Abstract
The Dof (DNA binding with One Finger) family of single zinc finger proteins is a family of plant-specific transcription factors. These transcription factors have a variety of important functions in different biological processes in plants. In the current study, we identified 26 Dof family genes in moso bamboo (Phyllostachys heterocycla var. pubescens). A complete overview of PhDof genes in moso bamboo is presented, including the gene structures, phylogeny, protein motifs and expression patterns. Phylogenetic analysis of the 26 PhDof proteins identified four classes constituting seven clusters (A, B1, C1, C2, D1, D2 and D3). In addition, a comparative analysis between the Dof genes in moso bamboo, Arabidopsis (Arabidopsis thaliana L.) and rice (Oryza sativa L.) was also performed, and several putative paralogous and orthologous genes were identified. The exon numbers in Dof genes ranged from one to three in many plants; however, the exon number in PhDofs ranged from one to four. The PhDof genes displayed differential expression in different parts of the shoot and at different flower development stages. This study represents the first step towards a genome-wide analysis of the Dof genes in moso bamboo. Our study provides a useful reference for cloning and functional analysis of members of the Dof gene family in moso bamboo and other species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In plants, the transcriptional and post-transcriptional regulation of gene expression influences and controls many important biological processes, such as cellular morphogenesis, signal transduction and environmental stress responses (Riechmann et al. 2000).
Transcription factors (TFs) are important regulating proteins that bind specific DNA sequences in gene promoters to initiate a program of increased or decreased gene transcription (Latchman 1997). Therefore, the identification and functional characterization of TFs is essential for building predictive models of transcriptional regulatory networks. In the plant TF database, PlantTFDB v3.0, 129,288 TFs (~60 families) from 83 species have been identified systematically, based on bioinformatics analysis, of which 67 species have genome sequences, covering the main lineages of green plants (Jin et al. 2014a). Thus, PlantTFDB provides a resource for functional and evolutionary studies of plant TFs. The Arabidopsis genome encodes at least 1533 TFs, which account for about 5.9 % of its estimated total number of genes (Riechmann et al. 2000). For moso bamboo (Phyllostachys heterocycla var. pubescens), ~5.53 % of the 31,987 protein-coding genes (Peng et al. 2013) have been identified to encode 1768 putative TFs, which could be classified into 54 families (Jin et al. 2014a).
The Dof (DNA-binding with one finger) proteins belong to the zinc finger superfamily, and contain a highly conserved DNA-binding C2C2-type-zinc-like motif named the Dof domain, which comprises 52 amino acid residues (Yanagisawa 1995). Dofs play critical roles as transcriptional regulators in plant growth and development. In 1993, the first two Dof proteins were identified from maize by Yanagisawa and Izui. Subsequently, numerous Dof genes were cloned or predicted from genome databases in plants, such as single-celled green algae (Chlamydomonas reinhardtii), moss (Physcomitrella patens), fern (Selaginella moellendorffii) and gymnosperms (Pinus taeda) to higher angiosperms, including 37, 30, 41, 26, 78, 31, 18, 28, 27, 34, 1, 8, 19 and 8, Dof genes in Arabidopsis (Lijavetzky et al. 2003), rice (Lijavetzky et al. 2003), poplar (Yang et al. 2006a, b), barley (Moreno-Risueno et al. 2007b), soybean (Guo and Qiu 2013), bread wheat (Shaw et al. 2009), maize (Jiang et al. 2012), sorghum (Kushwaha et al. 2011), Brachypodium distachyon (Hernando-Amado et al. 2012), tomato (Cai et al. 2013), algae, fern, moss (Shigyo et al. 2007) and gymnosperms (Moreno-Risueno et al. 2007b), respectively. As the development of genomic sequencing and bioinformatics technology expands rapidly, more and more Dof family members will be identified, emphasizing their critical role in plant development.
Dof protein binding elements have been discovered in many plant-specific promoter sequences. It has been suggested that the Dof proteins have diverse roles in the regulation of specific biological processes unique to plant development, such as carbon–nitrogen metabolism in maize (Yanagisawa 2000), pea (Pisum sativum) (Tanaka et al. 2009), wheat (Kumar et al. 2009), P. taeda (Rueda-Lopez et al. 2008) and P. patens (Park et al. 2003; Imaizumi et al. 2005; Ward et al. 2005; Sawa et al. 2007; Fornara et al. 2009); photoresponse and photoperiodic control of flowering in Arabidopsis (Rueda-Lopez et al. 2008), rice (Iwamoto et al. 2009; Li et al. 2009) and Jatropha curcas (Yang et al. 2010, 2011); floral organ and pollen development Arabidopsis (Wei et al. 2010) and maize (Chen et al. 2012); seed development and germination in Arabidopsis (Papi et al. 2000; Gualberti et al. 2002; Gabriele et al. 2010; Rizza et al. 2011; Rueda-Romero et al. 2012), soybean (Wang et al. 2007), maize (Vicente-Carbajosa et al. 1997, Marzabal et al. 2008), barley (Mena et al. 1998; Diaz et al. 2002; Mena et al. 2002; Moreno-Risueno et al. 2007b), wheat (Dong et al. 2007), rice (Washio 2003; Kawakatsu and Takaiwa 2010; Gaur et al. 2011); synthesis of secondary metabolites in Arabidopsis (Skirycz et al. 2006, 2007); guard cell-specific gene regulation in Arabidopsis (Cominelli et al. 2011) and potato (Plesch et al. 2001); vascular development in Arabidopsis (Konishi and Yanagisawa 2007; Guo et al. 2009; Gardiner et al. 2010); defensive reaction in Arabidopsis (Kang and Singh 2000; Kang et al. 2003), and auxin-response regulation in Cucurbita moschata (Kisu et al. 1998; Baumann et al. 1999).
