glucan loading. In addition, conversions to monomeric xylose declined more than monomeric glucose, which is consistent with another study (Jørgensen et al. 2007).
Monomeric and oligomeric sugar analysis of hydrolysate at different glucan loadings (1%, 3%, and 6%). Enzyme complex: Spezyme® CP (15 FPU/g glucan), Novozyme™ 188 (64 pNPGU/g glucan), Multifect® xylanase (2.67 g protein/g glucan), Multifect® pectinase (3.65 g protein/g glucan). Enzyme loading, 6% glucan loading; temperature, 50 °C; shaking speed, 150 rpm (1% glucan loading), 250 rpm (3% and 6% glucan loading). Values are means of duplicate experiments
Acid hydrolysis was conducted to determine the production of oligomeric sugars in enzymatic hydrolysate and washed streams. At 1% glucan loading, the concentrations of glucose and xylose oligomers were 0.7 and 1.2 g/L after 168 h of hydrolysis, which corresponds to 4.8% and 15.6% conversion, respectively. With increasing glucan loading (from 1% to 6%), we could see an increase in oligomeric sugars. The glucan conversion to oligomers was 8.5% and 9.9% after 168 h of hydrolysis at 3% and 6% glucan loading, respectively. The xylan conversion to oligomers increased almost linearly with glucan loading. At 3% and 6% glucan loading, the xylan conversion reached 21.8% and 33.2% after 168 h of hydrolysis, accounting for 24.0% of the total glucan conversion and 37.0% of the total xylan conversion, respectively. If oligomeric sugars were included, glucan conversion reached 89.6%, 90.2%, and 80.6% after 168 h of hydrolysis for 1%, 3%, and 6% glucan loading, respectively, while xylan conversion was up to 99.9%, 97.6%, and 89.6% after 168 h of hydrolysis for 1%, 3%, and 6% glucan loading, respectively.
Fermentations of RS hydrolysate using different ethanologenic strains
RS hydrolysate at 6% glucan loading was tested for fermentability using three yeast strains including S. cerevisiae 424A(LNH-ST), P. stipitis FPL-061, and P. stipitis DX-26. Fermentation was conducted in shake flasks with reaction volume of 70 mL at the initial cell density of 0.28 g dw/L. AFEX-treated RS showed high fermentability for co-fermentation of glucose and xylose by these three yeast strains. As illustrated in Fig. 4, glucose and xylose could be consumed simultaneously using all the three ethanologenic strains. Glucose (initially 59.6 g/L) can be consumed to a level of 1.2 g/L within 18 h using S. cerevisiae 424A(LNH-ST). During the same time, about 32.0% of xylose was consumed. At the end of the fermentation process (144 h), all of the glucose and 69.2% of the xylose were utilized by S. cerevisiae 424A(LNH-ST), resulting in an final ethanol concentration of 37.0 g/L (Table 2). P. stipitis FPL-061 and P. stipitis DX-26 also exhibited high ability to digest xylose. About 90% of monomeric xylose was consumed by P. stipitis FPL-061 and P. stipitis DX-26. At the end of the fermentation process (144 h), the final concentration of xylose using P. stipitis FPL-061 and P. stipitis DX-26 reached about 1.8 and 2.8 g/L, respectively, which is far below 7.4 g/L that was reached by using S. cerevisiae 424A(LNH-ST). However, the maximum ethanol concentrations reached by these two P. stipitis strains were only 29.7 and 27.6 g/L, respectively.
Fermentation of rice straw hydrolysate (6% glucan loading) using three different yeast strains. RS hydrolysate was from enzymatic hydrolysis at 6% glucan loading using enzyme complex shown in Fig. 3. Fermentation parameters: temperature, 30 °C; shaking speed, 150 rpm; initial cell density, 0.28 g dry wt/L. Values are means of duplicate experiments
Discussion
Compared with other agricultural residues, RS has a high content of ash and lignin, which makes it more recalcitrant and harder for enzymes to access (Chang and Holtzapple 2000; Jin and Chen 2006; Soest 2006). However, as a valuable inorganic resource in the semiconductor industry (Goodman 1996; Farone and Cuzens 1996), 10% to 14% of silica from RS is left behind after hydrolysis, which holds great potential for valuable by-product production over other lignocellulosic materials. As a dry-to-dry process, AFEX pretreatment was shown to be an effective pretreatment method for generating a highly fermentable hydrolysate after enzyme hydrolysis, while less than 3% sugar was lost during pretreatment (Table 1).
