Abstract
Khasi mandarin is one of the most remunerative crop in North Eastern Hills region of India playing a very critical role in the socio-economic upliftment of the people. The orchards of this region were found to express the typical symptoms of citrus tristeza virus (CTV). An extensive survey of the Khasi mandarin orchard for six North Eastern states of India namely Arunachal Pradesh, Meghalaya, Assam, Nagaland, Sikkim and Tripura, was carried out to establish the identity of these viruses using ELISA and PCR/RT-PCR techniques. Out of 300 Khasi mandarin tree samples collected from the six states, 172 were found to be positive for CTV infection by DAS-ELISA indicating 57.33% overall CTV disease incidence. Results revealed presence of CTV in all the surveyed states showing a maximum incidence of 66.00% in Arunachal Pradesh followed by 62.00% in Assam, 60.00% in Meghalaya and Nagaland, 54.00% in Sikkim and 42.33% in Tripura. Higher CTV concentration was recorded in the age group > 15 years (69.09%) followed by 10–15 (57%) and 5–10 years (43.33%). However, this study, to the best of our knowledge, is the first report for the detection of CTV in Khasi mandarin from Arunachal Pradesh, Nagaland and Tripura and also the first authentic survey of overall disease incidence of CTV Khasi mandarins from the six major mandarin growing North Eastern states of India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus is considered to be one of the most remunerative fruit crops of India, having a lasting niche in the international trade and world finance. The North Eastern part of India is recognized as one of the primary gene centre or natural home [1, 10, 19] and reservoir of numerous Citrus species including mandarin orange [14, 21]. Nearly 1600 ha scattered over nine states namely Assam, Arunachal Pradesh, Meghalaya, Manipur, Mizoram, Nagaland, Tripura, Sikkim and the Darjeeling district of West Bengal are under mandarin orange cultivation, where this high value crop contributes significantly to the small farm economy [12].
In India diseases caused by virus and virus-like pathogens remain to be one of the most potential threats to commercial citrus industry. Owing to the problem of citrus decline, the average yield of orange in India is very low (11.6 MT/HA) as compared to other countries like USA (32.6 MT/HA), Brazil (24.7 MT/HA) and China (13.7 MT/HA). The economic importance of these pathogens derives largely from their ability to cause systemic diseases and to persist in the vegetative parts of the plant for as long as these remain alive. Hence losses are not confined to the season in which infection occurs, but continue as long as the infected plants are in culture. Approximately, sixteen pathogens are known to infect citrus in India, among which, citrus tristeza virus (CTV), Indian citrus ringspot virus (ICRSV), citrus yellow mosaic virus (CiYMV), citrus exocortis viroid and citrus greening bacterium are of serious concern [11]. Among these viruses, CTV is most devastating and play a significant role in causing citrus decline in India [4, 22, 24].
Moreover, mandarins of North Eastern region which is generally known as Khasi mandarin, also known as Sohniamptra in Khasi, Humoptira or Komola in Assamese, Komla in Bengali and Manipuri languages [20]. In this part of India Khasi mandarin have got less attention towards the infecting viruses and very limited information as well as experimental data is available for the incidence and spread of these viruses. Only three reports for CTV in Khasi Mandarin out of which one from Sikkim [16] and two reports from Assam and Meghalaya [4, 6] for the entire northeast region of India which are not sufficient for the true representation of viral incidence of CTV for whole North Eastern states of India.
The conventional biodiagnosis has its own limitation due to it’s less reliably and time consuming. Moreover, biodiagnosis is not suitable for survey because the sample size during survey is usually very large and almost impossible to handle with this method. On the other hand, the protein based enzyme linked immuno-sorbent assay (ELISA) using pathogen specific polyclonal antibodies has revolutionized the detection test, making it feasible to test large number of samples [7, 16]. Now a days, nucleic acid based detection by PCR/RT-PCR techniques are being used for the reliable and rapid diagnosis of citrus virus [4, 9].
