Abstract
The spined shoulder bug, Podisus maculiventris, is a generalist predator studied for its biocontrol potential. Despite our growing understanding of gland development, the conditions that elicit releases are largely unknown. To determine if male age or gland development affects the chemical composition and release behavior, we dissected adult male bugs and profiled the chemical composition of the male DAG 1, 7, and 14 d post-eclosion. To determine if gland development is related to sexual maturity, we counted the number of sperm present in the seminal vesicles at the same time points. Finally, we measured the diurnal release patterns of different aged males and in various male-female combinations. We observed that newly eclosed adults have under-developed glands and male seminal vesicles contained few sperm. One week post-eclosion the DAG contained previously reported semiochemical compounds and males contained many sperm. Mirroring the trend in reproductive maturation and gland development, the number of semiochemical releases increased with age and the majority of releases followed a scotophase pattern unaffected by sexual composition. These findings link male age to 1) dorsal abdominal gland development 2) release behavior and 3) sexual maturity, which will help our understanding of when these olfactory cues are present for other organisms, like prey, to perceive. Given the results, releasing adults that are at least 1 week post eclosion will maximize the non-consumptive effects of this biocontrol agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insect predators play a critical role in food web dynamics and pest management (Polis and Strong 1996; Tipping et al. 1999; De Clercq et al. 2003). Often, the focus of predators’ role in trophic interactions is on the consumptive effect predators have on prey. Another way predators interact with prey is by eliciting non-consumptive responses that increase preys’ chances of survival (Sih 1980; Lima and Dill 1990; Werner and Peacor 2003). Non-consumptive effects are often mediated via sensory cues (Weissburg et al. 2014), but little work has been done to understand how predator development affects the delivery of cues. Knowing this type of information will improve our ability to make predictions about the effects of predators on prey. Olfaction is one of the most heavily relied-upon sensory modality used by insect predators and prey. Pentatomids, aptly named stink bugs, produce a diverse array of chemical compounds and the use of chemical communication is assumed to be especially pronounced in this group.
The spined soldier bug, Podisus maculiventris (Say) (Hemiptera : Pentatomidae), is a common omnivorous stink bug that is distributed across North America (Legaspi and Legaspi 2007) and used for augmentative biocontrol in the United States and Europe (Biever and Chauvin 1992; Hough-Goldstein and McPherson 1996). A wealth of research has been conducted on this stink bug species to determine how to use it effectively for biological control (Tipping et al. 1999), with a particular focus on its chemical ecology. This species produces numerous volatile chemicals that are released via a series of exocrine glands (Aflitto and Thaler 2020; Aldrich 1988; Hermann and Thaler 2014; Sant’Ana et al. 1997). The dorsal abdominal gland (DAG) is of specific interest to chemical ecologist as adult male bugs can release large amounts (>1 mg) of semiochemical from it into their surrounding environment (Aldrich 1995). This strong olfactory cue has long intrigued chemical ecologists, raising questions regarding its importance in driving inter- and intraspecific interactions. Recent work has highlighted the importance of interspecific detection of the DAG semiochemical. For example, Aflitto and Thaler 2020; Hermann and Thaler 2014, demonstrated that Colorado potato beetles (Leptinotarsa decemlineata (Say)), an economically important crop pest of solanaceous plants, consume less plant tissue in the presence of DAG semiochemicals. Adding to the intrigue is the fact that many other organisms are known to have receptors that detect one or more of the compounds released from the DAG blend, including certain plants (Bate and Rothstein 1998; Farag and Pare 2002).
The contents of the male DAG have been extensively characterized (Aldrich et al. 1978; Aldrich 1995, 1985, 1988; Sant’Ana et al. 1997). Both male and female bugs have a DAG, however it is many times smaller in females (Aldrich et al. 1978) and they only share a single compound in common (Aldrich et al. 1984b). The male DAG is the largest exocrine gland and the largest organ in their body. When airborne, the DAG compounds aggregate nymphs and adults, especially as overwintering bugs emerge in the spring when the pheromone plays a role in mate attraction (Aldrich and Cantelo 1999). The nymphal stages are also attracted to a synthetic blend of the three most abundant compounds in the DAG, (E)-2-hexenal, α-terpineol, and benzyl alcohol (Sant’Ana et al. 1997). While all motile stages are attracted to the DAG semiochemical, the benefit to the individual releasing the volatile cue is unknown.
