Abstract
The objective is to compare the consequences of routine visualization (RV) and the application of intermitted (I-IONM), standardized (S-IONM), and continuous monitoring (C-IONM) of recurrent laryngeal nerve (RLN) management. RV includes that 698 RLNs managed solely with visual identification. In a second period 777, RLNs were handled by the I-IONM. The third period 768 RLNs monitoring was performed according to the standards. C-IONM via VN stimulation included 626 RLNs. The following issues were analyzed and compared per each period study: RLN identification rate, branching detection, assessment of NRLN, intraoperative recognizable nerve damage, stage thyroidectomy rate, transient or definitive lesions, bilateral nerve palsy, and recovery time. Significance for nerve identification rate was achieved (p = 0.03) when the statistical analysis was applied between RV vs. S-IONM and C-IONM. Extralaryngeal bifurcation was identified in 21, 44, 43, and 46 of RLN dissected, respectively, per period (p = 0.005). The incidence of paralysis in identified and unidentified RLN was 3.8 % (107/2806) and 82 % (52/63), respectively. Rates of temporary/permanent RLNP were 16.7/1.7, 5/1.1, 4.5/1, and 3.1/0 % per period study, respectively (p = 0.07). Recognizable intraoperatively nerve damage was, respectively, 15, 45, 100, and 100 % for period study (p = 0.03). The recovery of injured nerves was significantly faster in C-IONM group. S-IONM and C-IONM cumulate 40-stage procedures. The standardized technique, guidelines adherences, and C-IONM allowed to (1) increase RLN identification; (2) reduce the severity of injuries in terms of (a) reset bilateral RLNP, (b) faster recovery time, and (c) lower definitive RLNP; (3) gather detection of branching and NRLN; (4) recognize nerve stress; and (5) cumulate stage procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The management of the recurrent laryngeal nerve (RLN) in thyroid surgery has undergone to revision and modifications [1, 2]. Procedures to reduce the rate of RLN palsy (RLNP) have been the subject of investigation [3–21].
Between 1960 and 1980, the authors advocated that the RLN should not be foresee [3–5]. Bergamaschi, Wagner, and Wade indicated that the temporary and permanent RLNP rates were not statistically different whether or not the RLN had been exposed [3–5].
In 1984, Karlan et al. reported 1000 operations with nerve dissection without the occurrence of permanent RLNP [6]. Mattig et al. demonstrated the decreased permanent RLNP rates (from 6 to 0.8 %) after methodical nerve identification [7]. Subsequently, routine meticulous anatomic localization and exposal of RLN have been recommended as the standard of care [8–19].
In the late 1990s, intraoperative neuromonitoring (IONM) has been proposed as an adjunct to the prime visual identification of laryngeal nerves [20, 21]. IONM has been applied for the early functional confirmation of the RLN, assists dissection in the case of intertwining between the branches of the inferior thyroid artery (ITA) or distorted RLN, nerve branching detection, and non-RLN (NRLN) assessment, assists in the completeness of total thyroidectomy, and detects and elucidates the mechanism of nerve injury [22].
Furthermore, the IONM technology and technique have been the subject of adjustment. A standardized technique was introduced, which includes vagal nerve (VN) stimulation before (V1) and after (V2) resection [23, 24]. Currently, the technology allows the permanent monitoring of the RLN by the continuous VN stimulation [25].
Hence, five period phases may be outlined for RLN management: (1) thyroidectomies without RLN identification; (2) routine nerve exposure; (3) application of intermitted intraoperative neuromonitoring (I-IONM); (4) appositeness of the standardized IONM (S-IONM); and (5) continuous IONM (C-IONM).
A descriptive audit on the consequences of four different periods of RLN management is the objective of this study. The comparison included the following conducts: (1) thyroidectomy with routine visualization (RV) of RLN, (2) I-IONM, (3) S-IONM, and (4) C-IONM.
Materials and methods
From January 2002 to December 2014, 4143 patients underwent thyroidectomy at the first Division of Surgery, Department of Surgical Sciences and Human Morphology, and University of Insubria (Varese—Como, Italy) for the treatment of various thyroid diseases. The study was confined to 1521 patients treated by the same surgeon (GD), including 318 men and 1203 women, whose ages ranged from 5 to 91 years (mean, 42 years). Exclusion criteria from the statistics were the history of preoperative injury to laryngeal nerves (n.49), intentional resection of the RLN (n.13), laryngeal or tracheal resection (n.9), and pre- or postoperative laryngeal examination not complete (n.26). Exclusion criteria were also the procedures included in the learning curve of devices applied (n.152 for I-IONM, n.25 for S-IONM, and n.50 for C-IONM) and/or IONM accessories failures (n.7) and patients lost in follow-up (n.38) [26]. There were 1348 total thyroidectomies and 173 lobectomies providing 2869 nerves at risk (NAR) for examination (1401 right sided and 1468 left sided).
The study was approved by the local institutional review board, and written informed consent was obtained from each patient before surgery. No financial association exists between the authors and the commercial companies, whose nerve-monitoring systems are presented.
Surgical technique
Period 1. RV Routine identification of the RLN was performed since 1995 in this Division. This period 1 study includes years from 2001 to 2004 when the RLN was identified solely with the visual identification. The operative procedure for nerve identification was similar to that described in 1986 by Harness et al. [27]. The RLN was not identified early in the operative procedure. When dissection proceeded to the area of Berry’s ligament, the inferior laryngeal nerve was identified, where it coursed through the ligament or close to it. The nerve was always identified according to anatomical landmarks. When a large substernal goiter was encountered, the RLN was identified and traced from the recurrent nerve triangle as advocated by Lore [28].
