Abstract
The coccinellid beetle Anovia punica Gordon (Coleoptera: Coccinellidae: Noviini) is an important predator of the Colombian fluted scale, Crypticerya multicicatrices Kondo & Unruh (Hemiptera: Monophlebidae). In order to gather information on the biological traits of A. punica, we conducted a series of studies, including of the developmental time of each life history stage, estimation of life table parameters, and predation rates under laboratory conditions [25.1 ± 1.6°C, with 70.5 ± 7.3% RH, and natural light regime, approx. 12:12 (L:D) h]. Developmental stages of A. punica were categorized as follows: egg stage, four larval instars, prepupal instar, pupal instar, and adult. Developmental time from egg to adult emergence averaged 29.41 ± 1.85 days, and 47.6% of the eggs developed to adulthood. Female and male survival was 94.42 and 90 days, respectively. Life table parameters show that one female of A. punica is replaced by 86 females (R 0), the intrinsic growth rate (r m ) was 0.1115, the average generation time (T) was 40 days, and the doubling time (D t ) was 6.2 days. The life table parameters suggest that A. punica can be used as a potential predator of C. multicicatrices and, more importantly, provided baseline information for a mass-rearing protocol. This is the first detailed study on the biology of A. punica that reports the potential of this predator as a biological control agent for scale insects of the tribe Iceryini.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Colombian fluted scale, Crypticerya multicicatrices Kondo & Unruh (Hemiptera: Coccomorpha: Monophlebidae) (Kondo & Unruh 2009), is a phytophagous and polyphagous insect native to continental Colombia, and reported as an invasive pest on the islands of San Andrés and Providencia from 2010–2013 (Kondo et al 2012). Due to the lack of natural enemies on the island, C. multicicatrices showed a rapid population growth that affected more than 150 species of plants, including some species of economic importance such as avocados, Persea americana (Lauraceae); coconut, Cocos nucifera (Arecaceae); soursop, Anona muricata (Annonaceae); guava, Psidium guajava (Myrtaceae); and mango, Mangifera indica (Anacardiaceae) (Kondo et al 2012, 2014, Silva-Gómez et al 2013).
Crypticerya multicicatrices sucks the sap from leaves, branches, and fruits of the host plant and produces honeydew that promotes the development of sooty molds. The presence of sooty molds over the commercial parts of plants causes damage that reduces the quality of the product, decreases the photosynthetic rate, and in severe attacks may cause the death of the plant (Kondo et al 2012). These same authors also discussed the need to implement a classical biological control program as a way to mitigate the environmental impact of this invasive pest, which led to the search for natural enemies of C. multicicatrices in continental Colombia. Fortunately, in 2013, C. multicicatrices on San Andrés island was controlled by a coccinellid, initially identified as Anovia sp. (Coleoptera: Coccinellidae), through a fortuitous and efficient classical biological control event (Kondo et al 2014). This voracious predator, now identified as Anovia punica Gordon (González & Kondo 2014), was first found in February 2013 on the island of San Andrés and only 8 months from its first sighting, this predator had spread throughout the island, decimating the populations of C. multicicatrices to the point that it was very difficult to find even a specimen (Kondo et al 2014). All known preys of A. punica are scale insects that belong to the tribe Iceryini (Hemiptera: Coccomorpha: Monophlebidae) (González & Kondo 2014). Besides C. multicicatrices, A. punica has been previously reported feeding on Icerya purchasi Maskell (Monophlebidae) and Crypticerya montserratensis (Riley & Howard 1890) (Gordon 1972) (Monophlebidae). Thus, it is likely that this predator may feed on other species of fluted scales in other regions of Central and South America (González & Kondo 2014).
Coccinellids, or lady beetles, of the tribe Noviini (Coleoptera: Coccinellidae) are known for their effectiveness in the control of scale insects, and most of the literature related to Noviini focuses on this aspect (Forrester 2008). Larvae and adults are voracious predators exclusively of scale insects of the family Monophlebidae (Forrester 2008). In addition, the tribe Noviini is diverse and consists of approximately 80 species divided into three genera: Anovia Casey, Novius Mulsant, and Rodolia (Mulsant) (Forrester et al 2009), many of which have a great importance as biological control agents. For example, the most famous reported case is the introduction of Rodolia cardinalis (Mulsant) to the State of California (USA) in 1889, to control the cottony cushion scale I. purchasi, an iceryine scale that caused large losses to the citrus industry in California (Caltagirone & Doutt 1989). Another example is the release of R. cardinalis to control I. purchasi in the Galapagos Islands in 2002 (Causton 2004). After 3 months, the population of I. purchasi decreased by 99–100%, possibly together with the effect of high rainfall in the period of study. Another noviine coccinellid, Anovia circumclusa (Gorham), has been reported as a natural enemy of the introduced pest Crypticerya genistae Hempel (Monophlebidae) in the State of Florida, USA (Forrester et al 2009).