Here, we investigate four factors that may influence the presence of DAG semiochemical in the environment: 1) adult male age, 2) sexual maturity, 3) time of day, and 4) interactions with conspecifics. Emissions from the DAG are controlled by the opening and closing of an ostiole located on the abdominal dorsum of bugs. However, the factors that elicit releases from the gland are not well described. More evidence linking the DAG development with other physiological processes such as sexual maturation may uncover a more nuanced use of this semiochemical cue and contribute to a greater understanding of the ecological effects of this source of chemical information in a system. Ontogentic changes in P. maculiventris gland development from the immature to adult stage has been well studied (Aldrich et al. 1984b). However, less is known about changes in gland volume and composition that occur during the adult stage in P. maculiventris, and more broadly Pentatomids (see Mcbrien et al. 2001; Cribb et al. 2006). To better understand how adult age affects gland development and emissions, we investigated how the amount and composition of DAG chemicals change from the point of adult male eclosion through sexual maturity (ca. 7 d) and into middle age (14 d old). To learn more about the potential congruencies between gland development and sexual maturity, we measured the size of DAGs of these three age classes, removed, extracted, and analyzed their contents. We then quantified the number of sperm (our proxy for sexual maturity) in their seminal vesicles. Finally, to understand the behavioral and diurnal conditions that elicit the release from the DAG, we used the same age classes mentioned above, paired a male bug with either another male or a female, and measured the total number of volatile chemical releases over 24 h.
Methods and Materials
Insects
P. maculiventris were maintained in a laboratory colony and were provided an ad libitum diet of Tenebrio molitor L. larvae and greenhouse-grown 2–5 week-old Solanum tuberosum L. plants (cv. Yukon Gold). The colony was maintained at 25 ± 2 °C, ambient relative humidity and a 16:8 h L:D cycle.
Male Gland Development over Time
To better understand the relationship between male DAG maturity and sexual development, we characterized the size and chemical composition of the contents of the male DAG at three time points. To do this, we generated discrete cohorts of fifth-instar nymphs weekly for the three weeks leading up to the start of the experiment. This provided a supply of adults that were 1 d post-eclosion (young), 7 d post-eclosion (mid), and 14 d post-eclosion (old). Each cohort was kept in a separate cage and once eclosed, all females were removed from the cups to ensure no males mated with females.
Individual males were removed from their respective colony (age class) and anesthetized with CO2. Insects were then weighed to the nearest mg and we measured and recorded their length and width at their widest point using digital calipers. Specimens were then dissected under PBS (buffer) with the aid of a microscope. We removed the DAG (Fig. 1) and measured its length and width at the widest points before quickly transferring it to a 2 ml auto sampler vial containing 200 μL of dichloromethane. We measured five glands from each of the 3 weekly cohorts, for a total of 15 replicates per age class. All gland samples were stored at −80 °C until GC-MS analysis.
An aliquat (100 µl) of each DAG extract was transferred to 150 µl GCMS vial inserts contained within autosampler vials. Tetralin (10 µl, 90 ng/mL) was added to each sample as an internal standard. All samples were run on an Agilent GC (6890n, Agilent Technologies, column DB-5MS, Agilent Technologies) and a JEOL MS (JEOL USA, Inc. Peabody, MA). Helium was used as the carrier gas and the temperature ramp cycle was: 45 °C for 2 mins, 145 °C at a rate of 10 °C/min, 270 °C at a rate of 25 °C/min. Inlet temperature was 230 °C and the transfer temperature was 270 °C. Because the male DAG contains many compounds at a wide range of concentrations, we ran each sample using a split setup at 3 sensitivities. This allowed us to accurately quantify the focal analytes at the variable amounts present in the age cohorts. High sensitivity was set at 400v with no attenuation and a 1.0 μL injection. Medium sensitivity was set to 300v with no attenuation and a 1.0 μL injection. Low sensitivity was set to 300v with an attenuation of 4 and a 0.2 μL injection. The peak area of each compound was calculated and made relative to the internal standard peak to obtain the concentration of the detected compounds. Preliminary compound identified was done by searching the mass spectra of a peak in NIST MS library.