An optimal enzyme cocktail containing sufficient activities for both cellulose and hemicellulose was added to improve saccharification efficiency. In one of our previous studies, AFEX [at a ratio of 2:1 ammonia to biomass (w/w), 40% moisture content, 90 °C]-treated RS could achieve a maximum of 70–80% glucan conversion using a much higher cellulase loading of 75 FPU/g glucan (Gollapalli et al. 2002). In this study, the addition of Multifect® xylanase at 2.67 mg protein/g glucan and Multifect® pectinase at 3.65 mg protein/g glucan was optimized to greatly increase sugar conversion of AFEX-treated RS. Glucan and xylan conversion to monomeric sugars reached 81.7% and 75.8% even at 3% glucan loading. Furthermore, our cellulase usage was only one-fifth of that (15 FPU/g glucan loading) in the previous study. Even at 6% glucan loading (the equivalent of 17.8% solid loading), about 80.6% of glucan conversions and 89.6% of xylan conversions (including monomeric and oligomeric sugars) were achieved in the hydrolysate.
Hemicellulose is thought to restrict the access of cellulose by enzymes in pretreated biomass (Berlin et al. 2007). The enzyme cocktail with appropriate cellulase and hemicellulase activities can work together synergistically to attack typical parts of cellulose fiber. Positive effects were observed by supplementation of the cellulase mixture with these commercial xylanase and pectinase-enriched enzymes. Although Spezyme® CP contains hemicellulase activity of 1% (by mass; Bradshaw et al. 2007), it is still regarded as possibly deficient in xylan-hydrolyzing activity (Dien et al. 2008). In this study, the addition of Multifect® xylanase at 2.67 mg protein/g glucan and Multifect® pectinase at 3.65 mg protein/g glucan resulted in a higher saccharification efficiency than that given by cellulase (Spezyme® CP) alone.
In order to make cellulosic ethanol production more economically feasible, high solid loading is required to produce a high concentration of sugars for subsequent fermentation. However, higher glucan loading gave even greater decreases in the conversions to monomeric sugars, while conversions to oligomeric sugars increased with greater glucan loading (Fig. 3). At high solid loading, mixing and liquefaction (mass transfer issue) are thought to restrict the access of enzymes to the solid substrate (Jørgensen et al. 2007). AFEX pretreatment greatly improves substrate digestibility. In this study, AFEX-treated RS was liquefied within 8 h even at 6% glucan loading. An agitation speed of 250 rpm was chosen to improve mixing performance for the saccharification process at 3% and 6% glucan loading. Cellulases and β-glucosidases are susceptible to shear stress, though β-glucosidases were shown to be more sensitive to shear stress than cellulase (Tengborg et al. 2001). This may explain why the percentage of oligomeric glucose was higher in the hydrolysate at 3% and 6% glucan loading than at the 1% glucan loading. The current available commercial enzyme cocktail lacks sufficient hemicellulose activity; the hemicellulosic sheath needs to be penetrated to allow access to the biomass microfibrils (Chandawat et al. 2007). This results in lower conversions to monomeric xylose at a higher glucan loading. At 6% glucan loading, oligomeric glucose and xylose after 168 h of hydrolysis accounted for 12.3% of the total glucose and 37.0% of the total xylose, respectively. Further improvements will require the utilization of oligomeric sugars in hydrolysate. Therefore, there are two ways to solve this problem: develop optimized enzyme formulations to fully convert oligomeric sugars to monomeric sugars or construct engineered strains to produce appropriate cellulase and hemicellulase that metabolize oligomeric sugars.