Currently, there are no reports or studies on the life history of A. punica, and to our knowledge, the present work is also the first detailed study of the biology of A. punica.
Although it has been documented that C. multicicatrices was controlled successfully by A. punica on San Andrés Island (Kondo et al 2014), the process behind this case of classical biological control has not been assessed. The present study aimed to confirm the efficacy of this predator by assessing the following: developmental time of each growth stage, adult longevity, reproductive potential, fecundity, and other ecological parameters of the predator A. punica when offered individuals of C. multicicatrices as prey. Such biological information on this noviine coccinellid will be of great importance when assessing the efficacy of this predator in biological control efforts against fluted scale insects and other monophlebids.
Materials and Methods
Study location
The study was conducted between May and December 2014 at the Colombian Corporation for Agricultural Research (Corpoica), Palmira Research Station, located in the municipality of Palmira, Valle del Cauca, Colombia, 03°31′17″N, 76°18′25″W, ca. 1000 m a.s.l. We conducted several biological studies, including diagnoses of the different developmental stages and their duration, life cycle, estimation of life table parameters, and predation rates under laboratory conditions [25.1 ± 1.6°C, with 70.5 ± 7.3% RH, and natural light regime, approx. 12:12 (L:D) h]. Environmental data were measured with a Datalogger (CEM, DT 171, Shenzhen Everbest Machinery Industry Co., Ltd., Shenzhen, China).
Rearing of Anovia punica
Sealed plastic containers (33 cm length × 23 cm width × 13 cm height) with two circular holes (10 cm in diameter) on the lid and covered with fine mesh for ventilation were used as rearing chambers. Individuals of A. punica and its host, C. multicicatrices, were collected from urban trees in the city of Cali, Colombia, where the presence of both prey and predators were observed. Collecting field trips were carried out once a week from May to December 2014. Larvae and pupae of A. punica were placed in rearing chambers to monitor the emergence of adults. Emerged adults of A. punica were collected and transferred to new rearing chambers, where 60–80 female and male individuals were placed per chamber to allow the oviposition of the female lady beetles. Twigs infested with C. multicicatrices were placed within each container to serve as food and oviposition substrate for the beetles. Every week, adults of A. punica were transferred to new rearing chambers with fresh prey and new substrate for oviposition, until all adults of A. punica had died. Eggs collected from the containers were monitored until the beetles reached the adult stage and these were separated into additional rearing chambers.
Developmental stages and life cycle duration of A. punica
In order to determine the development time of the immature stages of A. punica, 20 pairs of newly emerged adults were transferred to rearing chambers, which contained twigs of Caesalpinia pluviosa var. peltophoroides (Fabaceae) infested with second- and third-instar nymphs of C. multicicatrices. Due to the similarity between the eggs of A. punica and C. multicicatrices, no adults of the host iceryine were used as food and/or as an oviposition substrate in order to avoid confusion between the eggs of both insect species. The body surface of each nymph of C. multicicatrices, which is covered by white wax, was visually checked for the presence of eggs of A. punica. Infested nymphs were transferred to Petri dishes containing moistened filter paper. A cohort of 105 eggs of A. punica was used to study development and survival. Eggs, larvae, and adults were monitored daily to determine the survival and length of each developmental stage and the sex ratio of emerging adults. Observations were done under a stereoscope with a camera (Leica EZ4 HD Leica Microsystems, Wetzlar, Germany) to obtain measurements and photographic records that were processed when necessary using the software Adobe ® Photoshop ® (Adobe Systems, Inc., San Jose, CA).
Fecundity and longevity of A. punica
To determine the fecundity of A. punica, 85 pairs were evaluated by placing them in Petri dishes containing moistened filter paper and a twig of C. pluviosa var. peltophoroides infested with nymphs of C. multicicatrices. Three to eight pairs of A. punica, each less than 16 h after emergence, were placed in each Petri dish. Infested twigs were replaced daily, and eggs of A. punica were counted from the surface of the hosts on the removed twigs. Oviposition data from A. punica were recorded daily until the death of the last female coccinellid. To determine the average lifespan of the predator, daily observations were made by recording whether coccinellids were dead or alive, until the day of the death of the last individual.