Keeping this background into account, an extensive survey of the Khasi mandarin orchard for six North Eastern states of India was carried out with the objectives of establishing the identity of these viruses by using ELISA and PCR/RT-PCR techniques to enumerate the viral disease incidence of CTV in Khasi mandarins. However, present studies contain first authentic report of overall disease incidence of CTV in Khasi mandarins from major mandarin growing areas of North Eastern states of India.
Materials and methods
Survey of citrus orchards, collection of samples and detection of viruses
Survey and investigation was conducted during 2015 for six states viz., Arunachal Pradesh, Meghalaya (From Khasi Hills Division), Assam, Nagaland, Sikkim and Tripura which are considered as major Khasi mandarin growing North Eastern states of India. Three locations from each state were surveyed for CTV, infected plants. Suspected CTV infected samples from Khasi mandarin (Citrus reticulata Blanco) were collected from both commercial and home stead gardens of mandarin orange trees on the basis of visual observation from the age group of 5–10, 11–15 and above 15 years trees. One twig from each of the selected trees showing symptoms of leaf yellowing and vein clearing for CTV [5] were collected for testing of probable CTV infection and further study. Altogether three hundred plant samples of mandarin orange trees were collected from 18 locations during the field survey in the year 2015 (Supplementary Table 1). The characteristic symptoms of CTV viz., leaf yellowing and vein clearing were observed in most of the surveyed samples and categorised on a three-scale rating as severe (+++), moderate (++) and mild (+) (Supplementary Table 1).
Enzyme linked immunosorbent assay (ELISA)
DAS ELISA was performed using polyclonal antisera for CTV [8] using ELISA Kit procured from Agdia (USA). About 200–300 mg new leaves and tender bark tissue of citrus twigs were taken in pestle and mortar and ground to fine powder with liquid nitrogen and homogenized in extraction buffer at ratio of 1:10 (w/v) for virus antigen preparation. Coating buffer was used to dilute capture antibody in the ratio of 1:100 and dispensed 100 μl in each well of ELISA plate and incubate overnight at 4 °C in humid box. The wells were emptied and carefully washed three times for 3 min each with 100 μl wash buffer in each well every time to remove unbound capture antibody. Leaf extract was added and incubated for 2 h at 37 °C by wrapping in humid box. The wells were then carefully washed three times for 3 min each with 100 μl wash buffer in each well every time to remove tissue debris of the leaves followed by addition of 100 μl alkaline phosphatase conjugated antibody and the detection antibody diluted in the conjugate buffer in the ratio of 1:100 to each well and the plate was incubated for 2 h at 37 °C. After 2 h plate was again washed three times for 3 min each with 100 μl wash buffer in each well every time to remove unbound conjugate antibody. Plate was dried after the last wash, and then 100 μl of para-nitrophenol (PNP) prepared with 0.5 mg/ml in the substrate buffer were added to each well. The CTV virus was detected by the appearance of yellow colour in the particular well. The reaction was stopped by adding 50 μl of 3 M NaOH in each well and the absorbance was measured at 405 nm in ELISA plate reader (Bio Rad) using 405 nm wavelength after 15–30 min of addition of substrate. The absorbance value found three times higher as compared to healthy tissues was considered as CTV positive [17]. Percentage of disease incidence was calculated using standard method: number of samples infected divided by number of samples tested and multiplied by 100.
RNA isolation, first strand synthesis and RT-PCR amplification for CTV
Total RNA was isolated from the mandarin leaves and bark sample using RNeasy® Plant Mini Kit (Qiagen Inc., Valencia CA, USA) following the manufacturer’s protocol.