Linking Male Sexual Maturity to Gland Development
To determine how male age and sexual maturity correlates to gland development, we removed one seminal vesicle from each individual immediately after removing their DAG. Seminal vesicles were placed individually in 2 mL centrifuge tubes containing 1 mL of PBS. We gently ruptured the vesicle with fine forceps to release sperm. We made three serial dilutions (1:5, 1:10, and 1:20) of the original 1 mL stock to ensure that we had a concentration of sperm that could be easily quantified. Ten μl of each dilution were then pipetted on 15-well Multitest slides (MP Biomedicals, LLC) with predefined 3 mm diameter circles. We counted the number of sperm from 2 wells for 10 individuals from each age cohort and used the average of the two estimates per seminal vesicle for statistical analyses after accounting for dilutions. Two slides were lost from the 2-wk-old treatment, brining our total number of replicates for that treatment down to eight.
Semiochemical Release Frequency
To determine the effect of male age on DAG release frequency, two ‘young’ males (<1 day post eclosion), or two ‘mid–old’ males (7–14 days post eclosion) were paired and placed in 237 mL glass jars which were part of the olfactometer described in Aflitto and Thaler (2020). The olfactometer was set to a constant airflow flow of charcoal-filtered air at 50 mL/min over the pair of bugs and then over a metal-oxide volitle organic compound (VOC) sensor (IONTIK SemioSensor, Ithaca, NY). The sensors log total VOC, time, humidity, and temperature to a microSD card every 1 sec; an equivalent of 86,400 data writes per 24 h replicate. The SemioSensor detects total VOC fluctuations at a high sample rate and is not intended to provide identification of specific compounds. The experiment was performed on four different days (one replicate per day) with new pairs of males of each age cohort on each day.
To determine whether the presence of females influenced male release behavior, the numbers of releases of pairs of same-aged males (7-14 d, N = 10 replicates), pairs of females (age-range: 6 reps) and mixed-sex pairs (1 male with 1 female, n=10 replicates) were measured. Treatments were assigned randomly to bugs and replicates were conducted on different days. Data was collected for 24 h for each replicate as described above. For all sensor data, blank runs containing no bugs were conducted as a negative control to confirm that the VOC detector only captured data in the presence of insects. Preliminary testing indicated that a DAG release elicited sensor deflections ≥0.2 percent from the baseline readings when no insects were present. This helped filter out other VOC sources coming from the bugs such as defecation and smaller exocrine gland releases.
Statistical Analyses
All analyses were completed using R statistical software (Core Team R 2014). Linear mixed-effects models were used to analyze gland and body size comparisons and release behavior. We coded age as a fixed effect and date of measurement as a random effect. Linear models were used to assess chemical compound differences and for the seminal vesicle comparison with age as a fixed effect. Linear mixed effects models were used for semiochemical release behavior comparisons, where treatment and time of day were included as fixed effects and date as a random effect. Post hoc analyses were performed using estimated marginal means. Data was transformed where necessary to meet the linear model assumptions of normality and homogeneous variance. Mixed models were analyzed using the lme4 package (Bates et al. 2015).
Results
Male Gland Development over Time
The size of the DAG increased significantly as newly-eclosed adult males matured (F2,47 = 41.61, p = <0.001). We observed that 1 d-old bugs had substantially smaller DAGs compared to the 7 and 14 d-old males, which did not differ from each other (Figs. 1 and 2). Interestingly, while the mass of each age class of adults was similar (F2,99 = 0.011, p = 0.9), the body size (length x width) differed significantly (F2,102 = 13.57, p = <0.001). Fourteen day-old adults were slightly smaller (5% smaller) than the younger age groups. We hypothesize that this minor size difference is due to the exoskeleton transitioning from callow to a fully hardened state.
n (a) Mean dorsal abdominal gland (DAG) size (length x width) of 1-, 7-, and 14-d old male Podisus maculiventris adults; (b) sperm count from the seminal vesicles of each age cohort. Age is represented by days since eclosure; light grey points show individual data points, black filled points are the mean and bars are the standard error of the mean
Linking Male Sexual Maturity to Gland Development
The total sperm per male increased significantly with male age (F2,25 = 92.6, P < 0.0001) and each age cohort differed from each other (Fig. 2b). We found that the seminal vesicles of 1 d-old adult males contained few sperm; only a single individual contained any. Sperm counts increased in 7 d-old males and contained an average of 55,333 ± 9872 sperm per male, whereas 14 day-old males contained 101,125 ± 21,453 sperm per male.