Life table parameters of A. punica
The life table parameters of A. punica were estimated based on the data of fecundity and longevity using the jackknife method according to Maia et al (2000). The jackknife technique is a parametric method that reduces the bias in the estimation of the population and generates standard errors for a statistical parameter (Maia et al 2000). The life table parameters were calculated as follows: net reproductive rate R 0 = Σl x m x , where m x = fecundity for female at day x and l x = female longevity at day x; mean generation time T = Σxl x m x /R 0, where x = time interval; intrinsic rate of increase r m = lnR 0/T; and doubling time D t = ln2/r m (Andrewartha & Birch 1954, Silveira-Neto et al 1976, Rabinovich 1978, Southwood 1978, Maia et al 2000).
Predation rate of A. punica
This study was conducted in order to establish the consumption rate of the larvae and adults of A. punica on eggs of C. multicicatrices offered as prey. A pretest was carried out to define the number of eggs in the experiment. Two thousand four hundred eggs of C. multicicatrices were offered to five adults (1200 eggs) and five larvae (3–4th instar) (1200 eggs) of A. punica in the pretest (60 eggs/adult/day + 60 eggs/larva/day), and the numbers of eggs consumed were scored during the 4 days of observations. Both adults and fourth-instar larvae of A. punica consumed about the same number of eggs and never more than 20 eggs/day; thus, both stages were supplied with 25 eggs/day in order to provide them with plenty of eggs. Among the different larval stages, fourth-instar larvae consumed the most eggs because of their larger size.
Second-, third-, and fourth-instar larvae and adults of A. punica (n = 12 for each instar) were placed individually in Petri dishes covered with moistened filter paper and were fed with 25 eggs of C. multicicatrices. Information on egg consumption and larval development was daily recorded. Data recording on consumption by the larvae ended when the second- or third-instar larvae started molting or when the fourth-instar larvae reached the prepupal stage, since at this point they ceased feeding. Recently emerged adults of A. punica (n = 12) also were placed individually in Petri dishes and were supplied with 25 eggs of C. multicicatrices per day. Non-consumed eggs by both larvae and adults were discarded and replaced with a new batch of eggs each day. Given the difficulty of assessing the sex of adults of A. punica, the sex of individuals in the predation study was not taken into account. Data on predation of first-instar larvae was unavailable due to the high mortality at this stage. First-instar larvae are very small and fragile and were likely injured during manipulation. For the above reasons, the predation study was conducted using second- and older-instar larvae. However, it was noted that predation ranged from 3 to 6 eggs/day in three first-instar individuals that survived, and thus, the overall predation rate is actually higher than herein reported. With the recorded data, we calculated the age-specific predation rate (k x ), which is defined as the mean number of prey consumed by A. punica at age x. Following Chi & Yang (2003) and Yu et al (2005, 2013), with information on survival rate, we were able to calculate the age-specific net predation rate (q x ) defined as the weighted number of prey consumed by a predator of age x taking into consideration the survival rate of the predator at age x, calculated as q x = k x l x .
Statistical analyses
All the statistical analyses were done with the statistical program SAS (SAS Institute Inc. 2011). We used the post-hoc Tukey–Kramer test to compare the difference among treatments when necessary. Development time is expressed in units per day and changes in the development of the insects are assumed to occur at the midpoint of the interval of 24 h. Data on survival for males and females were analyzed using the LIFETEST procedure of SAS, through log-rank test (SAS Institute Inc. 2011).
Results and Discussion
Length and diagnoses of the developmental stages of A. punica
The average duration of the life cycle of A. punica from laying of an egg to emergence of an adult was 29.41 ± 1.85 days (Table 1, Fig 1). The developmental stages of A. punica were categorized during our study as follows: egg stage, four larval instars, prepupal stage, pupal stage, and adult (Figs 1, 2, 3).
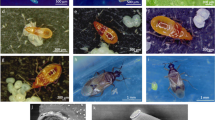
Immature stages of Anovia punica. A A recently oviposited egg of Anovia punica. B An egg close to hatching. C Eggs of A. punica laid between the body margin and ovisac of an adult individual of Crypticerya multicicatrices (see arrows). D Newly emerged fourth-instar larva, still devoid of wax; dorsal view. E Newly emerged fourth-instar larva; ventral view. F Fully developed fourth-instar larva with typical white wax; dorsal view. G Third-instar larva of A. punica (lower left) preying on a C. multicicatrices nymph (upper right). H Newly formed pupa. I Intermediate pupal stage. J Late pupal stage, showing same coloration as adults. Photos: C. Pinchao.