First-strand cDNA synthesis was done with the help of (M-MLV RT) enzyme (Promega Corp. Madison, WI, USA) following the manufacturer’s protocol. The PCR amplification was performed in 50 μl of reaction mixture containing five microliters of cDNA, 1 μl (5 u/μl) Taq DNA polymerase (Xcelris genomics, Ahmedabad, India), 5 μl 10 × PCR (250 mM KCl, 250 mM Tris–HCl (pH 9.0 at 25 °C), 2% Triton X 100 and 25 mM MgCl2) buffer, 0.5 μl of (25 mM MgCl2), 1 μl dNTPs (2.5 mM each of dATP, dCTP, dGTP and dTTP), 1 μl of (10 μM) coat protein gene specific forward primer KLM543: 5′-CTCTAGATCTTTTGAATTATGGACGAC-3′, reverse primer KLM544: 5′-CGCGAATTCAACAGATCAACGTGTGT-3′ [3] and 35.5 μl nuclease free water were added. PCR was performed using Veriti® 96-well Thermal Cycler (Applied Biosystems, USA). The thermal cycling profile for PCR was kept same as described by Biswas [3].
All the PCR products were analysed on 1% agarose gel at 80 V. 100 bp DNA ladder (Xcelris genomics, Ahmedabad, India) was used as the molecular weight standard. The gels were stained in ethidium bromide (0.5 µg/ml). The gels were photographed under a Gel Documentation system.
Results and discussion
Surveyed of citrus orchards by ELISA
Infection of CTV in citrus orchards surveyed from the 18 locations of 6 states of north east hill region in the present study was detected through ELISA and RT-PCR. The data from DAS-ELISA using polyclonal antisera to CTV revealed that sample from all the mandarin orchards collected were found to be infected by CTV, as the randomly collected samples showed positive reaction in DAS-ELISA (Supplementary Table 2).
Surveyed of citrus orchards through ELISA for CTV
The survey results indicated that the incidence of CTV were severe in the higher age group (> 15 years) whereas it was in moderate to mild form in lower age group (10–15 and 5–10 years) (Supplementary Table 1). The OD values of the samples at 405 nm varied depending upon samples. This variation may be due to different concentration of virus titer.
Out of 300 Khasi mandarin trees samples collected from Arunachal Pradesh, Meghalaya, Assam, Nagaland, Sikkim and Tripura, 172 trees were found to be positive for the CTV infection by DAS-ELISA (Supplementary Table 1) indicating 57.33% overall CTV disease incidence in these six North Eastern states. Results revealed presence of CTV in all the surveyed states of NE Region showing a high incidence of 66.00% in Arunachal Pradesh followed by 62.00% in Assam, 60.00% in Meghalaya and Nagaland, 54.00% in Sikkim and 42.33% in Tripura (Supplementary Table 1, Fig. 1a).
For Arunachal Pradesh 80.00–71.42% incidence was recorded in orchards located at Renging; 83.33–50.00% in Bodak village of East Siang district and 66.66–40.00% in Basar district (Supplementary Table 1). The age wise average infection was recorded maximum in the age group > 15 years (75%) followed by 10–15 (62.50%) and 5–10 years (57.14%) old plants (Fig. 1a). Whereas location wise average infection was recorded 75, 66.66 and 53.33% for Renging village, Bodak village of east Siang district and Basar district, respectively (Supplementary Table 1, Fig. 1b).
For Meghalaya 71.42–40.00% incidence was recorded in orchards located at Umsning of Ri Bhoi District; 80.00–40.00% in Barapani and 80.00–50.00% in Cherrapunjee of East Khasi Hills district (Supplementary Table 1). The age wise average infection was recorded maximum in the age group > 15 years (76.40%) followed by 10–15 (57.89%) and 5–10 years (42.85%) old plants (Fig. 1a). Whereas location wise avarage infection was recorded 55, 60 and 66.66% for Umsning of Ri Bhoi District, Barapani and Cherrapunjee of East Khasi Hills district respectively (Supplementary Table 1, Fig. 1b).