GC-MS Analysis
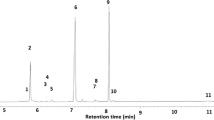
We found that the diversity and concentration of chemicals in the male DAG increased with age (1d vs. 7d: T = −3.90, df = 128, p = 0.0005) (1d vs. 14d: T = −4.87, df = 128, p < 0.0001) (7d vs. 14d: T = −1.49, df = 128, p = 0.30). The DAG of newly eclosed males was dominated by just 2 compounds; we found trace amounts of (E)-2-hexenal in all individuals and some males also contained tridecane (Fig. 3, Table 1). Older males had a more diverse array chemicals than younger males, and we observed nine different compounds. Six of those compounds in the mature DAG have been previously described as (E)-2-hexenal, benzyl alcohol, linalool, terpinen-4-ol, α-terpineol and tridecane (Table 1). The remaining three compounds remain to be identified definitively, however the mass spectra are consistent with monoterpenes (Table 1).
Representative gas chromatograms of the content of the dorsal abdominal glands (DAG) for each age cohort of P. maculiventris: (a) 1-day old, (b) 7 day-old, (c) 14 day-old; 10.36 min internal standard (tetralin), 5.12 min (E)-2-hexenal, 7.81 min benzyl alcohol, 8.12 min linalool, 9.14 min terpinen-4-ol, 10.87 min α-terpineol, 12.32 min tridecane; 7.99 min, 8.20 min, 8.23 min, 8.69 min unknown. Note the y axis scale differences
Frequency of Semiochemical Release
The study on how male age affected the number of releases from the DAG over the course of a day revealed a strong effect of male age (T = 6.57, df = 4, P = 0.003). We did not detect any chemical releases from the DAG during the test period when newly eclosed males were paired together. In contrast, we detected an average of 3.95 releases per hr from pairs of 7–14 day old males over the 24 h observation period. There was a strong diurnal pattern in releases from these pairs of older males (T = -7.31, df = 89, P<0.001) (Fig. 4), with 8.79 releases per hour during scotophase (Fig. 5), compared to 2.02 releases during photophase (Fig. 5). There were significantly fewer releases of semiochemicals from pairs of females (1.25 releases per 24 h) compared to male-female pairings (30.6 releases per 24 h; FF-MF: T = -3.99, df = 17, P = 0.003; FF-MM: T = -4.53, df = 17, P <0.001; Fig. 6). We did not find any evidence that the number of DAG releases from pairs of bugs was influenced by the precence of a female (MM/MF comparison: T= 0.643, df = 14, P = 0.799), and we detected similar numbers of DAG releases from pairs of males and mixed male-female pairs (Fig. 6).
Mean number of releases per hr for the old and young P. maculiventris male cohorts with standard error bars. Each dot represents the mean total number of sensor deflections (>0.02%) for a replicate male bug. The left panel is data collected when the lights were off, and the right is when the lights were on. Bars represent standard error of the mean
We detected significantly fewer releases from pairs of females (1.25 releases per 24 h) compared to male-female pairings (30.6 releases per 24 h; FF-MF: T = −3.99, df = 17, p = 0.003; FF-MM: T = −4.53, df = 17, p = 0.0008; Fig. 6). We did not find any evidence that the number of DAG releases from pairs of bugs was influenced by the presence of a female (MM/MF comparison: T = 0.643, df = 14, p = 0.799), and we detected similar numbers of DAG releases from pairs of males and mixed male-female pairs (Fig. 6).
Discussion
In this study we found that as male P. maculiventris mature their DAG increases in size, and the diversity and concentration of chemicals in the DAG also increased. These changes occurred in tandem with male sexual maturation. The glands of 1 d-old adult males contained only two main compounds and were nearly devoid of the compounds that dominated the profile of the older, more mature males that we analyzed. The chemical composition of the DAGs of 7 d-old adults contained the previously documented compounds presented in Aldrich et al. (1984b), and at 14 d-post eclosion we found the same suite of compounds, but they were more abundant. The gland development coincided with our proxy for sexual maturation, with the young age cohort containing only trace amounts of sperm in one individual, and 7 and 14 day adults having 55 thousand and 110 thousand sperm per male, respectively.
