Abstract
The high cost of commercial cellulases still hampers the economic competitiveness of the production of fuels and chemicals from lignocellulosic biomasses. This cost may be decreased by the on-site production of cellulases with the integrated use of the lignocellulosic biomass as carbon source. This integrated approach was evaluated in the present study whereby steam-pretreated sugarcane bagasse (SPSB) was used as carbon source for the production of cellulases by Trichoderma reesei Rut C30. The use of SPSB resulted in an enzyme preparation with a cellulase activity of 1.93 FPU/mL. Additionally, a significant β-glucosidase activity, of 0.37 BGU/mL, was achieved in buffered media, which is not a common feature of T. reesei culture supernatants, indicating the importance of pH control during enzyme production. The hydrolysis of SPSB with the laboratory-made mixture resulted in a glucose yield of 80%, which was equivalent to those observed for control experiments using commercial enzymes. It was shown that SPSB is a promising carbon source for the production of cellulases and β-glucosidases by T. reesei Rut C30 and that the enzyme preparation obtained is effective for the hydrolysis of SPSB without the need for β-glucosidases supplementation, supporting the on-site integrated approach to decrease the cost of the enzymatic hydrolysis of lignocellulosic biomass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last few years, several industrial facilities aiming at the production of cellulosic ethanol through the enzymatic hydrolysis of biomass have begun operating in different localities (Lynd et al. 2017). In Brazil, there are two facilities aiming at producing ethanol from sugarcane bagasse and straw, highly available feedstocks that amount to about 180 million tons of dry biomass per year (Ferreira-Leitão et al. 2010a; CONAB 2018). Nevertheless, this industry still faces several challenges, with economic studies showing that enzyme costs significantly affect the minimum ethanol selling price, resulting in a negative impact in the technology’s competitiveness in the biofuels market (Macrelli et al. 2012; Klein-Marcuschamer et al. 2012; Longati et al. 2018).
Industrial production of cellulases for lignocellulosic biomass hydrolysis is predominantly done by the companies Genencor (now part of DuPont) and Novozymes in a model of off-site centralized production and distribution (Singhania et al. 2017). Even though these companies have succeeded in lowering the costs associated with the production of cellulases, techno-economic analyses suggest that the on-site production of enzymes may further contribute to the necessary cost decrease (Liu et al. 2016). This is owing to the elimination of enzyme transportation costs and downstream steps (concentration and stabilization). Indeed, this approach has been shown feasible as cellulases have been already produced on site at a demonstration plant for the production of ethanol for sugarcane bagasse in Thailand (NEDO 2017). This is especially relevant to countries like Brazil, whose continental sizes are obstacles to the transportation of enzymes produced in an off-site centralized fashion.
Moreover, the choice of carbon source is a very important process parameter, since it has a significant influence on the cost of enzyme production (Klein-Marcuschamer et al. 2012) and on the final cellulase activity (Zhang et al. 2012; Silva et al. 2018). Pretreated lignocellulosic biomasses have been shown to have inducing effects on cellulase secretion, depending on the chosen pretreatment, even resulting in higher β-glucosidase production (Zheng et al. 2017). This is a quite interesting scenario, as the pretreated feedstock to be hydrolyzed would be readily available for the on-site cellulases production, reducing even more the production costs (Johnson 2016). Pretreatments based on the use of steam are considered effective for sugarcane bagasse, resulting in high glucose yields (Ferreira-Leitão et al. 2010b; Fockink et al. 2018). These pretreatments remove the hemicellulose, yielding a more exposed cellulose fraction to the action of cellulases, and are already being used in industrial cellulosic ethanol facilities (Salles-Filho et al. 2016).
Trichoderma reesei is the most widely studied fungus for cellulase production and the most commonly used species for industrial production of these enzymes (Peterson and Nevalainen 2012; Paloheimo et al. 2016). T. reesei Rut C30, probably the best-known mutant, was selected because of its catabolite derepression and its high protein titers (Montenecourt and Eveleigh 1979), which are about three times higher than those of the wild-type QM6a, selected by Mandels and Weber in the late 1960’s (Mandels and Weber 1969; Ryu and Mandels 1980). However, its secreted enzyme mixture has typically been reported as unbalanced regarding the cellulase:β-glucosidase ratio, which may limit the enzymatic hydrolysis of cellulose into glucose (Martins et al. 2008; Kovács et al. 2009; Zhao et al. 2018) and result in the accumulation of cellobiose, a dimer of glucose that causes cellulase inhibition (Gruno et al. 2004). To overcome this drawback, T. reesei cellulases are frequently supplemented with β-glucosidase from Aspergillus (Gottschalk et al. 2010; van den Brink et al. 2014), although the heterologous expression of β-glucosidase genes in T. reesei has also been studied (Zhang et al. 2012; Ellilä et al. 2017). Nevertheless, hardly any investigation has been done regarding the influence of the growth media on β-glucosidase production by T. reesei and the minimum cellulase:β-glucosidase ratio necessary for an efficient hydrolysis.