Bamboo is one of the most important non-timber forest products in the world, with high ecological, economic, edible and cultural value (Peng et al. 2010, p. 1013). The moso bamboo, a large woody bamboo, has one of the highest growth speeds in the world. However, moso bamboo has a rather striking life history, characterized by a prolonged vegetative phase lasting decades before flowering, which has hindered its genetic improvement. A high-quality draft genome sequence of moso bamboo should be published soon and represents a comprehensive genome dataset that will accelerate research into gene functions in moso bamboo. Despite the crucial roles of Dof proteins in transcriptional regulation of plant growth and development, little is known about this family in moso bamboo. In this study, we identified 26 Dof genes in the moso bamboo genome, named as PhDof 1–26. We then constructed a phylogenetic tree to evaluate the evolutionary relationships of Dof genes in moso bamboo. We also analysed the gene structures and conserved motifs. To identify the putative functions and evolution of PhDof genes, we performed phylogenetic analyses of the moso bamboo, Arabidopsis and rice Dof gene families and determined their expression profiles in moso bamboo. Thus systematic analysis provides a foundation for further functional dissection of PhDof genes, and could help elucidate Dof gene functions in other species.
Materials and methods
Identification of Dof genes in moso bamboo
We identified the members of the Dof genes family in moso bamboo using two approaches. First, the Dof sequences of Arabidopsis and rice were downloaded from the Arabidopsis genome TAIR database (http://www.arabidopsis.org/) and the rice genome annotation database (http://rice.plantbiology.msu.edu/). The protein sequences of the Dof domains were used to search for potential Dof-domain homolog hits in the whole genome sequence of moso bamboo, using BLASP searches against the protein profile, which has been published in the moso bamboo genome database (http://www.ncgr.ac.cn/bamboo). Additionally, hidden Markov model (HMM) searches (Finn et al. 2011) were performed locally in the moso bamboo database, using the Dof domain family HMM profile (PF02701). We subjected all the obtained protein sequences to domain analysis using the InterProScan (http://www.ebi.ac.uk/Tools/pfa/iprscan5/) and SMART (http://smart.embl-heidelberg.de/) tools, with the default parameters, to reveal the presence of Dof domains. Protein sequences lacking a Dof domain were rejected.
Multiple alignment and phylogenetic analysis
We performed alignments of Dof protein sequences using the Clustal X 2.1 program with its default settings (Larkin et al. 2007). Phylogenetic trees were constructed in the MEGA5.1 software using the Neighbor-Joining (NJ), Minimum Evolution (ME) and Maximum Likelihood (ML) methods (Tamura et al. 2011). We tested the reliability of the obtained trees using bootstrapping with 1000 replicates.
Genome structure and conserved motifs analysis
The GSDS (Gene Structure Display Server; http://gsds1.cbi.pku.edu.cn/) program was used to illustrate the exon/intron organization for individual Dof genes by comparing the coding sequences with their corresponding genomic DNA sequences from the moso bamboo genome database. The deduced amino-acid sequences of the PhDofs were analyzed using the online version of MEME 4.10.1 (http://meme-suite.org/tools/meme) for motif analysis. To identify conserved motifs in these sequences, selection of the maximum number of motifs was set to 25, with a minimum width of six and a maximum width of 120 amino acids, while other factors were set at default values.
mRNA sequencing and analysis
All the bamboo samples were taken from Baizhu Park, which is located at Yiyang city in Hunan province, China, on April 4, 2013. Samples from the tip of the shoot (ST), the middle part of shoot (SM), the base part of shoot (SB), and shoot sheaths (tip, middle, base of shoot sheaths mixtures, SS) were obtained from the wild P. heterocycla var. pubescens. Samples were collected from three plants whose heights were about 6.0 m. In all cases, samples were collected, immediately frozen in liquid nitrogen and stored at −80 °C until RNA extraction.
Total RNA was isolated from the plant tissues using an Easy-spin™ kit (Aidlab, Beijing, China), following the manufacturer’s instructions. Purified mRNA was chemically fragmented to 200–500 bp fragments. Next, we synthesized the fist- and second-strand cDNAs, followed by end repair and index adapter ligation. Finally, the resulting libraries were sequenced using an Illumina HiSeq™ 2000 (Illumina, San Diego, CA, USA) to generate paired-end sequences.
We conducted a gene expression analysis using Illumina RNA-Seq technology. The sequencing and assembly were performed at the Shanghai Hanyu Biotech Co. Transcriptome sequencing (RNA-Seq) data were generated using the Illumina HiSeq™ 2000 platform. Approximately 21957740, 20979560, 21704837 and 21759266 reads were generated from the four sample libraries (ST, SM, SB and SS), respectively. The adapters or low-quality reads, where the number of ‘N’ bases exceeded 5 %, were removed from the raw data. The reads were then mapped to genes and the genome of moso bamboo, allowing for a maximum of two mismatches. The gene expression values were normalized by the measure of reads per kilobase per million (RPKM). Finally, heat maps of gene expression from the four tissues were visualized using HemI 1.0 software (Deng et al. 2014).
Results
Dof gene family in moso bamboo
An HMM search with the Dof domain HMM profile (PF02701) and BLASTP using Arabidopsis and rice Dof protein as queries were used to identify moso bamboo Dof sequences. The obtained sequences were analyzed using InterProScan and SMART for the presence of the Dof domain. Twenty-six PhDof family genes were identified (Table 1), and all of them have a typical binding domain of 52 residues spanning a single C2/C2 zinc finger structure (DOF domain, Fig. S1). In the PlantTFDB (http://planttfdb.cbi.pku.edu.cn/), although 31 PhDof genes were identified, InterProScan and SMART analysis found that Dof domains were absent from five sequences (PH01000290G0170, PH01000789G0200, PH01000941G0150, PH01003477G0030, and PH01155840G0010). There was no standard annotation assigned to these newly identified genes; therefore, we named these PhDof genes PhDof-1 to PhDof-26, according to their location on the genome scaffolds. The names of the PhDof genes, the locus gene, the length, molecular weight (MW), isoelectric point (pI), and the grand average of hydropathicity (GRAVY) are shown in Table 1. The identified PhDof genes encode peptides ranging from 197 to 542 amino acids in length, with an average of 357.5.
To investigate the features of the homologous domain sequences, and the frequency of the most prevalent amino acids at each position within the moso bamboo Dof domain, multiple alignment analysis of the Dof domains from the 26 PhDofs was performed (Fig. 1). The Dof domain of moso bamboo was revealed to be a highly conserved sequence, and 25 out of 52 amino acids were 100 % conserved in all PhDof proteins, including four absolutely conserved cysteine residues that presumably coordinate the zinc ion. Other highly conserved residues in the moso bamboo Dof domains were Pro-4, Arg-5, Ser-8, Thr-11, Lys-12, Phe-13, Cys-14, Tyr-15, Asn-17, Asn-18, Gln-23, Pro-24, Arg-25, Arg-33, Trp-35, Thr-36, Gly-38, Gly-39, and Arg-42. These highly conserved residues were also nearly identical to the Dof domain proteins of other plants, such as soybean (Guo and Qiu 2013), sorghum (Kushwaha et al. 2011) and tomato (Cai et al. 2013). Moreover, eight other amino-acid residues showed variation in less than three sequences among all PhDofs.
