Abstract
Akodon montensis is abundant and widely distributed in the Atlantic Forest, which southern limits extends to northern Argentina, principally in the Misiones province. Chromosomal data from more centrally distributed populations in Brazil showed significant chromosome diversity; however, data from its southern boundaries are scarce. To explore the chromosomal diversity of this species, we conducted conventional chromosome staining, C-banding, CMA3/DAPI fluorochromes, and in situ hybridization with a telomere probe in samples from Argentina. Most specimens had the standard karyotype 2n = 24, although numerical variations due to aneuploidies and supernumerary (B) chromosomes were detected. We registered novel structural polymorphisms for pair 11 and sex chromosomes, and a rearrangement involving pairs 2 and 4, possibly due to a spontaneous chromosomal mutation. Most A. montensis females are homogametic with XX sex chromosomes, although XY and X0 females were observed. Most individuals carrying B chromosomes had 1 B and, to a lesser extent, 2 and 3 B. The chromosomal variability detected at the southern limit of A. montensis distribution is high and similar to other geographically distant populations, despite the fact that it could be a region recently colonized. Some of these variations are unique and could have originated independently in southern populations, while others are shared throughout the species distribution and may have originated earlier.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Akodon is among the most specious of sigmodontine rodents, with more than 40 species included in several species groups (Braun et al. 2008; Jayat et al. 2010; Coyner et al. 2013; Brandão et al. 2021). At the chromosomal level, this genus exhibits high variability regarding diploid numbers (2n) and autosomal fundamental numbers (FNa), with a broad range of variation between 2n = 10–44 and FNa = 14–44, respectively (González et al. 1998; Tiranti 1998; Silva and Yonenaga-Yassuda 1998; Christoff et al. 2000).
Akodon montensis is an abundant species with a wide distribution that comprises Brazil, Paraguay, and Argentina (Patton et al. 2015; Galliari and Pardiñas 2020). In Argentina, this species occurs in a few northeast Provinces, representing the southwestern boundaries of its distribution (Patton et al. 2015). This rodent inhabits the Interior Atlantic Forest, an important conservation area considered a biodiversity "hot spot". In Argentina, its distribution is mainly restricted to the Misiones province (Di Bitetti et al. 2003; Galindo-Leal and de Gusmão Câmara 2003). Phylogenetic studies place Akodon montensis within the A. cursor species group (Braun et al. 2008; Jayat et al. 2010; Coyner et al. 2013). The A. cursor species group is the most variable of the genus, demonstrating a complex chromosome evolution (Labaroni et al. in press). This group includes species with the highest 2n of the genus (2n = 44 in A. paranaensis and A. reigi) and the lowest 2n known for rodents (2n = 9-10 in A. aff. cursor). Akodon montensis has a standard karyotype with 2n = 24 and FNa = 42 reported for all the localities studied (Yonenaga-Yassuda et al. 1975; Kasahara and Yonenaga-Yassuda 1982; Yonenaga-Yassuda et al. 1992; Fagundes et al. 2000; Ventura et al. 2009; Malleret et al. 2016; Soares et al. 2018). However, the cytogenetic study effort has been disproportionate for different regions, being intense in some and almost absent in others. For instance, cytogenetic studies in southern Brazil reported significant variability regarding sex chromosomes, including sex reversions (fertile XY females), aneuploidy (X0 females) and polymorphisms for the morphology of the X chromosome. Moreover, different B chromosomes types were also described (Yonenaga-Yassuda et al. 1975; Kasahara and Yonenaga-Yassuda 1982; Yonenaga-Yassuda et al. 1992; Fagundes et al. 2000; Ventura et al. 2009; Soares et al. 2018). Conversely, there are no chromosomal data for populations of this species from central Brazil or Paraguay. The available information for A. montensis in Argentina is sparse and includes a small sampling size for a few populations of Misiones province. Liascovich and Reig (1989) studied four specimens from the Provincial Park "Islas Malvinas" (at present Parque Provincial Urugua-í) and reported a conserved standard karyotype. Later, Malleret et al. (2016) studied 31 specimens from five localities and described chromosome variation due to a trisomy, B chromosomes, XY females, and polymorphisms for the morphology of the X chromosome.
According to molecular evidence, the populations of A. montensis from Argentina and eastern Paraguay would represent a lineage derived from a recent expansion event of the Brazilian populations (Valdez and D'Elía 2013); however, the impact of this demographic event on the karyotypic diversity of the species is still unknown.
Knowledge of the chromosomal variability throughout the distribution range of Akodon montensis is essential to understand its complex evolution. This study analyzed specimens of this species by different cytogenetic approaches from localities found in Misiones Province, the southwest of its distribution.
