Abstract
The semaphorins and plexins comprise a family of cysteine-rich proteins implicated in control of nerve growth and development and regulation of the immune response. Our group and others have found that Semaphorin 4D (SEMA4D) and its receptor, Plexin-B1, play an important role in tumor-induced angiogenesis, with some neoplasms producing SEMA4D in a manner analogous to vascular endothelial growth factor (VEGF) in order to attract Plexin-B1-expressing endothelial cells into the tumor for the purpose of promoting growth and vascularity. While anti-VEGF strategies have been the focus of most angiogenesis inhibition research, such treatment can lead to upregulation of pro-angiogenic factors that can compensate for the loss of VEGF, eventually leading to failure of therapy. Here, we demonstrate that SEMA4D cooperates with VEGF to promote angiogenesis in malignancies and can perform the same function in a setting of VEGF blockade. We also show the potential value of inhibiting SEMA4D/Plexin-B1 signaling as a complementary mechanism to anti-VEGF treatment, particularly in VEGF inhibitor–resistant tumors, suggesting that this may represent a novel treatment for some cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular endothelial growth factor (VEGF) plays a unique role in physiological and pathological angiogenesis. VEGF promotes endothelial cell proliferation and migration, increases vascular permeability and inhibits apoptosis of endothelial cells lining newly formed vessels. There are numerous splice isoforms of VEGF that bind with varying degrees of affinity to VEGF receptors (VEGFR) on the surface of endothelial cells. Most of the angiogenic effects attributed to VEGF are a result of activation of VEGFR-2, which signals through the phosphatidyl-inositol 3 kinase (PI3K)/Akt pathway [1]. VEGFR-1, on the other hand, was initially thought to act as a decoy receptor that sequestered VEGF, rendering it less available to VEGFR-2 [2]. More recently, studies have suggested that VEGFR-1 has a functional role in angiogenesis as well [3].
VEGF transcription is stimulated by hypoxia-inducible factor (HIF)-1 binding to hypoxia response elements within the VEGF promoter [4]. It is therefore an important protein produced by rapidly expanding neoplasms that begin to outgrow their blood supply or are exposed to chemotherapy- or radiotherapy-induced hypoxia in order to attract blood vessels into the tumor for the purposes of promoting survival and metastasis. Several promising therapeutic strategies are in development that specifically exploits the dependence of tumors on VEGF production, and it has become a tempting target for neutralizing antibodies in the treatment of advanced neoplasms. However, it is known that other angiogenic factors such as SDF-1, FGF-1 and -2, PlGF, and MMP9 can be elevated in tumors following anti-VEGF therapy, allowing them to overcome angiogenesis inhibition approaches that target only one factor [5]. Therefore, combination therapy with multiple anti-angiogenic agents might be recommended for more effective treatment.
The semaphorins were originally identified as axon guidance factors and regulators of proliferation and activation of lymphocytes [6, 7]. Currently, more than 20 semaphorins have been identified, which are grouped into eight classes: classes 1 and 2 are invertebrate semaphorins, classes 3–7 are found in vertebrates, and an eighth class, class V, has been identified in some non-neurotropic DNA viruses. Plexins, which are the functional receptors for the semaphorins, share homology in their extracellular segment with the scatter factor receptors c-Met and RON. In humans, at least nine plexins have been identified and grouped into four families, A through D, most of which have been shown to mediate neuronal cell adhesion and contact, nerve fasciculation, and axon guidance [8]. Some semaphorins, such as those in class 3, require co-receptors, the neuropilins (NPs), to bind and initiate intracellular signaling through the plexins [9]. In the case of Semaphorin 4D (SEMA4D), signaling is mediated directly through binding to its single-pass transmembrane receptor, Plexin-B1.
In addition to their better-defined functions, a variety of semaphorins and plexins are known to play a critical role in blood vessel guidance and endothelial precursor cell homing during physiological and pathological blood vessel development, with the class 3 semaphorins being the most highly studied in this regard [10]. Class 3 semaphorins have been shown to act through their receptors, the NPs and the A-family plexins, to initiate signaling events in a variety of tissues that influence vascular morphogenesis and endothelial cell motility [11]. Our group and others have shown that SEMA4D is shed from the surface of tumor cells and acts through Plexin-B1 on endothelial cells to promote angiogenesis and tumor growth and survival [12–14]. Here, we demonstrate that SEMA4D is increased in the setting of VEGF inhibition in head and neck squamous cell carcinoma (HNSCC). Using in vivo and in vitro angiogenesis assays, RNA interference and blocking antibodies, we examine the contributions of the SEMA4D/Plexin-B1 system to tumor-induced angiogenesis when compared to VEGF and demonstrate that targeting these proteins might represent a complementary or parallel mode of treatment for anti-angiogenic therapy of HNSCC and other solid tumors exhibiting insensitivity to anti-VEGF therapy.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVEC, ATCC, Manassas, VA) were cultured in Endothelial Cell Medium-2 (EGM-2, Lonza). 293T cells (ATCC) and the HNSCC cell lines HN12 [15], 13, and 30 (gifts of Dr. J. Silvio Gutkind) were cultured in DMEM (Sigma, St. Louis, MO). Media for the HN lines were supplemented with 10 % fetal bovine serum and 100 units/ml penicillin/streptomycin/amphotericin B (Sigma). CT26 cells (ATCC) were cultured in RPMI-1640 (Mediatech, Manassas, VA) supplemented with 10 % fetal bovine serum and 100 units/ml penicillin/streptomycin/amphotericin B (Sigma).
Production of soluble SEMA4D
Soluble SEMA4D (sSEMA4D) was produced and purified as described previously [12]. Briefly, the extracellular portion of SEMA4D was subjected to PCR and the resulting product cloned into the plasmid pSecTag2B (Invitrogen, Carlsbad, CA). This construct was transfected into 293T cells growing in serum-free media. Media containing sSEMA4D was collected 65 h post-transfection and purified with TALON metal affinity resin (Clontech Laboratories, Palo Alto, CA) according to manufacturer’s instructions. Concentration and purity of the TALON eluates was determined by SDS PAGE analysis followed by silver staining (Amersham Life Science, Piscataway, NJ) and the Bio-Rad protein assay (Bio-Rad, Hercules, CA). In all cases, media collected from cells transfected with the empty pSecTag2B vector were used as control.
Immunoblot analysis
Cells were infected with the shRNA lentiviral constructs or treated as indicated and lysed in lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1 % NP 40) supplemented with protease inhibitors (0.5 mM phenylmethylsulfonyl fluoride, 1 μl/ml aprotinin and leupeptin, Sigma) and phosphatase inhibitors (2 mM NaF and 0.5 mM sodium orthovanadate, Sigma) for 15 min at 4 °C. After centrifugation, protein concentrations were measured using the Bio-Rad protein assay (Bio-Rad). 100 μg of protein from each sample was subjected to SDS–polyacrylamide gel electrophoresis and transferred onto a PVDF membrane (Immobilon P, Millipore Corp., Billerica, MA). The membranes were stained with Ponceau red (Sigma), cut into sections containing the proteins of interest, and then simultaneously probed for SEMA4D (BD Transduction Labs, BD Biosciences, Palo Alto, CA, Cat#: 610670), Plexin-B1 (Santa Cruz A8, Santa Cruz, CA), VEGFR-2 (Santa Cruz Biotech), VEGF (Santa Cruz Biotech), and the loading control, GAPDH (Sigma). Proteins were detected using the ECL chemiluminescence system (Pierce, Rockford, IL).