Dof domains are highly conserved across all Dof proteins in moso bamboo. The sequence logos are based on aligments of all moso bamboo Dof domains. Multiple alignment analysis of 26 typical moso bamboo Dof domains was performed with ClustalW. The bit score indicates the information content for each position in the sequence. Asterisks indicate the conserved cysteine residues (Cys) in the Dof domain
We analysed 16 species that, according to publications, contained 432 Dof proteins (Table 2). After the comparative genomic analysis, the number of Dof transcription factors in moso bamboo (26) was equal to that of barley (26) and exceeded that of Ricinus communis (21), Vitis vinifera (25), maize (18), P. patens (19), P. taeda (8), S. moellendorffii (8). It was, however, less than that of soybean (78), Arabidopsis (37), P. trichocarpa (41), tomato (34), rice (30), wheat (31), sorghum (28) and B. distachyon (27). In general, angiosperms have more Dof genes than gymnosperms, mosses, ferns and green algae. The genome size of moso bamboo (2.1 Gb) was less than that of maize (2.3 Gb), barley (5.1 Gb), wheat (17 Gb), and P. taeda (23.2 Gb), but greater than that of other species examined. We found that the number and genome size of the Dof genes showed no pattern among angiosperms, gymnosperm, moss, fern and green alga, which was the same for dicotyledons and monocotyledons. Although the genome size of barley (5.1 Gb) is 2.4 times that of moso bamboo (2.1 Gb), the two had equal numbers of Dof genes; however, the genome size of moso bamboo was 8.08 times larger than that of B. distachyon (260 Mb), and the two had similar numbers of Dof genes.
Phylogenetic, gene structure and conserved motif analysis of the Dof gene family in moso bamboo, Arabidopsis and rice
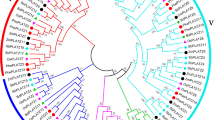
To investigate the molecular evolution and phylogenetic relationship among the Dof domain proteins in moso bamboo, Arabidopsis and rice, the 26 predicted PhDof proteins were subjected to multiple sequence alignment along with 36 Arabidopsis and 30 rice Dof proteins. Three unrooted phylogenetic trees were constructed using the NJ, ME and ML methods, based on the alignment of all the Dof amino-acid sequences. The tree topologies were similar, despite using different tree-building methods, except at the deep nodes (Fig. 2, Fig. S2). The NJ tree showed that all the Dof family proteins from the three higher plants were divided into four major clusters of orthologous groups and nine well-supported clades (A, B1, B2, C1, C2, C3 and D1, D2, D3; Fig. 2), similar to previous reports in Arabidopsis (Lijavetzky et al. 2003), soybean (Guo and Qiu 2013) and tomato (Cai et al. 2013). Among these, group D comprised the largest clade, containing 13 members and accounting for 50 % of the total Dof proteins. The other three groups contained two (Group A), three (Group B), and eight (Group D) members, respectively. Subgroup C3 comprised a species-specific group for Arabidopsis (monocotyledon), and subgroup D3 was specific for moso bamboo and rice (dicotyledons), similar to previous reports (Guo and Qiu 2013), indicating that there was a presumed gene loss event after the dicot–monocot split. Additionally, the B, C and D groups further clustered, forming a large clade, similar to previous reports for tomato and soybean, implying that they might have originated from a common ancestor by frequent gene duplication. Based on the phylogenetic tree, several putative paralogous (i.e. PhDof-7/PhDof-11, PhDof-9/PhDof-20 or PhDof-3/PhDof-15) and orthologous (i.e. AtDof5.6/PhDof-24/OsDof-7, AtDof2.4/PhDof-17/OsDof-16, AtDof1.1/PhDof-25/OsDof-15, or PhDof-13/OsDof-25/OsDof-24, PhDof-1/OsDof-2, AtDof5.2/PhDof-21) genes were identified.
Phylogenetic tree of all Dof domain-containing proteins from moso bamboo, Arabidopsis and rice. The deduced full-length amino-acid sequences of 26 moso bamboo, 36 Arabidopsis and 30 rice Dof genes were aligned by Clustal X 1.83 and the phylogenetic tree was constructed using MEGA 5.05 by the neighbor-joining method with 1000 bootstrap replicates. Each Dof subgroup is indicated by a specific color
Gene structural diversity is a possible mechanism for the evolution of multigene families. To gain further insight into the structural diversity of Dof genes, we compared the exon/intron organization in the coding sequences of individual PhDof genes. A detailed illustration of the exon/intron structures is shown in Fig. 3. According to the predicted structures, most of the Dof genes have one or two exons in Arabidopsis (Lijavetzky et al. 2003), B. distachyon (Hernando-Amado et al. 2012), cater bean (Jin et al. 2014b), rice (Lijavetzky et al. 2003), sorghum (Kushwaha et al. 2011) and tomato (Cai et al. 2013). By contrast, PhDofs have one to four exons. Among these genes, nine have one exon, ten have two exons, six have three exons and one gene (PhDof-16) contains four exons.
To reveal the diversification of Dof genes in moso bamboo, putative motifs were predicted by the program MEME (Multiple Em for Motif Elicitation), and 25 motifs were found in the 26 Dof proteins (Fig. 4; Table S2). Motif 1 was present in all the Dof proteins and represents the conserved Dof domain. Moreover, a number of common motifs were found in all moso bamboo Dofs (Table S2). As expected, most of the closely related members in the phylogenetic tree had common motif compositions. For example, there were no conserved motifs outside the Dof domain in subgroup A, B1 and D3, while motifs 2, 3, 4, 5, 6, 7, 8, 10, 11, 14, 16, 19, 20, 22, 23 and 25 appeared in nearly all the members of subgroup D1. In other subgroups, motifs 9 and 17 were specific to subgroup C1; motifs 12, 18, 21 and 24 were specific to subgroup C2; and motifs 13 were specific to subgroup D2. Thus, the phylogenetic tree was further supported by the comparative motif analyses of the deduced amino acid sequences of the Dof family proteins.