Period 2. Application of I-IONM The routine use of I-IONM begins in 2005. The period 2 includes years 2006–2008. The technique of I-IONM has been described previously [24, 26, 29]. The RLN was identified early as the first step in the operative procedure. Laryngeal nerves were located, mapped, and stimulated in the surgical field by the application of a sterile single-use pulse-generated monopolar stimulator probe (n.8,225,101, Medtronic Xomed) [24, 26, 29]. The probe delivers an electric current that ranged from 3 mA (for nerve identification), 1 mA (confirmation and monitoring), and 0.5 mA (dissection and branching identification) [24, 26, 29]. The identity of an intact nerve is confirmed through a series of audible acoustic signals generated by the machine [24, 26, 29]. In particular, we used the stimulator both prior (identification) and during (monitoring) the neural dissection. Efforts have been used to trace the whole cervical course of the RLN. The ITA was routinely visualized but not sealed until the nerve was exposed and isolated. The surgical procedure provided a caudal to cranial approach. Monitor (NIM2.0, Medtronic, Jacksonville, Florida, USA) was set with a reduced response threshold to identify a small response at 150 µV, stimulation rejection artifact at 2.6 ms, and pulsatile stimulus 100 µs duration at 4 Hz. Endotracheal EMG tube-based surface electrodes systems were applied. Size 5–8.5 internal diameter (ID) endotracheal tubes were used.
Period 3. S-IONM Starting end 2009, we modified the IONM technique, by introducing the standardized monitoring technique. The period includes years from 2010 to 2013. The S-IONM technique includes: (1) VN stimulation before thyroid dissection (V1); (2) RLN stimulation at the initial identification (R1); (3) RLN stimulation at the end of thyroid resection and complete haemostasis (R2); and (4) VN stimulation after complete thyroidectomy and haemostasis (V2) [23]. S-IONM was performed according to the standards of equipment setup, induction, and maintenance anesthesia, and corrected tube-positioning verification tests and EMG definitions described by the International Neurmonitoring Study Group (INMSG) Guidelines [24]. IONM equipment with an audio and graphic monitor documentation was used for the current study (NIM-Response 2.0 or 3.0 System, Medtronic, Jacksonville, Florida, USA). Proper endotracheal EMG tube-based surface electrode systems were verified by direct visualization after neck extension before operation and intraoperatively obtaining the first V1 stimulation value >500 μV [24]. The surgical dissection is always controlled by the early VN stimulation, i.e., V1 is for reference for dissection and RLN identification. Only in the absence of R1 EMG signal, we proceed to dissection. RLN research and confirmation are guided by V1 and R1 EMG signals. At an early stage of operation, VN was routinely stimulated at the level of inferior thyroid pole to ensure normal path of RLN. If there is a negative response from lower position but positive response from upper VN stimulation, it indicates the occurrence of a NRLN, and we localize its separation point and path [30–35]. We always test the RLN with a stimulation level of 1 mA. In the case, two structures run close together (anterior and posterior branches of the RLN, or a small artery and RLN), a false EMG signal can be induced by a shunt stimulus, and we lower the stimulation level to 0.5 mA. From the application of S-IONM, we implemented a strict standard operative procedure and modified our operative procedure according ulteriorly to INMSG Guidelines [24]. In all patients undergoing bilateral operation, we begin with the largest or with the cancerous-suspicious side without dissecting the contralateral side [24, 36, 37]. If the V2 IONM signal is lost at the end of the first side, we stop the procedure after the unilateral lobectomy, even if the RLN and VN are anatomically intact and regardless of malignancy [24, 36, 37].
Period 4. C-IONM C-IONM via VN stimulation was routinely introduced in 2013. This period study includes 2014 year. The technique of C-IONM has been described [38]. The Automatic Periodic Stimulating (APS, Medtronic, Jacksonville, Florida, USA) was used. After connecting the APS electrode with IONM system, baselines for the latency and amplitude of the evoked response were calibrated automatically to serve as control data. Stimulation frequency for C-IONM was set for every second, thus evaluating the RLN and VN constantly. The initial baseline responses should be greater than 500 μV [38–41]. An upper limit threshold for the latency (+10 %) and a lower limit threshold for amplitude (−50 %) were depicted as separate alarm lines [38–41]. Standardized intraoperative management for those NAR that experienced EMG changes (decrease amplitude and increate latency combined) was routinely applied and summarized in Fig. 1. The length of surgical standby was dictated until documented progressive latency reduction, and the amplitude increases up to the initial baseline normative data value obtained at the beginning of surgery.
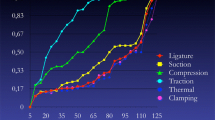
a Standardized intraoperative management for those NAR that experienced EMG changes (decrease amplitude and increate latency combined) was routinely applied in C-IONM period. Surgical maneuvers were modified when adverse electrophysiologic findings were noted. Intraoperative corticosteroids injection indeed. Length of surgical standby was dictated until documented progressive latency reduction, and the amplitude increases up to the initial baseline normative data value obtained at the beginning of surgery (mean 20 min ± 9, range 5–45 min). Six degrees of nerve at risk of injury are described, including type of EMG changes, potential mechanism, and surgical action proposed, and can be summarized in three phases: green (grade 0–1), yellow (2–3), and red (4–6) depending on RLNP prognosis 0, 0, and 7–100 %. b Example of C-IONM with critical RLN traction which developed RLNP
As for dissection techniques, the exclusive of IONM of these alternating changed over the 12 year study period with both ultrasonic and ligasure uses of energy device change.
