Abstract
Gliomas are one of the most common primary brain tumors in adults. They display aggressive invasiveness, are highly vascular, and have a poor prognosis. Plexin-B1 is involved in numerous cellular processes, especially cellular migration and angiogenesis. However, the role and regulatory mechanisms of Plexin-B1 in gliomas are not understood and were thus investigated in this study. By using multiple and diverse experimental techniques, we investigated cell apoptosis, mitochondrial membrane potential, cell migration and invasion, angiogenesis, PI3K and Akt phosphorylation, and also the levels of SRPK1 and αvβ3 in glioma cells and animal glioma tissues. The results indicated that Plexin-B1 expression in glioma cell lines is increased compared to normal human astrocytes. Plexin-B1 mediates RhoA/integrin αvβ3 involved in the PI3K/Akt pathway and SRPK1 to influence the growth of glioma cell, angiogenesis, and motility in vitro and in vivo. Thus, Plexin-B1 signaling regulates the Rho/αvβ3/PI3K/Akt pathway and SRPK1, which are involved in glioma invasiveness and angiogenesis. Therefore, the new drug research should focus on Plexin-B1 as a target for the treatment of glioma invasion and angiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the data of the Statistics Korea, new cases found to have the condition of brain or central nervous system tumors were 1592 persons, accounting for 0.7 % of 218,017 new cancer cases in 2011 [1]. Glioma as primary brain tumors has a lethal outcome and, thus, dismal prognosis. The current standard of care for gliomas is surgery, radiotherapy, and alkylating agent chemotherapy, which result in a 2-year survival rate of 15–26 % [2] and extremely frequent tumor recurrence. Research into the molecular mechanisms underlying glioma growth could deepen our understanding of the mechanisms of tumorigenesis.
Plexin-B1 is a high-affinity transmembrane receptor for semaphorin 4D (Sema4D), which is involved in numerous cellular processes related to neuronal guidance, the maturation and regulation of motility in the nervous system [3–5], bone formation [6], cellular migration [7], and angiogenesis [8]. Structural analysis of the intracellular side of Plexin-B1 revealed that it contains a Ras GTPase-activating protein (GAP) and Rho GTPase-binding protein domain (RBD), which can assemble components within the Rho family [7] and influence integrin-mediated adhesion and the PI3K/Akt pathway [9]. In a recent study, Zhang et al. examine the expression of Plexin-B1 in 110 cases of human glioma and compare its expression with tumor grade [10]. They find that Plexin-B1 protein is overexpressed in gliomas compared to non-neoplastic brain tissues, with increasing expression levels of Plexin-B1 correlating with tumor grade [10]. These results suggest that Plexin-B1 might participate in the carcinogenesis and progression of glioma. However, the specific roles and the relevant regulatory mechanisms of Plexin-B1 in the process of glioma remain unclear.
Integrins are a family of heterodimeric glycoprotein membrane receptors comprised of α and β subunits that bind to the extracellular matrix (ECM) and play a central role in regulating cell growth, migration, and tumor metastasis [11]. Integrin activation is essential for cell adhesion and migration, and several studies show that the Ras family of small GTPases regulates integrin activity [12]. Integrin αvβ3 is the most extensively studied integrin involved in angiogenesis and glioblastoma (GBM) radioresistance via the integrin linked kinase (ILK) and RhoB [13, 14].
Serine-arginine protein kinase 1 (SRPK1) participates in precursor mRNA translation, processing, and splicing, chromatin reconstruction, cell cycle progression, and the remodeling of cellular structures [15]. Further, SRPK1 can function as both an oncogene and a tumor suppressor by interfering with PHLPP-mediated dephosphorylation of Akt [16]. We previously confirmed that SRPK1 associates with glioma cell invasion and migration [17].
In this study, we observed the effect of Plexin-B1 in gliomas and its potential mechanism of action by evaluating several cellular effects. We found that the abnormal expression of Plexin-B1 in glioma cells influences cell apoptosis, migration, invasion, the actin cytoskeleton, and microtubule formation, and it exerts these effects via the Rho/αvβ3/PI3K/Akt pathway and regulating SRPK1. Our data highlight the importance of Plexin-B1 in influencing the survival, metastasis, and angiogenesis of glioma.
Materials and methods
Antibodies and reagents
We used the following antibodies: rabbit polyclonal antibodies against Sema4D, Plexin-B1, CD31, and GAPDH (abcam); mouse monoclonal antibodies against αv and SRPK1 (BD Biosciences) and Plexin-B1 (Santa Cruz Biotechnology); a rabbit polyclonal antibody sampler kit against RhoA, RhoB, and RhoC, and rabbit polyclonal antibodies against p-PI3K, Akt, and p-Akt (Cell Signaling Technology); a mouse monoclonal antibody against PI3K (abcam); a mouse monoclonal antibody against β3 (Millipore); a goat polyclonal secondary antibody to rabbit IgG-HRP (abcam); and goat secondary antibodies to rabbit FITC and to mouse dylight 549 (abbkine). F-actin was visualized with rhodamine phalloidin (cytoskeleton). Matrigel basement membrane matrix (BD Biosciences) was used in invasion and tube formation experiments. Cell apoptosis was evaluated using an Annexin V-FITC/PI apoptosis detection kit (KeyGEN). TUNEL Apoptosis Detection Kit was ordered from KeyGEN (KGA702). Immunohistochemistry was performed with a staining kit containing SABC goat secondary antibody to mouse and a rabbit immunohistochemistry staining kit (Boster). A mitochondrial membrane potential assay kit utilizing JC-1 was from Beyotime. Pierce protein A/G agarose and a BCA protein concentration determination kit were from Thermo. The mouse mAb IgG1 isotype control was from Cell Signaling Technology.