Short hairpin (sh) RNA and lentiviral infections
The shRNA sequences for SEMA4D, VEGF, Plexin-B1, and VEGFR-2 were obtained from Cold Spring Harbor Laboratory’s RNAi library (RNAi Central, http://cancan.cshl.edu/RNAi_central/RNAi.cgi?type=shRNA) [16, 17]. The sequence used as PCR templates for SEMA4D has been previously reported [18]. The sequence for Plexin-B1 shRNA was 5′-TGC TGT TGA CAG TGA GCG CGC CCA GTA TGT GGC CAA GAA CTA GTG AAG CCA CAG ATG TAG TTC TTG GCC ACA TAC TGG GCA TGC CTA CTG CCT CGG A-3′. The sequence used for VEGF was 5′-TGC TGT TGA CAG TGA GCG CGC TTC CTA CAG CAC AAC AAA TTA GTG AAG CCA CAG ATG TAA TTT GTT GTG CTG TAG GAA GCT TGC CTA CTG CCT CGG A-3′. The sequence used for VEGFR-2 was 5′-TGC TGT TGA CAG TGA GCG AGC TTG GCC CGG GAT ATT TAT ATA GTG AAG CCA CAG ATG TAT ATA AAT ATC CCG GGC CAA GCC TGC CTA CTG CCT CGG A-3′. Oligos were synthesized (Invitrogen) and cloned into pWPI GW, a Gateway-compatible CSCG-based lentiviral destination vector, as previously described [18–20]. Viral stocks were prepared and infections performed as previously reported [18, 21, 22]. Control infections were performed with viruses manufactured with empty expression vectors.
RNA isolation and PCR analysis
RNA was extracted from HN12, 13, and 30 whole cell lysates, control-infected or infected with VEGF shRNA-expressing lentivirus, and converted into cDNA using the AMV reverse-transcriptional system (Promega, Madison, WI) in the presence of random hexamers (Invitrogen). The cDNA was used for quantitative real-time PCR with specific gene primers as follows: SEMA4D (BC054500) forward: 5′-GTCTTCAAAGAAGGGCAACAGG-3′, reverse: 5′-GAGCATTTCAGTTCCGCTGTG-3′; and 18S forward: 5′-TTGACGGAAGGGCACCACCAG-3′, reverse: 5′-GCACCACCACCCACGGAATCG-3′. An MYIQ real-time PCR detection system and SYBR green PCR mix (Bio-Rad) were used to carry out real-time PCR. The relative abundance of SEMA4D transcript was quantified using the comparative Ct method with 18S as an internal control. All data were analyzed from three independent experiments and statistical significance validated by Student’s t test.
In vitro migration assay
Serum-free media conditioned by the indicated cells or containing 400 ng/ml sSEMA4D and/or 100 ng/ml VEGF, concentrations of protein chosen based upon prior published migration assays [12, 23–26], were placed in the bottom well of a Boyden chamber while serum-free media containing migrating HUVEC cells previously infected with lentiviruses encoding the appropriate shRNA were added to the top chamber. The two chambers were separated by a polyvinylpyrrolidone membrane (8 μ pore size, Osmonics; GE Water Technologies, Trevose, PA) and the migration assay performed as described [12]. Cell migration was expressed as membrane-staining intensity relative to the negative control wells containing 0.1 % BSA. 10 % FBS was used as the positive control. Each experiment was performed three times and average and standard deviation calculated.
In vivo tubulogenesis assay
HUVEC, untreated or previously infected with lentiviruses encoding the appropriate shRNA, where indicated, were grown in 35-mm plates coated with 150 μl of Cultrex basement membrane extract (Trevigen, Gaithersburg, MD) and incubated overnight in media containing 0.1 % BSA (negative control), 10 % FBS (positive control), 400 ng/ml sSEMA4D, 100 ng/ml VEGF, or both sSEMA4D and VEGF, based upon concentrations used in the migration assays, or in media conditioned by control-infected HN12 cells or cells infected with lentiviruses encoding the indicated shRNA constructs. Cells were then fixed in 0.5 % glutaraldehyde and photographed. Quantification of results was determined using NIH Image, measuring and summing the length of all tubular structures observed in 10 random fields for three independent experiments.
Directed in vivo angiogenesis assay (DIVAA)
A DIVAA assay (Trevigen) was performed as previously described [27] with modifications. Briefly, angioreactors were filled with 18 μl of Cultrex reconstituted basement membrane substrate (Trevigen) containing PBS (negative control), basic fibroblast growth factor (FGF, positive control), 250 ng of sSEMA4D or 100 ng VEGF, or both, and implanted subcutaneously into the flanks of immunocompromised nude mice. Starting at day 1 after surgery, the mice received i.p. injections of 100 μg of anti-VEGF antibody (R & D Systems Minneapolis, MN), 100 μg of anti-SEMA4D antibody (VX15, which reacts with both human and mouse SEMA4D Vaccinex, Rochester, NY), or both anti-SEMA4D and anti-VEGF antibodies, followed by further injections on days 2, 4, 6, and 8. As negative controls, the mice were treated with matching isotypes IgG4 and IgG2a. Control antibodies were added to the treatment groups to match the total amount of protein delivered in the combination group. The mice were killed on day 9 and the angioreactors removed, photographed, and processed with FITC-labeled Griffonia lectin (FITC-lectin), an endothelial cell selective reagent [28, 29], to quantify invasion of endothelial cells into the angioreactors. Fluorescence was determined in a plate reader as mean relative fluorescence units for four reactors.
Tumor cell injections and animal studies
2 × 106 HN12 cells, controls or infected ex vivo with lentiviruses coding for SEMA4D shRNA or VEGF shRNA, or 8 × 104 CT26 cells were resuspended in 100 μl of serum-free DMEM with an equal volume of liquid Cultrex basement membrane extract (Trevigen) and injected subcutaneously into immunocompromised nude mice. CT26 and some HN12 tumor cells were grown in mice receiving i.p. injections of 100 μg of anti-VEGF antibody (R & D Systems) or 100 μg of anti-SEMA4D antibody (Vaccinex), where indicated, post injection. As negative controls, tumor-bearing mice were also treated with IgG4 and IgG2a. Control antibodies were added to the treatment groups to match the total amount of protein delivered in the combination group. Tumor volume was measured throughout the duration of the experiment. After tumor growth had been recorded, animals were killed and tumors removed for final determination of weight and sectioning and processing for immunohistochemistry and immunofluorescence. All animal studies were approved by the University of Maryland Office of Animal Welfare, Institutional Animal Care and Use Committee, in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Immunofluorescence
Tumor tissues were processed for co-immunofluorescence as described [30]. Briefly, OCT-embedded 8-μm-thick frozen tissue sections were cut onto silanated glass slides, air-dried, and stored at −80 °C. Cryosections were thawed, hydrated, fixed, blocked in 10 % FBS, and incubated overnight at 4 °C with primary antibodies diluted in a 2 % BSA/0.1 % Tween 20 solution in PBS. The following primary antibodies were used: anti-CD31 (anti-PECAM, BD Pharmingen; 1:100 dilution); anti-PDGFRβ (Abcam, Cambridge, MA, 1:200 dilution). After washing with PBS, the slides were incubated with FITC-conjugated anti-rat (Sigma) and Texas red-conjugated anti-rabbit (Calbiochem, EMD Biosciences, San Diego, CA) secondary antibodies for 1 h at room temperature. Finally, the slides were mounted with Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Slides were examined with a Nikon Eclipse E800 microscope system. Blood vessel content was determined by counting the number of CD31-stained vessels in 10 fields at a magnification of 100× and calculating the average and standard deviation.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tumor tissues harvested from mice were processed for immunohistochemistry as previously described [30]. Briefly, tissues were deparaffinized, hydrated, rinsed with PBS, blocked in Power Block (BioGenex, Fremont CA), and incubated overnight at 4 °C with primary antibodies diluted in a 2 % BSA/0.1 % Tween 20 solution in PBS. The following antibodies were used: Anti-Ki-67 (Santa Cruz Biotech, 1:50 dilution); anti-CD31 (anti-PECAM, BD Pharmingen; 1:100 dilution); anti-cleaved caspase 3 (Cell Signaling, Danvers, MA, 1:200 dilution). The slides were then washed in PBS, incubated with biotinylated secondary antibody (Biotinylated Link Universal, DAKO North America) for 45 min, and treated with strepavidin-HRP (DAKO North America) for 30 min at room temperature. The slides were developed in 3,3-diaminobenzidine (FASTDAB tablets; Sigma), counterstained with dilute Mayer’s hematoxylin, dehydrated, and mounted. Images were taken with an Aperio ScanScope CS scanner (Aperio, Vista, CA).
