Abstract
Varroa destructor Anderson and Trueman (Acari: Varroidae) are ectoparasitic mites found in the western honeybee Apis mellifera Linnaeus (Hymenoptera: Apidae). Varroa destructor is classified into two haplotypes, i.e., Korea (K) and Japan (J), based on mtDNA sequences. Among these, V. destructor K haplotype is possibly a more severe threat to A. mellifera colonies. Previous studies collected both V. destructor haplotypes from honeybee colonies in Japan. However, no detailed surveillance of infestation of Japanese apiaries by V. destructor or identification of their genetic structure has been conducted to date. We surveyed V. destructor at 15 different Japanese apiaries of A. mellifera. Varroa destructor was collected from 14 Japanese apiaries, and all mites were classified as V. destructor K haplotype. Varroa destructor infestation of the Japanese honeybee A. cerana japonica Radoszkawsi (Hymenoptera: Apidae) was also analyzed. Varroa destructor K haplotype was predominant in A. cerana colonies. Despite the different host species, all collected V. destructor K haplotype samples were classified into a single haplogroup, i.e., K1-1/K1-2. These results indicate that A. mellifera and A. cerana were infested by the same V. destructor haplogroup. This is the first report detailing a survey on V. destructor prevalence and haplogroups among Japanese apiaries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Varroa destructor Anderson and Trueman (Acari: Varroidae) are known as ectoparasitic mites of the western honeybee Apis mellifera Linnaeus (Hymenoptera: Apidae). Varroa destructor proliferates in beehives, and it spends its phoretic phase on adult honeybees and reproductive phase on pupal honeybees (Dietemann et al. 2015; Evans and Cook 2018). Although V. destructor feeds on the hemolymph of immature and adult honeybees, a recent study demonstrated that V. destructor primarily feeds on the adipose tissue of honeybees (Ramsey et al. 2019). Varroa destructor transmits several viruses that cause severe diseases in honeybees such as deformed wing virus (Dainat et al. 2012; Hedtke et al. 2011; Martin 2001; Nazzi et al. 2012). Physical damage and disease transmission by V. destructor can cause fatalities not only of individual bees but also of entire honeybee colonies (Dainat et al. 2012; De Jong et al. 1982; Duay et al. 2002, 2003; Garedew et al. 2004; Guzmán-Novoa et al. 2010; Nazzi et al. 2012; Ratti et al. 2015; Schneider and Drescher 1987). Consequently, V. destructor infestation has become a major cause of colony loss, particularly winter colony loss (Branco et al. 1999; Shimanuki et al. 1994). As in other countries, V. destructor infestation caused varroosis in Japan (MAFF 2018) and may be responsible for winter colony loss. However, detailed surveillance of V. destructor infestation, such as its seasonal prevalence and infestation level in each colony, has not been conducted in Japan.
Varroa destructor was initially mistaken for V. jacobsoni Oudemans (Acari: Varroidae), which is the primary parasite of the eastern honeybee A. cerana Radoszkawsi (Hymenoptera: Apidae). Anderson and Truman (2000) clarified the morphological and genetic differences between V. destructor infesting A. mellifera and A. cerana colonies, and they termed the species that infests A. mellifera as V. destructor. Varroa destructor originally infested A. cerana colonies, but it has rarely caused severe damage to the host (Boot et al. 1997; Fries et al. 1996). It has since expanded its host range to A. mellifera colonies and has become a severe pest of the novel host. Japan is believed to be one of the locations where V. destructor has spread to A. mellifera colonies through the introduction of Western beekeeping (Sakai and Okada 1973; Yoshiyama and Kimura 2018). Another region is the far east of the former USSR (Crane 1978).
Genetic analysis demonstrated that these mites have different mtDNA sequences (Solignac et al. 2005), thereby being termed as J (Japan) or K (Korea or Russia in earlier studies) haplotypes according to the collection region. J and K haplotypes vary in terms of mtDNA cytochrome oxidase I (cox I) (Anderson and Truman 2000; Solignac et al. 2005). These differences have been investigated using randomly amplified polymorphic DNA or restriction fragment length polymorphism (RFLP)/polymerase chain reaction (PCR) of mtDNA (Anderson and Fuchs 1998; Kraus and Hunt 1995). Varroa destructor K haplotype is suspected to have a higher virulence and reproductive ability than its J haplotype (Delfinado-Baker 1988; Garrido et al. 2003; Strapazzon et al. 2009).