Material and methods
Study area and samples
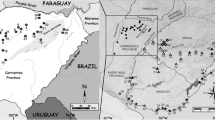
A total of 127 specimens of Akodon montensis were collected and analyzed cytogenetically (65 males, 62 females), of which 31 were previously analyzed by Malleret et al. (2016), (marked with an asterisk in supplementary material). The sampling was performed in nine localities of Misiones, Argentina (Fig. 1). Vouchers are housed in the mastozoological collection of the Instituto de Biología Subtropical (IBS-CONICET-UnaM, Misiones, Argentina) and Museo Argentino de Ciencias Naturales (MACN, Buenos Aires, Argentina). Catalogue numbers of studied specimens are presented in the supplementary material.
a Distribution of Akodon montensis (pink area). b Sampling localities in the Misiones Province: 1- Parque Nacional Iguazú, 2- Parque Provincial Urugua-í, 3- Puerto Esperanza, 4- Reserva Privada Forestal Belga, 5- Parque Provincial Piñalito, 6- Parque Ecológico y Camping Municipal del Valle del Cuña Pirú 7- Reserva Natural Osununú, 8- Campo San Juan, 9- Parque Provincial de la Sierra Ingeniero Raúl Martínez Crovetto. The map was created using SimpleMappr, an online tool to produce publication-quality point maps (https://www.simplemappr.net/)
Chromosome preparations
Chromosome preparations were obtained from bone marrow and testes, following Ford and Hamerton (1956) and Evans (1964), respectively. Slides were conventionally stained with 10% phosphate-buffered Giemsa (pH 6.8). The distribution of constitutive heterochromatin (CH) was studied through C-bands, according to Sumner (1972). In order to identify chromosome homology, preparations were stained with the fluorochromes DAPI (4,6-diamidino-2-phenylindole) and CMA3 (Chromomicine A3) (Schweizer 1976). Fluorescent in situ hybridization (FISH) was performed in six individuals carrying Bs (B+) with a Cy3-conjugated PNA pantelomeric probe (CCCTAA) obtained from PNA Bio (USA) according to the protocol provided by the supplier.
Results
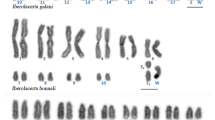
The standard karyotype of Akodon montensis consists of 2n = 24. Pairs 1 to 9 are large to medium, and pairs 10 and 11 are small; all chromosomes are bi-armed, except pair 10, which is acrocentric (Fig. 2a). This species shows a XX/XY sex chromosome determination system, where chromosome X is medium and the Y small, both acrocentric (Fig. 2a). We found a female with 2n = 24 from Martínez Crovetto whose karyotype was heteromorphic for pairs 2 and 4 (Fig. 2b). In this female, pair 2 consisted of a bi-armed and an acrocentric chromosome, and pair 4 of two bi-armed chromosomes of different sizes (Fig. 4c). We confirmed by fluorochrome staining (DAPI/CMA3) that this variation resulted from a rearrangement involving chromosomes of both pairs. Moreover, we found a polymorphism for the morphology of chromosomes of pair 11, causing variation in the FNa between specimens (FNa = 40 to 42). In most cases, pair 11 showed the standard morphology of two bi-armed chromosomes (Fig. 2e). In a less frequency, two acrocentrics or both morphologies occurred together in a heterozygous condition (Fig. 2g).
Conventional staining of mitotic chromosomes from A. montensis. a Karyotype of a male with the standard complement of 2n = 24. b Mitosis of a female with 2n = 24 and a chromosomal rearrangement between pairs 2 and 4 indicated by black arrowheads. c Metaphase of a female with 2n = 23 due to an X0 constitution. d Mitotic chromosomes from a male with 2n = 24 + 1B. e Metaphase of a male with 2n = 24 + 2B of different sizes. Pair 11 is indicated with arrowheads. f Metaphase of a male with 2n = 25 due to a trisomy in pair 11 (identified by black lines) and a B chromosome. g Mitosis of a female with 2n = 24 + 3B of different sizes and morphology. Pair 11 is indicated with arrowheads. Sex chromosomes are identified according to their morphology as Xa = acrocentric, Xs = subtelocentric, Ya = acrocentric, Ys = subtelocentric. B chromosomes were indicated with arrows as Bsm (medium submetacentric), Bss (small submetacentric) and Bas (small acrocentric). The bar corresponds to 10 μm
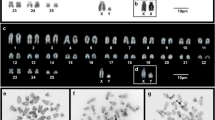
C-banding revealed heterochromatin in the pericentromeric regions of all chromosomes and the telomeric region of pairs 3 and 10 (Fig. 3). The banding pattern obtained with fluorochromes DAPI/CMA3 allowed the establishment of homologies between chromosomes. For instance, pairs 3 and 4 had DAPI+ pericentromeric bands, while pairs 1, 2 and 5 were DAPI–/CMA3+. Moreover, pairs 1, 8, and 9 had conspicuous DAPI+ interstitial bands; and pair 5 had a large DAPI+ distal band (Fig. 4a).