Statistical analysis
Student’s paired t tests were performed on means, and p values calculated: *, p < 0.05; **, p < 0.01.
Results
Semaphorin 4D increases in tumors in a background of VEGF inhibition
It is known that many different angiogenic factors are increased during cancer progression and development, sometimes in response to anti-VEGF therapy, eventually leading to treatment failure [5]. Our group and others have identified SEMA4D as a pro-angiogenic protein, shed by some solid tumors, that acts through Plexin-B1 on endothelial cells to enhance vessel growth into the developing malignancy [12–14]. Therefore, we looked for changes in SEMA4D protein and message in tumors and cell lines undergoing different anti-VEGF therapies to look for changes in the setting of VEGF blockade. Tumors comprised of HN12 cells infected with control lentiviruses or lentiviruses coding for VEGF short hairpin (sh) RNA, or grown in mice receiving anti-VEGF antibody injections, were harvested and processed to detect protein levels of SEMA4D. We noted high basal levels of SEMA4D, confirming our earlier findings [18, 31] but an increase in SEMA4D protein in tumors where VEGF had been silenced by shRNA when compared to controls (Fig. 1a, left, upper panel). Tumors harvested from anti-VEGF antibody-treated animals expressed higher levels of SEMA4D protein compared with IgG-treated controls (Fig. 1a, right, upper panel). Both tumors comprised of VEGF shRNA-infected HN12 cells and those harvested from mice receiving anti-VEGF antibody therapy expressed higher levels of SEMA4D message compared with their respective controls (Fig. 1b). To investigate these results in vitro and determine whether there could be a direct, microenvironment-independent feedback mechanism between VEGF and SEMA4D, we grew HN12, HN13, and HN30 cells in tissue culture, infected them with lentiviruses coding for VEGF shRNA and looked for changes in levels of SEMA4D protein. We confirmed VEGF knockdown in these cell lines (Fig. 1c, top row). As before, SEMA4D protein levels were increased in VEGF shRNA-infected cells versus control-infected cells (Fig. 1c, middle row). We again confirmed this effect at the transcriptional level, as VEGF shRNA-infected HN12, 13, and 30 cells expressed higher levels of SEMA4D message compared with controls (Fig. 1d). Taken together, these results suggest that SEMA4D, a pro-angiogenic protein strongly expressed in HNSCC cells and tumors, is further upregulated at the level of mRNA in HNSCC tumor tissues and cell lines where VEGF function is inhibited.
SEMA4D expression is elevated in HNSCC tumors and cell lines where VEGF function is inhibited by VEGF shRNA or anti-VEGF antibody. a Immunoblots for SEMA4D (upper panels) from tissue extracts of HN12 tumors comprised of control-infected cells (C) or cells infected with lentiviruses-expressing VEGF shRNA (VEGF shRNA, left panels) or from tumors where mice were treated with control antibody (IgG) or anti-VEGF antibody (anti-VEGF Ab) injections (right panels). b RNA was extracted from tumors from (a) to detect steady-state SEMA4D transcripts in a quantitative PCR analysis. The bar graph represents the ratio of SEMA4D mRNA to 18S mRNA, relative to controls, from three independent experiments (*p < 0.05). c Immunoblot for VEGF (upper panel) and SEMA4D (middle panel) from HN12, 13 and 30 cells growing in culture, infected with control lentivirus (C) or lentivirus-expressing VEGF shRNA (VEGF shRNA). For all immunoblots, GAPDH was used as the loading control (lower panels). d RNA was extracted to detect steady-state SEMA4D transcripts in a quantitative PCR analysis from HN12, 13, and 30 cells from (c). The bar graphs represent the ratio of SEMA4D mRNA to 18S mRNA, relative to controls, from three independent experiments (*p < 0.05)
A comparison of the in vitro pro-angiogenic effects of SEMA4D/Plexin-B1 and VEGF/VEGF-R2
Having established that SEMA4D increases in some anti-VEGF-treated tumors, we wanted to determine the in vitro angiogenic capacity of SEMA4D in comparison with VEGF. We employed HUVEC, control-infected or infected with Plexin-B1 shRNA-expressing lentiviruses or VEGFR-2 shRNA-expressing lentiviruses. Infected cells exhibited reduced expression of these proteins, respectively (Fig. 2a). When tested in a Boyden chamber migration assay, sSEMA4D acted as a strong promoter of cell migration compared with controls, but not when its receptor, Plexin-B1, was knocked down with the appropriate shRNA (Fig. 2b). VEGF induced a slightly stronger angiogenic response, which was abrogated in cells infected with VEGR-2 shRNA-expressing lentiviruses (Fig. 2b). When combined, sSEMA4D and VEGF promoted a more robust migratory response than either chemoattractant alone and greater than that seen in positive control cells migrating toward FBS. These results, which were statistically significant, are shown in the bar graph in Fig. 2c. We then looked for the ability of HUVEC growing on reconstituted basement membrane material to form tube-like capillary structures in tissue culture under identical conditions, which is indicative of a pro-angiogenic response. In both sSEMA4D and VEGF, HUVEC formed a capillary network, although not when infected with Plexin-B1 or VEGFR-2 shRNA-expressing lentiviruses, respectively (Fig. 2d). When combined, VEGF and sSEMA4D exhibited a greater response than either pro-angiogenic factor alone and greater than that seen in the positive control (Fig. 2d). These results are quantified in the bar graph in Fig. 2e. Taken together, these results indicate that SEMA4D alone can elicit a significant pro-angiogenic response in HUVEC similar to that of VEGF and that together SEMA4D and VEGF combine to yield a greater in vitro angiogenic phenotype compared with either factor alone.
