Abstract
Traditionally comparative cytogenetic studies are based mainly on banding patterns. Nevertheless, when dealing with species with highly rearranged genomes, as in Akodon species, or with other highly divergent species, cytogenetic comparisons of banding patterns prove inadequate. Hence, comparative chromosome painting has become the method of choice for genome comparisons at the cytogenetic level since it allows complete chromosome probes of a species to be hybridized in situ onto chromosomes of other species, detecting homologous genomic regions between them. In the present study, we have explored the highly rearranged complements of the Akodon species using reciprocal chromosome painting through species-specific chromosome probes obtained by chromosome sorting. The results revealed complete homology among the complements of Akodon sp. n. (ASP), 2n = 10; Akodon cursor (ACU), 2n = 15; Akodon montensis (AMO), 2n = 24; and Akodon paranaensis (APA), 2n = 44, and extensive chromosome rearrangements have been detected within the species with high precision. Robertsonian and tandem rearrangements, pericentric inversions and/or centromere repositioning, paracentric inversion, translocations, insertions, and breakpoints, where chromosomal rearrangements, seen to be favorable, were observed. Chromosome painting using the APA set of 21 autosomes plus X and Y revealed eight syntenic segments that are shared with A. montensis, A. cursor, and ASP, and one syntenic segment shared by A. montensis and A. cursor plus five exclusive chromosome associations for A. cursor and six for ASP chromosome X, except for the heterochromatin region of ASP X, and even chromosome Y shared complete homology among the species. These data indicate that all those closely related species have experienced a recent extensive process of autosomal rearrangement in which, except for ASP, there is still complete conservation of sex chromosomes homologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromosome studies of the species belonging to the rodent genus Akodon (Sigmodontinae, Akodontini) have revealed a great range in diploid numbers among the 29 described karyotypes so far, varying from 2n = 10 in Akodon sp. n. (ASP; Silva and Yonenaga-Yassuda 1998) to 2n = 46 in Akodon serrensis (Geise et al. 1998). Species of Akodon from Central, Northeastern, and Southeastern Brazil (Akodon sp. n. (ASP), 2n = 10; Akodon cursor (ACU), 2n = 14–16; Akodon montensis (AMO), 2n = 24; Akodon azarae, 2n = 38; Akodon lindberghi, 2n = 42; Akodon paranaensis (APA), 2n = 44; and A. serrensis, 2n = 46) have been studied using classic cytogenetics by G banding (Fagundes et al. 1997a,b; Geise et al. 1998; Silva and Yonenaga-Yassuda 1998; Ventura et al. 2006; Silva et al. 2006) and fluorescent in situ hybridization (FISH) with telomeric (Fagundes et al. 1997a,b; Silva and Yonenaga-Yassuda 1998; Vieira et al. 2004; Ventura et al. 2006) and species-specific probes (Fagundes et al. 1997b; Hass et al. 2008) aiming to identity chromosome rearrangements responsible for chromosome divergence. The results show that, in this group of species, complex rearrangements, including centric fusion and fission, Robertsonian and tandem rearrangements, inversions, conservation, and amplification of interstitial telomeric sequences (ITS) have occurred during chromosome evolution.
The A. cursor complex is comprised of several closely related species including A. paranaensis, Akodon mystax, Akodon reigi, with 2n = 44, and the cryptic A. montensis, A. cursor, and Akodon sp. n. (Silva et al. 2006; Smith and Patton 2007). In these last three species, conventional cytogenetics using G banding in conjunction with telomeric FISH were used to identify and localize chromosomal sequences involved in rearrangements. This has been an important strategy for detecting some mechanisms of chromosomal evolution.
A. cursor is characterized by an uncommon variability in diploid number (2n = 14–16) as well as in the number of autosomal arms (FN = 18–26). Complex rearrangements (pericentric inversion followed by centric fusion) that occur in homozygosis (2n = 14) or in heterozygosis (2n = 15), involving the autosomic pairs ACU 1 and ACU 3 from the 2n = 16 form, giving rise to a large metacentric ACU 1 + 3 from the 2n = 14 and 2n = 15 forms, and polymorphisms due to pericentric inversions, giving rise to acrocentric and submetacentric forms of pair ACU 2, acrocentric and metacentric of pair ACU 4, and acrocentric and submetacentric of pair ACU 6, are responsible for the high karyotypic variability in this species (Yonenaga 1972; Yonenaga et al. 1975; Yonenaga-Yassuda 1979; Sbalqueiro and Nascimento 1996; Christoff 1997; Fagundes et al. 1997a;Fagundes et al. 1998). The chromosome data and cytochrome b sequences that recover two reciprocal groups are the characters that separate this species from A. montensis (2n = 23–26; Kasahara and Yonenaga-Yassuda 1984; Geise et al. 2001) since they are morphologically indistinguishable. Both species occur in sympatry in regions located in the states of Rio de Janeiro, São Paulo, and Paraná, where sterile hybrids were found (Yonenaga 1972; Yonenaga et al. 1975; Yonenaga-Yassuda 1979; Sbalqueiro and Nascimento 1996; Christoff 1997; Fagundes et al. 1997a). Fagundes et al. (1997a, b) proposed that reduction of diploid number occurs in this species complex. This process was demonstrated by comparative cytogenetic analysis of G-banding patterns and by the presence of an ITS on the rearranged chromosome ACU 1 + 3, interpreted as a relic of the fusion event. Chromosome painting using the probe of chromosome 1 of 2n = 16 (ACU 1) obtained by microdissection confirmed the homology between chromosome ACU 1 and the long arm of the chromosome resulting from the fusion (ACU 1 + 3; Fagundes et al. 1997b). The probe was also hybridized to the closely related species A. montensis and showed that chromosome ACU 1 is homologous to three different biarmed autosomes of A. montensis, which was also suggested by previous analysis of G-banding patterns.