Therefore, the aim of the present work was to obtain a balanced cellulase and β-glucosidase enzyme preparation from Trichoderma reesei Rut C30 in an integrated production approach using steam-pretreated sugarcane bagasse (SPSB) as carbon source, as this substrate would be readily available in a cellulosic ethanol refinery for the on-site production of cellulases. The laboratory-made enzymes, together with commercial cocktails, were compared for their adequacy in hydrolyzing SPSB without supplementation of β-glucosidase.
Material and methods
Sugarcane bagasse and pretreatment conditions
Sugarcane bagasse was provided by the Centro de Tecnologia Canavieira (CTC), Piracicaba, São Paulo, Brazil and pretreated at the Department of Chemical Engineering, Lund University, Sweden. Prior to steam pretreatment, the bagasse was milled to < 2 mm and impregnated overnight with 3% CO2 at 5 °C (Ferreira-Leitão et al. 2010b). The impregnated material was steam-pretreated using a unit equipped with a 10-L reactor vessel at 220 °C for 5 min. The liquid fraction was separated and the resulting solid stream, called here steam-pretreated sugarcane bagasse (SPSB), was characterized according to standard procedures (Sluiter et al. 2012) and its composition was 59.1% glucan, 4.0% xylan and 31.2% lignin. The pretreated biomass was dried at 60 °C and stored in plastic bags in a refrigerator before further use.
Microorganisms maintenance and propagation
Trichoderma reesei Rut C30 (ATCC 56765) stock cultures were maintained on agar plates containing potato-dextrose agar. After incubation at 30 °C for 7 days, these were observed as greenish sporulating cultures. To prepare the submerged culture inoculum, conidia were suspended in 5 mL of sterile water, and 1 mL of this suspension (106 spores) was transferred to a 500-mL Erlenmeyer flask containing 100 mL of sterile Mandels medium (Mandels and Weber 1969). The inoculum culture was incubated at 30 °C and 200 rpm for 4 days.
Aspergillus awamori (2B.361 U2/1, classified by the Commonwealth Mycological Institute in the Aspergillus niger complex) maintenance and spore suspension preparation were performed as described for T. reesei Rut C30, but with blackish sporulating cultures. Inoculum preparation was completed by 2 days of cultivation at 30 °C and 200 rpm in sterile growth media containing (in g dry matter /L): 1.2 NaNO3, 3.0 KH2PO4, 6.0 K2HPO4, 0.2 MgSO4·7H2O, 0.05 CaCl2, 12 yeast extract and 30 native sugarcane bagasse as carbon source.
Enzyme production
For enzyme production with Trichoderma reesei Rut C30, 30 mL of the mycelium suspension from the inoculum cultures was used to initiate growth in a 1-L flask containing 300 mL of sterile growth medium as follows (in g dry matter/L): 0.3 urea, 1.4 (NH4)2SO4, 2.0 KH2PO4, 0.3 CaCl2, 0.3 MgSO4·7H2O, 6.0 yeast extract and 30 of either lactose (LAC, purity > 98%—Merck, Darmstadt, Germany), wheat bran (WB—Jasmine Alimentos, Paraná, Brazil) or SPSB as carbon source, plus 0.6% (v/v) corn steep liquor. Media were supplemented with trace elements as follows (in mg dry matter/L): 5 FeSO4·7H2O, 20 CoCl2, 1.6 MnSO4 and 1.4 ZnSO4. Media were buffered with 100 mM sodium phosphate buffer pH 6.0. Unbuffered fermentations with lactose as the carbon source were also carried out. The production cultures were incubated in an orbital shaker at 30 ºC and 200 rpm up to 7 days. Samples (10 mL) were taken every 24 h for the measurement of filter paper (FPase) and β-glucosidase activities and the determination of pH.
For enzyme production with Aspergillus awamori, a total volume of 30 mL of mycelium suspension, obtained from inoculum cultures, was used to initiate growth in a 1-L flask containing 300 mL of the sterile fresh medium described in the previous section. The culture was incubated in an orbital shaker at 30 ºC and 200 rpm up to 7 days.
