Abstract
For degradation of sugarcane bagasse (SCB), several enzymes are needed but β-glucosidase is rate limiting in cellulose hydrolysis. Since different microorganisms synthetize characteristic pool of enzymes, mixing extracts produced by different species may increase hydrolytic efficiency due to synergism between enzymes in cocktails. This paper reports the study of β-glucosidase production in solid state cultivation (SSC) of two filamentous fungi, thermophilic Thermoascus aurantiacus and mesophilic Trichoderma reesei, and application of the enzymatic extracts on non-pretreated SCB saccharification. Enzyme extract obtained from the thermophilic fungus presented higher β-glucosidase and FPU activities (1.8 U/mL and 10 FPU/mL) than the one from mesophilic (0.2 U/mL and 6 FPU/mL). Optimal SCB hydrolysis was achieved when applying enzymatic cocktail composed of equal volumes of both fungal extracts (3.6 FPU/gSCB, filter paper units per gram SCB, 2.25 FPU/gSCB provided by extract from T. aurantiacus and 1.35 FPU/gSCB from T. reesei) at 65 °C. The hydrolysis yield applying the enzyme cocktail, 124 mg total reducing sugars (TRS) per gSCB, was higher than any yield achieved when using the enzyme extracts separately (105 mgTRS/gSCB using 12.5 FPU per gSCB from T. aurantiacus at 65 °C; 79 mgTRS/gSCB using 7.5 FPU per gSCB from T. reesei at 45 °C). Therefore, the use of the cocktail (3.6 FPU/gSCB) at 65 °C released 18 and 57% more TRS respectively than when extracts from T. aurantiacus or from T. reesei were applied alone, respectively, even reducing enzyme load (FPU) by 70%, corroborating the synergistic effect when both extracts are used together.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Global concern about depletion of fossil fuel deposits and greenhouse gas effects demands research on renewable energy alternatives. Hence, large-scale production of clean fuels, such as bioethanol, is attracting worldwide interest. Assessments for 2030 estimate potential production of lignocellulosic or second generation ethanol (E2G) enough to provide 1019 J of energy or 10.5% of current transport fuel demand, using 25% of global forestry and agricultural residues for biofuel production [1].
In Brazil, sugarcane bagasse (SCB) is an abundant agro-industrial by-product for conversion to E2G, since it is a potential source of fermentable sugars [2]. During the 2017/2018 season, nearly 641 million tons of sugarcane (Saccharum officinarum) were harvested in Brazil [3], generating around 160 million tons of SCB. However, production of E2G from SCB is limited by the recalcitrance of this biomass. The most eco-friendly alternative to produce biofuels is via an enzymatic route, which requires cellulolytic and hemicellulolytic enzymes to depolymerize the biomass into fermentable sugars. Nevertheless, the high cost of commercial enzymes is a key economic factor in E2G production via an enzymatic route [4].
Some strategies are being applied that aim to reduce the effective cost of cellulases, such as the use of cheap raw materials for its production or the use of the enzymes in their crude form, without purification [5]. In this context, studies on obtaining cellulases and hemicellulases and optimizing production processes are very important.
Solid state cultivation (SSC) is a bioprocess that provides high concentrations of enzymes. SSC offers environmentally friendly and economical technology with simple process operations, thereby lowering capital and operational costs. However, SSC still requires further studies for it to become a viable industrial process, comprising selection of microorganisms and substrates [6, 7].
Filamentous fungi yield high activities of cellulolytic and hemicellulolytic enzymes by SSC because this bioprocess mimics the natural habitat of those microorganisms that release high levels of enzymes to an extracellular solid medium [6]. Utilization of agro-industrial by-products as substrates is another great advantage of SSC due to the low cost of these substrates [7].
Nevertheless, direct quantification of biomass in SSC systems is not feasible due to the impossibility of separating the various components of the cultivated media (substrate, microorganism, enzymes, and others), particularly for filamentous fungi, since their hyphae strongly bind to the particle surface and may penetrate far inside the particles. Hence, indirect methods of estimating biomass must be used in SSC. Some of them are based on quantification of specific constituents of microbial cells (e.g., chitin, ergosterol, nucleic acids, and proteins) and of biological activity (e.g., ATP production, enzyme activity, nutrient consumption, and respiration gases) [8]. The Kjeldahl method, based on estimation of total nitrogen, can be used for indirect estimative of fungal growth kinetics in SSC [9].
The role of enzymatic cocktails in performing the hydrolysis of cellulose and hemicellulose has called attention. The efficiency with which cellulose is hydrolyzed by enzymes depends on many factors, such as characteristics of the substrate and nature of the enzymatic systems. Recent research has focused on the optimization of the enzymatic profile employed in the process by using enzymatic cocktails as one of the factors to improve both the yield and the rate of enzymatic hydrolysis [10].
The utilization of abundantly available lignocellulosic biomass requires an efficient cellulolytic enzyme system. It is known that the complete degradation of complex molecules such as cellulose requires several enzymes acting together. The action of cellulolytic enzymes depends on at least three hydrolases: endoglucanase, exoglucanase, and β-glucosidase, with the last being essential to avoid an inhibitory effect of cellobiose [10]. Considering that different microorganisms synthetize a characteristic pool of enzymes even when cultivated using the same substrate and under the same conditions, the formulation of enzymatic cocktails obtained by mixing extracts produced by different microorganisms is an alternative to increase the efficiency of the enzymatic hydrolysis, since different enzymes can act synergistically [11].
Based on our previous results and on the literature, two species of fungi are expected to present good potential for producing high levels of cellulases and hemicellulases, the thermophilic T. aurantiacus and the mesophilic T. reesei [12]. T. aurantiacus is a good producer of endoglucanase and endoxylanase, but produces low exoglucanase activity [12]. This thermophilic fungus is also a good source of β-glucosidases [13]. T. reesei is recognized for its ability to produce several extracellular enzyme systems involved in the hydrolysis of polysaccharides, but it has a deficiency in β-glucosidases [10]. Thus, an increase in hydrolysis efficiency can be expected by looking for synergism between enzymes from both these fungi.
