Abstract
Sprouty (SPRY) proteins are well-characterized factors that inhibit receptor tyrosine kinase (RTK)-mediated activation of cellular signaling pathways. The down-regulation of SPRY4 expression has been reported in human ovarian cancer. However, the specific roles and mechanisms by which SPRY4 affects ovarian cancer progression are completely unknown. Amphiregulin (AREG) binds exclusively to the epidermal growth factor receptor (EGFR) and has been considered to be a dominant autocrine/paracrine EGFR ligand in ovarian cancer. In the present study, we first examined the effects of AREG on SPRY4 expression and the possible underlying molecular mechanisms involved in this process in two human ovarian cancer cell lines. Our results demonstrated that treatment with AREG up-regulated SPRY4 expression by activating the ERK1/2 signaling pathway. In addition, we showed that small interfering RNA (siRNA)-mediated knockdown of SPRY4 attenuated the AREG-induced down-regulation of E-cadherin by inhibiting the expression of SNAIL but not SLUG. In contrast, overexpression of SPRY4 enhanced AREG-induced down-regulation of E-cadherin by increasing the expression of SNAIL. Moreover, SPRY4 knockdown attenuated AREG-induced cell migration and invasion. Overexpression of SPRY4 enhanced AREG-induced cell invasion. This study reveals that SPRY4 is involved in EGFR-mediated human ovarian cancer progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sprouty (SPRY), which can antagonize fibroblast growth factor (FGF)-regulated tracheal development, was first identified in Drosophila [1]. Four mammalian SPRY genes (SPRY1–4) with sequence similarity to Drosophila SPRY have been identified [2]. In adult and mouse embryos, SPRY1, SPRY2, and SPRY4 are present in various tissues and organs, whereas the expression of SPRY3 is restricted to the brain and testes in adults [2, 3]. To date, many studies have demonstrated that, similar to Drosophila SPRY, mammalian SPRY inhibits the activation of the ERK1/2 signaling pathway in response to a wide range of growth factors [4]. Moreover, increasing evidence has indicated that there is aberrant expression of SPRY in different types of human cancer, and SPRY is involved in the regulation of tumorigenesis [5].
Although ovarian cancer accounts for only approximately 3 % of all cancers among women, it remains the primary cause of death from gynecological malignancies in developed countries due to the lack of effective screening methods and a paucity of symptoms during the early stages of the disease. The majority of women with ovarian cancer are diagnosed at a late stage when the cancer has spread beyond the confines of the ovary. Overexpression of the epidermal growth factor receptor (EGFR) has been detected in ovarian cancer and is associated with more aggressive clinical behavior and a poor prognosis [6]. Multiple cognate ligands have been shown to bind to and activate the EGFR, while only epidermal growth factor (EGF), amphiregulin (AREG) and transforming growth factor-α (TGF-α) bind to the EGFR exclusively [7]. In ovarian cancer tissues, cell lines, and peritoneal fluid, the expression levels of AREG are significantly higher than those of EGF or TGF-α, which indicates that AREG is the most important autocrine/paracrine ligand of the EGFR [8, 9].
We have shown that treating human ovarian cancer cells with AREG induces cell invasion by down-regulating E-cadherin expression [10, 11]. In addition, activation of ERK1/2 signaling is involved in EGFR-mediated down-regulation of E-cadherin and cell invasion in ovarian cancer cells [11–14]. Importantly, recent studies by our and other groups have demonstrated that the expression levels of SPRY4 are down-regulated in ovarian cancer cell lines and clinical tissue samples, which suggest that SPRY4 has an important role in ovarian cancer progression [15, 16]. However, thus far, the function of SPRY4 in ovarian cancer is completely unknown. In addition, whether AREG can regulate SPRY4 expression needs to be determined. Given the importance of SPRY proteins in regulating ERK1/2 activity, the present study was designed to investigate the effects of AREG on SPRY4 expression and to clarify the role of SPRY4 in AREG-induced down-regulation of E-cadherin and cell invasion in human ovarian cancer.
Materials and methods
Cell culture
The SKOV3 human ovarian cancer cell line was obtained from the American Type Culture Collection (Manassas, VA). The OVCAR5 human ovarian cancer line was kindly provided by Dr. T.C. Hamilton (Fox Chase Cancer Center, Philadelphia, PA). Cells were grown in a 1:1 (v/v) mixture of M199/MCDB105 medium (Sigma, Oakville, ON) supplemented with 10 % fetal bovine serum (FBS; Hyclone Laboratories Inc., Logan, UT). The cultures were maintained at 37 °C in a humidified 5 % CO2 atmosphere.