C-banding of mitotic chromosomes of A. montensis. a Metaphase of a female with 2n = 24 + 1B. b Mitosis of a female with 2n = 24 + 1B. c Metaphase of a male with 2n = 24 + 1B. d Mitosis of a male with 2n = 24 + 1B. e Metaphase of a male with 2n = 24 + 2B. f Chromosomal complement of a female with 2n = 24 + 3B. Fine arrows indicate pair 11 with positive staining. Black lines indicate chromosome pairs 3 and 10 with telomeric bands. B chromosomes were indicated with arrows and identified as in fig. 2. Sex chromosomes are identified according to their morphology, as in fig. 2. The bar corresponds to 10 μm
Chromosomes of A. montensis stained with the specific base fluorochromes DAPI/CMA3. a Karyotype of a male with 2n = 24 + 1B stained with DAPI. b Karyotype of a female with 2n = 24 + 1B stained with CMA3. c Karyotype of a female with 2n = 24. Brackets indicate the chromosomal arm involved in the rearrangement
FISH experiments with the telomeric probe revealed signals at the ends of all chromosomes (Fig. 6a-b). Besides, in five specimens, interstitial telomeric sequences (ITS) were observed in the pericentromeric regions of pair 3, and one had an additional ITS on both homologues of pair 2 (Fig. 6a). Also, we observed one specimen without ITS (Fig. 6b). Differences in the size and intensity of ITS were detected, being those of pair 2 larger and more intense (Fig. 6a).
The sex chromosomes were analyzed in 117 specimens (57 females and 60 males), and both X and Y showed variation. Of all females analyzed, 48 were XX and 9 had heteromorphic XY chromosomes. Two variants for the morphology of the X were observed, acrocentric (Xa) and subtelocentric (Xs), being present as XaXa (N = 38), XaXs (N = 9) and XsXs (N = 1). On the other hand, the Y chromosome showed an acrocentric (Ya) or subtelocentric (Ys) morphology. In males, the chromosomal constitutions were XaYa (N = 53), XaYs (N = 4) and XsYa (N = 3), and in XY females were XsYa (N = 7), XaYa (N = 1) and XsYs (N = 1).
All variants for X and Y had differences in their C-banding pattern. Both X showed a small pericentromeric C band (Fig. 3), although Xs also showed heterochromatin in the short arm (Fig. 3b-c). On the other hand, the Ya was whole heterochromatic, whereas the Ys had a C band restricted to the pericentromeric region (Fig. 3c-e). With fluorochromes, X variants had CMA3+ pericentromeric heterochromatin (Fig. 4b) and two interstitials DAPI+ bands (Fig. 4a). The short arm of the Xs did not show differential staining either for DAPI or CMA3 (Fig. 5a). The Y chromosome was DAPI+, regardless of its morphology (Fig. 4a; 5a, c).
a Chromosomal complement of a male with 2n = 24 + 1B. b Metaphase of a female with 2n = 24 + 1B. c Metaphase of a male with 2n = 24 + 2B. d Chromosomal complement of a female with 2n = 24 + 3B. The inset shows the B chromosomes with a neutral band in the pericentromeric region. B chromosomes were indicated with arrows and identified as in fig. 2. Sex chromosomes are identified according to their morphology, as in fig. 2. The bar corresponds to 10 μm
Of the total specimens studied, 101 had a 2n = 24 (Fig. 2a) and 26 showed a variable number due to B chromosomes (N = 24) or/and aneuploidy (N = 2). In the latter, we found a monosomic female of Martínez Crovetto with 2n = 23 with one X chromosome (Fig. 2c); and one trisomic male of Campo San Juan with 2n = 25 + B with three chromosomes 11 (described by Malleret et al., 2016, Fig. 2f).
B chromosomes (Bs) were found in individuals from all localities, including males and females, and they showed different morphologies and sizes (Fig. 2d-g; Table 1). Of the total of specimens, 22 had 1B (Fig. 2d), 2 had 2Bs (Fig. 2e), and 1 had 3Bs (Fig. 2g; Table 1). Besides, a specimen carrying 1B was also trisomic for pair 11 (Fig. 2f; Table 1).
Based on B chromosome morphology and size, we detected different types of Bs. A submetacentric and medium B chromosome, similar in size to pair 9, called Bsm (Fig. 2d-g), a submetacentric and small, similar to pair 10, called Bss (Fig. 2e), and one acrocentric and smaller than pair 11, called Bas (Fig. 2g). These Bs were found at different frequencies in different populations, and different types of Bs could be found in a specimen (Fig. 2e, g; Table 1). The Bsm was found in 21 individuals (84%) and present in all populations. The Bss was less frequent and found in 5 individuals (20%), alone in 4 individuals or with the Bsm in one individual (Fig. 2e). The Bas chromosome was found in a single specimen (4%) with 3Bs (Fig. 2g).
