Abstract
This paper is a compilation of notes on 142 fungal taxa, including five new families, 20 new genera, and 100 new species, representing a wide taxonomic and geographic range. The new families, Ascocylindricaceae, Caryosporaceae and Wicklowiaceae (Ascomycota) are introduced based on their distinct lineages and unique morphology. The new Dothideomycete genera Pseudomassariosphaeria (Amniculicolaceae), Heracleicola, Neodidymella and Pseudomicrosphaeriopsis (Didymellaceae), Pseudopithomyces (Didymosphaeriaceae), Brunneoclavispora, Neolophiostoma and Sulcosporium (Halotthiaceae), Lophiohelichrysum (Lophiostomataceae), Galliicola, Populocrescentia and Vagicola (Phaeosphaeriaceae), Ascocylindrica (Ascocylindricaceae), Elongatopedicellata (Roussoellaceae), Pseudoasteromassaria (Latoruaceae) and Pseudomonodictys (Macrodiplodiopsidaceae) are introduced. The newly described species of Dothideomycetes (Ascomycota) are Pseudomassariosphaeria bromicola (Amniculicolaceae), Flammeascoma lignicola (Anteagloniaceae), Ascocylindrica marina (Ascocylindricaceae), Lembosia xyliae (Asterinaceae), Diplodia crataegicola and Diplodia galiicola (Botryosphaeriaceae), Caryospora aquatica (Caryosporaceae), Heracleicola premilcurensis and Neodidymella thailandicum (Didymellaceae), Pseudopithomyces palmicola (Didymosphaeriaceae), Floricola viticola (Floricolaceae), Brunneoclavispora bambusae, Neolophiostoma pigmentatum and Sulcosporium thailandica (Halotthiaceae), Pseudoasteromassaria fagi (Latoruaceae), Keissleriella dactylidicola (Lentitheciaceae), Lophiohelichrysum helichrysi (Lophiostomataceae), Aquasubmersa japonica (Lophiotremataceae), Pseudomonodictys tectonae (Macrodiplodiopsidaceae), Microthyrium buxicola and Tumidispora shoreae (Microthyriaceae), Alloleptosphaeria clematidis, Allophaeosphaeria cytisi, Allophaeosphaeria subcylindrospora, Dematiopleospora luzulae, Entodesmium artemisiae, Galiicola pseudophaeosphaeria, Loratospora luzulae, Nodulosphaeria senecionis, Ophiosphaerella aquaticus, Populocrescentia forlicesenensis and Vagicola vagans (Phaeosphaeriaceae), Elongatopedicellata lignicola, Roussoella magnatum and Roussoella angustior (Roussoellaceae) and Shrungabeeja longiappendiculata (Tetraploasphaeriaceae). The new combinations Pseudomassariosphaeria grandispora, Austropleospora archidendri, Pseudopithomyces chartarum, Pseudopithomyces maydicus, Pseudopithomyces sacchari, Vagicola vagans, Punctulariopsis cremeoalbida and Punctulariopsis efibulata Dothideomycetes. The new genera Dictyosporella (Annulatascaceae), and Tinhaudeus (Halosphaeriaceae) are introduced in Sordariomycetes (Ascomycota) while Dictyosporella aquatica (Annulatascaceae), Chaetosphaeria rivularia (Chaetosphaeriaceae), Beauveria gryllotalpidicola and Beauveria loeiensis (Cordycipitaceae), Seimatosporium sorbi and Seimatosporium pseudorosarum (Discosiaceae), Colletotrichum aciculare, Colletotrichum fusiforme and Colletotrichum hymenocallidicola (Glomerellaceae), Tinhaudeus formosanus (Halosphaeriaceae), Pestalotiopsis subshorea and Pestalotiopsis dracaenea (Pestalotiopsiceae), Phaeoacremonium tectonae (Togniniaceae), Cytospora parasitica and Cytospora tanaitica (Valsaceae), Annulohypoxylon palmicola, Biscogniauxia effusae and Nemania fusoideis (Xylariaceae) are introduced as novel species to order Sordariomycetes. The newly described species of Eurotiomycetes are Mycocalicium hyaloparvicellulum (Mycocaliciaceae). Acarospora septentrionalis and Acarospora castaneocarpa (Acarosporaceae), Chapsa multicarpa and Fissurina carassensis (Graphidaceae), Sticta fuscotomentosa and Sticta subfilicinella (Lobariaceae) are newly introduced in class Lecanoromycetes. In class Pezizomycetes, Helvella pseudolacunosa and Helvella rugosa (Helvellaceae) are introduced as new species. The new families, Dendrominiaceae and Neoantrodiellaceae (Basidiomycota) are introduced together with a new genus Neoantrodiella (Neoantrodiellaceae), here based on both morphology coupled with molecular data. In the class Agaricomycetes, Agaricus pseudolangei, Agaricus haematinus, Agaricus atrodiscus and Agaricus exilissimus (Agaricaceae), Amanita melleialba, Amanita pseudosychnopyramis and Amanita subparvipantherina (Amanitaceae), Entoloma calabrum, Cora barbulata, Dictyonema gomezianum and Inocybe granulosa (Inocybaceae), Xerocomellus sarnarii (Boletaceae), Cantharellus eucalyptorum, Cantharellus nigrescens, Cantharellus tricolor and Cantharellus variabilicolor (Cantharellaceae), Cortinarius alboamarescens, Cortinarius brunneoalbus, Cortinarius ochroamarus, Cortinarius putorius and Cortinarius seidlii (Cortinariaceae), Hymenochaete micropora and Hymenochaete subporioides (Hymenochaetaceae), Xylodon ramicida (Schizoporaceae), Colospora andalasii (Polyporaceae), Russula guangxiensis and Russula hakkae (Russulaceae), Tremella dirinariae, Tremella graphidis and Tremella pyrenulae (Tremellaceae) are introduced. Four new combinations Neoantrodiella gypsea, Neoantrodiella thujae (Neoantrodiellaceae), Punctulariopsis cremeoalbida, Punctulariopsis efibulata (Punctulariaceae) are also introduced here for the division Basidiomycota. Furthermore Absidia caatinguensis, Absidia koreana and Gongronella koreana (Cunninghamellaceae), Mortierella pisiformis and Mortierella formosana (Mortierellaceae) are newly introduced in the Zygomycota, while Neocallimastix cameroonii and Piromyces irregularis (Neocallimastigaceae) are introduced in the Neocallimastigomycota. Reference specimens or changes in classification and notes are provided for Alternaria ethzedia, Cucurbitaria ephedricola, Austropleospora, Austropleospora archidendri, Byssosphaeria rhodomphala, Lophiostoma caulium, Pseudopithomyces maydicus, Massariosphaeria, Neomassariosphaeria and Pestalotiopsis montellica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Table of Contents
Ascomycota
Pezizomycotina Class Dothideomycetes
Amniculicolaceae
-
111.
Pseudomassariosphaeria Phukhamsakda, Ariyawansa, Camporesi & K.D. Hyde, gen. nov.
-
112.
Pseudomassariosphaeria bromicola Phukhamsakda, Ariyawansa, Camporesi & K.D. Hyde, sp. nov.
-
113.
Pseudomassariosphaeria grandispora (Sacc.) Phukhamsakda, Ariyawansa & K.D. Hyde, comb. nov.
Anteagloniaceae
-
114.
Flammeascoma lignicola Boonmee & K.D. Hyde, sp. nov.
Ascocylindricaceae
-
115.
Ascocylindricaceae Abdel-Wahab, Bahkali, E.B.G. Jones, Ariyawansa & K.D. Hyde, fam. nov.
-
116.
Ascocylindrica Abdel-Wahab, Bahkali & E.B.G. Jones, gen. nov.
-
117.
Ascocylindrica marina Abdel-Wahab, Bahkali & E.B.G. Jones, sp. nov.
Asterinaceae
-
118.
Lembosia xyliae X.Y. Zeng & K.D. Hyde, sp. nov.
Botryosphaeriaceae
-
119.
Diplodia crataegicola Dissanayake, Camporesi & K.D. Hyde, sp. nov.
-
120.
Diplodia galiicola Dissanayake, Camporesi & K.D. Hyde, sp. nov.
Caryosporaceae
-
121.
Caryosporaceae H. Zhang, K.D. Hyde & Ariyawansa, fam. nov.
-
122.
Caryospora aquatica H. Zhang, K.D. Hyde & Ariyawansa, sp. nov.
Cucubitariaceae
-
123.
Cucurbitaria ephedricola Esfand.
Didymellaceae
-
124.
Heracleicola Tibpromma, Camporesi & K.D. Hyde, gen. nov.
-
125.
Heracleicola premilcurensis Tibpromma, Camporesi & K.D. Hyde, sp. nov.
-
126.
Neodidymella Phookamsak, R.H. Perera & K.D. Hyde, gen. nov.
-
127.
Neodidymella thailandicum Phookamsak, R.H. Perera & K.D. Hyde, sp. nov.
Didymosphaeriaceae
-
128.
Austropleospora R.G. Shivas & L. Morin
-
129.
Austropleospora archidendri (Verkley et al.) Ariyawansa & K.D. Hyde, comb. nov.
-
130.
Pseudopithomyces Ariyawansa & K.D. Hyde, gen. nov.
-
131.
Pseudopithomyces chartarum (Berk. & M.A. Curtis) J.F. Li, Ariyawansa & K.D. Hyde, comb. nov.
-
132.
Pseudopithomyces palmicola J.F. Li, Ariyawansa & K.D. Hyde, sp. nov.
-
133.
Pseudopithomyces maydicus (Sacc.) J.F. Li, Ariyawansa & K.D. Hyde, comb. nov.
-
134.
Pseudopithomyces sacchari (Speg.) Ariyawansa & K.D. Hyde, comb. nov.
Floricolaceae
-
135.
Floricola viticola Phukhamsakda, Camporesi & K.D. Hyde, sp. nov.
Halotthiaceae
-
136.
Brunneoclavispora Phookamsak & K.D. Hyde, gen. nov.
-
137.
Brunneoclavispora bambusae Phookamsak & K.D. Hyde, sp. nov.
-
138.
Neolophiostoma S. Boonmee & K.D. Hyde, gen. nov.
-
139.
Neolophiostoma pigmentatum Boonmee & K.D. Hyde, sp. nov.
-
140.
Sulcosporium Phookamsak & K.D. Hyde, gen. nov.
-
141.
Sulcosporium thailandica Phookamsak & K.D. Hyde, sp. nov.
Latoruaceae
-
142.
Pseudoasteromassaria Matsumura & Kaz. Tanaka, gen. nov.
-
143.
Pseudoasteromassaria fagi Matsumura & Kaz. Tanaka, sp. nov.
Lentitheciaceae
-
144.
Keissleriella dactylidicola Mapook, Camporesi & K.D. Hyde, sp. nov.
Lindgomycetaceae
-
145.
Neomassariosphaeria Y. Zhang et al.
Lophiostomataceae
-
146.
Lophiostoma caulium (Fr.) Ces. & De Not.
-
147.
Lophiohelichrysum Dayarathne, Camporesi & K.D. Hyde, gen. nov.
-
148.
Lophiohelichrysum helichrysi Dayarathne, Camporesi & K.D. Hyde, sp. nov.
Lophiotremataceae
-
149.
Aquasubmersa japonica A. Hashim. & Kaz. Tanaka, sp. nov.
Macrodiplodiopsidaceae
-
150.
Pseudomonodictys Doilom, Ariyawansa, D.J. Bhat & K.D. Hyde, gen nov.
-
151.
Pseudomonodictys tectonae Doilom, Ariyawansa, D.J. Bhat & K.D. Hyde, sp. nov.
Melanommataceae
-
152.
Byssosphaeria rhodomphala (Berk.) Cooke
Microthyriaceae
-
153.
Microthyrium buxicola Hongsanan & K.D. Hyde, sp. nov.
-
154.
Tumidispora Hongsanan & K.D. Hyde, gen. nov.
-
155.
Tumidispora shoreae Hongsanan & K.D. Hyde, sp. nov.
Phaeosphaeriaceae
-
156.
Alloleptosphaeria clematidis Wanasinghe, Camporesi, E.B.G. Jones & K.D. Hyde, sp. nov.
-
157.
Allophaeosphaeria cytisi Wanasinghe, Camporesi, E.B.G. Jones & K.D. Hyde, sp. nov.
-
158.
Allophaeosphaeria subcylindrospora W.J. Li, Camporesi & K.D. Hyde, sp. nov.
-
159.
Dematiopleospora luzulae Wanasinghe, Camporesi, E.B.G. Jones & K.D. Hyde, sp. nov.
-
160.
Entodesmium artemisiae Konta, Bulgakov & K D Hyde, sp. nov.
-
161.
Galiicola Tibpromma, Camporesi & K.D. Hyde, gen. nov.
-
162.
Galiicola pseudophaeosphaeria Tibpromma, Camporesi & K.D. Hyde, sp. nov.
-
163.
Loratospora luzulae Jayasiri, Camporesi & K.D. Hyde, sp. nov.
-
164.
Nodulosphaeria senecionis Chethana, Camporesi, E.B.G. Jones & K.D. Hyde, sp. nov.
-
165.
Ophiosphaerella aquaticus Z.L. Luo, H.Y. Su & K.D. Hyde, sp. nov.
-
166.
Populocrescentia Wanasinghe, E.B.G. Jones &K.D. Hyde, gen. nov.
-
167.
Populocrescentia forlicesenensis Wanasinghe, Camporesi, E.B.G. Jones & K.D. Hyde, sp. nov.
-
168.
Vagicola Chethana & K.D. Hyde, gen. nov.
-
169.
Vagicola vagans (Niessl) O. Eriksson, Chethana & K.D. Hyde, comb. nov.
Pleosporaceae
-
170.
A lternaria ethzedia E.G. Simmons
Roussoellaceae
-
171.
Elongatopedicellata J.F. Zhang, J.K. Liu, K.D. Hyde & Z.Y. Liu, gen. nov.
-
172.
Elongatopedicellata lignicola J.F. Zhang, J.K. Liu, K.D. Hyde & Z.Y. Liu, sp. nov.
-
173.
Roussoella magnatum D.Q. Dai & K.D. Hyde, sp. nov.
-
174.
Roussoella angustior D.Q. Dai & K.D. Hyde, sp. nov.
Tetraploasphaeriaceae
-
175.
Shrungabeeja longiappendiculata S. Sommai, U. Pinruan, S. Nuankaew & S. Suetrong, sp. nov. Thyridariaceae
-
176.
Massariosphaeria
Wicklowiaceae
-
177.
Wicklowiaceae Ariyawansa & K.D. Hyde, fam. nov.
Class Eurotiomycetes
Mycocaliciaceae
-
178.
Mycocalicium hyaloparvicellulum Daranagama & K.D. Hyde, sp. nov.
Class Lecanoromycetes
Acarosporaceae
-
179.
Acarospora septentrionalis M. Westb. & Wedin, sp. nov.
-
180.
Acarospora castaneocarpa M. Westb. & Wedin, sp. nov.
Graphidaceae
-
181.
Chapsa multicarpa Lücking, Parnmen & Lumbsch, sp. nov.
-
182.
Fissurina carassensis Lücking, Parnmen & Lumbsch, sp. nov.
Lobariaceae
-
183.
Sticta fuscotomentosa Moncada, Coca & Lücking, sp. nov.
-
184.
Sticta subfilicinella Moncada, Coca & Lücking, sp. nov.
Class Pezizomycetes
Helvellaceae
-
185.
Helvella pseudolacunosa Q. Zhao & K.D. Hyde, sp. nov.
-
186.
Helvella rugosa Q. Zhao & K.D. Hyde, sp. nov.
Class Sordariomycetes
Annulatascaceae
-
187.
Dictyosporella Abdel-Aziz, gen. nov.
-
188.
Dictyosporella aquatica Abdel-Aziz, sp. nov.
Chaetosphaeriaceae
-
189.
Chaetosphaeria rivularia Réblová & J. Fourn., sp. nov.
Cordycipitaceae
-
190.
Beauveria gryllotalpidicola Luangsa-ard, Ridkaew & Tasan., sp. nov.
-
191.
Beauveria loeiensis Luangsa-ard, Ridkaew & Tasan., sp. nov.
Discosiaceae
-
192.
Seimatosporium sorbi Wijayawardene, Camporesi & K.D. Hyde, sp. nov.
-
193.
Seimatosporium pseudorosarum Wijayawardene, Camporesi & K.D. Hyde, sp. nov.
Glomerellaceae
-
194.
Colletotrichum aciculare Jayawardena, Y. Than, N. Tangthir., K.D. Hyde, sp. nov.
-
195.
Colletotrichum fusiforme Jayawardena, J. Bhat, N. Tangthir., K.D. Hyde, sp. nov.
-
196.
Colletotrichum hymenocallidicola Chethana, Tangthirasunun, Jayawardena & K.D. Hyde, sp. nov.
Halosphaeriaceae
-
197.
Tinhaudeus K.L. Pang, S.Y. Guo & E.B.G. Jones, gen. nov.
-
198.
Tinhaudeus formosanus K.L. Pang, S.Y. Guo & E.B.G. Jones, sp. nov.
Pestalotiopsiceae
-
199.
Pestalotiopsis subshorea Yong Wang bis, Y. Song, K. Geng & K.D. Hyde, sp. nov.
-
200.
Pestalotiopsis dracaenea Yong Wang bis, Y. Song, K. Geng & K.D. Hyde, sp. nov. Wang Yong
-
201.
Pestalotiopsis montellica (Sacc. & Voglino) Tak.Kobay. Togniniaceae
-
202.
Phaeoacremonium tectonae Doilom & K.D. Hyde, sp. nov.
Valsaceae
-
203.
Cytospora parasitica Norphanphoun, Bulgakov & K.D. Hyde, sp. nov.
-
204.
Cytospora tanaitica Norphanphoun, Bulgakov & K.D. Hyde, sp. nov.
Xylariaceae
-
205.
Annulohypoxylon palmicola J.K Liu & K.D Hyde, sp. nov.
-
206.
Biscogniauxia effusae Q.R. Li, J.C. Kang & K.D. Hyde, sp. nov.
-
207.
Nemania fusoideis Q.R. Li, J.C. Kang & K.D. Hyde, sp. nov.
Basidiomycota
Class Agaricomycetes
Subclass Agaricomycetidae
Order Agaricales
Agaricaceae
-
208.
Agaricus pseudolangei K.D. Hyde & R.L. Zhao, sp. nov.
-
209.
Agaricus haematinus K.D. Hyde & R.L. Zhao, sp. nov.
-
210.
Agaricus atrodiscus L.J. Chen, Callac, R.L. Zhao & K.D. Hyde, sp. nov.
-
211.
Agaricus exilissimus L.J. Chen, Callac, R.L. Zhao & K.D. Hyde, sp. nov.
Amanitaceae
-
212.
Amanita melleialba Zhu L. Yang, Q. Cai & Yang Y. Cui, sp. nov.
-
213.
Amanita pseudosychnopyramis Yang Y. Cui, Q. Cai & Zhu L. Yang, sp. nov.
-
214.
Amanita subparvipantherina Zhu L. Yang, Q. Cai & Yang Y. Cui, sp. nov.
Entolomataceae
-
215.
Entoloma calabrum Battistin, Marsico, Vizzini, Vila & Ercole, sp. nov.
Hygrophoraceae
-
216.
Cora barbulata Lücking, Dal-Forno & Lawrey, sp. nov.
-
217.
Dictyonema gomezianum Lücking, Dal-Forno & Lawrey, sp. nov.
Inocybaceae
-
218.
Inocybe granulosa Jacobsson & E. Larss. sp. nov.
Order Boletales
Boletaceae
-
219.
Xerocomellus sarnarii Simonini, Vizzini & Eberhardt, sp. nov.
Order Cantharellales
Cantharellaceae
-
220.
Cantharellus eucalyptorum Buyck, Randrianjohany & V. Hofstetter sp. nov.
-
221.
Cantharellus nigrescens Buyck, Randrianjohany & V. Hofstetter sp. nov.
-
222.
Cantharellus tricolor Buyck, Randrianjohany & V. Hofstetter sp. nov.
-
223.
Cantharellus variabilicolor Buyck, Randrianjohany & V. Hofstetter sp. nov.
Order Cortinariales
Cortinariaceae
-
224.
Cortinarius alboamarescens Kytöv., Niskanen & Liimat., sp. nov.
-
225.
Cortinarius brunneoalbus Ammirati, Liimat. & Niskanen, sp. nov.
-
226.
Cortinarius ochroamarus Niskanen, Kytöv. & Liimat., sp. nov.
-
227.
Cortinarius putorius Niskanen, Liimat. & Ammirati, sp. nov.
-
228.
Cortinarius seidlii Ammirati, Niskanen & Liimat., sp. nov.
Dendrominiaceae
-
229.
Dendrominiaceae Ghobad-Nejhad, fam. nov.
Punctulariaceae
-
230.
Punctulariopsis cremeoalbida (M.J. Larsen & Nakasone) Ghobad-Nejhad, comb. nov.
-
231.
Punctulariopsis efibulata (M.J. Larsen & Nakasone) Ghobad-Nejhad, comb. nov.
Order Hymenochaetales
Hymenochaetaceae
-
232.
Hymenochaete micropora L.W. Zhou & Y.C. Dai, sp. nov.
-
233.
Hymenochaete subporioides L.W. Zhou & Y.C. Dai, sp. nov.
Neoantrodiellaceae
-
234.
Neoantrodiellaceae Y.C. Dai, B.K. Cui, Jia J. Chen & H.S. Yuan, fam. nov.
-
235.
Neoantrodiella Y.C. Dai, B.K. Cui, Jia J. Chen & H.S. Yuan, gen. nov.
-
236.
Neoantrodiella gypsea (Yasuda) Y.C. Dai, B.K. Cui, Jia J. Chen & H.S. Yuan, comb. nov.
-
237.
Neoantrodiella thujae (Y.C. Dai & H.S. Yuan) Y.C. Dai, B.K. Cui, Jia J. Chen & H.S. Yuan, comb. nov.
Schizoporaceae
-
238.
Xylodon ramicida Spirin & Miettinen, sp. nov.
Order Polyporales Polyporaceae
-
239.
Colospora Miettinen & Spirin, gen. nov
-
240.
Colospora andalasii Miettinen & Spirin, sp. nov
Order Russulales
Russulaceae
-
241.
Russula guangxiensis G. J. Li, H.A. Wen & R.L. Zhao, sp. nov.
-
242.
Russula hakkae G. J. Li, H. A. Wen & R.L. Zhao, sp. nov.
Order Tremellales
Tremellaceae
-
243.
Tremella dirinariae Diederich, Millanes & Wedin, sp. nov.
-
244.
Tremella graphidis Diederich, Millanes, Wedin & Common, sp. nov.
-
245.
Tremella pyrenulae Diederich, Millanes, Wedin & Common, sp. nov.
Zygomycota
Mucoromycotina
Order Mucorales
Cunninghamellaceae
-
246.
Absidia caatinguensis D.X. Lima & A.L. Santiago, sp. nov.
-
247.
Absidia koreana H.B. Lee, H.W. Lee & T.T.T. Nguyen, sp. nov
-
248.
Gongronella koreana H.B. Lee & T.T.T. Nguyen, sp. nov
Mortierellaceae
-
249.
Mortierella pisiformis H.M. Ho, S.F. Wei & K. Voigt, sp. nov.
-
250.
Mortierella formosana S.F. Wei, H.M. Ho & K. Voigt, sp. nov.
Neocallimastigomycota
Neocallimastigomycetes
Order Neocallimastigales
Neocallimastigaceae
-
251.
Neocallimastix cameroonii G.W. Griff., Dollhofer, Veronika & Callaghan, Tony, sp. nov.
-
252.
Piromyces irregularis Fliegerová, K. Voigt & P.M. Kirk, sp. nov.
Introduction
This is the second in a series of papers where we provide notes on new species, reference specimens and other taxonomic changes.
Materials and methods
The phylogenetic analyses were performed based on up to date ex-type, or otherwise authentic sequence data available in GenBank as a concerted effort of multiple contributors listed in the authors section. New and reference species were sequenced based on the genomic DNA which was extracted from the growing mycelium. For lichenized and lichenicolous fungi and fungi not readily cultivatable, DNA was extracted directly from the ascomata or basidiomata. Gene sequences and genetic markers used for each genus were selected based on current publications and have commonly been used for each of the genera and families. Multiple sequence alignments were generated with MAFFT v. 6.864b (http://mafft.cbrc.jp/alignment/server/index.html) or BioEdit 7.0 (Hall 2004). The alignments were checked visually and improved manually where necessary. All introns and exons were aligned separately. Regions containing many leading or trailing gaps were removed from the alignments prior to tree building. The single gene alignments were then concatenated and used to construct the backbone trees of each group listed. The phylogenetic analyses were performed for maximum parsimony in PAUP v. 4.0b10 (Swofford 2002), maximum likelihood in CIPRES webportal (Miller et al. 2009) using RAxML v. 7.2.7 -HPC2 or RAxML 7.4.2 Black Box (Stamatakis 2006; Stamatakis et al. 2008) or RAxML GUI (Stamatakis 2006; Silvestro and Michalak 2011), PhyML 3.0 (Guindon et al. 2010) and Bayesian inference in MrBayes v. 3.2 (Huelsenbeck and Ronquist 2001) as specified in the legend of each phylogenetic tree. The trees used to represent each order, family and genus were analysed by multiple contributors based on the selection of genes in given publications under each description. The newly generated sequence data are listed in Table 1.
Colour terminology and alphanumeric codes are those of Kornerup and Wanscher (1978) and Seguy (1936). Faces of fungi numbers and Index Fungorum numbers were obtained as detailed in Jayasiri et al. (2015) and Index Fungorum (2015).
Results and discussion
Phylogeny
The data for the aligned sequence matrices for the trees obtained in the different studies are provided below. In the case that alignments of multi-genes are involved, the topologies of the obtained trees for each gene were compared manually to confirm that the overall tree topology of the individual datasets were similar to each other and to that of the tree obtained from the combined alignment.
Contributions to Ascomycota
Dothideomycetes
Amniculicolaceae Y. Zhang et al.
The family was introduced in Zhang et al. (2009a) and comprised Amniculicola, Rubicola and Neomassariosphaeria. Wanasinghe et al. (2015) added six new species to Murispora and provided a backbone tree to the family (Fig. 1). In this paper we transfer Neomassariosphaeria to Lindgomycetaceae and Massariosphaeria to Thyridariaceae and introduce a new genus Pseudomassariosphaeria. Members of this family are generally distributed in aquatic habitat as saprobes (Ingold 1942; Voglmayr 2004; Zhang et al. 2009a, b). The family Amniculicolaceae is characterized by ascomata with a rough black surface, usually staining the woody substrate purple, and short-pedicellate asci bearing hyaline, reddish-brown or pale, 1 to multi-septate or muriform ascospores, generally with a hyaline mucilaginous sheath (Zhang et al. 2009b).
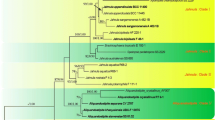
Phylogram generated from maximum likelihood analysis based on combined LSU, SSU, RPB2 and EF sequence data of Pleosporales. Maximum likelihood bootstrap support values greater than 50 % are near the nodes. The ex-type strains are in bold and the new isolates are in blue. The tree is rooted with Hysterium angustatum CBS 236.34
-
111.
Pseudomassariosphaeria Phukhamsakda, Ariyawansa, Camporesi & K.D. Hyde, gen. nov.
Index Fungorum number: IF551367; Facesoffungi number: FoF00916
Etymology: In reference to its similarity with Massariosphaeria.
Saprobic on dead stems. Sexual morph: Ascomata solitary, globose, erumpent or rarely superficial, black to dark brown, smooth-walled, papillate, ostiolate with periphyses. Peridium comprising two strata, outer stratum composed of 3–5 layers of light to dark brown cells of textura angularis, inner stratum comprising 1–2 layers of hyaline flattened cells. Hamathecium comprising 1.2–2.6 μm wide, filiform, anastomosing, hyaline, transversely septate, long, cellular pseudoparaphyses, embedded in a gelatinous matrix. Asci (7-)8-spored, bitunicate, fisitunicate, cylindrical to clavate, short pedicellate, apically rounded with an ocular chamber. Ascospores bi-seriate or overlapping, hyaline, fusiform to lunate, narrow towards the apex, 8–9-septate, constricted at septum, granulate, surrounded by a wide mucilaginous sheath. Asexual morph: Undetermined.
Type species: Pseudomassariosphaeria bromicola Phukhamsakda, Ariyawansa, Camporesi & K.D. Hyde
Notes: Pseudomassariosphaeria bromicola, is introduced from a dead terrestrial stem of Bromus sterilis L. (Poaceae). The ascospores are 21–36 × 4–9 μm, \( \overline{\mathrm{x}} \) = 31 × 7 μm, n = 50, and have fewer septa (8–9), and are not strongly constricted at the septa. Pseudomassariosphaeria bromicola is somewhat similar to Neomassariosphaeria grandispora sensu Zhang et al. (2009a). Both Pseudomassariosphaeria species group with Murispora rubicola with weak support, with various other genera in a well-supported subclade in the family Amniculicolaceae. The putatively named sequence of Pseudomassariosphaeria grandispora (Zhang et al. 2009a) is based on sequence data from a specimen collected from driftwood of Alnus glutinosa from the banks of Garonne River in France. In this French specimen (JF 07103), the ascospores are 38–42 × 6–6.8 μm, narrowly fusiform with acute ends, hyaline, 10-septate, each cell guttulate and strongly constricted at all septa, and have a wide gelatinous sheath (J. Fournier, pers. comm.). In the type protologue of Leptosphaeria grandispora Sacc. ascospores are reported as 45 × 8–9 μm, fusoid, hyaline and 10-septate and the host is Typha latifolia L. (Saccardo 1878). In collections of Massariosphaeria grandispora by Tanaka and Harada (2004), ascospores were 35–41 (−44) × 6.5–8 μm, 9- to 12-septate, hyaline, smooth-walled, granulate, with a sheath up to 20 μm wide, becoming brown at germination and the host was Phragmites australis.
-
112.
Pseudomassariosphaeria bromicola Phukhamsakda, Ariyawansa, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF551368; Facesoffungi number: FoF00917; Fig. 2
Pseudomassariosphaeria bromicola (holotype) a Ascomata on host surface b Section of ascomata c Section of partial peridium layer. The peridium comprising textura angularis d Hyaline pseudoparaphyses e Immature asci f, g Mature asci with short pedicels h–k Hyaline ascospores with visible mucilaginous sheath l Ascospore stained with Indian ink to show sheath m Germinating ascospore n, o Colonies on PDA from surface and reverse. Scale bar: a = 200 μm, b = 100 μm, c = 50 μm, d–g = 50 μm, e–g = 20 μm, h–k = 10 μm, l–m = 20 μm
Holotype: MFLU 15–1403
Saprobic on dead stem of Bromus sterilis. Sexual morph: Ascomata 205–249 μm high × 194–223 μm diam. (\( \overline{\mathrm{x}} \) = 233 × 208 μm, n = 10), on the surface of the host, solitary, scattered or gregarious, globose, erumpent or rarely superficial, black to dark brown, smooth, papillate, ostiolate with periphysoids. Peridium comprising two strata, outer stratum composed of 3–5 layers of dark brown to light brown cells of textura angularis, inner stratum comprising with 1–2 layers of hyaline flattened cells. Hamathecium of dense, 1.2–2.6 μm (\( \overline{\mathrm{x}} \) = 2 μm, n = 20), broad, filiform, anastomosing, hyaline, transversely septate, long, cellular psedoparaphyses, embedded in a gelatinous matrix. Asci 82–115 × 12–19 μm (\( \overline{\mathrm{x}} \) = 101 × 15 μm, n = 20), (7-)8-spored, bitunicate, fisitunicate, cylindrical to clavate, short pedicellate, apically round with ocular chamber up to 1–2 μm wide, 1–2 μm high. Ascospores 21–36 × 4–9 μm (\( \overline{\mathrm{x}} \) = 31 × 7 μm, n = 50), bi-seriate or overlapping, hyaline, fusiform to lunate, narrow towards the apex, 8–9-septate, constricted at septum, granulate, surrounded with wide mucilaginous sheath, 5–7 μm wide at apex and base, and 10–12 μm wide at sides. Asexual morph: Undetermined.
Culture characteristics: Colonies on PDA, reaching 4 cm diam. after 4 weeks at 16 °C, with dense mycelia, circular, rough margin white, becoming dark olive brown (4F8) after 2 weeks, flat on the surface, without aerial mycelium, producing black oil droplets after 4 weeks, lobate at lower margin, dark brown to black. Hyphae septate branched, containing small granules, brown.
Material examined: ITALY, Forlì-Cesena Province, Fiumicello-Premilcuore, on dead stem of Bromus sterilis (Poaceae), 15 June 2013, E. Camporesi (MFLU 15–1403, holotype), (isotype in HKAS, under the code of HKAS88969), ex-type living culture, MFLUCC 15–0031.
-
113.
Pseudomassariosphaeria grandispora (Sacc.) Phukhamsakda, Ariyawansa & K.D. Hyde, comb. nov.
Index Fungorum number: IF551442; Facesoffungi number: FoF01042
Basionym: Leptosphaeria grandispora Sacc., Michelia 1(no. 3): 341 (1878)
≡ Massariosphaeria grandispora (Sacc.) Leuchtm., Sydowia 37: 172 (1984)
Anteagloniaceae K.D. Hyde & A. Mapook
The family Anteagloniaceae was introduced in Hyde et al. (2013), while previously taxa in this family had been placed under Pleosporales genera incertae sedis. The type is Anteaglonium. The hysteriothecial ascomata of Anteaglonium are characteristic of Hysteriaceae, however, the genus clusters as a distinct lineage in Pleosporales (Mugambi and Huhndorf 2009b), showing a parallel evolution of hysteriothecial ascomata in the class Dothideomycetes. A similar topology was shown in the study of Schoch et al. (2009), Zhang et al. (2012a), Mugambi and Huhndorf (2009a) and was confirmed by Hyde et al. (2013) and Wijayawardene et al. (2014).
-
114.
Flammeascoma lignicola S. Boonmee & K.D. Hyde, sp. nov.
Index Fungorum number: IF551379; Facesoffungi number: FoF00852; Fig. 4
Etymology: Lignicola, referring to the habitat on wood.