Antibodies and reagents
The polyclonal anti-sprouty4 antibody (#ab7513) was obtained from Abcam (Toronto, ON). The monoclonal anti-E-cadherin antibody (#610181) was obtained from BD Biosciences (Mississauga, ON). The polyclonal anti-actin antibody (#sc-1615) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The polyclonal anti-ERK1/2 (#9102), anti-phospho-AKT (#9271), anti-AKT (#9272), anti-Slug (#9585), monoclonal anti-phospho-ERK1/2 (#9106), and anti-Snail (#3895) antibodies were obtained from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase-conjugated goat anti-mouse IgG and goat anti-rabbit IgG were obtained from Bio-Rad Laboratories (Hercules, CA). Horseradish peroxidase-conjugated donkey anti-goat IgG was obtained from Santa Cruz Biotechnology. AG1478 and LY294002 were obtained from Sigma. U0126 was obtained from Calbiochem (San Diego, CA). Recombinant human AREG was purchased from R&D Systems (Minneapolis, MN).
Small interfering RNA transfection and overexpression
For endogenous ERK1, ERK2, or SPRY4 knockdown, the cells were transfected with 50 nM ON-TARGETplus SMARTpool-specific small interfering RNA (siRNA) (Dharmacon Research, Inc., Lafayette, CO) using Lipofectamine RNAiMAX (Invitrogen, Burlington, ON). Non-targeting siCONTROL siRNA (Dharmacon) was used as a transfection control. To overexpress human SPRY4, 1 μg pXJ40-FLAG-SPRY4 vector or empty pXJ40-FLAG vector (gifts from Dr. Graeme R. Guy, Institute of Molecular and Cell Biology, Singapore) was transfected using Lipofectamine 3000 (Invitrogen).
Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted using the TRIzol reagent (Invitrogen, Life Technologies, Burlington, ON) according to the manufacturer’s instructions. Reverse transcription was performed with 3 μg RNA, random primers, and M-MLV reverse transcriptase (Promega, Madison, WI). The primers used for SYBR Green real-time quantitative PCR (RT-qPCR) were as follows: SPRY4, forward 5′-AGC CTG TAT TGA GCG GTT TG-3′ and reverse 5′-GGT CAA TGG GTA GGA TGG TG-3′; E-cadherin, forward 5′-ACA GCC CCG CCT TAT GAT T-3′ and reverse 5′-TCG GAA CCG CTT CCT TCA-3′; SNAIL, forward 5′-CCC CAA TCG GAA GCC TAA CT-3′ and reverse 5′- GCT GGA AGG TAA ACT CTG GAT TAG A-3′; SLUG, forward 5′-TTC GGA CCC ACA CAT TAC CT-3′ and reverse 5′-GCA GTG AGG GCA AGA AAA AG-3′; and GAPDH, forward 5′-GAG TCA ACG GAT TTG GTC GT-3′ and reverse 5′-GAC AAG CTT CCC GTT CTC AG-3′. RT-qPCR was performed using an Applied Biosystems 7300 Real-Time PCR System (Perkin-Elmer) equipped with a 96-well optical reaction plate. All RT-qPCR results represent the means from at least three independent experiments conducted in triplicate. The relative quantification of the messenger RNA (mRNA) levels was performed by the comparative Ct method using GAPDH as the reference gene and the formula 2−∆∆Ct.
Western blot
Cells were lysed in lysis buffer (Cell Signaling Technology), and the protein concentrations were determined using a DC protein assay kit with BSA as the standard (Bio-Rad Laboratories). Equal amounts of protein were separated by SDS polyacrylamide gel electrophoresis and were transferred to PVDF membranes. After being blocked with Tris-buffered saline (TBS) containing 5 % non-fat dry milk for 1 h, the membranes were incubated overnight at 4 °C with primary antibodies, followed by incubation with the HRP-conjugated secondary antibody. Immunoreactive bands were detected with an enhanced chemiluminescent substrate (Pierce, Rockford, IL).