In the current study, we produced cellulases from both fungi on SSC using agro-industrial by-products as substrates, focusing on the maximization of β-glucosidase production and its relation with growth kinetics. As these fungi present different adaptive and physiological responses (secreted enzymatic activity), we have also studied different compositions of solid medium, based on enzyme production and on specific growth rates. The enzyme extracts and an enzymatic cocktail were applied for the hydrolysis of SCB in order to test the expected synergistic effect.
2 Materials and methods
2.1 Microorganisms and substrates
The thermophilic fungus T. aurantiacus CBMAI756 and the mesophilic T. reesei QM9414 were used. They belong to the collection of the Laboratory of Biochemistry and Applied Microbiology of the Institute of Biosciences, Letters and Exact Sciences (IBILCE) of the São Paulo State University (UNESP). They were grown in Dextrose-Sabouraud-Agar (DSA), T. aurantiacus at 45 °C for 48 h and T. reesei at 28 °C for 168 h.
The culture media for the SSC experiments contained sugarcane bagasse (SCB) and wheat bran (WB). SCB was kindly provided by Usina Vale (Onda Verde-SP, Brazil) and was ground in a knife mill and sieved. The fraction used was that which passed through a sieve with 3-mm openings but was retained by one with 1.44 mm openings. This fraction was washed with tap water to remove dirt and remaining sugar and dried to constant weight in a convective oven at 60 °C. It was then placed in plastic bags and stored at 5 °C. The wheat bran (Cerealista Alvorada, São José do Rio Preto-SP, Brazil) was purchased from a local retailer and was not handled prior to use.
2.2 SSC experiments for β-glucosidase production
SSC experiments were done in plastic bags 12 cm × 20 cm with a circular opening 3.6 cm in diameter closed with a cotton wool plug, in order to allow gas exchange and avoid contamination. Each plastic bag received 5 g of substrate (dry weight). The sets of plastic bags and substrates were sterilized in an autoclave at 121 °C for 20 min. For inoculation, spores were suspended with nutrient solution enriched with mineral salts [14]. The spore concentration was fixed at 107 spores/mL, using a hemocytometer, and 1 mL was added to each bag. The moisture content of the substrate was adjusted by adding nutrient solution to the solid media. T. aurantiacus was cultivated at 50 °C for 120 h; T. reesei at 28 °C for 168 h.
Some plastic bags were destined for β-glucosidase activity quantification along and at the end of cultivation, as well as some bags were used for indirect measurements of fungal biomass. Each plastic bag was an independent sample. Three identical bags (triplicates) were used for each quantification.
Minitab 15.0 (Minitab Inc., State College, USA) was used for experimental design and statistical analysis, in which the response was the β-glucosidase activity. The chosen experimental design for solid state cultivation was a full-factorial design with random blocks and two factors with three levels for each factor. The factors were (i) moisture content of the substrate, with levels 70, 75, and 80% (wet basis) and (ii) composition of the substrate, with levels SCB:WB 1:1, 3:1, and 9:1 w/w. The fungi were treated as blocks of the design. ANOVA analysis and means comparisons using the Tukey test were performed in order to find the conditions that provided the highest β-glucosidase activities. All statistical significance levels were 95%.
2.3 Enzymatic activity quantification
For samples destined for enzymatic activity quantification (along and at the end of cultivation), distilled water was added to the fermented material (20 mL/g of initial dry substrate) after 120 h for T. aurantiacus and after 168 h for T. reesei, followed by agitation at 100 rpm for 30 min at room temperature and centrifugation at 104g for 15 min at 5 °C. The supernatant was denoted as enzymatic raw extract.
The carboxymethylcellulase (CMCase) and filter paper (FPU) activities were determined following IUPAC procedures [15]. To determine CMCase, 0.1 mL of appropriately diluted enzyme (to ensure the initial activity rate) was incubated with 0.9 mL of a suspension containing 4% of carboxymethylcellulose (Sigma-Aldrich, St. Louis, USA). In the case of FPU activity determination, the substrate was 50 mg (1 × 6 cm strip) of Whatman no. 1 filter paper. Carboxymethylcellulase (CMC) were dissolved in 0.1 M acetate buffer, pH 5.0. For filter paper, 0.9 mL buffer and 0.1 mL enzyme extract were used as well. After incubation at 60 °C for 10 min for CMCase and for 60 min for FPU, the amount of total reducing sugars (TRS) released were determined by 3,5-dinitrosalicylic acid (DNS) [16], as further detailed below. Controls were prepared with the enzyme extract being added after the boiling step of the referred methodology, in order to discount TRS possibly present in enzyme extracts prior to the reaction. One unit of enzyme activity (U) was defined as that amount which released 1 μmol of glucose per minute under the reaction conditions, by using glucose standard curves at 540 nm.
β-Glucosidase activity was quantified according to Leite et al. [17] at 45 °C for T. reesei and 65 °C for T. aurantiacus. The reaction mixture was 50 μL of the enzymatic extract, 250 μL of 100 mM sodium acetate buffer pH 5.0, and 250 μL of 4 mM4-nitrophenyl β-d-glucopyranoside (PNPG, Sigma). After 10 min, the reaction was stopped by the addition of 2 mL of 2 M sodium carbonate. One unit of enzymatic activity was the quantity of enzyme needed to release 1 μmol of p-nitrophenol per minute of reaction. A standard curve of p-nitrophenol at 410 nm was used.
2.4 Indirect measurement for biomass estimative
According to the Kjeldahl method, indirect measurement of biomass content in the fermented material along cultivation was based on total nitrogen [9]. The total nitrogen content of the fermented material was quantified by Kjeldahl method and then the total protein content was calculated by multiplying the nitrogen content by 6.25. The fungal protein content was obtained by subtracting the total protein content at given time interval from time zero (i.e., immediately after inoculation).
The logistic model of microbial growth was fitted to the estimated data of biomass concentration based on total protein content. Non-linear least square method optimized by Levenberg-Marquardt algorithm was used by the software Origin 6.0 (Microcal Software Inc., Northampton, USA). According to Viccini et al. [18], this model best represents growth in many SSC systems, being given by:
where X is the actual biomass concentration; Xm is the maximum biomass concentration; μ is the maximum specific growth rate constant; t is time. In the current paper, X is expressed as mg of estimated fungal biomass per gram of fermented material.