Holotype: MFLU 10-0061
Saprobic on dead wood in terrestrial habitats. Sexual morph: Ascomata (173-)278–362 μm high × 108–259 μm diam. (\( \overline{x} \) = 297 × 192 μm, n = 5), immersed, erumpent through the host tissue at maturity, scattered, subglobose to ovoid, carbonaceous, dark brown, with a small, ca. 9–10 μm diam. ostiole. Peridium 30–40 μm wide, comprising carbonaceous, occluded dark cells, easily cracking. Hamathecium comprising 0.5–1 μm wide, numerous, filiform, septate, branched, pseudoparaphyses, anastomosing between and above the asci. Asci (121-)128–163 × 18–24.5 μm (\( \overline{x} \) = 143 × 21.5 μm, n = 20), 8-spored, bitunicate, cylindrical to subclavate, apedicellate, rounded at the apex, with an ocular chamber. Ascospores 46–55 × 10–13 μm (\( \overline{x} \) = 52 × 11.5 μm, n = 20), overlapping biseriate, ellipsoid-fusiform, tapering towards the sub-acute ends, slightly curved, 1-septate, not constricted at the septum, hyaline, later becoming olivaceous-brown at maturity, containing two refractive globules when immature, lacking a mucilaginous sheath, smooth-walled. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on MEA within 36 h. Colonies growing on MEA, rather slow-growing, reaching 0.2 mm diam. in 1 week at 28 °C. Mycelium superficial, felty, gummy, edge undulate, brownish grey, olive brown.
Material examined: THAILAND, Chiang Mai, Mae Taeng, Huai Nam Dang, on dead wood of Pinus L. (Pinaceae), 8 September 2009, Saranyaphat Boonmee, HND-01 (MFLU 10-0061, holotype); ex-type living culture, MFLUCC 10-0128, IFRDCC 2200.
Notes: Phylogenetic analysis of LSU and TEF sequence data indicates that Flammeascoma lignicola belongs in Anteagloniaceae (Figs. 1 and 3). Flammeascoma lignicola is closely related to F. bambusae Phookamsak & K.D. Hyde (Liu et al. 2015) with moderate bootstrap support. However, F. lignicola differs from F. bambusae in having uniloculate, carbonaceous ascomata, in lacking a sheath and in having larger ascospores (Fig. 4).
Phylogram generated from Maximum Parsimony analysis based on combined LSU and EF sequence data of Anteagloniaceae and other related families. Parsimony bootstrap support values for MP ≥ 70 % are shown above the nodes and Bayesian posterior probabilities ≥95 % are indicated in bold branches. The tree is rooted with Delitschia winteri CBS 225.62 (Delitschiaceae). All ex-types and reference strains are in bold and new isolate is in blue
Flammeascoma lignicola (holotype). a Specimens b, c Appearance of ascomata on wood and close up of ascoma erumpent through the host tissues d Section of ascoma and peridium e Pseudoparaphyses f g Asci. h–j Ascospores—note j stained in India Ink k Germinating ascospore l Colony on MEA. Scale bars: b = 200 μm, c–d = 100 μm, e = 5 μm, f–g = 50 μm, h–k = 20 μm, l = 10 mm
-
115.
Ascocylindricaceae Abdel-Wahab, Bahkali, E.B.G. Jones, Ariyawansa & K.D. Hyde, fam. nov.
Index Fungorum number: IF551416; Facesoffungi number: FoF01041
Saprobic on lignicolous substrates in marine habitats. Sexual morph: Ascomata small (less than 300 μm), scattered, immersed, erumpent to superficial, globose to subglobose, dark-brown to black, papillate, ostiolate, periphysate. Peridium thin. Pseudoparaphyses trabeculate, embedded in mucilage, numerous, septate, branched. Asci 8-spored, bitunicate, fissitunicate, cylindrical, short pedicellate, uniseriate to overlapping uniseriate, with ocular chamber, developing at the base of the ascomatal venter. Ascospores ellipsoidal, dark-brown to black, one-septate, constricted at the septum, rough and ornamented, small. Asexual morph: Undetermined.
Type genus: Ascocylindrica Abdel-Wahab, Bahkali & E.B.G. Jones
Notes: The family Ascocylindricaceae is introduced to accommodate the monotypic marine genus, Ascocylindrica, based on both phylogeny and morphology. Ascocylindricaceae forms a well-supported clade within the suborder Pleosporineae, sister to the monotypic marine family Halojulellaceae (Fig. 1). Marine taxa in the order Pleosporales belong to 21 families (Jones et al. 2009, 2015) of which many new families were recently established to accommodate marine taxa, i.e., Aigialaceae, Biatriosporaceae, Halojulellaceae, Halotthiaceae and Salsugineaceae (Hyde et al. 2013; Jones et al. 2015). A marine taxon with small ascomata, cylindrical asci and bi-celled dark brown to black ascospores was recently collected from Saudi Arabia mangroves and its 18 and 28 s rDNA sequenced. Phylogenetic analyses of both genes placed the taxon in a new lineage in Pleosporales and is described herein as a new genus and family.
-
116.
Ascocylindrica Abdel-Wahab, Bahkali & E.B.G. Jones, gen. nov.
Index Fungorum number: IF551414; Facesoffungi number: FoF00954
Etymology: In reference to the cylindrical asci.
Saprobic on drift and submerged mangrove wood. Sexual morph: Ascomata globose to subglobose, immersed, erumpent to superficial, solitary, ostiolate, papillate, periphysate, coriaceous, dark-brown to black. Peridium comprising two strata, outer stratum a thin, black, amorphous layer, inner stratum comprising hyaline thick-walled cells arranged in a textura angularis. Hamathecium comprising numerous, 0.5–1 μm wide, septate, branched, trabeculate pseudoparaphyses, within a gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate, cylindrical, short pedicellate, apically rounded, with a wide, shallow ocular chamber. Ascospores uniseriate to overlapping uniseriate, dark-brown to black, 1-septate. Asexual morph: Undetermined.
Type species: Ascocylindrica marina Abdel-Wahab, Bahkali & E.B.G. Jones
Notes: The two Ascocylindrica marina isolates form a new lineage in Pleosporales (Fig. 1). Ascocylindrica marina closely resembles Halokirschsteiniothelia maritima (Linder) Boonmee & K.D. Hyde in having small ascomata and bi-celled brown ascospores. However, the latter species has subconical ascomata with a flattened base, clavate to oblong ellipsoidal asci and longer, smooth ascospores, with a submedian septum. Phylogenetic analyses placed H. maritima in the family Mytilinidiaceae (Suetrong et al. 2009; Boonmee et al. 2012; Fig. 1). Ascocylindrica marina is reminiscent of Didymosphaeria species. The genus Didymosphaeria, typified by D. futilis (Berk. & Br.) Rehm, is mainly confined to herbaceous stems and its species have immersed clypeate ascomata, a two-layered peridium that is cellular on the inside, and pseudostromatous and filamentous on the outside; asci without an ocular chamber or ring structure and brown, smooth ascospores with one central septum (Scheinpflug 1958; Eriksson 1981; Hawksworth 1985; Barr 1987; Kohlmeyer and Volkmann-Kohlmeyer 1990). Didymosphaeria futilis is distantly placed from Ascocylindrica marina (Fig. 1). However, the phylogenetic placement of D. futilis is confusing with putatively named strains of D. futilis obtained from GenBank clustered in different families (Zhang et al. 2012a; Ariyawansa et al. 2014a, b). Fresh collections of D. futilis are needed so that molecular data can be used to validate the natural taxonomic affinities of this genus (Ariyawansa et al. 2014a, b). Schatz (1984) established the genus Lautitia for the marine species Didymosphaeria danica (Berl.) I.M. Wilson & Knoyle. Lautitia danica (Berl.) S. Schatz is a parasite of the alga Chondrus crispus Stackh. and has larger asci and ascospores than Ascocylindrica marina.
Bicrouania is a genus of Melanommataceae with superficial ascomata, a peridium of textura epidermoidea and with a hymenial one-celled alga in the dome of the locule (Kohlmeyer and Volkmann-Kohlmeyer 1990). Lineolata rhizophorae (Kohlm. & E. Kohlm.) Kohlm. & Volkm.-Kohlm. differs from Ascocylindrica marina in having a multi-layered refractive ascal ring (Kohlmeyer and Volkmann-Kohlmeyer 1990). Verruculina enalia Kohlm.) Kohlm. & Volkm.-Kohlm. differs from Ascocylindrica marina in having carbonaceous, clypeate ascomata and ascospores with small, hyaline tubercles at each apex (Kohlmeyer and Kohlmeyer 1979) and is phylogenetically distant from A. marina, where it groups with Ulospora bilgramii (D. Hawksw. et al.) D. Hawksw. et al. and Neotestudina rosatii Segretain & Destombes, in the family Testudinaceae (Suetrong et al. 2009).
-
117.
Ascocylindrica marina Abdel-Wahab, Bahkali & E.B.G. Jones, sp. nov.
Index Fungorum number: IF551415; Facesoffungi number: FoF00955; Fig. 5
Ascocylindrica marina (holotype). a Vertical section of ascoma b Magnified part of the vertical section of the ascoma showing the peridium structure c, d, Immature asci e Ocular chamber in ascus f, g Mature ascus h Fissitunicate dehiscence in ascus i–k Ascospores. Scale bars: a = 50 μm, b–d, f–g = 7 μm, h = 15 μm, i–k = 5 μm
Etymology: In reference to the habitat where the fungus was first collected.
Holotype: MFLU 15-0750
Saprobic on drift and submerged mangrove wood. Sexual morph: Ascomata 90–240 μm diam. (\( \overline{x} \) = 148.6 μm, n = 10), globose to subglobose, immersed, erumpent to superficial, solitary, coriaceous, dark-brown to black, ostiolate. Ostiole papillate, 75–187 μm long, 50–122 μm wide, ostiolar canal 31–54 μm wide, filled with hypha-like periphyses, that are embedded in a gel. Peridium 8–21 μm wide, comprising two strata, outer stratum a thin, black, amorphous layer, inner stratum comprising 4–6 layers of hyaline thick-walled cells arranged in a textura angularis. Hamathecium comprising numerous, 0.5–1 μm wide, septate, branched, trabeculate pseudoparaphyses, embedded in a gelatinous matrix. Asci 62–97 × 8–12 μm (\( \overline{x} \) = 75 × 10.1 μm, n = 15), 8-spored, bitunicate, fissitunicate, cylindrical, short pedicellate, apically rounded, with a wide, shallow ocular chamber. Ascospores 11–14 × 5–7 μm (\( \overline{x} \) = 13.03 × 6.1 μm, n = 50), uniseriate to overlapping uniseriate, dark-brown to black, 1-septate, wall, thick, rough and ornamented. Asexual morph: Undetermined.
Culture characteristics: Colonies on PDA reaching a 25–30 mm radius after 15 days at 25 °C, with white to gray aerial and immersed mycelium, brown from below and producing fertile ascomata with dimensions similar to those recorded on natural wood.
Material examined: SAUDI ARABIA, Arabian Gulf, Al-Khobar City, on decayed wood at a sandy beach, 28 March 2013, M.A. Abdel-Wahab (MFLU 15-0750, holotype); ex-type living culture, MFLUCC 15-0750; SAUDI ARABIA, Al-Lith City, Al-Lith mangrove, on submerged decayed wood of the mangrove tree Avicennia marina (Forssk.) Vierh., 7 April 2015, M.A. Abdel-Wahab (MFLU 15-1508, paratype); EGYPT, Red Sea, Marsa Alam City, Marsa Alam mangrove, on balsa wood incubated with the pure culture of the fungus, and was originally isolated from submerged decayed wood of the mangrove tree Avicennia marina, 9 December 2003, M.A. Abdel-Wahab (MFLU 15-1509, paratype).
Asterinaceae Hansf.
The order Asterinales and family Asterinaceae has been revised by Hongsanan et al. (2014b) and Lembosia was regarded as a valid genus in the group.
Lembosia Lév., Annls Sci. Nat., Bot., sér. 3 3: 58 (1845)
Notes: Lembosia was established by Leveillé (1845) with descriptions of L. dendrochili, L. drimydis, L. macula and L. tenella and has been detailed in Hongsanan et al. (2014b). The genus is characterized by oval, elongated thyriothecia with X- or Y-shaped, or longitudinal dehiscence and with lateral appressoria on the hyphae (Hosagoudar and Goos 1991; Hosagoudar 2012). Hosagoudar (2012) segregated the family Lembosiaceae based on these characters and included Lembosia, while Hongsanan et al. (2014b) referred the genus in Asterinaceae and treated Lembosiaceae as a synonym. There are 173 epithets in Lembosia (Index Fungorum 2015), but sequence data is available for only two species in GenBank. Previous introduction of species in this genus were based on morphology; partly because Lembosia species have never before been cultured.
Lembosia species found on Fabaceae include L. dalbergiicola, L. erythrophlaei, L. hormosiana, L. humboldtiae, L. humboldtiicola, L. humboldtiigena, L. ormosiae, L. ormosiicola, L. sclerolobii, L. sophorae and L. verrucosa. Lembosia xyliae is the only species known on Xylia (Mimosoideae subfamily of Fabaceae), and is most similar to L. erythrophlaei. However it differs in having smaller appressoria and densely grouped ascomata. In the phylogenetic analyses (Fig. 6), there are two clades in the family Asterinaceae. One clade includes the type species of Asterina (Guatimosim et al. 2015), while the other clade includes our collection and several other species of Asterinaceae. Lembosia xyliae clustered with L. albersii with 100 % bootstrap and 1.00 posterior probabilities support.
Phylogram generated from Bayesian inference analyses of LSU sequenced data of taxa of Asterinaceae. Bootstrap values higher than 50 % are shown on the left, while values of Bayesian posterior probabilities above 90 % are shown on the right. The ex-type strains are in bold, the new isolates are in blue. The scale bar indicates 0.03 changes. The tree is rooted with Venturia inaequalis CBS 176.42
-
118.
Lembosia xyliae X.Y. Zeng & K.D. Hyde, sp. nov.
Index Fungorum number: IF551345; Facesoffungi number: FoF00933; Fig. 7
Lembosia xyliae (holotype) a Host b Habit c Ascomata on fallen dried leaves d Squash mount of ascoma e Ascoma in longitudinal section f Ascoma in cross section g Peridium h–j Immature to mature asci k–n Immature to mature ascospores o Germinating ascospore. Scale Bars: c–e = 100 μm, f–g = 50 μm, h–j = 20 μm, k–n = 10 μm
Etymology: From the host Xylia on which the taxon was observed.
Holotype: MFLU 14-0004
Epiphytic on leaves, forming circular or irregular blackened areas on the host surface. Colonies epiphyllous, circular, dense, single to confluent. Hyphae superficial, straight to substraight, dark brown, branching alternate to opposite at acute to wide angles, reticulate. Appressoria 1 celled, alternate, straight to substraight, lateral, antrorse. Sexual morph: Thyriothecia (315-)350–500(−724) × 200–300 μm (\( \overline{x} \) = 400 × 250 μm, n = 20), elongate, dimidiate to tripartite, soft, dense, borne on the surface of mycelium. Asci 40–60 × 30–50 μm (\( \overline{x} \) = 50 × 40 μm, n = 30), 8-spored, bitunicate, ellipsoid to subglobose, sessile. Ascospores (11-)25–32 × (5-)10–17 μm (\( \overline{x} \) = 28 × 13 μm, n = 30), 3–5-seriate, hyaline, 2-celled, constricted at the septum, obovoid to ellipsoid, lower cell slightly longer and narrower, with two oil drops in each cell when immature, becoming brown at maturity. Asexual morph: Undetermined.
Material examined: THAILAND, Chiang Rai, Mae Fah Luang University, on leaves of Xylia sp. (Fabaceae), 18 January 2014, XY Zeng (MFLU 14-0004, holotype).
Botryosphaeriaceae Theiss. & Syd.
The order Botryosphaeriales was reviewed by Liu et al. (2012b) and is not discussed further here.
Diplodia Fr., in Montagne, Annls Sci. Nat., Bot., sér. 2 1: 302 (1834)
Diplodia species are known to be pathogens, endophytes and saprobes on a wide range of woody hosts (Crous et al. 2006; Slippers and Wingfield 2007; Phillips et al. 2008). The genus was introduced by Montagne (1834) and typified by D. mutila (Fr.) Mont., which has hyaline, aseptate conidia that can become brown and septate with age. Diplodia comprises a large genus with more than 1000 names currently recognized. A search of MycoBank (2015) revealed 1339 names, while a search of Index Fungorum (2015) lists 1247 names. Based on up to date holotype or ex-type sequence data available in GenBank, Hyde et al. (2014) provided a backbone tree for 20 Diplodia species. Most of Diplodia species were defined on the basis of host association (Phillips et al. 2008). Host association is not a reliable feature for species differentiation in the Botryosphaeriaceae, nonetheless many species in Diplodia do show some host preference (Slippers et al. 2004). Phillips et al. (2013) stated that there are two distinct conidial morphologies in Diplodia species. The first type of conidia is initially hyaline and aseptate and later becomes pale to dark brown and 1-septate. Pigmentation is often delayed and in some species dark conidia are never seen. In the second type, the conidia become pigmented at an early stage of development, even they are still enclosed within the pycnidia and these conidia only rarely become septate. These two morphological groups are supported by two distinct phylogenetic lineages (Phillips et al. 2013, Fig. 8).
Phylogram generated from RAxML based on combined ITS, EF and β-tubulin sequenced data for Diplodia species. Maximum likelihood bootstrap support values and Bayesian posterior probabilities greater than 50 % and 0.90 are indicated above the nodes. Only ex-type and voucher strains are used and the new isolates are in blue. The tree is rooted with Endomelanconiopsis endophytica CBS 120397
-
119.
Diplodia crataegicola Dissanayake, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF551316; Facesoffungi number: FoF00885; Fig. 9 Etymology: Referring to the host Crataegus sp.
Diplodia crataegicola (holotype) a, b Conidiomata on host substrate c, d Cross section of conidiomata e, g Immature and mature conidia attached to conidiogenous cells h Immature conidium i–k Mature conidia l Germinating conidia m Spermatogenous cells and spermatia. Scale bars: a = 2 mm, b = 500 μm, c = 100 μm, d = 75 μm, e, f = 50 μm, g–m = 15 μm
Holotype: MFLU 15-1311
Saprobic on dead branch of Crataegus sp. Sexual morph: Undetermined. Asexual morph: Conidiomata 220–265 μm high × 260–380 μm diam. (\( \overline{x} \) = 245 × 340 μm, n = 10), pycnidial, stromatic, solitary or clustered, immersed in the host, erumpent at maturity, dark brown to black, ostiolate, apapillate. Peridium 25–35 μm wide, outer and inner layers composed of dark brown and thin-walled hyaline textura angularis. Conidiogenous cells 10–22 μm high × 4–6 μm wide, hyaline, thin-walled, smooth, cylindrical, swollen at the base, discrete, producing a single conidium at the apex. Conidia 11–16 × 6–10 μm (\( \overline{x} \) = 14 × 9 μm, n = 50), aseptate, globose to subglobose, widest in the center, with rounded apex, initially hyaline, becoming dark brown before release from the pycnidia, wall moderately thick, externally smooth, internally roughened. Spermatia rod-shaped with obtuse ends, hyaline, thin-walled, smooth, 3–5 × 1.5–2 μm.
Cultural characteristics: Conidia germinating on WA within 12 h and germ tubes produced from lower end. Colonies growing on PDA, covering the entire plate in 5 days at 28 °C, mycelium grey to olivaceous black at the surface and olivaceous black from below.
Material examined: ITALY, Province of Forlì-Cesena [FC], Passo del Barbotto–Mercato Saraceno, on dead branch of Crataegus sp. (Rosaceae), 3 November 2012, E. Camporesi, IT 875 (MFLU 15-1311, holotype), ex-type living cultures, MFLUCC 15–0648, KUMCC15-0075, CFTCC 15-0002.
Notes: Conidial length of all reported Diplodia species range from 21.5 to 52.5 μm and width vary from 10 to 22 μm (Phillips et al. 2013). The small conidia of Diplodia crataegicola (14 × 9 μm, L/W ratio = 1.55) clearly distinguish this species from all other reported species (Fig. 9). Diplodia crataegicola is phylogenetically most closely related to D. seriata (Fig. 8), and the two species can be separated on the shapes and dimensions of their conidia.
-
120.
Diplodia galiicola Dissanayake, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF551315; Facesoffungi number: FoF00884; Fig. 10 Etymology: Referring to the host Galium sp.
Diplodia galiicola (holotype) a, b Conidiomata on host substrate c Cross section of conidioma d Mature and immature conidia with conidiogenous cells e Immature conidia attached to conidiogenous cells f Mature conidia attached to conidiogenous cells g, h Mature and immature conidia i, j Immature conidia k, l Mature conidia. Scale bars: c = 200 μm, d–l = 20 μm
Holotype: MFLU 15-1310
Saprobic on stem of Galium sp. Sexual morph: Undetermined. Asexual morph: Conidiomata 320–385 μm high × 425–490 μm diam. (\( \overline{x} \) = 350 × 470 μm, n = 10), stromatic, solitary, immersed in the host, dark brown to black, slightly depressed, uniloculate, globose to subglobose, ostiole central, apapillate. Peridium 25–35 μm wide, outer layer composed of dark brown cells of textura angularis, inner layers of thin-walled hyaline cells of textura angularis. Conidiogenous cells 8–15 μm high × 5–7 μm wide, hyaline, thin-walled, smooth, cylindrical, swollen at the base, discrete, producing a single conidium at the apex. Conidia 17–23 × 11–13 μm (\( \overline{x} \) = 20 × 12 μm, n = 50), initially hyaline, becoming dark brown, moderately thick-walled, wall externally smooth, roughened on the inner surface, aseptate, ovoid, widest in the center, apex obtuse, base truncate or rounded.
Cultural characteristics: Conidia germinating on WA within 24 h and germ tubes produced from lower end. Colonies growing on PDA, covering the entire plate in 1 week at 28 °C, developing dense aerial mycelium with age, become pale olivaceous-grey to olivaceous black at the surface, and olivaceous black from below.
Material examined: ITALY, Province of Forlì-Cesena [FC], Strada San Zeno, Galeata, on dead stem of Galium sp. (Rubiaceae), 30 October 2013, E. Camporesi, IT 1495 (MFLU 15-1310, holotype), ex-type living cultures, MFLUCC 15–0647, KUMCC15-0074, CFTCC 15-0001.
Notes: In the combined phylogenetic analysis (ITS, EF1-α and β-tubulin genes), Diplodia galiicola is phylogenetically most closely related to D. seriata with high bootstrap support (1.0 Bayesian posterior probability) (Fig. 8). The conidia of D. galiicola differ from D. seriata in being shorter. The average conidia of D. seriata are longer than or equal to 25 μm (Phillips et al. 2013), while the conidia of D. galiicola are never 25 μm long (Fig. 10).
-
121.
Caryosporaceae Huang Zhang, K.D. Hyde & Ariyawansa, fam. nov.
Index Fungorum number: IF551417; Facesoffungi number: FoF00957
Saprobic on submerged wood in freshwater or mangrove habitats or on decaying terrestrial seeds. Sexual morph: Ascomata pseudothecial, erumpent, superficial, hemispherical, large, dark brown to black, carbonaceous, ostiolate, solitary or clustered. Ostiole central, circular, brown to black. Peridium thick, carbonized, dark brown, composed of rectangular, often occluded cells. Hamathecium comprising numerous, narrow (less than 1 μm wide), hyaline, trabeculate, anastomosing pseudoparaphyses, embedded in a gelatinous matrix. Asci 8-spored, bitunicate, broadly cylindrical to clavate, pedicellate, with an ocular chamber. Ascospores 1–3-seriate, relatively large, hyaline when young, hyaline or brown when mature, 1 − (−3)-septate, constricted at the central septa, broad-fusiform, ovoid or ellipsoidal, ends often papillate, often with polar germ pores at each end, with relatively thick walls, smooth-walled, with or without a mucilaginous sheath. Asexual morph: Undetermined.
Type genus: Caryospora De Not., Micr. Ital. Nov. 9: 7 (1855)
Notes: The family Caryosporaceae is established here to accommodate two marine genera Acrocordiopsis and Caryospora, which forms a distinct lineage in the order Pleosporales (Fig. 1). In previous studies (Zhang et al. 2012a, b; Hyde et al. 2013) Acrocordiopsis formed a relatively poorly supported clade with Salsuginea, but these studies did not include molecular data of Caryospora for the phylogenetic analysis. In this study Acrocordiopsis forms relatively highly supported clade with Caryospora, while Salsuginea forms a sister clade to Aigialaceae. Morphologically, Caryosporaceae is characterized by large, erumpent and conical to hemisphaerical ascomata. The structure of the ascomata is most similar to Astrosphaeriella and Trematosphaeria. However, the ascospores are broadly fusiform, with relatively thick walls in Caryospora, elongate-fusiform and thin-walled in Astrosphaeriella and fusoid and thin-walled in Trematosphaeria (Boise 1985; Hyde and Frohlich 1998; Liu et al. 2011). Acrocordiopsis patilii is a marine taxon. Caryospora aquatica and the putative strain of C. minima are from freshwater. This suggests that Caryosporaceae might be used for taxa often found in aquatic habitats as well as those from peach stones.
Caryospora De Not., Micr. Ital. Nov. 9: 7 (1855)
Caryospora was placed in Phaeophragmiae due to its terminal septa (Jeffers 1940) and later in Zopfiaceae in several studies (Lumbsch and Huhndorf 2010; Hyde et al. 2013). However, very little phylogenetic data is available for species of Caryospora (Cai and Hyde 2007). Three species of Caryospora, i.e., C. minima Jeffers, C. callicarpa (Curr.) Nitschke ex Fuckel and C. obclavata Raja & Shearer are known from freshwater.
Type species: Caryospora putaminum (Schwein.) De Not., Micr. Ital., Dec. 9: 7 (1855) De Not., Micr. Ital. Nov. 9: 7 (1855)
-
122.
Caryospora aquatica Huang Zhang, K.D. Hyde & Ariyawansa, sp. nov.
Index Fungorum number: IF551418; Facesoffungi number: FoF00958; Fig. 11
Caryospora aquatica (holotype) a, b Appearance of ascomata on the host surface. Note the plume of ascospores in b. c Section of an ascoma on wood d Section of ascoma e Peridium f Base of ascoma g Different ages of asci h Young ascus with trabeculate pseudoparaphyses i Nearly mature ascus j Mature ascus with anastomosing pseudoparaphyses (arrowed) k Trabeculate pseudoparaphyses l Anastomosing pseudoparaphyses m, n, o Ascospores. Note the polar germ pores in o. Scale bars: a, b, c, g = 200 μm, d, f = 100 μm, e, h, i, j = 50 μm, k, l = 10 μm, m, n, o = 30 μm
Etymology: in reference to the aquatic habitat.
Holotype: MFLU 11-1083
Saprobic on submerged wood. Sexual morph: Ascomata 350–600 μm high, 450–750 μm diam., solitary or clustered, erumpent, nearly superficial, hemisphaerical, base flattened, dark brown to black, carbonaceous, with ostiolate papilla. Papillae 50 μm long, 150 μm diam., apex truncate. Ostiole central, relatively broad, up to 0.1 mm diam., circular, brown to black. Peridium up to 90 μm wide at the sides and 10 μm wide at the base, strongly carbonized, composed of a black amorphous layer that cannot be differentiated, whose cells are rectangular, uninucleate, and often occluded. Hamathecium comprising numerous, up to 1 μm wide, filiform, trabeculate, anastomosing pseudoparaphyses, embedded in a gelatinous matrix. Asci 160–190 × 60–80 μm (\( \overline{x} \) = 179.6 × 63.4 μm, n = 12), 8-spored, bitunicate, fissitunicate, broadly cylindric-clavate, usually slightly curved, pedicellate, apically rounded, with an ocular chamber. Ascospores 44–52 × 20–27 μm (\( \overline{x} \) = 47.4 × 23.4 μm, n = 15), 2 − 3-seriate, 1-septate, broad-fusiform and hyaline when young, becoming irregularly diamond-shaped and dark brown at maturity, ends acute, slightly constricted at the septum, with polar germ pores at each end, relatively thick-walled, smooth-walled, with large globule in each cell, surrounded by narrow thin gelatinous mucilaginous sheath. Asexual morph: Undetermined.
Material examined: THAILAND, Chiang Rai Province, Hui Kang Pla Waterfall, on submerged wood, 18 January 2010, Huang Zhang (MFLU 11-1083, holotype); ex-type culture, MFLUCC 11-0008.
Notes: Caryospora minima Jeffers was first mentioned by Ellis and Everhart (1892) from peach stones, but they thought it was an 8-spored version of the type species of Caryospora, C. putaminum. Jeffers (1940) redescribed it as a new species when studying C. putaminum. The perithecia in C. minima are smaller than in C. putaminum (400–750 vs. 500–1200 μm). In this paper, we introduce a new species C. aquatica from submerged wood in freshwater. Caryospora aquatica is similar to the type species, C. putaminum in having erumpent, superficial, dark brown to black, carbonaceous, ostiolate ascomata, a thick and carbonized peridium, and relatively large and thick-walled ascospores, but can be distinguished in the smaller ascoma (350–600 vs. 500–1200 μm in C. putaminum) and 8-spored asci (2-spored in C. putaminum). Caryospora aquatica also differs from C. minima in its freshwater habitat. At maturity, the ascospores of C. minima are light brown with 3 septa, while in C. aquatica they become irregularly diamond-shaped and dark brown with polar germ pores. Caryospora easily produces perithecia in culture. Jeffers obtained perithecia in C. minima in peach-stone culture and we also found these in C. aquatica on PDA media. Under moist condition the spores easily discharge through the circular ostiole and form the plume on top of the perithecium.
In the phylogenetic tree (Fig. 1), C. aquatica clusters with a putatively named strain of C. minima, which was also isolated from submerged wood, and is sister to Acrocordiopsis clade. Caryospora has been collected from wood in freshwater on numerous occasions (Cai et al. 2003; Hyde et al. 1998; Kurniawati et al. 2010; Luo et al. 2004; Zhang et al. 2011) and was usually named as C. minima. The Caryospora species from freshwater are likely to comprise several taxa as has been shown for the freshwater species of Helicascus (Zhang et al. 2013a, b, 2014; Shearer 1993; Cai et al. 2003). This is the first time to confirm the natural placement of Caryospora species with verified strains, at the family level with molecular data.
Cucurbitariaceae G. Winter
The family Cucurbitariaceae was introduced by Winter (1885) and is typified by Cucurbitaria berberidis (Pers.) Gray. Cucurbitariaceae is characterized by aggregated ostiolate, ascomata on a basal stromatic structure, fissitunicate and cylindrical asci and pigmented, phragmosporous or muriform ascospores (Hyde et al. 2013). Recent studies based on molecular data have shown that the Cucurbitariaceae forms a well-supported clade in order Pleosporales (Doilom et al. 2013; Hyde et al. 2013).
Cucurbitaria Gray, Nat. Arr. Brit. Pl. (London) 1: 508, 519 (1821)
Cucurbitaria ephedricola was initially introduced as Fenestella ephedrae Rehm from material collected by G. Nevodovsky in Tbilisi Botanic Garden in 1913 on roots of Ephedra procera. Esfandiari (1947) placed the species in the genus Cucurbitaria where it fits the generic concept of Cucurbitaria in having black, papillate ascomata, that are grouped and immersed to erumpent on the substrate, and brown ascospores, with longitudinal and transverse septa, that are narrower at the median septum.
In our molecular analyses, Cucurbitaria ephedricola (MFLU 15-1516) forms a well-supported clade, sister to the C. berberidis clade, in the family Cucurbitariaceae (Fig. 12). Our material of C. ephedricola fits well with the description provided by Esfandiari (1947). In our collection of C. ephedricola the asci are slightly smaller than in the type, but peridium and ascospores are in the given size range (Esfandiari 1947). The ascomata, asci and ascospores of the reference specimen provided here are typical of the C. ephedricola protologue (Esfandiari 1947), but the location (Italy) differs. Thus we propose our collection as a reference specimen (sensu Ariyawansa et al. 2014c) until collections from the same host and location can be obtained.
Phylogram generated from maximum likelihood analysis based on combined LSU and SSU sequenced data from the family Cucurbitariaceae. Maximum likelihood bootstrap support values greater than 50 % are near the nodes. The ex-type strains are in bold and the new isolates are in blue. The tree is rooted with Pleospora herbarum MFLUCC 14-0261
-
123.
Cucurbitaria ephedricola Esfand., Sydowia 1(4–6): 162 (1947)
Facesoffungi number: FoF00934; Fig. 13
Cucurbitaria ephedricola (reference specimen) a Appearance of ascomata on the host tissue b Close up of ascoma c Section of ascoma d Peridium cells e Hamathecium of septate, cellular pseudoparaphyses f, g Asci h–k Mature yellowish brown ascospores. Scale bars: c = 200 μm, d = 50 μm, e = 10 μm, f, g = 20 μm, h–k = 5 μm
Saprobic on wood. Sexual morph: Ascomata 300–800 μm diam., immersed to erumpent, grouped, subglobose or flask-shaped and often flattened, with rounded papillate ostiole up to 50 μm diam., black. Peridium 60–100 μm wide, composed of 2–3 layers of pseudoparenchymatous cells, outer layer comprising of thick-walled, dark brown cells of textura angularis to textura globulosa, inner layer comprising of thin-walled, hyaline to pale brown, flattened cells of textura angularis. Hamathecium composed of 2 μm wide, numerous, hyaline, cellular, branched, septate pseudoparaphyses. Asci 150–200 × 13–20(−25) μm (\( \overline{x} \) = 176 × 16 μm, n = 20), 8-spored, bitunicate, fissitunicate, arising from the base of the inner ascomatal wall, numerous, cylindrical, with a broadly rounded apex with an ocular chamber. Ascospores 20–30(−35) × 10–15 μm, ellipsoidal, oblong ovoid, broadly fusiform, often asymmetrical, with obtuse to acute ends, straight or slightly curved, mostly 7-septate, sometimes 5- or 6- or 8- or 9-septate, with 1 or 2 longitudinal septa, often distinctly narrower at the median septum, initially colourless or yellowish, later olivaceous brown to dark brown. Asexual morph: undetermined.
Material examined: ITALY, Province of Forlì-Cesena [FC], Strada San Zeno, Galeata, on dead stem, 30 October 2013, E. Camporesi (MFLU 15–1516, reference specimen designate here).
Didymellaceae Gruyter et al.
The most recent treatment of Didymellaceae is that of Hyde et al. (2013)
-
124.
Heracleicola Tibpromma, Camporesi & K.D. Hyde, gen. nov.
Index Fungorum number: IF551380; Facesoffungi number: FoF00921
Etymology: refers to the host genus.
Saprobic on decaying plant stems. Sexual morph: Ascomata immersed, visible as shiny, raised dots on the host surface, vase-like, solitary or scattered, with central short papilla, dark brown to black. Peridium a single stratum, comprising relatively large, thick-walled, dark brown cells of textura angularis to textura globulosa. Hamathecium comprising numerous, 0.7–1.3 μm wide, long, filiform, frequently anastomosing, cellular, pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindric-clavate, short pedicellate, rounded at the apex, with a wide, shallow, ocular chamber. Ascospores overlapping 1–2-seriate, hyaline, 3-septate, fusiform, cell above central septum often enlarged, constricted at the septum. Asexual morph: undetermined.
Type species: Heracleicola premilcurensis Tibpromma, Camporesi & K.D. Hyde
Notes: Molecular data places Heracleicola in the family Didymellaceae with moderate support (Fig. 14). Its closest relatives are Didymella rabiei and Phoma medicaginis. Heracleicola is however, distinct in its vase-shaped ascomata and 4-celled hyaline ascospores and forms a distant clade from Didymella sensu stricto.