Varroa destructor J and K haplotypes were further classified into several haplogroups according to variations in cox I and III, ATP synthase 6 (atp 6), and cytochrome b (cyt b) sequences in mtDNA (Navajas et al. 2010) discovered following whole V. destructor mtDNA sequencing (Evans and Lopez 2002; Navajas et al. 2002). Varroa destructor J haplotype includes six haplogroups (J1-1–J1-6), whereas its K haplotype includes four haplogroups (K1-1–K1-4). Although Navajas et al. (2010) distinguished between K1-1 and K1-2, no differences were observed in terms of the registered sequences of cox I, cox III, atp 6, and cyt b between them. Therefore, they are described as K1-1/K1-2 in this study. Phylogenetic analysis of mtDNA sequences between these haplogroups showed that V. destructor K and J haplotypes formed distinct clades, supporting the fact that V. destructor K or J haplotype independently expanded their host range from A. cerana to A. mellifera (Navajas et al. 2010).
Varroa destructor is now globally dispersed, and its K haplotype represents the primary haplogroups worldwide. Studies conducted in the early twenty-first century reported that V. destructor K haplotype was widely detected in Asia, Europe, South and North America, and Oceania (New Zealand). Varroa destructor K haplotype infestations are still reported in several countries including Iran, Serbia, Benin, and Turkey (Farjamfar et al. 2018; Gajic et al. 2013, 2016; Kelomey et al. 2017; Warrit et al. 2015). Although V. destructor J haplotype has been found in a limited number of countries including Japan, Thailand, several countries in North and South America, and Spain (Anderson and Trueman 2000; de Guzman et al. 1997, 1999; Guerra et al. 2010; Maggi et al. 2012; Muñoz et al. 2008; Navajas et al. 2010; Solignac et al. 2005; Warrit et al. 2006), they have rarely been reported recently. However, the existence of V. destructor K haplotype has been reported in Brazil, which was first invaded by V. destructor J haplotype (Garrido et al. 2003; Strapazzon et al. 2009). In addition to V. destructor K and J haplotypes, regional V. destructor haplotypes have also been observed (Gajic et al. 2013, 2016; Navajas et al. 2010; Zhou et al. 2004), although their spread to other countries has not been reported.
In Japan, both these haplotypes have been collected from A. mellifera and A. cerana colonies (Navajas et al. 2010; Solignac et al. 2005). These reports determined that these haplotypes have genetic differences and are regionally distributed on a global scale. Navajas et al. (2010) analyzed three V. destructor samples collected from two A. mellifera and three A. cerana colonies in Japan during 1994–2000. The report showed that V. destructor samples collected from A. mellifera colonies were J or K haplotype (J1-6 in Tokyo and K1-1 in Tokyo), whereas those collected from A. cerana colonies were J haplotypes (J1-2 in Tokyo, J1-3 in Machida, and J1-4 in Shikoku) (Fig. 1a). Solignac et al. (2005) analyzed 1–19 V. destructor samples collected from two A. cerana and five A. mellifera colonies in Japan. Several colonies had single haplotypes: three A. mellifera colonies had V. destructor K haplotype (Yatsushiro, Yokohama, and Tokyo) and a single A. cerana colony had V. destructor J haplotype (Yatsushiro). The three remaining colonies had coexisting V. destructor K and J haplotypes in the same honeybee population (A. cerana colony in Machida and A. mellifera colonies in Machida and Noda). There were more K haplogroups than J haplogroups in these three colonies (Solignac et al. 2005). In addition, Techer et al. (2019) collected K1-1/K1-2 haplogroups from A. mellifera colonies in Okinawa Prefecture. Although the distribution of K haplogroups is irregular, no surveillance of the actual genetic structure of V. destructor in Japan has been conducted. To understand the significance of V. destructor infestation of A. mellifera colonies in Japan, apiaries across Japan were surveyed for V. destructor and the haplogroups of the collected V. destructor samples were determined. To the best of our knowledge, this is the first survey on the detailed genetic structure of V. destructor populations among Japanese apiaries.