The different B types showed variations in the content of heterochromatin. The Bsm presented two variants: one with a large block of heterochromatin comprising the pericentromeric region and a portion of the long arm (Fig. 3b, f), whereas the other variant has a small block of heterochromatin in the pericentromeric region (Fig. 3c, e). The Bss had a small heterochromatic block in the pericentromeric region (Fig. 3a, e). However, a single individual with 2n = 24 + 1Bss presented intraindividual variation for the C banding pattern. In this case, we identify the pattern previously described above for the Bss and a completely heterochromatic Bss (Fig. 3d). The Bas chromosome was euchromatic (Fig. 3f).
Bsm and Bss chromosomes showed a similar banding pattern with DAPI/CMA3 fluorochromes (Fig. 5). The interstitial region of the long arm had a DAPI+ band and the pericentromeric region was DAPI neutral, visible only in decondensed chromosomes (Fig. 5). With CMA3, these Bs did not have differential marks (Fig. 4b). Bas chromosome was DAPI+ (Fig. 5d). On the other hand, telomeric probes revealed signals only at both ends of the different types of Bs (Fig. 6a-b).
Fluorescent in situ hybridization with pantelomeric probe. a Metaphase of a female with 2n = 24 + 1B. Arrows indicate interstitial telomeric sequences in the pericentromeric region of chromosome pairs 2 and 3. b Metaphase of a female with 2n = 24 + 1B. The inset shows two different B chromosomes. B chromosomes and sex chromosomes were identified according to fig. 2
The meiosis of three B+ males was analyzed, one of them with 1B and the remaining two with 2Bs (Fig. 7). A total of 41 cells were studied, of which 11 corresponded to the pachytene phase, 25 to diakinesis, and 5 to metaphase II. In pachytene, a normal pairing of autosomes and sex chromosomes was observed in all individuals (Fig. 7a). In contrast, Bs were always observed as univalent (Fig. 7a). In diakinesis, 12 bivalents were identified (Fig. 7b-d). In this phase, the Bs were identified in 20 cells as univalent and without association with the chromosomes of the standard complement (Fig. 7b, d). Only five cells, two Bs were observed forming a bivalent in different individuals (Fig. 7c). In metaphase II, cells with 12, 13 and 14 chromosomes were observed (Fig. 7e-f).
Meiosis of A. montensis. a Pachytene of a specimen with 2n = 24 + 2B where 12 bivalents and B as univalents are observed. b Diakinesis of an individual with 2n = 24 + 1B where 12 bivalents and one B univalent are observed. c Diakinesis of a specimen with 2n = 24 + 2B where the B chromosomes are observed forming a bivalent. d Diakinesis of a specimen with 2n = 24 + 2B where the B chromosomes are observed as univalent. e Metaphase II with 13 chromosomes from a specimen with 2n = 24 + 2B. f Metaphase II with 14 chromosomes from an individual with 2n = 24 + 2B. Arrows indicate Bs chromosomes. Sex chromosomes are indicated as XY. The bar corresponds to 10 μm
Discussion
The standard karyotype of Akodon montensis
The regular karyotype of A. montensis is 2n = 24 (FNa = 42) with a sex chromosome system XX/XY. The general C-banding pattern of the autosomes is also conserved for the species, mainly restricted to centromeres and pericentromeric regions (Kasahara and Yonenaga-Yassuda 1982; Ortiz et al. 1998; Lisanti et al. 2001; Malleret et al. 2016; this study). Conversely, studies of an isolated population from southern Brazil showed centromeric CH only in pairs 2 and 9 (São Francisco do Sul Island, in Santa Catarina, Soares et al. 2018). In addition, distal C bands were identified in up to four chromosome pairs in Argentine populations (Malleret et al. 2016; this study), while in Brazil their detection was variable, being absent in some cases or present only in two pairs (Kasahara and Yonenaga-Yassuda 1982; Soares et al. 2018). These data suggest variation in the amount and distribution of heterochromatin between populations of A. montensis.
Since the patterns obtained by DAPI/CMA3 fluorescent banding are comparable to those obtained by G and R bands, we identified homology between the karyotypes of specimens from Brazil, previously studied with G-banding (Fagundes and Yonenaga-Yassuda 1998; Silva and Yonenaga-Yassuda 2004; Veyrunes et al. 2007), and the specimens from Argentine populations, analyzed with DAPI/CMA3 fluorochromes (Malleret et al. 2016; this study). On the other hand, the FISH experiments showed that the telomeric probe hybridized to both ends of all chromosomes, which is consistent with previous studies in Brazilian populations of this species and other rodent species (Lanzone et al. 2015; Soares et al. 2018; Labaroni et al. in press). Besides, we observed interstitial telomeric sequences (ITS) in two large chromosome pairs that were not previously described for the species (Soares et al. 2018). Interestingly, ITS were also reported in A. cursor, a cryptic species that is closely phylogenetically related to A. montensis (Fagundes et al. 1997). Within the genus, A. cursor and A. montensis have karyotypes with reduced chromosome numbers (Liascovich et al. 1989; Fagundez et al. 1998; Malleret et al. 2016; Soares et al. 2018), generated by different chromosomal rearrangements (Ventura et al. 2009). The presence of ITSs could reflect the ancient chromosome changes that occurred in the evolutionary history of these species, such as Robertsonian rearrangements (Slijepcevic 1998; Bolzán and Bianchi 2006; Ventura et al. 2006; Ruiz-Herrera et al. 2008); however, other mechanisms could explain the origin of ITS, such as the amplification and transposition of telomeric sequences (Meyne et al. 1990; Rovatsos et al. 2011). The apparent exclusivity of ITS in individuals of A. montensis from Argentine localities suggests independent processes involving these sequences leading to divergence between populations.