Phylogram generated from maximum likelihood analysis based on combined LSU, SSU and ITS sequenced data of the family Didymellaceae. Maximum likelihood bootstrap support values greater than 50 % are near the nodes. The ex-type strains are in bold and the new isolates are in blue. The tree is rooted with Phaeosphaeria oryzae CBS 110110
-
125.
Heracleicola premilcurensis Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF551381; Facesoffungi number: FoF00921; Fig. 15
Heracleicola premilcurensis (holotype) a, b Appearance of fungus on host surface c Cross section of ascoma d Ostiole e Section of peridium f Pseudoparaphyses g–i Asci j–l Ascospores m Germinating ascospore. Scale bars: a = 400 μm, b = 100 μm, c = 50 μm, d–e = 20 μm, f = 2 μm, g–i = 20 μm, j–l = 10 μm, m = 20 μm
Etymology: refers to the name of the municipality in Italy where the species was collected.
Holotype: MFLU 14-0725
Saprobic on decaying plant stem of Heracleum sphondylium. Sexual morph: Ascomata 211–272 μm high × 150–221 μm diam. (\( \overline{x} \) = 254 × 196 μm, n = 5), immersed, visible as shiny, raised dots on the host surface, vase-like, solitary or scattered, with central short papilla, dark brown to black. Peridium 37–43 μm wide, a single stratum, comprising relatively large (6–9 μm), thick-walled, dark brown cells of textura angularis to t. globulosa. Hamathecium comprising numerous, 0.7–1.3 μm wide, long, filiform, frequently anastomosing, cellular, pseudoparaphyses. Asci 50–94 × 11–16 μm (\( \overline{x} \) = 72 × 14 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindric-clavate, short pedicellate, rounded at the apex, with a wide, shallow, ocular chamber. Ascospores 23–36 × 6–9 μm (\( \overline{x} \) = 27 × 7 μm, n = 15), overlapping 1–2-seriate, hyaline, 3-septate, fusoid with rounded ends, cell above central septum often enlarged, constricted at the septum, guttulate, smooth-walled, lacking a mucilaginous sheath. Asexual morph: undetermined.
Culture characteristics: on MEA reaching 4 cm diam. after 1 week at 16 °C, later with dense mycelium, with irregular, rough margin, flattened, brown to black; hyphae septate, branched, light-brown, thick-walled.
Material examined: ITALY, Premilcuore, Province of Forlì-Cesena,, Valbura, on dead stem of Heracleum sphondylium (Apiaceae), 6 June 2014, Erio Camporesi IT1916 (MFLU 14-0725, holotype, HKAS, isotype); ex-type living culture, MFLUCC 14-0518, KUN; Ibid. (MFLU 15-1475, HKAS, isotypes); (MFLU 15-1476 bis, MFLU 15-1477 tris, paratypes).
-
126.
Neodidymella Phookamsak, R.H. Perera & K.D. Hyde, gen. nov.
Index Fungorum number: IF551389; Facesoffungi number: FoF00904
Etymology: The generic epithet “Neodidymella” refers to the similarity with Didymella.
Biotrophic or hemibiotrophic on living leaves. Sexual morph: Ascomata scattered, solitary to gregarious, visible as black dots on the lower epidermis, globose to subglobose, uni-loculate, glabrous, ostiole central, with pore-like opening. Peridium thin-walled, of equal thickness, composed of 2–3 layers of large, brown to dark brown, pseudoparenchymatous cells, arranged in a textura angularis. Hamathecium composed of dense, broad, distinctly septate, cellular pseudoparaphyses, constricted at the septum, branched, anastomosing at the apex, embedded in a gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate, broadly cylindrical to cylindric-clavate, sessile to subsessile, or short bulbous pedicel, apically rounded, with an indistinct ocular chamber. Ascospores overlapping bi-seriate, hyaline, ellipsoidal to clavate, or fusiform with rounded ends, 1-septate, slightly constricted at the septum, walls echinulate. Asexual morph: Undetermined.
Type species: Neodidymella thailandicum Phookamsak & K.D. Hyde
Notes: Neodidymella is introduced as a monotypic genus to accommodate a Dothideomycete species forming hyaline, didymosporous ascospores with pseudothecial ascomata. The genus belongs in the family Didymellaceae and forms a distinct lineage from Didymella sensu stricto with relatively low bootstrap support (Fig. 14). The family Didymellaceae was introduced by De Gruyter et al. (2009) to accommodate the sexual genera Didymella, Leptosphaerulina and Macroventuria, and the asexual genera Phoma and phoma-like species in Pleosporales (Zhang et al. 2012a; Hyde et al. 2013; Wijayawardene et al. 2014; Liu et al. 2015). The species of this family include various important plant pathogens such as Ascochyta, Didymella and Phoma (Kaiser et al. 2008; Aveskamp et al. 2010; De Gruyter et al. 2009, 2012; Zhang et al. 2012a; Hyde et al. 2013; Liu et al. 2015). Didymella, Leptosphaerulina and Macroventuria differ from Neodidymella in morphology. Hyde et al. (2013) examined the generic type of Didymellaceae, Didymella exigua (Niessl) Sacc. and described Didymella as lacking pseudoparaphyses. Neodidymella differs from Didymella in having pseudoparaphyses. Neodidymella differs from Leptosphaerulina in having didymosporous ascospores and producing pseudoparaphyses, while Leptosphaerulina species have dictyospores and lack pseudoparaphyses (Zhang et al. 2012a; Phookamsak et al. 2013). Neodidymella is most similar to Macroventuria due to its didymosporous ascospores and presence of pseudoparaphyses (Zhang et al. 2012a). However, Macroventuria differs from Neodidymella in having setose immersed to erumpent, nearly superficial ascomata, and fusiform ascospores. Neodidymella has immersed, glabrous ascomata, and ellipsoidal to clavate ascospores. Multigene phylogenetic analyses shows that Neodidymella forms a robust clade at base of the Didymella vitalbina Petr. clade (CBS 454.64 and MFLUCC 13-0877) and does not cluster with other sexual genera (Fig. 14).
Woudenberg et al. (2009) observed Didymella vitalbina in vitro on V8 media agar and described the morphological characters as producing superficial, globose to pyriform, perithecial ascomata with papilla; cylindrical asci with pseudoparaphyses and hyaline, ovoid to obpyriform, smooth-walled ascospores. According to the protologue of Didymella vitalbina (Petrak 1940) and Woudenberg’s description (2009), Neodidymella thailandicum is similar to D. vitalbina in its morphological characters. However, N. thailandicum has smaller ascomata, asci and ascospores (Petrak 1940; Woudenberg et al. 2009). Woudenberg et al. (2009) reported the asexual morph of D. vitalbina as Ascochyta vitalbae Briard & Har., while the asexual morph of N. thailandicum is undetermined. Based on the morphological characters, D. vitalbina might be related to N. thailandicum. However, these species do not cluster closely together in the tree, which may be a result of the limited number of taxa.
-
127.
Neodidymella thailandicum Phookamsak, R.H. Perera & K.D. Hyde, sp. nov.
Index Fungorum number: IF551390; Facesoffungi number: FoF00905; Fig. 16
Etymology: The generic epithet “thailandicum” refers to the country where the species was collected.
Holotype: MFLU 11-0176
Biotrophic or hemibiotrophic on living leaves of Calathea. Sexual morph: Ascomata 65–110 μm high, 80–130 μm diam., scattered, solitary to gregarious, visible as black dots on the lower epidermis, globose to subglobose, uni-loculate, glabrous, ostiole central, with pore-like opening. Peridium 8–20 μm wide, of equal thickness, composed of 2–3 layers of large, brown to dark brown, pseudoparenchymatous cells, arranged in a textura angularis. Hamathecium composed of dense, 2–5 μm wide, broad, cellular pseudoparaphyses, distinctly septate, constricted at the septum, branched, anastomosing at the apex, embedded in a gelatinous matrix. Asci (46–)50–60(−70) × (12–)13–15(−18) μm (\( \overline{x} \) = 54.8 × 13.8 μm, n = 25), initially 8-spored, bitunicate, fissitunicate, broadly cylindrical to cylindric-clavate, sessile to subsessile, or short, bulbous pedicellate, with furcate pedicel, apically rounded, with indistinct ocular chamber. Ascospores (11)12–13(−14) × 4–5(−6) μm (\( \overline{x} \) = 12.6 × 5.5 μm, n = 30), overlapping bi-seriate, hyaline, ellipsoidal to clavate, or fusiform with rounded ends, 1-septate, slightly constricted at the septum, wall echinulate. Asexual morph: Undetermined.
Culture characteristics: Colonies on MEA fast growing, 80–85 mm diam. after 2 weeks at 25–30 °C, colonies circular, medium dense, flat, smooth with entire edge, fairly fluffy to granular, slightly radiating; colonies in the upper part:white to cream at the margin, yellowish grey to pale yellowish at the middle and greyish yellow at the centre; reverse pale white to olive at the margin, yellowish greyish yellow at the centre; not producing pigmentation.
Material examined: THAILAND, Chiang Rai, Muang District, Pakha Village, on living leaves of Calathea sp. (Marantaceae), 4 August 2010, R. Phookamsak RP0056 (MFLU11-0176), living culture MFLUCC 11-0140, KUMCC.
Didymosphaeriaceae Munk
= Montagnulaceae M.E. Barr
The ascomycetous family Didymosphaeriaceae was introduced by Munk A (1953) and typified by the genus Didymosphaeria Fuckel. The family was characterized by 1-septate ascospores and trabeculate pseudoparaphyses which anastomosed mostly above the asci (Ariyawansa et al. 2014a, b). Ariyawansa et al. (2014b) synonymised Montagnulaceae under Didymosphaeriaceae which is the oldest name and has priority and provided an updated account for the family. Ariyawansa et al. (2014b) accepted 16 asexual and sexual genera based on analyses of concatenated internal transcribed spacer (ITS) with LSU, SSU and β-tubulin gene sequence data (Fig. 17).
Phylogram generated from maximum parsimony analysis based on combined LSU, SSU, β-tubulin and ITS sequenced data of Didymosphaeriaceae. Maximum parsimony bootstrap support values greater than 50 % and Bayesian posterior probabilities greater than 0.90 are near the nodes. The ex-type strains are in bold and the new isolates are in blue. The tree is rooted with Trematosphaeria pertusa CBS 122368
Austropleospora R.G. Shivas & L. Morin, in Morin et al., Fungal Diversity 40(1): 70 (2010).
Type species: Austropleospora osteospermi R.G. Shivas & L. Morin
-
128.
Austropleospora osteospermi R.G. Shivas & L. Morin, Morin et al., Fungal Diversity 40(1): 70 (2010).
Facesoffungi number: FoF00935; Fig. 18
Austropleospora osteospermi (holotype) a Herbarium material b Ascomata on host surface c Section through ascoma d Section of peridium e Cellular pseudoparaphyses f Mature bitunicate asci g Muriform ascospores h Section of pycnidium i Pycnidial wall j Conidiogenous cells and developing conidia k Brown 3-septate conidia. Scale bars: c = 50 μm, d = 25 μm, e, g = 10 μm, f = 25 μm, g = 5 μm, h = 20 μm, i–k = 5 μm
Parasitic on stem and leaves of Chrysanthemoides monilifera (L.) Norlindh. Sexual morph: Ascomata 75–110 × 130–200 μm (\( \overline{x} \) = 100 × 146 μm, n = 10), subglobose, sometimes slightly flattened, solitary or in groups, scattered, immersed immediately below the stem epidermis, ostiole 60–90 μm long, with a protruding neck. Peridium 8–18 μm (\( \overline{x} \) = 12 μm, n = 10) wide, composed of dark brown to black cells of textura angularis. Hamathecium of 2–3 μm wide, dense, filamentous, anastomosing, aseptate, hyaline pseudoparaphyses. Asci 75–120 × 13–18 μm (\( \overline{x} \) = 92 × 16 μm, n = 20), bitunicate, fissitunicate, 6–8-spored, cylindrical to clavate, with a short, broad, pedicel, rounded at the apex, with a minute ocular chamber. Ascospores 16.5 − 21× 6–8.4 μm (\( \overline{x} \) = 18 × 7.7 μm, n = 25), biseriate to overlapping uniseriate, yellowish brown, ellipsoidal, muriform, mostly with 3-transverse septa, 0–2 longitudinal septa, slightly constricted at median septum, not or very slightly constricted at other septa, apex rounded to slightly tapered, base tapered to rounded, smooth. Asexual morph: Conidiomata 75–110 × 100–130 μm (\( \overline{x} \) = 88 × 115 μm, n = 10), pycnidial, globose, superficial on stem, immersed in the host tissue and becoming erumpent at maturity, globose, dark brown in the erumpent part, with a single ostiole. Conidiomata wall 9–16 μm wide, brown to reddish-brown, thin-walled, comprising several layers with cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 10–12 × 2.5 − 3.5 μm (\( \overline{x} \) = 11 × 2 μm, n = 15), inconspicuously annellidic, discrete, cylindrical. Conidia 14–18 × 4.8 − 6.5 μm (\( \overline{x} \) = 15.8 × 5.7 μm, n = 25), cylindrical to narrowly ellipsoidal, initially hyaline and aseptate, becoming yellowish-brown at maturity, mostly transversely 1–3-septate, ends rounded.
Material examined: AUSTRALIA, New South Wales, Bellinger Head, Kalang River mouth, on stem and leaves of Chrysanthemoides monilifera ssp. rotundata (Asteraceae), 5 March 2008, L. Morin (BRIP 52234, holotype).
Notes: Austropleospora was introduced by Morin et al. (2010) to accommodate A. osteospermi and is monotypic. In the same study Shivas and Morin (in Morin et al. 2010) observed Hendersonia osteospermi Wakef. on the same host and identified it as the asexual morph of A. osteospermi both in culture and by DNA sequence analysis. Morin et al. (2010) placed Austropleospora in Pleosporales without assigning it to any family based on ITS sequence analysis. Thambugala et al. (2014) tentatively referred Austropleospora to Pleosporaceae based on morphological similarities but Ariyawansa et al. (2015) excluded Austropleospora from Pleosporaceae and tentatively referred it to Didymosphaeriaceae.
In the present study we found that the type strain of Austropleospora osteospermi forms a separate clade together with the type strain of Paraconiothyrium archidendri Verkley et al., sister to Paracamarosporium and Paraconiothyrium fuckelii (Sacc.) Verkley & Gruyter species complex clades in the family Didymosphaeriaceae. Thus we assign Austropleospora to the family Didymosphaeriaceae as a distinct genus. Austropleospora forms a distant clade from the generic type of Paraconiothyrium, P. estuarinum and this is also supported by morphology. Paraconiothyrium is paraphyletic within the family Didymosphaeriaceae.
We synonymise Paraconiothyrium archidendri under Austropleospora by giving the priority for the oldest epithet.
-
129.
Austropleospora archidendri (Verkley et al.) Ariyawansa & K. D. Hyde, comb. nov.
Facesoffungi number: FoF01038.
Basionym: Paraconiothyrium archidendri Verkley et al., in Verkley et al., Persoonia, Mol. Phyl. Evol. Fungi 32: 37 (2014).
-
130.
Pseudopithomyces Ariyawansa & K.D. Hyde, gen. nov.
Index Fungorum number: IF551392; Facesoffungi number: FoF00937
Etymology: Pseudo, refers to the similarity to Pithomyces.
Saprobic or parasitic on dead leaves, stems of plants and humans. Sexual morph: Undetermined. Asexual morph: Conidiophores micronematous or semi-macronematous, mononemous, flexuous, thin-walled, hyaline to subhyaline, aseptate, smooth, branch or unbranched, normally closely packed together. Conidiogenous cells arising perpendicularly from repent hyphae, monoblastic or blastic, closely aggregated, globose or subglobose, integrated, terminal, determinate, disintegrating at maturity to liberate conidia. Conidia acrogenous, dark fuligineous, septate to multi-septate, fusiform or subglobose, fusiform, verruculose to echinulate, walls ornamented, thin-walled, sometimes wide in the middle.
Type species: Pseudopithomyces chartarum (Berk. & M.A. Curtis) J.F. Li, Ariyawansa & K. D. Hyde
Notes: The genus Pithomyces is polyphyletic with species grouping in many different families in the Pleosporales such as Didymellaceae, Didymosphaeriaceae and Astrosphaeriellaceae (da Cunha 2014; Pratibha and Prabhugaonkar 2015). Pseudopithomyces is introduced as a new genus to accommodate Pithomyces chartarum (Berk. & M.A. Curtis) M.B. Ellis, P. maydicus (Sacc.) M.B. Ellis, P. sacchari (Speg.) M.B. Ellis and some unidentified Pithomyces strains, that group together in Didymosphaeriaceae. Morphological characters of Pseudopithomyces are similar to Pithomyces. The type species of the Pithomyces, P. flavus has been epitypified by Pratibha and Prabhugaonkar (2015), and the epitype strain of P. flavus (MTCC 12224) clustered in the family Astrosphaeriellaceae. Thus, these two genera are phylogenetically distinct.
Leptosphaerulina chartarum was mentioned as the sexual morph of Pithomyces chartarum (da Cunha 2014). da Cunha (2014) showed that the ex-type strain of Leptosphaerulina chartarum (CBS 329.86 T) clustered within the family Didymellaceae and did not belong in the Pithomyces chartarum clade or to be related to any of the Pithomyces lineages (da Cunha 2014).
In this study Pithomyces chartarum is selected as the generic type of Pseudopithomyces. Pseudopithomyces differs from Pithomyces in having echinulate or fusiform, verruculose dark conidia and producing brown to black colonies on the host. Pithomyces produces obovate to oblong, verruculose to spinulose, comparatively lighter conidia and forms whitish to yellowish colonies on the host (Ellis 1960; Pratibha and Prabhugaonkar 2015).
In the present study we introduced a novel species of Pseudopithomyces, Pse. palmicola and re-described Pse. maydicus from our fresh collection obtained from dead palm leaves in Northern Thailand (Fig. 19).
Phylogram generated from Bayesian analysis based on ITS and LSU sequence data. Bayesian posterior probabilities (PP) greater than 0.90 are indicated above or below the nodes. The ex-type and reference strains are in bold, the new isolates are in blue. The tree is rooted with Deniquelata barringtoniae (MFLUCC 11-0422)
-
131.
Pseudopithomyces chartarum (Berk. & M.A. Curtis) J.F. Li, Ariyawansa & K.D. Hyde, comb. nov.
Basionym: Sporidesmium chartarum Berk. & M.A. Curtis, in Berkeley, Grevillea 3(no. 26): 50 (1874).
≡ Pithomyces chartarum (Berk. & M.A. Curtis) M.B. Ellis, Mycol. Pap. 76: 13 (1960).
Index Fungorum number: IF551393; Facesoffungi number: FoF00938
Pathogenic on human or sheep skin. Colonies black, separate, later becoming confluent, up to 0.5 mm diam. Conidiophores 2–5 × 1.5–2.5 μm. micronematous or semi-macronematous, mononematous, branched and anastomosing, pale olive, smooth or occasionally verruculose. Conidiogenous cells integrated. Conidia 18–29 × 10–17 μm, acropleurogenous, mainly pleurogenous, broadly ellipsoid, 3-septate to multi-septate, mainly with 3–4 transverse septa and usually divided by longitudinal septum, often constricted at the septa, mid brown to dark brown, finely echinulate to verruculose, rhexolytic, smooth or occasionally verruculose, a small piece of the denticle invariably remains attached to the base of the conidium (Ellis 1971; Dingley 1962).
Notes: Pseudopithomyces chartarum was introduced as Sporidesmium chartarum by Berkeley and Curtis (1874). In this study we used the reference strain of Pse. chartarum for our phylogenetic analysis, which was used by da Cunha (2014). Our results clearly show that the strains of Pse. chartarum (MUCL 15905 and UBC F15184) forms a separate clade sister to the Pse. palmicola clade, including some other putative strains of Pse. chartarum (MUCL 4329, UTHSC 04 2495 and UTHSC 06 214), which we rename.
-
132.
Pseudopithomyces palmicola J.F. Li, Ariyawansa & K.D. Hyde, sp. nov.
Index Fungorum number: IF551421; Facesoffungi number: FoF00939; Fig. 20
Etymology: The name palmicola, refers to the common host name and Latin cola meaning loving.
Holotype: MFLU 15-1474
Saprobic on leaves of Acoelorrhaphe wrightii (Griseb. & H. Wendl.) H. Wendl. ex Becc. Sexual morph: Undetermined. Asexual morph: Colonies effuse, dark brown to black. Mycelium superficial or partly immersed on the substrate, composed of septate, branched, smooth, thin-walled, pale to grey hyphae. Conidiophores 3.6–6 × 3.2–3.5 μm (\( \overline{x} \) = 5.5 × 3.3 μm, n = 100), micronematous, mononemous, closely packed, hyaline, thin-walled, aseptate, smooth, branched, flexuous. Conidiogenous cells 3.5–5.5 × 2.5–3.5 μm (\( \overline{x} \) = 4.5 × 3.3 μm, n = 100), monoblastic, phialidic, integrated, terminal, determinate, hyaline, globose or subglobose. Conidia 21.5–30.5 × 10–16.5 μm (\( \overline{x} \) = 27.9 × 14.2 μm, n = 100), acrogenous, muriform, subglobose, 6–8-septate, verruculose to echinulate, amygdaliform or ovoid, thin-walled, light brown to brown.
Cultural characteristics: Conidia germinating on PDA within 14 h and germ tubes produced from end cells. Colonies growing on PDA, cottony, grey to brown, reaching 5 mm in 20 days at 25 °C, mycelium superficial, effuse, radially striate, with regular edge, hyphae pale to yellow white; asexual spores and sexual spores were not formed within 60 days.
Material examined: THAILAND, Chiang Rai Provice, Mae Fah Luang, on leaves of Acoelorrhaphe wrightii (Griseb. & H. Wendl.) H. Wendl. ex Becc. (Arecaceae), 26 January 2014, J.F. Li, H-4l (MFLU 15-1474, holotype), ex-type living culture, MFLUCC 14-0392, KUMCC 15-0100.
Notes: Phylogenetic analysis based on ITS and LSU sequence data showed that Pseudopithomyces palmicola is closely related to, but forms a distinct lineage from the Pse. chartarum clade (Fig. 19). Pseudopithomyces palmicola is similar to P. chartarum in having three constricted transverse septa and 1–2 longitudinal septa in the central cells. Pseudopithomyces palmicola differs from Pse. chartarum in having thick and hyaline conidiophores and mostly globose, pale brown conidia with slightly constricted septa, while Pse. palmicola has thin and heavily pigmented conidiophores with dark brown conidia which are comparatively more constricted at the septa (da Cunha 2014). Furthermore P. palmicola forms pale to yellow white colonies on PDA, while Pse. chartarum forms white to dark grey colonies on PDA (Supplementary Table 2).
-
133.
Pseudopithomyces maydicus (Sacc.) J.F. Li, Ariyawansa & K.D. Hyde, comb. nov.
Basionym: Clasterosporium maydicum Sacc., Nuovo G. bot. ital. 23(2): 213 (1916)
≡ Pithomyces maydicus (Sacc.) M.B. Ellis, Mycol. Pap. 76: 15 (1960)
For other synonyms see Index Fungorum (2015)
Index Fungorum number: IF551395; Facesoffungi number: FoF00940; Fig. 21
Saprobic on leaves of Acoelorrhaphe wrightii. Sexual morph: Undetermined. Asexual morph: Colonies effuse, dark brown to black. Mycelium superficial or partly immersed in the substrate, composed of septate, branched, smooth, thin-walled, white hyphae. Conidiophores 4–6 × 2.5–3 μm (\( \overline{x} \) = 4.4 × 2.7 μm, n = 100), semi-micronematous, mononemous, closely packed, hyaline to subhyaline, thin-walled, aseptate, smooth, unbranched, flexuous. Conidiogenous cells 3.5–5 × 1.4–4 μm (\( \overline{x} \) = 3.6 × 2.2 μm, n = 100), monoblastic, integrated, terminal, determinate, hyaline, globose or subglobose. Conidia 13.5–24.5 × 5.5–8.5 μm (\( \overline{x} \) = 21.8 × 5.6 μm, n = 100), wide in the middle, acrogenous, muriform, 3–4-septate, thin-walled, brown to dark brown, fusiform to pyriform, clavate.
Cultural characteristics: Conidia germinating on PDA within 14 h and germ tubes produced from apical cells. Colonies growing on PDA, hairy or cottony, white to dark grey, reaching 5 mm in 20 days at 25 °C, mycelium superficial, effuse, radially striate, with regular edge, hypha white to grey. Asexual spores and sexual spores not formed within 60 days.
Material examined: THAILAND, Chiang Rai Provice, Mae Fah Luang, on dead leaves of Acoelorrhaphe wrightii (Arecaceae), 26 Janurary 2014, J.F. Li, H-4e (MFLU 15-1473, reference specimen designated here), living culture, MFLUCC 14-0391, KUM-15-0101.
Notes: Pseudopithomyces maydicus is characterised by cylindrical or slightly clavate, pale brown to dark brown, verrucose conidia with two to three transverse septa, rarely with one oblique or longitudinal septum and in its white to grayish yellow colonies on PDA. The characters of our collection are typical with that of Clasterosporium maydicum (Ellis 1960). Furthermore our phylogeny also concluded that the putative strains of Pse. maydicus (UTHSC 06 3954 and UTHSC 06 1549) clustered with our strain (Fig. 19).
-
134.
Pseudopithomyces sacchari (Speg.) Ariyawansa & K.D. Hyde, comb. nov.
Basionym: Sporidesmium sacchari Speg., Anal. Mus. nac. B. Aires, Ser. 3 13: 443 (1911).
≡ Pithomyces sacchari (Speg.) M.B. Ellis, Mycol. Pap. 76: 17 (1960).
Index Fungorum number: IF551397
Floricolaceae Thambugala et al.
Notes: Floricola was introduced as a monotypic genus by Kohlmeyer and Volkmann-Kohlmeyer (2000) with F. striata Kohlm. & Volkm.-Kohlm. as the type species. Thambugala et al. (2015) noted that coelomycetous asexual morphs are characteristic of the family Floricolaceae. Floricola striata is characterized by immersed, pycnidial, ostiolate conidiomata, conidiophores that are reduced to phialidic conidiogenous cells and 3-septate, dark brown conidia. Floricola viticola is similar to F. striata except that conidiomata are larger and conidia smaller. Floricola striata grouped with Misturatosphaeria species in Lophiostomataceae (Hyde et al. 2013), but a further molecular study refers them to a new family Floricolaceae (Thambugala et al. 2015). Kohlmeyer and Volkmann-Kohlmeyer (2000) mentioned that F. striata frequently occurs on old inflorescences. Phylogenetic analysis (Fig. 22) supports the distinctness of this species and thus we introduced F. viticola as a new species.
Phylogram generated from maximum likelihood analysis based on combined LSU, ITS, EF and SSU sequenced data for the family Lophiostomataceae. Maximum likelihood bootstrap support values greater than 50 % are above the nodes. The ex-type strains are in bold and the new isolates are in blue. The tree is rooted to Melanomma pulvis-pyrius (strain CBS 124080)
-
135.
Floricola viticola Phukhamsakda, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF551366; Facesoffungi number: FoF00896; Fig. 23
Floricola viticola (holotype) a Habit of Floricola viticola on Vitis vinifera L. b Close up of conidiomata below surface of host c Vertical section through the conidioma d Part of conidiomata peridium e–j Developing state of conidiogenous cells k–n Conidia o–p Culture character on PDA. Scale bar: b = 200 μm, c = 100 μm, d = 50 μm, e–n = 5 μm
Etymology: named after the host genus, Vitis.
Holotype: MFLU 15–1404
Saprobic on dead branch of Vitis vinifera L. Sexual morph: Undetermined. Asexual morph: Conidiomata 220–239 μm high × 154–202 μm (\( \overline{x} \) = 237 × 177 μm, n = 10) diam., solitary, pycnidial, unilocular, scattered, immersed to erumpent, subglobose, dark brown to black. Pycnidial wall 13–35 μm (−40 μm at apex), externally merging with host tissues, composed of brown cells of textura angularis with a hyaline inner lining bearing conidiogenous cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 2–10 × 2–9 μm (\( \overline{x} \) = 4 × 5 μm, n = 20) diam., enteroblastic, phialidic, determinate, solitary, doliform, smooth-walled, hyaline. Conidia 7–12 × 2–6 μm (\( \overline{x} \) = 9 × 4 μm, n = 50), initially hyaline, dark brown at maturity, oblong, subfusiform, occasionally curved at the apex, with slight abscission scar at base of conidia, with 1–3 longitudinal septa, constricted at some septa, narrowly rounded at both ends, smooth-walled.
Cultural characteristics: Colonies on PDA reaching 20 mm diam. after 4 weeks at 16 °C, cream to orangish-white at the margins; reverse white to cream and orangish-white at the center, medium dense, circular, umbonate, fimbriate, fluffy, slightly radiating in the lower part, without diffusible pigments.
Material examined: ITALY, Forlì-Cesena Province, near Galeata, on dead branch of Vitis vinifera (Vitaceae). 16 October 2014, E. Camporesi (MFLU 15–1404, holotype), (isotype in HKAS, under the code of HKAS88971), ex-type living culture, MFLUCC 15–0039.
Halotthiaceae Ying Zhang et al.
The family Halotthiaceae was introduced by Zhang et al. (2013a, b) to accommodate freshwater and maritime Dothideomycetes. Halotthia, Mauritiana, Phaeoseptum and Pontoporeia were included in this family (Hyde et al. 2013; Zhang et al. 2013a, b; Wijayawardene et al. 2014). Halotthiaceae species were characterized by an aquatic habitat, large erumpent to superficial ascomata (>600 μm diam.), sometimes immersed in a pseudoclypeus, a thin-walled peridium, narrow pseudoparaphyses, and brown to dark brown, phragmosporous or dictyosporous ascospores (Hyde et al. 2013; Zhang et al. 2013a, b). Based on multigene phylogenetic analyses, the family formed a monophyletic clade close to Sporormiaceae, Roussoellaceae and Lophiostomataceae in Pleosporales (Suetrong et al. 2009; Zhang et al. 2012a, 2013a, b; Hyde et al. 2013; Wijayawardene et al. 2014). In this paper we introduce a new genus, Brunneoclavispora, to Halotthiaceae In this paper we introduce the novel genera Brunneoclavispora Neolophiostoma and Sulcosporium in Halotthiaceae, based on its phylogenetic placement and distinct characters (Fig. 1).
-
136.
Brunneoclavispora Phookamsak & K.D. Hyde, gen. nov.
Index Fungorum number: IF551326; Facesoffungi number: FoF00892
Etymology: The generic epithet “Brunneoclavispora” refers to the brown, clavate ascospores.
Saprobic on bamboo. Sexual morph: Ascomata solitary to gregarious, immersed in pseudoclypeus to superficial, raised, uni-loculate, elongate conical with a flattened base, ostiole central, with slit-like opening. Peridium thin-walled, composed of several layers, of brown to dark brown, pseudoparenchymatous cells, arranged in textura angularis to textura prismatica. Hamathecium composed of dense, narrow, cellular pseudoparaphyses, anastomosing. Asci 8-spored, bitunicate, fissitunicate, clavate, pedicelate, apically rounded with ocular chamber. Ascospores overlapping, uni- to bi-seriate, phragmosporous or dictyosporous, clavate with rounded upper part and tapered lower part, straight or slightly curved, brown to dark brown, septate, slightly constricted at the septum, smooth-walled. Asexual morph: Undetermined.
Type species: Brunneoclavispora bambusae Phookamsak & K.D. Hyde
Notes: Brunneoclavispora is introduced to accommodate a terrestial Dothideomycete species in Halothiaceae and is typified by B. bambusae Phookamsak & K.D. Hyde. The genus is unique in its clavate ascospores and combination of other characters. Ascospores of Brunneoclavispora are similar to species in Massariosphaeria in having large, clavate to fusiform, muriform or multi-septate ascospores (Crivelli 1983; Tanaka and Harada 2004; Wang et al. 2007). However, Brunneoclavispora differs from Massariosphaeria in its elongate conical ascomata, with a slit-like opening. Multigene phylogenetic analyses (Fig. 1), indicates that Brunneoclavispora belongs in Halotthiaceae, while Massariosphaeria species are polyphyletic and form clades in Amniculicolaceae, Lindgomycetaceae and Thyridariaceae (Wang et al. 2007; Hyde et al. 2013; Wijayawardene et al. 2014; this paper). Brunneoclavispora is similar to Phaeoseptum in Halotthiaceae but differs in its habitat, host, and ascospores with appendages. Brunneoclavispora is terrestial on bamboo, and has clavate, muriform ascospores (with 1–4 longitudinal septa) and a tail-like basal appendage. Phaeoseptum was found on Robinia pseudoacacia in freshwater fungi and has ellipsoidal to fusiform ascospores with longitudinal septa in each cell, and lacks appendages. In the phylogenetic tree (Fig. 1) Brunneoclavispora clusters in Hallothiaceae, close to Mauritiana rhizophorae.
-
137.
Brunneoclavispora bambusae Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF551327; Facesoffungi number: FoF00893; Fig. 24
Brunneoclavispora bambusae (holotype) a Ascomata visible as raised black oval areas, on host surface b Longitudinal section through an ascoma c Vertical section through an ascoma d, e Section through peridium f Pseudoparaphyses g–i Asci j–o Ascospores. Scale bars: b = 200 μm, c = 100 μm, d–i, m = 20 μm, j–o = 10 μm
Etymology: The specific epithet “bambusae” refers to the host.
Holotype MFLU 11-0213
Saprobic on bamboo. Sexual morph: Ascomata 220–330 μm high, 800–1100 μm diam., solitary to gregarious, immersed in pseudoclypeus, visible as raised darkened, elongate areas on host surface, uni-loculate, elongate conical with a flattened base, glabrous, ostiole central, with slit-like opening. Peridium 7–18.5 μm wide, of unequal thickness, slightly thick at the sides, composed of several layers of pseudoparenchymatous cells, outer layers comprising several layers of brown to dark brown cells, arranged in textura angularis to textura prismatica, inner layers comprising several layers of flattened, hyaline cells, arranged in textura prismatica to t. porrecta. Hamathecium composed of dense, 1–2.5 μm wide, cellular pseudoparaphyses, indistinctly septate, not constricted at the septum, anastomosing at the apex, embedded in a mucilagenous matrix. Asci (120–)130–170(−185) × (12–)13–15(−16) μm (\( \overline{x} \) = 147 × 13.9 μm, n = 25), 8-spored, bitunicate, fissitunicate, clavate, short to long pedicellate, apically rounded, with well-developed ocular chamber. Ascospores (23–)25–28(−30) × 5–7 μm (\( \overline{x} \) = 26.4 × 5.8 μm, n = 30), overlapping uni- to bi-seriate, brown to dark brown, clavate or fusuform, with rounded upper part and tapering lower part, straight or slightly curved, with 7–8 transverse septa, and 1–4 longitudinal septa, slightly constricted at the septum, deeply constricted at the third septum, the third cell usually large and having longitudinal septa, smooth-walled, with tail-like appendage at lower end (3–5 μm long). Asexual morph: Undetermined.