Enzyme assays and analysis methods
The activities of total cellulase (filter paper activity, FPase) and β-glucosidase were determined according to standard IUPAC procedures (Ghose 1987). One unit of FPase (FPU) corresponded to the release of 1 micromole of glucose per minute using an enzyme dilution providing 2 mg of glucose after 60 min assay reaction. One unit of β-glucosidase (BGU) was defined as the amount of enzyme that released 1 µmol of glucose in 1 min at 50 °C. Glucose concentrations for the β-glucosidase assay were measured using a biochemical analyzer (YSI 2700 Select, Yellow Springs, Ohio, USA).
Enzymatic hydrolysis of SPSB
Hydrolysis experiments were performed in 250-mL Erlenmeyer flasks containing 100 mL of the reaction mixture in sodium citrate buffer (50 mM), pH 5.0 and incubated at 50 °C and 200 rpm in an orbital shaker for 48 h. The reaction mixtures contained either 7.5% or 2.5% (w/v) SPSB, depending on the experiment, which had previously been dried overnight at 60 °C, plus the relevant enzyme pool. Enzymes, either produced in this work or commercially prepared (GC 220 and Spezyme® CP from Genencor®), were loaded into the reaction mixture with 15 FPU/g of dry biomass. The low solids content and high cellulase load were chosen to minimize mass transfer problems and allow the sole evaluation of the hydrolysis potential of the studied enzyme preparations.
The supernatants from the 7th day of cultivation were used as source of the enzymes produced on lactose, wheat bran or SPSB. For the experiments with different FPU:BGU ratios, a concentrated supernatant of the 7th day of cultivation in unbuffered lactose medium was used for the enzyme preparation with the 1:0.06 FPU:BGU ratio. A concentrated supernatant of the 7th day of cultivation in buffered lactose medium was used for the enzyme preparation with the 1:0.38 FPU:BGU ratio. This supernatant was supplemented with A. awamori enzymes, produced as described above, for obtaining mixtures with FPU:BGU ratios of 1:1 and 1:3. Enzyme concentration was performed in an Amicon 8200 stirred cell filtration module (Millipore, Billaica, USA) with a 10 kDa membrane.
Analysis of enzymatic hydrolysis samples
Monosaccharides were analyzed using an HPLC system equipped with a refraction index detector (RI-2031 Plus, Jasco Co., Japan) using an Aminex HPX-87P column (7.8 mm I.D. × 30 cm, Bio-Rad, USA) with a Carbo-P micro-guard cartridge (Bio-Rad, USA). Deionized water was used as the mobile phase at a flow rate of 1 mL/min at an 80 °C column temperature. Glucose concentrations were also measured using the Biochemistry Analyzer YSI 2700 Select. Hydrolysis experiments were carried out in triplicate.
Hydrolysis yields were calculated according to the Eq. (1):
where \({\mathrm{C}}_{\mathrm{glucose}}\) is the glucose concentration in the hydrolysates (g/L);\({\mathrm{C}}_{{\mathrm{glucose}}_{0}}\) is the initial glucose concentration in the hydrolysis assay; \({\mathrm{W}}_{\mathrm{bagasse}}\) is the total weight of bagasse in the hydrolysis assay (g); \({\mathrm{V}}_{0}\) is the initial volume of liquid (L); \({\mathrm{F}}_{\mathrm{glucan}}\) is the initial mass fraction of glucan in SPSB.
Statistical analysis
The main results from the triplicate experiments were compared using the Fisher’s LSD test (p ≤ 0.050) via Statistica® (version 7.0). Error bars were plotted based on the standard deviations obtained from the experimental replicates.
Results and discussion
Effect of SPSB on the production of FPase and β-glucosidase by Trichoderma reesei Rut C30
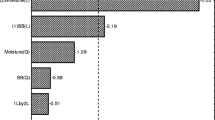
Trichoderma reesei was cultivated with steam-pretreated sugarcane bagasse (SPSB) as carbon source to assess the viability of using this material for enzyme production in an on-site integrated approach. Two other carbon sources, lactose and wheat bran, were used for comparison in simultaneous cultivations. The first is commonly used for cellulases production by T. reesei (Seiboth et al. 2007) and was chosen as a positive control against which to compare the enzyme production obtained with SPSB. Wheat bran is used for the production of β-glucosidade by Aspergillus sp. (Gottschalk et al. 2010) but is not used as the sole substrate for cellulases production, being chosen as a negative control. Figure 1 shows FPase and β-glucosidase production obtained in buffered media containing the different carbon sources.
