Abstract
Sexual maturation of male Bactrocera correcta (Bezzi) and age-related responses to β-caryophyllene (CP) and methyl eugenol (ME) were examined to identify effective attractants for sexually immature males of the species. Sexual maturation rates (100 × number of cages containing males which attained sexual maturity/total number of cages at a given day) over different ages were 0.0%, 6.7%, and 100.0% for 5-, 6-, and 13-day-old, respectively, and at all the different ages, more males were captured with CP than with ME. The capture rate (the predicted probability by logistic regression) with CP was 29.7% (30.6%) for 5-day-old males, which were still sexually immature, and 52.7% (38.2%) for 6-day-old males, for which the earliest sexual maturation was observed. In contrast, no 5-day-old males responded to ME (the probability was 12.6%), and the capture rate (the probability) of 6-day-old males was merely 0.3% (14.5%), while the rate gradually increased later. The present study revealed that certain males of B. correcta responded to CP prior to sexual maturation but not to ME under the laboratory conditions. These results imply that CP could be an effective attractant used in male annihilation techniques for B. correcta and that ME may not be useful.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bactrocera correcta (Bezzi) is a polyphagous pest that infests a wide range of economically significant fruits, such as guava, Psidium guajava L., mango, Mangifera indica L., peach, Amygdalus persica L., sapodilla, Manilkara zapota (L.) P. Royen, sea almond, Terminalia catappa L., etc. (Allwood et al. 1999; Tsuruta et al. 1997; White and Elson-Harris 1992). The species is widely distributed in Asian countries, such as China, India, Nepal, Pakistan, Sri Lanka, Thailand, and Vietnam, though it is not known to occur in Japan (Drew and Raghu 2002; Drew and Romig 2013; Wang 1996; White and Elson-Harris, 1992).
The species has a high potential for invasion of new areas. For example, a few adult males were trapped in California, USA, in 1986, although the species has not become established (Weems 1987). In addition, Liu et al. (2013) reported the recent spread of B. correcta within China. In Japan, import plant quarantine has frequently intercepted larvae from the species’ range on host plants carried by passengers. From 2010 to 2014, 193 cases were reported for B. correcta detection from fruits in baggage, which is the third largest number of fruit fly detections after those for Bactrocera latifrons (Hendel) and Bactrocera dorsalis (Hendel) species complex (PPS 2016). On the basis of a laboratory study using a Vietnamese population, Kamiji et al. (2014) found that B. correcta may develop and mature in any season throughout the year in the Nansei Islands of Japan, such as Amami and Okinawa. They also found that the number of generations per year of B. correcta in the Nansei Islands might be similar to those of B. dorsalis and Bactrocera cucurbitae (Coquillett), which had been established in the Nansei Islands. These results also imply that the risk of invasion of Japan by B. correcta may be high and the species may become established in the Nansei Islands.
Once established, B. correcta may cause serious economic problems. Vargas et al. (2015) ranked 73 Bactrocera species which have been reared from commercial and/or edible host fruits under four categories (category A–D), based on pest severity, host range, invasiveness, and frequency of infestation. On the basis of this analysis, B. correcta was categorized in category A. Category A includes widespread invasive polyphagous generalists, such as B. dorsalis and B. cucurbitae, or highly destructive specialists that have become established outside of their native range. To prevent invasion and establishment of B. correcta in the Nansei Islands, restrictive plant quarantine measures would have to be in place, and these would adversely affect both domestic and international trade. To minimize this effect, if an outbreak of B. correcta takes place, a proper eradication program would need to be implemented immediately.
It is well known that B. correcta males are attracted to methyl eugenol (ME) (Drew and Romig 2013; White and Elson-Harris 1992). B. dorsalis in the Nansei Islands was eradicated in 1986 after prolonged use of the male annihilation technique, in which ME was used as a male attractant (Nakamori et al. 1991; Yoshizawa 1997). One of the essential elements of successful eradication is to ensure that males respond to the applied lure before attaining sexual maturation (Iwahashi et al. 1996; Wong et al. 1989). While the use of ME is an inexpensive and convenient approach in comparison to the sterile insect technique, it is not known whether it would be effective in eradicating B. correcta, and the relationship between their response to ME and sexual maturation has not been studied.