The integrated form of Eq. (1), which was used in the fitting procedure, is:
where X0 is the initial biomass in the system (for t = 0). The parameters estimated from the experimental data were Xm, X0, and μ.
2.5 Hydrolysis of SCB
For hydrolysis, SCB fibers were milled to a particle size of 0.84 mm. Saccharification assays were performed with 0.2 g SCB in a thermostatic bath with orbital agitation. Minitab 15.0 (Minitab Inc., State College, USA) was used for generating and further analyzing a Box-Behnken experimental design, with 3 factors (total volume of enzyme, temperature of hydrolysis, and composition of the enzymes cocktail) with 3 levels for each factor (comprising 15 assays, 12 conditions and 3 tests on central point). For all assays, the final volume of the reaction was completed with 10 mL sodium acetate buffer 0.2 mol L−1 at pH 5.0.
The response of the hydrolysis tests was the quantity of total reducing sugars (TRS) released during the reaction. At the central point of experimental design, experiments varying the time of saccharification were also performed by taking independent samples every 4 h up to 24 h, in order to give the kinetics of saccharification under those conditions. Hydrolysis kinetic assays were also performed using 0.2 g SCB and applying 5 mL of the enzymatic extracts produced by T. aurantiacus and T. reesei at 55 °C, individually and combined (enzyme cocktail), to verify the synergistic effect. The experiments were performed under the same conditions described above, in order to give the kinetics of saccharification for the enzyme extracts alone and for the cocktail.
The release of TRS was determined according to Miller [16], using glucose as standard. The reaction was performed by adding 1.0 mL of the filtered sample and 1.0 mL of DNS reagent to the test tube and boiling for 10 min. During boiling, the DNS (3,5-dinitrosalicylic acid) reagent, when reacting with reducing sugars present in the medium, changes its initially yellow coloration to brown tones. The color intensity is directly proportional to the concentration of sugars in the medium. The coloring reaction is interrupted when the samples are transferred to an ice bath. 8.0 mL of distilled water was added, after which the absorbance was read at 540 nm. A calibration curve using glucose as a standard was plotted and the results were expressed as mg of reducing sugars/g of sugarcane bagasse.
Quantification of glucose, xylose, and cellobiose was carried out using a Dionex, ICS-5000 high-performance anion-exchange chromatography—with pulsed amperometric detection (HPAEC-PAD) (Thermo Scientific, Waltham, USA) with a CarboPac® PA-1 anion-exchange column with compartments at 25 °C and 30 °C, diluents prepared with ultrapure deionized water 18 MΩ and degassed with N2, with a flow of 1 mL min−1 with solvent A (ultrapure water) and B (500 mmol L−1 NaOH). Isocratic elution was used for column cleaning from 0 to 12 min with 95.2% A, from 12 to 16 min 100% B was used and from 16 to 30 min 95.2% of A was used to end the process [19]. The results were analyzed and quantified based on standard curves of glucose, xylose, and cellobiose (Sigma-Aldrich, St Louis, USA).
3 Results and discussion
3.1 β-Glucosidase activities and effect of the substrate composition and moisture content
Due to the different metabolisms of each microorganism and the variation in fermentative media, each fungus responded differently to medium stimuli, as a result of their different adaptation. For the thermophilic fungus, the minimum β-glucosidase activity (6.8 U/gss) was obtained with SCB:WB 1:1 w/w and 80% moisture content, while the maximum (16.8 U/gss) was found with SCB:WB 9:1 independent of moisture content (Table 1). Statistical analysis based on main effects plots suggested that β-glucosidase activity is significantly affected only by substrate composition. However, ANOVA analysis pointed out statistically significant interaction effects among substrate composition and moisture content in all treatments.
Selection of a proper substrate is another key aspect of SSC. Porous media act both as physical support and as a source of nutrients. However, in addition to supplying nutrients, the appropriate substrate is one that is also capable of inducing the production of the specific target enzyme [6]. Also, it is important to remember that an ideal porous matrix for SSC bioreactors should both fulfill the nutritional microbial needs and provide adequate physical structure for air percolation to guarantee efficient transfer of the respiratory gases and facilitate metabolic heat removal [20].
For the mesophilic fungus T. reesei, Table 1 shows minimum β-glucosidase activity (3.8 U/gss) for SCB:WB 3:1 w/w and 70% moisture content, while maximum activity (5.4 U/gss) was obtained with SCB:WB 1:1 w/w and 80% moisture content. Statistical analysis based on main effects plots suggested that, for T. reesei, β-glucosidase activity is significantly affected by both the substrate composition and the moisture content.
Grajek [21] evaluated β-glucosidase activity from the cultivation of T. aurantiacus on sugar beet pulp and the author found results similar to the ones in the current paper. Pinto [22] also obtained highest β-glucosidase activity (4.4 U/gss) in the solid cultivation of T. reesei on SCB:WB 1:1 w/w with 80% moisture content, although this was quantified after 216 h of cultivation.
Casciatori et al. [20] have studied filter paper activity (FPU) of a crude extract produced by same strain of T. reesei QM9414 cultivated in SCB:WB 1:1, 3:1, and 9:1 w/w and moisture contents 70, 75, and 80%. The authors found better results for SCB:WB 9:1 and 70% moisture content and attributed such a result to the structural properties of the porous media. These results, when compared to ours, show that the fermentation conditions influence the production of the target enzyme.
As already stated, the use of two fungi with different thermal behaviors aimed at comparing which system (fungus and temperature) enables reaching higher β-glucosidase activities [23]. Enzymatic extracts obtained from the cultivation of T. aurantiacus presented β-glucosidase activities approximately 3 times higher than the activities of the extracts obtained from T. reesei, reiterating that the thermophilic fungus is able to release enzymes which are more thermostable and have higher activities [23, 24]. Grajek [21, 25, 26] determined optimal temperature and pH for cellulase activities of enzymatic extracts obtained from several thermophilic fungi isolated from wood chip piles. T. aurantiacus (Miehe) produced the extracts with highest cellulases activities cultivated on solid media, reiterating the good adaptation of this thermophilic fungus to SSC [21, 22], which is in agreement with the results in the present study.