Cell lines, shRNA, and lentivirus infections
Human U251 and U87MG glioma cells were obtained from Wuhan Boster Biological Technology (China), the human astrocyte cell line HA-1800 was obtained from the Shanghai Cell Library of the Life Science Institute (China), and human umbilical vein endothelial cells (HUVECs) were a gift from the central laboratory of Binzhou Medical University. U251, U87 MG, and HUVECs were cultured in Dulbecco’s modified Eagle’s medium (DMEM, HyClone, USA) supplemented with 10 % fetal bovine serum (FBS, Gibco, USA) and 100 U/mL penicillin/streptomycin (Solarbio). The human astrocyte cell line HA-1800 was cultured in DMEM (low-glucose) with 15 % FBS. All cells were incubated at 37 °C in a 5 % CO2 incubator. To generate glioma cells and HUVECs with a stable knockdown of Plexin-B1 and SRPK1, we purchased short hairpin (sh) RNA lentiviral particles from Santa Cruz Biotechnology (sc-42176-V and sc-39235-V, respectively). Lentiviral particles generally contain three to five expression constructs, each encoding target-specific 19–25 nt (plus hairpin) shRNAs designed to knockdown gene expression. Briefly, cells were infected with lentiviruses to deliver shRNAs and a puromycin resistance gene. After selection, successful knockdown was verified by western blotting.
RNA extraction and quantitative real-time PCR
Total RNA extraction was performed using RNAiso Plus (Takara) and then reverse-transcribed using a Prime Script RT reagent Kit (Takara) according to the manufacturer’s instructions. Real-time quantitative PCR was used to detect the expression of SEMA4D, PLXNB1, SRPK1. Quantitative real-time PCR (qRT-PCR) was performed on an iQTM5 Real-Time PCR Optical System (Bio-Rad, USA) with software version 2.1 and the following primers: SEMA4D, 5′-GAGAAGCCTGGAACCCAAGT-3′ (forward) and 5′-TCAAAGCCTGTTAGCCATCC-3′ (reverse); Plexin-B1 (PLXNB1), 5′-TTTCCTATCAGTGCCAGACCT-3′ (forward) and 5′-TCACACCAGAACCAGTCAGC-3′ (reverse); SRPK1, 5′-TTCCTCAACTGTAGGTCAGTCATTC-3′ (forward) and 5′-TGTTCTTGCTCTTGTTCATCTTCAC-3′ (reverse); and GAPDH, 5′-GCACCGTCAAGGCTGAGAAC-3′ (forward) and 5′-TGGTGAAGACGCCAGTGGA-3′ (reverse). All primers were intron-spanning. Comparison of the expression of each gene between its control and stimulated states was determined with the 2−∆∆Ct method using GAPDH as a housekeeping gene [18]. Experiments were performed on three different cell preparations, and each run was analyzed in triplicate.
Cell apoptosis measurement via flow cytometry
We used an Annexin V-FITC/PI apoptosis detection kit to detect apoptosis among glioma cells infected with control or appropriate shRNAs. Flow cytometry analyses were performed on an Epics XL (Beckman Coulter) and processed using the associated software.
TUNEL method
TUNEL apoptosis detection kit was used to operate according to the kit instructions. The main process is as follows: slice dewaxing dehydration; 1 % TritonX-100 transparent liquid penetration; 3 % H2O2 blocking solution blocking of endogenous peroxidase (POD) (10 min); proteinase K permeability (30 min); with 50 μL TdT reaction liquid, 37 °C, 1 h; Streptavidin-HRP 0.50 μL mixed with 49.5 μL PBS 37 °C, 30 min, dark reaction; diaminobenzidine (DAB) coloration, hematoxylin, washed with water, dehydrated and transparent. Ultimately, the samples were sealed with neutral gum and visualized under a microscope. Cleavage of genomic DNA during apoptosis may yield DNA strand breaks; those can be identified by labeling free 3′-OH termini with modified nucleotides in an enzymatic reaction.
Mitochondrial membrane potential assay
The mitochondrial membrane potential of cells was measured using a kit with JC-1. Briefly, after the cells were loaded with JC-1 dyeing working liquid for 20 min at 37 °C, they were washed twice with buffer. The fluorescent dye was then excited at 490 nm, and the fluorescent intensities of both monomer and aggregated molecules were recorded at 590 nm with an inverted fluorescence microscope. Carbonylcyanide-p-chlorophenyl hydrazone (CCCP)-treated cells were used as a positive control. For most cells, treatment with 10 μM CCCP for 20 min typically results in after the complete loss of mitochondrial membrane potential.