Chromosomal variations in the karyotype of A. montensis
Different variations at the chromosomal level have been recorded for A. montensis. Some of these variants are widely distributed, including most of the populations, while others are restricted to specific localities (Yonenaga-Yassuda et al. 1975; Kasahara and Yonenaga-Yassuda 1982; Yonenaga-Yassuda et al. 1992; Fagundes et al. 2000; Ventura et al. 2009; Malleret et al. 2016; Soares et al. 2018; this study). We describe for this species a novel polymorphism for the standard bi-armed pair 11, possibly caused by a pericentric inversion observed only in Argentine populations. In Akodon, the A. cursor group shows marked reductions in 2n, which could be explained by cycles of chromosomal inversions and fusions. Ventura et al. (2009) recorded these rearrangements by chromosome painting in four Akodon species, which can explain the group's evolution of the lowest diploid number.
A single male specimen of Akodon montensis from Argentina was trisomic for pair 11 (Malleret et al. 2016; this study), a chromosome mutation rare in Akodon that was previously recorded in A. cursor (Fagundes et al. 1998). On the other hand, one female had a complex karyotype regarding chromosome pairs 2 and 4. The chromosome rearrangements involved in such variation are unknown. However, it is tempting to propose that it could result from the spontaneous fission of the short arm of one chromosome of pair 2 followed by a tandem translocation to pair 4. So far, this type of rearrangement has not been reported in the genus.
The sex chromosomes of A. montensis are polymorphic, both X and Y chromosomes showed two variants. The acrocentric X and Y (Xa and Ya) were recorded in previous studies and are the most frequent polymorphisms in Brazil and Argentina (Kasahara and Yonenaga-Yassuda 1982; Malleret et al. 2016; this study). Other Akodon species that are closely related to A. montensis also share acrocentrics X and Y, suggesting that this morphology would represent the plesiomorphic condition of the group (Silva and Yonenaga-Yassuda 1998; Fagundes et al. 1997; González et al. 1998; Christoff et al. 2000; Malleret et al. 2016; Soares et al. 2018; this study).
The Xs shows a short heterochromatic arm in the specimens of Argentina (Malleret et al. 2016; this study) that was not detected in specimens from Brazil (Kasahara and Yonenaga-Yassuda 1982). Similar variation is also observed for the Y chromosome, where its heterochromatin pattern is variable throughout the distribution of the species (Kasahara and Yonenaga-Yassuda 1982; Fagundes et al. 2000; this study). The subtelocentric Ys chromosome is restricted to Argentina. The differentiation of the X and Y and the low frequencies of some variants in Argentine populations suggest a recent origin in this region related to the heterochromatin addition-elimination process (John 1988; Steinemann and Steinemann 2000; Waters et al. 2007).
Supernumerary or B chromosomes are among the primary causes of karyotypic variability in A. montensis and are found in most populations. We found differences in the number of Bs per individual and populational prevalence. Most specimens B+ of this species show only one B (Kasahara and Yonenaga-Yassuda et al. 1982; Yonenaga-Yassuda et al. 1992; Malleret et al. 2016; Soares et al. 2018; this study). However, we registered specimens with 2 and 3 B for the first time in Argentina. In rodents and other vertebrates, variation in the frequency of Bs between population is a feature commonly observed (Silva and Yonenaga-Yassuda 2004; Vujošević et al. 2018). Notably, in A. montensis, the frequencies of specimens with Bs do not exceed 30% in almost all populations studied; however, two populations were identified with a high frequency of Bs. Soares et al. (2018) found a frequency of 75% of specimens with Bs in an island population in Santa Catarina. Similarly, in this study, B+ specimens represent almost 70% of the sample from Cuña Pirú (Misiones Province, Argentina). The different frequency of B chromosomes observed between population of A. montensis could be attributed to different factors that are currently unknown, such as ecological or historical factors, or even due to differences in the intrinsic characteristics of Bs between different localities, such as their transmission (Beukeboom, 1994; Camacho et al. 2000; Camacho 2005).