Comparison among the three ethanologenic strains reveals that S. cerevisiae 424A(LNH-ST) is the most promising candidate for RS hydrolysate with an ethanol volumetric productivity of 0.26 g/L/h. Higher xylose conversions but lower ethanol yields in P. stipitis may be attributed to continuous cell growth in these two Pichia strains. As illustrated in Fig. 4, S. cerevisiae 424A(LNH-ST) achieved a cell density of 4.86 g dw/L (OD600 = 8.83) at 24 h, which was 86% of the final cell density (5.62 g dw/L). Evidently, the majority of the sugar consumed was converted to ethanol rather than used to generate cell mass. Metabolic ethanol yield using this strain was 95.3% (Table 2). As fermentation proceeded, the cell density of the two P. stipitis strains continued to increase, which led to a maximum cell density of 11.46 g dw/L (OD600 = 20.9) by P. stipitis DX-26, followed by 11.14 g dw/L (OD600 = 20.3) of P. stipitis FPL-061 (Fig. 4). The maximum metabolic ethanol yields of P. stipitis FPL-061and P. stipitis DX-26 were 71.7% and 67.8%, respectively. From Fig. 4, we can also see that the ethanol concentration of the two P. stipitis strains decreased after 120 h, evidently due to ethanol reassimilation by P. stipitis in shake flask culture (Skoog et al. 1992). Carefully controlled micro-aeration is required for P. stipitis fermentation (Skoog and Hahn-Hagerdahl 1990; Sreenath and Jeffries 1997 ). Another report revealed that an ethanol yield of only 0.05 g/g was achieved in SSF of steamed pretreated bagasse without micro-aeration (Rudolf et al. 2008). The maximum specific ethanol productivity of P. stipitis has previous been reported at an oxygen consumption rate under 1 mmol/L/h. In our experiments, shake flasks were used for fermentations. Rigorous control of micro-aeration conditions could be achieved using fermentors in subsequent research.
Fermentations using S. cerevisiae 424A(LNH-ST) were conducted to determine the effects of different initial cell density on ethanol production, xylose consumption, etc. As illustrated in Fig. 5, both the consumption rate of glucose and consumption rate of xylose were enhanced by increased initial cell density. The highest ethanol concentration (39.3 g/L) and highest xylose consumption percentage (86.9%) were achieved with an initial OD600 of 8.0 (4.40 g dry wt/L), followed by an initial OD600 of 5.0 (38.5 g/L and 80.1%), and then an initial OD600 of 0.5 (37.0 g/L and 69.2%). Compared with the initial OD600 of 0.5, an increase of 4.1% and 6.2% in the final concentration of ethanol was observed using an initial OD600 of 5.0 and 8.0, respectively. Metabolic ethanol yields of S. cerevisiae 424A(LNH-ST) at an initial OD600 of 5.0 and 8.0 using this strain were 95.8% and 95.9%, respectively. However, our current results reveal that the concentration of xylitol was enhanced with an increase in initial cell density. The final xylitol concentration increased by 22.0% and 27.4% at an initial OD600 of 5.0 and 8.0, respectively, compared with 3.3 g/L at an initial OD600 of 0.5. Hydrolysate from lignocellulosic is generally regarded to be deficient of nutrients. However, our research strongly contradicts this perception. Nutrients provided by AFEX-treated biomass were sufficient to support robust yeast growth even at low initial cell density (Lau and Dale 2009).
Effect of initial cell density on fermentation using S. cerevisiae 424A(LNH-ST). RS hydrolysate was from enzymatic hydrolysis at 6% glucan loading using enzyme complex shown in Fig. 3. Fermentation parameters—30 °C; 150 rpm; initial cell density, 0.28, 2.75, and 4.40 g dry wt/L (equivalent of initial OD600 of 0.5, 5.0, and 8.0, respectively). Values are means of duplicate experiments
It is important to note that xylose utilization by S. cerevisiae is incomplete. This result is similar to our recent report regarding the fermentability of saccharified corn stover (Lau and Dale 2009). On the other hand, Pichia strains in this report did not appear to show this limitation (it consumed more xylose compared to S. cerevisiae).