Indication of surgery
Operative procedures for thyroid cancer, Graves’ disease, and recurrent goiter have higher RLNP rates in many reports [3, 42–45]. Therefore, for each study period, four groups of patients that had been classified according to the underlying diseases were studied: (1) primary benign thyroid disease (overall n = 941); (2) thyroid cancer (n = 293); (3) Graves’ disease (n = 189); or (4) reoperation (n = 98). Patients undergoing a first operation for nodular goiter, adenoma were included in the primary benign thyroid disease category. Individuals diagnosed with thyroid carcinoma from the final histopathology or those who underwent completion thyroidectomy for cancer were grouped with the thyroid cancer cases. Graves’ disease patient with thyroid cancer at the final histology was included in cancer group. Patients who had thyrotoxicosis, thyroiditis were included in the category Graves’ disease. Patients who had previously undergone a thyroid operation were included in the reoperation group.
Outcomes
The following issues were analyzed and compared per each period study:
-
RLN identification rate (measured as the proportion of positive identification of nerve vs. failure of identify);
-
RLN-branching detection (definite as the identification of any branching of the exposed RLN);
-
assessment of NRLN;
-
intraoperative recognizable nerve damage (ratio between the nerve lesions found and those not highlighted during surgery and positive postoperative laryngoscopy);
-
stage thyroidectomy rate (measured as the proportion of surgical procedures completed only on the first side due to V2 EMG signal lost at the end of the first side, although patient was planned for a total bilateral thyroidectomy);
-
transient or definitive RLN lesions (see below);
-
bilateral nerve palsy rate (per patient population);
-
RLNP recovery time (time interval from postoperative VCP to vocal function recovery during follow-up, in days).
Pre- and post-operative follow-up included for all patients directly laryngoscopy to check vocal cord mobility performed at 24 to 48 h before and within the second day postoperative after the surgical procedure by an independent laryngologist [46]. Any reduction in the movement of the cord was recorded as postoperative cord paralysis. For those patients with documented postoperative cord palsy, the repeated examinations were performed periodically, weekly, initially, and every 3–4 weeks thereafter until the recovery was achieved after the operation until full recovery of vocal cord function had been confirmed usually after speech therapy. RLN palsy was defined as permanent when there was no evidence of recovery within 12 months of surgery [46]. Hospital discharge was proposed if no signs of complications were present.
Statistical analysis
The measurement of the rate of RLNP is based on the NAR [47]. All patients’ data were collected in a prospective manner with a dedicated electronic Microsoft Office Access Data Base (Microsoft Corp, Redmond, Wash). Data are collected daily until the day of discharge, and then, there was a regular contact between the study coordinator and the participating specialist (laryngologist, endocrinologist, etc.). The data base is part of our Division’s quality-improvement program. The use of this database for clinical research has been approved by our Institutional review board. Outcome measures were: RLN identification rate; RLN-branching detection; assessment of NRLN; intraoperative recognizable nerve damage ratio; number of stage thyroidectomy; transient or definitive RLN lesions; uni- or bilateral; and RLNP recovery time. The statistical analysis was computed with SPSS, release 15.0 for Windows (SPSS Inc, Chicago-Ill, USA). The level of significance was set at p less than 0.05. All the efforts were made to avoid the sources of bias, such as the loss of individuals to follow-up during the study. The Chi-square test was used for the statistical analysis.
Results
There were no statistical difference between the four period groups study for the distribution of epidemiological characteristics, thyroid pathology, types of operations, the proportion of bilateral procedures, side, mean weight of the thyroid, mean size of dominant nodules, and NAR. A trend to an increased number of procedures per year and tumors is appreciable (not significant, data not shown). The mean hospital stay decreased: period 1 mean 4.3 ± 0.9 days vs. period 4 is 1.5 ± 0.7 days (p < 0.05). The mean operative time decreased: period 1 98.7 ± 29 min vs. period 4 is 71 ± 25 min (p < 0.05). Table 1 summarized the following findings.
Identification of RLN and its anatomical variations
The overall RLN identification rate was 97.8 %, i.e., 63 RLN were not identified during surgery in all the period study. There were no differences in statistics for RLN identification rate for any epidemiological characteristics, body mass index, side, types of operations, the proportion of bilateral procedures, mean weight of the thyroid, and mean size of dominant nodules (data not shown). The stratification of RLN identification rate for thyroid pathology was 99.4 % for primary benign thyroid disease, 97.9 % thyroid cancer, 97.6 % Graves’ disease, and 92.2 % reoperation (p = 0.04 primary benign thyroid disease vs. reoperation). In the first period study, RLN was identified in 93.2 % NAR. In the I-IONM group, the nerve was identified in the 97.9 %cases. In the S-IONM and C-IONM groups, the vagus nerve and the RLN were correctly localized and monitored in all the cases (100 %). The significance for nerve identification rate was achieved (p = 0.03) when the statistical analysis was applied between RV vs. S-IONM and RV vs. C-IONM.
The overall incidence of NRLN was 1.7 % (25/1401) of the right-sided nerves without a preoperative recognition. Combination with ipsilateral RLN and other non-recurrent types was not observed. Overall, the travelling patterns of the NRLNs could be classified as type 1 (45 %) and type 2 (55 %). There were no differences in statistics for NRLN identification rate for any characteristics. A trend of increase detection of NRLN is appreciated per period study: visual 0.8, I-IONM 1.9, S-IONM 2.2, and C-IONM 2.1 %.
An extralaryngeal RLN bifurcation was identified in 39 % overall. At least one extralaryngeal RLN bifurcation was identified in 21, 44, 43, and 46 % of RLN dissected, respectively, per period (p = 0.005). There were no differences in statistics for RLN-branching identification rate for any other characteristics.