Migration and invasion assays
Serum-free medium containing 10 % FBS (positive control) or 0.5 % bovine serum albumin (BSA, negative control) was placed in the bottom well of a transwell chamber (Corning), 200 L of 5 × 105/mL control-infected or lentivirus (expressing Plexin-B1 shRNA)-infected cells was then added in DMEM to the upper chamber coated with or without 100 L Matrigel, and the cells were incubated for 24 h at 37 °C. The cells were fixed in methanol and stained with 0.1 % crystal violet. Then, the transwell inserts were washed with 33 % acetic acid, and optical density (OD) values were measured at 570 nm. Each experiment was performed in triplicate, and the average and standard deviation were calculated.
Immunoblot analysis
Cells and tissues were lysed in RIPA lysis buffer (Beyotime). Protein levels were analyzed by immunoblotting and transferred onto polyvinylidene difluoride membranes after electrophoresis. Membranes were subsequently blocked in 5 % non-fat milk and then probed with antibodies at the concentrations recommended by the manufacturer (unless otherwise indicated) overnight at 4 °C. Visualization of protein bands was accomplished using horseradish peroxidase-coupled secondary antibodies, enhanced chemiluminescence (Thermo), and a ChemiScope3400 Mini system. Signals were then quantified with GeneTools analysis software, and the mean values of three independent experiments are indicated in figures.
Immunoprecipitation
Cells were lysed in buffer (see above), and proteins were immunoprecipitated from the cleared lysates by incubation overnight at 4 °C with antibody against Plexin-B1 or SRPK1. Immunocomplexes were recovered with immobilized protein A/G agarose. Lysates and anti-Plexin-B1 or anti-ARPK1 immunoprecipitates were analyzed by western blotting after SDS-PAGE (see above) and immunoblotted with anti-Plexin-B1, anti-RhoA, anti-RhoB, and anti-RhoC. Immunocomplexes were visualized with the ECL chemiluminescence system (see above).
Tumor cell injections and animal studies
BALB/c nude mice (4 weeks old) were purchased from Vital River Laboratories. U87 MG cells and control-infected or lentivirus (expressing Plexin-B1 shRNA or SRPK1 shRNA)-infected cells were resuspended in 100 μL serum-free DMEM at a density of 1.0 × 107/mL and subcutaneously injected into the flanks of immunocompromised nude mice. Tumor size was measured every 3 days and was calculated using the formula ab 2 /2, where a is the longest dimension of the tumor, and b is the width. Animals were euthanized and tumors removed for sectioning and processing 30 days after the injection. All animals were cared for and maintained under the supervision and guidelines of the BinZhou Medical University of Institutional Animal Care and Use Committee.
Immunohistochemistry
Cells were cultured in 6-well plates to a density of 1 × 105/mL and fixed with 4 % paraformaldehyde for 20 min at room temperature, washed, permeabilized with 3 % Triton X-100 for 15 min, incubated with primary antibodies (anti-Sema 4D and anti-Plexin-B1) for 2 h at 37 °C, visualized with DAB (Boster), and counterstained with hematoxylin. Ultimately, the samples were sealed with neutral gum and visualized under a microscope.
The tumor tissues harvested from mice, which were paraffin-embedded after formalin-fixing, were processed for immunohistochemistry. Then, slides were incubated in 3 % H2O2 to block endogenous peroxidase activity, blocked and further incubated with an antibody against CD31, and incubated with HRP-labeled secondary antibody. The sections were incubated with DAB. Finally, the slides were counterstained with hematoxylin and visualized under a microscope.
Tubulogenesis assay
HUVEC cells previously infected with lentiviruses encoding the appropriate shRNA or copGFP control lentiviral particles (where indicated) were grown in 96-well plates coated with 70 μL BD Matrigel and incubated overnight in media containing 0.1 % BSA (negative control) or 10 % FBS. Cells were then fixed in 0.5 % glutaraldehyde and photographed.
Cytoskeletal staining
Cells were crawling slices and fixed with 4 % paraformaldehyde, permeabilized with 0.5 % Triton X-100, incubated with rhodamine phalloidin for 40 min at room temperature, and DAPI-stained for 10 min at room temperature.
Fluorescence immunohistochemistry
Cells were crawling slices and fixed with 4 % paraformaldehyde, permeabilized with 0.5 % Triton X-100, incubated with primary antibodies (anti-SRPK1 and anti-Plexin-B1) for 2 h at 37 °C, visualized with goat secondary antibody to rabbit FITC or goat secondary antibody to mouse dylight 549 (abbkine), and counterstained with DAPI. Xenograft tumor tissues were fixed and then embedded in paraffin. After deparaffinization, they were incubated with 0.5 % Triton X-100 and blocked with 5 % BSA. Rabbit anti-human CD31 antibody and mouse anti-human SRPK1 and Plexin-B1 were added for overnight incubations, and an FITC-conjugated goat anti-rabbit IgG (H + L) Ab and a TRITC-conjugated goat anti-mouse IgG (H + L) Ab were added. Counterstaining was performed by incubating the specimens with DAPI. Tissue sections were mounted in glycerin and analyzed via laser scanning confocal microscopy (Leica).