Our results indicate that S. cerevisiae 424A(LNH-ST) and AFEX-treated RS can achieve excellent fermentation performance at low initial cell density (0.28 g dw/L), without washing, detoxification, or external nutrient supplementation. The mass balance of the whole process is illustrated in Fig. 6. The overall mass balance exhibited approximately 96.0% closure. Using optimized enzyme addition profiles, about 80.6% of glucose and 89.6% of xylose (including monomeric and oligomeric sugars) conversions were achieved in RS hydrolysate, even at 6% glucan loading. However, oligomeric glucose and xylose accounted for 12.3% of the total glucose and 37.0% of the total xylose, respectively. Conversion from oligomeric to monomeric sugars is a bottleneck and requires an optimized enzyme combination for further improvements. The final ethanol concentration at a low initial cell density (0.28 g dry wt/L) was 37.0 g/L, which is very close to the economically feasible benchmark (40 g/L). In one of our previous work, treating RS with white rot fungi prior to AFEX pretreatment (MMS/AFEX) could achieve 92% glucan and 55% xylan conversion, respectively. However, during this biological pretreatment, glucan and xylan contents were reduced by 23.2% and 20.1%, respectively (Balan et al. 2008). With the optimized addition of Multifect® Xylanase and Multifect® Pectinase in this study, 84.8% of glucan and 84.3% of xylan conversions to fermentable sugars were achieved at the same glucan loading. Even at high glucan loading (6%), glucan-to-glucose and xylan-to-xylose conversion could be 68.9% and 56.0%, respectively. Higher conversion to fermentable sugars implies higher concentration of ethanol; consequently, the costs on subsequent distillation unit operation were reduced.
Overall mass balance of the whole process, from rice straw to ethanol. AFEX pretreatment condition—80% moisture content (dwb), 1:1 ammonia loading, 140 °C, 30 min residence time. Enzymatic hydrolysis condition: Spezyme® CP (15 FPU/g glucan), Novozyme™ 188 (64 pNPGU/g glucan), Multifect® xylanase (2.67 g protein/g glucan), Multifect® pectinase (3.65 g protein/g glucan); 6% glucan loading (equivalent of 17.8% solid loading); 250 rpm, 50 °C. Fermentation condition: S. cerevisiae 424A(LNH-ST); 30 °C, 150 rpm. Values are means of duplicate experiments. Monomeric and oligomeric sugars in hydrolysate, washed streams, and solids were analyzed
Using RS as feedstock, AFEX as the pretreatment technology and S. cerevisiae 424A(LNH-ST) as the ethanologenic strain in separate hydrolysis and fermentation, we were able to achieve 175.6 g EtOH/kg untreated rice straw, at a final ethanol concentration of 37.0 g/L without washing pretreated biomass, detoxification, or nutrient supplementation. By integrating AFEX pretreatment, enzymatic hydrolysis, and fermentation at optimized conditions, biorefineries might make use of abundant supplies of RS.