SEMA4D and VEGF act together to promote endothelial cell migration and tube formation in vitro. a Immunoblot analysis for the SEMA4D receptor Plexin-B1 (left, upper panel) and the VEGF receptor VEGFR-2 (right, upper panel) in lysates from HUVEC infected with empty vector control lentivirus (C) or virus coding for the appropriate shRNA. GAPDH was used as a loading control (lower panels). b Control or shRNA-expressing lentivirus-infected HUVEC from (a) were examined in a Boyden chamber for migration toward 0.1 % BSA (negative control), 10 % FBS (positive control), SEMA4D, VEGF, or both. c The results of the migration assay, expressed as pixel intensity of scanned stained migration membranes relative to negative controls. Error bars represent the standard deviation from three independent experiments (*p < 0.05). d Control or shRNA-expressing lentivirus-infected HUVEC from (a) were plated on reconstituted basement membrane material in serum-free media with 0.1 % BSA (negative control), 10 % FBS (positive control), SEMA4D, VEGF or both and examined for formation of capillary tubes. e Quantification of the results of the tubulogenesis assay, measuring, and summing the length of all tubular structures observed in 10 random fields. Error bars represent the standard deviation from three independent experiments (*p < 0.05)
SEMA4D alone induces angiogenesis and cooperates with VEGF to elicit a stronger response in vivo
To compare the angiogenic effects of SEMA4D and VEGF in vivo, we performed an angiogenesis assay in immunocompromised nude mice implanted with silicon tubes (angioreactors) containing reconstituted basement membrane material mixed with PBS, basic fibroblast growth factor (FGF), sSEMA4D or VEGF, and receiving injections of isotype IgG control, anti-VEGF or anti-SEMA4D antibody, or both. FGF, which was used as a positive control, induced blood vessels to grow into the open end of the reactor while PBS failed to do so (Fig. 3a, left panel). Angioreactors containing either sSEMA4D or VEGF exhibited vessel growth into the open end of the reactor tube significantly above that for negative controls, with a slightly better response seen for VEGF (Fig. 3a, right panel). In vivo angiogenesis could be blocked for both factors with treatment with the corresponding blocking antibody. When present together, both sSEMA4D and VEGF elicited a more robust in vivo angiogenic response than either alone, but one that could be reduced by injections with both anti-VEGF- and anti-SEMA4D-blocking antibodies (Fig. 3a). These results are quantified in Fig. 3b, based upon FITC-lectin fluorescence binding to the endothelial cell contents of the angioreactors [27]. Taken together, these results show that SEMA4D can elicit a significant pro-angiogenic response in vivo similar to that of VEGF, even in the absence of VEGF, which would occur in the context of administration of anti-VEGF blocking antibody therapy. While SEMA4D and VEGF combine to yield a greater in vivo angiogenic response than either factor alone, the loss of angiogenesis observed when both inhibiting antibodies are used in combined therapy further suggests a role for blockade of both of these factors in the inhibition of pathological angiogenesis.
SEMA4D is a potent pro-angiogenic compound that cooperates with VEGF to enhance angiogenesis in vivo. a A DIVAA assay was performed in mice receiving injections of the indicated antibodies, measuring blood vessel growth into angioreactors containing reconstituted basement membrane material alone, or material mixed with VEGF, SEMA4D, or both. Negative and positive controls contain PBS and FGF, respectively (left panel). Photographs of representative angioreactors from the experiment are shown (right panels). b Quantification of blood vessel growth, as measured by FITC-lectin fluorescence (in arbitrary units, AU) from endothelial cell contents of each reactor, relative to negative controls (Y-axis). Error bars represent the standard deviation from four reactors (*p < 0.05; **p < 0.01)
Production of SEMA4D and VEGF by HNSCC contributes to an in vitro pro-angiogenic phenotype in HUVEC
As in lymphocytes [32] and platelets [33], SEMA4D is shed into the environment by tumor cells and acts on distant target tissues to exert its effects [13]. To specifically explore the effects on angiogenesis of tumor-produced SEMA4D relative to VEGF in vitro, we used HN12 cells, control-infected or infected with SEMA4D shRNA-expressing lentiviruses or VEGF shRNA-expressing lentiviruses. Infected cells exhibited reduced levels of protein compared with controls (Fig. 4a, top left and right panels). When the conditioned media from these cells were used as chemoattractants for HUVEC in a Boyden chamber migration assay, we observed that HUVEC migrated toward media from control-infected HN12 cells, but less so toward media from HN12 infected with lentiviruses coding for SEMA4D shRNA or VEGF shRNA (Fig. 4b). Media conditioned by HN12 cells infected with both SEMA4D and VEGF shRNA lentiviruses induced the least amount of HUVEC migration, comparable to that seen in negative controls (Fig. 4b). These results are quantified in the graph shown in Fig. 4c. We then performed a tubulogenesis assay on HUVEC growing on reconstituted basement membrane material in serum-free media conditioned by HN12 cells, control-infected or infected with lentiviruses coding for VEGF shRNA or SEMA4D shRNA. When cultured with 10 % FBS or in media conditioned by control-infected cells, HUVEC formed a recognizable capillary network, but less so when cultured with media conditioned by HN12 infected with SEMA4D shRNA-expressing lentiviruses or VEGF shRNA-expressing lentiviruses (Fig. 4d). Capillary formation was most inhibited when HUVEC were cultured in media conditioned by HN12 where both SEMA4D and VEGF were silenced (Fig. 4d). These results are quantified in the bar graph in Fig. 4e. Taken together, these results show that tumor-derived SEMA4D and VEGF are important in promoting HUVEC migration and capillary formation and that silencing both of these factors exerts a profound inhibition on in vitro angiogenesis.
SEMA4D and VEGF are produced by HNSCC and promote endothelial cell migration and tube formation in vitro.a Immunoblot analysis for SEMA4D (upper panel, left) and VEGF (upper right) in lysates from HN12 cells infected with empty vector control lentivirus (C) or virus coding for SEMA4D shRNA or VEGF shRNA, where indicated. GAPDH was used as a loading control (lower panels). b Boyden chamber migration assay in HUVEC toward 0.1 % BSA (negative control), 10 % FBS (positive control) or serum-free media conditioned by HN12 cells, control-infected or infected with lentivirus coding for SEMA4D shRNA, VEGF shRNA, or both. c Results of the migration assay from (b), quantified as the pixel intensity of scanned migration assay membranes relative to the negative control. Error bars represent the standard deviation from the averages from three wells (*p < 0.05; **p < 0.01). d HUVEC were plated on reconstituted basement membrane material in media containing 0.1 % BSA (negative control), 10 % FBS (positive control) or serum-free media conditioned by HN12 cells, control-infected or infected with lentivirus coding for SEMA4D shRNA, VEGF shRNA, or both, and examined for formation of capillary tubes. Representative photographs are shown. e Quantification of the results of the tubulogenesis assay, measuring, and summing the length of all tubular structures observed in 10 random fields. Error bars represent the standard deviation from three independent experiments (*p < 0.05; **p < 0.01)
HNSCC-mediated production of SEMA4D and VEGF cooperate to promote neoplastic cell proliferation, tumor growth and vascularity
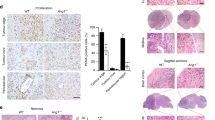
Since anti-VEGF therapy increased SEMA4D production in HNSCC tumors and cell lines and we observed SEMA4D to be strongly pro-angiogenic, similar to VEGF, we wanted to examine whether SEMA4D blockade augmented inhibition of tumor growth and vascularity seen in anti-VEGF therapy. We used the same populations of HN12 cells, control-infected or infected with VEGF shRNA or SEMA4D shRNA-expressing lentiviruses, injected subcutaneously into the flanks of immunocompromised nude mice. Tumor sizes were measured until kill, when they were photographed, weighed, and processed for immunohistochemistry and immunofluorescence. Tumors comprised of HN12 cells infected with SEMA4D shRNA-expressing lentiviruses were smaller than controls, whereas in HN12 cells infected with VEGF shRNA-expressing lentiviruses were slightly smaller still (Fig. 5a). In tumors comprised of cells that failed to express both SEMA4D and VEGF, growth was suppressed the greatest (Fig. 5a). Tumor volumes are shown graphically in Fig. 5b. Tumor weights were taken and show a similar pattern, with tumors that failed to express SEMA4D weighing about half that of controls and tumors that failed to express VEGF weighing slightly less (Fig. 5c). Tumors comprised of cells infected with lentiviruses expressing both SEMA4D and VEGF shRNA exhibited the greatest weight reduction (Fig. 5c). Knowing the influence of VEGF and SEMA4D on tumor-induced angiogenesis, we processed the tumor tissues for CD31 expression in immunofluorescence to look for vessel content. SEMA4D shRNA and VEGF shRNA-expressing lentiviruses both reduced blood vessel content of tumors compared to controls, with inhibition of VEGF showing a slightly greater response, but together exhibited a greater effect in decreasing tumor vascularity (Fig. 5d). These results are shown graphically in Fig. 5e. To determine the maturity of blood vessels in these tumors, we performed co-immunofluorescence to look for pericyte coverage of endothelial cells. We observed fewer and more immature blood vessels, as evidenced by a lack of pericyte coverage, in the stroma of tumors from HN12 cells infected with SEMA4D shRNA-expressing lentivirus compared with controls (Fig. 5f). The number of vessels in tumors comprised of VEGF shRNA-infected cells was also reduced but these vessels exhibited a mature phenotype, with well-formed pericyte sheaths surrounding the endothelial cells (Fig. 5f), as has been reported elsewhere [34], indicating a qualitative difference from vessels in tumors where SEMA4D expression is silenced. Importantly, vessels from tumors expressing both SEMA4D and VEGF shRNA exhibited an even greater reduction in vessel count and also lacked close pericyte association (Fig. 5f).