RLN lesions
Table 1 summarized the following findings. The identification and preservation of RLN were achieved in 96 % (2699/2806) NAR. The incidence of paralysis in identified and unidentified RLN was 3.8 % (107/2806) and 82 % (52/63), respectively. By excluding patients planned for unilateral procedure, 0.26 % (4/1348) patients developed bilateral cord paralysis. No mortality was observed. Bilateral paralysis developed in patients treated both for benign thyroid disease (n.1), thyroid cancer (n.1), Graves’ disease (n.1), and reoperation groups (n.1). Bilateral cord paralysis occurred in period 1 (n.3) and 2 (n.1). No bilateral vocal cord paralysis occurred during periods 3 and 4. Extensive follow-up revealed temporary bilateral paralysis in two patients, permanent bilateral n.1 patient, and permanent unilateral n.1. One bilateral paralysis required treatment with tracheostomy (period 1), maintained for 2 months, and followed by vocal cord surgery. Others required only conservative treatment with re-intubation for 48 h and subsequent protected extubation with high doses of cortisone.
The overall incidence of RLNP was 5.9 % of NAR, respectively. Because of insufficient numbers of permanent RLN palsies, a statistically significant result was not achieved in any variable analyzed. The temporary RLNP rate was significantly increased for reoperation group compared with the benign thyroid disease (p = 0.02). No permanent RLN injury occurred in C-IONM period. In detail, the overall rates of temporary/permanent RLNP were 16.7/1.7, 5/1.1, 4.5/1, and 3.1/0 % per period study, respectively.
Postoperative laryngoscopy revealed transient palsy in 2/25 (8 %) NRLN. RLNP was more frequently associated with branched than non-branched RLN (13.9 vs. 7.1 %; p = 0.02).
Recognizable nerve damage determined intraoperatively was, respectively, 15, 45, 100, and 100 % for period study. There were no differences in statistics for recognizable nerve damage for any epidemiological characteristics, body mass index, thyroid pathology, side, types of operations, the proportion of bilateral procedures, mean weight of the thyroid, and mean size of dominant nodules (data not shown).
Figure 2 describes the type of EMG changes, potential mechanism, surgical action, and RLNP prognosis according to the established standardized schema proposed for C-IONM.
Recovery time for RLNP stratified per studies period is depicted. Recovery of injured nerves was significantly faster in C-IONM group (p < 0.001). This result should be interpreted with caution. Possible hypothesis is a reduced severity of injuries of RLN in C-IONM group in terms of lower definitive RLNP, early intraoperative injection of corticosteroids, and no further damage to the nerve already stressed
The complete recovery of vocal cord function was documented in 141 of 170 NAR (82 %). The recovery time for RLNP stratified per studies period is depicted in Fig. 2 and ranged from 7 days to 9 months (mean, 35.2 days). The recovery of injured nerves was significantly faster in C-IONM group (p < 0.001). The recovery of RLN took up more than 6 months in two RLNP.
No patient in periods 1 and 2 underwent to stage thyroidectomy procedure. In periods 3 and 4, the identity of the 24 and 16 first-side-injured nerves, respectively, were confirmed through a negative EMG signal generated by the IONM machine by stimulating both the RLN and the vagus nerve (V2 post-thyroid resection stimulation). These patients were scheduled for a total thyroidectomy. The procedures were stopped after the first dominant side lobectomy to avoid the potential for bilateral vocal cord paralysis. Laryngoscopy performed at 48 h post.op confirmed RLNP in 39/40 nerves (97.5 %). All patients underwent completion thyroidectomies within 6 months from the first operation without sequel.
Discussion
The management of the RLN has renovated over time. Definitely is more methodical in both the pre-, intra-, and postoperative phase of surgery. Perfect conscience of nerve anatomy, surgeon training, experience, and high-volume activity in nerve dissection is prerequisite [1, 2, 48]. The importance of the recognition of VC movement by means of routine pre- and postoperative laryngeal examination has been widely accepted [49]. Yet, the treatment of voice changes has been confirmed to be beneficial as well [50]. The intraoperative management of RLN was been positively revised from its non-identification, routine recognition, and exposure, to the application of devices which have the task of confirm its relation with function [19]. Standards of care did change over time, and new approaches and innovations have been applied in thyroid surgery. We are in transition period from the era of visualization to the era of RLN neurophysiology and pathology. As a result, the current modern standards for RLN management consist of an extensive knowledge of dissection, surgeon maturity, routine visual identification and exposition of RLN, and pre- and postoperative laryngoscopy as well as possible intraoperative nerve functional verification. With the current technology, today, it is feasible to monitor intraoperatively (not longer postoperatively) the RLN function.
This descriptive audit evaluates the consequences of different RLN managements. Our hypothesis was that the introduction of new patterns of RLN care has resulted in new and different clinical consequences. Certainly, it is intricate to define the extent of these phase changes due to learning curve and technological changes. Thus, we have deliberately not considered periods of transition between different phases in the current audit. The collection of NAR data was perspective, and all NAR were subjected to methodical follow-up, excepting that the analysis was retrospective, and thus, the results should be interpreted with caution. It is certainly impractical to perform a prospective analysis of different approaches or technologies applied, due to the low patient and surgeon acceptance and the compliance of randomization and the high number of NAR demanded to achieve significance [47].
Therefore, the purpose of this descriptive analysis was to depict ordinarily the consequences of RLN management.
Standardization is useful for the application of a new technique and technology, to verify its accuracy, interoperability, safety, ratification, and repeatability, for training and education, to maintain or obtain better results [51, 52]. According to the present study, the application of a standardized, regulated, uniform technique, the proposed application of methodical VN stimulation, the pertinent proposition and adherence to published INMSG guidelines on use and interpretation of IONM, and the introduction of C-IONM to recognize impending RLN stress allowed to (1) increase the RLN identification rate; (2) reduce the severity of injuries of RLN in terms of (a) reset the bilateral VC paralysis, (b) faster recovery time, and (c) lower definitive RLNP; (3) gather detection of RLN branching and NRLN; (4) recognize intraoperatively nerve damage or stress; and (5) cumulate stage procedures (Table 1; Figs. 1, 2).