The morphology, size, and amounts of heterochromatin in the B chromosomes of A. montensis are variable. We found two submetacentric Bs, a medium and a small sized (Bsm and Bss, respectively), and a small acrocentric (Bas). In Misiones, the Bsm is the most frequent and widely distributed type of supernumerary, according to what is observed in other populations from Brazil (Kasahara and Yonenaga-Yassuda et al. 1982; Yonenaga-Yassuda et al. 1992). Soares et al. (2018) described four types of Bs for São Francisco do Sul Island (Santa Catarina, eastern Brazil); two of them would correspond to the Bsm and Bss detected in this study (Kasahara and Yonenaga-Yassuda et al. 1982; Yonenaga-Yassuda et al. 1992; Malleret et al. 2016; this work). The other two are apparently restricted to São Francisco do Sul Island. Moreover, the Bas reported in our study for Martinez Crovetto (Misiones, northern Argentina) corresponds to a novel morphological variant not previously observed in the species.
The characteristics in the heterochromatin composition of Bs are also shared between Argentine and Brazilian populations. Bs can be euchromatic with pericentromeric heterochromatin (Silva and Yonenaga-Yassuda 2004; Soares et al. 2018; this study) or, less frequently, heterochromatic (Kasahara 2009; this study); conversely, the Bas is the only morph that lacks heterochromatin. The particular characteristics of the Bas (morphology and chromatin pattern) differentiate this element from others suggesting that this variant would be restricted to southern Misiones populations in Argentina.
Soares et al. (2018) analyzed all B-variants studied by microdissection and chromosome painting, proposing a common origin for the Bs. The discovery of new variants in A. montensis through differentiation is a feature reported in other mammal species (Vujošević et al. 2018). Bs in Akodon would share a common origin with subsequent modifications. However, based on the reported variability and the lack of molecular studies to test homology between other studied populations, we cannot propose a conclusive hypothesis for Bs origin in this species.
Valdez and D' Elía (2013) presented a phylogeographic study of A. montensis in the Atlantic Forest. These authors based their work on the refuge hypothesis during Quaternary climatic fluctuations proposed by Carnaval and Moritz (2008). According to this, during the Last Glacial Maximum, forested areas of the Atlantic Forest were restricted to isolated refuges in central Brazil and later expanded to their current distribution. Valdez and D' Elía (2013) concluded that Argentine and northeast Paraguayan populations of A. montensis came from a recent expansion from a refuge in Brazil (São Paulo). Despite the recent colonization proposed for this region, in this study, we found an impressive chromosomal diversity for A. montensis, which corresponds to the southernmost range of the species (Misiones, Argentina). The variability found is comparable to that described for central Brazil, suggesting that chromosomal diversity does not decrease at the species boundaries. Besides, localities from Argentina show variations that are apparently restricted to southern localities. Interestingly, despite being a species that recently colonized Argentinian Atlantic Forest, A. montensis is the most abundant of this assemblage (Galliari and Pardiñas 2020).
The lack of knowledge about the genomic evolution of A. montensis should be overcome through high-throughput DNA sequencing technologies, for example, by studying individuals with different chromosome constitutions. Moreover, it would be interesting to study Paraguayan populations, for which cytogenetic information is still absent, to know if this part of the expansion replicates the same chromosome diversity pattern found in Argentina and Brazil and Argentina.
References
Beukeboom LW (1994) Bewildering Bs: an impression of the 1 st B-Chromosome Conference. Heredity 73:328–336
Bolzán AD, Bianchi MS (2006) Telomeres, interstitial telomeric repeat sequences, and chromosomal aberrations. Mutat Res 612:189–214
Brandão VM, Percequillo AR, D’Elía G, Paresque R, Carmignotto AP (2021) A new species of Akodon Meyen, 1833 (Rodentia: Cricetidae: Sigmodontinae) endemic from the Brazilian Cerrado. J Mammal 102(1):101–122
Braun JK, Coyner BS, Mares MA, Van Den Bussche RA (2008) Phylogenetic relationships of South American grass mice of the Akodon varius group (Rodentia, Cricetidae, Sigmodontinae) in South America. J Mammal 89:768–777