Production of SEMA4D by HNSCC cooperates with VEGF to promote tumor-induced angiogenesis. a HN12 cells infected with lentiviruses coding for control shRNA, SEMA4D shRNA, VEGF shRNA, or both were injected subcutaneously into nude mice along with a bolus of basement membrane extract. Representative tumors are shown at the time of kill. b The results of tumor volume measurement in cm3 are shown (n = 10; *p < 0.05). c The results of tumor weights in mg are shown (n = 10; *, p < 0.05; **p < 0.01). d Immunofluorescence for CD31 as a measure of vascular density from tumors derived from HN12 cells infected with a control lentivirus (control shRNA), lentivirus-expressing SEMA4D shRNA or VEGF shRNA, or both. Nuclei are stained with DAPI. e Results of measurement of vascular content from these tumors, determined by the average number of vessels in 10 high-power fields (hpf) of CD31-stained sections, are quantified in the bar graph (*p < 0.05; **p < 0.01). f Immunofluorescence for vessels (green, top row) and pericytes (red, middle row) in the same tumor populations, looking for association of pericytes with endothelial cells (merge, bottom row) as an indicator of vessel integrity and maturity. Tumors from cells-expressing SEMA4D shRNA exhibit reduced numbers of vessels that fail to associate with pericytes. Tumors comprised of HN12 cells infected with VEGF shRNA-expressing lentivirus also fail to induce robust blood vessel growth, but those vessels are closely associated with a pericyte sheath
We then processed these tumors to look at tumor cell growth, staining for Ki-67 expression, and apoptosis, using anti-cleaved caspase 3 antibody. Both shRNAs reduced proliferation of tumor cells, with SEMA4D shRNA exhibiting a slightly greater inhibition compared with VEGF shRNA (Fig. 6a). Together these two shRNA-expressing lentiviruses inhibited tumor cell proliferation greater than each one alone (Fig. 6a). These results are quantified in the bar graph in Fig. 6b. We then looked for the presence of cleaved caspase 3 fragment in these tumors, as an indicator of active apoptosis. At first, we observed very little cleaved caspase 3 in control-infected tumors and a much stronger signal in tumor tissues comprised of SEMA4D shRNA and VEGF shRNA-infected cells, either alone or together. Upon further examination we noted that the pattern of staining, instead of representing tumor cells, appeared to more closely resemble the distribution of blood vessels. Therefore, we performed immunohistochemistry on serial sections of these tumors for cleaved caspase 3 and CD31, looking for apoptosis that co-localized with endothelial cells. We observed very little cleaved caspase 3 in control-infected tumors (Fig. 6c, left column, lower panel). In tumors comprised of cells infected with SEMA4D shRNA or VEGF shRNA-expressing lentivirus, we observed more cleaved caspase 3 compared with controls, which corresponded with CD31 staining in the serial sections (Fig. 6c, second and third columns, respectively). The highest expression of cleaved caspase 3 was observed in tumors where the cells were infected with both types of lentiviruses (Fig. 6c, right column). These results are quantified in the bar graph in Fig. 6d. Taken together, these results suggest that SEMA4D and VEGF are produced by HNSCC for the purposes of enhancing vascularity, at least partly through protection of endothelial cells against apoptosis, in order to support tumor growth and survival, and that SEMA4D blockade enhances the anti-cancer activity of anti-VEGF therapy.
Production of SEMA4D and VEGF by HNSCC contributes to tumor cell proliferation and endothelial cell survival in tumor vasculature. a Immunohistochemistry for Ki-67, to measure proliferation of cells from tumors comprised of HN12 cells infected with control lentivirus (control shRNA), lentivirus-expressing SEMA4D shRNA, VEGF shRNA, or both. b Results of Ki-67 staining, expressed as percentage of positive cells observed from 10 high power fields. Error bars represent the standard deviation of the averages from three independent experiments (*p < 0.05; **p < 0.01). c The presence of cleaved caspase 3 (bottom row) was evaluated in tumors comprised of HN12 cells-expressing control shRNA, SEMA4D shRNA, VEGF shRNA or both, correlated with tumor sections stained for endothelial cells (CD31, top row). d Results of vascular apoptosis, expressed as percentage of vessels exhibiting cleaved caspase 3 observed from 10 high power fields, is shown in the bar graph (lower panel; p < 0.05; **p < 0.01)
Anti-SEMA4D therapy reduces tumor growth and vascularity of tumors resistant to anti-VEGF treatment
Because interference with SEMA4D functioning, either through blocking antibodies or shRNA knock down, could inhibit angiogenesis in vitro and in vivo and suppress the growth and vascularity of malignancies similar to that seen in VEGF/VEGFR-2 inhibition, we wanted to look at the ability of anti-SEMA4D therapy to rescue inhibition of tumor-induced angiogenesis and restrict tumor growth in the setting of loss of response to anti-VEGF therapy. Therefore, we injected CT26 tumor cells, which are resistant to anti-VEGF treatment [5], subcutaneously into host mice and treated with IgG control or anti-VEGF antibody. As expected, the tumors in anti-VEGF antibody-treated mice continued to grow at a rate similar to that seen in IgG-treated controls (Fig. 7a), reflecting a clinical situation where anti-VEGF treatment was unsuccessful and a different anti-angiogenic intervention was needed. Therefore, in two anti-VEGF antibody groups, treatment was changed to either IgG isotype control or anti-SEMA4D antibody starting on day 9. On day 18, the mice were killed and the tumors were photographed, weighed, and processed for immunofluorescence. We noted reduced sizes of tumors in mice switched to anti-SEMA4D antibody treatment compared with IgG controls or mice which received anti-VEGF antibody injections throughout the experiment (Fig. 7a). Tumor volumes are shown graphically in Fig. 7b and demonstrate that following the initial period of anti-VEGF therapy ‘failure,’ mice receiving anti-SEMA4D antibody injections during the latter half of the experiment exhibited significant reductions in tumor size compared with other groups (Fig. 7b). Tumor weights show a similar pattern, with tumors from mice receiving anti-VEGF antibody for the full 18 days and those receiving anti-VEGF antibody followed by IgG exhibiting similar weights to tumors from mice treated only with IgG (Fig. 7c). Tumor weights were reduced in mice receiving anti-SEMA4D antibody injections for the last 9 days (Fig. 7c). We then processed the tumors for CD31 expression in immunofluorescence to look for vessel content. Tumors from mice receiving either IgG or VEGF alone or anti-VEGF followed by IgG were highly vascular but there was a great reduction in vessel content in CT26 tumors harvested from mice where treatment was changed to anti-SEMA4D antibody injections (Fig. 7d). These results, which are shown graphically in Fig. 7e, suggest that anti-SEMA4D therapy could be a viable adjunct in the treatment of anti-VEGF therapy-resistant tumors.