3.2 Correlation between β-glucosidase activities and fungal growth kinetics
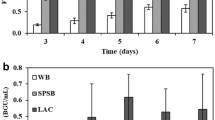
Figure 1a shows that, for T. aurantiacus cultivated under the previously determined, best conditions, during the phase of fast increase in biomass (exponential phase), there was a simultaneous increase in β-glucosidase activity. When the stationary phase of growth was reached, β-glucosidase activity also became almost constant, which means that enzyme activity production is associated with fungal growth. Logistic equation (Eq. 2), with substitution of X by P and of μ by k (here defined as a specific kinetics constant of β-glucosidase production), also fitted well (r2 = 0.95) to the experimental data of β-glucosidase activities over time. This reiterates the similarity between the curves of fungal growth and of enzyme activity kinetics, addressing the enzyme is a growth-associated product. Moreover, the fitted equation may be combined with SSC models and the fitting parameters values Pmax = 14.1 U/gss, P0 = 0.23 U/gss, and k = 0.080 h−1 can be useful for simulations and predictions of β-glucosidase yields when this cultivation is held at a pilot-scale, for instance.
Many authors reported linear relations between extracellular enzymes and microbial growth in SSC systems. For instance, Palma [27] correlated growth kinetics (estimates based on N-acetyl-glucosamine content) and kinetics of xylanase production of T. aurantiacus; the author observed association between biomass formation and enzyme production during the exponential phase, with enzymatic activity increasing with the increase of biomass, similar to that observed in the current paper. Kalogeris et al. [28] have also reported good correlation between T. aurantiacus growth on WB and production of cellulases and xylanase.
Similarly, for T. reesei cultivated on the best previously determined substrate composition and moisture content, Fig. 1b also shows simultaneous increases of fungal biomass and β-glucosidase activities at early stages of the cultivation. However, in the later stages, fungal biomass reached a constant value before β-glucosidase activities became constant. The logistic equation adapted to β-glucosidase production also fitted well (r2 = 0.97) to the experimental data of β-glucosidase activities over time of cultivation of T. reesei, with fitting parameters Pmax = 0.74 U/gss, P0 = 0.016 U/gss, and k = 0.040 h−1. In this case, the enzyme was non-growth-associated, but it was continuously produced independently of the stage of growth.
Studies on correlation between enzymatic activity and microbial growth are another interesting alternative for evaluating metabolic activity in SSC systems and it enables predicting process yields, mainly when enzymes are the interesting product [29].
Smits et al. [29] reported xylanase production was growth-associated under cultivation of T. reesei on WB, agreeing with the findings of the current paper, which supports an interpretation that the type of substrate, moisture, and microorganism can affect the hydrolytic enzyme production. On the above, monitoring fungal growth kinetics in SSC can indicate the stages of production of interesting metabolites from the cultivation, and with this, determine the right time to stop the growth, resulting in cost reduction. Moreover, kinetics parameters of fungal growth and of enzyme production are reliable for application in further modeling and simulation studies aimed at the design and operation of SSC bioreactors.
3.3 Saccharification of SCB by applying the raw enzymatic extracts
Prior to the application of the cellulolytic enzymes produced by T. aurantiacus CBMAI756 and T. reesei QM9414 on SCB saccharification, CMCase, FPU [15] and β-glucosidase [17] activities were determined (Table 2). It is possible to observe that enzymatic activities obtained for the extract produced by the mesophilic fungus were much lower than those obtained for the one produced by the thermophilic fungus under the best cultivation conditions, which is in agreement with De Cassia Pereira et al. [27].
Leite et al. [17], when evaluating the production of β-glucosidases by SSC employing the thermophilic fungus T. aurantiacus and the mesophilic fungus Aureobasidium pullulans on different substrates, had also reported that the thermophilic microorganism provided greater activities in comparison with the mesophilic fungus. The results also confirm that T. aurantiacus is well suited to the solid culture process and it is recognized for secreting high levels of cellulolytic enzymes into the medium [12].
The results of enzymatic activities CMCase (140.7 U/gss), FPU (10.1 U/gss), and β-glucosidase (35.7 U/gss) obtained in this study for T. aurantiacus are promising when compared with other works in the literature that used fungi of the same genus to produce enzymes on different substrates, in which CMCases and β-glucosidase activities ranged from 53.2 to 1709.0 U/gss and 8.9 to 105.0 U/gss, respectively [17, 28, 30].
Similarly, the results of activities obtained for the extract produced by the fungus T. reesei by solid cultivation, CMCase (73.7 U/gss), FPU (5.8 U/gss) and β-glucosidase (4.1 U/gss), are comparable to others found in the literature, considering that the results of the present study were similar to those reported by several authors for enzyme production, using fungi of the same genus on solid substrates, in which CMCase, FPU and β-glucosidase activities varied from 5.7 to 55.7 U/gss; 4.0 to 7.7 U/gss and 5.0 to 11.2 U/gss, respectively [31, 32].
Agreeing with our results, T. reesei is recognized for its remarkable ability to secrete a pool of saccharifying enzymes, although it has a deficiency in the production of β-glucosidases [10]. This deficiency in the expression of the β-glucosidase enzyme by the fungus T. reesei results in the accumulation of cellobiose, which inhibits the action of cellobiohydrolases and endoglucanases, decreasing the rate of hydrolysis [33]. Therefore, for the effective application in enzymatic saccharification of SCB, supplementation of the enzyme extract produced by T. reesei with β-glucosidase is necessary, in order to increase the conversion efficiency of cellulose to glucose [10].
Yields of total reducing sugars (TRS) released in the saccharification of SCB by applying the raw enzymatic extracts for 24 h, expressed as mg of TRS per gram of SCB, are presented in Table 3. It is possible to observe that the best result (approximately 124 mg TRS/g SCB) was obtained when applying 9 mL of the enzymes cocktail composed by equal parts of extracts from the cultivation of T. aurantiacus and T. reesei at 65 °C. This dosage is equivalent to an enzyme load of 3.6 FPU/gSCB, 2.25 FPU/gSCB provided by extract from T. aurantiacus and 1.35 FPU/gSCB by extract from T. reesei.