Silva and Yonenaga-Yassuda (1998) described for Akodon sp. n. the lowest diploid number for rodents. The specimens presented 2n = 9, 10 and FN = 14–16, with autosomal polymorphism due to pericentric inversion on pair ASP 3 and monosomy of the X chromosome. Preliminary morphological studies suggest that Akodon sp. n. is cryptic to A. cursor and A. montensis (Christoff, personal communication) since they are morphological indistinguishable. A comparison between the karyotype of Akodon sp. n. and A. cursor was made by Silva et al. (2006), but the authors were unable to establish complete correspondence between both complements since a distal part of pair 3 of the former species did not show homology to any chromosome pair of the latter using G banding. After telomeric FISH, both homologs of pairs ASP 1 and ASP 3 showed ITS, which did not coincide with the presumed breakpoints involved in the proposed chromosomal rearrangements. According to the authors, the ITS observed on the pericentromeric region of ASP 1 is related to the repetitive nature of the constitutive heterochromatin. The G-banding comparison between the karyotypes with 2n = 16 and 2n = 14 of A. cursor and the karyotype with 2n = 10 of Akodon sp. n. indicated that fewer steps and less complex rearrangements would be involved in the differentiation between the 2n = 16 and the 2n = 10 karyotypes than between 2n = 14 and 2n = 10, suggesting that a diploid number similar or larger than the 2n = 16 could be ancestral to the 2n = 10 of Akodon sp. n.
Traditionally, comparative cytogenetic studies are based mainly on banding patterns. Nevertheless, when dealing with species with highly rearranged genomes, as in Akodon species, or with other highly divergent species, cytogenetic comparisons of banding patterns prove inadequate (Chowdhary and Raudsepp 2001). Hence, comparative chromosome painting has become the method of choice for genome comparisons at the cytogenetic level since it allows complete chromosome probes of a species to be hybridized in situ onto chromosomes of other species, detecting homologous genomic regions between them. In the present study, the complex Akodon cytogenetic model based on reduction in diploid number (Fagundes et al. 1997a, b; Geise et al. 1998; Silva et al. 2006) was explored using reciprocal chromosome painting. The hybridization of species-specific chromosome probes, obtained by chromosome sorting, permitted the detection of extensive chromosomal rearrangements with high precision among the closely related species Akodon sp. n., A. cursor, A. montensis, and A. paranaensis. Chromosome paints from the latter species that presents 2n = 44, with most of the elements in acrocentric form, were particularly useful in demonstrating homologous regions with high resolution among the karyotypes with lower diploid number.
Material and methods
Specific painting probes were generated from flow-sorted chromosomes, in the Molecular Cytogenetics Laboratory, Department of Veterinary Medicine, University of Cambridge, UK, from chromosomes obtained from primary fibroblast cell lines of Akodon species: one from Akodon sp. n. (ASP, 2n = 10), two from A. cursor (ACU, 2n = 15 and 2n = 14), and one from APA (2n = 44) were each from male animals. These cell lines were established in the Laboratório de Citogenética de Vertebrados, Departamento de Genética Biologia Evolutiva do Instituto de Biociências da Universidade de São Paulo, Brazil. Spread metaphases of Akodon sp. n., A. cursor, and A. paranaensis were obtained from cell cultures and used to perform in situ hybridization. The A. montensis metaphases were obtained from in vivo bone marrow preparations following Ford and Hamerton (1956), with modifications. The specimen karyotypes in GTG-banding pattern are shown on Fig. 1.
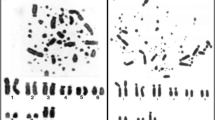
Akodon karyotypes in GTG-banding pattern. a A. paranaensis, 2n = 44 (modified from Sbalqueiro 1989); b A. montensis 2n = 24; c A. cursor, 2n = 15 with complex rearrangement involving ACU 1, ACU 3, and ACU 1 + 3, and heteromorphic ACU 2 d Akodon sp. n., 2n = 10 with heteromorphic ASP 3. Inset, sex chromosomes from another metaphase. Bars = 10 μm
The chromosomes of A. paranaensis were arranged in the karyotypes according to their position in the flow karyotype; the chromosomes of A. cursor were numbered as described by Fagundes et al. (1998) and Akodon sp. n. and A. montensis following Silva and Yonenaga-Yassuda (1998) and Fagundes et al. (1997b), respectively. The chromosome pairs were previously individually identified by G banding and were represented by ideograms. On the hybridized metaphases, the chromosomes were identified by 4′,6-diamidino-2-phenylindole (DAPI) staining.