Camacho JPM, Sharbel TF, Beukeboom LW (2000) B-chromosome evolution Philos Trans R Soc 355:163–178
Camacho JPM (2005) B chromosomes. In: Gregory TR (ed) The Evolution of the Genome. Elsevier, USA, pp 223–286
Carnaval AC, Moritz C (2008) Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest. J Biogeogr 35:1187–1201
Christoff AU, Fagundes V, Sbalqueiro YJ, Mattevi MS, Yonenaga-Yassuda Y (2000) Description of a new species of Akodon (Rodentia: Sigmodontinae) from Southern Brazil. J Mammal 81:838–851
Coyner BS, Braun JK, Mares MA, Van Den Bussche RA (2013) Taxonomic validity of species groups in the genus Akodon (Rodentia, Cricetidae). Zool Sc 42:335–350
Di Bitetti MS, Placci G, Dietz LA (2003) Una visión de biodiversidad para la Ecorregión del Bosque Atlántico del Alto Paraná: Diseño de un paisaje para la conservación de la biodiversidad y prioridades para las acciones de conservación. World Wildlife Fund, Washington, DC
Evans EP, Breckon G, Ford CE (1964) An air-drying method for meiotic preparations from mammalian testes. Cytogenetics 3:289–294
Fagundes V, Scalzi-Martin JM, Sims K, Hozier J, Yonenaga-Yassuda Y (1997) ZOO-FISH of a microdissection DNA library and G-banding patterns reveal the homeology between the Brazilian rodents Akodon cursor and A. montensis. Cytogenet Cell Genet 78:224–228
Fagundes V, Christoff AU, Yonenaga-Yassuda Y (1998) Extraordinary chromosomal polymorphisms with 28 different karyotypes in the neotropical species Akodon cursor (Muridae, Sigmodontinae), one of the smallest diploid number in rodents (2n = 16, 15 and 14). Hereditas 129:263–274
Fagundes V, Christoff A, Scalzi-Martin J, Hozier J, Moreira-Filho C, Yonenaga-Yassuda Y (2000) X;Y translocation revealed by chromosome microdissection and FISH infertile XY females in the Brazilian rodent Akodon montensis. Cytogenet Cell Genet 88:124–129
Ford CE, Hamerton JL (1956) A colchicine, hypotonic citrate, squash sequence for mammalian chromosomes. Stain Technol 31:247–251
Galindo-Leal C, Gusmão Câmara I (2003) The Atlantic Forest of South America: biodiversity status, threats and outlook. Island Press, Washington, USA
Galliari C, Pardiñas U (2020) Roedores Cricétidos de la provincia de Misiones (Argentina): síntesis de los datos obtenidos tras una década de muestreos esporádicos. Ecología en Bolivia 56(1):42–64
González EM, Langguth A, Oliveira LF (1998) A new species of Akodon from Uruguay and southern Brazil (Mammalia: Rodentia: Sigmodontinae). Comun Zool Mus Hist Nat Montevideo 191:1–8
Jayat JP, Ortiz PE, Salazar-Bravo J, Pardiñas UFJ, D'Elía G (2010) The Akodon boliviensis species group (Rodentia: Cricetidae: Sigmodontinae) in Argentina: species limits and distribution, with the description of a new entity. Zootaxa 2409:1–61
John B (1988) The biology of heterochromatin. In: Verma RS (ed) Heterochromatin: Molecular and structural aspects. Cambridge University Press, Cambridge, UK, London, New York, pp 1–128
Kasahara S, Yonenaga-Yassuda Y (1982) Chromosomal variability in Akodon sp. (Rodentia, Cricetidae). Cytologia 47:317–324
Kasahara S (2009) Introdução a Pesquisa em Citogenética de Vertebrados. In: Kassahara S (ed) Sociedade Brasileira de Genética, pp 9–160
Labaroni C, Paez Coll Mairhofer VA, Ojeda AA, Novillo A, Teta P, Jayat P, Ojeda RA, Buschiazzo LM, Cálcena EN, Bolzán AD, Lanzone C (In press) Revision and analysis of the chromosome variability in the speciose genus Akodon (Rodentia, Sigmodontinae), including new data from Argentina. An Acad Bras Ciênc
Lanzone C, Labaroni CA, Suárez N, Rodríguez D, Herrera ML, Bolzán AD (2015) Distribution of telomeric sequences (TTA GGG) n in rearranged chromosomes of phyllotine rodents (Cricetidae, Sigmodontinae). Cytogenet Genome Res 147:247–252
Liascovich RC, Reig OA (1989) Low Chromosomal number in Akodon cursor montensis Thomas, and karyologic confirmation of Akodon serrensis Thomas in Misiones Argentina. J Mammal 70:391–395
Lisanti JA, Pinna Senn E, Ortiz MI, Dalmasso G, Bella JL (2001) Characterization of the chromosomes of three species of Akodon (Rodentia, Sigmodontinae) by means of fluorochromes highly selective for DNA base composition. Cytologia 66:333–339
Malleret MM, Labaroni CA, García GV, Ferro JM, Martí DA, Lanzone C (2016) Chromosomal variation in Argentine populations of Akodon montensis Thomas, 1913 (Rodentia, Cricetidae, Sigmodontinae). Comp Cytogenet 10:129–140
Meyne J, Baker RJ, Hobart HH, Hsu TC, Ryder OA, Ward OD, Wiley JE, Wurster-Hill DH, Yates TL, Moyzis RK (1990) Distribution of nontelomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma 99:3–10