For Assam 83.33–40.00% incidence was recorded in orchards of Tinsukia District; 80.00–50.00% in Lakhimpur district and 62.50–57.14% in Jorhat district (Supplementary Table 1). The age wise average infection was recorded maximum in the age group > 15 years (72.22%) followed by 10–15 (61.11%) and 5–10 years (50%) old plants (Fig. 1a). Whereas location wise average infection was recorded 60, 60 and 66.66% for Jorhat District, Tinsukia District and Lakhimpur district respectively (Supplementary Table 1, Fig. 1b).
For Nagaland 80.00–50.00% incidence was recorded in orchards of Bade village of Dimapur District; 60.00–40.00% in Ralan village of Wokha District and 85.71–25.00% in Kohima District (Supplementary Table 1). The age wise average infection was recorded maximum in the age group > 15 years (68.42%) followed by 10–15 (58.82%) and 5–10 years (50%) old plants (Fig. 1a). Whereas location wise average infection was recorded 60, 53.33 and 66.66% for Bade village of Dimapur District, Ralan village of Wokha District and Kohima district respectively (Supplementary Table 1, Fig. 1b).
For Sikkim 62.50–37.50% incidence was recorded in orchards of Khamdong village of East Sikkim; 66.66–40.00% in Ligmoo at South Sikkim and 80.00–50.00% in Timbong at North Sikkim (Supplementary Table 1). The plants in the age group > 15 years recorded the maximum average infection (68.42%) followed by the plants in the age group 10–15 (50%) and 5–10 years (41.17%) (Fig. 1a). Whereas location wise average infection was recorded 50, 53.33 and 60% for Khamdong village of East Sikkim, Ligmoo at South Sikkim and Timbong at North Sikkim, respectively (Supplementary Table 1, Fig. 1b).
For Tripura 50.00–12.50% incidence was recorded in orchards of Amarpur at South Tripura; 80.00–40.00% in Longthorai Valley of Dhalai District and 50.00–25.00% in Knachanpur at North Tripura (Supplementary Table 1). The age wise average infection was recorded maximum in the age group > 15 years (52.94%) followed by 10–15 (50%) and 5–10 years (23.52%) old plants (Fig. 1a). Whereas location wise average infection was recorded 30, 60 and 40% for Amarpur at South Tripura, Longthorai Valley of Dhalai District and Kanchanpur at North Tripura (Supplementary Table 1, Fig. 1b).
However, a low average OD value and the low rate of infection in DAS-ELISA assay was recorded in the mandarin plants of Sikkim and Tripura which may due to some geographical isolation in hilly track or escape from vector feeding which corroborate the findings of Ghosh et al. [10] while working with CTV at Darjeeling Hills. Higher CTV concentration was recorded in the age group > 15 years followed by 10–15 and 5–10 years (Supplementary Table 1). The results also revealed that percentage of CTV infection was more in the higher age groups (Fig. 1a). CTV incidence was highest in > 15 year-old Khasi mandarin of Meghalaya followed by Arunachal Pradesh, Assam, Tripura, Nagaland and Sikkim (Supplementary Table 1, Fig. 1a).
In our study, the presence of a higher concentration of CTV was recorded in the older mandarin plants, which might be due to the increase of CTV concentration with age of the plant and multiple inoculations by aphids in older plants and this finding is further substantiated by the report of Ghosh et al. [13]. The occurrence of CTV was reported in some areas of Sikkim [16] and two reports from Assam and Meghalaya [4, 6]. Earlier work in this area was focused on the symptomatology, survey and characterization of the CTV. In the present study, more Khasi mandarin growing areas in North Eastern states were investigated which provided definite evidence of CTV spread in these states. CTV infection was recorded for the first time in Khasi mandarin from Arunachal Pradesh, Nagaland and Tripura states of North Eastern hills region according to our knowledge. Therefore, we can conclude from our findings that CTV distribution is widespread in the mandarin-growing areas of North Eastern hill region.