The whole chromosome-specific paints were made by degenerate oligonucleotide-primed polymerase chain reaction (DOP-PCR) on flow-sorted chromosomes (Telenius et al. 1992; Yang et al. 1995). The paints from each peak obtained from flow sorting were hybridized to metaphases of the same species to identify the chromosomes in each peak of the flow karyotype. Briefly, the chromosomes were prepared as described and stained with Hoechst 33258 (2 μg/ml) and chromomycin A3 (40 μg/ml) in the presence of magnesium sulfate (2.5 mmol/l) for 2 h. Sodium sulfate (25 mmol/l) and sodium citrate (10 mmol/l) were added 15 min prior to flow sorting. Chromosome sorting was performed using a dual-laser cell sorter (Mo-Flow system). About 400 chromosomes were sorted from each peak in the flow karyotypes. Chromosomes were sorted directly into PCR tubes containing 30 μl of distilled water. These samples were amplified by DOP-PCR using the primer 6 MW (Telenius et al. 1992). Primary PCR products were labeled either with biotin-16-dUTP (Boehringer Mannheim), fluorescein isothiocyanate (FITC)-12- dUTP (Amersham), or Cy3-dUTP by taking 1 μl of product to a second round of DOP-PCR using the same primer. In situ hybridization of painting probes was performed as previously described (Yang et al. 1995). Briefly, 14 ml of the hybridization buffer and 1 μl of labeled PCR product were denatured at 37°C for 30 min, dropped onto slides that were denatured in 70% formamide/2× saline sodium citrate (SSC) at 65°C for 2 min. The cross-species hybridization was performed for 48–72 h at 37°C. Posthybridization washes included 2 × 5-min incubations in 50% formamide/2× SSC at 42°C followed by 2 × 5-min incubations in 2× SSC and submerged for 4 min in 4 × T (100 ml 20× SSC + 400 ml H2O + 250 μl Triton X-100 Sigma-Aldrich). The biotinylated probes were detected with avidin-Cy3 for single-color or avidin-Cy5 for multicolor FISH whereas FITC-labeled probes were visualized with rabbit anti-FITC, followed by goat antirabbit antibody on both procedures. All slides were counterstained with DAPI diluted with Vectashield and analyzed under a Zeiss Axiophot fluorescence microscope equipped with software for image capture system (Isis karyotyping system, MetaSystems).
Results
Flow sorting of A. paranaensis, 2n = 44
The flow karyotype of A. paranaensis gave 19 peaks, and single chromosome paints were obtained from 16 peaks: 14 autosomes, chromosome X, and chromosome Y. Two peaks contain two autosomes (6 and 7; 18 and 19), and one peak contains three chromosomes (10, 11, and 12) as shown in the flow karyotype (Fig. 2a).
Flow karyotype of Akodon species. Chromosomes were sorted for DNA content and AT to GC base pair ratios after staining with Hoechst 33258 (vertical axis) and chromomycin-A3 (horizontal axis). a A. paranaensis, 2n = 44; b A. cursor, 2n = 15; c A. cursor, 2n = 14; d Akodon sp. n., 2n = 10. Chromosome 1 + 3 is omitted from b but shown in c
Flow sorting of A. cursor, 2n = 15 and 2n = 14
The flow karyotype of A. cursor, 2n = 15, gave nine peaks, and single chromosome paints were obtained from each one shown in the flow karyotype (Fig. 2b): five autosome pairs, two single chromosomes (1 and 3), chromosome X, and chromosome Y. The largest chromosome ACU 1 + 3 did not appear in this flow karyotype, and for this reason, another cell line, with 2n = 14, of A. cursor was used to obtain the ACU 1 + 3 autosome pair (Fig. 2c)
Flow sorting of Akodon sp. n., 2n = 10
The flow karyotype of Akodon sp. n. gave six peaks, and single chromosome paints were obtained from each one shown in the flow karyotype (Fig. 2d): four autosomes, chromosome X, and chromosome Y.
Hybridization of A. paranaensis probes on A. montensis, A. cursor, and Akodon sp. n. genomes
A. montensis genome
The hybridization of the A. paranaensis (2n = 44) probes to the genome of A. montensis (2n = 24) revealed 23 homologous segments (Fig. 3a). Four probes hybridized to whole chromosomes of A. montensis: APA 19 (AMO 10), APA 21 (AMO 11), APA X (AMO X), and APA Y (AMO Y; the last three are shown on Fig. 4a, d, g); three probes (APA 1, APA 20, and APA 3) hybridized to AMO 1 (Fig. 5a–c). The remaining chromosome paints hybridized to whole chromosomes arms of A. montensis: as depicted on Fig. 3a. This hybridization revealed that the 23 APA chromosomes are unbroken in AMO and organized either in nine syntenic segments: AMO 1 (APA 3/APA 20/APA 1), AMO 2 (APA 4/APA 2), AMO 3 (APA 7/APA 6), AMO 4 (APA 10/APA 5), AMO 5 (APA 9/APA 8), AMO 6 (APA 12/APA 11), AMO 7 (APA 16/APA 14), AMO 8 (APA 17/APA 15), and AMO 9 (APA 18/APA 13), or kept as whole chromosomes like in AMO 10, 11, X, and Y.
Ideograms of Akodon species karyotypes, based on G banding patterns, with cross-species homologies indicated on either side of each chromosome. APA on right side, ACU and/or ASP on left side. a
A. montensis 2n = 24; b
A. cursor, 2n = 15; c
Akodon sp. n., 2n = 10.  represent the nucleolar organizer regions (NORs). H = ASP X heterochromatin.
represent the nucleolar organizer regions (NORs). H = ASP X heterochromatin.  = ITS (yellow) and small insertions of ACU 7 (green) on ASP 1
= ITS (yellow) and small insertions of ACU 7 (green) on ASP 1
A. cursor genome
The hybridization of the A. paranaensis probes to the genome of A. cursor (2n = 15) revealed 24 homologous segments on the haploid set (Fig. 3b). Three paints of A. paranaensis were hybridized to whole chromosomes of A. cursor: APA 21 (ACU 7), APA X (ACU X), and APA Y (ACU Y; Fig. 4b, e, h). Nine probes (APA 1, APA 20, APA 3, APA 14, APA 16, APA 13, APA 18, APA 15, and APA 17) hybridized to ACU 1 + 3, with six corresponding to homologous parts of ACU 1 and, three corresponding to the homologous parts of ACU 3 (Fig. 5d–f), and APA 20 also exhibited a dot-like signal on proximal ACU (1 + 3)q (Fig. 5e); ACU 2 was painted by four probes and three probes (APA 9, APA 8, and APA 19) hybridized to ACU 5 (Fig. 6). Two chromosomes of A. cursor, ACU 4 and ACU 6, were hybridized by two probes. These hybridizations revealed that 21 APA chromosomes exist unbroken in ACU since APA 8 presents either a pericentric inversion or centromere repositioning as observed on ACU 5 (Fig. 6), and APA 20 is divided into two segments in ACU 1 + 3 (Fig. 5e). The same nine APA syntenic associations that were observed on A. montensis are present as well as another five associations that are specific for A. cursor: ACU 1q (APA 16/APA 13 and APA 18/APA 15), ACU 1 + 3 (APA 20/APA 14), ACU 2 (APA 7/APA12), and ACU 5 (APA 8/APA19).