The metal-oxide SemioSensor VOC detector was used to monitor release of semiochemicals over time. Although not able to distinguish specific semiochemicals, this device records total VOC every second to give a much higher temporal resolution than any other method and can be operated continuously through day and night periods. The sensor detected the greatest number of VOC emissions from the DAG of male P. maculiventris in the late evening to early morning (20:00-6:00h). Previous research on the timing of volatile emissions from the DAG were largely restricted to day-time releases (Aldrich et al. 1984b). Emissions of semiochemicals can occur during the day and for some males no releases were detected. Newly-eclosed males, with their underdeveloped glands, do not release volatiles (Aldrich et al. 1984b). This suggests that at least a subset of releases are used in either mate-calling or sex recognition. The delayed release of semiochemicals in non-sexually mature males is similar to what has been shown in another Pentatomid bug, brown marmorated stink bug, Halyomorpha halys, where adults only begin emitting semiochemical after approximately 13 days (Harris et al. 2015). Another Pentatomid, Piezodorus hybneri, is known to not release semiochemical until sexually mature (Endo et al. 2007). Additional work is needed to confirm which specific compounds are being released in the evening and that they come from the DAG.
Releases of semiochemicals and attraction to semiochemicals during the scotophase has been shown in other Pentatomids (Krupke et al. 2006). For example, H. halys releases the aliphatic alkane defense compound, tridecane, in greater amounts in the evening and night (Harris et al. 2015). Interestingly, knowing that diurnal prey detect and respond to DAG semiochemicals, a scotophase release pattern may help P. maculiventris evade detection by its prey. The night-time release may also have additional benefits such as evading detection of its own predators and parasitoids, and releasing at a time when the air is cooler and more humid, allowing the compounds to remain in the environment at a higher concentration than when it is hot and dry).
These findings provide additional insights into the ontogenetic changes in male stink bugs and the timing of release from the DAG, which is the first described aggregation pheromone in true bugs (Aldrich et al. 1978). Aldrich et al. conducted a field trial using a synthetic chemical blend composed of the most abundant compounds in the male DAG, (E)-2-hexenal, α-terpineol, linalool, terpinene-4-01, and benzyl alcohol (Aldrich et al. 1984a). They reported that the blend was attractive to both males and females, and they confirmed that these chemicals function as a long-range aggregation pheromone. Learning that the development of the DAG semiochemical coincides with sexual maturation in males provides support for the hypothesis that the gland is used to attract mates and as an aggregation pheromone. Although both males and females are attracted to the pheromone, males that are attracted may be exploiting the signal of conspecifics in the hopes of finding a mate.
Interestingly, 1 d-old adults were the only age cohort that we found to contain the alkane tridecane. This compound is found in immature stink bug DAG’s and may be residual material left over from the nymphal stages (Aldrich et al. 1984a, b). Tridecane has been described as an important semiochemical for another Pentatomid, Nezara viridula, where the nymphal stages release tridecane as an aggregation pheromone to remain clustered (Lockwood and Story 1985). The amount of tridecane increases as individuals mature through the five nymphal stadia and higher volumes of the compound in the air elicits anti-aggregation behavior, causing the later nymphal stages to disperse. Others have found that tridecane, in addition to (E)-2-hexenal, are effective repellents of other arthropods. The Pentatomid Coridius janus, releases a defense secretion containing the bioactive components of tridecane and (E)-2-hexenal, which repelled 3 herbivorous insects (Gunawardena and Herath 1991). The presence of tridecane in the newly eclosed males in our study might provide a level of protection from other insects while they are callow and quite fragile, however more research is needed to confirm that it is actually released by newly eclosed adults before this hypothesis can be substantiated.