Migration and invasion assay
Cell culture inserts (24-well, pore size 8 μm; BD Biosciences) were seeded with 1 × 105 cells in 250 μl of medium with 0.1 % FBS. Uncoated inserts were used for migration assays whereas inserts pre-coated with growth factor reduced Matrigel (50 μl, 1 mg/ml; BD Biosciences) were used for invasion assays. Medium with 10 % FBS (750 μl) was added to the lower chamber and served as a chemotactic agent. After 24- (migration) or 48-h (invasion) incubation, non-migrating/invading cells were wiped from the upper side of the membrane and cells on the lower side were fixed in cold methanol and air-dried. The cell nuclei were stained with crystal violet (Sigma), and the cell numbers were counted. Each individual experiment was performed with triplicate inserts, and five microscopic fields were counted per insert.
Statistical analysis
The results are presented as the means ± SEM of at least three independent experiments. The results were analyzed by a one-way ANOVA and Tukey’s multiple comparison test using the PRISM software. Significant differences were defined by values of P < 0.05.
Results
Amphiregulin up-regulates SPRY4 expression in human ovarian cancer cells
To examine the effects of AREG on SPRY4 expression, SKOV3 human ovarian cancer cells were treated with 10 ng/ml AREG for different periods of time, and the SPRY4 mRNA levels were examined by RT-qPCR. As shown in Fig. 1a, the SPRY4 mRNA levels were significantly up-regulated by treatment with AREG, with the maximum level observed 3 h after AREG treatment. In the human follicular fluid, the concentration of AREG was ∼100 ng/ml [17]. Therefore, we next examined the effects of different concentrations of AREG (1, 10, and 100 ng/ml) on SPRY4 expression. As shown in Fig. 1b, c, treatment with 1 ng/ml AREG did not significantly up-regulate the SPRY4 mRNA or protein levels. However, treatment with 10 and 100 ng/ml AREG led to comparable stimulatory effects on the SPRY4 mRNA and protein levels. Therefore, 10 ng/ml AREG was used in the subsequent experiments. In addition, treatment with AREG up-regulated SPRY4 protein levels in a time-dependent manner (Fig. 1d). Moreover, the stimulatory effect of AREG on SPRY4 protein expression was further confirmed in another human ovarian cancer cell line, OVCAR5 (Fig. 1e).
AREG up-regulates SPRY4 expression in human ovarian cancer cells. a SKOV3 cells were treated with 10 ng/ml AREG for different time periods; then, the SPRY4 mRNA levels were examined by RT-qPCR. The level of SPRY4 mRNA at each time point was normalized to the GAPDH mRNA level at the same time point. b, c SKOV3 cells were treated for 3 h with vehicle control or with different concentrations of AREG; then, the SPRY4 mRNA (b) and protein (c) levels were examined by RT-qPCR and Western blot, respectively. d, e SKOV3 (d) and OVCAR5 (e) cells were treated with 10 ng/ml AREG for different time periods; then, the SPRY4 protein levels were examined by Western blot. The results are expressed as the means ± SEM of at least three independent experiments. Values without a common letter are significantly different (P < 0.05)
Amphiregulin up-regulates SPRY4 expression by activating ERK1/2 signaling
Consistent with our previous study [11], treatment with AREG activated ERK1/2 and AKT signaling pathways in human ovarian cancer cells (Fig. 2a). Like EGF, AREG has been shown to bind exclusively to the EGFR [18]. To confirm the involvement of EGFR in the AREG-induced up-regulation of SPRY4, we used a specific EGFR tyrosine kinase inhibitor, AG1478, to block the function of the EGFR. As shown in Fig. 2b, pretreatment with AG1478 abolished the stimulatory effect of AREG on the SPRY4 protein expression in both SKOV3 and OVCAR5 cells. To examine whether the ERK1/2 or PI3K/AKT signaling pathway is required for AREG-induced up-regulation of SPRY4 expression, a MEK inhibitor, U0126, and a PI3K inhibitor, LY294002, were used to block the activity of ERK1/2 and AKT, respectively. The Western blot results showed that inhibition of ERK1/2 signaling attenuated the AREG-induced up-regulation of the SPRY4 protein levels in both SKOV3 and OVCAR5 cells. However, the stimulatory effect of AREG on the SPRY4 protein levels was not affected by the inhibition of PI3K/AKT signaling (Fig. 2b).