Akodon sp. n. genome
The hybridization of the A. paranaensis probes to the genome of Akodon sp. n. (2n = 10) revealed 26 homologous segments (Fig. 3c). Three paints of A. paranaensis were hybridized to whole chromosomes of Akodon sp. n.: APA 21 (ASP 4), APA X (ASP X), and APA Y (ASP Y; Fig. 4c, f, i). Nine probes hybridized to ASP 1, being five probes on ASP1p and four probes on ASP1q; APA 19 hybridized two segments on ASP1p, and APA 17 hybridized two segments on ASP 1q. Five probes hybridized to ASP 2; and six probes hybridized to ASP 3; however, APA 8 hybridized to two segments on this chromosome. These hybridizations revealed that Akodon sp. n. possesses 19 unbroken APA chromosomes. The rearranged chromosomes are as follows: APA 19 that painted two segments on ASP 1p, APA 17 that painted two segments on ASP 1q, APA 3 that presents either a pericentric inversion or centromere repositioning, and APA 8 that also presents either a pericentric inversion or centromere repositioning, and it is represented by two segments of ASP 3q. This species shares with A. montensis and with A. cursor eight autosomal syntenic associations; the syntenic association APA 16/APA 14, shared by AMO and ACU, is not observed on ASP. Six specific associations are observed on Akodon sp. n.: ASP 1p (APA 18/APA 14/APA 19/APA 16/APA 19/APA 5), ASP 1q (APA 10/APA 15 and APA 17/APA 15); ASP 2p (APA 4/APA 3), ASP 3q (APA 7/APA 8), and (APA 8/APA12). There were no APA syntenic associations shared exclusively by ASP and ACU. ASP Y and ASP X were the only chromosomes that presented whole homology to a single paint.
Hybridization of A. cursor probes on A. montensis and Akodon sp. n. genomes
A. montensis genome
The hybridization of A. cursor probes to the genome of A. montensis revealed 13 homologous segments (Fig. 3a). Six probes hybridized to whole chromosomes of A. montensis: ACU 3 (AMO 1), ACU 4 (AMO 2), ACU 6 (AMO 4), ACU 7 (AMO 11), ACU X (AMO X), and ACU Y (AMO Y). ACU 1 hybridized to three chromosomes of A. montensis (AMO 7, AMO 8, and AMO 9); ACU 2 hybridized to two chromosomes, AMO 3 and AMO 6; ACU 5 hybridized to AMO 5 and AMO 10.
Akodon sp. n. genome
The hybridization of A. cursor probes using single-color and multicolor FISH to the genome of Akodon sp. n. revealed 18 homologous segments (Figs. 3c, 7, and 8). Two probes hybridized to whole chromosomes of Akodon sp. n.: ACU X (ASP X) and ACU Y (ASP Y). Two probes hybridized to more than one chromosome of Akodon sp. n.: ACU 5 exhibited four hybridization signals, two being segments on ASP 1p, one segment on ASP 3q, and one small distal segment on ASP 4p (Fig. 8a, c, d); ACU 7 hybridized, besides chromosome ASP 4, to two small segments on ASP 1, one on ASP 1p, and another on ASP 1q (Figs. 8b, c). ACU 6 hybridized to one segment on ASP 1. Paint ACU 1 painted four segments on ASP 1: three on ASP 1p and one on ASP 1q. ACU 2 painted two segments on ASP 3. Two probes, ACU 4 and ACU 3, hybridized to ASP 2, the latter to part of the short arm and the latter to proximal part of the short arm and to whole ASP 2q.
Multicolour FISH of the A. cursor complement on the four autosomes of Akodon sp. n. Homologies to ACU complement are indicated on the right of each colored segment. Asterisk indicates ACU chromosomes that presented more than one homologous segment. The segments homologous to part of ACU 7 on ASP 1p and q is not evident
Akodon sp. n. metaphase painted with a ACU 5 and b ACU 7. c ASP 1 on the left hybridized with telomeric sequences and on the right with multicolour FISH; d ASP 3 on the left hybridized with telomeric sequences and on the right with multicolour FISH. Multicolour FISH corresponds to: blue ACU 1, green ACU 7, orange ACU 5, red ACU 6, and white ACU 2. Note that the ITS in c corresponds to the ACU 5 (orange) and in d to the junction between ACU 2 (white) and ACU 5 (orange). The red signals on ASP 1 b correspond to the green signals on c. The probes used for FISH are indicated at each upper right corner, and the painted chromosomes are shown in italics
No APA or ACU probes used in this work hybridized to the heterochromatic region of the X chromosome of Akodon sp. n. The hybridization of the ASP X to Akodon sp. n. revealed, besides whole ASP X, signals on the heterochromatic pericentromeric regions of ASP 1 and ASP 2 (Fig. 9). Paint ASP 4 (homologous to ACU 7) did not exhibit additional signals on ASP 1.
Hybridization of Akodon sp. n. probes on the A. montensis and A. cursor genomes
A. montensis genome
The hybridization of Akodon sp. n. probes to the genome of A. montensis revealed 13 homologous segments (Fig. 3a). Three paints hybridized to single whole chromosomes: ASP 4 (AMO 11), ASP X (AMO X), and ASP Y (AMO Y). ASP 1 hybridized to five whole chromosomes: AMO 4, AMO 7, AMO 8, AMO 9, and AMO 10; ASP 2 hybridized to two whole chromosomes: AMO 1 and AMO 2; ASP 3 hybridized to three whole chromosomes: AMO 3, AMO 5, and AMO 6.