Perrone et al. [19] performed hydrolysis using the commercial enzyme Prozyn® (São Paulo, Brazil), with a cellulase dosage of 32 FPU per gram of bagasse (dry basis) in sugarcane bagasse without pretreatment, and obtained 15 mg/g glucose, which corresponds to a glucose yield of 3%. Moretti et al. [34] performed hydrolysis by applying enzymes produced by the fungus Myceliophthora thermophila M.7.7. by solid culture and also the commercial enzyme Celluclast 1.5 L (Novozymes®) in untreated sugarcane bagasse (control). The authors reported that 17.9 mg/g and 27.5 mg/g, respectively, were obtained, corresponding to yields of 3 and 3.1%. Therefore, the results obtained in the present work are promising when compared to those reported in the literature for enzymatic hydrolysis of untreated sugarcane bagasse.
Statistical analysis of the Box-Behnken design pointed out a significant influence of the quadratic term of volume of enzymes. One may also observe that when the enzymatic extract produced from the cultivation of T. aurantiacus is applied (T. aurantiacus/T. reesei 1:0 v/v), TRS increase as temperature increases, since the enzymes from this thermophilic fungus do not act well at 45 °C, reducing the efficiency of the hydrolysis. On the other hand, when the enzymatic extract produced from the cultivation of T. reesei is applied (T. aurantiacus/T. reesei 0:1 v/v), TRS decrease as temperature increases, due to the start of thermal denaturation of the enzymes from the mesophilic strain at 65 °C. Such observations are reflected by a near-significant (p = 0.097) effect of interaction between hydrolysis temperature and composition of the enzymes cocktail.
Cellulase preparations from T. reesei usually have to be supplemented with β-glucosidase from other sources for cellulose saccharification on an industrial scale [35]. In this context, tests with T. aurantiacus/T. reesei 1:1 v/v at 65 °C may have released more TRS due to the synergistic effect between exo and endoglucanases from T. reesei and β-glucosidase from T. aurantiacus, since the extract from the thermophilic fungus presents high β-glucosidase activity at 65 °C, resulting in a near-significant effect of quadratic term of composition of the cocktail (p = 0.137).
T. reesei is known to produce at least two exoglucanases (CBHI and CBHII), five endoglucanases (EGI, EGII, EGIII, EGIV, and EGV), and two β-glucosidases (BGLI and BGLII) [36]. CBHI and CBHII and EGI and EGII are produced in an approximate proportion of 6:2:1:1 (w/w), i.e., endoglucanases represent only 20% of the cellulase system produced by T. reesei. However, exo and endoglucanases comprise 90% of the system, while β-glucosidases secreted by this fungus represent less than 1% of the cellulolytic extract produced. Once cellobiose—the major product of CBHI and CHBII activity—inhibits the activity of the CBHs and EGs [36] and T. reesei produces β-glucosidases at low levels, cellulase preparations from T. reesei usually have to be supplemented with β-glucosidase from other sources for cellulose saccharification on industrial scale [35, 36]. In this context, tests with T. aurantiacus/T. reesei 1:1 v/v at 65 °C have released more TRS probably due to the synergistic effect between CBHs and EGs from T. reesei and β-glucosidase from T. aurantiacus, since the extract from the thermophilic fungus presented high β-glucosidase activity at this temperature, as also reported by Leite et al. [17] and to a near-significant influence of the quadratic term of composition of the enzymes cocktail (p = 0.137). This increase in hydrolysis seen with this enzymatic cocktail could also be partially due to the presence in of oxidative enzymes of the GH61 family, which have recently been shown [37, 38] to play a key role in the cellulosic biomass hydrolysis (not accessed in this work).
By anion-exchange chromatography, it has been possible to evaluate the sugar content of the hydrolysates of SCB using isolated extracts of T. aurantiacus and of T. reesei or by a mixture of them in a cocktail (Fig. 2). Xylose content did not differ significantly between the hydrolysates, while glucose content was significantly higher in the hydrolysate resulting from the application of the cocktail. An increase of approximately 20% in the amount of glucose was achieved if the cocktail is compared with the extract from T. aurantiacus alone, while glucose concentration increases around 100% when the cocktail is compared to the extract from T. reesei alone. Comparing the application of the extract from T. aurantiacus alone with the one from T. reesei alone, as well as the glucose content being 75% higher in the hydrolysate using enzymes of the thermophilic fungus, the cellobiose content (dimers which were not converted into glucose) draws attention. In the hydrolysate using enzymes from T. reesei, the concentration of cellobiose exceeds the glucose concentration by around 50%. This is a consequence of the β-glucosidase deficiency of the extract from the mesophilic fungus. Cellobiose is a disaccharide considered a cellulase inhibitor [10], leading to incomplete hydrolysis of the cellulose. As already mentioned, T. reesei is reported to be deficient in synthetizing β-glucosidases [10], which are the enzymes responsible for cleaving cellobiose residues into glucose. As that extract contains a small amount of these enzymes, cellobiose accumulates in the reaction medium, impairing the overall efficiency of the enzymatic hydrolysis process.
Sugar contents (HPAEC-PAD) in hydrolysates of SCB by isolate and mixed extracts, 5 mL of enzyme, 24 h, 55 °C; (grayscale rectangle) cellobiose; (black rectangle) glucose; (white rectangle) xylose. For T. aurantiacus, hydrolysis with 12.5 FPU/gSCB at 65 °C; for T. reesei, 7.5 FPU/gSCB at 45 °C; for cocktail, 3.6 FPU/gSCB at 65 °C
With the objective of verifying the synergistic effect between the enzymes contained in the pool secreted by each fungus in SSC, at the central point (5 mL, 1:1 and 55 °C), experiments varying time of saccharification were performed by taking independent samples every 4 h over 24 h for determination of the hydrolysis kinetic of SCB. It is observed that TRS released increases over time (Fig. 3a).