Schematic distributions of the conserved motifs among the defined gene cluster. Motifs in the deduced amino-acid sequences of the 26 PhDofs were identified using MEME software. The relative position of each identified motif in all Dof proteins is shown. Each motif is represented by a colored block with a number. Multilevel consensus sequences for the MEME-defined motifs are listed in Table S2
Moreover, because most of the Arabidopsis and other plants Dof genes with similar functions tended to fall into one subgroup, moso bamboo Dof genes in the same subgroup may have similar functions. Therefore, another unrooted phylogenetic tree was constructed using the NJ method, based on the Dof amino-acid sequences for which the functions had been identified in many plants (Fig. S3).
In subgroup A, PhDof-22 and PhDof-25 clustered with AtDof1.1 (OBP2), which is involved in regulating glucosinolate biosynthesis (Skirycz et al. 2006), laying the foundation for the study of moso bamboo secondary metabolism. PhDof-22 also is also closely related with GmDof, which plays important role in regulating the synthesis of lipids in soybean seeds (Wang et al. 2007) and TaDof-1, whose overexpression could improve the utilization rate of nitrogen in wheat (Kumar et al. 2009; Fig. S3).
In subgroup B1, three moso bamboo Dof genes (PhDof-16, PhDof-17 and PhDof-18) clustered with AtDof3.6 (OBP3), which modulates phytochrome and cryptochrome signaling in Arabidopsis (Ward et al. 2005), and StDof1, which controls guard cell-specific gene expression in tomato (Plesch et al. 2001). These data will add to the design of tailor-made guard cell promoters as a further tool in molecular engineering of guard cell function and, hence, the control of stomatal carbon dioxide (CO2) uptake and water loss in crop plants.
In subgroup C1, four moso bamboo Dof genes (PhDof-2, PhDof-12, PhDof-14 and PhDof-24) clustered with AtDof5.6 (HCA2), which is expressed specifically in cells at an early stage of vascular tissue development (Guo et al. 2009).
In subgroup C2, four moso bamboo Dof genes (PhDof-1, PhDof-6, PhDof-8 and PhDof-10) clustered with AtDof3.7 (DAG1), AtDof2.5 (DAG2), OsDof-10 (RPBF) and HvSAD, PsDOF-7, which are indirectly or directly involved in carbohydrate metabolism (sugar and thiol status, and seed storage protein accumulation) (Kawakatsu and Takaiwa 2010; de Dios Barajas-López et al. 2012) and seed development (Gualberti et al. 2002; Moreno-Risueno et al. 2007a).
In subgroup D1, nine moso bamboo Dof genes (PhDof-4, PhDof-5, PhDof-7, PhDof-9, PhDof-1, PhDof19, PhDof-20, PhDof-21 and PhDof-26) clustered with AtDof5.5 (CDF1), AtDof5.2 (CDF2), AtDof3.3 (CDF3), AtDof1.5 (COG1), AtDof1.10 (CDF5), JcDof-1, JcDof-3, and OsDof-12, which are associated with the light-mediated circadian clock and regulation of flowering in Arabidopsis (Imaizumi et al. 2005; Sawa et al. 2007; Fornara et al. 2009; Song et al. 2012), J. curcas (Yang et al. 2010, 2011) and rice (Iwamoto et al. 2009; Li et al. 2009).
In subgroup D2, two moso bamboo Dof genes (PhDof-3 and PhDof-15) clustered with AtDof3.4 (OBP1), which specifically increases the binding of the OBF proteins to ocs element sequences, raising the possibility that interactions between these proteins are important for the activity of the 35 s promoter (Zhang et al. 1995), and AtDof5.8, which is the upstream regulator of ANAC069 and is responsive to abiotic stress (He et al. 2015). In addition, the AtDof5.8 promoter activity was specifically detected in the cells of prospective veins in leaf primordia of seedlings and cotyledons of developing embryos, and the vascular tissue of developing flower buds. The AtDof5.8 promoter showed strong activity in advance of perceptible procambium formation. Thus, AtDof5.8 might function in early, but different, processes in vascular development (Konishi and Yanagisawa 2007).
In subgroup D3, two moso bamboo Dof genes (PhDof-13 and PhDof-23) clustered with ZmDof-1 and ZmDof-2. Transgenic expression of the maize ZmDof-1 gene in rice enhanced carbon and nitrogen assimilation under low-nitrogen conditions (Kurai et al. 2011). Moreover, ZmDof-1 is involved in light-regulated gene expression and has distinct activities in greening and the etiolated protoplast. Both ZmDof-1 and ZmDof-2 specifically interact with the promoter of the phosphoenolpyruvate carboxylase gene to enhance or repress its promoter activity, respectively (Yanagisawa and Sheen 1998; Yanagisawa 2000).
Expression profiles of Dof genes in bamboo shoots and flowers
The growth of the moso bamboo shoot is rapid and steady, and in suitable spring conditions, at the peak of its growth, the shoot can grow by as much as 100 cm within 24 h. Moreover, bamboo shoots are a traditional vegetable and natural health food in China (Peng et al. 2010, 2013). The expression analysis of Dof genes in bamboo shoots has important implications for moso bamboo genetic studies during the fast growth of shoots and provides potential gene candidates for further research.
High-throughput sequencing and gene expression analyses were performed on four moso bamboo shoot tissues, and the RNA-seq data generated is a useful resource for studying gene expression profiles. Based on the RPKM transcriptomic data of Dof genes in four moso bamboo samples (Table S4), the expression patterns of the 26 moso bamboo Dof genes were analyzed (Fig. 5). Two genes (PhDof-5 and PhDof-23) had very low expression in all four tissues. This might be because these genes have some other functions during the bamboo development process. Only four genes, PhDof-6, PhDof-8, PhDof-13 and PhDof-15, showed high expression levels (RPKM > 100) among the four tissues. Half of the PhDof genes showed a certain degree of tissue specificity, with five genes being abundantly expressed in the shoot tops, seven genes being abundantly expressed in the bottom shoots and 1 gene being abundantly expressed in the shoot sheaths. No Dof genes showed abundant expression levels in the middle shoots.