A maximum FPase activity of 1.93 FPU/mL was achieved when T. reesei was cultivated on either SPSB or lactose as carbon source and a 70% lower activity was measured for the cultivation on wheat bran. The low cellulose content of wheat bran, of around 11% (w/w) (Kamal-Eldin et al. 2009), in comparison to the SPSB’s cellulose content, of 60% (w/w), could be associated to the poor enzyme production. Even though wheat bran has been reported as beneficial for cellulase production by T. reesei in submerged fermentations, it is usually used as a complement to other carbon sources (Yu et al. 1998; Lian et al. 2013). The FPase activity found in the present study for SPSB compares favorably with previous studies, being higher, for instance, than the values reported by Delabona et al. (2012) and Silva et al. (2018), of 0.98 FPU/mL and 0.15 FPU/mL in delignified steam-pretreated sugarcane bagasse and acid-pretreated sugarcane bagasse, respectively.
Overall, lactose was the best carbon source for cellulase production, with peak activities reached around the 4th day of cultivation. This finding confirms the ability of lactose to induce cellulase production in T. reesei (Seiboth et al. 2007; Porciuncula et al. 2013). Nevertheless, SPSB proved to be a promising carbon source for cellulase production with only a slightly lower productivity, reaching peak activities between the 6th and 7th days of cultivation. Maximum FPase activities reached in the cultivations with either lactose or SPSB showed no statistical difference (p > 0.05). Even though the β-glucosidase activity achieved with SPSB was lower than that for the lactose medium (p < 0.05), both carbon sources resulted in significant levels for this fungus (0.37 and 0.62 BGU/mL, respectively) when compared to reported values in literature, of < 0.3 IU/ml (Kovács et al. 2009; Zhao et al. 2018).
Assessment of the necessity of β-glucosidase supplementation in T. reesei supernatants
While using lactose as a control substrate for cellulases production by T. reesei in the present study, an unexpected high level of β-glucosidase activity was found, even though several works have reported T. reesei as an ineffective microorganism for breaking down lignocellulosic substrates into glucose molecules due to its low β-glucosidase production (Martins et al. 2008; Kovács et al. 2009; Zhao et al. 2018).
Nevertheless, some researches indicate that the amount of β-glucosidase in the culture filtrate of T. reesei Rut C30 may depend on the pH of the culture (Juhász et al. 2005a; Li et al. 2013). The high β-glucosidase activity observed in the present work was obtained in buffered medium containing lactose with an initial pH of 6.0 and a final pH of 5.8. Thus, to better understand the effect of pH control in the production of β-glucosidases by T. reesei Rut C30, this fungus was cultivated in unbuffered media containing lactose. This investigation was carried out with lactose instead of SPSB as the first is a more commonly used carbon source for enzyme production by T. reesei, being known for its cellulase-inducing effects. It was observed that the pH dropped to 2.5 and plateaued at this value from the 3rd to the 7th cultivation day. Under these conditions, the peak β-glucosidase activity corresponded to 0.02 BGU/mL, suggesting that low pH is an adverse condition for the enzyme production or stability. Interestingly, cellulase production was independent of pH control and a similar FPase activity, of 2.0 FPU/mL, was measured in both the lactose unbuffered and buffered medium.
Juhász et al. (2005a) attributed the higher β-glucosidase activity levels in buffered medium to the presence of maleic acid in the buffering system rather than to the actual culture pH. However, in the present study using phosphate buffer, with no maleic acid addition to the cultivation media, similar results were observed. Li et al. (2013) also observed a higher β-glucosidase production by T. reesei when pH was controlled with succinate buffer or under continuous adjustment with acid and base addition, corroborating that pH plays an important role in β-glucosidase production by T. reesei. Moreover, a recent study evaluated the relationship between pH and gene regulation in T. reesei (Häkkinen et al. 2015). The authors found that the expression of genes encoding the major cellulases in T. reesei was not significantly affected under different pH conditions, which is in good accordance with the results reported in the present work, in which the same FPase levels were found for buffered and unbuffered conditions. However, they observed that a gene encoding a candidate β-glucosidase displayed higher expression levels at pH 6 than at pH 3. Therefore, pH values higher than 3 seem to up-regulate the genes encoding β-glucosidase in T. reesei, resulting in a higher production of this enzyme with no effect on the production of FPase.