Among the various possible ways to detect the plant viruses, ELISA is a cost effective widely used method for the fast detection of CTV in infected plants in all the citrus growing countries [15]. The technique utilizes the ability of antibodies raised in animals to recognize proteins, usually the coat protein, of the virus of interest [26]. It is swift and effective method for the detection of CTV and can be used for a large number of samples at a time. In this study ELISA efficiently detected the CTV from various Khasi mandarin orchards.
Confirmation of CTV by PCR/RT-PCR
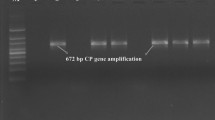
However, ELISA has many limitations which restrict its use for accurate detection of plant virus especially when the virus titer is low or the virus strains are closely related [23]. Therefore, nucleic acid based polymerase chain reaction (PCR/RT-PCR) is a method of choice for indexing of different viruses to overcome this problem [18, 25]. We have doubly confirmed the authenticity of the ELISA test result with the help of RT-PCR by taking 20 random samples from each states having negative and the borderline optical density value (just 3 time of negative control) considered as positive sample and we found the intense DNA band of approximately ~ 672 bp (Fig. 2) for the borderline positive sample and negative results for ELISA negative result. The RT-PCR using specific primes has been reported to be a sensitive technique and reliable technique to detect the CTV in mandarin plants [2, 15].
The present study on the basis of above findings concludes that CTV is the most important and wildly distributed viral disease in all these six states. The above findings conclude CTV is the most important and widely distributed disease in all these six states. Accurate virus indexing methods are necessary for making decision on virus eradication or management programmes. ELISA is effectively detects CTV infection, is a quick, reliable and effective detection method, and can be used for large number of sample at a time. For sake of the precision PCR and RT-PCR are extremely sensitive, requires minimal skill to perform and obviously the best method. These sensitive and effective indexing techniques will subsequently be helpful in the establishment of a sustainable citrus industry in the region. In the present study, more Khasi mandarin growing areas in North Eastern states were investigated which provided definite evidence of CTV spread in these states. CTV infection was recorded in Khasi mandarin from Arunachal Pradesh, Nagaland and Tripura for the first time from this North Eastern hills region.
References
Ahlawat YS. Virus and virus—like diseases affecting citrus in India—a step forward to management of die—back complex. Indian Phytopathol. 2005;3:257–68.
Biswas KK. Molecular diagnosis of Citrus tristeza virus in mandarin (Citrus reticulata) orchards of hills of West Bengal. Indian J Virol. 2008;19:26–31.
Biswas KK. Molecular characterization of Citrus tristeza virus isolates from the North Eastern Himalayan region of India. Arch Virol. 2010;155:959–63.
Biswas KK, Tarafdar A, Sharma SK, Singh JK, Dwivedi S, Biswas K, Jayakumar BK. Current status of Citrus tristeza virus incidence and its spatial distribution in citrus growing geographical zones of India. Indian J Agric Sci. 2014;2:184–9.
Borah M, Nath PD, Saikia AK. Serological detection of Citrus tristeza virus affecting citrus tree species in Assam. Indian Phytopathol. 2012;3:289–93.
Borah M, Nath PD, Saikia AK. Biological and serological techniques for detection of Citrus tristeza virus affecting Citrus species of Assam, India. Afr J Agric Res. 2014;52:3804–10.
Chakraborty NK, Ahlawat YS. Indexing of cross protected and non-cross protected citrus cultivars/species for Citrus tristeza virus by enzyme linked immunosorbent assays. Plant Dis Res. 2001;16:10–6.
Clark MF. Adams AN Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 1977;34:475–83.
Edson B, Aranzazu M, Nieves C, Antonio O, de Ana L, Eduardo V, Jordi PP, Mariano C. Quantitative detection of Citrus tristeza virus in plant tissues and single aphids by real-time RT-PCR. Eur J Plant Pathol. 2008;120:177–88.