References
Balan V, da Costa Sousa L, Chundawat SP, Vismeh R, Jones AD, Dale BE (2008) Mushroom spent straw: a potential substrate for an ethanol-based biorefinery. J Ind Microbiol Biotech 35:293–301
Bayrock DP, Ingledew WM (2001) Application of multistage continuous fermentation for production of fuel alcohol by very-high-gravity fermentation technology. J Ind Microbiol Biotech 27:87–93
Belkacemi K, Turcotte G, Halleux D, Savoie P (1998) Ethanol production from AFEX-treated forages and agricultural residues. Appl Biochem Biotechnol 70–72:441–462
Berlin A, Maximenko V, Gilkes N, Saddler J (2007) Optimization of enzyme complexes for lignocellulose hydrolysis. Biotechnol Bioeng 97:287–296
Bothast RJ, Schlicher MA (2005) Biotechnological processes for conversion of corn into ethanol. Appl Microbiol Biotechnol 67:19–25
Bradshaw TC, Alizadeh H, Teymouri F, Balan V, Dale BE (2007) Ammonia fiber expansion pretreatment and enzymatic hydrolysis on two different growth stages of reed canarygrass. Appl Biochem Biotechnol 137–140:395–405
Byung-Hwan U, Hanly TR (2008) High-solid enzymatic hydrolysis and fermentation of solka floc into ethanol. J Microbiol Biotechnol 18:1257–1265
Chandawat SPS, Balan V, Dale BE (2007) Effect of particle size based separation of milled corn stover on AFEX pretreatment and enzymatic digestibility. Biotechnol Bioeng 96:219–231
Chang VS, Holtzapple MT (2000) Fundamental factors affecting biomass enzymatic reactivity. Appl Biochem Biotechnol 84–86:5–37
Cohen R, Persky L, Hadar Y (2002) Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Appl Microbiol Biotechnol 58:582–594
Dien BS, Ximenes EA, O'Bryan PJ, Moniruzzaman M, Li XL, Balan V, Dale B, Cotta MA (2008) Enzyme characterization for hydrolysis of AFEX and liquid hot-water pretreated distillers' grains and their conversion to ethanol. Bioresour Technol 99:5216–5225
Eriksson T, Karlsson J, Tjerneld F (2002) A model explaining declining rate in hydrolysis of lignocellulosic substrates with cellobiohydrolase I (Cel7A) and endoglucanase I (Cel7B) of Trichoderma reesei. Appl Biochem Biotechnol 101:41–60
Fan ZL, South C, Lyford K, Munsie J, van Walsum P, Lynd LR (2003) Conversion of paper sludge to ethanol in a semicontinuous solids-fed reactor. Bioprocess Biosyst Eng 26:93–101
Farone WA, Cuzens JE (1996) Method of producing sugars using strong acid hydrolysis of cellulosic and hemicellulosic materials. US Patent 5,562,777
Farrell AE, Plevin RJ, Turner BT, Jones AD, O'Hare M, Kammen DM (2006) Ethanol can contribute to energy and environmental goals. Science 311:506–508
Forrest L, Williams J, Collin I, Holtzer R, Lindberg D, Maben L, Merz J, Parsons G, Waite W (1997) Report of the Advisory Committee on alternatives to rice straw burning. California Air Resources Board and California Department of Food and Agriculture, Sacramento, CA
Gollapalli LE, Dale BE, Rivers DM (2002) Predicting digestibility of ammonia fiber explosion (AFEX) treated rice straw. Appl Biochem Biotechnol 98–100:23–35
Goodman EF (1996) Moisture absorbing materials and methods of production. US Patent 5,503,931
Ho NWY, Chen ZD (1997) Stable recombinant yeasts capable of effective fermentation of both glucose and xylose. PCT Patent No. WO97/42307
Jin SY, Chen HZ (2006) Structure properties and enzymatic hydrolysis of rice straw. Process Biochem 41:1261–1264
Jørgensen H, Vibe-Pedersen J, Larsen J, Felby C (2007) Liquefaction of lignocellulose at high-solids concentrations. Biotechnol Bioeng 96:862–870
Lau MW, Dale BE (2009) Cellulosic ethanol production from AFEX-treated corn stover using Saccharomyces cereviasiae 424A(LNH-ST). Proc Natl Acad Sci USA 106:1368–1373
Lau MW, Dale BE, Balan V (2008) Ethanolic fermentation of hydrolysates from ammonia fiber expansion (AFEX) treated corn stover and distillers grain without detoxification and external nutrient supplementation. Biotechnol Bioeng 99:529–539
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69:627–642