Some studies suggest the possible use of β-caryophyllene (CP) as an attractant for B. correcta. Tokushima et al. (2010) reported that many wild males of B. correcta stored large quantities of CP in the rectal gland. In addition, CP traps were reportedly seven times more effective than ME traps in capturing B. correcta males in a field test in Thailand during 2012–2013 (Tan et al., unpublished data).
In the present study, we examine the age-dependent response of B. correcta males to CP and ME, particularly relative to their sexual maturation. The information gathered is crucial for assessing the value of CP and ME in male annihilation programs against this species.
Materials and methods
The present study includes two stages: (1) male sexual maturation test and (2) comparison test of the two attractants’ effectiveness.
Test insects
The insects used in the present study were originally from Vietnam and then maintained in Japan. In both locations, the colony was reared in the laboratory for over 6 years or approximately 40 generations (i.e., 12 total years of laboratory rearing). This was done under permission from the Minister of Agriculture, Forestry and Fisheries of Japan in 2010 (import permit No. 21Y-1299). The colony was taken from the stock culture originally collected in Dong Nai Province, Tien Giang Province, and Dong Thap Province in Vietnam in 2004.
After introduction into Japan, the flies were kept in a regular screen cage (30 × 30 × 60 cm, with a cloth-sleeved opening on one side) in a rearing facility (KOITOTRON-PCSH-2SP; Koito Electric Industries, Japan) at Research Division, Yokohama Plant Protection Station under constant conditions (25 ± 1 °C, 60 ± 10% relative humidity, and photoperiod of 14:10 light/dark, with two 1-h twilight phases at the beginning and end of the light phase).
The larval diet consists of 160 mL water, 5 mL HCl (3.5%), 0.19 g sorbic acid, 12.5 g dried yeast (EBIOS; Asahi Food & Healthcare, Japan), 8.8 g sugar, 6.3 g toilet paper, and 43.8 g wheat bran. The adult flies were provided water and diet of 4:1 mixture of sugar and hydrolyzed yeast (AY65; Asahi Food & Healthcare, Japan) ad libitum.
Male sexual maturation
On the basis of preliminary observations, male sexual maturation was measured as mating frequency using groups of 10 males. Preliminary observations showed that mature females housed with 4-day-old and younger males do not produce fertile eggs. Consequently, our observations dealt with 5-day-old and older males.
The test was designed so that virgin, sexually mature females were placed with males for one evening twilight period [when mating takes place (Christenson and Foote 1960)] and replaced with a different set each day, as described in the following paragraphs.
Ten 0-day-old B. correcta males (i.e., immediately after the morning emergence) and five mature virgin females (over 30 days old) were released into a small screen cage (15 × 15 × 22 cm, with a cloth-sleeved opening on one side) with sufficient water and adult diet in the morning of day 0 (emergence day of males). On the morning of the 6th day, the five females which had stayed with males (until 5 days old at last evening twilight period) were transferred from the small screen cage to another small screen cage without males. After that, a new set of five mature virgin females was introduced into the initial small screen cage for a 1-day stay, including the evening twilight period, where ten 6-day-old males remained.
Likewise, on every morning of the following days until the 15th, a new set of five mature virgin females was replaced. On the morning of the 15th day, the final set of five females, which had stayed with 14-day-old males (at last evening twilight period), was transferred to another small screen cage.
All the sets of five females, which were transferred to separate cages without males, were provided with water and adult diet and also egging receptacles [apparatus for collecting eggs, which is a 35-mm film case with 15 pinholes, containing and coated with a small amount of lemon juice, reported by Kamiji et al. (2014)]. The eggs were collected to observe hatching every day for 3 weeks. For a given cage, the earliest male age that resulted in production of fertile eggs was defined as the “sexual maturation period”. For example, if the females laid any fertilized eggs during a 3-week-long observation period and if they shared evening twilight with the 7-day-old males, then the sexual maturation period of the cage will be named as “7-day-old” (in this case, females which had shared evening twilight with the 5- and 6-day-old males in the same cage did not lay fertilized eggs).
In the present study, on the basis of the results of all 15 cages (replicates), the daily sexual maturation rate was calculated as 100 × the number of cages containing males which attained sexual maturity at a given day (at least 5 days old)/15 (total number of cages at a given day)”.
Comparison of male response to CP and ME
As a bioassay of male response to CP and ME, the males captured in a simple trap (with CP or ME applied to) (Fig. 1) were counted over ages (1–14 and over 30 days old). This bioassay was conducted in an air-conditioned laboratory maintained at 25 ± 1 °C.