Ghosh SP. Citrus fruits. Delhi: Indian Council of Agricultural Research; 2007.
Ghosh DK, Ahlawat YS. Recent advances in developing disease diagnostics and management of major virus and virus-like pathogens infecting citrus in India. XVIII Conference of the IOCV. Citrus Research and Technology, Cordeirópolis, v. 31, Suplemento. 2010; 1–129: 68.
Ghosh SP, Singh RB. Citrus in south Asia. FAO/RAPA Publication No. 1993/24. Bangkok, Thailand. 1993; p. 70.
Ghosh A, Das A, Pun KB, Kumar R, Meena R, Baranwal VK. Present status of Citrus tristeza virus infecting Citrus spp. in Darjeeling hills and its detection in different plant parts. Phytoparasitica. 2014;42:381–6.
Hazarika TK. Citrus genetic diversity of northeast India, their distribution ecogeography and ecobiology. Genet Resour Crop Evol. 2012;59:1267–80.
Hilf ME, Garnsey SM. Citrus tristeza virus in Florida: a synthesis of historical, and contemporary biological, serological and genetic data. In: Proceedings of 15th Conference of International Organ Citrus Virology, IOCV, Riverside, CA; 2002. p. 13–20.
Kishore K, Rahman H, Kalita H, Pandey B, Minika N. Prevalence of Citrus tristeza virus in mandarin of Sikkim Himalayan Region. Indian J Virol. 2010;21:140–3.
Lbida B, Bennani A, Serrhini MN, Zemzami M. Biological, serological and molecular characterization of three isolates of Citrus tristeza clostero virus introduced into Morocco. EPPO Bull. 2005;35:511–7.
Ram R, Verma N, Singh AK, Singh L, Hallan V, Zaidi AA. Indexing and production of virus-free chrysanthemums. Biol Plant. 2005;49:149–52.
Ray BK, Deka PC. Numerical taxonomic study of different mandarin oranges using morphological characters. Indian J Genet Plant Breed. 2000;60:227–32.
Singh VB. Fruits of N.E. region. New Delhi: Wiley; 1990.
Singh HP, Chadha KL. Genetic resources of Citrus. In: Chadha KL, Pareek OP, editors. Advances in horticulture: fruit crops, vol. 1., Malhotra Publishing HouseIndia: New Delhi; 1993. p. 95–121.
Singh JK, Tarafdar A, Sharma SK, Biswas KK. Evidence of recombinant Citrus tristeza virus isolate occurring in acid lime cv. Pant lemon orchard in Uttarakhand Terai region of northern Himalaya in India. Indian J Virol. 2013;24(1):35–41.
Steel E, Barker I, Danks C, Coates D, Boonham N. Agrobacterium tumefaciens mediated transient expression as a tool for antigen production for Cucurbit yellow stunting disorder virus. J Virol Methods. 2010;163:222–8.
Tarafdar A, Godara S, Dwivedi S, Jayakumar BK, Biswas KK. Characterization of Citrus tristeza virus and determination of genetic variability in North-east and South India. Indian Phytopathol. 2013;66(3):302–7.
Verma N, Ram R, Zaidi AA. In vitro production of Prunus necrotic ringspot virus-free begonias through chemo-and thermotherapy. Sci Hortic. 2005;103:239–47.
Webster CG, Wylie SJ, Jones MGK. Diagnosis of plant viral pathogens. Curr Sci. 2004;86:1604–7.
Acknowledgements
Authors acknowledge the laboratory as well as library assistance from BCKV, West Bengal. We further are thankful to the reviewers and the editor of the article for their critical comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, A.K., Meetei, N.T., Singh, B.K. et al. High incidence of citrus tristeza virus in mandarin (Citrus reticulata) in North-East states of India. VirusDis. 28, 401–407 (2017). https://doi.org/10.1007/s13337-017-0411-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-017-0411-7