For every analysis time, higher efficiency of hydrolysis was obtained when applying the enzymatic cocktail (Fig. 3a). Concerning the individual extracts, the one produced by T. reesei was more efficient than the one produced by T. aurantiacus up to 4 h but then it was overtaken by the extract produced by the thermophilic fungus and even more so by the enzymatic cocktail. This suggests a partial denaturation of some enzymes secreted by the mesophilic fungus is likely over increasing time of exposure at 55 °C. The cocktail of extracts produced by fungi with different thermal preferences and its application at an intermediate temperature achieved a synergistic effect between the enzymes secreted by both fungi, resulting in an improvement in the efficiency of the saccharification of SCB. Concerning the hydrolysis kinetics, similar trends have been reported by Bi et al. [39].
According to Lopes et al. [40], mixtures of varied enzymes from different sources usually lead to an increase in hydrolysis rate. This was observed in the present work, which showed a 26% increase compared to T. aurantiacus and 59% to T. reesei when the enzymatic cocktail was applied. Braga et al. [41] supplemented a commercial preparation (Celluclast 1.5 L; Novozymes) with 30% of the crude extract from Aspergillus oryzae P21C3, a good producer of xylanase and feruloylesterases, resulting in an enhancement of 36% in the conversion of cellulose from pretreated sugarcane bagasse.
Arias et al. [42] in the enzymatic breakdown of sugarcane bagasse by crude extracts from Trichoderma harzianum, Penicillium funiculosum, and A. niger obtained 42, 66, and 1%, respectively. On the other hand, the optimized cocktail comprising 50% of the crude extract from P. funiculosum, and 35 and 15% of the crude extracts from A. niger and T. harzianum, respectively, showed a hydrolysis yield of sugarcane bagasse of 91%, confirming the synergism of enzymes in the hydrolysis. In another study, an optimized enzymatic cocktail developed to hydrolyze sugarcane bagasse, comprising 80% T. reesei fraction, 10% endoglucanase (recombinant enzyme from Bacillus subtilis), and 10% BGL (from A. niger), converted 72% of cellulose present in hydrothermally pretreated bagasse [43]. The lower conversions achieved in the current work are very likely related to the use of untreated sugarcane bagasse with lower enzymatic loads.
The local derivatives of the experimental curve of TRS as a function of time gave the rate of TRS release (Fig. 3b). From 8 h of hydrolysis on, the rate of reaction decreases and remains almost constant, likely due to both partial thermal denaturation of the enzymes and gradual reduction of substrate concentration.
Kaschuk et al. [5] studied the production of reducing sugars obtained from the enzymatic hydrolysis of sisal pulp, microcrystalline cellulose and filter paper using cellulases from Aspergillus sp. CBMAI 1198. The authors reported that for microcrystalline cellulose and sisal pulp, the amounts of reducing sugars produced were close, and a large increase in the production was observed up to 10 h, followed by a deceleration up to 48 h. However, for the enzymatic hydrolysis of the filter paper, higher amounts of reducing sugars were produced from the beginning of the reaction until 23 h, when a deceleration was observed. This deceleration observed has already been reported in other studies, and may be attributed to changes in the substrates or related to the increase in reaction products (glucose and oligosaccharides) that can inhibit the enzyme cellulases in the reaction medium [5].
An exponential decay curve was fitted to the experimental points, resulting in the following Eq. (3):
where d(TRS)/dt|0 is the initial reaction rate; A is a pre-exponential factor and 1/n is a constant on the exponential argument.
The parameters for the fitting of exponential decay to the hydrolysis kinetics were: d(TRS)/dt|0 = 1.07; A = 3.85 and n = 5.34 (r2 = 0.86) for T. aurantiacus; d(TRS)/dt|0 = − 3.87; A = 8.89 and n = 15.76 (r2 = 0.86) for T. reesei; d(TRS)/dt|0 = 1.67; A = 5.95 and n = 4.61 (r2 = 0.94) for the enzymatic cocktail.
The parameters of the fitting of exponential decay to hydrolysis kinetics are useful parameters for design and optimization of the hydrolysis operation in terms of its extension to bioreactor use, as well as for simulation of the process.
Based on the saccharification results, applying combined raw enzymatic extracts of T. aurantiacus and T. reesei for enzymatic hydrolysis of SCB is very interesting in the production of ethanol 2G, since a reasonably acceptable level of TRS is released, which can then be used for yeast fermentation.
4 Conclusions
The enzymatic extract produced by T. aurantiacus presents higher β-glucosidase, CMCase and filter paper activities than the one produced by T. reesei, hence it can be said the thermophilic fungus is better suited to the SSC process. Total protein content quantification enabled the estimation of fungal biomass data and fitting the logistic model to describe the growth. For both fungi, comparing kinetics of estimated fungal growth and of β-glucosidase activity, T. aurantiacus shows a growth-associated production and T. reesei shows an enzyme production independent of stage. Optimal saccharification of SCB was achieved by using 9 mL of an enzymatic cocktail composed of equal volumes of both fungal extracts, totaling 3.6 FPU/gSCB, at 65 °C. Once higher efficiency of hydrolysis was obtained for the application of the enzymatic cocktail with lower enzyme load, a synergy between enzymes from both extracts is addressed. The major contributions of this work are twofold: hydrolytic enzyme production is related to fungal growth, so it can express process kinetics; and a low-cost in-house produced enzymatic cocktail, using a combination of T. reesei and T. aurantiacus enzymes, is effective for lignocellulose saccharification at a high temperature and with low enzyme load.