Culture characteristics: Colonies on PDA slow growing, reaching 12–18 mm diam. after 4 weeks at 25–30 °C, colonies irregular, dense, low convex, dull with undulate edge, velvety, slightly radiating, redially furrowed, colonies from above white to grey (5A1–5E1) at the margin, orange grey to grey-orangish (5B2–5B3) in the middle, and light brown to yellowish brown at the centre (5D6–5E6); from below yellowish brown to blackish brown (5 F8–6G8), not producing pigment on PDA, but producing dark brown pigment in V8 media and yellowish white to yellowish grey pigment in MEA.
Material examined: THAILAND, Chiang Rai Province, Mae Jun District, Huai Kang Pla Waterfall, on dead stem of bamboo, 25 October 2010, R. Phookamsak RP0093 (MFLU 11-0213, holotype), ex-type living culture, MFLUCC 11-0177, BCC.
-
138.
Neolophiostoma S. Boonmee & K.D. Hyde, gen. nov.
Index Fungorum number: IF551404; Facesoffungi number: FoF00961
Etymology: Neolophiostoma, referring to a new genus similar to Lophiostoma.
Saprobic on dead wood in terrestrial habitats. Sexual morph: Ascomata immersed, scattered to gregarious, partially erumpent at maturity, subglobose, unilocular, black, apically carbonaceous, with wide papilla, ostiolate. Peridium 24.5–27 μm wide, composed of dark brown, carbonaceous, occluded cells. Hamathecium comprising 1–1.5 μm wide, filiform, septate, branched, pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical to subclavate, pedicellate or apedicellate, apically rounded, with an ocular chamber. Ascospores overlapping biseriate, hyaline, elongate-fusiform, narrow towards the sub-acute ends, 3–5-septate, constricted at the median septum. Asexual morph: Undetermined.
Type species: Neolophiostoma pigmentatum S. Boonmee & K.D. Hyde
Notes: Neolophiostoma is similar to Lophiostoma species in having immersed, ascomata with minute crest-like papilla and elongate-fusiform ascospores with a thin gelatinous sheath (Mugambi and Huhndorf 2009a; Hirayama and Tanaka 2011; Thambugala et al. 2015). The genus is typified by Neolophiostoma pigmentatum and is characterized by hyaline, fusiform ascospores and immersed ascomata with a carbonaceous peridium, and in producing gummy mycelia and reddish brown pigmentation in culture (Fig. 25k–l). Phylogenetic analysis places Neolophiostoma with Mauritiana rhizophorae (GU 371824), Pontoporeia biturbinata (GU 479796), Halotthia posidoniae (GU 371824), and two novel genera Brunneoclavispora and Sulcosporium with moderate support (Fig. 1).
Neolophiostoma pigmentatum (holotype) a Appearance of ascomata on substrate b Section of ascomata c Close up of carbonaceous peridium d Pseudoparaphyses e, f Asci g–i Ascospores j Germinating ascospore k–l Colonies on MEA; note pigmentation in aged culture. Scale bars: a = 500 μm, b = 100 μm, c, e–f = 20 μm, d = 5 μm, g–j = 10 μm, k–l = 10 mm
-
139.
Neolophiostoma pigmentatum S. Boonmee & K.D. Hyde, sp. nov.
Index Fungorum number: IF551405; Facesoffungi number: FoF00962; Fig. 25
Etymology: Pigmentatum, referring to the pigmentation produced in the culture media.
Holotype: MFLU 10-0062.
Saprobic on dead wood in terrestrial habitats. Sexual morph: Ascomata 146–231.5 μm high × 188–395 μm diam. (\( \overline{x} \) = 207 × 314 μm, n = 5), immersed, scattered to gregarious, partially erumpent at maturity, subglobose, unilocular, black, apically carbonaceous, with wide papilla, ostiolate. Peridium 24.5–27 μm wide, composed of dark brown, carbonaceous, occluded cells. Hamathecium comprising 1–1.5 μm wide, filiform, septate, branched, pseudoparaphyses. Asci 60–79 × 11–13.5 μm (\( \overline{x} \) = 68 × 12 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindrical to subclavate, narrow towards the base, pedicellate or apedicellate, apically rounded, with an ocular chamber. Ascospores 24–31 × 5–7 μm (\( \overline{x} \) = 28 × 6 μm, n = 20), overlapping biseriate, hyaline, elongate-fusiform, narrow towards the sub-acute ends, slightly curved, 3–5-septate, constricted at the median septum, surrounded by a thin gelatinous sheath, smooth-walled. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on MEA within 12 h. Colonies growing on MEA, reaching 5 mm diam. in 1 week at 28 °C. Mycelium superficial, slightly effuse, thinly hairy, with mucous, radially striate with lobate edge, brown to dark brown, with reddish brown pigmented in media after 60 days.
Material examined: THAILAND, Chiang Mai, Mae Taeng, Huai Nam Dang, on dead wood of unidentified plant, 8 September 2009, Saranyaphat Boonmee, HND-02 (MFLU 10-0062, holotype); ex-type living culture, MFLUCC 10-0129, BCC 52149, IFRDCC 2185.
-
140.
Sulcosporium Phookamsak & K.D. Hyde, gen. nov.
Index Fungorum number: IF551328; Facesoffungi number; FoF00894
Etymology: The generic epithet “Sulcosporium” refers to the striate ascospores.
Holotype: MFLU 11-0243
Pathogen on grasses, causing necrotic leaf spots. Sexual morph: Ascomata solitary, scattered, immersed, globose to subglobose, uni-loculate, membranous, with minute central papilla erumpent through host surface, ostiolate. Peridium thin, composed of several layers of thick-walled, brown to dark brown, pseudoparenchymatous cells, arranged in a textura angularis. Hamathecium comprising dense, 2–4 μm wide, cellular pseudoparaphyses, anastomosing at the apex, embedded in mucilagenous matrix. Asci 8-spored, bitunicate, fissitunicate, broadly fusiform to clavate, saccate or ampulliform, with a short blunt pedicel, apically rounded, with well-developed ocular chamber. Ascospores overlapping bi- to tri-seriate, initially hyaline, becoming very pale brown, ellipsoidal to fusiform, or slightly clavate, 1-septate. Asexual morph: Undetermined.
Type species: Sulcosporium thailandica Phookamsak & K.D. Hyde
Notes: Sulcosporium is introduced as a monotypic genus in to accommodate a Dothideomycete species in the family Halottiaceae, causing leaf spots on grasses, with immersed ascomata and two-celled, striate, thick-walled ascospores. The phylogenetic analyses show that Sulcosporium forms a well-supported clade, basal to the Mauritiana clade, in the family Halotthiaceae.
The species is most similar to Sulcispora (Shoemaker and Babcock 1989; Senanayake et al. 2015), which is unrelated as it belongs in Phaeosphaeriiaceae. Sulcosporium may be similar to Mixtura in causing leaf spot disease on Poaceae, and having pseudoparaphyses with saccate asci (Eriksson and Yue 1990; Phookamsak et al. 2014). However, Sulcosporium differs from Mixtura in having two-celled ascospores, while Mixtura has muriform ascospores.
-
141.
Sulcosporium thailandica Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF551330; Facesoffungi number: FoF00895; Fig. 26
Sulcosporium thailandica (holotype) a Lesions on host leaves b Papilla of ascomata visible as black spots on host surface c Vertical section through an ascoma d Section through peridium e Pseudoparaphyses stained in Melzer’s reagent f, g Asci h Ascus stained in Melzer’s reagent i–k Ascospores l Ascospore stained in Indian ink. Scale bars: c = 50 μm, e–h = 20 μm, d, i, j, k, l = 120 μm
Etymology: The generic epithet “thailandica” refers to the country where the fungus was first collected.
Holotype: MFLU 11-0243
Pathogen on Axonopus compresus (Sw.) P. Beauv., causing necrotic leaf spots. Lesions 3–5 cm long, usually forming from leaf margins, irregular in shape, visible as pale brown to brown regions, separated from healthy part of leaf by reddish brown margins. Sexual morph: Ascomata 70–130 μm high, 80–140 μm diam., solitary, scattered, immersed, globose to subglobose, uni-loculate, membranous, with minute central papilla erumpent through host surface, ostiolate. Peridium 8–20 μm wide, composed of several layers of thick-walled, brown to dark brown, pseudoparenchymatous cells, arranged in a textura angularis. Hamathecium comprising dense, 2–4 μm wide, cellular pseudoparaphyses, constricted at the septum, with distinct septa, anastomosing at the apex, embedded in mucilagenous matrix. Asci (70–)80–100(−110) × (24.5–)27–33(−36) μm (\( \overline{x} \) = 86.2 × 30.4 μm, n = 25), 8-spored, bitunicate, fissitunicate, broadly fusiform to clavate, saccate or ampulliform, with a short blunt pedicel, apically rounded, with well-developed ocular chamber. Ascospores (27–)29–35(−37) × 8–10 μm (\( \overline{x} \) = 33.4 × 9.8 μm, n = 30), overlapping bi- to tri-seriate, initially hyaline, becoming very pale brown, ellipsoidal to fusiform, or slightly clavate, with rounded ends, 1-septate, not constricted at the septum, rough, furrowed, thick-walled, slightly swollen above septa. Asexual morph: Undetermined.
Culture characteristics: Colonies on PDA slow growing, 20–28 mm diam. after 4 weeks at 25–30 °C, colonies irregular, sparse to mediun dense, flattened, slightly raised, smooth with fimbriate to rhizoid edge, thinly hairy to woolly, smooth, from above dark greenish to dark brown at the margin, grey to dark grey in the centre; reverse dark brown at the margin, black at the centre, not producing pigment in PDA.
Material examined: THAILAND, Chiang Rai Province, Muang District, Khun Korn Waterfall, on living leaves of Axonopus compresus (Poaceae), 21 June 2011, R. Phookamsak RP0125 (MFLU 11-0243, holotype), ex-type living culture, MFLUCC 12-0004, BCC.
Latoruaceae Crous
This family was introduced by Crous et al. (2015a, b) to accommodate Latorua and Polyschema in order Pleosporales. In this paper we add Pseudoasteromassaria to the family (Fig. 27).
-
142.
Pseudoasteromassaria Matsumura & Kaz. Tanaka, gen. nov.
Index Fungorum number: IF551448; Facesoffungi number: FoF00963
Etymology: Referring to the similarity of the sexual morph with that of Asteromassaria.
Parasitic on twigs. Sexual morph: Ascomata mostly scattered, immersed, compressed globose, with central, papillate ostiole. Peridium composed of two layers. Hamathecium comprising numerous, hyaline, septate, branched, cellular pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindric to clavate, pedicellate, apically rounded, with an ocular chamber. Ascospores 2–3 overlapping seriate, brown, fusiform, 1–3-septate. Asexual morph: Conidiomata pycnidial, scattered, immersed or superficial, globose, ostiolate. Peridium composed of 2 strata; outer stratum composed of 3–5 layers of polygonal, brown to pale brown cells; inner stratum composed of 2–3 layers of polygonal, hyaline cells. Conidiophores absent. Conidiogenous cells enteroblastic, phialidic, doliform to ampulliform. Conidia cylindric, truncate at the base, 6–7-septate, hyaline.
Type species: Pseudoasteromassaria fagi Matsumura & Kaz. Tanaka
Notes: Pseudoasteromassaria is superficially similar to Asteromassaria (Pleomassariaceae). The type species of Asteromassaria, A. macrospora (Desm.) Höhn., also has large ascomata and occurs on twigs of Fagus (Sivanesan 1984), but the asexual morph, which is referred to as Scolicosporium, is quite distinct from that of Pseudoasteromassaria in having acervular conidiomata and fusiform, pigmented conidia (Fischer 1944; Spooner and Kirk 1982). The globose pycnidia and cylindrical, hyaline conidia of Pseudoasteromassaria resemble those of Stagonospora in the family Massarinaceae, but the latter genus has a didymella-like sexual morph, with small ascomata (up to 300 μm diam.), clavate to fusoid-ellipsoidal asci, and hyaline ascospores, and occurs on leaves of Carex (Quaedvlieg et al. 2013).
-
143.
Pseudoasteromassaria fagi Matsumura & Kaz. Tanaka, sp. nov.
Index Fungorum number: IF551435; Facesoffungi number: FoF00964; Fig. 28
Pseudoasteromassaria fagi (a, b, f–j HHUF 30472; c–e, k HHUF 30471 holotype; l, m, p, s, u culture MAFF 245222; n, o, q, r, t culture MAFF 245221) a, b Appearance of ascomata on host surface c Ascoma in longitudinal section d Peridium e Pseudoparaphyses f, g Asci h Ascus apex i–l Ascospores m Germinating ascospore n, o Conidiomata on rice straw in culture p Conidioma in longitudinal section q Peridium r Conidiogenous cells s–u Conidia. Scale bars: a, b, n = 500 μm, o = 200 μm, c, p = 100 μm, d–m, q–u = 10 μm
Etymology: In reference to the host genus Fagus.
Holotype: HHUF 30471
Parasitic on twigs of Fagus crenata Blume. Sexual morph: Ascomata 250–310 μm high, 700–760 μm diam., mostly scattered, immersed, compressed globose in section, with central, papillate ostiole. Peridium 37.5–50 μm wide at the base, 45–100 μm wide at the sides, composed of two strata; outer stratum thin, composed of dark brown cells; inner stratum composed of 4–7 layers of hyaline, angular cells (7.5–15 × 5–13 μm). Hamathecium comprising numerous, 1–2.5 μm wide, hyaline, septate, branched, cellular pseudoparaphyses. Asci 139.5–195.5 × 35–40 μm (\( \overline{x} \) = 168.1 × 36.7 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindric to clavate, pedicellate (24.5–38.5 μm long), apically rounded, with an ocular chamber. Ascospores 38.5–47 × 11–15 μm (\( \overline{x} \) = 43.5 × 13.3 μm, n = 20), L/W 2.9–3.8 (\( \overline{x} \) = 3.3, n = 20), 2–3 overlapping seriate, brown, fusiform, mostly straight, 1–3-septate, thick-walled, verrucose, with a prominent surrounding sheath. Asexual morph: Conidiomata pycnidial, 230–340 μm high, 140–240 μm diam., scattered, immersed or superficial, globose, ostiolate. Peridium 30–52.5 μm wide, composed of 2 strata; outer stratum composed of 3–5 layers of polygonal, brown to pale brown cells (8–17.5 × 5–10 μm); inner stratum composed of 2–3 layers of polygonal, hyaline cells (8–10 × 4.5–6 μm). Conidiophores absent. Conidiogenous cells 6–16 × 5–7 μm, enteroblastic, phialidic, doliform to ampulliform. Conidia (52–)61–71 × 10–12.5 μm (\( \overline{x} \) = 66.1 × 11.6 μm, n = 20), L/W (4.3–)5.1–7.0 (\( \overline{x} \) = 5.8, n = 20), cylindric, truncate at the base, 6–7-septate, hyaline, smooth-walled, lacking a sheath.
Material examined: JAPAN, Aomori, Towada, Tsuta hot-spring, on twigs of Fagus crenata (Fagaceae), 6 November 2011, K. Tanaka, KT 2966 (HHUF 30471, holotype designated here); living culture, MAFF 245221; Kagoshima, Tarumizu, Mt. Oonogara, on twigs of Fagus crenata, 25 October 2013, K. Tanaka, KT 3432 (HHUF 30472, paratype), ex-type living culture, MAFF 245222.
Lentitheciaceae Y. Zhang et al.
The family Lentitheciaceae was introduced by Zhang et al. (2009b) and is typified by Lentithecium with L. fluviatile as the type species. The family also includes the genera Katumotoa, Keissleriella and Tingoldiago. Wanasinghe et al. (2014) introduced the new genus Murilentithecium from dead branches of Clematis vitalba to accommodate a single species with muriform ascospores and a coelomycetous asexual morph. Singtripop et al. (2015) introduced Keissleriella dactylidis from Dactylis sp., while Liu et al. (2015) introduced Keissleriella sparticola Singtripop & K.D. Hyde with hyaline muriform ascospores, in this family, from a dead stem of Dactylis sp. Phookamsak et al. (2015) introduced a new genus Poaceascoma helicoides Phookamsak & K.D. Hyde, as a single species with scolecospores. A phylogenetic tree for the family is presented in Fig. 29.
Phylogram generated from RAxML analysis based on combined LSU, SSU and EF sequence data with bootstrap support values greater than 50 % indicated above the nodes (ML, black) and Bayesian posterior probabilities (PP, red) ≥95 % indicated in bold branches. The ex-type strains are in bold; the new isolates in blue. The tree is rooted with Massarina eburnea (CBS 473.64)
-
144.
Keissleriella dactylidicola Mapook, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF551391; Facesoffungi number: FoF00941; Fig. 30
Keissleriella dactylidicola (holotype) a, b Appearance of oval ascomata on the host tissue c Section through ascoma d Ostiole with external, dark brown setae e Dark brown setae f Peridium g Pseudoparaphyses h–k Immature and mature asci l–n Ascospores o Ascospore in Indian ink surrounded by hyaline gelatinous sheath p Germinating ascospore. Scale bars: a = 500 μm, b = 200 μm, c = 50 μm, d, e, h–k = 20 μm, f, l–p = 10 μm, g = 5 μm
Etymology: The specific epithet dactylidicola is named after the host Dactylis.
Holotype: MFLU 15–1518
Saprobic on dead stem of Dactylis sp. Sexual morph: Ascomata (140–)160–210 high, (150–)200–230 μm diam. (\( \overline{x} \) = 176.5 × 203 μm, n = 5), superficial, solitary or scattered, oval to ellipsoidal, globose to subglobose, brown to dark brown, without a subiculum covering the host. Ostiole (15–)25–30 × 3–4 μm, slightly protruding, with apical dark brown setae, with blunt or acute apex. Peridium (14–)15–25 (−30) μm wide, comprising 2–4 layers, outer layer comprising irregular brown cells, inner layers comprising brown, flattened cells of textura angularis. Hamathecium comprising 1.5–2 μm wide, cylindrical to filiform, septate, branched, pseudoparaphyses. Asci (50–)60–80 × 8–10 μm (\( \overline{x} \) = 65.5 × 9 μm, n = 10), 8-spored, bitunicate, cylindric-clavate, slightly curved, with short bulbose pedicel, apically rounded, with a wide ocular chamber. Ascospores 15–19 × 4–5 μm (\( \overline{x} \) = 18.5 × 4 μm, n = 10), overlapping 1–2-seriate, hyaline, broadly fusiform, 1-septate, deeply constricted at the septum, widest at the middle and tapering towards the narrow ends, straight or slightly curved, guttulate, surrounded by hyaline, gelatinous sheath, observed only mounted in Indian ink. Asexual morph: Undetermined.
Material examined: ITALY, Arezzo Province, Papiano - Stia, on dead stem of Dactylis sp. (Poaceae), 27 August 2013, E. Camporesi (MFLU 15–1518, holotype), ex-type living cultures, MFLUCC 13–0866; ITALY, Arezzo Province, Papiano - Stia, on dead stem of Dactylis sp. (Poaceae), 16 September 2013, E. Camporesi (MFLU 15–1519, paratype).
Notes: Keissleriella dactylidicola was collected as a saprobe from dead stems of Dactylis sp. Keissleriella dactylidicola is similar to K. dactylidis but differs in having shorter, dark brown setae and in the size, shape and septation of the ascospores (Supplementary Table 3). Keissleriella dactylidicola is similar to K. caudata E. Müll. (Corbaz 1957) in having apical, dark brown setae and hyaline, 1-septate ascospores, with a sheath. It is distinct from K. caudata in having larger ascomata with dark brown setae and a hyaline, mucilaginous sheath surrounding the ascospores (Supplementary Table 3).
Lindgomycetaceae K. Hiray., Kaz. Tanaka & Shearer
Details of this family can be seen in Zhang et al. (2012a) and Hyde et al. (2013).
-
145.
Neomassariosphaeria Y. Zhang et al., in Zhang et al., Stud. Mycol. 64: 96 (2009b)
Type species: Neomassariosphaeria typhicola (P. Karst.) Y. Zhang et al., Stud. Mycol. 64: 96 (2009b)
Notes: Neomassariosphaeria was introduced by Zhang et al. (2009b) to accommodate Massariosphaeria typhicola (generic type) and M. grandispora in family Amniculicolaceae, because the generic type of Massariosphaeria, M. phaeospora, formed a distant clade outside the family Amniculicolaceae. Recent studies (Abdel-Aziz and Abdel-Wahab 2010; Hirayama et al. 2010; Raja et al. 2013), as well as our phylogenetic analyses (Fig. 1) showed that N. typhicola forms a distinct clade within the family Lindgomycetaceae, sister to the Lolia clade. Therefore in order to resolve the polyphyletic nature of Neomassariosphaeria in the order Pleosporales we transfer Neomassariosphaeria to the family Lindgomycetaceae. Furthermore we propose a novel genus, Psuedomassariosphaeria to accommodate Massariosphaeria grandispora in the family Amniculicolaceae.
Lophiostomataceae Sacc.
Thambugala et al. (2015) provided a revision of this family and introduce several new genera and species. The family is characterized by immersed to erumpent, carbonaceous to coriaceous ascomata, with rounded or slit-like ostioles, with a small to large, compressed, crest like apex and fusiform or ellipsoid to fusiform, 1 to multi-septate, or muriform ascospores (Thambugala et al. 2015). A backbone to the family is provided in this paper (Fig. 22).
-
146.
Lophiostoma caulium (Fr.) Ces. & De Not., Schem. di Classif. Sferiacei: 45 (1863)
Facesoffungi number: FoF00881; Fig. 31.
Lophiostoma caulium (reference specimen) a–c Appearance of immersed ascomata on host surface d, e Hand sections of ascomata. Note the ostiole, with a small to large, flat, crest-like apex f Peridium g. Hamathecium h–k Asci with ascospores l–p Ascospores o. Germinating ascospore. Scale bars: d, e = 100 μm, f, h–k = 20 μm, g = 10 μm, l–q = 10 μm
Saprobic on Salvia. Sexual morph: Ascomata 190–210 μm high × 122–190 μm diam. (\( \overline{\mathrm{x}} \) = 198 × 171 μm, n = 5), scattered to gregarious, semi-immersed to erumpent, dark brown to black, globose to subglobose, ostiolate. Ostiole 80–90 μm high, 83–110 μm diam. (\( \overline{\mathrm{x}} \) = 84 × 95 μm, n = 5), slit-like, with a small, to large, flat, crest-like apex, which is variable in shape; apex composed of pseudoparenchymatous cells. Peridium 19–23 μm thick at the sides, broad at the apex, thinner at the base, composed of 4–5 layers of brown to dark brown cells, arranged in a textura angularis, fusing at the outside with the host cells. Hamathecium of 1.5–2.5 μm diam., septate, long, anastomosing and branched, cellular pseudoparaphyses, embedded in gelatinous matrix. Asci 64–91 × 8–10 μm (\( \overline{\mathrm{x}} \) = 82 × 9 μm, n = 30), 8-spored, bitunicate, fissitunicate, cylindrical to clavate, with furcate pedicel, rounded at the apex, with an ocular chamber. Ascospores 20–24 × 4–6 μm (\( \overline{\mathrm{x}} \) = 22 × 5 μm, n = 30), uniseriate or overlapping biseriate, pale brown to dark brown, narrowly fusiform with acute ends, 3–5-septate, slightly constricted at each septum, with a distinct guttule in each cell, smooth-walled, with mucilaginous appendages, drawn out at the ends. Asexual morph: Coelomycetous. Conidiomata superficial, dark brown to black, globose, uniloculate, solitary to scattered. Pycnidial wall thick-walled, multi-layered, with inner layer comprising several cell-layers, comprising brown-walled cells of textura angularis. No spores were produced.
Culture characteristics: Ascospores germinating on MEA within 36 h. Colonies growing on MEA, rather slow growing, reaching 0.2 mm diam. in 1 week at 28 °C. Mycelium superficial, felty, gummy, grey.
Material examined: ITALY, Forlì-Cesena Province, Teodorano di Meldola, dead stem of Salvia sp., 13 October 2013, E. Camporesi (MFLU 15-1074, reference specimen designate here); ex-type living culture, MFLUCC 15-0176, BIOTEC.
Notes: Our material of Lophiostoma caulium (Fr.) Ces. de Not. fits well with the description provided by Cesati and De Notaris (1863). In our collection of L. caulium, the ascomata are slightly smaller than that in the type, but the size of peridium and ascospores are in the range given. The ascomata, asci and ascospores of our reference specimen provided here are also typical of the L. caulium. Our specimen of L. caulium was collected from a different host than that of the type, thus we propose this as reference specimens until collections from the same host and location can be obtained. In our phylogeny Lophiostoma caulium (MFLUCC 15-0176) forms a well-supported (BS 100) clade sister to Lophiostoma caulium “var. a” (JCM 17669) in the family Lophiostomataceae (Fig. 22).
-
147.
Lophiohelichrysum Dayarathne, Camporesi & K.D. Hyde, gen. nov.
Index Fungorum number: IF551400; Facesoffungi number: FoF00913
Etymology: In reference to a new genus in Lophiostomataceae and its host genus Helichrysum.
Saprobic on dead plant stems. Ascomata scattered to gregarious, semi-immersed to erumpent, coriaceous, dark brown to black, globose to subglobose, ostiolate, periphysate. Ostiole slit-like, with a flat, crest-like apex. Peridium comprising 4–5 layers of brown cells, arranged in a textura angularis, fusing at the outside with the host cells, where cells are occluded and black. Hamathecium comprising numerous, 0.5–1.5 μm wide, aseptate, long, filiform pseudoparaphyses, embedded in gelatinous matrix. Asci 8-spored, bitunicate, cylindric-clavate, pedicellate, rounded at the apex, with an ocular chamber. Ascospores overlapping biseriate, hyaline, faintly brown when mature, fusiform, 1-septate, asymmetrical, upper cell wider than lower cell. Asexual morph: undetermined.
Type species: Lophiohelichrysum helichrysi Dayarathne, Camporesi & K.D. Hyde
Notes: Lophiohelichrysum is clearly different from other genera of the family Lophiostomataceae based on molecular data and morphological characters. This monotypic genus is introduced to accommodate taxa characterized by semi-immersed to erumpent ascomata, with a flat, crest-like apex, and fusiform, 1-septate, hyaline ascospores. It differs from Lophiostoma macrostomum, the type of Lophiostoma having coriaceous ascomata, a peridium of textura angularis, 1-septate, asymmetrical, hyaline to faintly brown ascospores, lacking appendages (Thambugala et al. 2015).
-
148.
Lophiohelichrysum helichrysi Dayarathne, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF551401; Facesoffungi number: FoF00914; Fig. 32
Lophiohelichrysum helichrysi (holotype) a Specimen b Ascomata immersed to semi immersed in host tissue c Section through ascoma d Section through neck region e Section through peridium f–i Asci. j Pseudoparaphyses k–n Ascospores with polar appendages o Germinating ascospore p, q Culture on MEA, p from above, n from below. Scale bars: b = 200 μm, c = 100 μm, d, e, j = 20 μm, f–i = 50 μm, k–o = 20 μm
Etymology: named after the host genus on which the fungus occurs.
Holotype: MFLU 15–0175
Saprobic on dead stems of Helichrysum sp. Sexual morph: Ascomata 225–260 μm high, 140–200 μm diam., scattered to gregarious, semi-immersed to erumpent, coriaceous, dark brown to black, globose to subglobose, ostiolate, periphysate. Ostiole slit-like, with a flat, crest-like apex. Peridium 35–45 μm wide, comprising 4–5 layers of brown cells, arranged in a textura angularis, fusing at the outside with the host cells, where cells are occluded and black. Hamathecium comprising numerous, 0.5–1.5 μm wide, aseptate, long, filiform pseudoparaphyses, embedded in gelatinous matrix. Asci 45–100 × 11–20 μm (\( \overline{x} \) = 72.5 × 15.5 μm; n = 30), 8-spored, bitunicate, cylindric-clavate, pedicellate, rounded at the apex, with an ocular chamber. Ascospores 15–30 × 11–16 μm (\( \overline{x} \) = 22.5 × 13.5 μm; n = 30), overlapping biseriate, hyaline, faintly brown when mature, fusiform, 1-septate, asymmetrical, upper cell wider than lower cell, slightly constricted at the septum, with guttules in each cell, smooth-walled, without a mucilaginous sheath, 1–3-septate at germination. Asexual morph: Undetermined.
Culture characteristics: Colonies growing on MEA, reaching 8 cm diam. after 2 weeks at 20–25 °C, with circular, grey, dense, mycelium on the surface, reverse brown to black.
Material examined: ITALY, Province of Forlì-Cesena [FC], Santa Sofia, Collina di Pondo, on dead stem of Helichrysum sp. (Asteraceae), 7 March 2014, Erio Camporesi, IT-1296 (MFLU 15-0175, holotype), ex-type living culture, MFLUCC 15-0701; on dead stem of Helichrysum sp., 3 July 2014, E. Camporesi (MFLU 15-1500, paratype).
Lophiotremataceae K. Hiray. & Kaz. Tanaka
This family was introduced in Hirayama and Tanaka (2011) and presently includes only the genus Lophiotrema (Hyde et al. 2013). In this paper we show that Aquasubmersa also belongs in the family.
Aquasubmersa K.D. Hyde & Huang Zhang, Cryptog. Mycol. 33(3): 340 (2012)
Saprobic on dead wood. Sexual morph: Ascomata scattered to grouped, subglobose, immersed to semi-immersed, ostiolate, short papillate. Peridium composed of flattened, thin-walled cells of textura angularis, black at the outside. Hamathecium comprising numerous, hyaline, septate, branched pseudoparaphyses. Asci 8-spored, bitunicate, cylindrical, with a short pedicel, apically rounded, with an ocular chamber. Ascospores biseriate, hyaline, broadly fusiform with rounded ends, straight, with a mostly median septum, with gelatinous sheath. Asexual morph: Coelomycetous. Conidiomata pycnidial, globose to subglobose, scattered, semi-immersed, solitary, black to brown, ostiolate. Peridium 20–25 μm wide, composed of polygonal, hyaline to brown cells. Conidiophores reduced. Conidiogenous cells holoblastic, lageniform, hyaline, smooth. Conidia hyaline, ellipsoidal, smooth-walled.
Notes: The genus Aquasubmersa was established to accommodate a freshwater coelomycete, A. mircensis, by Zhang et al. (2012b). The genus was characterized by the pycnidia, which have papilla, a peridium composed of thin-walled cells, and unicellular conidia; no sexual morph was reported.
Phylogenetic analyses including 18S and 28S rDNA sequence data, placed A. mircensis in Pleosporales, but the familial position was not clarified (Zhang et al. 2012b). Our phylogenetic analysis suggested that this genus has a close phylogenetic affinity with Lophiotrema (Lophiotremataceae) (Fig. 1). Lophiotrema can be distinguished from Aquasubmersa by having a compressed, slit-like ostiole, and a peridium composed of parallel rows of elongate cells. This is the first report of sexual morph of Aquasubmersa.
-
149.
Aquasubmersa japonica A. Hashim. & Kaz. Tanaka, sp. nov.
Index Fungorum number: IF551422; Facesoffungi number: FoF00956; Fig. 33
Aquasubmersa japonica a, b Appearance of ascomata on substrate c Ascoma in longitudinal section d Peridium. e Pseudoparaphyses f Ascus g, h Ascospores with mucilaginous sheath i Ascospore in India ink j, k Conidiomata in culture l Conidioma in longitudinal section m Peridium of conidioma n Conidiogenous cell and immature conidium o–r Conidia. a, b, e, h, i from HHUF 30469; c, d, g from HHUF 30470; f from HHUF 30468; j–r from culture MAFF 245220. Scale bars: a, b = 200 μm, c, f, l = 20 μm, d, e, g–i, m–r = 10 μm, j, k = 300 μm
Etymology: named after the country of origin, Japan.
Holotype: HHUF 30469
Saprobic on dead wood. Sexual morph: Ascomata 180–210(−300) μm high, 115–175(−230) μm diam., scattered to grouped, subglobose, immersed to semi-immersed. Ostiole 10–16 μm high, short papillate, lacking a clypeus. Peridium 20–30 μm wide, composed of 4–7 layers of flattened, 8–12.5 × 2.5–5.5 μm, thin-walled cells of textura angularis, black at the outside. Hamathecium comprising numerous, 2–4.5 μm wide, hyaline, septate, branched pseudoparaphyses. Asci (60–)66–93 × (4.5–)5.5–8 μm (\( \overline{x} \) = 76 × 6.2 μm, n = 17), 8-spored, bitunicate, cylindrical, with a short pedicel (9–14 μm long), apically rounded, with an ocular chamber. Ascospores 20–26 × 4–5.5(−6) μm (\( \overline{x} \) = 23.2 × 4.6 μm, n = 65), L/W (3.7–)4–6(−6.5) (\( \overline{x} \) = 5.1, n = 65), biseriate, hyaline, broadly fusiform with rounded ends, straight, with a mostly median septum (0.45–0.54; \( \overline{x} \) = 0.50, n = 65), smooth, guttulate when young, with an entire sheath; sheath when fresh condition diffuse, gelatinous, up to 10 μm wide, later becoming sharply delimited firm sheath of 1.5–4 μm thick. Asexual morph: Coelomycetous. Conidiomata pycnidial, globose to subglobose, up to 215 μm high in section, 115–195 μm diam., scattered, semi-immersed, solitary, black to brown. Ostiole 53–80 μm high, short papillate, circular, dark-brown, central. Peridium 20–25 μm wide, composed of 9.5–12.5 × 3–7.5 μm, polygonal, hyaline to brown cells; Conidiophores reduced. Conidiogenous cells holoblastic, 9–12 × 5.5–6.5 μm, lageniform, hyaline, smooth. Conidia ellipsoidal, 17–22(−23) × (7–)8–9 μm (\( \overline{x} \) = 19.4 × 8.4 μm, n = 50), L/W 1.9–2.7 (\( \overline{x} \) = 2.3, n = 50), hyaline, smooth, guttulate when young.
Culture characteristics: Ascospores formed in culture are similar to those on natural substrate.
Material examined: JAPAN, Okinawa, Isl. Ishigaki, Banna Park, on dead wood, 16 July 2011, K. Tanaka & K. Hirayama KT 2862 (HHUF 30469, holotype), ex-type living culture, MAFF 245219; ibid. KT 2863 (HHUF 30470, paratype), living culture, MAFF 245220. JAPAN, Okinawa, Isl., Iriomote, Oomija River, on submerged wood, 12 July 2011, K. Tanaka & K. Hirayama KT 2813(HHUF 30468, paratype), living culture, MAFF 245218.