The egg
Eggs are elongated and oval in shape (Fig 2A) (0.64 ± 0.04 mm length and 0.32 ± 0.04 mm width, n = 32), being larger than the eggs of a closely related species, A. circumclusa (Gorham) as described by Forrester et al (2009). The eggs have a red coloration, with a granular surface and are covered by waxy exudate as reported for A. circumclusa (Forrester et al 2009). Prior to hatching, the eggs become whitish in color (Fig 2B), caused by the separation of the chorion from the embryo, forming an air space as reported for eggs of Scymnus (Neopullus) sinuanodulus Yu and Yao (Coleoptera: Coccinellidae) (Lu et al 2002). Eggs are oviposited on the body surface of second- and third-instar nymphs and adults of the host, C. multicicatrices, or on its ovisac (Fig 2C). In some cases, eggs were found on the surface of the leaves or twigs of the host plant of C. multicicatrices. Eggs were oviposited singly and it was common to find 1–3 eggs per individual of C. multicicatrices, although in a rare case, an individual with up to 11 eggs on its surface was observed. The eggs of A. punica have a very similar appearance to the eggs of C. multicicatrices and are very difficult to differentiate with the naked eye.
Larvae
Anovia punica has four larval instars, which do not show marked morphological differences except for size (Table 2, Fig 1). The characteristics of the larval instars are as follows: larvae campodeiform and red in color; body convex and ovoid, widest in the middle part (Fig 2D–G), membranous, with 10 segments (Fig 2E); dorsal surface lightly covered with setae and waxy exudate (Fig 2F, G), which hide most aspects of their external anatomy, as previously reported for A. circumclusa (Forrester et al 2009). In some species of coccinellids, wax threads are apparently exudates from the base of a spicule, which are used also as a support when wax threads grow upwards (Pope 1979). Wax threads in coccinellids may also have evolved as a physical defense against ant attacks (Pope 1979). In line with this, Majerus et al (2007) compiled evidence that suggests that waxy threads in coccinellid larvae may act as a barrier against ants trying to bite them. In addition, the sticky property of the wax can cause the ants to clean their mouthparts and therefore decrease the frequency of attacks (Völkl & Vohland 1996). In the same way, mimicry with their prey will be another adaptive function for avoidance of ant attacks, since the tending ants will ignore or will not be able to distinguish between the hemipterans that they tend and the coccinellid predators (Bach 1991).
Manipulation of larvae (also pupae and adults) causes the release of a red fluid, possibly containing carminic acid. The same response has been observed in larvae and adults of R. cardinalis, wherein the secreted fluid is used as a chemical defense (Forrester 2008). The secretion of unpleasant fluids, which are composed mainly by alkaloids, histamines, and pyrazines, is a well-studied defense mechanism against vertebrate and invertebrate predators of Coccinellidae (Majerus et al 2007). The newly emerged larvae (at each molt) lack the waxy processes and are completely red in color (Fig 2D, E), but after a few hours, they become fully covered with wax.
Larvae of A. punica and nymphs of C. multicicatrices may be mistaken at first glance due to similar body size and the white wax common to both species (Fig 2G). Larvae and adults of A. punica prefer feeding on eggs of C. multicicatrices, but may feed on other nymphal stages (Pinchao et al 2015). On the other hand, both larvae and adults of R. cardinalis feed on eggs and crawlers, and on the underside of young prey (Quezada & DeBach 1973, Pinchao et al 2015), with no apparent preference for eggs.
Prepupa
After the fourth instar, the beetle larva enters a prepupal stage (Fig 1). At this point, individuals secrete a red exudate to adhere the apex of the abdomen to the substrate, as has been reported for other species of the tribe Noviini (Vandenberg 2002, Forrester 2008). In addition, individuals in the prepupal stage lack movement and do not feed.
Pupa
The exarate pupa (4.17 ± 0.19 mm long and 3.04 ± 0.12 mm wide, n = 33) is partially covered by the last larval exuviae at the body margins (Fig 2H–J), as reported by Phuoc & Stehr (1974) for the Noviini tribe. The pupa is sedentary and attaches to the substrate using the exudate previously released in the prepupal stage. In the dark color morph used in the present study, the pupae have an initial red coloration and gradually darken to black (adult color) (Fig 2H–J). Individuals in the pupal stage do not feed.