Expression profiles of moso bamboo Dof genes in four samples. Transcriptome sequencing (next-generation RNA-seq) was employed to investigate expression patterns of Dof genes. The color scale is shown on the right, with blue indicating low expression levels while red indicates high levels. ST shoot tip, SM the middle part of the shoot, SB the base part of the shoot, and SS shoot sheaths (tip, middle, base of shoot sheaths mixtures)
Moso bamboo is an arborescent, perennial plant characterized by woody stems and a rather striking life history, such as flowering synchronously and dying collectively after flowering (Lin et al. 2010). Gao et al. (2014) characterized the floral transcriptome of moso bamboo at four flowering developmental stages (floral bud formation, inflorescence development, anthesis and flower withered stages) by transcriptome sequencing and RNA-seq analysis. The significance of differential transcript abundance of the 26 Dof family genes from Gao et al. (2014) data during the different flower developmental stages is shown in Table S5. All 26 PhDofs were significantly differentially expressed in the early stage of flowering, suggesting that PhDofs play important roles in the early stage of flower development. Most of them showed upregulated expression; nevertheless, four genes (PhDof-4, PhDof-5, PhDof-20 and PhDof-22) were downregulated, and showed period-specific expression in the floral bud formation stage. PhDof-2 and PhDof-25 showed the most differential expressions (fold-change values of approximately 6.93 and 6.29) followed by PhDof-3 (fold-change value approximately 5.12). They were all upregulated at the floral bud formation stage, although PhDof-3 and PhDof-25 had the opposite expression patterns at the inflorescence development stage. PhDof-3, PhDof-15, PhDof-19 and PhDof-25 were differentially expressed in the floral bud formation and inflorescence development stages, moreover, PhDof-3 and PhDof-15 showed similar expression trends, with upregulated expression at the floral bud formation stage and then downregulated expression at the inflorescence development stage. PhDof-5 and PhDof-12 were differentially expressed in the floral bud formation and the flower withered stages, and they were both downregulated during the stage representing the flower withering process. The only difference was that the expression of PhDof-5 decreased significantly during the floral bud formation process, while PhDof-12 was increased significantly at this stage. Only PhDof-10 was increased significantly during the first three phases (floral bud formation, inflorescence development and anthesis stage), with significant differential expression during the last flower withered stage. Nine PhDof genes (PhDof-6, PhDof-7, PhDof-9, PhDof-11, PhDof-14, PhDof-17, PhDof-21, PhDof-23 and PhDof-26) were all dramatically differentially expressed at the four flowering development stages, but showed different expression patterns.
Discussion
In this study, 26 PhDof genes were identified in moso bamboo. This number is similar to the Dof genes present in barley (26; Moreno-Risueno et al. 2007b), grape (25; Li et al. 2013), B. distachyon (27; Hernando-Amado et al. 2012), sorghum (28; Kushwaha et al. 2011); but it is much less than that in soybean (78; Guo and Qiu 2013), which currently has the largest number of identified Dof genes. Glycine max is an ancient polyploid (palaeopolyploid), whose whole genome duplications (WGD) occurred at approximately 59 and 13 million years ago, resulting in a highly duplicated genome with nearly 75 % of the genes present in multiple copies (Schmutz et al. 2010). These facts suggested that the WGDs of soybean facilitated the expansion of the Dof gene family.
Among rice, Arabidopsis, tomato, B. distachyon, cater bean and sorghum Dof genes, the organizations of the exon/intron structures are conserved: the number of introns in the Dof genes ranged from 0 to 2 (Lijavetzky et al. 2003; Kushwaha et al. 2011; Hernando-Amado et al. 2012; Cai et al. 2013; Jin et al. 2014b). However, in moso bamboo, the intron number in PhDofs ranged from 0 to 3 (PhDof-16 contained 3 introns. In Arabidopsis (Lijavetzky et al. 2003), rice (Lijavetzky et al. 2003), soybean (Guo and Qiu 2013), tomato (Cai et al. 2013), and many other plants, the most closely related Dof gene members in the same subgroup generally show the same exon/intron pattern, with the position and length of the introns being almost completely conserved within most subgroups. By contrast, the gene structure appeared to be more variable for the D2 and D3 PhDofs subgroups (Fig. 3), suggesting that there might be larger evolutionary variation among moso bamboo Dofs.
Our results also revealed that certain Dof genes might have specific functions during moso bamboo shoot development and flower development process. For example, PhDof-5 and PhDof-23 had very low expressions, with an RPKM < 1, in the four moso bamboo shoot tissues (Table S4), suggesting that they might do not participate in regulating the growth of bamboo shoots. However, they showed significant differential expression patterns during the flower development processes (Table S5). PhDof-5 showed downregulated expression at the floral bud formation stage, and might specificity negatively regulate the plant transition from the vegetative to the reproductive stage. PhDof-5 was clustered with PhDof-4 and OsDof12. Previous studies showed that a moso bamboo Dof (a homolog of OsDof12) might be active in the drought-Dof-MADS14-flowering pathway during bamboo flowering process under drought stress in Southern China (Peng et al. 2013); PhDof-4 was also downregulated at the floral bud formation stage; therefore, PhDof-4 or PhDof-5 might participate in the drought-Dof-MADS14-flowering pathway in moso bamboo. PhDof-23 might not participate in regulating the growth of bamboo shoots: it was obviously differentially expressed in the whole process of bamboo flower development, showed upregulated expression at the floral bud formation and inflorescence development stages, was specifically downregulated at the anthesis stage, and was then upregulated expression again at the withered stage. This expression pattern suggested that it might a key positive regulator for the early stages of floral development and withering, and negative regulator for flower opening.
Bamboo shoot shell extracts contain many kinds of biologically active substances that show significant antioxidative activity (Gao 2011). The expression of PhDof-15 was abundant in the shoot sheaths. Phylogenetic analysis showed that it was closely related to AtOBP1, which was identified as responsive to abiotic stress. Thus, PhDof-15 might be involved in producing antioxidants in shoot sheaths resist abiotic stress. Moreover, PhDof-15 was dramatically differentially expressed during the floral bud formation and inflorescence development processes. This result agreed with those of previous studies, which also showed that a drought-responsive PeDof gene was highly expressed in the floral tissues, especially in the early stage of flowering (Gao et al. 2014; Peng et al. 2013).