The routine application in monitored thyroidectomy of V1 was decisive [23, 24, 47]. V1 is reference for definite nerve identification, confirmation, and dissection, to prevent visual misidentification. De facto, the RLN identification rate was 100 % in S-IONM and C-IONM significantly higher than without VN stimulation (I-IONM and non-IONM). With the reference of V1 signal, Surgeon can identify and confirm the RLN. De facto, IONM, can be performed even if the recurrent laryngeal nerve is not identified by means of V1 as the reference of dissection and RLN identification. The result of this study seems to indicate that standardized IONM either by S-IONM or C-IONM is capable of increasing nerve identification rate. On the right side, V1 must be routinely stimulated at the level of inferior thyroid pole to ensure normal path of RLN [31–35]. The absence of a V1 EMG signal at the early stage is considered suggestive of the presence of a NRLN [31–35]. If there is a negative response from lower position but positive response from upper vagal stimulation, it indicates the occurrence of an NRLN.
It can be presumed that RLN-branching detection is much more a problem of anatomical knowledge rather than of IONM use. According to the present study, a standardized IONM technique significantly enhances the surgeon’s ability to identify branched RLN [53, 54]. Extralaryngeal RLN bifurcation was identified in 45 vs. 21 % of NAR operated with vs. without IONM (p = 0.005). Chiang extensively described the standardized IONM technique of how to identify and handle the anatomical variations of the RLN with the application of IONM [53]. RLN is tested with a stimulation level of 1 mA. In the case, two structures run close and a false EMG signal can be induced by a shunt stimulus [53]. In this context, it seems to be reasonable and adequate to lower the stimulation to 0.5 mA if one wants to differentiate a motor branch from a sensory branch, or a small artery from the RLN [53].
The datum that RLN palsy occurred in 85 % of non-recognized nerves is obvious, but this should simply not happen in thyroid surgery. It is no surprise that the unidentified RLN was at greater risk of damage than a nerve clearly seen, but nevertheless, the reduction in nerve damage rate, as a result of monitoring, was not significantly lower than when identification was purely visual and unaided electronically. However, the increase in recognizable intraoperative nerve damage consequent upon monitoring is potentially valuable and has clinical significance. V2 is essential for recognizing any RLN lesions and predicting nerve postoperative function [24]. Indeed, we attain a significant increase intraoperative identification of lesions not visible. Furthermore, INMSG guidelines [24] suggest if the V2 signal is lost at the end of the first side, to stop the procedure after the unilateral lobectomy, even if the RLN and VN are anatomically intact and regardless of indication of planned total thyroid resection. Several authors demonstrated that a failed V2 stimulation after resection of the first thyroid lobe is specific enough to reconsider the surgical strategy in patients with bilateral thyroid disease to surely prevent bilateral RLN palsy [36, 37]. Failed V2 stimulation in S-IONM and C-IONM period predicts the outcome of VC function during the operation which helped with intraoperative decision making as to whether to consider bilateral thyroid surgery. With this option, we did to reset the bilateral VC paralysis in S- and C-IONM periods. The reset of bilateral paralysis resulted in an increase of the procedures in two times (stage thyroidectomy) that form 0 to almost 7 % of planned total thyroidectomies.
The consistent standardization process is indeed enhanced by the introduction of C-IONM via continuous VN stimulation. C-IONM has utility in identifying real-time adverse concordant amplitude and latency changes (combined events), which can prompt the modification of the associated surgical maneuver and prevent RLN paralysis during thyroidectomy [39–41]. The authors report electrophysiologic parameters as waveform, amplitude decreased, and latency increases combined changes and relates these parameters to intraoperative surgical maneuvers that delineate nascent adverse but reversible electrophysiologic parameters to prevent nerve injury [39–41]. A dedicated scheme for RLN management in adverse situations according to EMG changes was applied during C-IONM thyroid surgery (Fig. 1). Surgical maneuvers were modified when adverse electrophysiologic findings were noted. Intraoperative corticosteroids injection indeed. Wang revealed that a dose of intraoperative corticosteroids did shorten the recovery time for patients suffering from temporary RLN palsy [55]. Six degrees of nerve at risk of injury are described, including type of EMG changes, potential mechanism, and surgical action proposed, and can be summarized in three phases: green (grade 0–1), yellow (2–3), and red (4–6) depending on RLNP prognosis 0, 0, and 7–100 %. According to our analysis, C-IONM reduced the severity of injuries of RLN in terms of faster recovery time, reset definitive RLNP, and lower RLNP (Table 1; Fig. 2). Different kinds of nerve injury have very different possibility of recovery [47]. For traction injury, it has the highest possibility of recovery. C-IONM can detect traction injury earlier than I-IONM [39–41]. Thus, the severity of nerve injury in C-IONM group will be less than that of I-IONM group. As to thermal injury or clamping injury, the possibility of RLN recovery is highly variable. No matter they were detected by I-IONM or C-IONM, and the severity of nerve injury will be the same. For future study, the comparison of RLNP recovery time between I-IONM and C-IONM should be stratified to the groups of traction injury, thermal injury, or clamping injury.
While the study does demonstrate some potential advantages to improvements in IONM, it has significant limitations.