Notes: The asexual characters of A. japonica show a good match with the generic concepts of Aquasubmersa. The conidia of A. japonica differ in being slightly larger than those of A. mircensis (18.3 × 9.4 μm; Zhang et al. 2012b). ITS sequence differences between our materials of A. japonica and the ex-type strain of A. mircensis (NR 121545) were found at 27–28 positions with three gaps.
Macrodiplodiopsidaceae Voglmayr et al.
This family was introduced by Crous et al. (2015a, b) to accommodate a single genus Macrodiplodiopsis. In this paper we add Pseudomonodictys to the family.
-
150.
Pseudomonodictys Doilom, Ariyawansa, D.J. Bhat & K.D. Hyde, gen nov.
Index Fungorum number: IF551348; Facesoffungi number: FoF00906
Etymology: In reference to the similarity to Monodictys.
Saprobic on dead wood. Sexual morph: Undetermined. Asexual morph: Colonies on natural substrate, superficial, scattered, solitary to gregarious, black. Conidiophores semi-macronematous to sometimes macronematous, septate, branched, pale brown to brown. Conidiogenous cells holoblastic, doliform, integrated, terminal or intercalary, indeterminate, pale brown. Conidia dictyosporous, muriform, top-shaped, reddish-brown to dark brown, solitary, with a protruding basal cell; truncate to slightly rounded at the base.
Notes: Pseudomonodictys is introduced as a monotypic genus with P. tectonae as the type species. The new genus is somewhat similar to Monodictys in having semi-macronematous, erect or flexuous, unbranched or irregularly branched conidiophores, holoblastic conidiogenous cells and dictyosporous conidia, but the conidia of Pseudomonodictys have granular contents and colonies on PDA produce red pigments which has not been reported for Monodictys species (Ellis 1971; Day et al. 2006). The conidiophores of Monodictys are mostly micronematous. There is no sequence data available for Monodictys putredinis, the type species of the genus Monodictys, in GenBank. Therefore, comparative phylogenetic analyses between Pseudomonodictys tectonae and the type of Monodictys cannot be carried out at this time. In this study, isolate MFLUCC 12-0552 (from P. tectonae) grouped next to, but separate from Macrodiplodiopsis desmazieri (strains CAP 24971, CBS 125026 and CPC 24648) with 73 % MLBP support in the combined LSU, SSU, EF and RPB2 phylogeny (Fig. 1). Phylogenetic analysis of combined SSU and ITS sequence data place Monodictys arctica within the Dothideomycetidae and grouping within species of Leptosphaeria (Day et al. 2006). Monodictys castanea shows affinities with the Sordariomycetidae (Barr and Huhndorf 2001). Monodictys pelagica has Nereiosproa cristata as its sexual morph, which groups in Halosphaeriaceae (Sordariomycetes, Mouzouras and Jones 1985; Jones et al. 2015). Our strain cannot be placed in Leptosphaeria or Sordariomycetidae (Fig. 1) and we therefore introduce the monotypic genus Pseudomonodictys in Macrodiplodiopsidaceae, Pleosporales with P. tectonae. Further representative taxa analyses and increased sampling as well as molecular studies are required in the future to strengthen the phylogenetic status of these taxa and confirm the polyphyletic nature of Monodictys.
Pseudomonodictys also shows some resemblance to dictyosporous genera such as Acrodictys and Dictyoarthrinium. It differs from Acrodictys in having semi-macronematous conidiophores producing several conidia, whereas in Dictyoarthrinium, as the name indicates, the terminal conidiogenous cell of macronematous conidiophores produces a muriform holoblastic conidium. Dictyoarthrinium differs from Pseudomonodictys by its basauxic conidiophores. The phylogenetic analyses support the distinction between these genera.
Type species: Pseudomonodictys tectonae Doilom, Ariyawansa, D.J. Bhat & K.D. Hyde
-
151.
Pseudomonodictys tectonae Doilom, Ariyawansa, D.J. Bhat & K.D. Hyde, sp. nov.
Index Fungorum number: IF551349; Facesoffungi number: FoF00907; Fig. 34
Pseudomonodictys tectonae (holotype) a Conidia on dead wood of Tectona grandis (arrows) b, c Mycelia bearing conidiophores d–i Conidia attached to conidiophores e–l Conidia m Germinating conidia n Seven day-old colony (from above) on PDA o Eight month-old colony (reverse view) on PDA p Close-up red pigment on PDA q Crystals of red pigment r Conidiophores producing immature conidia s Conidiophores t-w Conidiophores with conidia and immature conidia (arrow) x Chlamydospores. Scale bars: b–m, r, s, v–x = 10 μm, q, t, u = 20 μm
Etymology: Name refers to the host genus Tectona on which the fungus was collected.
Holotype: MFLU 15-1413
Saprobic on dead wood of Tectona grandis L.f. Sexual morph: Undetermined. Asexual morph: Colonies on natural substrate, superficial, scattered, solitary to gregarious, black. Conidiophores up to 60 μm long, 2.5–5 μm wide, semi-macronematous to sometimes macronematous, erect to slightly curved, septate, branched, slightly constricted at the septa, pale brown to brown. Conidiogenous cells holoblastic, doliform, integrated, terminal or intercalary, indeterminate, smooth to verrucose, pale brown. Conidia (13–) 16–17 (−22) × (14–) 16–19 (−25) diam. μm (\( \overline{x} \) = 16 × 17 μm, n = 20), dictyosporous, muriform, top-shaped, initially subglobose with 1–2 cells, becoming ellipsoidal to irregular with several cells, acropleurogenous, reddish-brown to dark brown, solitary, with granules, with a protruding basal cell; truncate to slightly rounded at the base.
Culture characteristics: Conidia germinating on PDA within 24 h. Germ tubes produced from the basal cells. Colonies on PDA reaching 21–23 mm diam. after 7 days in the dark at 25 °C (\( \overline{x} \) = 22.5 mm n = 5), with entire edge, raised, circular, superficial at the centre, velvety, medium dense, olive grey (3 F2) at the centre, white (3A1) at the edge after 7 days. Mycelium comprising 2–3 μm wide, partly superficial, verrucose, pale brown to dark brown, septate, branched hyphae. Conidiophores semi-macronematous to macronematous, erect to slightly curved, mostly creeping, initially subhyaline, later becoming brown to pale brown. Conidiogenous cells holoblastic, doliform, integrated, terminal, terminal or intercalary, indeterminate. Conidia (8–) 15–19 (−22) × (9–) 14–16 (−19) diam. μm (\( \overline{x} \) = 16 × 14 μm, n = 30), produced on aerial mycelium, dictyosporous, pale brown to reddish-brown, initially subglobose with 1–2 cells, becoming ellipsoidal or cylindrical to irregular with several cells, solitary or rarely catenate, with granular contents, with a protruding basal cell; truncate to slightly rounded at the base. Chlamydospores develop in culture, catenate, intercalary or terminal, initially hyaline and becoming brown to dark brown with maturity, subglobose to ellipsoidal, thick-walled, smooth. Mycelium with rod shaped, septate, erect hyphae, up to 60 μm long, 0.6–1.7 μm diam., producing red pigments in PDA medium.
Material examined: THAILAND, Chiang Rai Province, Mae Suai District, Mae Lao garden, on dead wood of Tectona grandis, 18 August 2012, M. Doilom MKT 68 (MFLU 15-1413, holotype), ex-type living culture, (MFLUCC 12-0552).
Melanommataceae G. Winter
Based on globose or depressed perithecial ascomata, bitunicate and fissitunicate asci, pigmented phragmosporous ascospores, as well as the trabeculate pseudoparaphyses, Winter (1885) introduced the family Melanommataceae, which is typified by Melanomma (Hyde et al. 2013; Tian et al. 2015). Barr (1983) treated Melanommataceae as a separate order, but recent molecular phylogenetic studies do not give any support to the separation of Melanommatales from Pleosporales (Liew et al. 2000; Mugambi and Huhndorf 2009a; Zhang et al. 2012a; Hyde et al. 2013). Currently, the family comprises 16 genera and is considered a well-supported family in the order Pleosporales, based on both phylogeny and morphology (Tian et al. 2015).
Byssosphaeria Cooke, Grevillea 7(no. 43): 84 (1879)
Byssosphaeria is a widespread melanommataceous genus containing approximately 20 species with pyrenochaeta-like coelomycetous asexual morphs (Kirk et al. 2001; Tian et al. 2015). The species are saprobic on decorticated wood, bark of fallen branches, old leaves and various other plant substrates (Kirk et al. 2001; Tian et al. 2015). Byssosphaeria was introduced by Cooke and Plowright (1879) based on its superficial ascomata, seated on a “tomentose subiculum of interwoven threads”, including various species in Sphaeria and Byssisedae, and was validly typified by B. keitii (Cooke and Plowright 1879). Byssosphaeria was assigned to Herpotrichia sensu lato, and Byssosphaeria schiedermayeriana was transferred to H. schiedermayeriana Fuckel (Zhang et al. 2012a). After studying Herpotrichia in North America, Barr (1984) accepted a relatively narrow generic concept, Herpotrichia sensu stricto, and revived Byssosphaeria; this proposal is supported by phylogenetic study (Mugambi and Huhndorf 2009a). Morphologically, B. keitii is characterized by large ascomata with orange to reddish, flat apices, and is closely related to B. rhodomphala (Berk.) Cooke (Barr 1984).
In our phylogeny Byssosphaeria rhodomphala and B. jamaicana, each represented by several collections including our strain MFLU 15-1501, occur in a strongly supported sister relationship (Fig. 35). Our material of B. rhodomphala fits well with the descriptions provided by Barr (1984) and Chen and Hsieh (2004). In B. rhodomphala the asci are slightly larger than that in the type, but the peridium and ascospores are in the given range (Barr 1984; Chen and Hsieh 2004). Byssosphaeria rhodomphala was collected from a different host and location to that of the protologue; thus we propose our collection as a reference specimen (sensu Ariyawansa et al. 2014c) until collections from the same host and location can be obtained for epitypification.
Phylogram generated from maximum likelihood analysis based on combined LSU and EF sequenced data of Byssosphaeria. Maximum likelihood bootstrap support values greater than 50 % and Bayesian posterior probabilities greater than 0.75 are near the nodes. The ex-type strains are in bold and the new isolates are in blue. The scale bar indicates 10 changes. The tree is rooted with Melanoma pulvis-pyrius CBS 183.55
-
152.
Byssosphaeria rhodomphala (Berk.) Cooke, Grevillea 15(no. 75): 81 (1887)
Facesoffungi number: FoF00942; Fig. 36
Byssosphaeria rhodomphala (reference specimen) a Habitat of the taxon on host substrate b Vertical section through ascoma. Note the reddish region around the ostiole c Close up of the peridium d Pseudoparaphyses e, f Asci h–k Ascospores. Scale bars: b = 200 μm, c = 50 μm, d = 10 μm, e–f = 10 μm, h–k = 20 μm
Saprobic on dead stems of Senecio spp. Sexual morph: Ascomata 360–500(−600) μm high × 300–500 μm diam. (\( \overline{x} \) = 450 × 470 μm, n = 10), scattered or in small groups, superficial with basal subiculum anchoring them to the substrate, globose, subglobose to turbinate, apapillate, with pore-like ostiole, ostiolar region flat, often orange or greenish. Peridium 20–55 μm wide, comprising two cell types, outer layer composed of brown, thick-walled cells of textura epidermoidea, inner layer composed of hyaline to pale brown cells of textura angularis. Hamathecium of dense, 0.5–1.5 μm broad, long trabeculate pseudoparaphyses, embedded in mucilage, anastomosing between and above the asci. Asci 90–120 × 10–14 μm (\( \overline{x} \) = 100 × 12 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindric-clavate, pedicel 15–20 (−53) μm long, immature asci usually with a longer and furcate pedicel, apically rounded with an ocular chamber. Ascospores 18–23 × 5.5–8 μm (\( \overline{x} \) = 20 × 6 μm, n = 40), overlapping biseriate above and uniseriate below, pale brown, fusoid to ellipsoidal with rounded ends, 1-septate, with hyaline, smooth-walled. Asexual morph: Undetermined.
Material examined: THAILAND, Chiang Rai Province, Bandu, on dead stem, 12 January 2012, H.A. Ariyawansa (MFLU 15-1501, reference specimen designate here).
Microthyriaceae Sacc.
The family Microthyriaceae was introduced by Saccardo (1883), with the generic type Microthyrium. The family is characterized by ascomata which appear as small black dots on host plants, which are the flattened ostiolate thyriothecia, with poorly developed bases and cells radiating from the prominent central ostiole. Asci are bitunicate and ascospores are hyaline to brown, 1-septate, and with or without ciliate appendages (Doidge 1942; Müller and von Arx 1962; Luttrell 1973; Hofmann and Piepenbring 2006; Hofmann 2010; Wu et al. 2011, 2014; Hyde et al. 2013; Hongsanan et al. 2014a). Combinations of morphological and phylogenetic analyses indicate that Microthyriaceae is a distinct family in Microthyriales, Dothideomycetes (Schoch et al. 2009; Hyde et al. 2013; Hongsanan et al. 2014a; Wijayawardene et al. 2014; Fig. 37).
Phylogram generated from maximum likelihood analysis based on combined LSU and SSU sequenced data. The first set of numbers above the nodes are maximum likelihood values above 50 % shown. The second set of numbers above the nodes are Bayesian posterior probabilities, with values above 0.9 shown. Strain numbers are indicated after species names. New sequence data are in blue bold, and ex-type strains are in black bold
-
153.
Microthyrium buxicola Hongsanan & K.D. Hyde, sp. nov.
Index Fungorum number: IF551429; Facesoffungi number FoF00943; Fig. 38
Microthyrium buxicola (holotype). a Substrate b Thyriothecia on the surface of leaves c, f Thyriothecium when viewed in squash mounts and close-up of ascal arrangement d Upper wall when viewed in squash mount e Prominent darkened ring around the central ostiole g, h Asci with 4-spores i, l Ascospores with appendages. Scale bars: c = 50 μm, d, i–l = 10 μm, e–h = 20 μm
Etymology: buxicola referring to the host on which the taxon was found.
Holotype: MFLU 15-0052
Epiphytic on the upper surface of dead fallen leaves, rarely on the lower surface, appearing as small black dots. Superficial hyphae absent. Sexual morph: Thyriothecia 190–240 μm diam. (\( \overline{x} \) = 215 μm, n = 5), superficial, mostly solitary, sometimes gregarious, light brown to brown, circular, flattened, basal peridium poorly developed, easily removed, with prominent, darker, central ostiole, pale brown and meandering and branched at the margin. Upper wall comprising brown cells of textura angularis, radiating in parallel lines from center to the outer rim. Pseudoparaphyses not seen. Asci 20–32 × 10–12 μm (\( \overline{x} \) = 30 × 11 μm, n = 10), 4-spored, bitunicate, fissitunicate, fusiform to clavate, short pedicellate or apedicellate, apically rounded, with an ocular chamber. Ascospores 14–15 × 4–5 μm (\( \overline{x} \) = 14.4 × 4.6 μm, n = 10), 2–3-seriate, fusiform to ellipsoidal, hyaline, 1-septate, not constricted at the septum, or slightly constricted at the septum when in 10 % lactic acid, apical cell slightly wider and longer than lower cell, smooth-walled, 2–3-guttulate, with 3–6-appendages near the apex, and 3–4-appendages at the septum. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on PDA at 25–30 °C in 12 h of light/12 h of dark, at first hyaline to brownish, becoming brown to reddish. Colonies slow growing reaching 1.5 cm diam. after 10 days, colony superficial to erumpent, very thin, surface smooth, difficult to remove, darker at the center.
Material examined: ITALY, on leaves of Buxus sp. (Buxaceae), 9 December 2014, E. Camporesi (MFLU 15-0052, holotype); ibid., (KIB, isotype); ex-type living cultures MFLUCC 15-0212, MFLUCC 15-0213.
Notes: Microthyrium buxicola is most similar to M. nolinae A.W. Ramaley, but differs in lacking superficial hyphae (Ramaley 1999). Ascospores have up to 20 appendages around the upper cells in M. nolinae, while in M. buxicola there are 3–6-appendages around the upper cell and 3–4-appendages at the septum. The species is also similar to M. guadalupensis A.W. Ramaley. Microthyrium guadalupensis has ascospores with 1–4 appendages at both ends, unlike in M. buxicola. Combined gene analysis of LSU and SSU sequences data indicate that M. buxicola groups in the Microthyriaceae clade sister to Micropeltidaceae clade and is a distinct species (Fig. 37).
-
154.
Tumidispora Hongsanan & K.D. Hyde, gen. nov.
Index Fungorum number: IF551375; Facesoffungi number: FoF00944
Etymology: refers to the swollen cell of the ascospores.
Epiphytic on the upper surface of leaves, appearing as small black dots. Superficial hyphae absent. Sexual morph: Thyriothecia superficial, mostly solitary, light brown to brown, circular, flattened, basal peridium poorly developed, easily removed, darker around the central ostiole, dark brown towards the outer lighter rim, irregularly rounded at the margin. Upper wall comprising brown cells of textura angularis, radiating in slightly irregular, but parallel lines from center to the outer rim. Hamathecium comprising 2 μm wide, branched pseudoparaphyses, asci inclined towards the central ostiole. Asci 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, short pedicellate, apically rounded, with an ocular chamber. Ascospores 1–2-seriate, hyaline, narrowly fusiform with subpapillate ends, 1-septate, apical cell swollen and wider and subpapillate, basal cell subconical. Asexual morph: Undetermined.
Type species: Tumidispora shoreae Hongsanan & K.D. Hyde
Notes: Phylogenetic analyses of LSU and SSU sequence data indicate that Tumidispora is a distinct genus in Microthyriaceae which forms a well-supported clade sister to the Microthyrium clade (99 % ML, 1.0 PP support). Tumidispora shoreae is similar to Microthyriaceae in having superficial thyriothecia, with a central ostiole and hyaline, 1-septate ascospores. Tumidispora shoreae is most typical of Paramicrothyrium chinensis H.X. Wu & K.D. Hyde, but differs in having a prominent darkened area around the central ostiole and also in the shape of the ascospores. There are few sequences for species of Microthyriaceae in GenBank, thus Tumidispora is an important addition.
-
155.
Tumidispora shoreae Hongsanan & K.D. Hyde, sp. nov.
Index Fungorum number: IF551376; Facesoffungi number: FoF00945; Fig. 39
Tumidispora shoreae (holotype) a, b Thyriothecia on surface of leaf c Thyriothecium when viewed in squash mount d Upper wall of thyriothecium when viewed in squash mount e, f Central ostiole with darkened cells g Section through thyriothecium h Ascus with 8 ascospores i Ascus in Melzer’s reagent j Ascus in cotton blue reagent k Pseudoparaphyses l Pseudoparaphyses in cotton blue reagent m Ascospore in water n Ascospore in Melzer’s reagent o Ascospore in cotton blue reagent p Germinating ascospore. Scale bars: c, g = 100 μm, d–f, h–j = 20 μm, k–p = 10 μm
Etymology: shoreae referring to the host on which the taxon was collected.
Holotype: MFLU 15-1391
Epiphytic on the upper surface of leaves, appearing as small black dots. Superficial hyphae absent. Sexual morph: Thyriothecia 261–283 μm diam. (\( \overline{x} \) = 268 μm, n = 10), superficial, mostly solitary, light brown to brown, circular, flattened, basal peridium poorly developed, easily removed, darker around the central ostiole, dark brown towards the outer lighter rim, irregularly rounded at the margin. Upper wall comprising brown cells of textura angularis, radiating in slightly irregular, but parallel lines from center to the outer rim. Hamathecium comprising 2 μm wide, branched pseudoparaphyses, asci inclined towards the central ostiole. Asci 53–55 × 7–8 μm (\( \overline{x} \) = 54 × 7 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, short pedicellate, apically rounded, with an ocular chamber. Ascospores 14–15 × 4–5 μm (\( \overline{x} \) = 14 × 5 μm, n = 10), 1–2-seriate, hyaline, narrowly fusiform with subpapillate ends, 1-septate, constricted at the septum, apical cell swollen and wider and subpapillate, basal cell subconical, smooth-walled. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on PDA at 28 °C for 12 h of light/12 h of dark, hyaline to grayish at first, becoming gray. Colonies slow growing, reaching 1.5 cm diam. after 5 days, colony erumpent, velvety, difficult to remove, with dark grey to black mycelium at margin, greyish to grey at the center.
Material examined: THAILAND, Chiang Rai, Doi Mae Salong (Site 1), on dead leaves of Shorea sp. (Dipterocarpaceae), 18 July 2012, N. Tangteerasunan DMSL13 (MFLU 15-1391, holotype); ibid. (KIB, isotype); ex-type living culture, MFLUCC 12-0409, CPC 21385.
Notes: Tumidispora shoreae is most similar to Paramicrothyrium chinensis in having similar thyriothecia and hyaline, 1-septate ascospores. Tumidispora shoreae has brown to dark brown thyriothecia, with a prominent darkened area around the central ostiole, and 1–2-seriate ascospores which are strongly constricted at the septum, with an apical swollen cell, which is wider than the lower cell and with a subpapillate apex. Paramicrothyrium chinensis has black thyriothecia, and uniseriate ascospores which are not strongly constricted at the septum (Wu et al. 2011).
Phaeosphaeriaceae M.E. Barr
The family Phaeosphaeriaceae (Pleosporales) was introduced by Barr (1979) and typified by Phaeosphaeria with P. oryzae as the type species (Zhang et al. 2012a; Hyde et al. 2013; Phookamsak et al. 2014). Members of this family include plant pathogens, saprobes and endophytes associated with a wide variety of plant hosts, especially monocotyledons, although some have also been reported from dicotyledonous hosts (Zhang et al. 2012a; Hyde et al. 2013; Ariyawansa et al. 2014a, b, c; Phookamsak et al. 2014). Phaeosphaeriaceae has often been confused with Leptosphaeriaceae and Didymosphaeriaceae (Khashnobish and Shearer 1996; Zhang et al. 2012a; Hyde et al. 2013; Phookamsak et al. 2014). Barr (1979) introduced the family with 15 genera, which now has been increased to more than 35 sexual and asexual genera (Hyde et al. 2013; Phookamsak et al. 2014). Various phylogenetic studies have shown that Phaeosphaeriaceae is a heterogeneous group of taxa and recent studies have introduced several new genera and transferred some genera to other families (Zhang et al. 2012a; Hyde et al. 2013; Phookamsak et al. 2014; Trakunyingcharoen et al. 2014; Crous et al. 2015a, b; Ertz et al. 2015). In the present study, a backbone tree for the family is presented (Fig. 40) including the genera Allophaeosphaeria, Ampelomyces, Chaetosphaeronema, Dematiopleospora, Didymocyrtis, Entodesmium, Leptospora, Loratospora, Muriophaeosphaeria, Neosetophoma, Neostagonospora, Nodulosphaeria, Ophiobolus, Ophiosphaerella, Paraphoma, Parastagonospora, Phaeosphaeria, Phaeosphaeriopsis, Sclerostagonospora, Septoriella, Setomelanomma, Setophoma, Stagonospora, Vrystaatia, Wojnowicia, Xenophoma, Wojnowiciella and Xenoseptoria.
Phylogram generated from maximum likelihood analysis based on combined LSU and ITS sequenced data of Phaeosphaeriaceae. Maximum parsimony and maximum likelihood bootstrap support values greater than 50 % and Bayesian posterior probabilities greater than 0.75 are near the nodes. The ex-type strains are in bold and the new isolates are in blue. The scale bar indicates 0.1 changes. The tree is rooted with Didymella exigua CBS 183.55
Allophaeosphaeria Ariyawansa et al., in Liu et al., Fungal Diversity 72(1): 137 (2015)
The genus Allophaeosphaeria was introduced by Liu et al. (2015) to accommodate A. muriformia Ariyawansa et al. and A. dactylidis Wanasinghe et al. Allophaeosphaeria also resembles many species of Phaeosphaeria in having a peridium comprising 2–3 layers of brown to dark brown cells of textura angularis and multi-septate ascospores with a gelatinous sheath, but differs in having muriform ascospores (Liu et al. 2015). Multigene phylogenetic analyses (ITS, LSU and SSU sequence data) indicates that Allophaeosphaeria belongs in Phaeosphaeriaceae, but is distinct from Phaeosphaeria sensu stricto (Fig. 40). A key to Allophaeosphaeria species is provided.
-
156.
Allophaeosphaeria cytisi Wanasinghe, Camporesi, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF551409; Facesoffungi number: FoF00946; Fig. 41
Allophaeosphaeria cytisi (holotype) a Appearance of ascomata on host substrate. Note the papilla with white circle around the ostiole b Section of ascoma c Close up of ostiole d Peridium e Pseudoparaphyses f–i Asci j–o Ascospores. Scale bars: b = 200 μm, c, d = 50 μm, e = 5 μm, f–i = 20 μm, j–o = 10 μm
Etymology: Name reflects the host genus Cytisus, from which the species was collected.
Holotype: MFLU 15-1502
Saprobic on dead herbaceous branches. Sexual morph: Ascomata 500–550 μm high, 400–450 μm diam. (\( \overline{\mathrm{x}} \) = 526.3 × 423.3 μm, n = 10), solitary, scattered, immersed to erumpent, obpyriform, dark brown to black, coriaceous, ostiolate. Ostiole 190–220 μm high, 150–200 μm diam. (\( \overline{\mathrm{x}} \) = 206.4 × 172 μm, n = 5), papillate, black, smooth, filled with hyaline cells, appearing as a white ring around ostiole. Peridium 10–20 μm wide at the base, 20–40 μm wide in sides, comprising 6–8 layers, outer layer heavily pigmented, comprising blackish to dark brown, thick-walled cells of textura angularis, inner layer composed of brown, thin-walled cells of textura angularis. Hamathecium comprising numerous, 2–2.5 μm (n = 30) wide, filamentous, branched, septate, pseudoparaphyses. Asci 110–140 × 10–15 μm (\( \overline{\mathrm{x}} \) = 124.2 × 13.1 μm, n = 40), 8-spored, bitunicate, fissitunicate, cylindrical, pedicel furcate and up to 15–25 μm long, rounded and thick-walled at the apex, with a ocular chamber. Ascospores 20–25 × 8–10 μm (\( \overline{\mathrm{x}} \) = 21.5 × 9.3 μm, n = 50), mostly uniseriate, initially hyaline, becoming yellowish brown at maturity, ellipsoidal, muriform with 6–8 transverse septa and 3–7 vertical septa, strongly constricted at the central septa, weakly constricted at the other septa, with conical and narrowly rounded ends, lacking a mucilaginous sheath. Asexual morph: Undetermined.
Culture characteristics: Colonies on PDA reaching 20–25 mm diam. in 21 days, olivaceous-grey in center, pale olivaceous-grey in outer region, spreading with moderate aerial mycelium, and smooth, even margins; olivaceous-grey from below.
Material examined: Italy, Arezzo Province, Casuccia di Micheli in Quota, dead and hanging branches of Cytisus sp. (Fabaceae), 20 June 2012, E. Camporesi (MFLU 15-1502, holotype), ex-type living culture, MFLUCC 15-0649.
Notes: Allophaeosphaeria cytisi has muriform ascospores and cylindrical asci similar to those characterized in Cucurbitaria, Camarosporium arezzoensis Tibpromma et al., Mycoporum elabens (A. Massal.) Flot. ex Nyl. and Teichospora trabicola Fuckel. Multigene analyses of ITS, LSU and SSU sequence data (Fig. 40) indicate that A. cytisi belongs in Phaeosphaeriaceae. Allophaeosphaeria cytisi forms a distinct clade from Phaeosphaeria sensu stricto. Allophaeosphaeria cytisi differs from A. clematidis and A. dactylidis in its uniseriate ascospores arranged in cylindrical asci, and having 6–8 transverse septa and 3–7 vertical septa, while A. clematidis and A. dactylidis have cylindric-clavate asci with 1–2-seriate ascospores having 4–6 transverse septa, 3–4 vertical septa or 3–5 transverse septa, with 1–3 vertical septa, respectively. Allophaeosphaeria muriformia differs from A. clematidis in having overlapping 2–3-seriate, comparatively large (\( \overline{\mathrm{x}} \) = 56 × 26 μm), oblong to narrowly oblong ascospores.
-
157.
Allophaeosphaeria clematidis Wanasinghe, Camporesi, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF551408; Facesoffungi number: FoF00947; Fig. 42
Etymology: Name reflects the host genus Clematis, from which the species was collected.
Holotype: MFLU 15-1505
Saprobic on dead branches of Clematis vitalba L. Sexual morph: Ascomata 110–130 μm high, 150–180 μm diam. (\( \overline{\mathrm{x}} \) = 124.1 × 166 μm, n = 10), superficial, solitary, scattered, broadly oblong and flattened, dark brown to black, coriaceous, ostiolate. Ostiole 25–35 μm high, 25–40 μm diam. (\( \overline{\mathrm{x}} \) = 29.5 × 32.7 μm, n = 5), papillate, black, smooth, comprising short, hyaline setae. Peridium 4–6 μm wide at the base, 9–10 μm wide in sides, comprising 3–4 layers, of heavily pigmented, thin-walled, blackish to dark brown, cells of textura angularis. Hamathecium comprising numerous, 2–2.5 μm (n = 30) wide, filamentous, branched, septate, pseudoparaphyses. Asci 60–100 × 15–30 μm (\( \overline{\mathrm{x}} \) = 79.7 × 20.3 μm, n = 40), 8-spored, bitunicate, fissitunicate, cylindric-clavate, short pedicellate, rounded and thick-walled at the apex, with an ocular chamber. Ascospores 20–25 × 8–10 μm (\( \overline{x} \) = 23.2 × 9.1 μm, n = 50), overlapping 1–2-seriate, initially hyaline, becoming brown at maturity, mostly ellipsoidal, curved, muriform with 4–6 transverse septa, and 3–4 vertical septa, strongly constricted at the central septa, the cells above the central septum wider, weakly constricted at the other septa, with conical, narrowly rounded ends, lacking a mucilaginous sheath. Asexual morph: Undetermined.
Culture characteristics: Colonies on PDA reaching 30–35 mm diam. in 21 days, surface dirty white, spreading with moderate aerial mycelium, and smooth even, margins; reverse pale luteous.
Material examined: Italy, Forlì-Cesena Province, Fiumicello in Premilcuore, dead and hanging branches of Clematis vitalba (Ranunculaceae), 5 December 2013, E. Camporesi (MFLU 15-1505, holotype), ex-type living culture, MFLUCC 14-0652.
Notes: Allophaeosphaeria clematidis differs from A. dactylidis in having 23.2 × 9.1 μm, mostly ellipsoidal, curved ascospores, with 4–6 transverse septa, and 3–4 vertical septa, while A. dactylidis has 18.2 × 6.3 μm, ellipsoidal to subfusiform, ascospores with 3–5 transverse septa, and 1–3 vertical septa. Allophaeosphaeria muriformia differs from A. clematidis in having large, oblong to narrowly oblong, 56 × 26 μm ascospores.
Key to species of Allophaeosphaeria
-
1. Peridium comprising two cell layers, outwardly heavily pigmented, inwardly hyaline .............................................2
-
1. Peridium comprising a single heavily pigmented cell layer ........................................................3
-
2. Ascospores ellipsoidal, with 4–6 transverse septa, and 3–4 vertical septa ...................... A. clematidis
-
2. Ascospores subfusiform, with 3–5 trans-verse septa, and 1–3 vertical septa .............. A. dactylidis
-
3. Ascospores mostly uniseriate, with 6–8 transverse septa, and 3–7 vertical septa ........................................ A. cytisi
-
3. Ascospores mostly overlapping 2–3-seriate, with 2–3 transverse septa, and 1–2 vertical septa ....................................................... A. muriformia
-
158.
Allophaeosphaeria subcylindrospora W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF551396; Facesoffungi number: FoF00948; Fig. 43
Allophaeosphaeria subcylindrospora (holotype) a Specimen showing appearance of taxon on host b Surface view of conidioma c, d Vertical section of conidioma with conidiogenous cells and developing conidia e Surface view of ostiole f Section of peridium g Germinating conidia h–k Conidia l Culture on PDA. Scale bars: b = 100 μm, c–d = 50 μm, e–f = 10 μm, g = 20 μm, h, i, k = 5 μm, l = 25 μm
Etymology: In reference to the subcylindrical conidia.
Holotype: MFLU 15–0697
Saprobic on dead stem of Dactylis glomerata L. (Poaceae), forming conspicuous, small, rounded, black fruiting bodies. Sexual morph: Undetermined. Asexual morph: Coelomycetous. Conidiomata 65–70 μm high, 83–93 μm diam., pycnidial, solitary, gregarious or confluent, subepidermal, immersed to semi-immersed, globose, pale brown to brown, unilocular, ostiolate. Wall of conidiomata 6–11 μm wide, composed of 4–5-layers, thin-walled cells of textura angularis, with cells of outer 1–2 layers pale brown, gradually merging with inner 1–2 hyaline layers. Ostiole 5–7 μm wide, rounded, central, comprising dark brown to black cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, subcylindrical, hyaline, arising from inner region of conidiomata. Conidia 14–22 × 1.6–3.7 μm (\( \overline{x} \) = 19 × 2.6 μm; n = 30), fusiform to subcylindrical, with an obtuse and narrow apex, and a truncate base, pale brown, mostly 3-septate, occasionally 1-septate, not constricted at septa, thick-walled, smooth-walled.
Culture characteristics: Colonies on PDA flat, circular, with dull green margin, mouse-grey in the middle and pale mouse-grey at the center; reverse dull green.
Material examined: ITALY, Province of Trento [TN], Val di Sole, Cogolo, on dead stem of Dactylis glomerata (Poaceae), 30 June 2012, Erio Camporesi, IT-520 (MFLU 15–0697, holotype); ex-type living culture, MFLUCC 13-0380, KUMCC 15–0090; ibid. (KUN! HKAS 89500, isotype).
Notes: In the present paper, we introduce an asexual morph taxon, A. subcylindrospora, which is phylogenetically close to, but distinct from A. muriformia (Fig. 31). Allophaeosphaeria lacks any known asexual morph (Liu et al. 2015). Allophaeosphaeria subcylindrospora resembles P. papayae (Speg.) Quaedvl. et al. in having pycnidial, brown, globose conidiomata and subcylindrical, pale brown conidia with an obtuse apex, and truncate base. However, A. subcylindrospora is further characterized by cylindrical conidiogenous cells, and mostly 3-septate, occasionally 1-septate conidia, which are shorter than P. papaya (15–)26–32(−35) × (2.5–)3 μm (Quaedvlieg et al. 2013).
Dematiopleospora Wanasinghe et al., Crypt. Mycol. 35: 110 (2014)
Dematiopleospora was introduced by Wanasinghe et al. (2014) to accommodate D. mariae Wanasinghe et al. The genus is characterized by thick, brown, periphyses in the ostiole, superficial ascomata and muriform ascospores. Multigene phylogenetic analyses indicated that Dematiopleospora belongs to Phaeosphaeriaceae which forms a separate clade from Phaeosphaeria species and groups with Chaetosphaeronema, Entodesmium, Loratospora and Nodulosphaeria (Phookamsak et al. 2014; Wanasinghe et al. 2014; Fig. 40).