In this study, PhDof-1, PhDof-6, and PhDof-8 showed different site-specific middle–to–high expression levels in bamboo shoots (Table S4). Moreover, PhDof-6 was differentially expressed at four flower development stages, and PhDof-1 and PhDof-8 were period-specifically differentially expressed at the floral bud formation stage (Table S5). In addition, they clustered with DAG1, DAG2, RPBF, HvSAD and PsDOF-7, which are indirectly or directly involved in carbohydrate metabolism (Kawakatsu and Takaiwa 2010; de Dios Barajas-López et al. 2012). PhDof-1, PhDof-6 and PhDof-8 might be site-specifically or period-specifically involve in carbohydrate metabolism for rapid bamboo shoots growth and flower development process, respectively. During the flower development process, PhDof-2, PhDof-3 and PhDof-25 showed the largest differential expressions at the early stage, suggesting that they all positively regulated floral bud formation. The phylogenetics analysis results showed that PhDof-25 clustered with AtDof1.1 (OBP2), PhDof-2 clustered with AtDof5.6 (HCA2), and PhDof-3 clustered with AtDof5.8, which has been reported to involved in regulating glucosinolate biosynthesis (Skirycz et al. 2006). AtDof5.8 is expressed specifically in cells at an early stage of vascular tissue development (Guo et al. 2009; Konishi and Yanagisawa 2007), and is responsive to abiotic stress (He et al. 2015), respectively. These results will help build a foundation for the study of moso bamboo secondary metabolism, vascular tissue development and abiotic stress responses.
Genes in the same group, with similar expression patterns, might have conserved functions. PhDof-7 and PhDof-11 had similar expression patterns in bamboo shoots but showed different patterns in flower development stages. However, some Dof members in the same subgroups had totally different expression patterns. For example, in the D3 subgroup, PhDof-13 showed specific expression in the base of the shoots, while PhDof-23 showed very low expression in all four shoot samples; even some paralogous genes with highly identical amino acid sequences had totally opposite expression patterns. For example, for the PhDof-3/PhDof-15 paralogous gene pair, PhDof-3 was mainly expressed in the base part of the shoots, while PhDof-15 was rarely expressed there, suggesting that they might participate in the same process through different ways. This phenomenon was also observed in an expression analysis of the soybean Dof family genes (Guo and Qiu 2013). The results revealed that the Dof family genes show functional and regulative diversity, even among the paralogous genes, despite having highly similar amino acid sequences.
Conclusions
In this study, we conducted a detailed analysis of the Dof gene family in moso bamboo, including genome-wide identification, phylogeny, gene structure, protein motifs and expression pattern analyses. These results will form the basis for future gene-cloning and functional analysis to unravel the role of Dof genes in the fast growth and floral development of moso bamboo.
References
Baumann K, De Paolis A, Costantino P, Gualberti G (1999) The DNA binding site of the Dof protein NtBBF1 is essential for tissue-specific and auxin-regulated expression of the rolB oncogene in plants. Plant Cell 11:323–333
Cai X, Zhang Y, Zhang C, Zhang T, Hu T, Ye J, Zhang J, Wang T, Li H, Ye Z (2013) Genome-wide analysis of plant-specific Dof transcription factor family in tomato. J Integr Plant Biol 6:552–566
Chen X, Wang D, Liu C, Wang M, Wang T, Zhao Q, Yu J (2012) Maize transcription factor Zmdof1 involves in the regulation of Zm401 gene. Plant Growth Regul 66:271–284
Cominelli E, Galbiati M, Albertini A, Fornara F, Conti L, Coupland G, Tonelli C (2011) DOF-binding sites additively contribute to guard cell-specificity of AtMYB60 promoter. BMC Plant Biol 11:162
de Dios Barajas-López J, Tezycka J, Travaglia CN, Serrato AJ, Chueca A, Thormählen I, Geigenberger P, Sahrawy M (2012) Expression of the chloroplast thioredoxins f and m is linked to shortterm changes in the sugar and thiol status in leaves of Pisum sativum. J Exp Bot 13:4887–4990
Deng W, Wang Y, Liu Z, Cheng H, Xue Y (2014) HemI: a toolkit for illustrating heatmaps. PLoS one 11:e111988
Diaz I, Vicente-Carbajosa J, Abraham Z, Martinez M, Moneda II, Carbonero P (2002) The GAMYB protein from barley interacts with the DOF transcription factor BPBF and activates endosperm-specific genes during seed development. Plant J 4:453–464
Dong G, Ni Z, Yao Y, Sun XN, Qi X (2007) Wheat Dof transcription factor WPBF interacts with TaQM and activates transcription of an alpha-gliadin gene during wheat seed development. Plant Mol Biol 63:73–84
Finn RD, Clements J, Eddy SR (2011) HMMER web server: interactive sequence similarity searching. Nucleic Acids Res 39:29–37
Fornara F, Panigrahi KCS, Gissot L, Sauerbrunn N, Ruhl M, Jarillo JA, Coupland G (2009) Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev Cell 17:75–86
Gabriele S, Rizza A, Martone J, Circelli P, Costantino P, Vittorioso P (2010) The Dof protein DAG1 mediates PIL5 activity on seed germination by negatively regulating GA biosynthetic gene AtGA3ox1. Plant J 61:312–323
Gao X (2011) Study on the components and biological activity of bamboo shoot shell extractions. Dissertation, Beijing Forestry University
Gao J, Zhang Y, Zhang CL, Qi FY, Li XP, Mu SH, Peng ZH (2014) Characterization of the floral transcriptome of moso bamboo (Phyllostachys edulis) at different flowering developmental stages by transcriptome sequencing and RNA-Seq analysis. PLoS one 9:e98910
Gardiner J, Sherr I, Scarpella E (2010) Expression of DOF genes identifies early stages of vascular development in Arabidopsis leaves. Int J Dev Biol 54:1389–1396
Gaur VS, Singh US, Kumar A (2011) Transcriptional profiling and in silico analysis of Dof transcription factor gene family for understanding their regulation during seed development of rice (Oryza sativa L.). Mol Biol Rep 38:2827–2848