Distribution of Varroa destructor haplotypes and haplogroups in Japan. a Summary of V. destructor haplotypes and haplogroups reported by Navajas et al. (2010) and Solignac et al. (2005). *1: data from the study by Navajas et al. (2010). *2: data from the study by Solignac et al. (2005). bVarroadestructor haplogroups determined in the current study. The numbers in parentheses represent the numbers of apiaries infested with V. destructor. Black areas indicate the surveillance locations for Apis mellifera (Am) colonies, and gray areas indicate surveillance locations for A. cerana (Ac) colonies
Materials and methods
Collection of V. destructor from honeybee colonies
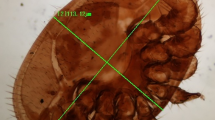
Varroa destructor females were collected from July to October 2018 from 15 different commercial apiaries located in Hokkaido, Fukushima, and Osaka Prefectures in Japan (Table 1, Fig. 1b). Phoretic V. destructor samples were collected using a modified sugar roll test (Ogihara et al. submitted). In brief, 1 cup (200 ml, approximately 500 honeybees) of adult honeybees was collected from individual beehives. Honeybees were put into a plastic jar (240 mm long, 65 mm I.D.) containing 100 g of powdered sugar (Uehara, Tokyo, Japan) and shaken for 1 min. Dislodged V. destructor and powdered sugar were sifted through a rough mesh (3 mm) attached to a hole punched on the jar lid. Varroa destructor samples were collected using a fine screen and counted. Then 9–12 beehives were surveyed at each apiary. For genetic analysis, V. destructor samples were collected from capped cells in a honeycomb frame when small numbers of V. destructor samples were obtained using the sugar roll test.
Varroa destructor-infested A. cerana colonies were kindly gifted by Dr. Taro Maeda (Institute of Agrobiological Sciences, National Agriculture and Food Research Organization). A. cerana beehives are located at Tsukuba and Kashima (Ibaraki Prefecture), Inuyama (Aichi Prefecture), Matsumuto (Nagano Prefecture), and Matsue (Shimane Prefecture; Fig. 1b). Only a single V. destructor was obtained from each location. The Japanese map shown in Fig. 1 was constructed using Excel for Office 365 MSO (Microsoft, Redmond, WA, USA).
Analysis of V. destructor haplotypes and haplogroups by RFLP/PCR and sequencing
Genomic DNA was extracted from individual V. destructor using the Easy DNA Extraction Kit version 2 (Kaneka Corporation, Tokyo, Japan). Varroa destructor haplotypes were determined using RFLP/PCR, which was a slightly modified form of the method reported by Solignac et al. (2005). The obtained genomic DNA was amplified with GoTaq Green Master Mix (Promega, Fitchburg, USA) using COIF-long and COIR primers (Table 2). PCR conditions were as follows: denaturing at 95 °C for 2 min, 35 cycles of denaturing at 95 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 30 s. PCR products were digested with the restriction enzyme Sac I (Takara Bio Inc., Shiga, Japan) at 37 °C. Digestion was confirmed using electrophoresis.
To determine the V. destructor haplogroups in Japan, cox I, cox III, atp 6, and cyt b mtDNA sequences were determined using the primers described by Navajas et al. (2010). PCR was performed using KOD FX Neo (TOYOBO, Osaka, Japan). PCR conditions were as follows: denaturing at 94 °C for 2 min, 35 cycles of denaturing at 98 °C for 10 s, annealing at 50 °C for 30 s, and extension at 68 °C for 45 s. Obtained PCR products were purified using the QIAquick PCR Purification Kit, QIAGEN II Gel Extraction Kit (QIAGEN, Hilden, Germany), or Ethachinmate (Nippon gene, Tokyo, Japan). Then direct sequencing was performed using the Big Dye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, USA) on an ABI 3500xl Genetic Analyzer (Applied Biosystems, Waltham, USA).
Statistical analysis
Spearman’s rank correlation analysis was performed using R software (version 3.5.1; R Developmental Core Team 2013). Correlation analysis between the average number of dislodged V. destructor among the infested colonies at each apiary and the ratio of infested honeybee colonies at each apiary were analyzed using Spearman’s rank correlation method.
Results
Surveillance of V. destructor infestation at Japanese apiaries
Varroa destructor samples were collected from A. mellifera colonies located at 15 different commercial apiaries using modified phoretic mite detection. In Japan, many beekeepers migrate to Hokkaido Prefecture in summer for honey collection and colony rearing. Therefore, surveillance was primarily conducted in Hokkaido Prefecture (10 apiaries). Four apiaries in Fukushima Prefecture and one apiary in Osaka Prefecture were also surveyed. These apiaries were not shifted to Hokkaido Prefecture (Table 1). Varroa destructor samples were collected from 14 apiaries (Fig. 2), except for the Hokkaido 7 apiary. The number of V. destructor dislodged by each sugar roll test was greater in the later season (Fig. 2). Similar to the number of dislodged V. destructor, the ratio of V. destructor-infested honeybee colonies at each apiary was higher in autumn than in summer (Fig. 2). The average number of dislodged V. destructor among the infested honeybee colonies correlated with the ratio of V. destructor-infested honeybee colonies at the apiaries (ρ = 0.90, p < 0.01; Fig. 3), suggesting that V. destructor populations increase in summer and autumn.