From an applied perspective, gaining a more complete understanding of the effects of the DAG semiochemical on crop pests will allow growers to better leverage the volatile cue for plant protection against herbivores. Hermann and Thaler (2014) showed that the presence of adult male P. maculiventris contained in breathable bags changed L. decemlineata behavior; beetles exposed to stink bug semiochemicals in the field reduced the rate of potato leaf consumption. However, the long-term use of live predators as a semiochemical source can elicit a variable response by L. decemlineata over and across seasons (Aflitto and Thaler 2020). The use of a synthetic DAG mixture in release devices, which provide a more consistent release of the semiochemical, has been shown to produce a more reliable response, causing L. decemlineata to avoid plots containing DAG emitters and reduce plant damage (Aflitto and Thaler 2020). In 2020, a synthetic blend of the male DAG was approved as an organic crop treatment to reduce L. decemlineata plant damage (Aflitto and Thaler 2020). This difference between the response of potato beetles to live stink bugs and synthetic semiochemical emitters raises questions about the consistency of cue release from the live stink bugs and the conditions that elicit their release. Or results indicate that in order to maximize the olfactory-induced non-consumptive effects of P. maculiventris, male adults that are at least 7 days past exclusion should be used.
References
Aflitto NC, Thaler JS (2020) Predator pheromone elicits a temporally dependent non-consumptive effect in prey. Ecol Entomol 1–10. https://doi.org/10.1111/een.12905
Aflitto NC, Thaler JS (2021) Shared semiochemicals in a tri-trophic system benefit crop plants. J Chem Ecol PREPRINT:
Aldrich JR (1985) Pheromone of a true bug (Hemiptera- Heteroptera): attractant for the predator, Podisus Maculiventris, and Kairomonal effects. Walter de Gruyter & Co., Berlin-New York
Aldrich JR (1988) Chemistry and biological activity of pentatomid sex pheromones. Biol Active Nat Prod 417–431. https://doi.org/10.1021/bk-1988-0380.ch028
Aldrich JR (1995) Chemical communication in the true bugs and parasitoid exploitation. Chem Ecol Insects 2:318–363. https://doi.org/10.1007/978-1-4615-1765-8_9
Aldrich JA, Cantelo W (1999) Suppression of Colorado potato beetle infestation by pheromone-mediated augmentation of the predatory spined soldier bug, Podisus maculiventris (say) (Heteroptera: Pentatomidae). Agric For Entomol 1:209–217. https://doi.org/10.1046/j.1461-9563.1999.00026.x
Aldrich JA, Blum MS, Lloyd H, Fales HM (1978) Pentatomid natural products. J Chem Ecol 4:161–172
Aldrich JR, Kochansky JP, Lusby WR, Sexton JD (1984a) Semiochemicals from a predaceous stink bug, Podisus maculiventris (Hemiptera: Pentatomidae). J Washington Acad Sci 74:39–46
Aldrich JR, Kochansky J, Abrams C (1984b) Attractant for a beneficial insect and its parasitoids: pheromone of the predatory spined soldier bug, Podisus maculiventris (Hemiptera: Pentatomidae). Environ Entomol 13:1031–1036
Bate NJ, Rothstein SJ (1998) C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J 16:561–569
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Biever KD, Chauvin RL (1992) Suppression of the Colorado potato beetle ( Coleoptera : Chrysomelidae ) with augmentative releases of predaceous stink bugs ( Hemiptera : Pentatomidae ). J Econ Entomol 85:720–726
Cribb BW, Siriwardana KN, Walter GH (2006) Unicellular pheromone glands of the Pentatomid bug Nezara viridula (Heteroptera: Insecta): Ultrastructure,Classification, and proposed function. J Morphol 267:831–840. https://doi.org/10.1002/jmor
Core Team, R (2014) R: A language and environment for statistical computing. R Foundation for Statistical Com, Vienna
De Clercq P, Peeters I, Vergauwe G, Thas O (2003) Interaction between Podisus maculiventris and Harmonia axyridis, two predators used in augmentative biological control in greenhouse crops. BioControl 48:39–55. https://doi.org/10.1023/A:1021219714684