Likely, most of the improved results in different periods in the study depend more upon the increased experience rather than upon use of IONM; this should also be accepted and induce to less assertive conclusions. The study may just represents the surgeon’ sequential experience and would have more weight if it included the experience of the other surgeons at his institution. It is difficult to separate enhanced single surgeon results that come from continued operative experience and improved results that stem from technical advancements with IONM. The IONM results are all significantly better compared to the initial poor 16.7 % RLN injury rate with visual identification only. Historical control always implies a time bias which is difficult to overcome. There seems to be a significant time bias in the present study spanning over such a long period of time. In particular, outcome from the first period (visualization alone) does skew the statistical analysis and makes comparison with the neuromonitoring periods very arguable. Moreover, some years that were not included in the study are related to “learning curves” with the IONM hardware.
A major problem with the study is that it is completely uncontrolled and, therefore, subjects to many biases that could impact the results. The major limitation of the study is of being retrospective with comparisons made between different consecutive time periods. This design is inevitably prone to bias in terms of surgeon experience, changing clinical practice and improved technology and surgical techniques. These weaknesses can only be resolved by a prospective randomized study. One issue is that this study involves the experience of a single surgeon. Most surgeons get better with experience. As such, the outcomes over time might have improved slightly, because the surgeon gained more experience and essentially became a better surgeon. The fact that 7 % of RLN were not visually identified during the first period illustrates that the surgeon was probably less experienced during that time. In addition, a large number of these unidentified nerves were injured. This is clearly a matter of experience, since many studies have shown that the incidence of RLN injury is no different based on IONM usage.
Moreover, the study involved 1521 patients operated on by the same surgeon over a 12-year period. Approximately 225 patients were excluded from the analysis, because they were apparently during the “learning curves” for the various techniques. We excluded 152 patients for intermittent IONM, 25 patients for the standard IONM, and 50 patients for continuous IONM as “learning curve.” The number of patients chosen for “learning curve” inclusion was not arbitrary but related to the validity of the previous results [26]. This is a problem, since we are talking about an experienced thyroid surgeon. If the RLN injury rates were higher during a learning period (for an experienced thyroid surgeon), this would be an important outcome to factor into the equation and might impact the risk/benefit analysis for the various surgical approaches.
One of the main or perhaps only purported advantages of IONM is that the surgeon can identify a loss of RLN signal and terminate the operation, thus avoiding the potential need for a tracheostomy. In this series, there was only one tracheostomy needed (that is 1 out of approximately 1400 cases) in period 1. One must then assess the risk/benefit associated with potentially preventing one tracheostomy with the added risk and cost associated with the IONM approach and the extra operations in all the patients who had their total thyroidectomy procedures aborted due to loss of signal. This analysis was not performed in the current study. The authors have already commented on the costs of the various IONM approaches [56]. These costs should also be factored, as one analyzes the efficacy of utilizing a new technology.
97.5 % of laringoscopy confirmation of the loss of signal is higher and not concordant with other study and may be related again with the consistent standardization process [57].
The hospital stay decreased from 4.3 to 1.5 days during the study period. The decrease in hospital stay for the study may be a significant improvement for Authors Institution, but it is difficult to attribute this to change in RLN monitoring techniques. Other institutions at the same time have reported very good results with just 23 h observation periods or even with same-day surgery discharge involving no hospital stay. This decrease in hospital stay illustrates the advances in thyroid surgery and peri-operative care that occurred during the study period. These advances were independent of IONM and, therefore, highlight the issue that improvements in outcomes with the RLN may also have occurred independent of the IONM [58, 59].
Finally, it would be interesting to perform a prospective randomized trial, involving surgeons experienced with each of the approaches.
Conclusion
The study reports outcomes in regard to the rates of RLN identification, injury, and recovery in four different time frames in which the surgical practice evolved from just visualization to the use of IONM to eventual C-IONM. The authors conclude that the application of a standardized technique with C-IONM led to an increase in RLN identification rates, a reduction in severity of RLN injuries, and faster recovery times for RLN function. It also led to an increase in the number of staged procedures.
References
Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H (2003) The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 133(2):180–185