A. cursor genome
The hybridization of Akodon sp. n. probes to the genome of A. cursor (2n = 15) revealed ten homologous segments (Fig. 3b). Three paints hybridized to single whole chromosomes of A. cursor: ASP 4 (ACU 7), ASP X (ACU X), and ASP Y (ACU Y). ASP 1 painted four segments: whole ACU 1 and ACU 6, ACU 1 + 3q, and a distal segment on ACU 5q. The ASP 2 painted three segments: whole ACU 4 and ACU 3 and ACU 1 + 3p. ASP 3 painted whole chromosome ACU 2 plus ACU 5p and the proximal part of ACU 5q.
The figures numbered 10 and 11 will be used to illustrate some points of the following discussion.
Discussion
Chromosome painting, using A. paranaensis (2n = 44) probes, established with great precision the homology between the Akodon species studied herein and revealed a large number of chromosome rearrangements involved in chromosomal diversification among Akodon sp. n., A. cursor, A. montensis, and A. paranaensis.
Autosomal polymorphisms of A. cursor and Akodon sp. n.
Autosomal polymorphism due to pericentric inversions of ACU 2, ACU 4, and ACU 6, observed in homozygous and heterozygous forms, and complex rearrangements involving pericentric inversion followed by centric fusion of ACU 1 and ACU 3 are observed on A. cursor (2n = 14–16 and FN = 18–26) from different Brazilian localities (Yonenaga 1972; Yonenaga et al. 1975; Yonenaga-Yassuda 1979; Sbalqueiro and Nascimento 1996; Fagundes et al. 1997a; Fagundes et al. 1998).
Akodon sp. n. (2n = 10 and FN = 14–16) presents autosomal polymorphism of ASP 3 due to pericentric inversion resulting either in acrocentric or submetacentric forms (Silva and Yonenaga-Yassuda 1998).
According to Fagundes et al. (1997a), the position of an ITS on the short arms of the big metacentric ACU 1 + 3 suggested the conservation of the whole ACU 1 and the loss of centromeric and telomeric segments of ACU 3. Our results, on the other hand, reveal a dot-like segment on the pericentromeric ACU (1 + 3)q after hybridization of APA 20, confirming the conservation of the whole ACU 3 after pericentric inversion followed by centric fusion (Fig. 10).
a ACU 1 + 3 and ACU 1 chromosomes painted with ACU 1 paint. Note that there is no homology between ACU 1 and pericentromeric region indicated by arrows. b ACU 1 + 3 and ACU 1 chromosomes painted with APA 20 paint. Note that the dot-like signal on the pericentromeric region of ACU 1 + 3q confirms the complete inclusion of whole ACU 3 after inversion and centric fusion
The regions of ACU 2 and ASP 3, involved in pericentric inversion in both A. cursor and Akodon sp., are homologous and correspond in greater part to APA 6, localized in A. montensis, A. cursor, and Akodon sp. n., forming the syntenic association APA 6/APA 7.
The segments of ACU 4 and ACU 6, also involved in the same kind of rearrangement, are homologous to the syntenic associations APA 4/APA 2 and APA 10/APA 5, respectively. The majority of the inverted segment corresponds to APA 4 in ACU 4 and to APA 10 in ACU 6.
Chromosomal rearrangements among Akodon species by chromosome painting
The following discussion is based on the reduction of diploid number, from 2n = 44 in A. paranaensis to 2n = 10 in Akodon sp. n., being the species that accumulates the largest number of rearrangements detected by chromosome painting. The eight syntenic associations shared among A. montensis, A. cursor, and Akodon sp. n., as well as the syntenic association APA 16/APA 14 shared by AMO and ACU and the specific associations of A. cursor and Akodon sp. n., all established by APA specific probes, are considered.
A. paranaensis, 2n = 44, compared to A. montensis, 2n = 24
Nine APA syntenic associations have been described in the A. montensis complement. Eight of them are exclusively related to Robertsonian rearrangements since the association APA 3/APA 20/APA 1 (AMO 1) is related to one Robertsonian rearrangement plus, as we can observe on AMO 1q, one tandem rearrangement involving APA 1 and APA 20. This rearrangement, or syntenic association (APA 1/APA 20), was previously described by Ventura et al. (2006) by G-banding and telomeric FISH comparison for the species A. lindberghi, 2n = 42; A. boliviensis, 2n = 40; and A. azarae, 2n = 38 and was conserved in all species of Akodon reported so far with a diploid number lower than 2n = 44, including Akodon sp. n., A. cursor, and A. montensis in the present report. A. azarae is the single species that presents ITS coincident to the rearrangement site (Vieira et al. 2004; Ventura et al. 2006).
A. montensis, 2n = 24, compared to A. cursor, 2n = 14 and 2n = 16
The nine APA syntenic segments shared between these species show that the differentiation between the A. montensis karyotype and the 2n = 14 or 2n = 16 A. cursor karyotype, respectively, is due to at least five pericentric inversions, four tandem rearrangements, and one Robertsonian rearrangement, in the first case, and due to three inversions and four tandem rearrangements in the second case. Both cases present a small pericentric inversion or centromere repositioning involving AMO 5q and ACU 5 related to the APA 8 homologous segments (Fig. 3a).
The tandem rearrangements gave rise to the A. cursor-specific APA associations, APA 16/APA 13, APA 18/APA 15, APA 7/APA 12, and APA 8/APA 19, and the Robertsonian rearrangement gave rise to APA 3/APA 20/APA 14.