Several reports have indicated that the threshold for the number of V. destructor for winter colony loss is 4–8 phoretic V. destructor per 100 honeybees in autumn (Currie and Gatien 2006; Gatien and Currie 2003; Strange and Sheppard 2001). Therefore, we set a strict benchmark of 20 dislodged V. destructor per single sugar roll test for predicting winter colony loss. In July and August, < 20 V. destructor individuals were dislodged in a single sugar roll test, except for the Hokkaido 10 apiary. In contrast, cases with > 20 V. destructor per sugar roll test increased at the apiaries surveyed during September and October. In a severe case, 162 V. destructor individuals were detected in a single sugar roll test at the Fukushima 2 apiary. These colonies had a high probability of winter colony loss.
Genetic analysis of V. destructor haplotypes and haplogroups in Japan
Varroa destructor haplotypes were investigated using RFLP/PCR for mtDNA cox I (Solignac et al. 2005). All V. destructor samples collected in this study were of V. destructor K haplotype (Table 3). K1-1/K1-2, K1-3, and K1-4 showed variation in terms of cox I mtDNA sequence (Navajas et al. 2010). Therefore, the V. destructor haplogroups collected were investigated using mtDNA sequencing. All V. destructor samples collected in this study had mtDNA sequences identical to those of K1-1/K1-2 (Table 3). Partial sequences of atp 6 to cox III, and cyt b were also determined to distinguish other regional V. destructor haplogroups (Navajas et al. 2010). However, no variations were observed for these mtDNA sequences. These data demonstrate that V. destructor infesting A. mellifera at Japanese apiaries were of V. destructor K1-1/K1-2 and were identical among all apiaries (Fig. 1b).
Varroa destructor infesting A. cerana was also obtained, and the haplotypes were analyzed using the same method as used for A. mellifera colonies. Varroa destructor samples collected from A. cerana colonies in Tsukuba, Kashima, Inuyama, and Matsue were V. destructor K1-1/K1-2 (Table 3, Fig. 1b). No sequence variations within atp 6 to cox III or cyt b were found in these mites. This indicates that A. mellifera and A. cerana colonies were infested with the same V. destructor K1-1/K1-2 haplogroup. Only one V. destructor collected from Matsue (Shimane Prefecture) was V. destructor J haplotype. The mtDNA sequences of cox I, atp 6 to cox III, and cyt b indicated that the haplogroup of V. destructor collected from Matsue was J1-3 with no differences compared to the original J1-3 (Table 3). Although we used a small number of samples, these results indicate that K1-1/K1-2 is the dominant V. destructor haplogroup causing damage to A. cerana in Japan (Fig. 1b).
Discussion
In this study, we conducted a survey on V. destructor infestation at 15 different Japanese apiaries. Although V. destructor has previously been collected from A. mellifera and A. cerana colonies (Navajas et al. 2010; Solignac et al. 2005; Techer et al. 2019), a field survey on V. destructor among Japanese apiaries has not been conducted previously. We could collect V. destructor from almost all investigated apiaries. The number of detected V. destructor in the colonies and the ratio of infested colonies at the apiaries were correlated and greater in autumn surveillance than that in summer (Fig. 3), indicating that V. destructor prevalence increased in autumn. The number of V. destructor increased in summer to autumn in Japan, which has also been reported in other countries (Currie and Gatien 2006; Gatien and Currie 2003; Martin 2001). Some honeybee colonies had severe V. destructor infestations with more than 20 V. destructor detected using the sugar roll test. These colonies had V. destructor infestation that exceeded the benchmark for V. destructor number in terms of winter colony loss (Currie and Gatien 2006; Gatien and Currie 2003; Strange and Sheppard 2001); therefore, they had the potential to undergo winter colony loss by V. destructor infestation.
Varroa destructor infestation is compounded at an apiary in several manners such as drifting and robbing (Boecking and Genersch 2008). Varroa destructor infestation has been shown to disrupt the orientation of foragers and increase the frequency of drifters (Kralj and Fuchs 2006; Kralj et al. 2007), with highly infested colonies beginning to accept drifters (Forfert et al. 2015). Varroa destructor can serve as a vector for several viruses causing severe diseases such as deformed wing virus and acute paralysis virus (Boecking and Genersch 2008). Colonies with severe mite infestations appear to be the source of V. destructor and related disease at apiaries. Early treatment before an increase in V. destructor population in honeybee colonies is necessary to prevent the high probability of winter colony loss (van Dooremalen et al. 2012). In Japan, V. destructor population is controlled with the acaricides τ-fluvalinate and amitraz. However, these acaricides cannot be used during honey production. It is common for beekeepers in Japan to continue honey production during September, which may allow an increase in V. destructor population that exceeds the threshold for winter colony loss. Monitoring V. destructor population and early acaricide-based treatment before an increase in its population may prevent severe V. destructor infestation.