-
159.
Dematiopleospora luzulae Wanasinghe, Camporesi, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF551410; Facesoffungi number: FoF00949; Fig. 44
Etymology: Name reflects the host genus Luzula from which the species was collected.
Holotype: MFLU 15-1503
Saprobic on dead stem of Luzula sp. Sexual morph: Ascomata 140–160 μm high, 175–200 μm diam. (\( \overline{\mathrm{x}} \) = 148.4 × 184.5 μm, n = 10), immersed, solitary, scattered, globose, brown to dark brown, coriaceous, fusing with the host tissue, ostiolate. Ostiole 25–40 μm high, 30–50 μm diam. (\( \overline{\mathrm{x}} \) = 29.5 × 36.2 μm, n = 5), black, smooth, upper covering of ostiole, comprising short, light brown setae-like structures. Peridium 10–20 μm wide at the base, 10–15 μm wide at the sides, comprising 3–4 cell layers of thin-walled, brown cells of textura angularis, inwardly lighter. Hamathecium comprising numerous, 2–3 μm (n = 30) wide, filamentous, branched, septate, pseudoparaphyses. Asci 70–80 × 15–20 μm (\( \overline{\mathrm{x}} \) = 76.4 × 18.1 μm, n = 40), 8-spored, bitunicate, fissitunicate, cylindric-clavate, short pedicellate, rounded and thick-walled at the apex, with an ocular chamber. Ascospores 15–20 × 7–10 μm (\( \overline{\mathrm{x}} \) = 19.3 × 8.6 μm, n = 50), overlapping biseriate, initially hyaline, becoming brown at maturity, ellipsoidal to oval, some curved, muriform with 4–6 transverse septate and 3–4 vertical septa, strongly constricted at the central septa, the two cells rows above the central septum wider, weakly constricted at the other septa, conical and narrowly rounded at the ends, surrounded by a thick, hyaline, mucilaginous sheath. Asexual morph: Undetermined.
Culture characteristics: Colonies on PDA reaching 30–35 mm diam. in 21 days, olivaceous-grey, spreading with moderate aerial mycelium, with smooth, even, margins; reverse iron-grey.
Material examined: Italy, Trento Province, Mezzana, Marilleva 1400, on dead stem of Luzula sp. (Juncaceae), 1 August 2013, E. Camporesi (MFLU 15-1503, holotype); ex-type living culture, MFLUCC 14-0932.
Notes: Dematiopleospora luzulae is introduced as a second species of Dematiopleospora and has muriform ascospores similar to those characterized in Pleospora (Zhang et al. 2009a, b). Dematiopleospora luzulae resembles D. mariae in having brown, periphyses in the ostiole, a peridium comprising brown to dark brown cells of textura angularis and muriform ascospores, but differs in having ascospores without light end cells.
Entodesmium Riess, Hedwigia 1(6): 28 (1854)
Entodesmium was introduced by Reiss (1854) and is typified by E. rude Riess. Entodesmium is characterized by immersed, globose ascomata, and filiform, multi-septate ascospores, some breaking into part spores (Shoemaker 1984; Barr 1992a; Zhang et al. 2012a; Phookamsak et al. 2014). Our new species, Entodesmium artemisiae has a close phylogenetic relationship with E. rude (Fig. 40). However, our new species differs in host, ascomata, periphyses, asci and/or ascospore characters. No species of Entodesmium have been described from Artemisia sp. (Index Fungorum 2015).
-
160.
Entodesmium artemisiae S. Konta, Bulgakov & K.D. Hyde. sp. nov.
Index Fungorum number: IF551450; Facesoffungi number: FoF00950; Fig. 45
Entodesmium artemisiae (holotype) a Appearance of ascomata on host substrate b Close up of ascomata which are erumpent through host epidermis c Cell arrangement of peridium d Section of ascoma e Periphyses f Pseudoparaphyses g–j Asci k–n Ascospores o Germinating ascospore. Scale bars: a = 500 μm, b = 200 μm, c = 20 μm, d = 50 μm, e–f = 20 μm, g–j = 50 μm, k–n = 20 μm, o = 50 μm
Etymology: The specific epithet refers to the host genus Artemisia.
Holotype: MFLU 15-0007
Saprobic on on dead stems of Artemisia. Sexual morph: Ascomata 205–292 μm wide, 177–258 μm high (\( \overline{x} \) = 231.3 μm × 207 μm), scattered, solitary, dark brown, semi-immersed to erumpent, subglobose, ostiolate. Ostiole lined with hyaline periphyses. Peridium 26–41 μm wide (\( \overline{x} \) = 35.08 μm), comprising two strata, outer stratum comprising black, thick-walled, occluded cells, inner stratum comprising 3–4 layers of black, thick-walled cells of textura angularis. Hamathecium comprising numerous, 1.5–2.5 μm wide (\( \overline{x} \) = 2 μm), filamentous, branched, septate pseudoparaphyses. Asci 80.5–112.5 × 9–14 μm (\( \overline{x} \) = 95.6 × 11.22 μm, n = 20), 8-spored, bitunicate, cylindric-clavate, pedicellate, apically rounded, with an ocular chamber. Ascospores 61.5–96.5 × 2.5–4 μm (\( \overline{x} \) = 82.96 × 2.13 μm, n = 40), fasciculate, initially hyaline, becoming yellowish brown to brown at maturity, filiform, slightly curved, widest and curved approximately 1/3rd from apex, 13–20-septate, lacking a sheath or appendages, not breaking into part spores. Asexual morph: Undetermined.
Culture characteristics: Colonies on MEA, reaching 5.5 cm diam. after 2 weeks at 16 °C, olive brown in the middle, yellowish white at the edges, and reverse olive brown at the margins, medium dense, irregular, flattened to slightly raised, with rough surface, crenated radiating margins, floccose to fairly fluffy, brown pigment produced in agar.
Material examined: RUSSIA, Rostov region, Shakhty City, Salty Hollow, stony steppe, dead stems of Artemisia campestris L., 3 June 2014, T. Bulgakov, T109 (MFLU 15-0007, holotype); ex-type living culture, MFLUCC 14-1156.
-
161.
Galiicola Tibpromma, Camporesi & K.D. Hyde, gen. nov.
Index Fungorum number: IF551383; Facesoffungi number: FoF00923
Etymology: refers to the host genus from which the taxon was collected.
Saprobic on Galium. Sexual morph: Ascomata solitary, semi-immersed or slightly erumpent though the host surface, appearing as black dots on the host surface globose to subglobose, with a small central ostiole. Ostiole short, shiny, apapillate. Peridium composed of 3–5 layers of thin-walled, yellow to orange brown, flattened cells. Hamathecium comprising numerous 1.7–2.3 μm wide, filamentous, branched, anastomosing, septate pseudoparaphyses. Asci 8-spored, bitunicate, cylindric-clavate, with short pedicel, apex rounded an ocular chamber. Ascospores overlapping 1−3-seriate, initially hyaline, becoming orange brown at maturity, elongate fusiform, with 4–5 transverse septate, some with 1–2 vertical septa, constricted at central septum, cell above central septum slightly swollen. Asexual morph: Undetermined.
Type species: Galiicola pseudophaeosphaeria Tibpromma, Camporesi & K.D. Hyde
Notes: The genus Galliicola belongs in the family Phaeosphaeriaceae (Fig. 40) where it is basal to Xenophoma. The genus is unique in Phaeosphaeriaceae in having a peridium of 3–5 layers of thin-walled, yellow to orange brown, flattened cells and orange-brown ascospores.
-
162.
Galiicola pseudophaeosphaeria Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF551384; Facesoffungi number: FoF00924; Fig. 46
Etymology: Refers to the similarity to Phaeosphaeria.
Holotype: MFLU 15-1027
Saprobic on Galium. Sexual morph: Ascomata 109–137 μm high, 134–148 μm diam. (\( \overline{\mathrm{x}} \) = 127 × 142 μm, n = 5), scattered or sometimes clustered, solitary, semi-immersed or slightly erumpent though the host surface, appearing as black dots on the host surface, globose to subglobose, with a central ostiole. Ostiole short, shiny, apapillate. Peridium 6–10 μm, a single stratum composed of 3–5 layers of thin-walled, yellow to orange brown, flattened cells. Hamathecium comprising numerous, 1.7–2.3 μm, filamentous, branched, anastomosing, septate pseudoparaphyses. Asci 45–62 × 7–9 μm (\( \overline{\mathrm{x}} \) = 53 × 8 μm, n = 15), 8-spored, bitunicate, fissitunicate, cylindric-clavate, with short bulbous pedicel, apex rounded with an ocular chamber. Ascospores 15–18 × 3–5 μm (\( \overline{\mathrm{x}} \) = 16 × 4 μm, n = 15), overlapping 1–3-seriate, initially hyaline, becoming orange brown at maturity, elongate fusiform with acute ends, some slightly curved, with 4–5 transversely septate, some with 1–2 vertical septa, constricted at the central septum, cell above central septum slightly swollen, lacking a mucilaginous sheath. Asexual morph: Undetermined.
Culture characteristics: on MEA reaching 2 cm diam. after 2 weeks at 16 °C, later with dense mycelium, with circular colony, rough entire margin, surface smooth but raised; hyphae septate, branched, hyaline to light-brown, thick-walled.
Material examined: ITALY, Forlì Province, Selva di Ladino, on dead stem of Galium sp. (Rubiaceae), 19 April 2014, E. Camporesi IT1916 (MFLU 15-1027, holotype); ex-type living culture, MFLUCC 14-0524; ibid. (MFLU 15-1487, KUM, isotypes); (MFLU 15-1498bis, MFLU 15-1499tris, paratypes).
-
163.
Loratospora luzulae Jayasiri, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum Number: IF551325; Facesoffungi number: FoF00882; Fig. 47
Etymology: In reference to host genus.
Holotype: 15-1394
Saprobic on dead stem of Luzula nivea (Juncaceae). Sexual morph: Ascomata 112–120 × 93–100 μm (\( \overline{\mathrm{x}} \) = 98 × 115 μm, n = 5), scattered to clustered, solitary, immersed, visible as small, black spots on the host surface, uni-loculate, globose to subglobose, glabrous, brown to dark brown, ostiole central, with a minute papilla containing hyaline periphyses. Peridium 8–20 μm wide, of unequal thickness, thickened at the apex, composed of several layers of brown to dark brown, pseudoparenchymatous cells, arranged in a textura globulosa. Hamathecium of 1.5–2 μm wide, septate, long, cellular pseudoparaphyses, embedded in a gelatinous matrix. Asci 64–75 × 14–16 μm (\( \overline{\mathrm{x}} \) = 70 × 15 μm, n = 10), 8-spored, bitunicate, fissitunicate, short pedicellate, apically rounded and thick-walled, with an ocular chamber. Ascospores 14–19 × 4–7 μm (\( \overline{\mathrm{x}} \) = 16 × 4.6 μm, n = 30), overlapping 2–4-seriate, initially hyaline to subhyaline, pale yellowish at maturity, fusiform with rounded ends, 3-septate, slightly curved, constricted at the septa, wall rough, surrounded by a mucilaginous sheath. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on MEA within 36 h. Colonies growing on MEA, slow-growing, reaching 0.5 mm diam. in 1 week at 28 °C. Mycelium superficial, felty, grey.
Material examined: ITALY, Province of Forlì-Cesena [FC], Campigna-Santa Sofia, on dead stems of Luzula nivea (Nathh.) DC (Juncaceae), 8 June 2014, E. Camporesi, IT 1918 (MFLU 15-1394, holotype, BBH), ex-type living culture MFLUCC 14-0826, BCC.
Notes: Loratospora was introduced by Kohlmeyer and Volkmann-Kohlmeyer (1993) as a monotypic genus, with Loratospora aestuarii Kohlm. & Volkm.-Kohlm. Suetrong et al. (2009) transferred Loratospora to Phaeosphaeriaceae based on phylogenetic data, whereas based on morphology it had previously been placed in Planistromellaceae (Barr 1996). This group was not well-resolved in Phookamsak et al. (2014). In this study we introduce a new species to the genus because it is morphologically and phylogenetically different to Loratospora aestuarii. Loratospora luzulae forms a separate branch with Loratospora aestuarii in the phylogenetic analysis (Fig. 40).
Nodulosphaeria Rabenh., Klotzschii Herb. Viv. Mycol., Edn 2: no. 725 (in sched.) (1858)
Nodulosphaeria was established by Rabenhorst (1858) to accommodate species with ascomata with setae in the papilla and ascospores with three or more transverse septa with an enlarged cell near to the apex (Barr 1992; Holm 1961). The genus was previously considered as a synonym of Leptosphaeria (Clements and Shear 1931). However, Holm (1957) reinstated the genus Nodulosphaeria and N. hirta Rabenh. was selected as the type species (Zhang et al. 2012a; Phookamsak et al. 2014). Identification of species belonging in this genus has been complicated due to the unavailability of molecular data (Shoemaker 1984; Zhang et al. 2012a; Ariyawansa et al. 2014c; Phookamsak et al. 2014). There are 64 epithets listed under Nodulosphaeria (Index Fungorum 2015), but sequence data is available for only a single species in GenBank (Phookamsak et al. 2014).
-
164.
Nodulosphaeria senecionis Chethana, Camporesi, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF551310; Facesoffungi number: FoF: 00871; Fig. 48
Nodulosphaeria senecionis (holotype) a, b Appearance of ascomata on the host substrate c Close up of ascoma d Section of the ascoma e Section of the ostiole with setae f Peridium g, h Immature asci i–k Mature asci l Hamathecium of septate, cellular pseudoparaphyses m Immature hyaline ascospore n–v Mature hyaline to yellowish brown ascospores. Scale bars: a = 500 μm, b, c = 200 μm, d = 100 μm, e = 25 μm, f, m–v = 10 μm, g–k = 20 μm, l = 5 μm
Etymology: The specific epithet senecionis is named after the host Senecio from which the taxon was collected.
Holotype: MFLU 15–1297
Saprobic on dead stems of Senecio spp. Sexual morph: Ascomata 189–275 μm high (including papilla), 257–299 μm diam. (\( \overline{x} \) = 238.31 × 284.06 μm, n = 10), scattered, solitary, semi-immersed to superficial, dark brown to black, globose to subglobose, wall rough, with hairs at the base, ostiole central, papillate. Papilla 36–47 μm high, 30–38 μm diam. (\( \overline{\mathrm{x}} \) = 34.1 × 39.9 μm, n = 10), protruding from substrate if immersed, with numerous, brown to dark brown, 25–40 μm long setae. Peridium 37–59 μm thick at side walls, up to 45–79 μm thick near the apex and 36–66 μm thick at the base, composed of 4–5 layers of thick-walled, dark brown cells of textura globularis. Hamathecium composed of numerous, 2–3 μm broad, filiform, anastomosing, septate, pseudoparaphyses, embedded in a gelatinous matrix. Asci 33–113 × 4–14 μm (\( \overline{x} \) = 82.93 × 10.89 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindric-clavate, short pedicellate, apically rounded, with an ocular chamber. Ascospores 30–45 × 3–5 μm (\( \overline{x} \) = 38.39 × 4.22 μm), overlapping biseriate to tri-seriate, initially hyaline, becoming yellowish brown at maturity, long fusiform to almost cylindrical, enlarged at the third cell from the apex, tapering towards the rounded ends, 8-septate, slightly curved, constricted at the third septum, smooth-walled, with small guttules and small, curved, cylindrical appendages at both ends. Asexual morph: Undetermined.
Material examined: ITALY, Forlì-Cesena Province, Fiumicello-Premilcuore, on dead stem of Senecio sp. (Asteraceae), 12 May 2013, E. Camporesi (MFLU 15–1297, holotype); ibid. (isotype in BBH); on dead stem of Senecio sp., 3 June 2013, E. Camporesi (MFLU 15–1312, paratype); ITALY. Province of Forlì-Cesena, Fiumicello-Premilcuore, on a dead stem of Centaurea sp. (Asteraceae), 8 July 2013, E. Camporesi (MFLU 15–1313).
Notes: Based on our phylogenetic analyses (Fig. 40) and morphological comparison, our isolate belongs in Nodulosphaeria in Phaeosphaeriaceae. Nodulosphaeria senecionis is distinct from N. hirta in having larger ascospores. Nodulosphaeria pellita (Fr.) Shoemaker, N. octoseptata (Wehm.) L. Holm, N. derasa (Berk. & Broome) L. Holm and Leptosphaeria senecionis (Fuckel) G. Winter have also been isolated from Senecio sp. and should be compared. Nodulosphaeria pellita and N. octoseptata differ from N. senecionis in having larger ascomata, longer ascospores constricted at the first septum, and with the fourth cell from the apex being enlarged (Shoemaker 1984). Nodulosphaeria derasa differs from N. senecionis in having ascospores, which are constricted at the first septum and with the fourth cell from the apex being enlarged (Shoemaker 1984). Leptosphaeria senecionis differs from Nodulosphaeria senecionis in having 3-septate, hyaline ascospores (Saccardo 1884). Nodulosphaeria centaureae differs from N. senecionis in having 6-septate ascospores (Shoemaker 1984).
Ophiosphaerella Speg., Anal. Mus. nac. B. Aires, Ser. 3 12: 401 (1909)
Ophiosphaerella is a confused genus lacking sequence data for the type species in GenBank (Phookamsak et al. 2014) with 11 species listed in Index Fungorum (2015). Sequence data is available for Ophiosphaerella agrostidis Dern et al. and Ophiosphaerella herpotricha (Fr.) J. Walker and our new species cluster with these taxa (Fig. 40).
-
165.
Ophiosphaerella aquaticus Z.L. Luo, H.Y. Su & K.D. Hyde, sp. nov.
Index Fungorum number: IF551441; Facesoffungi number: FoF00951; Fig. 49
Etymology: With reference to the aquatic habitat.
Holotype: MFLU 15–0071
Saprobic on decaying wood submerged in freshwater. Sexual morph: Ascomata 420–470 μm high, 265–360 μm diam., scattered, solitary, immersed to semi-immersed or erumpent through host tissue, with minute papilla, visible as raised, small black dots on host surface, uni-loculate, pyriform, pilous, dark brown to black, ostiole central, with periphyses. Peridium 20–37 μm wide, of unequal thickness, composed of several layers of pseudoparenchymatous cells, outer part comprising dark brown, occluded thickened cells, fusing with the host, inner part comprising several layers of thin-walled, flattened, brown-walled cells. Hamathecium composed of numerous, 3–6 μm wide, filamentous, septate pseudoparaphyses, embedded in a mucilaginous matrix, anastomosing at the apex. Asci 146–174 × 10–12.5 μm (\( \overline{\mathrm{x}} \) = 160 × 11 μm, n = 20), 8-spored, bitunicate, cylindrical to cylindric-clavate, short pedicellate, apically rounded, with an ocular chamber. Ascospores 143–162 × 2.5–3.5 μm (\( \overline{\mathrm{x}} \) = 152.5 × 3 μm, n = 20), fasciculate, scolecosporous, parallel or spiral, filiform, pale brown to brown, aseptate, smooth-walled. Asexual morph: Undertermined.
Material examined: THAILAND, Chiang Mai Province, saprobic on decaying wood submerged in a stream, November 2013, Z.L. Luo ZL-4 (MFLU 15–0071, holotype), ex-type culture, MFLUCC 14-0033, KIBCC, DLUCC; ibid. (HKAS 86446, isotype).
Notes: This scolecosporous Ophiosphaerella species was collected from a decaying stem of bamboo submerged in freshwater in Thailand. Ophiosphaerella aquaticus is characterized by semi-immersed, ascomata with a central ostiole, and filiform ascospores. In the phylogenetic analysis, O. aquaticus clusters with O. agrostidis in the family Phaeosphaeriaceae but in a distinct clades. Ophisphaerella aquaticus is compatible with O. agrostidis. However, O. aquaticus differs in having a pyriform ascomata, larger aseptate ascospores and in its aquatic habit (Phookamsak et al. 2014).
-
166.
Populocrescentia Wanasinghe, E.B.G. Jones & K.D. Hyde, gen. nov.
Index Fungorum number: IF551411; Facesoffungi number: FoF00952
Etymology: from the Latin crescit, meaning growing on, referring to its ecological habit, growing on Populus.
Saprobic on dead herbaceous branches in terrestrial habitats. Sexual morph: Ascomata solitary, scattered, immersed to semi-erumpent, globose or subglobose, dark brown to black, coriaceous, cupulate when dry, ostiolate. Ostiole papillate, black, smooth, ostiolar canal filled with sparse periphyses. Peridium comprising 6–8 layers, outer part heavily pigmented, thick-walled, comprising blackish to dark brown cells of textura angularis, inner part composed of hyaline, thin-walled cells of textura angularis. Hamathecium comprising 2–3 μm wide, filamentous, branched septate, cellular pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, short pedicellate, rounded and thick-walled at the apex, with a minute ocular chamber. Ascospores overlapping 1–2-seriate, initially hyaline, becoming yellowish brown at maturity, broad fusiform, muriform, deeply constricted at the middle septum, slightly constricted at the remaining septa. Asexual morph: Undetermined.
Type species: Populocrescentia forlicesenensis Wanasinghe, Camporesi, E.B.G. Jones & K.D. Hyde
Notes: Populocrescentia is introduced as a new genus in the family Phaeosphaeriaceae with P. forlicesenensis as the type species. Both morphology and phylogeny (Fig. 40) indicate this is a distinct genus from Dematiopleospora and Allophaeosphaeria (Wanasinghe et al. 2014; Liu et al. 2015). Allophaeosphaeria differs from Populocrescentia in its peridium structure and papillate ostioles. Populocrescentia shares most similarities with Dematiopleospora in having flat, cupulate, globose to subglobose ascomata, a minute papilla, cylindrical asci and muriform ascospores. Dematiopleospora however, differs from Populocrescentia in having a wide, brown, periphysate ostiole, subfusiform ascospores with the upper part widest and light end cells. This is also phylogenetically supported as Populocrescentia forlicesenensis forms a distant clade from these taxa (Fig. 40).
-
167.
Populocrescentia forlicesenensis Wanasinghe, Camporesi, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF551412; Facesoffungi number: FoF00953; Fig. 50
Etymology: Name reflects the locality, Forlì-Cesena Province, where this species was collected.
Holotype: MFLU 15-1504
Saprobic on dead herbaceous branches of Populus nigra L. Sexual morph: Ascomata 150–270 μm high, 200–250 μm diam. (\( \overline{\mathrm{x}} \) = 213 × 234.7 μm, n = 10), superficial, solitary, scattered, broadly oval, dark brown to black, coriaceous, cupulate when dry, ostiolate. Ostiole 30–60 μm high, 25–50 μm diam. (\( \overline{\mathrm{x}} \) = 45 × 38.3 μm, n = 5), papillate, black, smooth, with short, light brown setae. Peridium 10–20 μm wide at the base, 20–35 μm wide at the sides, comprising 6–8 layers, outer part heavily pigmented, thick-walled, comprising blackish to dark brown cells of textura angularis, inner part composed of hyaline, thin-walled cells of textura angularis. Hamathecium comprising numerous, 2–3 μm (n = 30) wide, filamentous, branched, septate, pseudoparaphyses. Asci 90–110 × 14–20 μm (\( \overline{\mathrm{x}} \) = 100.5 × 16.5 μm, n = 40), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, pedicellate, rounded and thick-walled at the apex, with an ocular chamber. Ascospores 17–22 × 8–10 μm (\( \overline{\mathrm{x}} \) = 19.7 × 8.7 μm, n = 50), overlapping 1–2-seriate, initially hyaline, becoming yellowish brown at maturity, broadly fusiform, lower part of spore longer, muriform, with 4–6 transverse septa and 3–4 vertical septa, constricted at the central septum, cells above central septum slightly swollen, weakly constricted at the other septa, ends conical, basal cell often lighter, lacking a mucilaginous sheath. Asexual morph: Undetermined.
Culture characteristics: Colonies on PDA reaching 30–35 mm diam. in 21 days, greenish-grey in the centre, with dirty white outer region, spreading with moderate aerial mycelium, and smooth, even margins; reverse rust coloured.
Material examined. Italy, Forlì-Cesena Province, Bleda in Santa Sofia, dead and hanging branches of Populus nigra (Salicaceae), 11 October 2013, E. Camporesi (MFLU 15-1504, holotype), ex-type living culture, MFLUCC 14-0651.
-
168.
Vagicola Chethana & K.D. Hyde, gen. nov.
= Phaeosphaeria subgen. Vagispora Shoemaker & Babcock, Can. J. Bot. 67: 1500–1599 (1989)
Index Fungorum number: IF551346; Facesoffungi number: FoF: 00908.
Etymology: The subgenus Vagispora is moved to genus rank.
Saprobic on grass stems. Sexual morph: Ascomata scattered, immersed, mostly subepidermal, broadly ellipsoidal to globose, smooth-walled. Papilla central, terete, flush to papillate, intraepidermal, composed of brown polygonal cells around ostiole, lacking periphyses. Peridium surface composed of cells of textura angularis, lateral layers uniform, thick, composed of brown cells of textura prismatica. Hamathecium composed of numerous, 1–3 μm wide, pseudoparaphyses, with thin septa, and with slime coating. Asci 8-spored, bitunicate, fissitunicate, cylindrical, pedicellate. Ascospores overlapping biseriate, yellowish brown, with L/W greater than or equal to 3, narrowly fusiform, enlarged at the third cell from the apex, multi-septate, straight or slightly curved, slightly constricted at the first septum, not constricted at other septa, with a prominent sheath (description modified from Shoemaker and Babcock (1989)). Asexual morph: Undetermined.
Type species: Vagicola vagans (Niessl) O. Eriksson, Chethana & K.D. Hyde
Notes: The subgenus Vagicola (Shoemaker and Babcock 1989) is raised to generic rank to accommodate Phaeosphaeria vagans. In various molecular studies (Zhang et al. 2012a; Hyde et al. 2013; Phookamsak et al. 2014), as well as in the present study (Fig. 40) a strain of this taxon (CBS 604. 86) clustered away from Phaeosphaeria sensu stricto. This strain was isolated from Calamagrostis arundinacea (Poaceae) in Sweden by K. & L. Holm. Other collections linked to this strain (CBS 604. 86) is ETH 9280. Another voucher material for this strain has been isolated from Ammophila arenaria (Poaceae), Skåne, Åhus par., Yngsjö, Sweden by K. & L. Holm on 15 April 1989. The herbarium material is stored in UPS herbarium (UPS:BOT:F–169873) and cultures are deposited at CBS 114119, UPSC 2908 and UPSC 2907.
-
169.
Vagicola vagans (Niessl) O. Eriksson, Chethana & K.D. Hyde, comb. nov.
Index Fungorum number: IF551347; Facesoffungi number: FoF: 00909.
Basionym: Pleospora vagans Niessl, Verh. Naturf. Ver. Briinn 14: 174. 1876
≡ Phaeosphaeria vagans (Niessl) O.E. Erikss., Ark. Bot. 6: 430 (1967)
Saprobic on grass stems. Sexual morph: Ascomata 130–180 μm high, 250–350 μm diam., scattered, immersed, subepidermal, smooth, brown mycelium ramifying in the host tissue, broadly ellipsoidal to globose, smooth-walled. Ostiole papillate, 15–30 μm long, 20–25 μm wide, central, terete, flush to papillate, intraepidermal, of 2–6 layers of brown polygonal 4–7 × 3–6 μm cells around 10–50 μm diam., ostiole without periphyses. Peridium surface composed of cells of textura angularis, lateral layers uniform, 12–18 μm thick, composed of brown, 3–6 layers of textura prismatica, 6–11 × 3–5 μm pseudoparenchymatous cells, thicker above the smaller darker cells. Hamathecium composed of numerous, 1–3 μm broad, paraphyses with thin septa at 10–to 30 μm intervals without guttules and with thin copious slime coating. Asci 60–110 × 12–20 μm, 8-spored, bitunicate, fissitunicate, numerous, cylindrical and short pedicellate. Ascospores 23–28 × 8–10 μm (L/W 3), overlapping biseriate, yellowish brown, narrowly fusiform, enlarged at the third cell from the apex, 5-septate, straight or slightly curved, slightly constricted at the first septum which is sub-median, not constricted at the other septa, smooth-walled, without guttules and with a prominent sharply delimited 2–8 μm mucilaginous sheath (description from Shoemaker and Babcock 1989). Asexual morph: Undetermined.
Illustrations: Shoemaker and Babcock 1989
Pleosporaceae Nitschke
Multi-gene phylogenetic analyses have shown that the familial placement of Pleosporaceae with respect to other families in order Pleosporales is valid (Hyde et al. 2013; Ariyawansa et al. 2015). Taxa in Alternaria, Bipolaris, Stemphylium and phoma-like species are more common asexual morphs in Pleosporaceae and can be saprobic or parasitic on various hosts (Ariyawansa et al. 2015). Ariyawansa et al. (2015) accepted 22 genera in the family based on the analysis of combined 18S nrDNA, 28S nrDNA, ITS, GAPDH, RPB2 and EF sequence data.
Alternaria Nees, Syst. Pilze (Würzburg): 72 (1816) [1816–17]
Notes: Alternaria was introduced by Nees (1816) and is a ubiquitous genus that comprises saprobic, endophytic and pathogenic species, associated with a wide variety of substrates (Woudenberg et al. 2013; Ariyawansa et al. 2015). Based on analysis of combined GAPDH, RPB2 and EF sequence data, Woudenberg et al. (2013) and Ariyawansa et al. (2015) concluded that the Alternaria clade contains 24 internal clades and six monotypic lineages, the grouping of which are recognised as Alternaria.
Alternaria ethzedia was introduced by Simmons (1986) and named as the sexual morph Lewia ethzedia. Our strain (MFLUCC 13–0404) groups together with the type strain of Alternaria ethzedia (CBS 197.86) in section Infectoriae and our collection is from the same host genus (Brassica) as the holotype. Therefore we treat our isolate as A. ethzedia (≡ Lewia ethzedia) and illustrate the sexual morph here which has not been previously well-illustrated. Alternaria ethzedia is similar to A. murispora Ariyawansa & K.D. Hyde in having (4–6–)8-spored asci, ellipsoid to fusoid, muriform, brown ascospores with a mucilaginous sheath, but they differ as A. murispora has a two-layered peridium with heavily pigmented to hyaline cells and pale, dark brown ascospores which are constricted at the three major septa (Fig. 51).
Phylogram generated from Maximum Likelihood (RAxML) analysis based on combined LSU, ITS, EF and RPB2 sequence data of Pleosporaceae. Maximum likelihood bootstrap support values greater than 50 % are indicated above and below the nodes, and the ex-type strains are in bold; the new isolates are in blue. The tree is rooted with Pleospora herbarum (CBS 191.86)
-
170.
Alternaria ethzedia E.G. Simmons, Mycotaxon 25(1): 300 (1986)
≡ Lewia ethzedia E.G. Simmons, Mycotaxon 25(1): 299 (1986)
Facesoffungi number: FoF00918; Fig. 52
Saprobic on dead stem. Sexual morph: Ascomata 96–162 μm high × 138–226 μm diam. (\( \overline{x} \) = 131 × 193 μm, n = 10), scattered to clustered, semi-immersed to erumpent, dark brown to black, globose to subglobose, coriaceous, ostiolate. Ostiole central, papillate. Peridium 20–38 μm (\( \overline{x} \) = 26.8 μm, n = 10) wide, comprising 3–5 layers of light to dark brown thick-walled cells of textura angularis. Hamathecium of 1.5–3 μm wide, cellular, septate pseudoparaphyses, anastomosing between and above asci. Asci 73–90 × 11.5–13.5 μm (\( \overline{x} \) = 82 × 12.5 μm, n = 20), (4–6–)8-spored, bitunicate, fissitunicate, cylindrical to subcylindrical, straight or somewhat curved, with a short pedicel, apically rounded, with a minute ocular chamber. Ascospores 15.5–20 × 7–8.5 μm (\( \overline{x} \) = 18 × 7.8 μm, n = 30), overlapping uni to biseriate, pale brown, ellipsoid to fusoid, muriform, initially with 3 transverse septa, become 4–5 at maturity, central 4 rows with a single longitudinal septum, constricted at the central septum, slightly constricted at the other septa, smooth-walled, surrounded by a mucilaginous sheath. Asexual morph: see asexual morph description in Simmons (1986).
Material examined: ITALY, Forlì-Cesena Province, Fiumicello di Premilcuore, on dead stem of Brassica nigra (Brassicaceae), 13 January 2013, Erio Camporesi IT 1007 (MFLU 15–1411), ex-type living culture, MFLUCC 13–0404, CFTCC.
Roussoellaceae J.K. Liu et al.
Roussoellaceae was introduced by Liu et al. (2014) and is characterized by immersed, gregarious, clypeate, ascostromata, cylindrical, bitunicate, asci and brown, 2-celled, ornamented ascospores. Wijayawardene et al. (2014) included five genera, viz. Appendispora, Cytoplea, Neoroussoella, Roussoella and Roussoellopsis, in this family and in this paper we introduce a new sexual genus, Elongatopedicellata, based on morphology and phylogeny (Figs. 1 and 53). Members of Roussoellaceae mostly occur on monocotyledons such as bamboo and palms (Liu et al. 2014). Crous et al. (2014) however, described a new species, Roussoella acacia Crous & M.J. Wingf., from Acacia. The asexual morphs of this family are linked to Cytoplea, Melanconiopsis and Neomelanconium (Liu et al. 2014). These three genera have aseptate conidia according to Sutton (1980), whereas in this study, two new asexual morph species of Roussoella with 1-septate conidia are described and illustrated. A phylogenetic tree, based on combined SSU, LSU and EF sequence data for the genus Roussoella, is presented in (Figs. 1 and 53).
Phylogenetic tree generated from maximum likelihood (lnL = −2561.341099) revealed by RAxML (GTR + G model) based on combined SSU, LSU and EF sequence data. MP/ML values (>50 %) resulting from 1000 bootstrap replicates are given at the nodes, and branches with Bayesian posterior probabilities greater than 0.90 are given in bold. The original isolate codes are noted after the species names. The tree is rooted to Lindgomyces angustiascus (G202_1a2). Ex-type strains are in bold, new isolates are in blue
-
171.
Elongatopedicellata J.F. Zhang, J.K. Liu, K.D. Hyde & Z.Y. Liu, gen. nov.
Index Fungorum number: IF551484; Facesoffungi number: FoF00959
Etymology: in reference to the Latin, to asci which have a long pedicel.
Saprobic on the dead wood. Sexual morph: Ascomata solitary to gregarious, scattered, immersed or erumpent, uni-loculate, subglobose to obpyriform, coriaceous, with papillate ostiole. Peridium 14–21 μm wide, composed of several layers of brown to dark brown, thick-walled cells, arranged in a textura angularis. Hamathecium composed of 1–2 μm wide, filiform pseudoparaphyses, anastomosing between and above the asci, embedded in a gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate, fusiform-clavate, with a long pedicel, apically rounded, with a well-developed ocular chamber. Ascospores 1–3 overlapping seriate, hyaline, fusiform, 1-septate, constricted at the septum, upper cell shorter and wider, lower cell long and narrow. Asexual morph: Undetermined.