There is total homology maintenance between the autosomes ACU 3 and AMO 1, ACU 4 and AMO 2, ACU 6 and AMO 4, and ACU 7 and AMO 11.
A. cursor, 2n = 16, compared to Akodon sp. n., 2n = 10
Silva and Yonenaga-Yassuda (1998) compared GTG bands of the 2n = 10 and 2n = 16 karyotypes and demonstrated that fewer steps were involved in the differentiation between these karyotypes than between 2n = 10 and 2n = 14. The analysis suggested that a similar karyotype or a diploid number higher than 2n = 16 gave rise to the lowest chromosome number of Akodon sp. n. (2n = 10). Because of this, the discussion is based on the chromosome painting comparison between the 2n = 16 of A. cursor and the 2n = 10 of Akodon sp. n.
The results of chromosome painting with A. cursor paints on Akodon sp. n after single-color and multicolor FISH indicated that: (1) tandem rearrangements involving ACU 3 and ACU 4 (acrocentric form) gave rise to ASP 2; (2) pericentric inversion or centromere repositioning resulted in a change in morphology of ACU 3p (when comparing ACU 3 and its homologous part on ASP 2); (3) chromosome breakage of ACU 5 into three segments: the first segment was translocated to ACU 6p (acrocentric form), the second segment was inserted in ACU 2q, giving rise to ASP 3, and the third segment was translocated to ASP 4p. After that, break and insertion of a small segment, homologous to ACU 5, occurred on ASP 1p; (4) insertion of the ACU 5/ACU 6 segment on ACU 1 gave risen to ASP 1; (5) break of ACU 7 and insertion of two small segments on ASP 1p and q.
However, when the larger number of chromosomes paints from APA (a species with a higher diploid number) was used for hybridization in both species, a greater number of rearrangements between A. cursor and Akodon sp. n. were much more evident. The association APA 14/APA 16/APA 13/APA 18 is observed on ACU 1. When these homologous segments are compared with Akodon sp. n., the association APA 18/APA 14 is observed on ASP 1p. Chromosome breaks that could allow the insertion of the segment composed by APA 13/APA 18 in a distal position relative to the segment APA 14/APA 16, explains the association APA 18/APA 14 on Akodon sp. n.
Chromosome inversions or centromere repositioning could explain the morphological change in the homologous part of APA 3 on ACU 3 and on ASP 2 (Fig. 11).
It was also possible to identify that the part of ACU 5 that is inserted into ASP 1p is homologous to APA 19, and that the part inserted into ASP 3q is homologous to APA 8/APA 9/APA 8. This presumes an earlier pericentric inversion of the segment APA 8/APA 9 in chromosome ACU 5, in which the distal part is homologous to the whole APA 9 and two proximal segments, including the centromere, that are homologous to APA 8.
The segment of ACU 5, homologous to APA 19, may have been originally translocated to the short arm of ACU 6 (a segment that is homologous to APA 10), although APA 19/APA 5 is the association observed on the pericentromeric region of ASP 1. A paracentric inversion involving the segment APA 10/APA 5 could have occurred in ASP 1q, giving rise to the association APA 19/APA 5 now observed.
It is noticed that the breakage of ACU 1, where the segment ACU 5/ACU 6 was inserted giving rise to ASP 1, has occurred between the segments homologous to APA 15 and APA 18. After this process, the functional centromere of ASP 1 is the one kept by the segment ACU 5/ACU 6 and inserted in ACU 1.
Chromosome break and insertion of a part of the segment homologous to chromosome APA 17 could explain the two hybridization signals produced by APA 17 probe on the distal portion of ASP 1, and the same rearrangement could explain the small interstitial segment of ACU 5 (APA 19) on ASP1p (Fig. 8c).
Sex chromosomes
The reciprocal chromosome painting revealed total homology between the X chromosomes of A. paranaensis, A. montensis, and A. cursor.
The hybridization using APA X and ACU X probes painted the long arm of ASP X. The X chromosome of Akodon sp. n. bears a pericentromeric block of heterochromatin (Silva and Yonenaga-Yassuda 1998) that is unique to this species and does not share homology with any other paint. This heterochromatin is specific and presents homology to the heterochromatin existent on pericentromeric region of ASP 1 and ASP 2, as shown by hybridization of ASP X. This result highlights the complexity concerning the chromosome rearrangements of the Akodon sp. n. karyotype.
The Y chromosome preserves total homology among the species, indicating that these four Akodon species are closely related. Usually, among more distantly related species, this degree of Y chromosome homology is lost (Glas et al. 1999; Muller et al. 1999; Yang et al. 2003; Tian et al. 2004; Li et al. 2004).
Final considerations
Breakpoints, where chromosome rearrangements seen to be favorable, were noticed:
-
1.
The tandem fusion point of AMO 9 and AMO 8 observed on ACU 1, delineated by the hybridization of APA 18 and APA 15, is related to the occurrence of five chromosome breaks. This is the same site involved on the process of break and insertion, related to segment ACU 5/ACU 6, as noticed on ASP 1, and it is also the site of insertion of two segments of ACU 7: between the syntenic associations APA 13/APA 18 and APA 14/APA 16 on ASP 1p and between the syntenic associations APA 5/APA 10 and APA 15/APA 17 on ASP 1q. The site of insertion of ACU 7 is coincident with the point of insertion of the segment APA 13/APA 18 in a distal position on ASP 1p and with the point of insertion of small portion of the segment APA 17 on ASP 1q.
-
2.
The tandem fusion point of AMO 10 and AMO 5 observed on ACU 5 is delineated by the hybridization of APA 8 and APA 19. This is the same site involved on break and translocation of part of ACU 5 (APA 19) to the short arm of ACU 6 as observed on ASP 1.
-
3.