Bliss RD, Gauger PG, Delbridge LW (2000) Surgeon’s approach to the thyroid gland: surgical anatomy and the importance of technique. World J Surg 24(8):891–897
Wagner HE, Seiler C (1994) Recurrent laryngeal nerve palsy after thyroid gland surgery. Br J Surg 81:226–228 (8)
Wade JSH (1955) Vulnerability of the recurrent laryngeal nerves at thyroidectomy. Br J Surg 43:164–179 (9)
Bergamaschi R, Becouarn G, Ronceray J, Arnaud JP (1998) Morbidity of thyroid surgery. Am J Surg 176:71–75
Karlan MS, Catz B, Dunkelman D, Uyeda RY, Gleischman S (1984) A safe technique for thyroidectomy with complete nerve dissection and parathyroid preservation. Head Neck Surg 6:1014–1019 (11)
Mattig H, Bildat D, Metzger B (1998) Reducing the rate of recurrent nerve paralysis by routine exposure of the nerves in thyroid gland operation. Zentralbl Chir 123:17–20
Jatzko GR, Lisborg PH, Muller MG, Wette VM (1994) Recurrent nerve palsy after thyroid operations-principal nerve identification and a literature review. Surgery 115:139–144 (13)
Lo CY, Kwok KF, Yuen PW (2000) A prospective evaluation of recurrent laryngeal nerve paralysis during thyroidectomy. Arch Surg 135:204–207 (14)
Steurer M, Passler C, Denk DM, Schneider B, Niederle B, Bigenzahn W (2002) Advantages of recurrent laryngeal nerve identification in thyroidectomy and parathyroidectomy and the importance of preoperative and postoperative laryngoscopic examination in more than 1000 nerves at risk. Laryngoscope 112:124–133 (15)
Seiler CA, Glaser C, Wagner HE (1996) Thyroid gland surgery in an endemic region. World J Surg 20:593–597 (16)
Liu Q, Djuricin G, Prinz RA (1998) Total thyroidectomy for benign thyroid disease. Surgery 123:2–7 (17)
Chonkich GD, Petti GH Jr, Goral W (1987) Total thyroidectomy in the treatment of thyroid disease. Laryngoscope 97:897–900 (18)
Dener C (2002) Complication rates after operations for benign thyroid disease. Acta Otolaryngol 122:679–683 (19)
Reeve TS, Delbridge L, Cohen A, Crummer P (1987) Total thyroidectomy: the preferred option for multinodular goiter. Ann Surg 206:782–786
Jacobs JK, Aland JW, Ballinger JF (1983) Total thyroidectomy: a review of 213 patients. Ann Surg 197:542–549 (21)
Thompson NW, Oslen WR, Hoffman GL (1973) The continuing development of the technique of thyroidectomy. Surgery 73:913–927 (22)
Tovi F, Noyek AM, Chapnik JS, Freeman JL (1989) Safety of total thyroidectomy: review of 100 consecutive cases. Laryngoscope 99:1233–1237
Chiang FY, Wang LF, Huang YF, Lee KW, Kuo WR (2005) Recurrent laryngeal nerve palsy after thyroidectomy with routine identification of the recurrent laryngeal nerve. Surgery 137(3):342–347
Lamade W, Fogel W, Rieke K, Senninger N, Herfarth C (1996) Intraoperative monitoring of the recurrent laryngeal nerve. A new method. Chirurg. 67(4):451–454
Thomusch O, Dralle H (2000) Advantages of intraoperative neuromonitoring in thyroid gland operations. Dtsch Med Wochenschr 125(24):774
Ahmed M (2013) Aurangzeb, Abbas S, Boota M, Ashfaq M, Rashid AZ, Qureshi MA, Iqbal N. Should we routinely expose recurrent laryngeal nerve(s) during thyroid surgery? J Coll Physicians Surg Pak 23(3):186–189
Chiang FY, Lee KW, Chen HC, Chen HY, Lu IC, Kuo WR, Hsieh MC, Wu CW (2010) Standardization of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid operation. World J Surg 34(2):223–229
Randolph GW, Dralle H, International Intraoperative Monitoring Study Group, Abdullah H, Barczynski M, Bellantone R, Brauckhoff M, Carnaille B, Cherenko S, Chiang FY, Dionigi G, Finck C, Hartl D, Kamani D, Lorenz K, Miccoli P, Mihai R, Miyauchi A, Orloff L, Perrier N, Poveda MD, Romanchishen A, Serpell J, Sitges-Serra A, Sloan T, Van Slycke S, Snyder S, Takami H, Volpi E, Woodson G (2011) Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 121(Suppl 1):S1–S16
Schneider R, Przybyl J, Pliquett U, Hermann M, Wehner M, Pietsch UC, König F, Hauss J, Jonas S, Leinung S (2010) A new vagal anchor electrode for real-time monitoring of the recurrent laryngeal nerve. Am J Surg 199(4):507–514
Jonas J (2010) Continuous vagal nerve stimulation for recurrent laryngeal nerve protection in thyroid surgery. Eur Surg Res 44(3–4):185–191
Harness JK, Fung L, Thompson NW, Burney RE, McLeod MK (1986) Total thyroidectomy: complications and technique. World J Surg 10:781–786 (2)
Lore JM Jr (1983) Practical anatomical considerations in thyroid tumor surgery. Arch Otolaryngol 109:568–574
Sun H, Tian W, Jiang K, Chiang F, Wang P, Huang T, Zhu J, Qin J, Liu X (2015) Clinical guidelines on intraoperative neuromonitoring during thyroid and parathyroid surgery. Ann Transl Med. 3(15):213
Brauckhoff M, Walls G, Brauckhoff K, Thanh PN, Thomusch O, Dralle H (2002) Identification of the non-recurrent inferior laryngeal nerve using intraoperative neurostimulation. Langenbecks Arch Surg. 386(7):482–487 (Epub 2001 Oct 27)
Dionigi G, Kim HY, Wu CW, Lavazza M, Ferrari C, Leotta A, Spampatti S, Rovera F, Rausei S, Boni L, Chiang FY (2013) Vagus nerve stimulation for standardized monitoring: technical notes for conventional and endoscopic thyroidectomy. Surg Technol Int. 23:95–103 (PubMed PMID: 23860931)
Wu CW, Dionigi G, Chen HC, Chen HY, Lee KW, Lu IC, Chang PY, Hsiao PJ, Ho KY, Chiang FY (2013) Vagal nerve stimulation without dissecting the carotid sheath during intraoperative neuromonitoring of the recurrent laryngeal nerve in thyroid surgery. Head Neck 35(10):1443–1447. doi:10.1002/hed.23154 (Epub 2012 Sep18)
Medniuk A, Bareisiene D, Ahmad I (2014) Nerve integrity monitor tubes for thyroid surgery. Anaesthesia 69(3):287–288