Type species: Elongatopedicellata lignicola J.F. Zhang, J.K. Liu, K.D. Hyde & Z.Y. Liu
Notes: This taxon is morphologically typical of Didymosphaeria, however, the phylogenetic analysis showed that the new taxon has a close relationship with Roussoellaceae (Fig. 1). Currently, Roussoellaceae comprises the genera Neoroussoella, Roussoella and Roussoellopsis. Phylogenetically, this taxa differs from Roussoellaceae in having cellular pseudoparaphyses and unequal ascospores surrounded with a gelatinous sheath, while Roussoellaceae has trabeculate pseudoparaphyses and brown, 2-celled, ornamented ascospores (Liu et al. 2014). We therefore introduce a new genus Elongatopedicellata in Roussoellaceae to accommodate E. lignicola.
-
172.
Elongatopedicellata lignicola J.F. Zhang, J.K. Liu, K.D. Hyde & Z.Y. Liu, sp. nov.
Index Fungorum number: IF551485; Facesoffungi number: FoF00960 Fig. 54
Elongatopedicellata lignicola (holotype) a, b Appearance of ascomata on host surface c Section of peridium d Vertical section through ascoma e Pseudoparaphyses f Germinating spore g–j Asci k–o Ascospores p Ascospore in Indian ink showing sheath. Scale bars: b = 200 μm, c, h, j = 10 μm, d = 50 μm, e–g, i, k–p = 5 μm
Etymology: from the Latin lignin and cola, in reference to its habit on wood.
Holotype: MFLU 15-2717
Saprobic on the dead wood. Sexual morph: Ascomata 294–387 μm high (including neck), 187–225 μm diam., solitary to gregarious, scattered, immersed or erumpent, uni-loculate, subglobose to obpyriform, coriaceous, visible on host surface as raised, dark spots, with papillate ostiole. Ostiole central, with pore-like opening and papilla to long neck. Peridium 14–21 μm wide, of unequal thickness, slightly thin at the base, composed of several layers of brown to dark brown, thick-walled cells, arranged in a textura angularis. Hamathecium composed of 1–2 μm wide, filiform pseudoparaphyses, anastomosing between and above the asci, embedded in a gelatinous matrix. Asci (59.5–)63–132(−137) × (9.5–)10–12.5(−13.5) μm (\( \overline{\mathrm{x}} \) = 98.7 × 12.2 μm, n = 25), 8-spored, bitunicate, fissitunicate, fusiform-clavate, with a long foot-like or knob-like pedicel, apically rounded, with a well-developed ocular chamber. Ascospores (18.5–)19–21(−22) × (4.9)–5.2–6(−6.3) μm (\( \overline{\mathrm{x}} \) = 20.3 × 5.8 μm, n = 30), 1–3 overlapping seriate, hyaline, fusiform with narrow ends, 1-septate, constricted at the septum, upper cell shorter and wider, lower cell long and narrow, slightly curved, surrounded by a mucilaginous sheath. Asexual morph: Undetermined.
Material examined: THAILAND, Chiang Rai, Muang District, Mae Chang Hot Spring, on dead branch, 25 November, 2014, JF Zhang (MFLU 15-2717, holotype); ex-type living culture, MFLUCC 15-0642, CFTCC.
Culture characteristics: Colonies on PDA fast growing, reaching 35–38 mm diam. after 2 weeks at 25 °C, cream to pale gray at the margin, buff pigment produced at the center, reverse cream to pale yellowish white at the margin, yellowish to pale reddish at the center, slighting radiating, medium dense, raised, dull with undulate edge, fairy fluffy at the margins, separating from agar.
-
173.
Roussoella magnatum D.Q. Dai & K.D. Hyde, sp. nov.
Index Fungorum number: IF551342; Facesoffungi number: FoF00902; Fig. 55
Roussoella magnatum (holotype) a Conidiomata on bamboo culms b, c Conidiomata in culture d Conidiomata after cutting away top part e Conidiogenous inner layer f, g Conidiogenous cells h–j Paraphyses k–n Conidia o Germinated conidium p Conidia q, r Cultures on PDA after 60 days. Scale bars: a–c = 1 mm, d = 100 μm, e = 50 μm, f–k, m, n = 10 μm, l = 5 μm
Etymology: The he Latin, magna, meaning large, in reference to the large conidiomata.
Holotype: MFLU 15–1213
Saprobic on bamboo culms, forming dark spots on the host surface, with pustule-like raised conidiomata. Sexual morph: Undetermined. Asexual morph: Conidiomata 2–3 mm long, 1–2 mm wide, 300–500 μm high, eustromatic, immersed in the host tissue, partly erumpent when mature, solitary, scattered, multi-loculate, ellipsoid to wide-fusiform, coriaceous, with fissure-like ostiole. Conidiomatal wall thin, 15–30 μm wide, with dark brown to hyaline, conidiogenous, inner layer, comprising cells of textura angularis. Paraphyses 45–80 μm long, 2–5.5 μm wide, hyaline, septate, straight to flexuous, wide at base. Conidiophores 6–15.5 × 4–7 μm (\( \overline{x} \) = 12.1 × 5.1 μm, n = 10), cylindrical, subglobose to irregular, smooth, hyaline, occasionally branched. Conidiogenous cells 5–22 × 2.5–7 μm (\( \overline{x} \) = 12.6 × 4.8 μm, n = 20), enteroblastic, phialidic, cylindrical to subglobose, or ampulliform, determinate, discrete, smooth, hyaline. Macroconidia 16.5–21 × 5.5–6 μm (\( \overline{x} \) = 18.9 × 5.6 μm, n = 20), pale brown to dark brown, 1-septate, cylindrical, obtuse at both ends, occasionally truncate at the lower end, smooth to slightly warty, straight, with many guttules. Microconidia 2.5–4 × 1.5–2.5 μm (\( \overline{x} \) = 3.4 × 2.1 μm, n = 20), subglobose, hyaline, smooth, guttulate.
Culture characteristics: Ascospores germinating on PDA within 24 h and germ tubes produced from lower cell. Colonies growing slowly on PDA, reaching 5 mm in 60 days at 28 °C, circular, floccose, with uneven margin, white from above, yellowish brown to dark brown from below. Mycelium immersed and superficial in the media, composed of branched, septate, smooth-walled, hyaline aerial hyphae and verrucose, dark brown hyphae near or within the media, producing black conidial masses and subglobose to irregular conidiomata with multi-locules in the center and at the margin.
Material examined: THAILAND, Phang-Nga, Doi Nang Hong, Tham Thong Lang, Thap Put District, 8°32′11″N 98°33′35″E, on dead culm of bamboo, 6 December 2014, K.D. Hyde DDQ00282 (MFLU 15–1213, holotype); Ibid. (KUN, HKAS 88720, isotype), ex-type living culture at MFLUCC 15–0185, KUMCC.
Notes: Roussoella magnatum is characterized by large, black, eustromatic, ellipsoid to wide fusiform conidiomata, and dark, warty, 1-septate conidia. This new taxon forms 2–3 × 1–2 mm conidiomata on the host. Such large conidiomata are not so far observed in any other species of Roussoella (Hyde et al. 1996; Liu et al. 2014).
-
174.
Roussoella angustior D.Q. Dai & K.D. Hyde, sp. nov.
Index Fungorum number: IF551344; Facesoffungi number: FoF00903; Fig. 56
Roussoella angustior (holotype) a–c Conidiomata on bamboo culms d Conidiomata after cutting away top part e, f Conidial masses on PDA g Conidiogenous inner layer h–k Paraphyses and conidiogenous cells l, m Conidia n Germinating conidium o Micro-conidia p, q Cultures on PDA after 60 days. Scale bars: a = 5 mm, b, e, f = 1 mm, c, d = 500 μm, g = 50 μm, h–o = 10 μm
Etymology: Refers to the narrow conidia.
Holotype: MFLU 15–1214
Saprobic on bamboo culms, forming darkened spots on the host surface with pustule-like raised conidiomata. Sexual morph: Undetermined. Asexual morph: Conidiomata 1–2.5 mm long, 0.8–1.2 mm wide, 300–500 μm high, eustromatic, immersed in the host tissue, partly erumpent when mature, solitary, scattered, multi-loculate, ellipsoid to wide fusiform, coriaceous. Conidiomatal wall 15–30 μm wide, with dark brown to hyaline conidiogenous inner layer, comprising cells of textura angularis. Paraphyses 15–35 μm long, 2–3.5 μm wide, hyaline, septate, straight to curved. Conidiophores 2–6.5 × 2.5–3.5 μm (\( \overline{x} \) = 4.4 × 3.2 μm, n = 10), short, cylindrical, smooth, hyaline. Conidiogenous cells 2–10 × 2.5–3.5 μm (\( \overline{x} \) = 5.9 × 3.3 μm, n = 20), enteroblastic, phialidic, cylindrical to ampulliform, determinate, discrete, smooth, hyaline. Macroconidia 17.5–24 × 3.5–5 μm (\( \overline{x} \) = 19.6 × 4.2 μm, n = 20), pale brown to dark brown, 1-septate, cylindrical, obtuse at both ends, occasionally truncate at lower end, smooth, straight, with many guttules. Microconidia 3.5–4.5 × 1.5–2.5 μm (\( \overline{x} \) = 4.1 × 2.2 μm, n = 20), subglobose, hyaline, smooth, guttulate.
Culture characteristics: Ascospores germinating on PDA within 24 h and germ tubes produced from lower cell. Colonies growing slowly on PDA, reaching 7 mm in 60 days at 28 °C, circular, floccose, with uneven margin, white from above, yellowish brown to dark brown from below. Mycelium immersed and superficial in the media, composed of branched, septate, smooth-walled, hyaline aerial hyphae and verrucose, dark brown hyphae near or within the media, producing a few black conidial masses.
Material examined: THAILAND, Phang-Nga, Doi Nang Hong, Tham Thong Lang, Thap Put District, 8°32′11″N 98°33′35″E, on dead culm of bamboo, 6 December 2014, K.D. Hyde DDQ00286 (MFLU 15–1214, holotype); Ibid. (KUN, HKAS 88721, isotype), ex-type living culture at MFLUCC 15–0186, KUMCC.
Notes: This new taxon is morphologically and phylogenetically close to Roussoella magnatum in having black, ellipsoid to wide fusiform conidiomata, hyaline, septate pseudoparaphyses and brown, cylindrical, 1-septate conidia. However, R. angustior has narrower (19.6 × 4.2 μm vs. 18.9 × 5.6 μm) and smooth-walled conidia. These two species are phylogenetically separated by the combined genes analyses (Figs. 1 and 53). Roussoella species usually produce short (not more than 15 μm long), oblong to ellipsoidal conidia (Hyde et al. 1996; Liu et al. 2014). The new species collected by us from bamboo has longer (more than 16.5 μm long), cylindrical conidia. (Crous et al. 2014) described R. acacia with 7–10 × 4–5 μm conidia from Acacia. Moreover, in these two new taxa, R. magnatum and R. angustior, microconidia and macroconidia are formed in the same conidiomata. Such characters are not reported in other species in the genus Roussoella (Hyde et al. 1996; Crous et al. 2014; Liu et al. 2014).
Tetraplosphaeriaceae Kaz. Tanaka & K. Hiray.
The family Tetraplosphaeriaceae was introduced by Tanaka et al. (2009). Members of the family are mainly isolated from bamboo or grasses and include Polyplosphaeria, Pseudotetraploa, Quadricrura, Tetraplosphaeria and Triplosphaeria. Members of this family are characterized by immersed to superficial, globose to subglobose ascomata, cellular or trabeculate pseudoparaphyses, cylindrical to clavate asci, and narrowly fusiform to broadly cylindrical, 1–3-septate, hyaline to pale brown ascospores, surrounded by an entire mucilaginous sheath or narrow appendage-like sheath. A phylogenetic tree for the family is presented in Figs. 57 and 58.
One of three MPTS inferred from combined SSU and LSU rDNA sequence data of Dothideomycetes, generated with maximum parsimony. Maximum parsimony (BSMP, left) and likelihood (BSML, right) bootstrap values >50 % are given above the nodes. Bayesian posterior probabilities >0.95 are given below each node (BYPP). The internodes that are highly supported by all bootstrap (100 %) and posterior probabilities (1.00) are shown as a thicker line
One of 26 MPTS inferred from ITS rDNA sequence data from Tetraplosphaeriaceae generated with maximum parsimony. Maximum parsimony (BSMP, left) and likelihood (BSML, right) bootstrap values >50 % are given above the nodes. Bayesian posterior probabilities >0.95 are given below each node (BYPP). The internodes that are highly supported by all bootstrap (100 %) and posterior probabilities (1.00) are shown as a thicker line
The genus Shrungabeeja was established by Rao and Reddy (1981), and was collected from dead culms of Bambusa sp. and three species (S. begonia K. Zhang & X.G. Zhang, S. melicopes K. Zhang & X.G. Zhang and S. vadirajensis V.G. Rao & K.A. Reddy) were accepted by Zhang et al. (2009d). This genus is typified by S. vadirajensis. The genus is characterised by effuse, dark, hairy colonies, distinct, dark coloured conidiophores and integrated, monoblastic, lageniform, conidiogenous cells that produce solitary, aseptate, subglobose or turbinate conidia with filiform or horn-like appendages.
-
175.
Shrungabeeja longiappendiculata Sommai, Pinruan, Nuankaew & Suetrong, sp. nov.
Index Fungorum Number: IF551322; Facesoffungi number: FoF00967; Fig. 59
Shrungabeeja longiappendiculata (holotype) a, b Colony on PDA, a from above, b from below c Sporulation on PDA d Germinating conidium (arrowed) e Colonies on natural substratum f Conidiophore with mature conidium g Conidiophore with developing conidium h Conidiophore showing percurrent proliferation (arrowed) i–l Conidia with appendages. Scale bars: a–c = 10 mm, d, g, h, i–l = 50 μm, e–f = 10 μm
Etymology: longiappendiculata referring to the long appendages.
Holotype: BBH 39513
Saprobic on dead culm of Bambusa sp. in evergreen forest. Colonies effuse, brown to dark brown. Mycelium partly superficial, partly immersed in the substratum, composed of branched, septate, pale brown, smooth-walled hyphae, 2–4 μm wide. Sexual morph: Undetermined. Asexual morph: Conidiophores macronematous, mononematous, erect, straight or flexuous, unbranched, smooth, thick-walled, 132.5–225 μm long, 11.25–15 μm wide at the base, 6.25–10 μm wide in the middle, up to 5 μm wide at the apex, 3–4-septate. Conidiogenous cells monoblastic, terminal, lageniform, determinate or percurrent, pale brown to brown, smooth, 12.5–25 μm long, 6–7.5 μm broad at base, up to 5–7.5 μm. broad at apex. Conidia solitary, dry, acrogenous, subglobose or turbinate, aseptate, hollow, pedicellate, with 4–7 filiform appendages in the anterior region which are continuous or sometimes 4–21-septate, smooth, pale brown to brown, conidia 32.5–50 μm long, 40–60 μm wide, 5–7.5 μm broad at the base, broader than long, appendages (47.5–82.5) 100–360 (400–480) μm long, 5–7.5 μm broad at base, and 1.25 μm at apex.
Culture characteristics: Colonies on PDA attaining 4–4.5 cm diam. after 20 days at room temperature (25 °C), cottony, grey, with entire margin, reverse almost black, no pigment produced, sporulating.
Material examined: THAILAND, Nakhon Nayok Province, on dead culm of Bambusa sp. (Poaceae), 3 September 2014, S. Sommai (BBH 39513, holotype); ex-type living culture, BCC 76463.
Notes: Shrungabeeja species have dark conidiophores with integrated, terminal, monoblastic, determinate or percurrent conidiogenous cells and lageniform, acrogenous, solitary, aseptate, globose or turbinate conidia, with filiform or horn-like appendages (Rao and Reddy 1981). There are no known sexual morphs. Shrungabeeja longiappendiculata differs from all species of Shrungabeeja as it has 40–60 × 32.5–50 μm conidia, with 4–7 very long appendages. Maximum parsimony, likelihood and Bayesian analyses of combined SSU and LSU gene region of 80 taxa from the Dothideomycetes place Shrungabeeja longiappendiculata in Tetraplosphaeriaceae with Tetraplosphaeria and Pseudotetraploa species with 63 % BSMP, 78 % BSBL and 1.00 B.P. (Fig. 57). ITS rDNA gene analysis shows two isolates of Shrungabeeja longiappendiculata clustered with species of Triplosphaeria and Quadricrura and Polyplosphaeria fusca in Tetraplosphaeriaceae (Fig. 58). Based on the above morphological and molecular characteristics, Shrungabeeja longiappendiculata is introduced as a new species and placed in Tetraplosphaeriaceae.
Thyridariaceae Q. Tian & K.D. Hyde
This family was introduced by Hyde et al. (2013).
-
176.
Massariosphaeria (E. Müll.) Crivelli, Diss. Eidgenöss. Techn. Hochschule Zürich 7318: 141 (1983)
Type species: Massariosphaeria phaeospora (E. Müll.) Crivelli, Ueber die Heterogene Ascomycetengattung Pleospora Rabh.; Vorschlag für eine Aufteilung (Diss. Eid genössischen Technischen Hochschule Zürich 7318): 141 (1983)
Basionym: Leptosphaeria phaeospora E. Müll., Sydowia 4(1–6): 208 (1950)
Notes: The genus Massariosphaeria was established by Müller (1950) as a section of Leptosphaeria based on its large, thick-walled ascospores, with a mucilaginous sheath, as well as its ascomata with a thick apex. Crivelli (1983) treated Massariosphaeria as a separate genus characterized by its wide peridial apex, comprising thick-walled cells, compressed to round papilla, and relatively large, thick-walled, reddish brown to brown, multi-septate to dictyosporous ascospores, usually surrounded by a sheath (Crivelli 1983; Huhndorf et al. 1990; Leuchtmann 1984). Crivelli (1983) noted that species of Massariosphaeria often stain the woody substrate (or culture) purple.
Recent studies based on multigene phylogenies revealed that Massariosphaeria is not monophyletic and results are in disagreement with existing morphological-based classification schemes (Wang et al. 2007). Characters, such as ascomata shape and ascospore morphology and staining the woody substrate (or culture) purple have evolved more than once within the families of Pleosporales (Abdel-Aziz and Abdel-Wahab 2010; Shoemaker and Babcock 1989; Tanaka and Harada 2004; Wang et al. 2007; Zhang et al. 2009a, b, 2012a, b).
Based on DNA sequence data in combination with a review of literature, as well as the present study (Fig. 1), we conclude that the generic type of Massariosphaeria, M. phaeospora (E. Müll.) forms a well-supported clade within the family Thyridariaceae (Hyde et al. 2013; Zhang et al. 2009b, 2012a, b). Therefore we place Massariosphaeria in Thyridariaceae. Other species in this genus need to be sequenced to establish their affinities.
-
177.
Wicklowiaceae Ariyawansa & K.D. Hyde, fam. nov.
Index Fungorum number: IF551445; Facesoffungi number: FoF00966
Saprobic on submerged decorticated woody debris in aquatic habitats. Sexual morph: Ascomata immersed, becoming erumpent, solitary to gregarious, appearing as a black, oval to circular, shallow, crater-like depressions on the substrate, subglobose, ostiolate. Peridium comprising 4–5 layers of small pseudoparenchymatous cells, arranged in a textura angularis, fusing at the outside with the host cells. Hamathecium of dense, septate, cellular pseudoparaphyses, embedded in a gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate, broadly clavate, pedicellate, rounded at the apex, with a wide, shallow, ocular chamber. Ascospores overlapping 2–3-seriate, hyaline, ellipsoidal, 1-septate, slightly constricted at the septum, surrounded by a gelatinous sheath that expands in water; with a gelatinous curtain extending downwards from the base, that fragments into filamentous appendages, forming a subapical fringe. Asexual morph: Undetermined.
Type genus: Wicklowia Raja et al., in Raja et al., Mycoscience 51(3): 210 (2010).
Notes: The new family Wicklowiaceae is introduced here to accommodate the monotypic freshwater ascomycetous genus Wicklowia (Raja et al. 2010). The genus is characterized by subglobose, immersed to erumpent, black ascomata, a peridium composed of 4–5 layers of darkened pseudoparenchymatous cells and cellular pseudoparaphyses in a gelatinous matrix with broadly clavate asci bearing cylindrical, hyaline, one-septate ascospores having rounded apices and surrounded by a gelatinous sheath (Raja et al. 2010). Raja et al. (2010) placed the genus in the order Pleosporales based on 28S rDNA sequence data, without assigning it to any family.
The strains of Wicklowia aquatica (F76-2T and AF289-1) form a well-supported clade basal to the familial clade of Lindgomycetaceae (Fig. 1). A similar topology was shown in the study of Schoch et al. (2009), Zhang et al. (2012a), Hyde et al. (2013). We therefore introduce a new family to accommodate this distinct lineage.
Type: Wicklowia Raja et al., in Raja et al., Mycoscience 51(3): 210 (2010).
Type species: Wicklowia aquatica Raja et al., in Raja et al., Mycoscience 51(3): 210 (2010)
Eurotiomycetes
Mycocaliciaceae A.F.W. Schmidt
Mycocaliciaceae is a family of calicioid fungi and a heterogeneous grouping of ascomycetes included in the order Mycocaliciales with Mycocalicium as the type genus (Angeles Vinuesa et al. 2001). This family represents ascomycetes, with small, usually stalked apothecia, where both lichenized and non–lichenized species are involved (Rikkinen 2003). This family includes Chaenothecopsis, Mycocalicium, Phaeocalicium and Stenocybe (Lumbsch and Huhndorf 2010). The members of Mycocaliciaceae are saprophytic, parasitic on free-living algae or lichens, or parasymbiotic with lichens. Most species inhabit the bark of old trees or wood and occur in cool-temperate and temperate regions, but some occur in subtropical and tropical areas (Tibell and Titov 1995) (Fig. 60).
Phylogram inferred from likelihood analysis of genus Mycocalicium using ITS sequence data. Strain/culture numbers are given following the taxon names. The bootstrap support values from likelihood analysis >70 % from 1000 RAxML replicates are shown above the branches. The tree is rooted with Chaenothecopsis nana and C. pusilla (out group). The new species is highlighted in blue
Mycocalicium Vain., Acta Soc. Fauna Flora fenn. 7(no. 2): 182 (1890)
The genus was introduced by Vainio (1890) to accommodate species from Calicium which are not lichenized. Schmidt (1970) emended the generic concepts in the Mycocaliciaceae, thus characterized Mycocalicium as having non-septate spores, short asci, periclinal hyphae in the ascoma stalk, and an evenly thickened ascus apex. However with the discovery of more species this characterization was not consistent and a delimitation of Mycocalicium from Phaeocalicium and Chaenothecopsis as suggested by Schmidt was not reliable (Angeles Vinuesa et al. 2001). There are 52 epithets listed in Mycocalicium (Index Fungorum 2015).
-
178.
Mycocalicium hyaloparvicellulum Daranagama & K. D. Hyde, sp. nov.
Index Fungorum number: IF551202; Facesoffungi number: FoF00707; Figs. 61 and 62
Mycocalicium hyaloparvicellulum (holotype) a, b Habit on wood c, d Vertical section of apothecium showing the exciple e, f Ascospores in water with hyaline dwarf cell at the side of the spore g Ascus in water h Ascus in Melzer’s reagent showing apical thickening. Scale bars: a = 1 cm, b = 1500 μm, c, d = 500 μm, e–h = 10 μm
Mycocalicium hyaloparvicellulum (ex-type culture) Colony on OA after 4 weeks a Reverse of the colony b Oil droplets deposited on the mycelium masses c Sporulating regions after 14 days d Sporulating regions after 21 days e, f Sporulating regions after 30 days g, h Development of conidiophores i, j Conidiogenous cells k Immature conidial chains l Mature conidiogenous cells with attached conidia m Conidial chains. Scale bars: g–m = 10 μm
Etymology: Refers to the hyaline small celled ascospores; hyalo = hyaline; parvus = small; cellula = cell.
Holotype: MFLU 15-0667
Saprobic on cone of Pinus halepensis Miller. Sexual morph: Thallus crustaceous, solitary, erumpent, consist with a head and stipe. Apothecia 1000–1100 μm high (\( \overline{x} \) = 1083 μm, n = 10), black, subglobose. Capitulum shiny, black, lenticular to subsphaerical, often irregular, with a granular surface. Hypothecium 80–95 μm wide (\( \overline{x} \) = 88 μm, n = 10), dark brown to olivaceous brown. Excipulum 15–26 μm wide (\( \overline{x} \) = 21 μm, n = 10), light brown, poorly–developed, arranged as a continuation of the outer layers of the stalk, comprising a few cell layers of textura irregulata, darker than the hyphae from the stalk. Stalk 1300–1500 μm high (\( \overline{x} \) = 1487 μm, n = 10), brown to pale brown, consisting of light brown periclinal parallel hyphae, with a gelatinous coat. Asci 56–83 × 4.3–5.8 μm (\( \overline{x} \) = 72.5 × 4.8 μm, n = 20), 8–spored, unitunicate, cylindrical, short pedicellate, apex strongly thickened, round, inconspicuously bluing in Meltzer’s reagent, apical apparatus, globose. Ascospores 10–15 × 4–6 μm (\( \overline{x} \) = 12.9 × 5.3 μm, n = 20), uniseriate, ellipsoidal, one pointed end and rounded end, light brown, with a hyaline dwarf cell at one side (upper side), smooth-walled, with thin mucilaginous sheath. Asexual morph: Conidiophores 55–80 × 2.6–3.4 μm (\( \overline{x} \) = 77 × 3.1 μm, n = 20), some are longer, emerging through the thick mycelium, which formed into hyphal aggregates, immature conidiophores hyaline, becoming light brown at maturity, complex, dichotomously branched, septate. Conidiogenous cells 10–14 × 3.2–4.5 μm (\( \overline{x} \) = 12.9 × 4.3 μm, n = 20), terminal, single, cylindrical and elongated, with smoothly curved apex. Conidia 5.2–7.5 μm diam. (\( \overline{x} \) = 6.3 μm, n = 20), light brown, globose, always occurs as conidial chains of 4–5 conidia, rarely observed single conidia.
Culture characteristics: Colonies on Difco OA plates reaching 5 cm edge Petri dish in 1 month at 25–28 °C, at first citrine or grey olivaceous, felty, zonate, with diffuse margins, after three weeks become olivaceous and isebelline and zonate; reverse turning brown and then to dark brown almost black, zonate.
Material examined: ITALY, Cosenza Province, Rocca Imperiale, on cone of Pinus halepensis (Pinaceae), 5 April 2013, Lugaresi Renzo (MFLU 15-0667, holotype), living culture, MFLUCC 14-0169.
Notes: Mycocalicium hyaloparvicellulum has a close relationship with Mycocalicium subtile (Pers.) Szatala, but differs in apothecium size, colour and structure of stalk, ascus size and spore dimensions and colour (Muniz and Hladun 2007). Like Mycocalicium llimonae, M. hyaloparvicellulum was also isolated from a cone of Pinus halepensis. Mycocalicium llimonae is a saprobe on pine cones on dry branches of Pinus halepensis and several other Pinus spp. (Muniz and Hladun 2007). Mycocalicium llimonae differs from M. hyaloparvicellulum in having smaller apothecia with hyaline stalks. In the phylogenetic analysis (Fig. 60), M. hyaloparvicellulum is a distinct species with high bootstrap support within the Mycocalicium clade.
Lecanoromycetes
Acarosporaceae Zahlbr.
Two major phylogenetic studies covering this group were published recently (Miadlikowska et al. 2014; Westberg et al. 2015). The phylogeny presented in Fig. 63 is based on the matrix of Westberg et al. (2015), where citations of samples and sequences used can be found. The two new species described here all belong to the Acarospora glaucocarpa group (including e.g., A. cervina and A. badiofusca), which is not part of Acarospora in the strict sense (type: A. schleicheri). The morphologically defined A. glaucocarpa group is nested with species currently placed in Sarcogyne. The new species are provisionally placed in Acarospora according to the current morphological concept of the genus, but will likely not be classified here in the future. A generic revision is, however, not possible at this stage.
Phylogram generated from maximum likelihood analysis (implemented in RAxMLGUI 1.3) based on combined ITS, nuLSU, mtSSU and beta-tubulin sequenced data of the family Acarosporaceae. Maximum likelihood bootstrap values ≥70 % are indicated over the branches. Newly described species are in blue. The tree is rooted with Pycnora sorophora
-
179.
Acarospora septentrionalis M. Westb. & Wedin, sp. nov.
MycoBank number: MB 813043; Facesoffungi number: FoF00927; Fig. 64a–e
a–e Acarospora septentrionalis a Norway, Westberg 08-148 (S holotype) b Iceland, Fröberg (LD) c Sweden, Westberg 3064 (LD) d, e (holotype) Section through thallus showing continuous algal layer, a paraplectenchymatous cortex and thick, uneven epinecral layer f–i Acarospora castaneocarpa f–i Finland, Pykälä 26880 (H holotype) g, h Section through apothecium showing dense layer of crystals in the cortex and anatomy of the true exciple i Spores. Scale bars: a–c, f = 1 mm, c = 50 μm, e = 10 μm, g = 50 μm, h–i = 10 μm. Photographs by M. Westberg
Etymology: In reference to the northern distribution of the species.
Holotype: NORWAY, Sogn og Fjordane, Årdal, along Tindeveien between Øvre Årdal and Turtagrø, UTM (WGS84) 32V 0431788 6807104, alt. 984 m, on cement, 12 June 2008, Martin Westberg 08-148 (S F132535).
Synonym: Acarospora badiofusca v. glaucocarpoides H. Magn., Göteborgs Kungl. Vetensk. Samhälles handl., ser. 4, 28: 117 (1924). Type: Sweden. Torne Lappmark, Jukkasjärvi par., prope Torne träsk, Jebrinjokk, 600 m. 16 July 1919, Magnusson 3310 (UPS L-522388, lectotype, here designated).
Lichenized, photobiont a chlorococcoid alga. Thallus epilithic, areolate to rarely subsquamulose; areoles rounded at first, becoming irregular in outline, to 0.55 mm thick, 0.3–0.8(−1.5) mm across, the largest becoming subsquamulose with edges raised from the substrate; upper surface pale brown to medium brown, smooth, mat, epruinose, but sometimes with large, pruina-like flakes formed by detaching epinecral layer; epinecral layer present, colourless, 0–40 μm thick; cortex, paraplectenchymatous, 25–60 μm thick, uppermost cell-layer reddish brown, below colourless, cells distinct, rounded to elongated or somewhat irregular, lumina up to 7 μm across; algal layer continuous; medulla white, hyphae with rounded to elongated, thin-walled cells, lower cortex lacking. Apothecia lecanoroid, at first immersed with a sunken disc, soon expanding and becoming raised and often darker brown than the thallus (in a similar way to A. badiofusca); disc, brown, at first concolourous with the thallus, later often becoming darker brown, plane, round, epruinose, 0.3–1 mm diam.; true exciple at first invisible from the outside, becoming distinct in later stages and then concolourous with the disc, I–, in section prosoplectenchymatous, below the hymenium composed of elongated, narrow, rather indistinct cells forming a very compact layer, to c. 25 μm thick, towards the surface expanding, becoming fan-shaped and composed of small, rounded to somewhat elongated cells, the uppermost cells rounded, 2–4 μm wide, laterally merging with the cortex; epithecium, medium brown–reddish brown; hymenium colourless, I+ blue, 65–90 μm tall; paraphyses, easily distinguished in water, 1.5–2 μm wide in mid hymenium, tips cylindrical to weakly clavate, rarely up to 5 μm wide; subhymenial layers colourless, wedge-shaped, up to 80 μm tall centrally, composed of small, more or less rounded cells; with numerous, colourless oil-drops. Asci 70–80 × 15–18 μm (few well-developed seen), narrowly clavate, >100-spored. Ascospores colourless, simple, narrowly ellipsoid–ellipsoid, 3–5 × 1.5–2 μm. Pycnidia rare; conidia ellipsoid–ovoid, 2–2.5 × 1.5 μm. All spot tests negative.
Material examined: ICELAND, Nordur-Island, East side of Eyjafjörður, on ledge above a brook, 10 June 2007, L. Fröberg (LD); NORWAY, Finnmark: Varanger Peninsula, Vardø, Bukkemoltangen, 1 July 2014, M. Westberg VAR043 (S F263442); Varanger Peninsula, Båtsfjord, Skogdalen, 2 July 2014, M. Westberg VAR048 (S F263443); SWEDEN, Lule Lappmark, Jokkmokk par., Padjelanta National Park, north side of lake Virihaure (Virihávrre), at the foot of Mt Allak, c. 3.5 km SSW of the peak Allaktjåhkkå and c. 10 km N of Staloluokta, 590 m alt., 31 July 2004, M. Westberg 3064 (LD); Torne Lappmark: Jukkasjärvi par., Jebrinjokk, 400 m, 16 July 1919, Magnusson 3310 (UPS L-514336); near Torneträsk, Nuolja. 2 August 1921, Magnusson 6199a (UPS L-514869).
Ecology and distribution: Acarospora septentrionalis is currently known from the montane regions of northern Scandinavian and from Iceland. It has been collected on calcareous schist, dolomite and cement.
Notes: Magnusson (1924) first described this taxon as a variety of A. badiofusca (var. glaucocarpoides), but later changed his mind and considered it to be a peculiar form of A. glaucocarpa (Magnusson 1929). We find that this is a morphologically distinct taxon and molecular data support the distinction of A. septentrionalis as a species. The epithet glaucocarpoides is not appropriate to use due to the already existing name Sarcogyne glaucocarpoides Flagey (1896) and hence we describe it as a new species here. It is somewhat similar to A. badiofusca, especially when the apothecia becomes superficial and darker than the surrounding thallus. Acarospora badiofusca, however, usually has larger and more distinctly sessile apothecia, which typically become dark brown to almost black. The paraphyses in A. badiofusca are wider (2–3 μm) and the spores are also wider (2–3 μm). The molecular data clearly show that A. septentrionalis is not conspecific with A. badiofusca or its close relative A. cervina. Acarospora septentrionalis may also be confused with A. glaucocarpa. However, A. septentrionalis (like A. badiofusca), has an algal layer that is continuous and not interrupted by hyphal bundles as in A. glaucocarpa and some other species (Knudsen et al. 2014). A further difference to A. glaucocarpa is the paraplectenchymatous cortex consisting of rounded, thin-walled cells. Acarospora glaucocarpa on the other hand has a prosoplectenchymatous cortex of irregular hyphae with narrow lumina.
-
180.
Acarospora castaneocarpa M. Westb. & Wedin, sp. nov.
MycoBank number: MB 813044; Facesoffungi number: FoF00928; Fig. 64f–i.
Etymology: In reference to the chest-nut coloured ascocarps.