Statistics
The data are expressed as means and standard deviations. The experiments shown were repeated three times with similar results. Statistical analyses between two groups were performed by unpaired two-tailed Student’s t test (*P < 0.05, **P < 0.01).
Results
Sema4D and Plexin-B1 are overexpressed in glioma cells
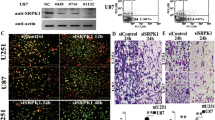
It was unknown if Sema4D and Plexin-B1 are expressed in glioma cells. Therefore, we performed experiments to investigate their expression. Immunohistochemical staining of normal glial and glioma cells revealed that Sema4D and Plexin-B1 have significantly different expression levels between these cell types (Fig. 1a). Therefore, we further investigated the expression of Sema4D and Plexin-B1 between normal glial and glioma cells at the protein and gene levels. We found that compared to the glial cell group, both the protein and mRNA levels of Sema4D and Plexin-B1 are significantly increased in glioma cells (Fig. 1b, c). These differences suggested that Sema4D–Plexin-B1 signaling may play some role in glioma development.
Sema4D and Plexin-B1 are overexpressed in glioma cells. a Upregulation of Sema4D and Plexin-B1 expression is shown in U251 and U87 MG cells. Objective ×200, scale bars 50 m. b Western blot results showing the levels of Sema4D and Plexin-B1 in normal glial and glioma cells; GAPDH was used as an internal loading control. Band intensity was quantified using NIH Scion Image and normalized to GAPDH. Bar chart is the quantitation of relative expression of Sema4D and Plexin-B1 relative to GAPDH. c Bar graph showing summary analyses of Sema4D and Plexin-B1 with glioma cells by RT-PCR compared to normal glial cells, respectively. d RT-PCR verified the successful knockdown of Plexin-B1 in glioma cells. e Western blotting showing that Plexin-B1 was successfully depleted. All error bars, ±SEM. All quantitative data were generated from a minimum of three replicates. **P < 0.01 versus normal group; Student’s t test
Genetic ablation of Plexin-B1 induces glioma cell apoptosis and reduces glioma cell motility
We depleted Plexin-B1 in human glioma cells using lentiviral-delivered shRNA. After puromycin selection, successful knockdown was verified by RT-PCR and western blotting (Fig. 1d, e). This knockdown of Plexin-B1 significantly increased the apoptosis of glioma cells (Fig. 2a, b). Next, an annexin V/PI staining assay was used to assess U251 and U87 MG cells. The results suggest that early apoptotic cells are prevalent in the Plexin-B1 knockdown group compared to the mock-vehicle control group (Fig. 2a). Furthermore, we found that Plexin-B1 influences glioma cell apoptosis in vitro using a mitochondrial membrane potential assay. Control cells exhibited numerous stained mitochondria that emitted red-orange fluorescence, which is indicative of a normal high membrane potential (Fig. 2b). At low mitochondrial membrane potential, JC-1 cannot be gathered in the mitochondrial matrix; JC-1 is monomer at this time, can produce green fluorescence. CCCP-treated positive control cells emitted green fluorescence. The experimental cells exhibited numerous stained mitochondria that emitted green-orange fluorescence, which is indicative of a low membrane potential.
Promotion of apoptosis and inhibition of motility of glioma cells. a Flow cytometry demonstrates that treating with Pleixn-B1 shRNA can induce glioma cell apoptosis. AA1, necrotic cells; AA2, dead/late apoptotic cells; AA3, normal cells; and AA4, early apoptotic cells. b Control cells exhibiting numerous stained mitochondria that emitted red-orange fluorescence, which is indicative of normal high membrane potential. CCCP-treated positive control cells emitted green fluorescence. The experimental cells exhibited numerous stained mitochondria that emitted green-orange fluorescence, which is indicative of a low membrane potential. c Matrigel transwell assay demonstrating that Plexin-B1 shRNA inhibits the invasion of glioma cells induced by 10 % FBS over 24 h. d Transwell assay demonstrating that Plexin-B1 shRNA inhibits the migration of glioma cells induced by 10 % FBS over 24 h. Error bars represent the standard error. All error bars, ±SEM. All quantitative data were generated from a minimum of three replicates. **P < 0.01 versus normal group; Student’s t test
To elucidate the relationship between cellular invasion ability and Plexin-B1, we measured cellular invasion and migration into an artificial model of the extracellular matrix [19]. Compared to the control group, Plexin-B1 shRNA-treated glioma cells displayed significantly decreased invasion (P < 0.01, Fig. 2c) and migration (P < 0.01, Fig. 2d). These results indicate that inhibition of Plexin-B1 expression decreases the motility of glioma cells.