ERK1/2 signaling is required for the AREG-induced up-regulation of SPRY4 expression. a SKOV3 and OVCAR5 cells were treated with 10 ng/ml AREG for 5, 10, and 30 min; then, the levels of p-ERK1/2 and p-AKT were examined by Western blot. b SKOV3 and OVCAR5 cells were treated with vehicle control (DMSO), 10 μM AG1478, 10 μM U0126, or 10 μM LY294002 for 30 min and then were treated with 10 ng/ml AREG for 3 h. The protein levels of SPRY4 were examined by Western blot. The results are expressed as the means ± SEM of at least three independent experiments. Values without a common letter are significantly different (P < 0.05)
Knockdown of ERK1/2 attenuates amphiregulin-induced up-regulation of SPRY4 expression
To further confirm the involvement of ERK1/2 signaling in AREG-induced SPRY4 expression and avoid off-target effects of the pharmacological inhibitor, the endogenous expression of ERK1/2 was knocked down by transfection with specific siRNAs. As shown in Fig. 3, ERK1/2 siRNAs specifically down-regulated ERK1/2 protein levels in both SKOV3 and OVCAR5 cells. In addition, knockdown of ERK1/2 attenuated the AREG-induced up-regulation of SPRY4 protein levels. These results clearly indicated that the AREG-induced up-regulation of SPRY4 expression in human ovarian cancer cells is mediated by the ERK1/2 signaling pathway.
Knockdown of ERK1/2 attenuates AREG-induced up-regulation of SPRY4 expression. SKOV3 and OVCAR5 cells were transfected for 48 h with 50 nM control siRNA (siCtrl) or ERK1/2 siRNAs (siERK1/2) and then treated with 10 ng/ml of AREG for 3 h. The protein levels of SPRY4 and ERK1/2 were examined by Western blot. The results are expressed as the means ± SEM of at least three independent experiments. Values without a common letter are significantly different (P < 0.05)
Knockdown of SPRY4 attenuates amphiregulin-induced down-regulation of E-cadherin expression
To examine the role of SPRY4 in the AREG-induced down-regulation of E-cadherin, a siRNA-mediated knockdown approach was used to block the function of SPRY4. As shown in Fig. 4a, transfecting SKOV3 and OVCAR5 cells with SPRY4 siRNA not only knocked down the endogenous SPRY4 mRNA levels but also eliminated the AREG-induced up-regulation of the SPRY4 mRNA levels. Importantly, SPRY4 knockdown did not affect the basal mRNA levels of E-cadherin but significantly attenuated the AREG-induced down-regulation of the E-cadherin mRNA levels. Similarly, Western blot results showed that knockdown of SPRY4 attenuated AREG-induced down-regulation of E-cadherin protein levels in both SKOV3 and OVCAR5 cells (Fig. 4b).
Knocking down SPRY4 attenuates the AREG-induced down-regulation of E-cadherin. a, b SKOV3 and OVCAR5 cells were transfected for 48 h with 50 nM control siRNA (siCtrl) or SPRY4 siRNA (siSPRY4) and then treated with 10 ng/ml of AREG for 24 h. The mRNA (a) and protein (b) levels of E-cadherin and SPRY4 were examined by RT-qPCR and Western blot, respectively. The results are expressed as the means ± SEM of at least three independent experiments. Values without a common letter are significantly different (P < 0.05)
Overexpression of SPRY4 enhances amphiregulin-induced down-regulation of E-cadherin expression
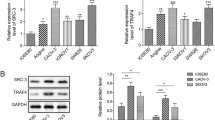
Next, we performed forced-expression studies to further confirm the role of SPRY4 in AREG-induced down-regulation of E-cadherin. As shown in Fig. 5, SKOV3 and OVCAR5 cells transfected with vector encoding human SPRY4 had significantly increased SPRY4 protein levels compared to cells transfected with empty vector. In addition, overexpression of SPRY4 enhanced AREG-induced down-regulation of E-cadherin protein levels. These results clearly indicate that SPRY4 is required for AREG-induced down-regulation of E-cadherin in human ovarian cancer cells.