Anti-SEMA4D therapy reduces growth and vascularity of tumors resistant to anti-VEGF treatment. a CT26 cells were injected subcutaneously into nude mice along with a bolus of basement membrane extract. Control mice were treated with IgG isotype control (IgG) or anti-VEGF antibody (anti-VEGF) for 18 days. Treatment for some mice receiving anti-VEGF antibody was changed to IgG (anti-VEGF + IgG) or anti-SEMA4D antibody (anti-VEGF + anti-SEMA4D) starting at day 9, extending until the end of the experiment (day 18). Representative tumors are shown at the time of kill. b The results of tumor volume measurement in cm3 are shown (n = 10; *p < 0.05; **p < 0.01). c The results of tumor weights in mg in mice treated with IgG or anti-VEGF antibody for the duration of the experiment or in mice treated with anti-VEGF antibody from days 1 to 9 and then switched to IgG or anti-SEMA4D antibody injections for days 9–18 (n = 10; *p < 0.05). d Immunofluorescence for CD31 as a measure of vascular density from tumors derived from CT26 cells. e Results of measurement of vascular content from these tumors determined by the average number of vessels in 10 high-power fields (hpf) for CD31-stained sections (*p < 0.05)
Discussion
The role of VEGF- and VEGFR-2 in tumor-induced angiogenesis has been well characterized. As a result, these proteins have been targeted as part of a treatment strategy for different neoplasms. Unfortunately, anti-VEGF therapies have failed to live up to expectations or extend cancer patient survival for more than a few months [35, 36]. It is now recognized that other angiogenic factors are upregulated during cancer progression and development, sometimes as a direct response to anti-VEGF therapy, and that failure of anti-angiogenic treatment can often be traced to the induction of these factors. Their identification is important, as they could also be targeted concurrently with VEGF in order to overcome anti-angiogenic evasion. Here, we show that anti-VEGF therapy increases SEMA4D production in tumor tissues and in cell culture and provide evidence that SEMA4D-mediated induction of angiogenesis plays a significant role in the growth and vascularity of HNSCC and possibly other cancers. Indeed, while SEMA4D cooperates with VEGF to promote angiogenesis and tumor progression, it also exerts significant effects when acting alone, as might occur in the background of anti-VEGF therapy.
There is great interest in determining the mechanism of how interference with VEGF upregulates other pro-angiogenic factors [37]. One possibility is that anti-VEGF therapy causes severe in intratumoral hypoxia, which could stimulate HIF-1-mediated production of other angiogenic factors by tumor cells. Fischer, et al. [5] have shown that VEGF blockade increases hypoxia and subsequently transcription of the pro-angiogenic genes Sdf-1, Fgf-1, Fgf-2, and Plgf. There is evidence in the literature that plexins and semaphorins are also regulated by changes in oxygen tension [38–40], while we recently have shown dramatic upregulation of SEMA4D protein and mRNA occurring in a HIF-dependent manner [31]. The results presented here demonstrate that increases in SEMA4D occur at the transcriptional level, so hypoxia and its associated transcription factors may in fact play a role. However, not all pro-angiogenic factors are influenced by hypoxia and not all anti-angiogenic interventions result in intratumoral hypoxia and increased levels of active HIF-1, casting doubt on a ‘global’ mechanism for hypoxic regulation for pro-angiogenic genes, including that of semaphorins. An alternative mechanism of angiogenic control might be contributions of pro-angiogenic and pro-inflammatory mediators from tumor associated macrophages (TAMs) and cancer-associated fibroblasts (CAFs) [41, 42]. TAMs account for the majority of the infiltrating inflammatory cells in a tumor mass and play an important role during tumor progression [43], producing factors that promote angiogenesis not only in mouse tumor xenograft models [44] but also in human cancers [45]. CT26 tumors likely are resistant to anti-VEGF treatment because they induce macrophage infiltration [5]. Since TAMs have been shown to produce SEMA4D, which contributes significantly to tumor-induced angiogenesis [41], perhaps this is another way anti-SEMA4D treatment successfully opposes induction of angiogenesis and could explain why SEMA4D blocking antibodies were so effective in reducing the growth and vascularity of CT26 tumors in our experiments.
We observed elevated SEMA4D expression in response to loss of VEGF not only in a mouse model but also in vitro, suggesting a host or microenvironment-independent feedback mechanism where cancer cells directly respond to changes in VEGF levels. While we have not detected VEGFR expression in most HNSCC cell lines examined (data not shown), cancer cells do in fact express another VEGF receptor, neuropilin-1 (NP-1), which has become a valid anti-tumor target in a variety of malignancies [46]. NPs mostly function as co-receptors that interact with other cell surface proteins to generate a signal. For example, VEGF engagement of NP-1 activates Met [47], a cell surface receptor homologous to the semaphorins and plexins that engages numerous downstream pathways, including NF-κB. In turn, NF-κB has been shown to inhibit HIF-1 activity [48], both in its ability to block HIF-1 transactivation of hypoxia responsive genes [49] and at the level of expression of the HIF-1α protein subunit [50]. Interestingly, there is evidence of direct NF-κB control over the HIF-1β promoter as well [51], a finding consistent with our data demonstrating elevated HIF-1β protein levels in HNSCC cells with silenced VEGF (unpublished observations). Therefore, we propose a possible mechanism where loss of VEGF signaling through NP-1 results in alleviation of NF-κB-mediated repression of HIF (either at the level of HIF activity or HIF-1α and β protein levels), and subsequent activation of SEMA4D transcription. Indeed, we have determined that Plexin-B1 itself activates NF-κB in a process necessary for angiogenesis [52], a pathway that could also serve as a negative feedback loop to turn off SEMA4D signaling in normal cells and tissues where appropriate. However, there are likely numerous feedback mechanisms between VEGF, SEMA4D and other pro-angiogenic factors that remain unexplored.
We have previously demonstrated that SEMA4D is expressed by many different cell lines and aggressive carcinomas [18] and that it is shed by tumor cells into the surrounding environment in a manner analogous to VEGF to act on endothelial cells and promote angiogenesis [13]. Here, when we compared the pro-angiogenic effects of SEMA4D with VEGF directly, we showed that SEMA4D potently induced angiogenesis to levels similar to that seem for VEGF. However, there appears to be some differences in the effects on tumor growth and vascularity between these two proteins. Interfering with SEMA4D and Plexin-B1 inhibited HUVEC migration and tube formation in vitro, as well as inhibiting in vivo angiogenesis, to levels comparable to that seen in anti-VEGF therapy, while VEGF blockade was slightly more effective in reducing tumor weight and volume. This is likely due to differences in signaling between their respective receptors, with VEGFR-2 in general inducing a more potent pro-angiogenic effect than Plexin-B1. However, it also possible that SEMA4D and Plexin-B1 might have effects on tumor growth directly, mediated by co-activation of the tyrosine kinase receptor Met [53], as previously noted. While we have not thoroughly studied the proliferative effects of SEMA4D on tumor cells themselves, we did note a greater decrease in Ki-67 staining from tumors where SEMA4D was silenced, compared with those comprised of cells infected with VEGF shRNA-expressing virus. There is evidence in the literature showing that tumors expressing high levels of SEMA4D are more aggressive, more difficult to treat, and have a poor prognosis, without specifically describing any effect on angiogenesis [54, 55]. A recent study of Semaphorin 3E (SEMA3E)/Plexin-D1 signaling suggests that these proteins are able to act in an autocrine or paracrine manner on tumor cells to enhance invasiveness but do so without affecting tumor size or vascularity, as SEMA3E is actually anti-angiogenic [56].