Plexin-B1 associates with RhoA and activates downstream signaling to induce cytoskeletal disassembly in glioma cell lines
To investigate whether the observed effects on cell motility were related to cytoskeletal alterations, we investigated the effect of Plexin-B1 shRNA transfection on the cytoskeleton via rhodamine phalloidin and confocal microscopy. We found that, compared to the control group, Plexin-B1 shRNA induced structural cytoskeletal disassembly in the Plexin-B1 shRNA-transfected group (Fig. 3a). To determine if Plexin-B1 exerts its effects via the Rho family, extracts from glioma cells were immunoprecipitated with RhoA, RhoB, and RhoC antibodies. Immunoblotting revealed association of the intracellular domain of Plexin-B1 with RhoA but not RhoB or RhoC in U251 cells (Fig. 3b).
Plexin-B1 associates with RhoA and activates downstream signaling to induce migration and invasion in glioma cell lines. a Slides of glioma cells with or without Plexin-B1 shRNA lentivirus were photographed after immunofluorescent staining and rhodamine-phalloidin staining. Successful Plexin-B1 (green) knockdown was verified by immunofluorescent staining. Images were acquired with a laser scanning confocal microscopy. Each experiment was repeated at least twice. b Immunoblotting revealed the association of the intracellular domain of Plexin-B1 with RhoA but not RhoB or RhoC in U251 cells. c Loss of Plexin-B1 expression in U251 and U87 MG cells resulted in a strong inhibition of αvβ3 integrin, RhoA, and SRPK1 activity, while the RhoB and RhoC activity was not altered. d SRPK1 shRNA lentiviral particle-infected glioma cells and successful knockdown was verified by RT-PCR and western blotting. **P < 0.01, versus control group. Data are represented as mean ± SEM. e Knockdown of SRPK1 did not associate with αvβ3 integrin, RhoA, RhoB, or RhoC
A previous study demonstrates that Plexin-B1 utilizes RhoA and Rho kinase to promote the integrin-dependent activation of Akt and ERK, as well as endothelial cell motility [20]. To investigate the downstream effects of PlexinB1/Rho signaling in glioma cells, we examined glioma cell extracts. Loss of Plexin-B1 expression in glioma cells resulted in a strong inhibition of αvβ3 integrin, RhoA, and SRPK1 activity, while RhoB and RhoC activity were not altered (Fig. 3c).
SRPK1 involvement in Plexin-B1 signaling was an unanticipated result. To explore the relationship between SRPK1 and Plexin-B1, we purchased SRPK1 shRNA lentiviral particles and infected glioma cells. After puromycin selection, successful knockdown was verified by RT-PCR and western blotting (Fig. 3d). However, we did not observe a similar phenomenon in the SRPK1 shRNA group as seen with the Plexin-B1 shRNA (Fig. 3e). Thus, Plexin-B1 associates with RhoA and activates integrin signaling to induce motility in glioma cell lines.
Plexin-B1 mediates the expression of both RhoA/integrin αvβ3 involved in the PI3K/Akt pathway and SRPK1 to influence glioma cell growth in vivo
To determine the effect of loss of expression of Plexin-B1 and SRPK1 inhibition on tumor cell growth in vivo, we implanted normal, lentivirus control, Plexin-B1 shRNA, or SRPK1 shRNA U87 MG cells into nude mice and measured the rates of tumor formation. Ablation of Plexin-B1 and SRPK1 strongly suppressed tumor formation in this xenograft model (Fig. 4a, b). Similarly, we found that knockdown of Plexin-B1 and SRPK1 significantly increased the apoptosis of glioma cells (Fig. 4e). The result of the TUNEL test indicates that both Plexin-B1 and SRPK1 could induce apoptosis in vivo. We found that only Akt phosphorylation was decreased in the Plexin-B1 shRNA and SRPK1 shRNA groups (Fig. 4c). We then examined the cross talk of Plexin-B1 and SRPK1 signaling in tumor tissue. Similar to the above, loss of Plexin-B1 expression in tumor tissues resulted in a strong inhibition of αvβ3 integrin, RhoA, and SRPK1 activity, while RhoB and RhoC activities were not altered. Further, SRPK1 did not influence the protein levels of Plexin-B1, αvβ3 integrin, or Rho (Fig. 4d).