The tandem fusion point of AMO 3 and AMO 6 observed on ACU 2 is delineated by the hybridization of APA 7 and APA 12. This is the same site involved on the process of break followed by insertion of part of ACU 5 as observed on ASP 3.
The ITS described by Silva and Yonenaga-Yassuda (1998) at ASP 3q seems to be located at the junction between the breakpoint of ACU 2q and the inserted ACU 5 (Figs. 3c and 8d), and another on ASP 1p is near to the homologous APA 19 (ACU 5) interstitial segment (Fig. 3c and 8c). Besides, ITS colocalized with heterochromatin was detected in the pericentromeric region of ASP 1. Although extremely rearranged, only three ITS were detected using conventional commercial telomeric probes. Further experiments using a more sensitive FISH analysis with a longer synthetic (TTAGGG)n probe, might show previously undetected patterns of ITS [see Ruiz-Herrera et al. (2008), for review on telomeric repeats].
The chromosome painting using the APA set of 21 autosomes plus X and Y exhibited eight syntenic associations that are shared with A. montensis, A. cursor, and Akodon sp. n. and one syntenic association (APA 16/APA 14) that is shared exclusively by A. montensis and A. cursor, plus five exclusive syntenic associations for A. cursor and six for Akodon sp. n.
Hass et al. (2008) established chromosome homology maps between Mus musculus and five rodent species, among them A. cursor, A. montensis, and A. paranaensis, by chromosome painting using mouse-chromosome-specific probes. The grouping of AMO + ACU as recovered by the authors is also supported by the present data on Akodon species. Regarding Akodon sp. n., Silva et al. (2006) recovered this species as a sister group to A. cursor, and their relationship with A. montensis was unresolved. The present data also appear to place ASP closer to AMO and ACU than to APA.
Reciprocal chromosome painting revealed complete homology among the Akodon sp. n., 2n = 10; A. cursor, 2n = 15; A. montensis, 2n = 24; and A. paranaensis, 2n = 44, and a large number of chromosomal rearrangements have been highlighted among the four complements, including Robertsonian and tandem rearrangements, pericentric inversions and/or centromere repositioning, translocations, and insertions, and the occurrence of breakpoints which were observed where chromosome rearrangements seem to be favored. It was possible to demonstrate that the X chromosomes, except for the heterochromatin region of ASP X that is specific and presents rearrangements involving the heterochromatic pericentric regions of ASP 1 and ASP 2, and even chromosome Y are conserved among the species.
The probe set obtained from the complement of A. paranaensis, 2n = 44, composed in the majority by acrocentric elements, was useful as chromosome markers, especially regarding the large chromosomes of Akodon sp. n., and enabled the detection of intrachromosomal rearrangements that have occurred during the process of diploid number reduction.
The Y chromosome homology shared among these species, despite the extremely rearranged complements, indicates that these are closely related species that have experienced a recent, rapid, and intensive process of autosomal rearrangement, in which there is still complete genome conservation of the Y chromosome.
Abbreviations
- ACU:
-
Akodon cursor
- AMO:
-
Akodon montensis
- APA:
-
Akodon paranaensis
- ASP:
-
Akodon sp. n.
- DOP-PCR:
-
Degenerate oligonucleotide-primed PCR
- FISH:
-
Fluorescent in situ hybridization
- FN:
-
Fundamental number of autosomal arms
- ITS:
-
Interstitial telomeric sequences
- PCR:
-
Polymerase chain reaction
References
Chowdhary BP, Raudsepp T (2001) Chromosome painting in farm, pet and wild animal species. Methods Cell Sci 23:37–55
Christoff AU (1997) Contribuição à sistemática das espécies do gênero Akodon (Rodentia, Sigmodontinae) do leste do Brasil: estudos anatômicos, citogenéticos e de distribuição geográfica. Tese de Doutorado. Departamento de Biologia, Instituto de Biociências, Universidade de São Paulo, São Paulo
Fagundes V, Vianna-Morgante AM, Yonenaga-Yassuda Y (1997a) Telomeric sequences localization and G-banding patterns in the identification of a polymorphic chromosomal rearrangement in the rodent Akodon cursor (2n = 14, 15 and 16). Chromosome Res 5:228–232
Fagundes V, Scalzi-Martin JM, Sims K, Hozier J, Yonenaga-Yassuda Y (1997b) ZOO-FISH of a microdissection DNA library and between the Brazilian rodents Akodon cursor and A. montensis. Cytogenet Cell Genet 78:224–228
Fagundes V, Christoff AU, Yonenaga-Yassuda Y (1998) Extraordinary chromosomal polymorphism with 28 different karyotypes in the neotropical species Akodon cursor (Muridae, Sigmodontinae), one of the smallest number in rodents (2n = 16, 15 and 14). Hereditas 129:263–247
Ford CE, Hamerton JL (1956) A colchicine hypotonic citrate squash sequence for mammalian chromosomes. Stain Technol 31:247–251
Geise L, Canavez FC, Seuánez HN (1998) Comparative karyology in Akodon (Rodentia, Sigmodontinae) from Southwestern Brazil. J Hered 89:158–163
Geise L, Smith MF, Patton JL (2001) Diversification of genus Akodon (Rodentia: Sigmodontinae) in southeastern South America: mitochondrial DNA sequence analysis. J Mammal 82:92–101