Gualberti G, Papi M, Bellucci L, Ricci I, Bouche D, Camilleri C, Costantino P, Vittorioso P (2002) Mutations in the Dof zinc finger genes DAG2 and DAG1 influence with opposite effects the germination of Arabidopsis seeds. Plant Cell 14:1253–1263
Guo Y, Qiu L (2013) Genome-wide analysis of the Dof transcription factor gene family reveals soybean-specific duplicable and functional characteristics. PLoS one 9:e76809
Guo Y, Qin G, Gu H, Qu L (2009) Dof5.6/HCA2, a Dof transcription factor gene, regulates interfascicular cambium formation and vascular tissue development in Arabidopsis. Plant Cell 21:3518–3534
He L, Su C, Wang Y, Wei Z (2015) ATDOF5.8 protein is the upstream regulator of ANAC069 and is responsive to abiotic stress. Biochimie 110:17–24
Hernando-Amado S, González-Calle V, Carbonero P, Barrero-Sicilia C (2012) The family of DOF transcription factors in Brachypodium distachyon: phylogenetic comparison with rice and barley DOFs and expression profiling. BMC Plant Biol 12:202
Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA (2005) FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 309:293
Iwamoto M, Higo K, Takano M (2009) Circadian clock- and phytochrome-regulated Dof-like gene, Rdd1, is associated with grain size in rice. Plant Cell Environ 32:592–603
Jiang Y, Zeng B, Zhao H, Zhang M, Xie S, Lai J (2012) Genome-wide transcription factor gene prediction and their expressional tissue-specificities in maize. J Integr Plant Biol 9:616–630
Jin J, Zhang H, Kong L, Gao G, Luo J (2014a) PlantTFDB 3.0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res 42:D1182–D1187
Jin Z, Chandrasekaran U, Liu A (2014b) Genome-wide analysis of the Dof transcription factors in castor bean (Ricinus communis L.). Genes Genom 36:527–537
Kang H, Singh KB (2000) Characterization of salicylic acid-responsive, Arabidopsis Dof domain proteins: overexpression of OBP3 leads to growth defects. Plant J 4:329–339
Kang H, Foley RC, Onate-Sanchez L, Lin C, Singh KB (2003) Target genes for OBP3, a Dof transcription factor, include novel basic helix-loop-helix domain proteins inducible by salicylic acid. Plant J 35:362–372
Kawakatsu T, Takaiwa F (2010) Differences in transcriptional regulatory mechanisms functioning for free lysine content and seed storage protein accumulation in rice grain. Plant Cell Physiol 12:1964–1974
Kisu Y, Ono T, Shimofurutani N, Suzuki M, Esaka M (1998) Characterization and expression of a new class of zinc finger protein that binds to silencer region of ascorbate oxidase gene. Plant Cell Physiol 10:1054–1064
Konishi M, Yanagisawa S (2007) Sequential activation of two Dof transcription factor gene promoters during vascular development in Arabidopsis thaliana. Plant Physiol and Biochem 45:623–629
Kumar R, Taware R, Gaur VS, Guru SK, Kumar A (2009) Influence of nitrogen on the expression of TaDof1 transcription factor in wheat and its relationship with photo synthetic and ammonium assimilating efficiency. Mol Biol Rep 36:2209–2220
Kurai T, Wakayama M, Abiko T, Yanagisawa S, Aoki N, Ohsugi R (2011) Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotechnol J 9:826–837
Kushwaha H, Gupta S, Singh VK, Rastogi S, Yadav D (2011) Genome wide identification of Dof transcription factor gene family in sorghum and its comparative phylogenetic analysis with rice and Arabidopsis. Mol Biol Rep 38:5037–5053
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R et al (2007) Clustal W and Clustal X version 2.0. Bioinformatics. Bioinformatics 21:2947–2948
Latchman DS (1997) Transcription factors: an overview. Int J Biochem Cell Biol 29:1305–1312
Li D, Yang C, Li X, Gan Q, Zhao X, Zhu L (2009) Functional characterization of rice OsDof12. Planta 229:1159–1169
Li CH, Cai B, Lou XM, Tang R (2013) Genome-wide analysis of the Dof transcription factor gene family in grape. J Yangzhou Univ (Agric Life Sci Ed) 4:99–103
Lijavetzky D, Carbonero P, Vicente-Carbajosa J (2003) Genome-wide comparative phylogenetic analysis of the rice and Arabidopsis Dof gene families. BMC Evol Biol 3:17
Lin XC, Chow TY, Chen HH, Liu CC, Chou SJ, Huang BL, Kuo CI, Wen CK, Huang LC, Fang W (2010) Understanding bamboo flowering based on large-scale analysis of expressed sequence tags. Genet Mol Res 9:1085–1093
Marzabal P, Gas E, Fontanet P, Vicente-Carbajosa J, Torrent M, Ludevid MD (2008) The maize Dof protein PBF activates transcription of γ-zein during maize seed development. Plant Mol Biol 67:441–454
Mena M, Vicente-Carbajosa J, Schmidt RJ, Carbonero P (1998) An endosperm-specific DOF protein from barley, highly conserved in wheat, binds to and activates transcription from the prolamin-box of a native B-hordein promoter in barley endosperm. Plant J 1:53–62
Mena M, Cejudo FJ, Isabel-Lamoneda I, Carbonero P (2002) A role for the DOF transcription factor BPBF in the regulation of gibberellin-responsive genes in barley aleurone. Plant Physiol 130:111–119
Moreno-Risueno MA, Dıaz I, Carrillo L, Fuentes R, Carbonero P (2007a) The HvDOF19 transcription factor mediates the abscisic acid-dependent repression of hydrolase genes in germinating barley aleurone. Plant J 51:352–365
Moreno-Risueno MÁ, Martínez M, Vicente-Carbajosa J, Carbonero P (2007b) The family of DOF transcription factors: from green unicellular algae to vascular plants. Mol Genet Genom 277:379–390
Papi M, Sabatini S, Bouchez D, Camilleri C, Costantino P, Vittorioso P (2000) Identification and disruption of an Arabidopsis zinc finger gene controlling seed germination. Gene Dev 14:28–33
Park DH, Lim PO, Kim JS, Cho DS, Hong SH, Nam HG (2003) The Arabidopsis COG1 gene encodes a Dof domain transcription factor and negatively regulates phytochrome signaling. Plant J 34:161–171
Peng ZH, Lu TT, Li LB, Liu X, Gao ZM, Hu T, Yang X, Feng Q, Guan J, Weng QJ et al (2010) Genome-wide characterization of the biggest grass, bamboo, based on 10,608 putative full-length cDNA sequences. BMC Plant Biol 10:116