References
OECD/IEA (2010) Sustainable production of second-generation biofuels. https://www.iea.org/publications/freepublications/publication/second_generation_biofuels.pdf. Accessed 23 June 2017
Jeon YJ, Xun Z, Rogers PL (2010) Comparative evaluations of cellulosic raw materials for second generation bioethanol production. Lett Appl Microbiol 51:518–524. https://doi.org/10.1111/j.1472-765X.2010.02923.x
UNICA (2018) União da Indústria de Cana-de-Açúcar. https://www.unicadata.com.br/historico-de-producao-e-moagem.php. Accessed 05 Aug 2018
Mishima D, Tateda M, Ike M, Fujita M (2006) Comparative study on chemical pretreatments to accelerate enzymatic hydrolysis of aquatic macrophyte biomass used in water purification processes. Bioresour Technol 97:2166–2172. https://doi.org/10.1016/j.biortech.2005.09.029
Kaschuk JJ, Santos DA, Frollini E, Canduri F, Porto ALM (2019) Influence of pH, temperature, and sisal pulp on the production of cellulases from Aspergillus sp. CBMAI 1198 and hydrolysis of cellulosic materials with different hemicelluloses content, crystallinity, and average molar mass. Biomass Conv Bioref 1–12. https://doi.org/10.1007/s13399-019-00440-2
Pandey A (2003) Solid-state fermentation. Biochem Eng J 13:81–84. https://doi.org/10.1016/S1369-703X(02)00121-3
Pandey A, Soccol CR, Mitchell D (2000) New developments in solid state fermentation: I-bioprocesses and products. Process Biochem 35:1153–1169. https://doi.org/10.1016/S0032-9592(00)00152-7
Desgranges C, Vergoignan C, Georges M, Durand A (1991) Biomass estimation in solid state fermentation. 1. Manual biochemical methods. Appl Microbiol Biotechnol 35:200–205. https://doi.org/10.1007/BF00184687
Jiang B, Tsao R, Li Y, Miao M (2014) Food safety: food analysis technologies/techniques. In: Van Alfen NK (ed) Encyclopedia of Agriculture and Food Systems, 2nd edn. Academic Press, pp 273–288. https://doi.org/10.1016/B978-0-444-52512-3.00052-8
Florencio C, Badino AC, Farinas CS (2017) Desafios relacionados à produção e aplicação das enzimas celulolíticas na hidrólise da biomassa lignocelulósica. Quim Nova 40:1082–1093. https://doi.org/10.21577/0100-4042.20170104
Sørensen HR, Pedersen S, Jørgensen CT, Meyer AS (2007) Enzymatic hydrolysis of wheat arabinoxylan by a recombinant “minimal” enzyme cocktail containing beta-xylosidase and novel endo-1, 4-beta-xylanase and alpha-l-arabinofuranosidase activities. Biotechnol Prog 23:100–107. https://doi.org/10.1021/bp0601701
Da Silva R, Lago ES, Merheb CW, Macchione MM, Park YK (2005) Production of xylanase and CMCase on solid state fermentation in different residues by Thermoascus aurantiacus Miehe. Braz J Microbiol 36:235–241. https://doi.org/10.1590/S1517-83822005000300006
McClendon SD, Batth T, Petzold CJ, Adams PD, Simmons BA, Singer SW (2012) Thermoascus aurantiacus is a promising source of enzymes for biomass deconstruction under thermophilic conditions. Biotechnol Biofuels 5:54. https://doi.org/10.1186/1754-6834-5-54
Zanelato AI, Shiota VM, Gomes E, Thoméo JC (2012) Endoglucanase production with the newly isolated Myceliophtora sp. i-1d3b in a packed bed solid state fermentor. Braz J Microbiol 43:1536–1544. https://doi.org/10.1590/S1517-83822012000400038
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Leite RSR, Alves-Prado HF, Cabral H, Pagnocca FC, Gomes E, Da Silva R (2008) Production and characteristics comparison of crude β-glucosidases produced by microorganisms Thermoascus aurantiacus e Aureobasidium pullulans in agricultural wastes. Enzym Microb Technol 43:391–395. https://doi.org/10.1016/j.enzmictec.2008.07.006
Viccini G, Mitchell DA, Boit SD, Gern JC, da Rosa AS, Costa RM, Dalsenter FDH, Von Meien OF, Krieger N (2001) Analysis of growth kinetic profiles in solid-state fermentation. Food Technol Biotechnol 39:271–294
Perrone OM, Colombari FM, Rossi JS, Moretti MMS, Bordignon SE, Nunes CCC, Gomes E, Boscolo M, Da Silva R (2016) Ozonolysis combined with ultrasound as a pretreatment of sugarcane bagasse: effect on the enzymatic saccharification and the physical and chemical characteristics of the substrate. Bioresour Technol 218:69–76. https://doi.org/10.1016/j.biortech.2016.06.072
Casciatori FP, Laurentino CL, Taboga SR, Casciatori PA, Thoméo JC (2014) Structural properties of beds packed with agro-industrial solid by-products applicable for solid-state fermentation: experimental data and effects on process performance. Chem Eng J 255:214–224. https://doi.org/10.1016/j.cej.2014.06.040
Grajek W (1987a) Comparative studies on the production of cellulases by thermophilic fungi in submerged and solid state fermentation. Appl Microbiol Biotechnol 26:126–129. https://doi.org/10.1007/BF00253895
Pinto TOP (2010) Cellulolytic enzymes production by fungi Thermoascus aurantiacus CBMAI 756, Thermomyces lanuginosus, Trichoderma reesei QM9414 and Penicillium viridicatum RFC3 and application in saccharification of sugarcane bagasse with different pretreatments. Dissertation, São Paulo State University
Gomes E, Umsza-Guez MA, Martin N, Silva R (2007) Enzimas termoestáveis: fontes, produção e aplicação industrial. Quim Nova 30:136–145. https://doi.org/10.1590/S0100-40422007000100025
De Cassia PJ, Paganini Marques N, Rodrigues A, Brito de Oliveira T, Boscolo M, Da Silva R, Gomes E, Bocchini Martins DA (2015) Thermophilic fungi as new sources for production of cellulases and xylanases with potential use in sugarcane bagasse saccharification. J Appl Microbiol 118:928–939. https://doi.org/10.1111/jam.12757