Adults
Adults (Fig 3A–C, E, F) have an average length of 2.95 ± 0.18 mm (range 2.5–3.3 mm) and an average width of 2.47 ± 0.15 mm (range 2.1–2.8 mm) (n = 52). Body is oval and hemispherical in shape; head is not visible from above (Fig 3A); semi-decumbent pubescence (Gordon 1972); abdomen with incomplete post-coxal line; with six ventrites, progressively closer; the last ventrite in males with slightly emarginate apex (Fig 3G), apex of females not emarginate (Fig 3D) (Gordon 1972). Basal lobe of male genitalia abruptly narrows at one third of its length from distal end; basal lobe shorter than parameters (Fig 3I) (González & Kondo 2014). The curvature of the tube of the sipho is quite variable, ranging from almost absent (as illustrated in Gordon 1972) to highly curved, forming a letter “C” shape (Fig 3H); however, the curvature of the sipho may be affected by the dissection treatment (González & Kondo 2014). Coxites in A. punica females have sclerotized apices that are used to scrape off the wax of its host (e.g., the ovisac of C. multicicatrices) in order to aid in the anchoring of oviposited eggs to the surface of the host. This novel function of the coxites has not been reported previously in Coccinellidae.
Adults of A. punica are extremely polymorphic, ranging from light brown, dark brown, purple, black opaque, or black with tinges of shiny green or blue; with color patterns composed of reddish or brown areas on black background or black areas on brown or reddish background; these patterns are usually found on the front edge and the lateral third of the pronotum and scutellum, and the lateral edges of the elytra; often forming a band of variable width and length submarginally or marginally on the elytra or sometimes found at the basal area of the elytra near the scutellum (González & Kondo 2014). Specimens used in the present study have a black design with shiny blue elytra.
Longevity, fecundity, and life table parameters of Anovia punica
Fifty individuals reached the adult stage, representing 47.6% of the initial cohort (n = 105 eggs). The adults lived on average 71.24 ± 22.4 days under laboratory conditions (Table 1). The female to male sex ratio was 1:1. The population of A. punica exhibited a type III survival curve with a high mortality rate (about 40%) during the egg and first-larval (larva I) developmental stages. According to Rabinovich (1980), in this model, animal populations with well-defined larval instars often suffer from relatively high mortalities at very young stages of development or in the transitions of one way of life to another. After surviving the first-larval instar, the probability of reaching the adult stage increases. This information is very useful for the implementation of a mass-rearing program for A. punica, since improvement of rearing conditions at early developmental stages would lead to a decrease in the mortality of A. punica populations.
Longevity among adult males and females of A. punica was not significantly different (log-rank χ 2 = 30.3658, df = 1, p < 0.5453) under laboratory conditions. The average survival time for females was 94.42 days [95% confidence interval (CI) 81–124 days, n = 26] and the average male survival time was 90 days (95% CI 79–121 days, n = 24).
The maximum fecundity was reached during the first month after emergence as adult females (Fig 4). Over the entire lifespan, a female laid an average of 366 ± 114 eggs. During the first 15 days, females had low mortality (10%) and then (up to day 40) had a recorded mortality of 80% (Fig 4).
Life table parameters provide an overall description of the development, survival, and fecundity of a certain population. Within the evaluated parameters, the intrinsic rate of increase is the most important, since it allows comparison of the population growth potential under specific climatic and food conditions (Southwood & Henderson 2000). In this study, the mean value for the intrinsic rate of natural increase (r m ) was 0.1115 female/day (95% CI 0.09–0.13), and this value coincides with that of lambda 1.12 (95% CI 1.09–1.13). By comparing this ecological value with that recorded by Sotelo & Kondo (2016) (r m = 0.05) for C. multicicatrices, we can suggest that A. punica would potentially act as an efficient biological control agent since there is a twofold increase in the r m parameter compared to its host. Likewise, the mean value for the net reproductive rate (R 0) was estimated as 86.1 (95% CI 63.6–108.6). This means that, on average, each female is replaced by 86 females in one generation under controlled conditions. The value for the mean generation time (T) was 39.9 days (95% CI 35.1–44.5) which means that A. punica would have an average of 9 generations per year, which is very important in terms of biological control efficacy against C. multicicatrices, since this species was recorded to have a total life cycle of 5 months (Sotelo & Kondo 2016). In addition, prey eggs in ovisacs will be available as food resource for A. punica for at least 3 months (Sotelo & Kondo 2016). Finally, the mean value for the doubling time (D t ) was 6.18 days (95% CI 5.20–7.16), meaning that the original population will double in number after 1 week.