Peng Z, Lu Y, Li L, Zhao Q, Feng Q, Gao ZM, Lu HY, Hu T, Yao N, Liu KY et al (2013) The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nature Genet 4:456–463
Plesch G, Ehrhardt T, Mueller-Roeber B (2001) Involvement of TAAAG elements suggests a role for Dof transcription factors in guard cell-specific gene expression. Plant J 4:455–464
Riechmann JL, Heard J, Martin G, Reuber L, Jiang CZ, Keddie J, Adam L, Pineda Ratcliffe J, Samaha RR et al (2000) Arabidopsis transcription factors genome-wide comparative analysis among eukaryotes. Science 290:2105–2110
Rizza A, Boccaccini A, Lopez-Vidriero I, Costantino P, Vittorioso P (2011) Inactivation of the ELIP1 and ELIP2 genes affects Arabidopsis seed germination. New Phytol 190:896–905
Rueda-Lopez M, Crespillo R, Canovas FM, Avila C (2008) Differential regulation of two glutamine synthetase genes by a single Dof transcription factor. Plant J 56:73–85
Rueda-Romero P, Barrero-Sicilia C, Gomez-Cadenas A, Carbonero P, Onate-Sanchez L (2012) Arabidopsis thaliana DOF6 negatively affects germination in non-after-ripened seeds and interacts with TCP14. J Exp Bot 5:1937–1949
Sawa M, Nusinow DA, Kay SA, Imaizumi T (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318:261–265
Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W (2010) Genome sequence of the palaeopolyploid soybean. Nature 463:178–183
Shaw LM, McIntyre CL, Gresshoff PM, Xue G (2009) Members of the Dof transcription factor family in Triticum aestivum are associated with light-mediated gene regulation. Funct Integr Genom 9:485–498
Shigyo M, Tabei N, Yoneyama T, Yanagisawa S (2007) Evolutionary processes during the formation of the plant-specific Dof transcription factor family. Plant Cell Physiol 1:179–185
Skirycz A, Reichelt M, Burow M, Birkemeyer C, Rolcik J, Kopka J, Zanor MI, Gershenzon J, Strnad M, Szopa J et al (2006) DOF transcription factor AtDof1.1 (OBP2) is part of a regulatory network controlling glucosinolate biosynthesis in Arabidopsis. Plant J 47:10–24
Skirycz A, Jozefczuk S, Stobiecki M, Muth D, Zanor MI, Witt I, Mueller-Roeber B (2007) Transcription factor AtDOF4.2 affects phenylpropanoid metabolism in Arabidopsis thaliana. New Phytol 175:425–438
Song YH, Smith RW, To BJ, Millar AJ, Imaizumi T (2012) FKF1 conveys crucial timing information for CONSTANS stabilization in the photoperiodic flowering. Science 6084:1045–1049
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Abiko T, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony. Mol Biol Evol 10:2731–2739
Tanaka M, Takahata Y, Nakayama H, Nakatan M, Tahara M (2009) Altered carbohydrate metabolism in the storage roots of sweetpotato plants overexpressing the SRF1 gene, which encodes a Dof zinc finger transcription factor. Planta 230:737–746
Vicente-Carbajosa J, Moose SP, Parsons RL, Schmidt RJ (1997) A maize zinc-finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator Opaque2. Proc Natl Acad Sci USA 94:7685–7690
Wang H, Zhang B, Hao Y, Huang J, Tian A, Liao Y, Zhang J, Chen S (2007) The soybean Dof-type transcription factor genes, GmDof4 and GmDof11, enhance lipid content in the seeds of transgenic Arabidopsis plants. Plant J 52:716–729
Ward JM, Cufr CA, Denzel MA, Neff MM (2005) The Dof transcription factor OBP3 modulates phytochrome and cryptochrome signaling in Arabidopsis. Plant Cell 17:475–485
Washio K (2003) Functional dissections between GAMYB and Dof transcription factors suggest a role for protein-protein associations in the gibberellin-mediated expression of the RAmy1A gene in the rice aleurone. Plant Physiol 133:850–863
Wei P, Tan F, Gao X, Zhang X, Wang G, Xu H, Li L, Chen J, Wang X (2010) Over-expression of AtDOF4.7, an Arabidopsis DOF family transcription factor, induces floral organ abscission deficiency in Arabidopsis. Plant Physiol 153:1031–1045
Yanagisawa S (1995) A novel DNA-binding domain that may form a single zinc finger motif. Nucleic Acids Res 17:3403–3410
Yanagisawa S (2000) Dof1 and Dof2 transcription factors are associated with expression of multiple genes involved in carbon metabolism in maize. Plant J 3:281–288
Yanagisawa S, Sheen J (1998) Involvement of maize Dof zinc finger proteins in tissue-specific and light-regulated gene expression. Plant Cell 10:75–89
Yang XH, Tuskan GA, Cheng ZM (2006a) Divergence of the dof gene families in poplar, Arabidopsis and rice suggests multiple modes of gene evolution after duplication. Plant Physiol 142:820–830
Yang X, Tuskan GA, Cheng Z (2006b) Divergence of the Dof gene families in poplar, Arabidopsis, and rice suggests multiple modes of gene evolution after duplication. Plant Physiol 142:820–830
Yang J, Yang M, Wang D, Chen F, Shen S (2010) JcDof1 a Dof transcription factor gene, is associated with the light-mediated circadian clock in Jatropha curcas. Physiol Plantarum 139:324–334
Yang J, Yang M, Zhang W, Chen F, Shen S (2011) A putative flowering-time-related Dof transcription factor gene, JcDof3, is controlled by the circadian clock in Jatropha curcas. Plant Sci 181:667–674
Zhang B, Chen W, Foley RC, Büttner M, Singh KB (1995) Interactions between distinct types of DNA binding proteins enhance binding to ocs element promoter. Plant Cell 7:2241–2252
Acknowledgments
This work was supported by the Forestry Project of the Ministry of Science and Technology of the People’s Republic of China (Grant 200704001 to Z. J.), and the Chinese Academy of Sciences (KSCX2-YW-G-034 to B. H.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that no competing interests exist.
Additional information
Tao Wang and Jin-Jun Yue have contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, T., Yue, JJ., Wang, XJ. et al. Genome-wide identification and characterization of the Dof gene family in moso bamboo (Phyllostachys heterocycla var. pubescens). Genes Genom 38, 733–745 (2016). https://doi.org/10.1007/s13258-016-0418-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-016-0418-2