Plexin-B1 mediates both RhoA/integrin αvβ3 involved in the PI3K/Akt pathway and SRPK1 to influence glioma cell growth in vivo. a Normal, lentivirus control, Plexin-B1 shRNA, or SRPK1 shRNA U87 MG cells (1.0 × 107 cells/mouse) were transplanted into BALB/c nude mice. The images of the extracted tumors are shown. b The tumor volume was measured every 3 days. Data shown are the means ± SEM (n = 6). Significant differences are shown: **P < 0.01. There was no difference in the normal group compared to the control group. Significant differences are shown in the normal group versus the Plexin-B1 shRNA group. The same results were observed in the SRPK1 shRNA group and normal group. c Western blotting was used to detect tumor tissue. Akt phosphorylation was decreased both in the Plexin-B1 shRNA group and the SRPK1 shRNA group. PI3K phosphorylation was decreased only in the Plexin-B1 shRNA group. d Knockdown of Plexin-B1 expression in tumor tissues resulted in a strong inhibition of αvβ3 integrin, RhoA, and SRPK1 activity; RhoB and RhoC activities were not altered. SRPK1 did not influence the protein levels of Plexin-B1, αvβ3 integrin, and Rho. e The result of TUNEL test indicate that both Plexin-B1 and SRPK1 could induce apoptosis in vivo. Brown was TUNEL-positive apoptotic cells, blue was normal cells which were stained by hematoxylin
Plexin-B1/RhoA and SRPK1 signaling mediates glioma angiogenesis in vivo and in vitro
Tumor tissue immunofluorescence was analyzed to determine the localization of Plexin-B1, SRPK1, and CD31 expression in all groups (Fig. 5a, b). We found that Plexin-B1 and SRPK1 are strongly decreased in the Plexin-B1 shRNA group and SRPK1 shRNA group, respectively. We used CD31, a representative endothelial cell marker, to detect blood vessels. Paraffin sections of the tumors were prepared and immunostained for CD31 to examine microvessel density (MVD; vessel area/mm2) in the tumors. The MVD was significantly reduced in the Plexin-B1 shRNA tumors compared to the normal and control vector tumors. The MVD was also reduced in the SRPK1 shRNA group, though not significantly (Fig. 5c). We then performed a tubulogenesis assay, growing HUVECs on Matrigel in identical conditions as those used in the migration assays. Compared to the control group, the capillary network was incompletely formed in the Plexin-B1 shRNA and SRPK1 shRNA groups (Fig. 5d). In Fig. 6, we show a model for Sema4D/Plexin-B1-mediated signaling leading to angiogenesis and glioma cell migration by two routes: (1) Sema4D–PlexinB1/RhoA Integrin αvβ3 activation triggers the PI3K/Akt pathway to regulate apoptosis, cell motility, and angiogenesis in glioma cells; and (2) Plexin-B1 can regulate SRPK1 to phosphorylate Akt and influence glioma progression (Fig. 6).
Plexin-B1/ RhoA and SRPK1 signaling mediates angiogenesis in vivo and in vitro. a Tumor tissue immunofluorescence was performed to localize the expression of Plexin-B1 (red) and CD31 (green) in all groups. b Tumor tissue immunofluorescence was performed to localize the expression of SRPK1 (red) and CD31 (green) in all groups. c Paraffin sections of tumor tissues extracted from the mice implanted with U87 MG cells transfected with control vector, Plexin-B1 shRNA, SRPK1 shRNA, and buffer were prepared and immunohistochemically stained for CD31 (black arrows) to examine the microvessel density (MVD) in the tumors. Bars show the mean ± SEM (n = 3). *P < 0.05, **P < 0.01, versus control group. d Tubulogenesis assays were performed with HUVECs transfected with a co-GFP control vector or Plexin-B1 shRNA-GFP and SRPK1 shRNA-GFP. Compared to the control group, the capillary network was incompletely formed in the Plexin-B1 shRNA and SRPK1 shRNA groups
Model for Sema4D/Plexin-B1-mediated signaling leading to angiogenesis and glioma cell migration by two ways. (1) Sema4D–Plexin-B1 interactions promote phosphorylation of Plexin-B1 and the downstream effector RhoA, which is required for Sema4D–Plexin-B1-mediated cell migration and invasive growth. Plexin-B1-mediated attraction of ECs requires Rho, which then phosphorylates integrin to induce actomyosin stress fiber contraction and to direct the assembly of focal adhesion complexes. Integrin αvβ3 activation triggers the PI3K/Akt pathway to regulate apoptosis, cell motility, and angiogenesis in glioma. (2) In addition, Plexin-B1 can regulate SRPK1 to phosphorylate Akt and influence glioma progression
Discussion
The lethal outcome and dismal prognosis of glioma are due to the aggressive invasiveness and high vascular of gliomas. In this study, we focused on these two characteristics in an attempt to determine their molecular mechanisms and provide treatment strategies. Gliomas preferentially invade along white matter tracts such as the corpus callosum [21], suggesting the involvement of guidance molecules such as semaphorins in the invasion [22].