Ortiz MI, Dalmasso G, Dezi R, Pinna Senn E, Lisanti JA (1998) A C-band polymorphism of the X chromosome in Akodon azarae (Rodentia, Cricetidae). Cytologia 63:365–369
Patton JL, Pardiñas UF, D’Elía G (2015) Mammals of South America. Volume 2. Rodents. University of Chicago Press, Chicago, Illinois
Rovatsos MT, Marchal JA, Romero-Fernández I, Fernández FJ, Giagia Athanosopoulou EB, Sánchez A (2011) Rapid, independent, and extensive amplification of telomeric repeats in pericentromeric regions in karyotypes of arvicoline rodents. Chromosome Res 19:869–882
Ruiz-Herrera A, Nergadze SG, Santagostino M, Giulotto E (2008) Telomeric repeats far from the ends: mechanisms of origin and role in evolution. Cytogenet Genome Res 122:219–228
Schweizer D (1976) Reverse Fluorescent Chromosome Banding with Chromomycin and DAPI. Chromosoma 58:307–324
Silva MJ, Yonenaga-Yassuda Y (1998) Karyotype and chromosomal polymorphism of an undescribed Akodon from Central Brazil, a species with the lowest known diploid chromosome number in rodents. Cytogenet Cell Genet 81:46–50
Silva MJJ, Yonenaga-Yassuda Y (2004) B chromosomes in Brazilian rodents. Cytogenet Genome Res 106:257–263
Slijepcevic P (1998) Telomeres and mechanisms of Robertsonian fusion. Chromosoma 107:136–140
Soares AA, Castro JP, Balieiro P, Dornelles S, Degrandi TM, Sbalqueiro IJ, Ferreira Artoni R, Hass I (2018) B Chromosome Diversity and Repetitive Sequence Distribution in an Isolated Population of Akodon montensis (Rodentia, Sigmodontinae). Cytogenet Genome Res 154:79–85
Steinemann M, Steinemann S (2000) Common mechanisms of Y chromosome evolution. Genetica 109:105–111
Sumner AT (1972) A simple technic for demonstrating centromeric heterochromatin. Exp Cell Res 75:304–306
Tiranti SI (1998) Chromosomal variation in the scrub mouse Akodon molinae (Rodentia: Sigmodontinae) in central Argentina. Texas JSc 50:223–238
Valdez L, D'Elía G (2013) Differentiation in the Atlantic Forest: phylogeography of Akodon montensis (Rodentia, Sigmodontinae) and the Carnaval-Moritz model of Pleistocene refugia. J Mammal 94:911–922
Ventura K, Silva MJJ, Fagundes V, Christoff UA, YonenagaYassuda Y (2006) Non-telomeric sites as evidence of chromosomal rearrangement and repetitive (TTAGGG)n arrays in heterochromatic and achromatic regions in four species of Akodon (Rodentia, Muridae). Cytogenet Genome Res 115:169–175
Ventura K, O'Brien PCM, Yonenaga-Yassuda Y, Ferguson-Smith MA (2009) Chromosome homologies of the highly rearranged karyotypes of four Akodon species (Rodentia, Cricetidae) resolved by reciprocal chromosome painting: the evolution of the lowest diploid number in rodents. Chromosome Res 17:1063–1078
Veyrunes F, Watson J, Robinson TJ, Britton-Davidian J (2007) Accumulation of rare sex chromosome rearrangements in the African pygmy mouse, Mus (Nannomys) minutoides: a whole-arm reciprocal translocation (WART) involving an X-autosome fusion. Chromosome Res 15:223–230
Vujošević M, Rajičić M, Blagojević J (2018) B Chromosomes in Populations of Mammals Revisited. Genes 9:487
Waters PD, Wallis MC, Marshall Graves JA (2007) Mammalian sex Origin and evolution of the Y chromosome and SRY. Semin Cell Dev Biol 18:389–400
Yonenaga-Yassuda Y, Kasahara S, Almeida EJC, Peracchi AL (1975) Chromosomal banding patterns in Akodon arviculoides (2n = 14), Akodon sp. (2n = 24 and 25) and two male hybrids with 19 chromosomes. Cytogenet Cell Genet 15:388–399
Yonenaga-Yassuda Y, Assis MFL, Kasahara S (1992) Variability of the nucleolus organizes regions and the presence of the rDNA genes in the supernumerary chromosome of Akodon aff. arviculoides (Cricetidae, Rodentia). Caryologia 45:163–174
Acknowledgements
We thank the anonymous referees for the substantial contribution to this work. The authors thank to the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Funding
This research has been partially funded by Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (ANPCyT), Préstamo BID 2016 PICT N° 537.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Communicated by: Jan M. Wójcik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Labaroni, C.A., Ferro, J.M., Buschiazzo, L. et al. Extraordinary chromosome diversity in the southernmost populations of the montane grass mouse Akodon montensis (Cricetidae, Sigmodontinae). Mamm Res 68, 355–365 (2023). https://doi.org/10.1007/s13364-023-00687-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-023-00687-1