The V. destructor haplotypes collected in this study were analyzed. Varroa destructor K haplotype was dominant in Japanese honeybee colonies. Varroa destructor K1-1 and J1-6 have previously been collected from A. mellifera colonies in Japan (Navajas et al. 2010). However, we demonstrated that V. destructor K1-1/K1-2 could be detected in A. mellifera colonies across all sampling locations. Although several reports have shown regional variations in mtDNA sequences (Gajic et al. 2013; Navajas et al. 2010; Zhou et al. 2004), no regional variations were found in the current study. In addition, Techer et al. (2019) reported that V. destructor in Okinawa Prefecture was V. destructor K1-1/K1-2, indicating this haplogroup is dominant in A. mellifera colonies in Japan. In contrast, V. destructor J haplotype appears to be decreasing at Japanese apiaries. Replacement of J haplotype with K haplotype has been reported in Brazil, concurrent with an increase in the fertilization rate of V. destructor observed in A. mellifera colonies (Garrido et al. 2003; Strapazzon et al. 2009). This suggests that V. destructor K haplotype has more severe impacts on A. mellifera colonies. The current results indicate that V. destructor K1-1/K1-2 has a higher infectivity than V. destructor J haplotype and can cause a loss of V. destructor diversity.
In Japan, A. mellifera colonies travel across various locations. Some A. mellifera colonies travel for the promotion of pollination for crop production such as strawberry horticulture, whereas some travel for migratory beekeeping practices (Yoshiyama and Kimura 2018). Hokkaido Prefecture is the major area for migratory beekeeping in Japan. Seventeen percent of all western honeybee colonies in Japan migrate to Hokkaido Prefecture from summer to autumn for honey production and for colony rearing (Livestock Farming Promotion Division, Bureau for Promotion of Agricultural Production of Hokkaido Pref 2018). Indeed, beekeepers represented in the current study migrated to Hokkaido Prefecture from all areas of Japan (Table 1). Varroadestructor K1-1/K1-2 might disseminate to other areas where the colonies once migrated. Moreover, V. destructor collected from apiaries located in Fukushima and Osaka Prefectures, which did not migrate to Hokkaido Prefecture, were also shown to be V. destructor K1-1/K1-2. Varroa destructor K1-1/K1-2 might rapidly spread throughout Japan. Information concerning V. destructor haplogroups present at other locations is lacking. Therefore, nationwide studies must be conducted to determine the most prevalent V. destructor haplotypes to prevent further damage to A. mellifera colonies. Furthermore, global surveillance of V. destructor is required to identify the most highly infective V. destructor haplogroups.
In addition to A. mellifera, the current study investigated the dominant V. destructor haplogroups infesting A. cerana colonies in Japan. Although A. cerana is infested by V. destructor, they are resistant to V. destructor infestations (Boot et al. 1997; Fries et al. 1996). Surveillance of V. destructor in A. cerana colonies via sugar roll tests is not advisable because A. cerana colonies are quick to abscond when they experience any type of stress. Therefore, fewer numbers of V. destructor infesting A. cerana were investigated in this study. The dominant V. destructor haplogroup collected from A. cerana was V. destructor K1-1/K1-2. A single V. destructor collected at Matsue was determined to be V. destructor J1-3 (Fig. 1b). Navajas et al. (2010) collected V. destructor J1-2, 1–3, and 1–4 from A. cerana colonies in Tokyo, Machida, and Shikoku, respectively (Fig. 1a). Although the sample size was small, the genetic analysis of V. destructor in the current study indicated V. destructor K1-1/K1-2 infestation of A. cerana colonies (Fig. 1b). Loss of genetic variation may occur even in A. cerana colonies. The current results suggest that the spread of V. destructor K1-1/K1-2 to A. cerana colonies has already occurred probably due to the interactions between A. cerana and A. mellifera. Further analysis of the genetic structure of V. destructor using microsatellite DNA (Beaurepaire et al. 2015; Dynes et al. 2016; Roberts et al. 2015; Solignac et al. 2003, 2005) is necessary to understand the process of single haplogroup spread in Japan to prevent severe V. destructor infestations.