In the past decade, multiple reports show that Plexin-B1, the high affinity transmembrane receptor for Sema4D, regulates various cellular processes and plays a crucial role in tumor progression. Rho family proteins act as key regulators of cell migration and mediate glioma cell metastasis [23]. Plexin-B1 is induced by Sema4D to activate PDZ-RhoGEF to mediate RhoA activation, ensuring formation of focal adhesion complexes and increased integrin-dependent signaling networks that result in the activation of PI3K and Akt, as well as promote endothelial cell motility [20]. Migrating cells are highly polarized, with complex regulatory pathways that spatially and temporally integrate their component processes, including anchoring to the ECM and detachment of the trailing end. Integrin-mediated cell adhesions respond to changes in ECM properties, cytoskeletal organization, cell migration, and signaling processes; they contribute to tissue morphogenesis and coherence; and they provide cells with vital environmental cues [24]. Zhang et al. found that Plexin-B1 protein is overexpressed in gliomas compared to non-neoplastic brain tissues, with increasing expression levels of Plexin-B1 correlated with tumor grade [10]. These results suggest that Plexin-B1 might participate in the carcinogenesis and progression of glioma. In our study, we observed the overexpression of Sema4D and Plexin-B1 in glioma cells (Fig. 1). Furthermore, we depleted Plexin-B1 in human glioma cells using lentiviral-delivered shRNA to determine the role of Plexin-B1 in glioma progression. As Fig. 2 shows, knockdown of Plexin-B1 expression induced glioma cell apoptosis and reduced motility. We found that Plexin-B1 induces RhoGEF to regulate RhoA involved in actin activation and focal adhesion. However, knockdown of Plexin-B1 expression did not affect RhoB or RhoC activation, though it downregulated the expression of αvβ3 integrin.
However, conflicting data have been reported regarding the correlation of Plexin-B1 expression levels and cancer progression. Some groups report that Plexin-B1 acts as a tumor promoter, having higher expression levels in breast and ovarian cancers [7, 25]. Other studies show a downregulation of Plexin-B1 expression in melanoma and renal carcinoma [26, 27]. The same phenomenon also occurs with SRPK1. What leads to these significant differences?
SRPK1, as a class of enzymes, can mediate hyperphosphorylation of the oncogenic RNA-binding protein SRSF1 to regulate splicing of VEGF and promote angiogenesis [28]. A recent study finds that SRPK1 can function as both an oncogene and a tumor suppressor by interfering with PHLPP-mediated dephosphorylation of Akt [16]. Our study showed that knockdown of Plexin-B1 both decreases αvβ3 integrin activation and downregulates SRPK1 expression.
To investigate the relevant mechanism between Plexin-B1 and SRPK1, we assessed the activation of PI3K and Akt. As shown above, Plexin-B1 can regulate SRPK1 to influence the Akt pathway. These findings reveal that SRPK1 activity is dependent on upstream regulation. They also provide evidence for the interpretation of the different expression levels of Plexin-B1 in different tumors. Blood vessels are a critical substratum for glioma cell migration and growth. Indeed, anti-angiogenesis is already an important strategy for the treatment of gliomas. A recent report by Zhou et al. (2014) suggests that the semaphorin 4D-Plexin-B1-RhoA signaling axis recruits pericytes and regulates vascular permeability [29]. In addition, αvβ3-integrin and vascular endothelial growth factor receptor 2 (VEGFR2) synergistically interact in endothelial cells to promote angiogenesis [30]. Here, we identified the interaction of Plexin-B1 and αvβ3 integrin via the RhoA signaling pathway, providing new mechanism for glioma angiogenesis. Strikingly, knockdown Plexin-B1 induced a significant downregulation of angiogenesis compared to the control group (Fig. 5). These exciting results prompted us to explore its specific mechanism.
In conclusion, we first observed the tumor promoting effect of Plexin-B1 and highlighted the possible mechanism in glioma. Here, we demonstrated that Plexin-B1 regulates the RhoA/αvβ3 signaling axis to enter the PI3K/Akt pathway and also activates SRPK1 to influence Akt signaling, which is involved in glioma invasiveness and angiogenesis. Our study reveals the role of Plexin-B1 on glioma progression and sheds light on its regulatory mechanism. Our results further indicate that Plexin-B1 can be used as a marker for tumor diagnosis, increasing the discovery rate of tumor, improving patients’ long-term survival rate in advance. Plexin-B1 can also be used as therapeutic targets for glioma, inhibiting the growth, invasion, and migration of tumor cell, and causes clear boundaries with the normal surrounding tissue, making the surgery more thorough.
References
Statistics Korea. Statistic of mortality (2014) http://www.nso.go.kr/.
Soderberg-Naucler C, Rahbar A, Stragliotto G. Survival in patients with glioblastoma receiving valganciclovir. N Engl J Med. 2013;369:985–6.
Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80.
Vodrazka P, Korostylev A, Hirschberg A, Swiercz JM, Worzfeld T, Deng S, et al. The semaphorin 4D-plexin-B signalling complex regulates dendritic and axonal complexity in developing neurons via diverse pathways. Eur J Neurosci. 2009;30:1193–208.
Giacobini P, Messina A, Morello F, Ferraris N, Corso S, Penachioni J, et al. Semaphorin 4D regulates gonadotropin hormone-releasing hormone-1 neuronal migration through PlexinB1-Met complex. J Cell Biol. 2008;183:555–66.
Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17:1473–80.
Worzfeld T, Swiercz JM, Looso M, Straub BK, Sivaraj KK, Offermanns S. ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis. J Clin Invest. 2012;122:1296–305.