Overexpression of SPRY4 enhances the AREG-induced down-regulation of E-cadherin. SKOV3 and OVCAR5 cells were transfected for 48 h with 1 μg control vector (Vector) or SPRY4 overexpression vector (SPRY4) and then treated with 10 ng/ml of AREG for 24 h. The protein levels of E-cadherin and SPRY4 were examined by Western blot. The results are expressed as the means ± SEM of at least three independent experiments. Values without a common letter are significantly different (P < 0.05)
Knockdown of SPRY4 attenuates amphiregulin-induced up-regulation of SNAIL expression
SNAIL and SLUG are the best characterized transcriptional repressors of E-cadherin [19]. Our previous studies have shown that treatment of SKOV3 cells with AREG up-regulates SNAIL and SLUG expression, and both SNAIL and SLUG are required for the AREG-induced down-regulation of E-cadherin [10, 11]. Therefore, we examined whether knocking down SPRY4 affects the AREG-mediated up-regulation of SNAIL and SLUG. As shown in Fig. 6a, consistent with our previous studies, treatment with AREG up-regulated the SNAIL and SLUG mRNA levels in both SKOV3 and OVCAR5 cells. Interestingly, SPRY4 knockdown attenuated the AREG-induced up-regulation of the SNAIL mRNA levels, but not those of SLUG. Consistent with RT-qPCR results, in both SKOV3 and OVCAR5 cells, Western blot results showed that knockdown of SPRY4 attenuated the AREG-induced up-regulation of the SNAIL protein levels. The AREG-induced SLUG protein levels were not affected by SPRY4 knockdown (Fig. 6b).
Knocking down SPRY4 attenuates the AREG-induced up-regulation of SNAIL. a, b SKOV3 and OVCAR5 cells were transfected for 48 h with 50 nM control siRNA (siCtrl) or SPRY4 siRNA (siSPRY4) and then treated with 10 ng/ml of AREG for 3 h. The mRNA (a) and protein (b) levels of SNAIL, SLUG, and SPRY4 were examined by RT-qPCR and Western blot, respectively. The results are expressed as the means ± SEM of at least three independent experiments. Values without a common letter are significantly different (P < 0.05)
Overexpression of SPRY4 enhances amphiregulin-induced up-regulation of SNAIL expression
Similarly, we performed forced-expression studies to further confirm the role of SPRY4 in AREG-induced up-regulation of SNAIL and SLUG. As shown in Fig. 7, SKOV3 and OVCAR5 cells transfected with vector encoding human SPRY4 had significantly increased SPRY4 protein levels compared to cells transfected with empty vector. In addition, overexpression of SPRY4 enhanced AREG-induced up-regulation of SNAIL protein levels. The AREG-induced SLUG protein levels were not affected by SPRY4 overexpression.
Overexpression of SPRY4 enhances the AREG-induced up-regulation of SNAIL. SKOV3 and OVCAR5 cells were transfected for 48 h with 1 μg control vector (Vector) or SPRY4 overexpression vector (SPRY4) and then treated with 10 ng/ml of AREG for 3 h. The protein levels of SNAIL, SLUG, and SPRY4 were examined by Western blot. The results are expressed as the means ± SEM of at least three independent experiments. Values without a common letter are significantly different (P < 0.05)
SPRY4 is required for amphiregulin-induced cell migration and invasion
We have previously shown that down-regulating E-cadherin expression promotes human ovarian cancer cell invasion [10, 11, 20]. We next examined whether SPRY4 knockdown also affects the AREG-induced cell migration and invasion. As shown in Fig. 8a, treatment with AREG induced cell migration in both SKOV3 and OVCAR5 cells. SPRY4 knockdown did not affect the basal level of cell migration but significantly attenuated the AREG-induced cell migration. Similarly, in both cell lines, AREG treatment induced cell invasion and the AREG-induced cell invasion was attenuated by knockdown of SPRY4 (Fig. 8b). Moreover, overexpression of SPRY4 enhanced AREG-induced cell invasion in both SKOV3 and OVCAR5 cells (Fig. 8c).