Further differences between the effects of VEGF and SEMA4D on tumors were reflected in differences in vessel maturity. Vessels from tumors composed of cells infected with VEGF shRNA-expressing lentivirus demonstrated a close association with pericytes, similar to what others have reported [34], while tumors composed of cells infected with SEMA4D shRNA-expressing lentiviruses, or of cells infected with both lentiviruses, lacked this organization. We are not sure of the significance of these findings, except that vessels not associated with pericyte sheaths are more immature, leaky, less capable of remodeling, and may be more reliant on growth factors for survival. If blocking SEMA4D function inhibits vascular maturation and remodeling, it could render vessels more reliant on VEGF for survival and thus more susceptible to anti-VEGF antibody or VEGF-trap approaches in some sort of combined therapy. In fact, we did observe enhanced endothelial cell apoptosis in tumors where both SEMA4D and VEGF were silenced, which resulted in greatly reduced tumor size. Finally, anti-SEMA4D antibody partially rescued growth inhibition in anti-VEGF therapy-resistant CT26 tumors, which was reflected in decreased blood vessel content, suggesting that SEMA4D blockade could be an excellent form of treatment for some malignancies concurrent with anti-VEGF therapy or when anti-VEGF therapy has failed. Anti-SEMA4D therapy also would likely be very safe, as Plexin-B1 signaling is redundant in normal vascular development [57] but as we have shown contributes significantly to angiogenesis in malignancies.
In conclusion, we show that the ability of tumors to overcome or evade anti-angiogenic therapies is at least in part determined by the pro-angiogenic protein SEMA4D. In this study, we show that SEMA4D contributes to tumor-induced angiogenesis and that SEMA4D blockade enhances the anti-cancer activity of anti-VEGF treatments. Interference with SEMA4D-mediated pathways could be a viable adjunct to anti-VEGF therapy.
Change history
10 March 2020
The Editors-in-Chief have retracted this article [1] following an investigation by the University of Maryland. The institution found that in Figures 1B and 1D, the cell lines are different and all published histograms show SEMA4D mRNA level whereas Excel data have two histograms showing SEMA4D expression and two histograms showing VEGF expression. In Figure 2B, the metadata for one image shows different treatment conditions than those reported in the article. The published image labelled VEGF + VEGFR-2 shRNA has a metadata label of S4d-plexinB1 shRNA2. In Figure 2E, statistical significance was shown in the published figure for four comparisons, but upon recalculation, one comparison noted as significant was not. In Figure 6A, the lower left image is labelled VEGF shRNA in the published figure, but the metadata label is S4DshRNA-HN121-20X. In Figure 6C, specifically, within columns 2-4, for each antibody used for immunocytochemistry, the three images have been swapped so that the original images do not match the shRNA labels in the figure (the labels for the two antibodies were correct). In Figure 7D, the first published image is labelled as IgG in the paper, but the metadata show a label of Restore (V+S).tif. The third published image has a label of anti-VEGF IgG, and the metadata show a label of con sh.tif. Due to these errors, the Editors-in-Chief have found that the results are no longer reliable.
10 March 2020
The Editors-in-Chief have retracted this article [1] following an investigation by the University of Maryland. The institution found that in Figures 1B and 1D, the cell lines are different and all published histograms show SEMA4D mRNA level whereas Excel data have two histograms showing SEMA4D expression and two histograms showing VEGF expression. In Figure 2B, the metadata for one image shows different treatment conditions than those reported in the article. The published image labelled ���VEGF + VEGFR-2 shRNA��� has a metadata label of S4d-plexinB1 shRNA2���. In Figure 2E, statistical significance was shown in the published figure for four comparisons, but upon recalculation, one comparison noted as significant was not. In Figure 6A, the lower left image is labelled ���VEGF shRNA��� in the published figure, but the metadata label is ���S4DshRNA-HN121-20X���. In Figure 6C, specifically, within columns 2-4, for each antibody used for immunocytochemistry, the three images have been swapped so that the original images do not match the shRNA labels in the figure (the labels for the two antibodies were correct). In Figure 7D, the first published image is labelled as ���IgG��� in the paper, but the metadata show a label of ���Restore (V+S).tif���. The third published image has a label of ���anti-VEGF IgG���, and the metadata show a label of ���con sh.tif���. Due to these errors, the Editors-in-Chief have found that the results are no longer reliable.
References
Zachary I (2003) Vascular endothelial growth factor and anti-angiogenic peptides as therapeutic and investigational molecules. IDrugs 6:224–231
Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M (1998) Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA 95:9349–9354
Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7:1194–1201
Semenza GL (2001) Regulation of hypoxia-induced angiogenesis: a chaperone escorts VEGF to the dance. J Clin Invest 108:39–40
Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N, Giacca M, Stassen JM, Dewerchin M, Collen D, Carmeliet P (2007) Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131:463–475
Kumanogoh A, Kikutani H (2001) The CD100–CD72 interaction: a novel mechanism of immune regulation. Trends Immunol 22:670–676
Hall KT, Boumsell L, Schultze JL, Boussiotis VA, Dorfman DM, Cardoso AA, Bensussan A, Nadler LM, Freeman GJ (1996) Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc Natl Acad Sci USA 93:11780–11785
Carmeliet P, Tessier-Lavigne M (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436:193–200
Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM (1999) Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99:59–69
Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van NP, Fishman MC, Childs S, Epstein JA, Weinstein BM (2004) Semaphorin–plexin signaling guides patterning of the developing vasculature. Dev Cell 7:117–123
Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M (2002) Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci USA 99:3657–3662
Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS (2004) Class IV semaphorins promote angiogenesis by stimulating rho-initiated pathways through plexin-B. Cancer Res 64:5212–5224
Basile JR, Holmbeck K, Bugge TH, Gutkind JS (2007) MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem 282:6899–6905
Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, Bussolino F, Giordano S (2005) Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood 105:4321–4329
Cardinali M, Pietraszkiewicz H, Ensley JF, Robbins KC (1995) Tyrosine phosphorylation as a marker for aberrantly regulated growth-promoting pathways in cell lines derived from head and neck malignancies. Int J Cancer 61:98–103
Siolas D, Lerner C, Burchard J, Ge W, Linsley PS, Paddison PJ, Hannon GJ, Cleary MA (2005) Synthetic shRNAs as potent RNAi triggers. Nat Biotechnol 23:227–231
Hannon GJ, Conklin DS (2004) RNA interference by short hairpin RNAs expressed in vertebrate cells. Methods Mol Biol 257:255–266
Basile JR, Castilho RM, Williams VP, Gutkind JS (2006) Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. PNAS 103:9017–9022
Paddison PJ, Caudy AA, Sachidanandam R, Hannon GJ (2004) Short hairpin activated gene silencing in mammalian cells. Methods Mol Biol 265:85–100