Zhou H, Binmadi NO, Yang YH, Proia P, Basile JR. Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression. Angiogenesis. 2012;15:391–407.
Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–70.
Zhang Y, Li Q, Zhuang R, Gao Z, Liu J, Li J, et al. Plexin-B1: a potential diagnostic biomarker for glioma and a future target for glioma immunotherapy. J Neuroimmunol. 2012;252:113–7.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22.
Kinbara K, Goldfinger LE, Hansen M, Chou FL, Ginsberg MH. Ras GTPases: integrins’ friends or foes? Nat Rev Mol Cell Biol. 2003;4:767–76.
Skuli N, Monferran S, Delmas C, Favre G, Bonnet J, Toulas C, et al. Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for hypoxia regulation in glioblastoma. Cancer Res. 2009;69:3308–16.
Ellegala DB, Leong-Poi H, Carpenter JE, Klibanov AL, Kaul S, Shaffrey ME, et al. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation. 2003;108:336–41.
Zhou B, Li Y, Deng Q, Wang H, Wang Y, Cai B, et al. SRPK1 contributes to malignancy of hepatocellular carcinoma through a possible mechanism involving PI3K/Akt. Mol Cell Biochem. 2013;379:191–9.
Wang P, Zhou Z, Hu A, Ponte de Albuquerque C, Zhou Y, Hong L, et al. Both decreased and increased SRPK1 levels promote cancer by interfering with PHLPP-mediated dephosphorylation of Akt. Mol Cell. 2014;54:378–91.
Wu Q, Chang Y, Zhang L, Zhang Y, Tian T, Feng G, et al. SRPK1 dissimilarly impacts on the growth, metastasis, chemosensitivity and angiogenesis of glioma in normoxic and hypoxic conditions. J Cancer. 2013;4:727–35.
Dussault AA, Pouliot M. Rapid and simple comparison of messenger RNA levels using real-time PCR. Biol Proced Online. 2006;8:1–10.
Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26.
Basile JR, Gavard J, Gutkind JS. Plexin-B1 utilizes RhoA and Rho kinase to promote the integrin-dependent activation of Akt and ERK and endothelial cell motility. J Biol Chem. 2007;282:34888–95.
Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–69.
Neufeld G, Shraga-Heled N, Lange T, Guttmann-Raviv N, Herzog Y, Kessler O. Semaphorins in cancer. Front Biosci. 2005;10:751–60.
Goldberg L, Kloog Y. A Ras inhibitor tilts the balance between Rac and Rho and blocks phosphatidylinositol 3-kinase-dependent glioblastoma cell migration. Cancer Res. 2006;66:11709–17.
Wolfenson H, Lavelin I, Geiger B. Dynamic regulation of the structure and functions of integrin adhesions. Dev Cell. 2013;24:447–58.
Ye S, Hao X, Zhou T, Wu M, Wei J, Wang Y, et al. Plexin-B1 silencing inhibits ovarian cancer cell migration and invasion. BMC Cancer. 2010;10:611.
Argast GM, Croy CH, Couts KL, Zhang Z, Litman E, Chan DC, et al. Plexin B1 is repressed by oncogenic B-Raf signaling and functions as a tumor suppressor in melanoma cells. Oncogene. 2009;28:2697–709.
Gomez Roman JJ, Garay GO, Saenz P, Escuredo K, Sanz Ibayondo C, Gutkind S, et al. Plexin B1 is downregulated in renal cell carcinomas and modulates cell growth. Transl Res. 2008;151:134–40.
Amin EM, Oltean S, Hua J, Gammons MV, Hamdollah-Zadeh M, Welsh GI, et al. WT1 mutants reveal SRPK1 to be a downstream angiogenesis target by altering VEGF splicing. Cancer Cell. 2011;20:768–80.
Zhou H, Yang YH, Basile JR. The Semaphorin 4D-Plexin-B1-RhoA signaling axis recruits pericytes and regulates vascular permeability through endothelial production of PDGF-B and ANGPTL4. Angiogenesis. 2014;17:261–74.
Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res. 2007;101:570–80.
Acknowledgments
This study was supported, in part, by the Key Project of the National Natural Science Foundation of Shandong Province (ZR 2009CL004), the China Postdoctoral Science Foundation (20100481466), the Foundation of Taishan Scholar (tshw20110575), the Pharmaceutical Health Science and Technology Development Program of Shandong Province (2011QZ001, 2013G0021816), the National Natural Science Foundation of China (81171142/H0910, 81271092, 61427807), a Project of the Shandong Province Higher Educational Science and Technology Program (J11LF61), and the Program of Major Research and Development Institutions in Fujian Province (2012I2014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Yingwei Chang and Li Li contributed to this work equally and should be considered as co-first authors
Rights and permissions
About this article
Cite this article
Chang, Y., Li, L., Zhang, L. et al. Plexin-B1 indirectly affects glioma invasiveness and angiogenesis by regulating the RhoA/αvβ3 signaling pathway and SRPK1. Tumor Biol. 37, 11225–11236 (2016). https://doi.org/10.1007/s13277-016-4849-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4849-9