The possibility of upregulating β-glucosidase production in T. reesei puts in check the common assumption that T. reesei preparations need to be supplemented with this enzyme for an effective hydrolysis process. In order to better understand the influence of the FPU:BGU ratio on the hydrolysis of SPSB, a T. reesei preparation with an FPU:BGU ratio of 1:0.38, produced on lactose, was supplemented with an Aspergillus awamori enzyme pool produced on sugarcane bagasse containing high levels of β-glucosidase (8.2 BGU/mL) and almost null FPase activity (0.06 FPU/mL), such that the final blends showed FPU:BGU ratios of 1:1 and 1:3, which are within the ranges commonly used in other studies (Pryor and Nahar 2015). Additional hydrolysis assays using enzymes produced in an unbuffered lactose medium, with a FPU:BGU of 1:0.06, were simultaneously carried out. The time course for glucose accumulation in experiments using the enzyme preparations with different FPU:BGU ratios are shown in Fig. 2.
Glucose concentrations obtained during the hydrolysis of 7.5% (w/v) steam-pretreated sugarcane bagasse (SPSB) using enzymes secreted by T. reesei Rut C30 when cultivated in unbuffered lactose medium (1:0.06), buffered lactose medium (1:0.38), and the latter supplemented with Aspergillus awamori enzymes (1:1 and 1:3). Numbers in parenthesis represent the FPU:BGU ratio. Hydrolysis media were prepared using citrate buffer at pH 5 to eliminate the interference of the different pH values of the enzymatic preparations used
The use of an enzyme preparation with an FPU:BGU ratio of 1:0.38, produced in a buffered medium, resulted in a final glucose concentration two times higher than that obtained when using its unbuffered counterpart, suggesting that the use of a buffered medium for cellulase production results in a more adequate cellulosic complex for the hydrolysis of SPSB. As expected, a cellobiose accumulation of 8.3 g/L was observed in the hydrolysis using the enzyme with an FPU:BGU ratio of 1:0.06, which most likely inhibited the action of cellulases.
Interestingly, the preparations with higher FPU:BGU ratios gave similar glucose yields after 48 h of hydrolysis (p > 0.05). Nevertheless, hydrolysis rates up to 24 h were higher in the experiments with an increased β-glucosidase activity (p < 0.05), suggesting that a small supplementation could be beneficial, especially in experiments with higher solids loadings. This result is in good agreement with those obtained by Pallapolu et al. (2011), who reported a beneficial effect of β-glucosidase supplementation only up to 24 h of hydrolysis for substrates poor on xylan, such as SPSB. For xylan-rich substrates, the authors found higher final hydrolysis yields with the supplementation and attributed it to accessory enzymes present in the β-glucosidase preparations, such as xylanases.
In the present study, a FPU:BGU ratio as low as 1:0.38 was effective for the hydrolysis of SPSB. A similar result was obtained by Pryor and Nahar (2015) with acid-treated corn cob and stover; the authors found that a 1:0.2 FPU:CBU (corresponding to a 1:0.4 FPU:BGU in the present study) was sufficient for the hydrolysis of these materials in low solids loadings.
Effectiveness of enzymes produced on SPSB for enzymatic hydrolysis of SPSB
The hydrolytic efficiency of the culture supernatant of T. reesei grown in SPSB was evaluated in standard hydrolysis experiments alongside those obtained in the cultivations with lactose (LAC) or wheat bran (WB) and commercial cellulases in order to evaluate the effectiveness of the enzymes produced on SPSB for the hydrolysis of this substrate. Since an FPU:BGU ratio of 1:0.38 was found sufficient for an effective hydrolysis of SPSB, none of the tested preparations was supplemented with β-glucosidade. Table 1 presents the hydrolysis yields achieved with each enzyme preparation.
Previous reports indicate an advantage in using enzymes produced on the same substrate that would be further hydrolyzed (Juhász et al. 2005b; Zhang et al. 2012). The present study, however, reported similar hydrolytic performance for enzymes produced on either lactose, wheat bran or SPSB (p > 0.05). Our data are in good agreement with those of Jørgensen and Olsson (2006), who reported that the hydrolysis yields for pretreated spruce were not affected regardless of the use of enzymes produced on different substrates, although the activity profile of the enzyme preparations could vary.
When comparing the enzyme preparation produced on SPSB with the commercial preparations, it was observed a slightly lower efficiency of the SPSB enzyme mixture during the first 6 h of hydrolysis (p < 0.05); after 24 h, the SPSB mixture showed statistically similar performance to the GC 220 preparation (p > 0.05), but the glucose yield obtained with it was still lower than that for Spezyme CP (p < 0.05). Nevertheless, the 48 h glucose yields for the SPSB preparation and that for the commercial enzymes were similar (p > 0.05), even though the SPSB preparation had the lowest FPU:BGU ratio. Once more, a low FPU:BGU ratio, of 1:0.45, was sufficient for an effective hydrolysis in low solids loadings.