Grajek W (1986) Temperature and pH optimum of enzyme activities produced by cellulolytic thermophilic fungi in batch and solid-state celulases. Biotechnol Lett 8:587–590. https://doi.org/10.1007/BF01028089
Grajek W (1987b) Production of D-xylanases by thermophilic fungi using different methods of culture. Biotechnol Lett 9:353–356. https://doi.org/10.1007/BF01025803
Palma MB (2003) Xylanases production by Thermoascus aurantiacus in solid statecultivation. Thesis, Federal University of Santa Catarina
Kalogeris E, Christakopoulos P, Katapodis P, Alexiou A, Vlachou S, Kekos D, Macris BJ (2003a) Production and characterization of cellulolytic enzymes from the thermophilic fungus Thermoascus aurantiacus under solid state cultivation of agricultural wastes. Process Biochem 38:1099–1104. https://doi.org/10.1016/S0032-9592(02)00242-X
Smits JP, Rinzema A, Tramper J, Van Sonsbeek HM, Knol W (1996) Solid-state fermentation of wheat bran by Trichoderma reesei QM9414: substrate composition changes, C balance, enzyme production, growth and kinetics. Appl Microbiol Biotechnol 46:489–498. https://doi.org/10.1007/s002530050849
Kalogeris E, Iniotaki F, Topakas E, Christakopoulos P, Kekos D, Macris BJ (2003b) Performance of an intermittent agitation rotating drum type bioreactor for solid-state fermentation of wheat straw. Bioresour Technol 86:207–213. https://doi.org/10.1016/S0960-8524(02)00175-X
Basso TP, Gallo CR, Basso LC (2010) Atividade celulolítica de fungos isolados de bagaço de cana-de-açúcar e madeira em decomposição. Rev Agropec Bras 45:1282–1289. https://doi.org/10.1590/S0100-204X2010001100008
King BC, Donnelly MK, Bergstrom GC, Walker LP, Gibson DM (2009) An optimized microplate assay system for quantitative evaluation of plant cell wall-degrading enzyme activity of fungal culture extracts. Biotechnol Bioeng 102:1033–1044. https://doi.org/10.1002/bit.22151
Kovács K, Szakacs G, Zacchi G (2009) Comparative enzymatic hydrolysis of pretreated spruce by supernatants, whole fermentation broths and washed mycelia of Trichoderma reesei and Trichoderma atroviride. Bioresour Technol 100:1350–1357. https://doi.org/10.1016/j.biortech.2008.08.006
Moretti MMS, Bocchini-Martins DA, Nunes CCC, Villena MA, Perrone OM, Da Silva R, Boscolo M, Gomes E (2014) Pretreatment of sugarcane bagasse with microwaves irradiation and its effects on the structure and on enzymatic hydrolysis. Appl Energy 122:189–195. https://doi.org/10.1016/j.apenergy.2014.02.020
Margeot A, Hahn-Hagerdal B, Edlund M, Slade R, Monot F (2009) New improvements for lignocellulosic ethanol. Curr Opin Biotechnol 20:372–380. https://doi.org/10.1016/j.copbio.2009.05.009
Lynd LR, Weimer PJ, Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577. https://doi.org/10.1128/MMBR.66.3.506-577.2002
Jung S, Song Y, Kim HM, Bae HJ (2015) Enhanced lignocellulosic biomass hydrolysis by oxidative lytic polysaccharide monooxygenases (LPMOs) GH61 from Gloeophyllum trabeum. Enzym Microb Technol 77:38–45. https://doi.org/10.1016/j.enzmictec.2015.05.006
Ezeilo UR, Zakaria II, Huyop F, Wahab RA (2017) Enzymatic breakdown of lignocellulosic biomass: the role of glycosyl hydrolases and lytic polysaccharide monooxygenases. Biotechnol Biotechnol Equip 31:647–662. https://doi.org/10.1080/13102818.2017.1330124
Bi S, Peng L, Chen K, Zhu Z (2016) Enhanced enzymatic saccharification of sugarcane bagasse pretreated by combining O2 and NaOH. Bioresour Technol 214:692–699. https://doi.org/10.1016/j.biortech.2016.05.041
Lopes AM, Ferreira Filho EX, Moreira LRS (2018) An update on enzymatic cocktails for lignocellulose breakdown. J Appl Microbiol 125:632–645. https://doi.org/10.1111/jam.13923
Braga CMP, Delabona PDS, Lima DJDS, Paixão DAA, Pradella JGDC, Farinas CS (2014) Addition of feruloyl esterase and xylanase produced onsite improves sugarcane bagasse hydrolysis. Bioresour Technol 170:316–324. https://doi.org/10.1016/j.biortech.2014.07.115
Arias JM, Modesto LFA, Polikarpov I, Pereira-Jr N (2016) Design of an enzyme cocktail consisting of different fungal platforms for efficient hydrolysis of sugarcane bagasse: optimization and synergism studies. Biotechnol Prog 32:1222–1229. https://doi.org/10.1002/btpr.2306
Bussamra BC, Freitas S, Costa AC (2015) Improvement on sugar cane bagasse hydrolysis using enzymatic mixture designed cocktail. Bioresour Technol 187:173–181. https://doi.org/10.1016/j.biortech.2015.03.117
Acknowledgments
The authors would like to acknowledge the National Council for the Improvement of Higher Education (CAPES) and the São Paulo Research Foundation (FAPESP) for their financial support and scholarships [grant numbers 2010/12624-0, 2011/21239-6, 2011/07453-5, 2012/02768-0, 2013/01756-1, 2017/16482-5, 2018/00996-2] and the National Council for Scientific and Technological Development (CNPQ) [grant number 426578/2016-3].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Thermophilic fungus yielded more β-glucosidase than the mesophilic one, in a shorter time;
• Fungal biomass was well estimated based on total protein content by the Kjeldahl method;
• β-Glucosidase activity production was strongly and directly associated with fungal growth;
• Enzymes in cocktail have a synergistic effect on the hydrolysis of sugarcane bagasse.
Rights and permissions
About this article
Cite this article
Frassatto, P.A.C., Casciatori, F.P., Thoméo, J.C. et al. β-Glucosidase production by Trichoderma reesei and Thermoascus aurantiacus by solid state cultivation and application of enzymatic cocktail for saccharification of sugarcane bagasse. Biomass Conv. Bioref. 11, 503–513 (2021). https://doi.org/10.1007/s13399-020-00608-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00608-1