The simple trap mentioned above was devised to conduct bioassays of the male response to CP and ME in regular screen cages, as follows: (1) a glass funnel and a 200-mL Erlenmeyer flask were connected together with Parafilm (Bemis Flexible Packaging, USA); (2) 5 µL of CP or ME was applied to the inner stem wall of the glass funnel with a micropipette. The top of the funnel was 90 mm in diameter, and the funnel stem was about 60 mm long; the outside diameter of the funnel stem was about 9 mm, and the inside was about 6 mm.
In the afternoon (1400–1600) of the day before the bioassay, groups of 20 males of a given age were released in regular screen cages for acclimatization and provided with water and diet. At 0930, a simple trap with CP or ME was installed in the regular screen cage, and trap catches were recorded at 1030. Flies were scored as effective catches if they were found at the stem of the funnel or the inside of Erlenmeyer flask. Another simple trap without CP or ME was placed at the same time in each regular screen cage as a control. In the present study, 15 replicates were conducted for males of each age (i.e., each day old).
Data analyses
A generalized linear model with binominal distribution and logit link, i.e., logistic regression model, was used to analyze the effects of male age, attractant (CP or ME), and their interaction on the response/non-response of B. correcta males. The male age, the attractant, and their interaction were used as independent variables; the male response/non-response was used as the dependent variable.
The predicted probabilities of the male response of B. correcta to the simple trap baited with CP or ME at different ages (1–14 and 31 days old) were calculated using the aforementioned logistic regression model.
The computer program JMP version 11 (Institute SAS 2013) was used for the analyses.
Results and discussion
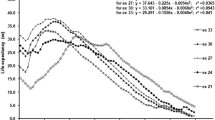
Results of the sexual maturation test (daily sexual maturation rates) are presented in Fig. 2. Amongst 15 cages, the earliest male sexual maturation period was 6-day-old. The latest one was 13-day-old. The mean male age of male sexual maturation period of all cages was 8.8-day-old. Daily sexual maturation rates were 0.0%, 6.7%, 20.0%, 46.7%, 73.3%, 86.7%, 93.3%, and 100.0% for 5, 6, 7, 8, 9, 10, 11, and 13-day-old, respectively.
Daily sexual maturation rate (filled diamonds connected with a dotted line) of B. correcta males and their mean capture rate to a simple trap baited with CP (open circles) and ME (open triangles). The prediction curves of the response probability for CP and ME are solid and break lines, respectively. The asterisk represents the actual mean capture rate at over 30 days old
In the sexual maturation test of the present study, if males attained sexual maturation and mated with females, the eggs which were produced by the females might not hatch owing to factors such as cryptic female choice and genetic incompatibility of the male and female. Therefore, there is a possibility that the male sexual maturation period was overestimated. In addition, if only one male in a particular cage was mature and fertilized eggs were discovered, that cage was labelled “sexually mature” in the present study. Therefore, there is a possibility that male sexual maturation period was underestimated. For these reasons, our estimate of the male sexual maturation period may lack a high degree of precision.
The mean capture rate of B. correcta males in traps baited with CP or ME at different ages is presented in Fig. 2, and the results of logistic regression to analyze the effects of male age, attractant, and their interaction on the response/non-response of B. correcta males are presented in Fig. 2 and Table 1.
The mean capture number (the capture rate) with CP increased from 0.6 (3.0%) to 3.4 (17.0%) as the age advanced from 1 to 4 days old; although their capture rates were less than 20%, some males responded to the trap with CP at all ages from 1 to 4 days old. The mean capture number (the capture rate) with CP increased from 5.9 (29.7%) to 18.1 (90.3%) as the age advanced from 5 to 14 days old, and was 18.8 (94.0%) for over 30-day-olds; the capture rate increased sharply after 5 days old, and the rate exceeded 90% for 14-day-olds and over 30-day-olds. No fly was caught in the control trap (without CP) in all replicates.
No B. correcta male responded to the trap baited with ME until flies reached 5 days old, and the mean capture number (the capture rate) with ME was 0.1 (0.3%) for 6-day-olds. The mean capture number (the capture rate) increased from 0.9 (4.7%) to 12.9 (64.7%) as the age advanced from 7 to 14 days old, and was 14.8 (74.0%) for over 30-day-olds; although the capture rate gradually increased after 7 days old, the rate was only 74.0% even for over 30-day-olds. One fly was caught in the control trap (without ME) in all replicates.