Some studies using the integrated enzyme production approach with T. reesei did not report hydrolysis yields (Silva et al. 2018), not allowing a proper comparison of the results, as similar FPase activities may not directly correspond to similar hydrolysis abilities. Moreover, the reported results usually indicate low glucose yields for cellulase production from lignocellulosic biomasses (Juhász et al. 2005b; Sipos et al. 2010), unless when using a β-glucosidase supplementation (Juhász et al. 2005b). Thus, the results obtained in the present study, where SPSB was used for on-site enzyme production and no β-glucosidase supplementation was necessary, compares favorably to previous works.
Therefore, we concluded that the necessity of β-glucosidase supplementation for biomass hydrolysis using the enzyme preparation produced by T. reesei Rut C30 may be lower than it is usually reported or even unnecessary depending on the cultivation conditions, and that SPSB was susceptible to hydrolysis using enzymes produced by T. reesei in an integrated approach, using SPSB as carbon source, without supplementation. Future studies should evaluate the minimum β-glucosidase loading necessary for achieving high hydrolysis rates and yields in conditions of high solids loadings in order to assess the real necessity for this supplementation and guide the formulation of enzymes mixtures produced on-site. Indeed, the identification of the correct amount of cellulases and β-glucosidase would be valuable to the further cost reduction of the integrated enzyme production and biomass hydrolysis processes. Even if a supplementation is found to be necessary, the present study indicates that the on-site integrated production of β-glucosidases would also be possible with Aspergillus awamori, as the supplementations done were carried out using A. awamori enzymatic preparations obtained during cultivation on sugarcane bagasse.
Conclusions
This study showed that T. reesei Rut C30 is able to produce a balanced enzyme preparation for the hydrolysis of SPSB when grown in a buffered medium with SPSB as carbon source. With this strategy, it was possible to exclude, or at least greatly reduce, the need for β-glucosidase supplementation, decreasing the relative cost of enzymes in the hydrolysis process. The effectiveness of SPSB as a carbon source to produce T. reesei cellulases and β-glucosidases is advantageous considering the on-site integrated enzyme production approach, as this substrate would be promptly available in the sugar mill, reducing the logistical costs for substrate and enzyme transportation.
References
CONAB (2018) Boletim Cana 4 Levantamento. pp 17–18. https://www.conab.gov.br/info-agro/safras/cana
da Delabona PS, Farinas CS, da Silva MR et al (2012) Use of a new Trichoderma harzianum strain isolated from the Amazon rainforest with pretreated sugar cane bagasse for on-site cellulase production. Bioresour Technol 107:517–521. https://doi.org/10.1016/j.biortech.2011.12.048
de Porciuncula JO, Furukawa T, Shida Y et al (2013) Identification of major facilitator transporters involved in cellulase production during lactose culture of Trichoderma reesei PC-3-7. Biosci Biotechnol Biochem 77:1014–1022. https://doi.org/10.1271/bbb.120992
Ellilä S, Fonseca L, Uchima C et al (2017) Development of a low-cost cellulase production process using Trichoderma reesei for Brazilian biorefineries. Biotechnol Biofuels 10:30. https://doi.org/10.1186/s13068-017-0717-0
Ferreira-Leitão V, Gottschalk LMF, Ferrara MA et al (2010a) Biomass residues in Brazil: availability and potential uses. Waste Biomass Valorization 1:65–76. https://doi.org/10.1007/s12649-010-9008-8
Ferreira-Leitão V, Perrone C, Rodrigues J et al (2010b) An approach to the utilisation of CO2 as impregnating agent in steam pretreatment of sugar cane bagasse and leaves for ethanol production. Biotechnol Biofuels 3:7. https://doi.org/10.1186/1754-6834-3-7
Fockink DH, Sánchez JH, Ramos LP (2018) Comprehensive analysis of sugarcane bagasse steam explosion using autocatalysis and dilute acid hydrolysis (H3PO4 and H2SO4) at equivalent combined severity factors. Ind Crops Prod 123:563–572. https://doi.org/10.1016/j.indcrop.2018.07.017
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Gottschalk LMF, Oliveira RA, da Bon EPS (2010) Cellulases, xylanases, β-glucosidase and ferulic acid esterase produced by Trichoderma and Aspergillus act synergistically in the hydrolysis of sugarcane bagasse. Biochem Eng J 51:72–78. https://doi.org/10.1016/j.bej.2010.05.003