References
Anderson DL, Fuchs S (1998) Two genetically distinct populations of Varroa jacobsoni with contrasting reproductive abilities on Apis mellifera. J Apic Res 37:69–78. https://doi.org/10.1080/00218839.1998.11100957
Anderson DL, Trueman JW (2000) Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp Appl Acarol 24:165–189
Beaurepaire AL, Truong TA, Fajardo AC et al (2015) Host specificity in the honeybee parasitic mite, Varroa spp in Apis mellifera and Apis cerana. PLoS ONE 10:e0135103. https://doi.org/10.1371/journal.pone.0135103
Boecking O, Genersch E (2008) Varroosis—the ongoing crisis in bee keeping. J Verbrauch Lebensm 3:221–228. https://doi.org/10.1007/s00003-008-0331-y
Boot WJ, Tan NQ, Dien PC et al (1997) Reproductive success of Varroa jacobsoni in brood of its original host, Apis cerana, in comparison to that of its new host, A. mellifera (Hymenoptera: Apidae). B Entomol Res 87:119–126. https://doi.org/10.1017/S0007485300027255
Branco MR, Kidd NAC, Pickard RS (1999) Development of Varroa jacobsoni in colonies of Apis mellifera iberica in a Mediterranean climate. Apidologie 30:491–503
Crane E (1978) The Varroa mite. Bee World 59:164. https://doi.org/10.1080/0005772X.1978.11097718
Currie RW, Gatien P (2006) Timing acaricide treatments to prevent Varroa destructor (Acari: Varroidae) from causing economic damage to honey bee colonies. Can Entomol 138:238–252
Dainat B, Evans JD, Chen YP et al (2012) Dead or alive: deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Appl Environ Microbiol 78:981–987. https://doi.org/10.1128/AEM.06537-11
de Guzman LI, Rinderer TE, Stelzer JA (1997) DNA evidence of the origin of Varroa jacobsoni Oudemans in the Americas. Biochem Genet 35:327–335. https://doi.org/10.1023/A:1021821821728
de Guzman LI, Rinderer TE, Stelzer JA (1999) Occurrence of two genotypes of Varroa in North America. Apidologie 30:31–36
De Jong D, De Jong PH, Gonçalves LS (1982) Weight loss and other damage to developing worker honeybees from infestation with V. jacobsoni. J Apic Res 21:165–216. https://doi.org/10.1080/00218839.1982.11100535
Dietemann V, Nazzi F, Martin SJ et al (2015) Standard methods for Varroa research. J Apic Res 52:1–54. https://doi.org/10.3896/IBRA.1.52.1.09
Delfinado-Baker (1988) Variability and biotypes of Varroa jacobsoni Oudemans. Am Bee J 128:567–568
Duay P, De Jong D, Engels W (2002) Decreased flight performance and sperm production in drones of the honeybee (Apis mellifera) slightly infested by Varroa destructor mites during pupal development. Genet Mol Res 1:227–232
Duay P, De Jong D, Engels W (2003) Weight loss in drone pupae (Apis mellifera) multiply infested by Varroa destructor mites. Apidologie 34:61–65. https://doi.org/10.1051/apido:2002052
Dynes TL, De Roode JC, Lyons JI et al (2016) Fine scale population genetic structure of Varroa destructor, an ectoparasitic mite of the honey bee (Apis mellifera). Apidologie 48:93–101. https://doi.org/10.1007/s13592-016-0453-7
Evans JD, Cook SC (2018) Genetics and physiology of Varroa mites. Curr Opin Insect Sci 26:130–135. https://doi.org/10.1016/j.cois.2018.02.005
Evans JD, Lopez DL (2002) Complete mitochondrial DNA sequence of the important honey bee pest, Varroa destructor (Acari: Varroidae). Exp Appl Acarol 27:69–78
Farjamfar M, Saboori A, Gonzalez-Cabrera J, Hernandez Rodriguez CS (2018) Genetic variability and pyrethroid susceptibility of the parasitic honey bee mite Varroa destructor (Acari: Varroidae) in Iran. Exp Appl Acarol 76:139–148. https://doi.org/10.1007/s10493-018-0296-1
Forfert N, Natsopoulou ME, Frey E et al (2015) Parasites and pathogens of the honeybee (Apis mellifera) and their influence on inter-colonial transmission. PLoS ONE 10:1–14. https://doi.org/10.1371/journal.pone.0140337
Fries I, Huazhen W, Wei S, Jin CS (1996) Grooming behavior and damaged mites (Varroa jacobsoni) in Apis cerana cerana and Apis mellifera ligustica. Apidologie 27:3–11. https://doi.org/10.1051/apido:19960101
Gajić B, Radulović Ž, Stevanović J et al (2013) Variability of the honey bee mite Varroa destructor in Serbia, based on mtDNA analysis. Exp Appl Acarol 61:97–105. https://doi.org/10.1007/s10493-013-9683-9