Endo N, Yasuda T, Matsukura K et al (2007) Possible function of Piezodorus hybneri (Heteroptera: Pentatomidae) male pheromone: effects of adult age and diapause on sexual maturity and pheromone production. Appl Entomol Zool 42:637–641. https://doi.org/10.1303/aez.2007.637
Farag MA, Pare PW (2002) C6-Green leaf volatiles trigger local and systemic VOCemissions in tomato. Phytochemistry 61:545–554
Gunawardena HM, Herath KB (1991) Significance of medium chain n-alkanes as accompanying compounds in hemipteran defensive secretions: an investigation based on the defensive secretion of Coridius janus. J Chem Ecol 17:2449–2458
Harris C, Abubeker S, Yu M et al (2015) Semiochemical production and laboratory behavior response of the brown marmorated stink bug, Halyomorpha Halys. PLoS One 10:. https://doi.org/10.1371/journal.pone.0140876
Hermann SL, Thaler JS (2014) Prey perception of predation risk: volatile chemical cues mediate non-consumptive effects of a predator on a herbivorous insect. Oecologia 176:669–676. https://doi.org/10.1007/s00442-014-3069-5
Hough-Goldstein J, McPherson D (1996) Comparison of Perillus bioculatus and Podisus maculiventris (Hemiptera: Pentatomidae) as potential control agents of the Colorado potato beetle (Coleoptera: Chrysomelidae). J Econ Entomol 89:1116–1123. https://doi.org/10.1093/jee/89.5.1116
Krupke CH, Jones VP, Brunner JF (2006) Diel periodicity of Euschistus conspersus (Heteroptera: Pentatomidae) aggregation, mating, and feeding. Ann Entomol Soc Am 99:169–174. https://doi.org/10.1603/0013-8746(2006)099[0169:DPOECH]2.0.CO;2
Legaspi JC, Legaspi BC (2007) Bioclimatic model of the spined soldier bug (Heteroptera: Pentatomidae) using CLIMEX: testing model predictions at two spatial scales. J Entomol Sci 42:533–547. https://doi.org/10.18474/0749-8004-42.4.533
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. https://doi.org/10.1139/z90-092
Lockwood JA, Story RN (1985) Bifunctional pheromone in the first instar of the southern green stink bug, Nezara viridula (L.) (Hemiptera: Pentatornidae): its characterization and interaction with other stimuli. Ann Entomol Soc Am 78:474–479. https://doi.org/10.1093/aesa/78.4.474
Mcbrien HL, Millar JG, Gottlieb L et al (2001) Male-produced sex attractant pheromone of the green stink bug, Acrosternum hilare (say). J Chem Ecol 27:1821–1839. https://doi.org/10.1023/A:1010460709535
Polis GA, Strong D (1996) Food web complexity and community dynamics. Am Soc Nat 147:813–846
Sant’Ana J, Bruni R, Abdul-Baki AA, Aldrich JR (1997) Pheromone-induced movement of nymphs of the predator, Podisus maculiventris (Heteroptera: Pentatomidae). Biol Control 10:123–128. https://doi.org/10.1006/bcon.1997.0549
Sih A (1980) Optimal behavior : can foragers balance two conflicting demands? Sci New Ser 210:1041–1043. https://doi.org/10.1126/science.210.4473.1041
Tipping PW, Holko CA, Abdul-Baki AA, Aldrich JR (1999) Evaluating Edovum puttleri Grissell and Podisus maculiventris (say) for augmentative biological control of Colorado potato beetle in tomatoes. Biol Control 16:35–42
Werner EE, Peacor SD (2003) A review of trait-mediated indirect interactions in ecological communities. Spec Featur Ecol 84:1083–1100
Weissburg M, Smee DL, Ferner MC (2014) The sensory ecology of nonconsumptive predator effects. The American Naturalist 184(2):141–157
Acknowledgments
We are grateful for the director of the NMR Facility at Cornell University, Ivan Keresztes for his assistance with the GC-MS analysis. Thanks to Ari Grele and Tait Stevenson for their help with dissections.
Funding
This project was funded by the NSF Graduate Research Fellowship Program (GRFP) (DGE-1650441) awarded to N. Aflitto.
Author information
Authors and Affiliations
Contributions
N.A., A.D., T.U., and J.T. conceived and planned the experiments. N.A., A.D., T.U. carried out the experiments. N.A. took the lead in writing the manuscript. All authors provided feedback, comments, and edits.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aflitto, N.C., Dittmar, A.G., Ugine, T.A. et al. Semiochemical Release and Ontogenetic Changes in a Primary Scent Gland of Podisus maculiventris. J Chem Ecol 49, 428–436 (2023). https://doi.org/10.1007/s10886-023-01411-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-023-01411-8