Results of the logistic regression analysis indicate that the effects of male age and attractant on the response/non-response of Bactrocera correcta male are interactive (Table 1). However, the predicted probability for the response to CP was always greater than the one to ME during all observed age period (1-14 and over 30-day-old), while the difference varied with male ages (Fig. 2).
The capture rate with CP of B. correcta males was 29.7%, and the predicted probability by the logistic regression model for the male response to CP was 30.6% at 5 days old, which was before sexual maturation. The capture rate was 52.7%, and the predicted probability was 38.2% at 6 days old, which was the earliest age of sexual maturation. In contrast, no B. correcta male responded to ME from 1 to 5 days old, and the predicted probability with ME was 12.6% at 5 days old. The capture rate with ME was 0.3%, and the predicted probability was 14.5% at 6 days old.
The present study revealed that, under laboratory conditions, males of B. correcta responded to CP, but not ME, prior to sexual maturation. The relevance of sexual maturation for successful eradication with the male annihilation technique was suggested by Iwahashi et al. (1996) and Wong et al. (1989), and Shelly et al. (2008) later reported that B. dorsalis males were attracted to ME even before attaining sexual maturity. In the present study, B. correcta males responded to the trap with CP at all ages, and the capture rates (the predicted probability for the male response) were 29.7% (30.6%) before sexual maturation (at 5 days old) and 52.7% (38.2%) at 6 days old when sexual maturation rate was still low number (6.7%). On the basis of this observation, CP is a good attractant candidate for B. correcta, as it appears to be effective before sexual maturation.
Wong et al. (1986) reported that laboratory-reared adults of B. cucurbitae reach sexual maturation earlier than wild flies. In the present study, the colony had been maintained in the laboratory over 12 years; therefore, the age of sexual maturation may be earlier than that of the wild population. If there is a phenomenon that the response to CP for laboratory-reared population differs from the response for the wild population before sexual maturation, the result of the present study does not necessarily apply to the wild population.
ME is a well-known male attractant for B. correcta, and its use might be considered in eradication plans in case of a B. correcta invasion in Japan. However, the present study concludes that CP could be a better attractant for B. correcta males, attracting them before their sexual maturation and being substantially more effective than ME under laboratory conditions.
In order to evaluate the efficiency and to endorse the use of CP in actual eradication, the age-related response of male B. correcta to traps baited with CP should be tested using field cages such as those shown by Shelly et al. (2008) and Umeya et al. (1973). If the result is similar to that of the present study, further trap surveys with wild B. correcta in the field are needed. To this end, it is essential to have proper methods to identify sexual maturation status/degree of males trapped for assessing possibility of application of the male annihilation technique. The ejaculatory apodeme of B. correcta males could be an indicator for this purpose as it develops with age after eclosion (Kaneda et al. unpublished data).
In addition, in the present study, it was found that the mean capture numbers (the predicted probability for the response) of B. correcta males with CP were greater than those with ME at all ages. This finding may lead to further consideration for possible CP use other than eradication as discussed above. ME has been often used in monitoring surveillance in order to detect accidental introduction of B. dorsalis for early control actions, being recognized as an effective attractant for the species. Umeya et al. (1973), for example, reported that over 90% of mature males of B. dorsalis responded to ME traps in laboratory conditions (rearing cages). Since the mean capture rate (the predicted probability for the response) of mature males of B. correcta (over 30 days old) to CP in the present study was 94.0% (99.9%), which is similar to the level reported by Umeya et al. (1973), CP use should be considered in monitoring surveillance to detect B. correcta.