Lu P, Davis BP, Hendrick J, Jeffries TW (1998) Cloning and disruption of the beta-isopropylmalate dehydrogenase gene (LEU2) of Pichia stipitis with URA3 and recovery of the double auxotroph. Appl Microbiol Biotechnol 49:141–146
Lu Y, Wang Y, Xu G, Chu J, Zhuang Y, Zhang S (2008) Influence of high solid concentration on enzymatic hydrolysis and fermentation of steam-exploded corn stover biomass. Appl Biochem Biotechnol. doi:https://doi.org/10.1007/s12010-008-8306-0
Lübbert A, Jøgensen BS (2001) Bioreactor performance: a more scientific approach for practice. J Biotechnol 85:187–212
Mansfield SD, Mooney C, Saddler JN (1999) Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol Prog 15:804–816
NREL (2004) Laboratory analytical procedure (LAP). http://www.nrel.gov/biomass/analytical_procedures.html
Rudolf A, Baudel H, Zacchi G, Hahn-Hägerdal B, Lidén G (2008) Simultaneous saccharification and fermentation of steam-pretreated bagasse using Saccharomyces cerevisiae TMB3400 and Pichia stipitis CBS6054. Biotechnol Bioeng 99:783–790
Sedlak M, Ho NW (2004) Production of ethanol from cellulosic biomass hydrolysates using genetically engineered Saccharomyces yeast capable of cofermenting glucose and xylose. Appl Biochem Biotechnol 113–116:403–416
Skoog K, Hahn-Hagerdahl B (1990) Effect of oxygenation on xylose fermentation by Pichia stipitis. Appl Environ Microbiol 56:3389–3394
Skoog K, Hahn-Hägerdal B, Degn H, Jacobsen JP, Jacobsen HS (1992) Ethanol reassimilation and ethanol tolerance in Pichia stipitis CBS 6054 as studied by C nuclear magnetic resonance spectroscopy. Appl Environ Microbiol 58:2552–2558
Soest PJV (2006) Rice straw, the role of silica and treatments to improve quality. Anim Feed Sci Technol 130:137–171
Sreenath HK, Jeffries TW (1997) Diminished respirative growth and enhanced assimilative sugar uptake result in higher specific fermentation rates by the mutant Pichia stipitis FPL-061. Appl Biochem Biotechnol 63–65:109–116
Tengborg C, Galbe M, Zacchi G (2001) Influence of enzyme loading and physical parameters on the enzymatic hydrolysis of steam-pretreated softwood. Biotechnol Prog 17:110–117
Teymouri F, Laureano-Perez L, Alizadeh H, Dale BE (2006) Optimization of the ammonia fiber explosion (AFEX) treatment parameters for enzymatic hydrolysis of corn stover. Bioresour Technol 96:2014–2018
Wingren A, Galbe M, Zacchi G (2003) Techno-economic evaluation of producing ethanol from softwood: comparison of SSF and SHF and identification of bottlenecks. Biotechnol Prog 19:1109–1117
Xiao ZZ, Zhang X, Gregg DJ, Saddler JN (2004) Effects of sugar inhibition on cellulases and beta-glucosidase during enzymatic hydrolysis of softwood substrates. Appl Biochem Biotechnol 113–116:1115–1126
Zacchi G, Axelsson A (1989) Economic evaluation of preconcentration in production of ethanol from dilute sugar solutions. Biotechnol Bioeng 34:223–233
Acknowledgments
We would like to thank Dr. Thomas W. Jeffries for providing the engineered Pichia stipitis strains. We deeply appreciate the valuable suggestions given by both Leonardo daCosta Sousa and Shishir Chundawat during the course of this experiment. The authors are grateful for the financial support from the Michigan State University Research Foundation through SPG grants and the National Natural Science Foundation of China (Key Program Grant No. 20736006), the National Basic Research Program of China (“973” Program: 2007CB714301), the International Collaboration Project of MOST(2006DFA62400), and key projects in the National Science & Technology Pillar Program (No. 2007BAD42B02). We would like to thank Genencor for generously supplying Spezyme® CP cellulase, Multifect® xylanase, and Multifect® pectinase for our research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, C., Lau, M.W., Balan, V. et al. Optimization of enzymatic hydrolysis and ethanol fermentation from AFEX-treated rice straw. Appl Microbiol Biotechnol 84, 667–676 (2009). https://doi.org/10.1007/s00253-009-2001-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2001-0