Phelan E, Schneider R, Lorenz K, Dralle H, Kamani D, Potenza A, Sritharan N, Shin J, Randolph G (2014) Continuous vagal IONM prevents recurrent laryngeal nerve paralysis by revealing initial EMG changes of impending neuropraxic injury: a prospective, multicenter study. Laryngoscope 124(6):1498–1505
Chiang FY, Lu IC, Tsai CJ, Hsiao PJ, Lee KW, Wu CW (2012) Detecting and identifying nonrecurrent laryngeal nerve with the application of intraoperative neuromonitoring during thyroid and parathyroid operation. Am J Otolaryngol 33(1):1–5
Dralle H, Sekulla C, Lorenz K, Nguyen Thanh P, Schneider R, Machens A (2012) Loss of the nerve monitoring signal during bilateral thyroid surgery. Br J Surg 99(8):1089–1095
Dionigi G, Frattini F (2013) Staged thyroidectomy: time to consider intraoperative neuromonitoring as standard of care. Thyroid. 23(7):906–908
Dionigi G, Chiang FY, Hui S, Wu CW, Xiaoli L, Ferrari CC, Mangano A, Lianos GD, Leotta A, Lavazza M, Frattini F, Annoni M, Rausei S, Boni L, Kim HY (2015) Continuous intraoperative neuromonitoring (C-IONM) technique with the automatic periodic stimulating (APS) accessory for conventional and endoscopic thyroid surgery. Surg Technol Int. 26:101–114
Sritharan N, Chase M, Kamani D, Randolph M, Randolph GW (2015) The vagus nerve, recurrent laryngeal nerve, and external branch of the superior laryngeal nerve have unique latencies allowing for intraoperative documentation of intact neural function during thyroid surgery. Laryngoscope. 125(2):E84–E89
Schneider R, Bures C, Lorenz K, Dralle H, Freissmuth M, Hermann M (2013) Evolution of nerve injury with unexpected EMG signal recovery in thyroid surgery using continuous intraoperative neuromonitoring. World J Surg 37(2):364–368. doi:10.1007/s00268-012-1853-0 (PubMed PMID: 23188536)
Schneider R, Randolph GW, Sekulla C, Phelan E, Thanh PN, Bucher M, Machens A, Dralle H, Lorenz K (2013) Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 35(11):1591–1598
Scanlon EF, Kellogg JE, Winchester DP, Larson RH (1981) The morbidity of total thyroidectomy. Arch Surg 116:568–571
Katz AD, Bronson D (1978) Total thyroidectomy: the indications and results of 630 cases. Am J Surg 136:450–454
Martensson H, Terins J (1985) Recurrent laryngeal nerve palsy in thyroid gland surgery related to operations and nerves at risk. Arch Surg 120:475–477
Perzik SL (1976) The place of total thyroidectomy in the management of 909 patients with thyroid disease. Am J Surg 132:480–483
Lee CY, Long KL, Eldridge RJ, Davenport DL, Sloan DA (2014) Preoperative laryngoscopy in thyroid surgery: do patients’ subjective voice complaints matter? Surgery 156(6):1477–1482
Dralle H, Sekulla C, Lorenz K, Brauckhoff M, Machens A, German IONM (2008) Study Group. Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg 32(7):1358–1366
Kandil E, Noureldine SI, Abbas A, Tufano RP (2013) The impact of surgical volume on patient outcomes following thyroid surgery. Surgery 154(6):1346–1352 (discussion 1352–3)
Randolph GW (2010) The importance of pre- and postoperative laryngeal examination for thyroid surgery. Thyroid 20(5):453–458
Lee SW, Kim JW, Chung CH, Mok JO, Shim SS, Koh YW, Choi EC (2010) Utility of injection laryngoplasty in the management of post-thyroidectomy vocal cord paralysis. Thyroid 20(5):513–517
Dralle H, Lorenz K (2010) Intraoperative neuromonitoring of thyroid gland operations: surgical standards and aspects of expert assessment. Chirurg 81(7):612–619. doi:10.1007/s00104-009-1882-x (German)
González-Sánchez C, Franch-Arcas G, Gómez-Alonso A (2013) Morbidity following thyroid surgery: does surgeon volume matter? Langenbecks Arch Surg 398(3):419–422
Chiang FY, Lu IC, Chen HC, Chen HY, Tsai CJ, Hsiao PJ, Lee KW, Wu CW (2010) Anatomical variations of recurrent laryngeal nerve during thyroid surgery: how to identify and handle the variations with intraoperative neuromonitoring. Kaohsiung J Med Sci 26(11):575–583
Barczyński M, Konturek A, Stopa M, Hubalewska-Dydejczyk A, Richter P, Nowak W (2011) Clinical value of intraoperative neuromonitoring of the recurrent laryngeal nerves in improving outcomes of surgery for well-differentiated thyroid cancer. Pol Przegl Chir. 83(4):196–203
Wang LF, Lee KW, Kuo WR, Wu CW, Lu SP, Chiang FY (2006) The efficacy of intraoperative corticosteroids in recurrent laryngeal nerve palsy after thyroid surgery. World J Surg 30(3):299–303
Pisanu A, Porceddu G, Podda M, Cois A, Uccheddu A (2014) Systematic review with meta-analysis of studies comparing intraoperative neuromonitoring of recurrent laryngeal nerves versus visualization alone during thyroidectomy. J Surg Res 188(1):152–156
Sitges-Serra A, Fontané J, Dueñas JP, Duque CS, Lorente L, Trillo L, Sancho JJ (2013) Prospective study on loss of signal on the first side during neuromonitoring of the recurrent laryngeal nerve in total thyroidectomy. Br J Surg 100(5):662–666
Dionigi G, Bacuzzi A, Barczynski M, Biondi A, Boni L, Chiang FY, Dralle H, Randolph GW, Rausei S, Sacco R, Sitges-Serra A (2011) Implementation of systematic neuromonitoring training for thyroid surgery. Updates Surg. 63(3):201–207
Frattini F, Mangano A, Boni L, Rausei S, Biondi A, Dionigi G (2010) Intraoperative neuromonitoring for thyroid malignancy surgery: technical notes and results from a retrospective series. Updates Surg 62(3–4):183–187
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Anuwong, A., Lavazza, M., Kim, H.Y. et al. Recurrent laryngeal nerve management in thyroid surgery: consequences of routine visualization, application of intermittent, standardized and continuous nerve monitoring. Updates Surg 68, 331–341 (2016). https://doi.org/10.1007/s13304-016-0393-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-016-0393-9