Life history parameters have been extensively studied for aphidophagous coccinellids, including Hippodamia variegata (Goeze) (Farhadi et al 2011), Harmonia dimidiata (Fabricius) (Yu et al 2013), Scymnus apetzi Mulsant, S. subvillosus (Goeze), Exochomus nigromaculatus (Goeze) (Atlihan & Kaydan 2002), and Lemnia biplagiata (Swartz) (Yu et al 2005). In the case of biological control efforts against scale insects, there are numerous studies on the success of R. cardinalis as a biological control agent. More specifically, an extensive review by Van Driesche et al (2010) focused on how R. cardinalis has been repeatedly and extensively used to control the polyphagous cottony cushion scale, I. purchasi, since the coccinellid develops exclusively on monophlebines (Hemiptera: Monophlebidae), principally Icerya species (Causton et al 2004). However, no information is currently available on the biological control efficacy for any species of Anovia. Results of this study will contribute to a better understanding of biological aspects and life history parameters of A. punica. We include information that may be useful in estimating the potential success of A. punica when used as a biological control agent, measured in terms of predation.
Predation rate
Measurements of daily consumption of eggs of C. multicicatrices by the larvae of A. punica increased as a result of the size of the larvae. Fourth-instar larvae presented the highest daily consumption average compared to larvae of earlier instars (F = 24.45, P < 0.001) (Table 3). Larval predation rate increased significantly from age 9 to 11 days. Second-instar larvae that were molting to the third instar ceased feeding (C. Pinchao, personal observation). However, the same behavior was not observed for third-instar larvae molting to fourth-instar larvae. Third- and fourth-instar larvae showed a significant increase in the predation rate from age 13 to 19 days. In general, this coccinellid has three non-predatory periods, namely, the egg stage, second- to third-instar larval molting period, and the collective prepupal and pupal stages. These non-predatory periods formed three gaps in the predation rate (Fig 5).
Age-specific predation rate (k x ), age-specific net predation rate (q x ), and survival (l x ) of several developmental stages of Anovia punica on eggs of its host, Crypticerya multicicatrices, under laboratory conditions [temp 25.6 ± 1.7°C, HR 67.8 ± 8.1%, and natural light regime, approx. 12:12 (L:D) h]. Age of the predator is counted from birth. (l x ) of several developmental stages of Anovia punica on eggs of its host, Crypticerya multicicatrices, under laboratory conditions [temp 25.6 ± 1.7°C, HR 67.8 ± 8.1%, and natural light regime, approx. 12:12 (L:D) h]. Age of the predator is counted from birth.
Regarding the total eggs consumed by each larval instar, fourth-instar larvae consumed more eggs compared to smaller second- and third-instar larvae (F = 12.26 P < 0.001) (Table 3). In addition, adults of A. punica consumed an average of 13.6 ± 2.3 eggs of C. multicicatrices per day. The first day after an adult’s emergence, a consumption of 16.5 ± 2.1 eggs of C. multicicatrices was observed. Consumption rate increased gradually until day 5 after emergence and then stabilized at 12–13 eggs per day (Fig 5). During the entire larval period, an individual of A. punica consumed 101.5 ± 11.1 eggs of its host, C. multicicatrices.
Due to a lack of availability of eggs of the host reared under laboratory conditions (i.e., viable and non-parasitized eggs), we limited the study to the first 15 days after the emergence of adults of A. punica, which prevented us from having an accurate comparison between larval and adult predation rate. By calculating the mean daily predation rate for the interval between age 0 to 45 days (counted from birth), we were able to estimate the net predation rate of A. punica. Chi & Yang (2003) calculated the net predation rate (C 0) as follows: C 0 = Σk x l x . In this study, because the data on predation were recorded only to age 45 days, we obtained a partial net predation rate C 0 = 174.5 ± 9.3 eggs of C. multicicatrices per individual beetle. However, the studies on development here presented show that individuals of A. punica can live close to 100 days (counted from birth); therefore, additional studies are required to estimate a more accurate predation rate. As mentioned before, larvae and adults of A. punica use the same food resource, preferring to feed on eggs of C. multicicatrices, which would represent a continuous control of the fluted scale. However, it is necessary to consider that A. punica may feed on other nymphal stages of C. multicicatrices (Pinchao et al 2015).
Although population growth parameters obtained here are indicative of the effect of the predator population on the prey population, it is necessary to consider a population dynamics study through the use of more complex models. Thereby, we expect to evaluate the prey population levels according to the predation level in terms of stage specific predation rate and also the specific vulnerability of the prey stage as suggested by several authors (Hassell 1978, Chi and Yang, 2003, Yu et al 2005, 2013). Aspects such as food resource availability for prey, predator searching efficiency, predator conversion (i.e., ability to turn food into offspring), and the predator’s functional response (i.e., rate of prey capture as a function of prey abundance) also must be considered in future studies for this promising biological control agent.