Gruno M, Väljamäe P, Pettersson G, Johansson G (2004) Inhibition of the Trichoderma reesei cellulases by cellobiose is strongly dependent on the nature of the substrate. Biotechnol Bioeng 86:503–511. https://doi.org/10.1002/bit.10838
Häkkinen M, Sivasiddarthan D, Aro N et al (2015) The effects of extracellular pH and of the transcriptional regulator PACI on the transcriptome of Trichoderma reesei. Microb Cell Fact 14:63. https://doi.org/10.1186/s12934-015-0247-z
Johnson E (2016) Integrated enzyme production lowers the cost of cellulosic ethanol. Biofuels Bioprod Biorefining 10:164–174. https://doi.org/10.1002/bbb.1634
Jørgensen H, Olsson L (2006) Production of cellulases by Penicillium brasilianum IBT 20888—effect of substrate on hydrolytic performance. Enzyme Microb Technol 38:381–390. https://doi.org/10.1016/j.enzmictec.2005.06.018
Juhász T, Egyházi A, Réczey K (2005a) β-Glucosidase production by Trichoderma reesei. Appl Biochem Biotechnol 121:0243–0254. https://doi.org/10.1385/ABAB:121:1-3:0243
Juhász T, Szengyel Z, Réczey K et al (2005b) Characterization of cellulases and hemicellulases produced by Trichoderma reesei on various carbon sources. Process Biochem 40:3519–3525. https://doi.org/10.1016/j.procbio.2005.03.057
Kamal-Eldin A, Lærke HN, Knudsen K-EB et al (2009) Physical, microscopic and chemical characterisation of industrial rye and wheat brans from the Nordic countries. Food Nutr Res 53:1912. https://doi.org/10.3402/fnr.v53i0.1912
Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA, Blanch HW (2012) The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng 109:1083–1087. https://doi.org/10.1002/bit.24370
Kovács K, Szakacs G, Zacchi G (2009) Comparative enzymatic hydrolysis of pretreated spruce by supernatants, whole fermentation broths and washed mycelia of Trichoderma reesei and Trichoderma atroviride. Bioresour Technol 100:1350–1357. https://doi.org/10.1016/j.biortech.2008.08.006
Li C, Yang Z, He Can Zhang R et al (2013) Effect of pH on cellulase production and morphology of Trichoderma reesei and the application in cellulosic material hydrolysis. J Biotechnol 168:470–477. https://doi.org/10.1016/j.jbiotec.2013.10.003
Lian X, Chao H, Wan feng P, et al (2013) Efficient cellulase production from low-cost substrates by Trichoderma reesei and its application on the enzymatic hydrolysis of corncob. Afr J Microbiol Res 7:5018–5024. https://doi.org/10.5897/AJMR12.1761
Liu G, Zhang J, Bao J (2016) Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous Aspen Plus modeling. Bioprocess Biosyst Eng 39:133–140. https://doi.org/10.1007/s00449-015-1497-1
Longati AA, Lino ARA, Giordano RC et al (2018) Defining research and development process targets through retro-techno-economic analysis: the sugarcane biorefinery case. Bioresour Technol 263:1–9. https://doi.org/10.1016/j.biortech.2018.04.102
Lynd LR, Liang X, Biddy MJ et al (2017) Cellulosic ethanol: status and innovation. Curr Opin Biotechnol 45:202–211. https://doi.org/10.1016/j.copbio.2017.03.008
Macrelli S, Mogensen J, Zacchi G (2012) Techno-economic evaluation of 2nd generation bioethanol production from sugar cane bagasse and leaves integrated with the sugar-based ethanol process. Biotechnol Biofuels 5:22. https://doi.org/10.1186/1754-6834-5-22
Mandels M, Weber J (1969) The production of cellulases. pp 391–414
Martins LF, Kolling D, Camassola M et al (2008) Comparison of Penicillium echinulatum and Trichoderma reesei cellulases in relation to their activity against various cellulosic substrates. Bioresour Technol 99:1417–1424. https://doi.org/10.1016/j.biortech.2007.01.060
Montenecourt BS, Eveleigh DE (1979) Selective screening methods for the isolation of high yielding cellulase mutants of Trichoderma reesei. pp 289–301
NEDO (2017) NEDO Demonstrates the effectiveness of a bioethanol production technology that utilizes bagasse as a feedstock in Thailand. http://www.nedo.go.jp/english/news/AA5en_100238.html. Accessed 7 Sep 2020
Pallapolu VR, Lee YY, Garlock RJ et al (2011) Effects of enzyme loading and β-glucosidase supplementation on enzymatic hydrolysis of switchgrass processed by leading pretreatment technologies. Bioresour Technol 102:11115–11120. https://doi.org/10.1016/j.biortech.2011.03.085