SPRY4 knockdown attenuates AREG-induced cell migration and invasion. a, b SKOV3 and OVCAR5 cells were transfected with 50 nM control siRNA (siCtrl) or SPRY4 siRNA (siSPRY4) for 48 h. After transfection, the cells were treated with 10 ng/ml AREG and the effects of AREG on cell migration (a) and invasion (b) were examined by the Transwell migration/invasion assay. c SKOV3 and OVCAR5 cells were transfected with 1 μg control vector (Vector) or SPRY4 overexpression vector (SPRY4) for 48 h. After transfection, the cells were treated with 10 ng/ml AREG and the effects of AREG on cell invasion were examined by the Transwell invasion assay. The results are expressed as the means ± SEM of at least three independent experiments. Values without a common letter are significantly different (P < 0.05)
Discussion
Although the down-regulation of SPRY4 has been reported in human ovarian cancer, the regulation and function of SPRY4 remain unknown. It has been shown that SPRY4 expression can be rapidly up-regulated in human and mouse fibroblasts by treatment with serum and EGF, FGF, insulin growth factor (IGF) or platelet-derived growth factor (PDGF) [21–24]. However, the effects of an EGFR ligand, AREG, on SPRY4 expression were unknown. In the present study, we showed that treatment of two human ovarian cancer cell lines with AREG up-regulated the SPRY4 expression. Many studies have demonstrated that ERK1/2 is the major signaling pathway that mediates the growth factor-induced up-regulation of SPRY4 [22–24]. Consistent with the previous findings obtained using pharmacological inhibitors, our results showed that ERK1/2, but not the PI3K/AKT signaling pathway, was required for AREG-induced up-regulation of SPRY4 in ovarian cancer cells.
Generally, SPRY proteins are able to inhibit the receptor tyrosine kinase (RTK)-mediated ERK1/2 activation [25]. Interesting, the inhibitory function of SPRY4 has been shown to be ligand-specific. In human embryonic kidney fibroblasts and mouse embryonic fibroblasts, SPRY4 inhibits the FGF-induced activation of ERK1/2 but enhances the EGF-induced ERK1/2 activation [23, 26]. Given the binding specificity of AREG to the EGFR, these results suggest that SPRY4 may enhance the effects of AREG. Indeed, our loss- and gain-of-function experiments showed that SPRY4 is required for the AREG-induced down-regulation of E-cadherin and ovarian cancer cell invasion. In human ovarian cancer, not only the EGFR but also many other RTKs are involved in disease progression, and targeting RTKs has been applied for the clinical treatment of ovarian cancer patients [27, 28]. Therefore, although the loss of SPRY4 attenuates EGFR-mediated ERK1/2 activation and cellular functions, it may also enhance other RTK-mediated cellular functions, which could contribute to the progression of ovarian cancer. Interestingly, the overexpression of SPRY4 has been shown to inhibit the basal cell migration and invasion of human prostate and lung cancers [29, 30]. However, the effects of SPRY4 overexpression on growth factor-induced cancer cell migration and invasion were not examined in those studies. In the present study, our results showed that knocking down SPRY4 did not affect the basal cell invasiveness but did attenuate the AREG-induced invasion of ovarian cancer cells. Moreover, overexpression of SPRY4 enhanced the AREG-induced cell invasion. These results indicate that SPRY4 acts in a context-dependent manner. Thus, further studies will be needed to clarify the role of SPRY4 in regulating the progression of different human cancers.
SPRY proteins have proved to exert divergent effects, and the role of different SPRY proteins in regulating of cancer progression is associated with complexity and even controversy [5]. To the best of our knowledge, the functions of SPRY protein in human ovarian cancer progression remain largely unknown. In SKOV3 cells, overexpression of SPRY1 suppresses cell proliferation, migration, and invasion [31]. Our previous study shows that, in SKOV3 cells, the basal levels of E-cadherin expression or cell invasiveness are not affected by overexpression of SPRY2. However, the SPRY2 overexpression attenuates EGF-induced down-regulation of E-cadherin and cell invasion [16]. These results indicate that SPRY2 antagonizes the effects of EGF on the E-cadherin down-regulation and cell invasion. Interestingly, the current study demonstrated that SPRY4 was required for the AREG-induced down-regulation of E-cadherin and cell invasion. Given the exclusive binding between EGF/AREG and EGFR, our studies indicated that, in human ovarian cancer cells, SPRY2 and SPRY4 have opposing effect on regulation of EGFR-mediated E-cadherin down-regulation and cell invasion. Our previous study and a recent immunohistochemical study show that SPRY2 and SPRY4 proteins are significantly down-regulated in human ovarian cancer cell lines and clinical tissue samples [15, 16]. Importantly, patients with the low expressing of SPRY2, but not SPRY4, have significantly poorer survival than those with high expression of SPRY2 [15]. These results suggest that the roles of SPRY2 in regulating human ovarian cancer progression may be more important than that of SPRY4. However, the details of the molecular mechanisms underlying the different roles of SPRY2 and SPRY4 in regulation of E-cadherin expression and cell invasion in human ovarian cancer remain unknown and warrant further investigation.