Conclusions
In this study, we obtained basic information about the morphology and duration of the developmental stages of A. punica, a coccinellid with larvae and adults that are voracious predators of the scale insect C. multicicatrices. Basic information provided by the life cycle, predation rate, and life table parameters obtained herein suggests that A. punica may be considered as a candidate for use in future control programs as a biological control agent against C. multicicatrices and other pestiferous iceryine scales. Further studies are needed to elucidate the predator-prey relationship of A. punica and C. multicicatrices, by looking at the predator preference at different developmental stages of the prey in order to have a better understanding of the predator-prey dynamics under field conditions.
References
Andrewartha HG, Birch LC (1954) The distribution and abundance of animals. University of Chicago, Chicago, p 169
Atlihan R, Kaydan MB (2002) Development, survival and reproduction of three coccinellids feeding on Hyalopterus pruni (Geoffer) (Homoptera: Aphididae). Turk J Agric For 26:119–124
Bach CE (1991) Direct and indirect interactions between ants (Pheidole megacephala), scales (Coccus viridis) and plants (Pluchea indica). Oecologia 87:233–239
Caltagirone LE, Doutt RL (1989) The history of the vedalia beetle importation to California and its impact on the development of biological control. Annu Rev Entomol 34:1–16
Causton CE (2004) Predicting the field prey range of an introduced predator, Rodolia cardinalis Mulsant, in the Galápagos. p 195–223. In: Van Driesche RG, Reardon R (eds) Assessing host ranges for parasitoids and predators used for classical biological control: a guide to best practice. FHTET-2004-03, United States Department of Agriculture Forest Service, Morgantown p 242
Causton CE, Lincango MP, Poulsom TGA (2004) Feeding range studies of Rodolia cardinalis (Mulsant), candidate biological control agent of Icerya purchasi Maskell in the Galápagos Islands. Biol Control 29:315–325
Chi H, Yang TC (2003) Two-sex life table and predation rate of Propylaea japonica Thunberg (Coleoptera: Coccinellidae) fed on Myzus persicae (Sulzer) (Homoptera: Aphididae). Environ Entomol 32:327–333
Farhadi R, Allahyari H, Chi H (2011) Life table and predation capacity of Hippodamia variegata (Coleoptera: Coccinellidae) feeding on Aphis fabae (Hemiptera: Aphididae). Biol Control 59:83–89
Forrester JA (2008) Sacred systematics: the Noviini of the world (Coleoptera: Coccinellidae). (Ph.D. Thesis). Universidad de Georgia. Georgia, USA, p 236
Forrester JA, Vandenberg NJ, McHugh JV (2009) Redescription of Anovia circumclusa (Gorham) (Coleoptera: Coccinellidae: Noviini), with first description of the egg, larva, and pupa, and notes on adult intraspecific elytral pattern variation. Zootaxa 2112:25–40
González G, Kondo T (2014) Geographical distribution and phenotypic variation of Anovia punica Gordon (Coleoptera: Coccinellidae: Noviini), a predatory ladybeetle of fluted scales (Hemiptera: Coccoidea: Monophlebidae). Insecta Mun 0398:1–6
Gordon RD (1972) The tribe Noviini in the new world (Coleoptera: Coccinellidae). J Wash Acad Sci 62:23–31
Hassell MP (1978) The dynamics of arthropod predator prey system. Princeton University Press, Princeton, p 248
Kondo T, Unruh C (2009) A new pest species of Crypticerya Cockerell (Hemiptera: Monophlebidae) from Colombia, with a key to species of the tribe Iceryini found in South America. Neotrop Entomol 38:92–100
Kondo T, Gullan P, Ramos-Portilla AA (2012) Report of new invasive scale insects (Hemiptera: Coccoidea), Crypticerya multicicatrices Kondo & Unruh (Monophlebidae) and Maconellicoccus hirsutus (Green) (Pseudococcidae), on the islands of San Andres and Providencia, Colombia, with an updated taxonomic key to iceryine scale insects of South America. Insecta Mun 0265:1–17
Kondo T, Gullan P, González G (2014) An overview of a fortuitous and efficient biological control of the Colombian fluted scale, Crypticerya multicicatrices Kondo & Unruh (Hemiptera: Monophlebidae: Iceryini), on San Andres island, Colombia. Acta Zool Bulg Suppl 6:87–93
Lu W, Souphanya P, Montgomery ME (2002) Description of immature stages of Scymnus (Neopullus) sinuanodulus Yu and Yao (Coleoptera: Coccinellidae) with notes on life history. Coleopt Bull 56:127–141