Paloheimo M, Haarmann T, Mäkinen S, Vehmaanperä J (2016) Production of industrial enzymes in Trichoderma reesei. In: Dattenböck C, Schmoll M (eds) Gene expression systems in fungi advancements and applications. Springer, Berlin, pp 23–57
Peterson R, Nevalainen H (2012) Trichoderma reesei RUT-C30—thirty years of strain improvement. Microbiology 158:58–68. https://doi.org/10.1099/mic.0.054031-0
Pryor SW, Nahar N (2015) β-glucosidase supplementation during biomass hydrolysis: how low can we go? Biomass Bioenerg 80:298–302. https://doi.org/10.1016/j.biombioe.2015.06.005
Ryu DDY, Mandels M (1980) Cellulases: biosynthesis and applications. Enzyme Microb Technol 2:91–102. https://doi.org/10.1016/0141-0229(80)90063-0
Salles-Filho S, Bin A, Castro PFD et al (2016) Innovation in the Brazilian bioethanol sector: questioning leadership. In: Global bioethanol. Elsevier, Amsterdam. pp 122–141
Seiboth B, Pakdaman BS, Hartl L, Kubicek CP (2007) Lactose metabolism in filamentous fungi: how to deal with an unknown substrate. Fungal Biol Rev 21:42–48. https://doi.org/10.1016/j.fbr.2007.02.006
Silva DF, Hergesel LM, Campioni TS et al (2018) Evaluation of different biological and chemical treatments in agroindustrial residues for the production of fungal glucanases and xylanases. Process Biochem 67:29–37. https://doi.org/10.1016/j.procbio.2018.02.008
Singhania RR, Adsul M, Pandey A, Patel AK (2017) Cellulases. In: Current developments in biotechnology and bioengineering. Elsevier, Amsterdam. pp 73–101
Sipos B, Benkő Z, Dienes D et al (2010) Characterisation of specific activities and hydrolytic properties of cell-wall-degrading enzymes produced by Trichoderma reesei Rut C30 on different carbon sources. Appl Biochem Biotechnol 161:347–364. https://doi.org/10.1007/s12010-009-8824-4
Sluiter A, Hames B, Ruiz R, et al (2012) NREL/TP-510–42618 analytical procedure—determination of structural carbohydrates and lignin in Biomass. Lab Anal Proced 17. NREL/TP-510-42618
van den Brink J, Maitan-Alfenas GP, Zou G et al (2014) Synergistic effect of Aspergillus niger and Trichoderma reesei enzyme sets on the saccharification of wheat straw and sugarcane bagasse. Biotechnol J 9:1329–1338. https://doi.org/10.1002/biot.201400317
Yu X-B, Yun HS, Koo Y-M (1998) Production of cellulase by Trichoderma reesei Rut C30 in wheat bran-containing media. J Microbiol Biotechnol 8:208–213
Zhang L, Liu Y, Niu X et al (2012) Effects of acid and alkali treated lignocellulosic materials on cellulase/xylanase production by Trichoderma reesei Rut C-30 and corresponding enzymatic hydrolysis. Biomass Bioenerg 37:16–24. https://doi.org/10.1016/j.biombioe.2011.12.044
Zhao C, Deng L, Fang H (2018) Mixed culture of recombinant Trichoderma reesei and Aspergillus niger for cellulase production to increase the cellulose degrading capability. Biomass Bioenerg 112:93–98. https://doi.org/10.1016/j.biombioe.2018.03.001
Zheng W, Chen X, Xue Y et al (2017) The influence of soluble polysaccharides derived from rice straw upon cellulase production by Trichoderma reesei. Process Biochem 61:130–136. https://doi.org/10.1016/j.procbio.2017.06.007
Acknowledgements
The authors would like to thank Dr. Clarissa Perrone for providing the samples of steam-pretreated sugarcane bagasse.
Funding
The present work was conducted without any specific funding.
Author information
Authors and Affiliations
Contributions
Marcella Fernandes de Souza contributed through the analysis and interpretation of data and the drafting of the manuscript. Elba Pinto da Silva Bon contributed with the critical revision of the manuscript regarding its coherence and scientific contribution. Ayla Sant’Ana da Silva contributed through the conception and design of the experiments, the collection, assembly, analysis and interpretation of data and the final revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflicts of interest.
Consent for publication
All authors have consented to publish the present work.
Dta availability
Data will be made available upon request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza, M.F., da Silva Bon, E.P. & da Silva, A.S. Production of cellulases and β-glucosidases by Trichoderma reesei Rut C30 using steam-pretreated sugarcane bagasse: an integrated approach for onsite enzyme production. Braz. J. Chem. Eng. 38, 435–442 (2021). https://doi.org/10.1007/s43153-021-00114-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-021-00114-5