References
Allwood AJ, Chinajariyawong A, Drew RAI et al (1999) Host plant records for fruit flies (Diptera: Tephritidae) in Southeast Asia. Raffles Bull Zool Suppl 7:1–92
Christenson LD, Foote RH (1960) Biology of fruit flies. Annu Rev Entomol 5:171–192
Drew RAI, Raghu S (2002) The fruit fly fauna (Diptera: Tephritidae: Dacinae) of the rainforest habitat of the Western Ghats, India. Raffles Bull Zool 50:327–352
Drew RAI, Romig MC (2013) Tropical fruit flies (Tephritidae: Dacinae) of South-East Asia. CAB International, UK
Institute SAS (2013) JMP: statistical discovery, version 11. SAS Institute, USA
Iwahashi O, Syamusdin-Subahar TS, Sastrodihardjo S (1996) Attractiveness of methyl eugenol to the fruit fly Bactrocera carambolae (Diptera: Tephritidae) in Indonesia. Ann Entomol Soc Am 89:653–660
Kamiji T, Arakawa K, Kadoi M (2014) Effect of temperature on the development of a Vietnamese population of Bactrocera correcta (Bezzi) (Diptera: Tephritidae). Jpn J Environ Entomol Zool 25:101–109
Liu X, Jin Y, Ye H (2013) Recent spread and climatic ecological niche of the invasive guava fruit fly, Bactrocera correcta, in mainland China. J Pest Sci 86:449–458
Nakamori H, Nishimura M, Kakinohana H (1991) Eradication of the oriental fruit fly, Dacus dorsalis Hendel (Diptera: Tephritidae), from Miyako and Yaeyama Islands by the male annihilation method. In: Vijaysegaran S, Ibrahim AG (eds) Fruit Flies in the tropics: Proceedings of the First International Symposium. Malaysian Agricultural Research and Development Institute, and Malaysian Plant Protection Society, Malaysia, pp 230–231
PPS (Plant Protection Station, MAFF) (2016) Plant quarantine statistics. http://www.maff.go.jp/pps/j/tokei/index.html. Accessed 20 Sep 2016
Shelly TE, Edu J, Pahio E et al (2008) Re-examining the relationship between sexual maturation and age of response to methyl eugenol in males of the oriental fruit fly. Entomol Exp Appl 128:380–388
Tokushima I, Orankanok W, Tan KH et al (2010) Accumulation of phenylpropanoid and sesquiterpenoid volatiles in male rectal pheromonal glands of the guava fruit fly, Bactrocera correcta. J Chem Ecol 36:1327–1334
Tsuruta K, White IM, Bandara HMJ et al (1997) A preliminary notes on the host-plants of fruit flies of the tribe Dacini (Diptera, Tephritidae) in Sri Lanka. Esakia 37:149–160
Umeya K, Sekiguchi Y, Ushio S (1973) The reproductive ability of the oriental fruit fly, Dacus dorsalis Hendel and the response of adults to methyl eugenol. Jpn J Appl Ent Zool 17:63–70 (in Japanese with English summary)
Vargas RI, Piñero JC, Leblanc L (2015) An overview of pest species of Bactrocera fruit flies (Diptera: Tephritidae) and the integration of biopesticides with other biological approaches for their management with a focus on the Pacific region. Insects 6:297–318
Wang XJ (1996) The fruit flies (Diptera: Tephritidae) of the East Asian region. Acta Zootaxon Sinica Suppl 21:1–419
Weems HV Jr (1987) Guava fruit fly, Dacus (Strumeta) correctus (Bezzi) (Diptera: Tephritidae). Entomol Circ 291:1–4
White IM, Elson-Harris MM (1992) Fruit flies of economic significance: their identification and bionomics. CAB International, UK
Wong TTY, McInnis DO, Nishimoto JI (1986) Melon fly (Diptera: Tephritidae): sexual maturation rates and mating responses of laboratory-reared and wild flies. Ann Entomol Soc Am 79:605–609
Wong TTY, McInnis DO, Nishimoto JI (1989) Relationship of sexual maturation rate to response of oriental fruit fly strains (Diptera: Tephritidae) to methyl eugenol. J Chem Ecol 15:1399–1405
Yoshizawa O (1997) Successful eradication programs on fruit flies in Japan. Res Bull Pl Prot Jpn Suppl 33:1–10
Acknowledgements
We deeply acknowledge Ritsuo Nishida (Professor Emeritus, Kyoto University) for the technical information on the male attractant in the present study and the critical reading on the manuscript. We are also grateful to Takuya Uehara (NIAS), and Yukio Yokoi, Hideaki Matsuura, Hiroyuki Adachi, Hiroshi Uematsu, and Ren Iwaizumi (Yokohama Plant Protection Station, MAFF) for their critical reading and helpful comments on the manuscript. We are also indebted to two anonymous reviewers for their critical comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamiji, T., Kaneda, M., Sasaki, M. et al. Sexual maturation of male Bactrocera correcta (Diptera: Tephritidae) and age-related responses to β-caryophyllene and methyl eugenol. Appl Entomol Zool 53, 41–46 (2018). https://doi.org/10.1007/s13355-017-0525-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-017-0525-9