Gajić B, Stevanović J, Radulović Ž et al (2016) Haplotype identification and detection of mitochondrial DNA heteroplasmy in Varroa destructor mites using ARMS and PCR–RFLP methods. Exp Appl Acarol 70:287–297. https://doi.org/10.1007/s10493-016-0086-6
Garedew A, Schmolz E, Lamprecht I (2004) The energy and nutritional demand of the parasitic life of the mite Varroa destructor. Apidologie 35:419–430. https://doi.org/10.1051/apido:2004032
Garrido C, Rosenkranz P, Paxton RJ, Gonçalves LS (2003) Temporal changes in Varroa destructor fertility and haplotype in Brazil. Apidologie 34:535–541. https://doi.org/10.1051/apido:2003041
Gatien P, Currie R (2003) Timing of acaricide treatments for control of low-level populations of Varroa destructor (Acari: Varroidae) and implications for colony performance of honey bees. Can Entomol 135:749–763. https://doi.org/10.4039/n02-086
Guerra JJ, Issa M, Carneiro F et al (2010) RAPD identification of Varroa destructor genotypes in Brazil and other regions of the Americas. Genet Mol Res 9:303–308. https://doi.org/10.4238/vol9-1gmr696
Guzmán-Novoa E, Eccles L, Calvete Y et al (2010) Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 41:443–450. https://doi.org/10.1051/apido/2009076
Hedtke K, Jensen PM, Jensen AB, Genersch E (2011) Evidence for emerging parasites and pathogens influencing outbreaks of stress-related diseases like chalkbrood. J Invertebr Pathol 108:167–173. https://doi.org/10.1016/j.jip.2011.08.006
Kelomey AE, Paraiso A, Sina H et al (2017) Genetic characterization of the honeybee ectoparasitic mite Varroa destructor from Benin (West Africa) using mitochondrial and microsatellite markers. Exp Appl Acarol 72:61–67. https://doi.org/10.1007/s10493-017-0141-y
Kralj J, Fuchs S (2006) Parasitic Varroa destructor mites influence flight duration and homing ability of infested Apis mellifera foragers. Apidologie 37:577–587. https://doi.org/10.1051/apido:2006040
Kralj J, Brockmann A, Fuchs S, Tautz J (2007) The parasitic mite Varroa destructor affects non-associative learning in honey bee foragers, Apis mellifera L. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 193:363–370. https://doi.org/10.1007/s00359-006-0192-8
Kraus B, Hunt G (1995) Differentiation of Varroa jacobsoni Oud populations by random amplification of polymorphic DNA (RAPD). Apidologie 26:283–290. https://doi.org/10.1051/apido:19950402
Livestock Farming Promotion Division, Bureau for Promotion of Agricultural Production of Hokkaido Pref. (2018) Status of beekeeping in Hokkaido Prefecture (Hokkaido no youhou wo meguru jyousei, in Japanese (https://www.pref.hokkaido.lg.jp/ns/tss/35/hachi/youhouwomegurujyousei.pdf)
MAFF (Ministry of Agriculture, Forestry and Fisheries) (2018) Annual report of monitored infectious diseases at act on domestic animal infectious diseases control (Kanshi densenbyou hassei nenpyou, in Japanese) (https://www.maff.go.jp/j/syouan/douei/kansi_densen/attach/pdf/kansi_densen-146.pdf)
Maggi M, Medici S, Quintana S et al (2012) Genetic structure of Varroa destructor populations infesting Apis mellifera colonies in Argentina. Exp Appl Acarol 56:309–318. https://doi.org/10.1007/s10493-012-9526-0
Martin SJ (2001) The role of Varroa and viral pathogens in the collapse of honeybee colonies: a modelling approach. J Appl Ecol 38:1082–1093. https://doi.org/10.2307/827245
Muñoz I, Garrido-Bailón E, Martín-Hernández R et al (2008) Genetic profile of Varroa destructor infesting Apis mellifera iberiensis colonies. J Apic Res 47:310–313. https://doi.org/10.1080/00218839.2008.11101480
Navajas M, Anderson DL, de Guzman LI et al (2010) New Asian types of Varroa destructor : a potential new threat for world apiculture. Apidologie 41:181–193. https://doi.org/10.1051/apido/2009068
Navajas M, Le Conte Y, Solignac M et al (2002) The complete sequence of the mitochondrial genome of the honeybee ectoparasite mite Varroa destructor (Acari: Mesostigmata). Mol Biol Evol 20:2313–2317. https://doi.org/10.1093/oxfordjournals.molbev.a004055
Nazzi F, Brown SP, Annoscia D et al (2012) Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. Plos Pathog 8:e1002735. https://doi.org/10.1371/journal.ppat.1002735