Glas R, de Leo AA, Delbridge ML, Reid K, Ferguson-Smith MA, O'Brien PCM, Westerman M, Graves JAM (1999) Chromosome painting in marsupials: genome conservation in the kangaroo family. Chromosome Res 7:167–176
Hass I, Sbalqueiro JI, Muller S (2008) Chromosomal phylogeny of four Akodontini species (Rodentia, Cricetidae) from Southern Brazil established by Zoo-FISH using Mus musculus (Muridae) painting probes. Chromosome Res 16(1):75–88
Kasahara S, Yonenaga-Yassuda Y (1984) A progress report of cytogenetic data on Brazilian rodents. Rev Bras Genet 63:156–159
Li T, O'Brien PCM, Biltueva L, Fu B, Wang J, Nie W, Ferguson-Smith MA, Graphodatsky AS, Yang F (2004) Evolution of genome organizations of squirrels (Sciuridae) revealed by cross-species chromosome painting. Chromosome Res 12:317–35
Muller S, Stanyon R, O'Brien PCM, Ferguson-Smith MA, Plesker R, Wienberg EJ (1999) Defining the ancestral karyotype of all primates by multidirectional chromosome painting between tree shrews, lemurs and humans. Chromosoma 108:393–400
Ruiz-Herrera A, Nergadze SG, Santagostino M, Giulotto E (2008) Telomeric repeats far from the ends: mechanisms of origin and role in evolution. Cytogenet Genome Res 122:219–228
Sbalqueiro IJ (1989) Análises cromossômicas e filogenéticas em algumas espécies de roedores da região sul do Brazil. PhD Thesis. Universidade Federal do Rio Grande do Sul. Porto Alegre. Brazil
Sbalqueiro JI, Nascimento AP (1996) Occurrence of Akodon cursor (Rodentia, Cricetidae) with 14, 15 and 16 chromosome cytotypes in the same geographic area in South Brazil. Braz J of Genet 19(4):565–569
Silva MJJ, Yonenaga-Yassuda Y (1998) Karyotype and chromosomal polymorphism of a undescribed Akodon from Central Brazil, a species with the lowest known diploid chromosome number in rodents. Cytogenet Cell Genet 81:46–50
Silva MJJ, Patton JL, Yonenaga-Yassuda Y (2006) Phylogenetic relationships and karyotype evolution in the sigmodontine rodent Akodon with (2n = 10 and 2n = 16) from Brazil. Genet Mol Biol 29(3):469–474
Smith MF, Patton JL (2007) Molecular phylogenetics and diversification of South American grass mice, genus Akodon. In: Kelt DA, Lessa EP, Salazar-Bravo J, Patton J (eds) The quintessential naturalist: honoring the life and legacy of Oliver P. Pearson. University of California Publications in Zoology, vol 34. University of California Press, Berkeley, pp 827–858
Telenius H, Pelmear A, Tunnacliffe A et al (1992) Cytogenetic analysis by chromosome painting using DOP-PCR amplified flow-sorted chromosomes. Genes Chromosomes Cancer 4:257–263
Tian Y, Nie W, Wang J, Ferguson-Smith MA, Yang F (2004) Chromosome evolution in bears: reconstructing phylogenetic relationships by cross-species chromosome painting. Chromosome Res 12:55–63
Ventura K, Silva MJJ, Fagundes V, Christoff UA, Yonenaga-Yassuda Y (2006) Non-telomeric sites as evidence of chromosomal rearrangement and repetitive (TTAGGG) n arrays in heterochromatic and achromatic regions in four species of Akodon (Rodentia, Muridae). Cytogenet Genome Res 115(2):169–175
Vieira A, Ortiz MI, Oinna-Senn E, Dalmasso G, Bella JL, Lisanti JA (2004) Chromosomal localization of telomeric sequences in three species of Akodon (Rodentia, Sigmodontinae). Cytogenet Genome Res 107:99–10
Yang F, Carter NP, Shi L, Ferguson-Smith MA (1995) A comparative study of karyotypes of muntjacs by chromosome painting. Chromosoma 103:642–652
Yang F, Alkalaeva EZ, Perelman PL, Pardini AT, Harrison WR, O’Brien PCM, Fu B, Graphodatsky AS, Ferguson-Smith MA, Robinson TJ (2003) Reciprocal chromosome painting among human, aardvark, and elephant (superorder Afrotheria) reveals the likely eutherian ancestral karyotype. PNAS 100:1062–1066
Yonenaga Y (1972) Chromosomal polymorphism in the rodent Akodon arviculoides ssp. (2n = 14) resulting from two pericentric inversions. Cytogenetics 11:448–499
Yonenaga Y, Kasahara S, Almeida EJC, Peracchi AL (1975) Chromosomal banding patterns in Akodon arviculoides (2n = 14), Akodon sp. (2n = 24 and 25), and two male hybrids with 19 chromosomes. Cytogenet Cell Genet 15:388–399
Yonenaga-Yassuda Y (1979) New karyotypes and somatic and germ-cell banding in Akodon arviculoides (Rodentia, Cricetidae). Cytogenet Cell Genet 23:241–249
Acknowledgements
We thank Dr. Willem Rens, Margaret Wallduck, Frances Lovell, and Laura Hirst for the valuable contributions during the lab work and data analysis. We also thank Dr. Maria José de Jesus Silva and Dr. Valéria Fagundes for the Akodon sp. n., A. cursor and A. montensis G-banding karyotypes and Silvia Sousa da Costa for technical assistance with the cell cultures. Permit for cell lines transportation was provided by IBAMA. Grants were received from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de do Amparo à Pesquisa Estado de São Paulo (FAPESP). The Cambridge Resource Center for Comparative Genomics was supported by a grant to MAFS from the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Fengtang Yang.
Rights and permissions
About this article
Cite this article
Ventura, K., O’Brien, P.C.M., Yonenaga-Yassuda, Y. et al. Chromosome homologies of the highly rearranged karyotypes of four Akodon species (Rodentia, Cricetidae) resolved by reciprocal chromosome painting: the evolution of the lowest diploid number in rodents. Chromosome Res 17, 1063–1078 (2009). https://doi.org/10.1007/s10577-009-9083-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-009-9083-5