SPRY2 has been shown to regulate E-cadherin expression by modulating its transcriptional repressors [16, 32]. However, it is currently unclear if the same is true for SPRY4. In this study, we showed that knocking down SPRY4 did not affect the basal levels of E-cadherin, SNAIL, or SLUG. Interestingly, SPRY4 knockdown attenuated the AREG-induced down-regulation of E-cadherin by inhibiting SNAIL, but not SLUG, expression. Moreover, overexpression of SPRY4 enhanced AREG-induced down-regulation of E-cadherin by increasing SNAIL, but not SLUG, expression. It has previously been shown that knocking down SPRY2 resulted in different inhibitory effects on SNAIL and SLUG expression in two human colorectal cancer cell lines [32]. These results suggest that the effects of SPRY proteins on E-cadherin transcriptional repressors are cell-type dependent. Future studies will be required to investigate in more detail of the molecular mechanisms underlying this regulation.
In summary, our results showed that treatment with the most abundant and important EGFR ligand, AREG, up-regulated the SPRY4 expression in human ovarian cancer cells by activating the ERK1/2 signaling pathway. In addition, using loss- and gain-of-function approaches, we showed that SPRY4 was required for the AREG-induced down-regulation of E-cadherin and cell invasion. These results provide for the first evidence of the involvement of SPRY4 in ovarian cancer progression. A better understanding of the mechanisms underlying the function and regulation of SPRYs may provide an opportunity to develop novel therapeutic strategies.
References
Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. Sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the drosophila airways. Cell. 1998;92:253–63.
Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, et al. Vertebrate sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–75.
Zhang S, Lin Y, Itaranta P, Yagi A, Vainio S. Expression of sprouty genes 1, 2 and 4 during mouse organogenesis. Mech Dev. 2001;109:367–70.
Guy GR, Jackson RA, Yusoff P, Chow SY. Sprouty proteins: modified modulators, matchmakers or missing links? J Endocrinol. 2009;203:191–202.
Masoumi-Moghaddam S, Amini A, Morris DL. The developing story of sprouty and cancer. Cancer Metastasis Rev. 2014;33:695–720.
Bartlett JM, Langdon SP, Simpson BJ, Stewart M, Katsaros D, Sismondi P, et al. The prognostic value of epidermal growth factor receptor mRNA expression in primary ovarian cancer. Br J Cancer. 1996;73:301–6.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37.
Yotsumoto F, Yagi H, Suzuki SO, Oki E, Tsujioka H, Hachisuga T, et al. Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem Biophys Res Commun. 2008;365:555–61.
Yagi H, Miyamoto S, Tanaka Y, Sonoda K, Kobayashi H, Kishikawa T, et al. Clinical significance of heparin-binding epidermal growth factor-like growth factor in peritoneal fluid of ovarian cancer. Br J Cancer. 2005;92:1737–45.
Qiu X, Cheng JC, Klausen C, Fan Q, Chang HM, So WK, et al. Transforming growth factor-alpha induces human ovarian cancer cell invasion by down-regulating e-cadherin in a snail-independent manner. Biochem Biophys Res Commun. 2015;461:128–35.
So WK, Fan Q, Lau MT, Qiu X, Cheng JC, Leung PC. Amphiregulin induces human ovarian cancer cell invasion by down-regulating e-cadherin expression. FEBS Lett. 2014;588:3998–4007.
Cheng JC, Auersperg N, Leung PC. EGF-induced EMT and invasiveness in serous borderline ovarian tumor cells: a possible step in the transition to low-grade serous carcinoma cells? PLoS ONE. 2012;7, e34071.
Cheng JC, Chang HM, Leung PC. Egr-1 mediates epidermal growth factor-induced downregulation of e-cadherin expression via slug in human ovarian cancer cells. Oncogene. 2013;32:1041–9.
Cheng JC, Chang HM, Leung PC. Epidermal growth factor induces human oviductal epithelial cell invasion by down-regulating e-cadherin expression. J Clin Endocrinol Metab. 2012;97:E1380–1389.
Masoumi-Moghaddam S, Amini A, Wei AQ, Robertson G, Morris DL. Sprouty 2 protein, but not sprouty 4, is an independent prognostic biomarker for human epithelial ovarian cancer. Int J Cancer J Int Cancer. 2015;137:560–70.
So WK, Cheng JC, Fan Q, Wong AS, Huntsman DG, Gilks CB, et al. Loss of sprouty2 in human high-grade serous ovarian carcinomas promotes EGF-induced e-cadherin down-regulation and cell invasion. FEBS Lett. 2015;589:302–9.