Maia A d HN, Luiz AJB, Campanhola C (2000) Statistical inference on associated fertility life table parameters using jackknife technique: computational aspects. J Econ Entomol 93:511–518
Majerus ME, Sloggett JJ, Godeau JF, Hemptinne JL (2007) Interactions between ants and aphidophagous and coccidophagous ladybirds. Popul Ecol 49:15–27
Phuoc DT, Stehr FW (1974) Morphology and taxonomy of the known pupae of Coccinellidae (Coleoptera) of North America, with a discussion of phylogenetic relationships. Contrib Am Entomol Inst 10:31–123
Pinchao EC, Kondo T, González G (2015) Rodolia cardinalis (Mulsant) (Coleoptera: Coccinellidae), a new predator of Crypticerya multicicatrices Kondo and Unruh (Hemiptera: Monophlebidae). Insecta Mun 0431:1–7
Pope RD (1979) Wax production by coccinellid larvae (Coleoptera). Syst Entomol 4:171–196
Quezada JR, DeBach P (1973) Bioecological studies of the cottony cushion scale, Icerya purchasi Mask., and its natural enemies Rodolia cardinalis Mul. and Cryptochaetum iceryae Will., in southern California. Hilgardia 41:631–688
Rabinovich JE (1978) Ecología de las Poblaciones Animales. Washington, Secretaría General de la Organización de los Estados Americanos, p 114
Rabinovich JE (1980) Introducción a la Ecología de Poblaciones Animales. Compañía Editorial Continental S.A, México, p 313
Riley CV, Howard LO (1890) Some new iceryas. Insect Life 3:92–106
SAS Institute Inc (2011) SAS procedures guide, versión 9.3. SAS Institute Inc, Cary
Silva-Gómez M, Quiroz-Gamboa J, Yepes F, Maya MF, Santos A, Hoyos-Carvajal L (2013) Incidence evaluation of Crypticerya multicicatrices and Maconellicoccus hirsutus in Colombian Seaflower Biosphere Reserve. Agric Sci 4:654–665
Silveira-Neto S, Nakano O, Barbin D (1976) Manual de Ecologia dos Insetos. Ceres, São Paulo, p 419
Sotelo P, Kondo T (2016) On the biology of the Colombian fluted scale, Crypticerya multicicatrices Kondo & Unruh (Hemiptera: Monophlebidae). Neotrop Entomol. https://doi.org/10.1007/s13744-016-0463-1
Southwood TRE (1978) Ecological methods with particular reference to the study of insects populations, 2nd edn. Chapman and Hall, London, p 524
Southwood TRE, Henderson PA (2000) Ecological methods. Blackwell, Oxford, p 575
Van Driesche RG, Carruthers RI, Center T, Hoddle MS, Hough-Goldstein J, Morin L, Smith L, Wagner DL et al (2010) Classical biological control for the protection of natural ecosystems. Biol Control Suppl 1:S2–S33
Vandenberg NJ (2002) Family 93. Coccinellidae Latreille 1807. In: Arnett RH, Thomas MC Jr, Skelley PE, Frank JH (eds) American Beetles Volume II: Scarabaeoidea through Curculionoidea. CRC Press LLC, Boca Raton, pp 371–389
Völkl W, Vohland K (1996) Wax covers in larvae of two Scymnus species: do they enhance coccinellid larval survival? Oecologia 107:498–503
Yu JZ, Chi H, Chen BH (2005) Life table and predation of Lemnia biplagiata (Coleoptera: Coccinellidae) fed on Aphis gossypii (Homoptera: Aphididae) with a proof on relationship among gross reproduction rate, net reproduction rate, and preadult survivorship. Ann Entomol Soc Am 98:475–482
Yu JZ, Chi H, Chen BH (2013) Comparison of the life-tables and predation rates of Harmonia dimidiata (F.) (Coleoptera: Coccinellidae) fed on Aphis gossypii Glover (Hemiptera: Aphididae) at different temperatures. Biol Control 64:1–9
Acknowledgments
Many thanks to Dr. Penny J. Gullan (The Australian National University, Canberra, Australia) for checking the English text and for useful comments. Thanks to anonymous reviewers for their useful comments that helped improve the manuscript. The authors thank the Colombian Ministry of Agriculture and Rural Development (MADR) that funded the research project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Christian S Torres – UFRPE
Rights and permissions
About this article
Cite this article
Pinchao, E.C., Sotelo, P., González, G. et al. Biological Data on Anovia punica Gordon (Coleoptera: Coccinellidae), a Predator of Crypticerya multicicatrices Kondo & Unruh (Hemiptera: Monophlebidae). Neotrop Entomol 47, 385–394 (2018). https://doi.org/10.1007/s13744-017-0561-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-017-0561-8