Paddison PJ, Silva JM, Conklin DS, Schlabach M, Li M, Aruleba S, Balija V, O’Shaughnessy A, Gnoj L, Scobie K, Chang K, Westbrook T, Cleary M, Sachidanandam R, McCombie WR, Elledge SJ, Hannon GJ (2004) A resource for large-scale RNA-interference-based screens in mammals. Nature 428:427–431
Naldini L, Blomer U, Gage FH, Trono D, Verma IM (1996) Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA 93:11382–11388
Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D (1996) In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263–267
Stone D, Phaneuf M, Sivamurthy N, LoGerfo FW, Quist WC (2002) A biologically active VEGF construct in vitro: implications for bioengineering-improved prosthetic vascular grafts. J Biomed Mater Res 59:160–165
Wang F, Yamauchi M, Muramatsu M, Osawa T, Tsuchida R, Shibuya M (2011) RACK1 regulates VEGF/Flt1-mediated cell migration via activation of a PI3 K/Akt pathway. J Biol Chem 286:9097–9106
Carretero-Ortega J, Walsh CT, Hernandez-Garcia R, Reyes-Cruz G, Brown JH, Vazquez-Prado J (2010) Phosphatidylinositol 3,4,5-triphosphate-dependent Rac exchanger 1 (P-Rex-1), a guanine nucleotide exchange factor for Rac, mediates angiogenic responses to stromal cell-derived factor-1/chemokine stromal cell derived factor-1 (SDF-1/CXCL-12) linked to Rac activation, endothelial cell migration, and in vitro angiogenesis. Mol Pharmacol 77:435–442
Mirzapoiazova T, Kolosova I, Usatyuk PV, Natarajan V, Verin AD (2006) Diverse effects of vascular endothelial growth factor on human pulmonary endothelial barrier and migration. Am J Physiol Lung Cell Mol Physiol 291:L718–L724
Guedez L, Rivera AM, Salloum R, Miller ML, Diegmueller JJ, Bungay PM, Stetler-Stevenson WG (2003) Quantitative assessment of angiogenic responses by the directed in vivo angiogenesis assay. Am J Pathol 162:1431–1439
Sahagun G, Moore SA, Fabry Z, Schelper RL, Hart MN (1989) Purification of murine endothelial cell cultures by flow cytometry using fluorescein-labeled griffonia simplicifolia agglutinin. Am J Pathol 134:1227–1232
Laitinen L (1987) Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. Histochem J 19:225–234
Amornphimoltham P, Sriuranpong V, Patel V, Benavides F, Conti CJ, Sauk J, Sausville EA, Molinolo AA, Gutkind JS (2004) Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: a potential target for UCN-01. Clin Cancer Res 10:4029–4037
Sun Q, Zhou H, Binmadi NO, Basile JR (2009) Hypoxia-inducible factor-1-mediated regulation of semaphorin 4D affects tumor growth and vascularity. J Biol Chem 284:32066–32074
Elhabazi A, Delaire S, Bensussan A, Boumsell L, Bismuth G (2001) Biological activity of soluble CD100. I. The extracellular region of CD100 is released from the surface of T lymphocytes by regulated proteolysis. J Immunol 166:4341–4347
Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, Tamagnone L, Wagner DD, Milla ME, Brass LF (2007) Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci USA 104:1621–1626
Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le Couter J, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ (2007) Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 11:53–67
Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91:1071–1121
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307
Ferrara N (2010) Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev 21:21–26
Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL (2002) MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell 109:887–900
Siebold C, Berrow N, Walter TS, Harlos K, Owens RJ, Stuart DI, Terman JR, Kolodkin AL, Pasterkamp RJ, Jones EY (2005) High-resolution structure of the catalytic region of MICAL (molecule interacting with CasL), a multidomain flavoenzyme-signaling molecule. Proc Natl Acad Sci USA 102:16836–16841
Ventura A, Pelicci PG (2002) Semaphorins: green light for redox signaling? Sci STKE 2002:PE44
Sierra JR, Corso S, Caione L, Cepero V, Conrotto P, Cignetti A, Piacibello W, Kumanogoh A, Kikutani H, Comoglio PM, Tamagnone L, Giordano S (2008) Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med 205:1673–1685
Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N (2009) PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 15:21–34
Albini A, Sporn MB (2007) The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer 7:139–147
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66:11238–11246
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4:71–78
Jubb AM, Strickland LA, Liu SD, Mak J, Schmidt M, Koeppen H (2012) Neuropilin-1 expression in cancer and development. J Pathol 226:50–60
Zhang S, Zhau HE, Osunkoya AO, Iqbal S, Yang X, Fan S, Chen Z, Wang R, Marshall FF, Chung LW, Wu D (2010) Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Mol Cancer 9:9
Mendonca DB, Mendonca G, Aragao FJ, Cooper LF (2011) NF-kappaB suppresses HIF-1alpha response by competing for P300 binding. Biochem Biophys Res Commun 404:997–1003
Shin DH, Li SH, Yang SW, Lee BL, Lee MK, Park JW (2009) Inhibitor of nuclear factor-kappaB alpha derepresses hypoxia-inducible factor-1 during moderate hypoxia by sequestering factor inhibiting hypoxia-inducible factor from hypoxia-inducible factor 1alpha. FEBS J 276:3470–3480
Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M (2008) NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453:807–811
van Uden P, Kenneth NS, Webster R, Muller HA, Mudie S, Rocha S (2011) Evolutionary conserved regulation of HIF-1beta by NF-kappaB. PLoS Genet 7:e1001285
Yang YH, Zhou H, Binmadi NO, Proia P, Basile JR (2011) Plexin-B1 activates NF-kappaB and IL-8 to promote a pro-angiogenic response in endothelial cells. PLoS ONE 6:e25826
Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM (2002) The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol 4:720–724
Ch’ng E, Tomita Y, Zhang B, He J, Hoshida Y, Qiu Y, Morii E, Nakamichi I, Hamada K, Ueda T, Aozasa K (2007) Prognostic significance of CD100 expression in soft tissue sarcoma. Cancer 110:164–172
Valente G, Nicotra G, Arrondini M, Castino R, Capparuccia L, Prat M, Kerim S, Tamagnone L, Isidoro C (2009) Co-expression of plexin-B1 and Met in human breast and ovary tumours enhances the risk of progression. Cell Oncol 31:423–436
Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, Risio M, Trusolino L, Weitz J, Schneider M, Mazzone M, Comoglio PM, Tamagnone L (2010) Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Invest 120:2684–2698
Fazzari P, Penachioni J, Gianola S, Rossi F, Eickholt BJ, Maina F, Alexopoulou L, Sottile A, Comoglio PM, Flavell RA, Tamagnone L (2007) Plexin-B1 plays a redundant role during mouse development and in tumour angiogenesis. BMC Dev Biol 7:55
Acknowledgments
The authors would like to thank Ernest Smith and Maurice Zauderer of Vaccinex, Inc., for providing the anti-SEMA4D antibody and offering technical support and Daniel Martin and Silvio Gutkind of the National Institute of Dental and Craniofacial Research, NIH, for contributing the head and neck cancer cell lines and assisting in the generation of shRNA lentiviruses. This work was supported by the National Cancer Institute grant R01-CA133162 to J.R.B.
Author information
Authors and Affiliations
Corresponding author
Additional information
The Editors-in-Chief have retracted this article following an investigation by the University of Maryland. The institution found problems in some figures. Due to these errors, the Editors-in-Chief have found that the results are no longer reliable.
About this article
Cite this article
Zhou, H., Binmadi, N.O., Yang, YH. et al. RETRACTED ARTICLE: Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression. Angiogenesis 15, 391–407 (2012). https://doi.org/10.1007/s10456-012-9268-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-012-9268-y