Inoue Y, Miyamoto S, Fukami T, Shirota K, Yotsumoto F, Kawarabayashi T. Amphiregulin is much more abundantly expressed than transforming growth factor-alpha and epidermal growth factor in human follicular fluid obtained from patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2009;91:1035–41.
Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–16.
Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28.
Cheng JC, Klausen C, Leung PC. Hydrogen peroxide mediates EGF-induced down-regulation of e-cadherin expression via p38 MAPK and snail in human ovarian cancer cells. Mol Endocrinol. 2010;24:1569–80.
Mayer CE, Haigl B, Jantscher F, Siegwart G, Grusch M, Berger W, et al. Bimodal expression of sprouty2 during the cell cycle is mediated by phase-specific Ras/MAPK and c-Cbl activities. Cell Mol Life Sci: CMLS. 2010;67:3299–311.
Ozaki K, Kadomoto R, Asato K, Tanimura S, Itoh N, Kohno M. ERK pathway positively regulates the expression of sprouty genes. Biochem Biophys Res Commun. 2001;285:1084–8.
Sasaki A, Taketomi T, Wakioka T, Kato R, Yoshimura A. Identification of a dominant negative mutant of sprouty that potentiates fibroblast growth factor- but not epidermal growth factor-induced ERK activation. J Biol Chem. 2001;276:36804–8.
Doriguzzi A, Haigl B, Gsur A, Sutterluty-Fall H. The increased sprouty4 expression in response to serum is transcriptionally controlled by specific protein 1. Int J Biochem Cell Biol. 2015;64:220–8.
Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54.
Taniguchi K, Ayada T, Ichiyama K, Kohno R, Yonemitsu Y, Minami Y, et al. Sprouty2 and sprouty4 are essential for embryonic morphogenesis and regulation of FGF signaling. Biochem Biophys Res Commun. 2007;352:896–902.
Klempner SJ, Myers AP, Mills GB, Westin SN. Clinical investigation of receptor and non-receptor tyrosine kinase inhibitors for the treatment of epithelial ovarian cancer. Expert Opin Pharmacother. 2013;14:2171–82.
Morotti M, Becker CM, Menada MV, Ferrero S. Targeting tyrosine-kinases in ovarian cancer. Expert Opin Investig Drugs. 2013;22:1265–79.
Tennis MA, Van Scoyk MM, Freeman SV, Vandervest KM, Nemenoff RA, Winn RA. Sprouty-4 inhibits transformed cell growth, migration and invasion, and epithelial-mesenchymal transition, and is regulated by Wnt7A through PPARgamma in non-small cell lung cancer. Mol Cancer Res: MCR. 2010;8:833–43.
Wang J, Thompson B, Ren C, Ittmann M, Kwabi-Addo B. Sprouty4, a suppressor of tumor cell motility, is down regulated by DNA methylation in human prostate cancer. Prostate. 2006;66:613–24.
Masoumi-Moghaddam S, Amini A, Ehteda A, Wei AQ, Morris DL. The expression of the sprouty 1 protein inversely correlates with growth, proliferation, migration and invasion of ovarian cancer cells. J Ovarian Res. 2014;7:61.
Zhang Q, Wei T, Shim K, Wright K, Xu K, Palka-Hamblin HL, Jurkevich A, Khare S: Atypical role of sprouty in colorectal cancer: sprouty repression inhibits epithelial-mesenchymal transition. Oncogene 2015.
Acknowledgments
We also thank Dr. Graeme R. Guy (The Institute of Molecular and Cell Biology, Singapore) for the generous gifts of pXJ40-FLAG and FLAG-SPRY4 plasmids. This work was supported by an operating grant from the Canadian Institutes of Health Research (#143317) to P.C.K.L. P.C.K.L. is a Scientist Level 3 of the Child and Family Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Grant support
This work was supported by an operating grant from the Canadian Institutes of Health Research (#143317) to P.C.K.L.
Rights and permissions
About this article
Cite this article
So, WK., Cheng, JC., Liu, Y. et al. Sprouty4 mediates amphiregulin-induced down-regulation of E-cadherin and cell invasion in human ovarian cancer cells. Tumor Biol. 37, 9197–9207 (2016). https://doi.org/10.1007/s13277-016-4790-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4790-y