Ogihara MH, Stoich M, Morimoto N, Yoshiyama M, Kimura K (2020) Roasted soybean flour as an alternative powder roll substrate for Varroa mite detection
R Development Core Team (2013) R: A Language and environment for statistical computing. R foundation for statistical computing, Vienna
Ramsey SD, Ochoa R, Bauchan G et al (2019) Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc Natl Acad Sci USA 116:1792–1801. https://doi.org/10.1073/pnas.1818371116
Ratti V, Kevan PG, Eberl HJ (2015) A mathematical model of the honeybee-Varroa destructor-acute bee paralysis virus system with seasonal effects. B Math Biol 77:1493–1520. https://doi.org/10.1007/s11538-015-0093-5
Roberts JMK, Anderson DL, Tay WT (2015) Multiple host shifts by the emerging honeybee parasite, Varroa jacobsoni. Mol Ecol 24:2379–2391. https://doi.org/10.1111/mec.13185
Sakai T, Okada I (1973) The present beekeeping in Japan. Glean Bee Cult 101:356–357
Schneider P, Drescher W (1987) The influence of Varroa jacobsoni Oud. on weight, development of weight and hypopharyngeal glands, and longevity of Apis mellifera L. Apidologie 18:101–110
Shimanuki H, Calderone NW, Knox DA (1994) Parasitic mite syndrome: the symptoms. Am Bee J 134:827–828
Solignac M, Cornuet J-M, Vautrin D et al (2005) The invasive Korea and Japan types of Varroa destructor, ectoparasitic mites of the Western honeybee (Apis mellifera), are two partly isolated clones. Proc Biol Sci 272:411–419. https://doi.org/10.1098/rspb.2004.2853
Solignac M, Vautrin D, Pizzo A et al (2003) Characterization of microsatellite markers for the apicultural pest Varroa destructor (Acari: Varroidae) and its relatives. Mol Ecol Notes 3:556–559. https://doi.org/10.1046/j.1471-8286.2003.00510.x
Strapazzon R, Carneiro FE, Guerra JCVJ, Moretto G (2009) Genetic characterization of the mite Varroa destructor (Acari: Varroidae) collected from honey bees Apis mellifera (Hymenoptera, Apidae) in the state of Santa Catarina, Brazil. Genet Mol Res 8:990–997. https://doi.org/10.4238/vol8-3gmr567
Strange JP, Sheppard WS (2001) Optimum timing of miticide applications for control of Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) in Washington State, USA. J Econ Entomol 94:1324–1331. https://doi.org/10.1603/0022-0493-94.6.1324
Techer MA, Rane RV, Grau ML et al (2019) Divergent evolutionary trajectories following speciation in two ectoparasitic honey bee mites. Commun biol 2:357. https://doi.org/10.1038/s42003-019-0606-0
van Dooremalen C, Gerritsen L, Cornelissen B et al (2012) Winter survival of individual honey bees and honey bee colonies depends on level of Varroa destructor infestation. PLoS ONE 7:e36285. https://doi.org/10.1371/journal.pone.0036285
Warrit N, Smith DR, Lekprayoon C (2006) Genetic subpopulations of Varroa mites and their Apis cerana hosts in Thailand. Apidologie 37:19–30. https://doi.org/10.1051/apido:2005051
Warrit N, Hagen TAR, Smith DR, Çakmak I (2015) A survey of Varroa destructor strains on Apis mellifera in Turkey. J Apic Res 43:190–191. https://doi.org/10.1080/00218839.2004.11101137
Yoshiyama M, Kimra K (2018) Bee diversity and current status of beekeeping in Japan. In: Chantawannakul P, Williams G, Neumann P (eds) Asian beekeeping in the twenty-first century. Springer, Berlin, pp 223–246
Zhou T, Anderson DL, Huang ZY et al (2004) Identification of Varroa mites (Acari: Varroidae) infesting Apis cerana and Apis mellifera in China. Apidologie 35:645–654. https://doi.org/10.1051/apido:2004059
Acknowledgements
We would like to express our gratitude to all beekeepers for allowing our surveillance and V. destructor collection. We are grateful to Dr. Taro Maeda (Institute of Agrobiological Sciences, National Agriculture and Food Research Organization) for providing V. destructor collected from A. cerana colonies. We want to thank Ms. Noriko Takano, Ms. Mai Wakasa, and Mr. Mitsugu Ebihara for surveillance preparation. The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ogihara, M.H., Yoshiyama, M., Morimoto, N. et al. Dominant honeybee colony infestation by Varroa destructor (Acari: Varroidae) K haplotype in Japan. Appl Entomol Zool 55, 189–197 (2020). https://doi.org/10.1007/s13355-020-00667-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-020-00667-w