Holotype: FINLAND, Varsinais-Suomi (Regio Aboensis), Lohja, Paavola, NE of Rautaniemi, stony SE-slope, young Pinus sylvestris plantation, on calcareous stone, 50 m alt., 21 May 2005, Juha Pykälä 26880 (H).
Lichenized, photobiont a chlorococcoid alga. Thallus only present as a thalline margin in the ascocarps. Apothecia lecanoroid, rounded or more often irregular in outline, 0.6–1.1 mm diam., margin distinct, raised above the disc, up to c. 0.12 mm thick, reddish brown, shiny, in the paratype with a bluish white pruina along the inner rim (adjacent to the disc); disc concolourous with the margin, flat, smooth, shiny, epruinose; true exciple indistinct from the outside, I–, prosoplectenchymatous, below the hymenium of thick-walled cells with narrow lumina, towards the surface expanding and becoming fan-shaped, composed of branching, narrow, elongated cells, becoming shorter and rounded towards the surface, uppermost cells rounded, up to c. 5 μm diam., with a reddish-brown colour; epithecium reddish brown; hymenium colourless, I+ blue, 65–80 μm tall; paraphyses, easily distinguished in water, 1–1.5 μm in mid hymenium, tips clavate, up to 4.5 μm wide, upper part of the top cell often reddish brown; subhymenial layers, colourless, flat (i.e., not forming a wedge-shaped structure), up to c. 40 μm thick, prosoplectenchymatous, compact, of small, indistinct cells, oil-drops colourless, small, very few seen; Asci clavate, multi-spored (no well-developed asci found). Ascospores 3.5–5 × 1–1.5 μm (only few spores found), colourless, simple, narrowly ellipsoid–cylindrical. Pycnidia not seen. All spot tests negative.
Material examined: FINLAND, Varsinais-Suomi (Regio Aboensis), Lohja, Torhola, 300 m N-NE of Kallioranta, young pine plantation, S-slope, small rock wall of calcareous rock, 60 m alt., 22 July 2004, Juha Pykälä 25280 (H).
Ecology and distribution: Acarospora castaneocarpa is so far only known from two localities near the lake Lohjanjärvi in southernmost Finland where it was collected on calcareous rocks in young Pinus sylvestris forests.
Notes: Surprisingly this characteristic species is closely related to Sarcogyne algoviae, a very different species with black, lecideine apothecia with a strongly carbonized margin. In A. castaneocarpa the margin of the apothecium has a cortical layer that is densely filled with small, colourless crystals (calcium oxalate?). We have not seen this character in any other species in Acarosporaceae and as far as we know this is a very unusual character in the family.
Graphidaceae Dumort.
Graphidaceae is the second largest family of lichenized fungi, after Parmeliaceae, with 2100 species (Rivas Plata et al. 2012; Lücking et al. 2014; Lumbsch and Lücking 2015). The family is chiefly tropical, with a few taxa occurring in extratropical regions. After a thorough phylogenetic revision, the family now also includes the previously separate family Thelotremataceae and comprises 77 genera, now excluding the separate family Gomphillaceae which had previously also been synonymized with Graphidaceae (Lumbsch and Lücking 2015). Lücking et al. (2014) predicted that the family might contain another 1800 undescribed species and 175 new species were recently described in a single, compiled work (Sohrabi et al. 2014). Here we described two further new species based on recent molecular phylogenetic analyses (Rivas Plata et al. 2013).
-
181.
Chapsa multicarpa Lücking, Parnmen & Lumbsch, sp. nov.
Index Fungorum number: IF551486; Facesoffungi number: FoF00968; Fig. 66a, c, e
Etymology: Referring to the usually aggregate ascomata.
Holotype: R. Lücking 24007 (RAMK).
Diagnosis: Differing from Chapsa leprocarpa and C. patens in the smaller, aggregate ascomata with layered excipulum.
Thallus corticolous on tree trunks and branches, endoperidermal, continuous, smooth to uneven, following the contours of the bark, brownish to greenish depending on bark type, opaque. Photobiont endoperidermal, Trentepohlia; cells rounded to irregular in outline, in irregular groups, yellowish green, 6–12 × 5–10 μm. Sexual morph: Ascomata aggregated, groups erumpent, angular to elongate, 0.5–1.5 mm across, usually composed of 2–5 individual ascomata; individual ascomata angular-rounded, 0.2–0.3 mm diam. and 0.15–0.18 mm high; disc mostly covered by concentric layers of excipula, grey-brown, thickly white-pruinose; proper margin distinct, white, forming several excipular layers; thalline margin formed by shallow, erect lobules, pale yellowish. Columella absent. Excipulum paraplectenchymatous, 15–20 μm wide, hyaline, fused with thalline margin; thalline margin 40–70 μm thick; hypothecium prosoplectenchymatous, 15–20 μm high, hyaline; hymenium 130–150 μm high, hyaline, clear; epithecium with large crystal clusters, 20–30 μm high, grey. Paraphyses unbranched, smooth; lateral paraphyses up to 15 μm long; asci ellipsoid to fusiform, 120–140 × 25–30 μm. Ascospores 1 per ascus, richly muriform, hyaline, ellipsoid to fusiform, with thin septa and rectangular lumina, 80–130 × 20–25 μm, I–. Asexual morph: Undetermined. Secondary chemistry: No substances detected by TLC.
Material examined: THAILAND, Nakhon Ratchasima, Khao Yai National Park, 200 km NE of Bangkok, Khao Keaw scenic lookout; 14° 22′ N, 101° 24′ E; 1140 m; tropical submontane moist evergreen forest dominated by Dipterocarpaceae; 13 March 2008; R. Lücking 24007 (RAMK holotype; F isotype); same locality and date, R. Lücking 24009 (F paratype).
Notes: Chapsa multicarpa is characterized by the small ascomata aggregate in irregular to linear groups, containing two to five individual ascomata, with a layered excipulum and large, muriform ascospores occurring singly in each ascus. Molecular sequence data place the new species in Chapsa sensu stricto (Parnmen et al. 2012; Rivas Plata et al. 2013), unsupported sister to the type species, C. indica A. Massal. (Fig. 65). The large, single, muriform ascospores and the lack of secondary substances would key out C. multicarpa close to C. leprocarpa (Nyl.) Frisch and C. patens (Nyl.) Frisch (Rivas Plata et al. 2010), but both these species have larger, solitary ascomata and the excipulum is not layered. Molecular sequence data including mtSSU, nuLSU and RPB2 also show that all three species are phylogenetically distinct (Rivas Plata et al. 2013; Fig. 65). Chapsa multicarpa also resembles Astrochapsa kalbii Poengsungnoen et al. (2014), which also features aggregate ascomata with a layered excipulum. However, the latter forms an epiperidermal thallus with a thin, loose cortex, the hymenium is formed in the outermost ring of the concentric excipulum layers (not in a central disc), and the ascospores are smaller (less than half the size) and with 1–2 per ascus. Phylogenetically, the two species are only distantly related, as A. kalbii is closely related to the type species of Astrochapsa, A. astroidea (Berk. & Broome) Parnmen et al. (2012; Poengsungnoen et al. 2014).
Best-scoring maximum likelihood tree with bootstrap support values computed with RAxML 8.2 of selected species of Graphidaceae subfamily Graphidoideae tribe Thelotremateae genus Chapsa sensu stricto, including the new species C. multicarpa, using Dyplolabia afzelii as the outgroup. For GenBank numbers based on voucher information indicated in the tree, see (Parnmen et al. 2012) and (Rivas Plata et al. 2013)
-
182.
Fissurina carassensis Lücking, Parnmen & Lumbsch, sp. nov.
Index Fungorum number: IF551487; Facesoffungi number: FoF00969; Fig. 66b, d, f
Etymology: Referring to the type locality.
Holotype: R. Lücking 31224a (SP).
Diagnosis: Differing from Fissurina dumastii in the apically smooth paraphyses and the more strongly I+ violet-blue ascospores, and from F. amazonica, F. coarctata and F. subcoarctata in the larger ascomata and I+ violet-blue ascospores.
Thallus corticolous on tree trunks, epiperidermal, up to 10 cm diam., continuous; surface smooth to uneven, mottled yellowish green to silvery grey, glossy; prothallus absent; thallus in section 70–130 μm thick, with prosoplectenchymatous cortex, 15–25 μm thick, photobiont layer 25–50 μm thick, and medulla, 30–50 μm thick, filled with numerous small, grey crystals that dissolve in K; large clusters of calcium oxalate crystals only present at the base of the ascomata. Photobiont Trentepohlia; cells rounded to irregular in outline, in irregular groups, yellowish green, 6–11 × 5–9 μm. Sexual morph: Ascomata lirellate, immersed-erumpent, irregularly branched and rather dense, with thin labia (fissurine), closed to very slightly gaping, 1–3 mm long, 0.15–0.25 mm broad, 0.1–0.13 mm high; disc more or less concealed; proper margin indistinct; thalline margin slightly ascending, entire, whitish to light yellowish grey. Excipulum orange-yellow, 20–30 μm wide, prosoplectenchymatous, fused with thalline margin; thalline margin 50–70 μm thick, anatomically similar to thallus; hypothecium prosoplectenchymatous, 10–15 μm high, hyaline; hymenium 70–80 μm high, hyaline, clear; epithecium indistinct, 5–10 μm high, hyaline. Paraphyses unbranched, smooth; periphysoids present, rather distinct, shallowly and indistinctly warty; asci cylindrical, 70–80 × 8–10 μm. Ascospores 8 per ascus, uniseriate, ellipsoid, 3-septate, 10–13 × 5–6 μm, 1.8–2.3 times as long as wide, hyaline, distoseptate with lens-shaped lumina, I+ violet-blue. Asexual morph: Undetermined. Secondary chemistry: No substances detected by TLC; medulla P–, microscopic section K–.
Material examined: BRAZIL, Minas Gerais, Serra do Caraça, Reserva Particular do Patrimônio Natural (RPPN) Santuário do Caraça, trail through forest to chapel; 20° 06′ S, 43° 29′ W, 1300–1400 m, Atlantic Rain Forest, relatively well-preserved gallery forest (mata de galeria or floresta ombrófila densa montana) along small river, 27 July 2010, R. Lücking 31224a (SP holotype; F isotype).
Notes: Fissurina carassensis belongs in a complex of species centered around F. dumastii Fée, characterized by immersed-erumpent, fissurine ascomata, 3-septate, very small ascospores, and lack of secondary substances. Within this complex, the new species is characterized by its distinctly I+, violet-blue ascospores, whereas in F. dumastii the ascospores are only weakly I+ violet-blue and in the other species of this complex, F. amazonica M. Cáceres et al. (2014) and F. coarctata Makhija and Adawadkar (2007), they are I–. Fissurina dumastii also differs in its apically spinulose paraphyses (Staiger 2002), whereas F. amazonica and F. coarctata have very small lirellae (up to 1 mm long only). Molecular sequence data show that F. carassensis and F. dumastii are phylogenetically distinct (Rivas Plata et al. 2013; Fig. 67).
Best-scoring maximum likelihood tree with bootstrap support values computed with RAxML 8.2 of selected species of Graphidaceae subfamily Fissurinoideae, including the new species Fissurina carassensis, using Dyplolabia afzelii as outgroup. For GenBank numbers based on voucher information indicated in the tree, see (Rivas Plata et al. 2013)
Lobariaceae Chevall.
The family Lobariaceae traditionally comprised the three genera Lobaria, Pseudocyphellaria and Sticta, but the genus concept has recently been revised to accept 12 genera (Moncada et al. 2013; Galloway 2015; Lumbsch and Lücking 2015). In addition, phylogenetic and morphological studies also suggest that species richness in this family is much higher than previously accepted and that names applied to supposedly widespread taxa, such as Lobariella crenulata (Hook. in Kunth) Yoshim., Pseudocyphellaria crocata (L.) Vain. and Sticta fuliginosa (Dicks.) Ach., actually represent many different, often unrelated species (Moncada et al. 2013, 2014a, b). Especially the genus Sticta is now believed to contain several hundred species (Moncada et al. 2014a), two of which are described here.
-
183.
Sticta fuscotomentosa Moncada, Coca & Lücking, sp. nov.
Index Fungorum number: IF551488; Facesoffungi number: FoF: 00929; Fig. 69a–d
Etymology: Referring to the superficial similarity with Sticta tomentosa.
Holotype: L.F. Coca 1207 (FAUC).
Diagnosis: Differing from Sticta tomentosa and S. leucoblepharis in the brown to dark tomentum and the 2–4 papillae on the cells of the basal membrane of the cyphellae.
Thallus epiphytic, corticolous. Primary photobiont a cyanobacterium (Nostoc). Basal stipe absent or indistinct. Thallus palmate to irregular, up to 10 cm across, moderately branched. Lobes laciniate to ligulate, ascendant to erect, adjacent to imbricate, plane to involute, with rounded tips; margins straight to sinuose; lobe internodes 4–12 mm long and (5–)10–20 mm broad. Upper surface even to shallowly foveolate (point-impressed), dark green when fresh and bluish grey in the herbarium, opaque to slightly glossy, glabrous, with scattered, irregular, cream-coloured maculae; cilia abundant, dark greyish brown, up to 1 mm long. Vegetative propagules absent. Apothecia abundant, mostly laminal, substipitate, up to 1.3 mm diam. and 330 μm high; disc reddish brown; margin entire, cream-coloured. Excipulum up to 120 μm broad; hymenium up to 100 μm high; epithecium up to 5 μm high, orange. Ascospores fusiform, 1–3-septate, 20–35 × 7–8 μm, hyaline. Medulla loose in surface view, whitish to cream-coloured, K+ yellow, C–, KC–, P–. Upper cortex paraplectenchymatous, 20–30 μm thick; upper layer with smaller, thicker-walled cells and lower layer with larger, thinner-walled cells; photobiont layer 10–20 μm thick; medulla 75–120 μm thick, with yellow-orange crystals; lower cortex paraplectenchymatous, 15–20 μm thick. Lower surface even to slightly undulate, whitish to cream-coloured, but becoming darker towards the center. Primary tomentum irregular, thick, becoming sparse and thin towards the margin, arachnoid to spongy, beige to dark greyish brown towards the center, hairs 120–750 μm long, forming fascicles of 12–20 unbranched, septate hyphae; secondary tomentum absent. Rhizines absent. Cyphellae abundant, 1–20 per cm2 towards the center and 41–60 per cm2 towards the margin, dispersed, angular-rounded, urceolate with wide pore, (0.25–)0.4–0.7(−1.3) mm diam., immersed to prominent but remaining below the level of the tomentum, with involute, whitish to cream-coloured, glabrous margin; basal membrane finely pubescent, with 2–4 papillae per cell, pale yellowish, K+ yellow, C–, KC–, P–. Pycnidia not observed. Secondary chemistry: No substances detected by TLC but medulla and basal membrane of the cyphellae with dispersed, orange-yellow pigment granules in anatomical sections.
Material examined: COLOMBIA, Risaralda, Mun. Santuario, Tatamá National Natural Park, close to Monte Zancudo, 2600 m, 13 January 2011, L.F. Coca et al. 1207 (FAUC holotype; UDBC isotype); Quindío, Mun. Salento, Navarco Alto trail, Piscícola station, trail to the line, 3000 m, 4 November 1991, J. Uribe & A. Bonilla 2173 (COL); Risaralda, Mun. Pereira, Otún Quimbaya Fauna and Flora Sanctuary, La Florida, La Suiza trail, 1820 m, 2 September 2003, B. Moncada & R. Dávila 1908b (UDBC); Tolima, Mun. Ibagué, Las Juntas, El Silencio to El Rancho road, 2600 m, 18 May 2008, B. Moncada 2531b (UDBC).
Distribution and Ecology: Sticta fuscotomentosa is thus far known from the Colombian Andes, in the upper montane cloud forest zone between 1820 and 3000 m altitude. The species is typically found epiphytically in shady to semi-exposed microsites.
Notes: Sticta fuscotomentosa belongs in the S. tomentosa clade (Moncada et al. 2014a, b; Fig. 68) and is most similar to S. tomentosa (Sw.) Ach. and S. leucoblepharis (Nyl.) Tuck. & Mont. and differs chiefly in the dark lower tomentum towards the thallus center and the dark marginal cilia (Moncada 2012). The apothecial margin is glabrous, whereas young apothecia in S. tomentosa and apothecia in S. leucoblepharis have tomentose margins.
Best-scoring maximum likelihood tree with bootstrap support values computed with RAxML 8.2 of selected species of Sticta (Lobariaceae), including the new species S. fuscotomentosa and S. subfilicinella. GenBank numbers and voucher information are indicated in the tree (see Moncada et al. 2014 for full tree)
-
184.
Sticta subfilicinella Moncada, Coca & Lücking, sp. nov.
Index Fungorum number: IF551576; Facesoffungi number: FoF00930; Fig. 69e–f
Etymology: Referring to the similarity and close relationship with Sticta filicinella.
Holotype: L.F. Coca 1110 (FAUC).
Diagnosis: Differing from Sticta filicinella in the absence of isidia.
Thallus epiphytic, corticolous. Primary photobiont a cyanobacterium (Nostoc). Basal stipe absent or indistinct. Thallus irregular to suborbicular, up to 5 cm across, moderately branched. Lobes laciniate to ligulate, ascendant, adjacent to imbricate, plane to canaliculate, with rounded, revolute tips; margins straight to sinuose; lobe internodes 4–10(−15) mm long and 4–10 mm broad. Upper surface even to shallowly scrobiculate-rugose, greenish grey when fresh and bluish to greenish grey in the herbarium, opaque to slightly glossy, glabrous, with abundant, irregular, cream-coloured maculae; cilia sparse to abundant, dark brown, up to 1 mm long. Vegetative propagules absent. Apothecia sparse, mostly submarginal, substipitate, up to 1.5 mm diam. and 330 μm high; disc orange; margin entire, cream-coloured to light yellowish. Excipulum up to 130 μm broad; hymenium up to 160 μm high; epithecium up to 5 μm high, orange. Ascospores not observed. Medulla loose in surface view, cream-coloured, K+ yellow, C–, KC–, P–. Upper cortex paraplectenchymatous, 30–45 μm thick, with uniform layers; photobiont layer 25–35 μm thick; medulla 50–120 μm thick, with yellow-orange crystals; lower cortex paraplectenchymatous, 15–30 μm thick. Lower surface undulate, cream-coloured to pale yellowish. Primary tomentum vein-like, thick, becoming sparse and thin towards the margin, spongy, dark brown, hairs 75–900 μm long, forming fascicles of 12–20 unbranched, septate hyphae; secondary tomentum absent. Rhizines absent. Cyphellae abundant, 21–40 per cm2 towards the center and 100–200 per cm2 towards the margin, dispersed, angular-rounded, thelotremoid to urceolate with narrow pore, 0.1–0.25 mm diam., erumpent to prominent but remaining below the level of the tomentum, with involute, cream-coloured to pale yellowish, glabrous margin; basal membrane finely pubescent, with papillum per cell, cream-coloured to pale yellowish, K+ yellow, C–, KC–, P–. Pycnidia not observed. Secondary chemistry: No substances detected by TLC but medulla and basal membrane of the cyphellae with dispersed, orange-yellow pigment granules in anatomical sections.
Material examined: COLOMBIA, Risaralda, Mun. Santuario, Tatamá National Natural Park, close to Monte Zancudo, 2600 m, 13 January 2011, L.F. Coca et al. 1110 (FAUC holotype; UDBC isotype); Caldas, Mun. Pacora, Filobonito trial, La Quinta section, 2123 m, 2010, L.F. Coca 177 (UDBC); Cundinamarca, Mun. Choachí, El Verjón, Matarredonda Ecological Park, 04° 34′ N, 74° 00′ W; 2900–3220 m, 8 May 2010, B. Moncada 3060 (UDBC); Magdalena, Mun. Santa Marta, Sierra Nevada de Santa Marta, Alto Buritaca transect, 2500 m, August 1977, G. van Reenen & O. Rangel 269 (COL, U); Same locality, Buritaca transect, 2500 m, August 1977, O. Rangel et al. 711 (COL, U); Nariño, Mun. Piedrancha, La Planada, San Isidro, S of Ricaurte (Pasto to Tumaco road), 1750 m, 2 June 1993, H. Sipman et al. 32849 (B, COL); Risaralda, Mun. Santuario, Tatamá National Natural Park, Planes de San Rafael, trail after hut, 2400 m, 12 January 2011, L.F. Coca et al. 1079 (FAUC); same locality, close to Monte Zancudo, 2600 m, 13 January 2011, L.F. Coca et al. 1139, 1213, 1214 (FAUC).
Distribution and Ecology: Sticta subfilicinella is thus far known from the Colombian Andes, in the upper montane cloud forest zone between 1750 and 3220 m altitude. The species is typically found epiphytically in semi-exposed microsites.
Distribution and Ecology: Sticta subfilicinella is thus far known from the Colombian Andes, in the upper montane cloud forest zone between 1750 and 3220 m altitude. The species is typically found epiphytically in semi-exposed microsites.
Notes: This new species belongs in a group characterized by an exclusively cyanobacterial photobiont forming small, thin, fragile grey thalli (often both fresh and in the herbarium), with marginal cilia, the lower tomentum often vein-like, and small, thelotremoid cyphellae. The lower margin is usually glabrous. This group is related to Sticta tomentosa and allies (Moncada et al. 2014a, b; Fig. 62). Most similar to Sticta subfilicinella is S. filicinella (Nyl.) Zahlbr., which produces abundant isidia instead of apothecia (Lumbsch et al. 2011; Moncada 2012). Another similar species is the recently described S. venosa Lücking et al. from Ecuador (Lumbsch et al. 2011), which has broader lobes and like S. filicinella also produces isidia.
Pezizomycetes
Helvellaceae Fr.
The family Helvellaceae was introduced by Fries (1822) to accommodate Helvella L.
-
185.
Helvella pseudolacunosa Q. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: 551446; Facesoffungi number: FoF00971; Fig. 71
Diagnosis: Pileus has a grey to dark grey, pinched hymenium, margin fused with stipe in several places, and a creamy, glabrous sterile surface. Stipe is enlarged at base, rounded longitudinal ribs, near the base with enlarged deep furrows, grey-white.
Etymology: Named because of the resemblance with Helvella lacunosa.
Holotype: CHINA, Neimenggu Province, Axiangshan County, alt. 180 m, 16 August 2013, Qi Zhao 2012 (HKAS 87594, GenBank number: KR 493476).
Symbiotic in the coniferous forests of Larix gmelinii (Rupr.) Rupr. or Pinus koraiensis Sieb. et Zucc. Sexual morph: Pileus saddle-shaped to irregularly lobed when young, irregularly lobed or brain-like when maturity, 1–4 cm high, 2–3 cm broad, pinched at apex, margin fused with stipe, hymenium glabrous, grey to dark grey, becoming black when dried, sterile surface glabrous, creamy, becoming yellowish when dried. Stipe 2–8 cm long, 0.7–1.5 cm broad, enlarged at base (up to 3 cm), round-shaped longitudinal ribs, near the base with enlarged deeply furrows, glabrous, grey-white when young, becoming grey with age, sometimes ochraceous along the upper portions of the stipe, white mycelium at base. Medullary excipulum 180–260 μm broad, textura intricata, hyaline, hyphae 3–5.5 μm broad, walls thickened. Ectal excipulum 120–220 μm broad, hyaline, terminal cells 16–40 × 10–20 μm, blue in cotton blue. Stipitipellis 40–55 μm, hyaline, composed of enlarged parenchymal cells, terminal cells 14–30 × 8–12 μm, walls thickened, blue in cotton blue. Asci 230–280 × 13–20 μm, pleurorhynchous, 8-spored, subcylindrical to clavate, blue in cotton blue. Ascospores [H2O] (14.5)15–19.5(20) × (9.5)10–12(12.5) μm [\( \overline{x} \) = 17.5 × 11 μm n = 40], ellipsoid, smooth. Paraphyses filiform, 3–4.5 μm broad, slightly exceeding the asci, brown, with a yellow refractive content in Melzer’s reagent, blue in cotton blue; apex 3.5–5 μm broad. Asexual morph: Undetermined.
Material examined: CHINA, Jilin Province, Antu County, alt. 200 m, 26 August 2006, B. Tolgor 4533 (HMJAU 4533 holotype).
Notes: The genus Helvella (Helvellaceae, Pezizomycotina) is regarded as common macrofungi in most temperate forests, and have been widely reported from Europe, North America, Asia and Australia; very little is known about the trophic status of these fungi (Abbott and Currah 1997; Hwang et al. 2015). Identification of the species belonging in this genus has often been based on ascoma shape and colour and the presence or absence of projecting hyphae on the sterile surface of the apothecium (Abbott and Currah 1997; Dissing 1966; Hwang et al. 2015; Weber 1972). There are 476 epithets listed under Helvella (Index Fungorum 2015), but few molecular data are available in GenBank (Landerose and Korf 2012; Nguyen et al. 2013).
Based on phylogenetic analyses and morphological comparison, our isolate belongs to H. lacunosa complex in Helvellaceae (Fig. 70). Helvella pseudolacunosa is similar to H. dryophila N.H. Nguyen & Vellinga, H. lacunosa Afzel.: Fr and H. vespertina N.H. Nguyen & Vellinga (Fig. 71). However, H. dryophila differs from H. pseudolacunosa in having a dark, almost black, squat pileus, light stipe, and forms ectomycorrhiza with Quercus species (Nguyen et al. 2013). Morphological differences between H. pseudolacunosa and H. lacunosa are subtle, the stipe of H. pseudolacunosa is usually enlarged at base, with rounded, longitudinal ribs, grey-white when young, ochraceous along the upper portions, while the latter species generally has a sharp-edged ribs, inside of stipe with longitudinal chambers, pale greyish to greyish-brown to nearly black (Dissing 1966). Helvella vespertina differs from H. pseudolacunosa by its pileus, three-lobes and wavy, gigantic stipe, and in forming ectomycorrhiza associations with conifers (Nguyen et al. 2013).
-
186.
Helvella rugosa Q. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: 551447; Facesoffungi number: FoF00972; Fig. 72
Diagnosis: Pileus has a pale to dark grey or greyish brown hymenium, margin reflexed and fused with stipe in several places, and a wrinkled-folded, white to pale sterile surface. Stipe is almost only has regularly longitudinal ribs, tapering downwards, greyish brown.
Etymology: Named because of its sterile surface wrinkled-folded.
Holotype: CHINA, Yunnan Province, Xishan County, Quercus spp. and bamboo forest, alt. 2600 m, 28 August 2009, Qi Zhao 481 (HKAS 75442).
Symbiotic in the coniferous forests of Quercus sp. Sexual morph: Pileus saddle-shaped to three-lobed, 1–2 cm high, 1–2 cm broad, margin reflexed and fused with stipe, hymenium glabrous, pale to dark grey or greyish brown when fresh, becoming black when dry. Sterile surface wrinkled-folded, white to pale when young, becoming yellowish when dried. Stipe 2–4 cm long, 0.4–0.7 cm broad, tapering downward, mainly longitudinal ribs and few lacunose, glabrous, greyish brown when young, becoming black when dry, white mycelium at base. Medullary excipulum 80–260 μm broad, textura intricata and textura prismatica, hyaline, hyphae 3–5.5 μm broad, enlarged cells 8–15 × 7–14 μm, walls thickened. Ectal excipulum 50–80 μm broad, outermost cells paliform, hyaline, terminal cells 16–40 × 8–16 μm. Stipitipellis 50–70 μm, hyaline, composed of textura prismatica, terminal cells 18–32 × 8–18 μm, walls thickened. Asci 220–260 × 13–17 μm, pleurorhynchous, 8-spored, subcylindrical to clavate, blue in cotton blue. Ascospores [in H2O] (15)15.5–18(18.5) × (9.5)10–11(11.5) μm [(\( \overline{x} \) = 17 × 10 μm n = 40)], ellipsoid, smooth, finely rugose under SEM. Paraphyses filiform, 4–5.5 μm broad, slightly exceeding the asci, brown, with a yellow refractive content in Melzer’s reagent, blue in cotton blue; apex enlarged, 6–8 μm broad. Asexual morph: Undetermined.
Additional material examined: CHINA, Yunnan Province, Xishan County, alt. 2600 m, 28 August 2009, Qi Zhao 482 (HKAS 87587 holotype).
Notes: Helvella rugosa can be easily recognized by its small fruiting body, pale to dark grey or greyish brown hymenium, wrinkled-folded, white to pale sterile surface, and a tapering downward, mainly longitudinal ribs stipe.
The gross morphology of H. rugosa is very similar to that of H. fusca Gillet and H. sulcata Afzel., because they have lobed apothecia, a ribbed stipe and a glabrous stipitipellis. However, H. fusca has a pale brown hymenium, with ribs on the sterile surface that are branched or unbranched, extending near to the margin, stipe equal or slightly enlarged at the base, and are associated with Populus spp. (Landerose and Korf 2012). Helvella sulcata, has a pale to dark grey or greyish brown hymenium, a glabrous sterile surface and only the stipe has longitudinal ribs (Weber 1972).
Sordariomycetes
Annulatascaceae S.W. Wong et al.
Wong et al. (1998) introduced the family Annulatascaceae to accommodate saprobic ascomycetes mainly found on submerged wood in freshwater habitats. Species in the family are characterized by asci with massive, bipartite, apical ring and ascospores with or without sheaths or appendages (Wong et al. 1998). Phylogenetic analyses of ribosomal genes showed that Annulatascaceae is polyphyletic (Campbell and Shearer 2001, 2004; Raja et al. 2003; Abdel-Wahab et al. 2011). There is no asexual genus described in this family and most of the described sexual genera did not produce asexual morphs in culture. However, Zelski et al. (2011) described a new genus, Chaetorostrum, in the family, with a taeniolella-like asexual morph and Conlarium duplumascospora F. Liu & L. Cai produced helicoid conidia in culture (Liu et al. 2012a). During an ongoing study of freshwater fungi from River Nile in Egypt, a fungus with helicoid conidia was commonly recorded. Phylogenetic analyses of ribosomal loci place the new fungus (Dictyosporella aquatica) in the family Annulatascaceae, but distantly placed from all genera in the family.
-
187.
Dictyosporella Abdel-Aziz, gen. nov.
Index Fungorum number: IF551480; Facesoffungi number: FoF00973
Etymology: In reference to the septation of the conidia.
Saprobic on grass stem in freshwater. Sexual morph: Undetermined. Asexual morph: Mycelium superficial and immersed in the substrate, yellow-brown to brown, septate, branched. Conidiophores reduced. Conidiogenous cells holoblastic. Conidia helicoid when young and quickly becoming a mass of cells, brown to black, terminal and lateral, single, determinate. Conidial cells are generally similar in shape, colour and size that are globose, subglobose, rounded or with truncate base, brown to dark-brown.
Type species: Dictyosporella aquatica Abdel-Aziz
Notes: Dictyosporella aquatica was frequently recorded on submerged decaying stems of Phragmites australis in the River Nile in Egypt. Dictyosporella aquatica is reminiscent of Cirrenalia, a marine genus with helicoid conidia that is commonly collected on driftwood and mangrove wood (Jones et al. 2009). Phylogenetic studies placed the type species, C. macrocephala in Halosphaeriaceae (Tsui and Berbee 2006; Abdel-Wahab et al. 2010). Other species belong to Juncigenaceae, Lulworthiaceae, and Torpedosporaceae (Abdel-Wahab et al. 2010; Jones et al. 2014). Phylogenetic analyses of SSU (data not shown) and LSU rDNA placed D. aquatica in a weakly supported clade with Cyanoannulus petersenii Raja et al. in the family Annulatascaceae (Fig. 73). The monotypic genus Conlarium is characterized by helicoid conidia, similar to those of D. aquatica (Liu et al. 2012a), however, C. duplumascospora is distantly placed from D. aquatica in the family Annulatascaceae (Fig. 73). Most of the species described under Annulatascaceae are from freshwater habitats (Wong et al. 1998).
Phylogram generated from maximum likelihood analysis (PAUP 6) based on LSU sequence data of Dictyosporella aquatica and related taxa in Annulatascaceae. Representatives of the order Pezizales were used as outgroup. Maximum Likelihood bootstrap support values greater than 50 % are indicated. The numbers above or below the nodes indicate ML, PP and MP bootstrap value s ≥ 50 %. The new species, D. aquatica is in blue
-
188.
Dictyosporella aquatica Abdel-Aziz, sp. nov.
Index Fungorum number: IF551481; Facesoffungi number: FoF00974; Fig. 74
Etymology: From the Latin adjective aquaticus, in reference to the freshwater habitat of the fungus.
Holotype: 15-0751
Saprobic on decayed herbaceous stems in freshwater. Sexual morph: Undetermined. Asexual morph: Mycelium 2–4 μm diam., superficial and immersed in the substrate, yellow-brown to brown. Conidiophores reduced. Conidiogenous cells holoblastic. Conidia 12–22 μm (\( \overline{x} \) = 18 μm, n = 55) diam., helicoid when young, quickly becoming masses of cells, brown to black, terminal and lateral, single, determinate. Cells of conidia −13 μm (\( \overline{x} \) = 7.09 μm, n = 20) diam., generally increasing in size from basal to apical cells, but with similar colour, globose, subglobose, rounded or with truncate base, brown to dark brown. Number of conidial cells ranging between 4 and 18 (\( \overline{x} \) = 10.3 μm, n = 20).
Culture characteristics: Colonies on PDA reaching 20–25 mm diam. after 15 days at 25 °C, with grey to light brown mycelium, reverse brown, not sporulating in culture.
Material examined: EGYPT, Sohag, River Nile, on submerged decayed stem of Phragmites australis (Poaceae), 14 August 2014, F.A. Abdel-Aziz (CBS H-22127, holotype); Ibid. (MFLU 15-1510, isotype) ex-type living culture, MD1302 (Fig. 75).
Phylogenetic tree showing Thozetella clade comprising C. rivularia discerned in the phylogeny of the Chaetosphaeriales. Phylogram is generated from Maximum Likelihood analysis based on ITS and nuc28S rDNA sequences of originally 82 members of the Chaetosphaeriales. Maximum likelihood bootstrap support values greater than 50 % are indicated above the nodes. Four members of the Glomerellales were used to root the phylogeny
Chaetosphaeriaceae Réblová, M.E. Barr & Samuels
Chaetosphaeria Tul. & C. Tul., Select. fung. carpol. (Paris) 2: 252 (1863)
The genus is a lignicolous perithecial ascomycete with a world-wide distribution. It is characterized by non-stromatic, dark, papillate ascomata, persistent paraphyses, unitunicate asci with a shallow, refractive J- apical annulus and hyaline, ellipsoidal, fusiform to filiform, one to several-septate hyaline ascospores, although several species with versicolorous ascospores are also accommodated in the genus.
The systematics of Chaetosphaeria is complicated by the diversity of morphological characters of the asexual morphs. To date around 30 asexually reproducing genera of dematiaceous phialidic hyphomycetes have been experimentally linked with members of the Chaetosphaeriales or their relationship was suggested based on molecular data. The majority of these asexual morphs is linked with Chaetosphaeria, and they represent the greatest variability within the genus.
-
189.
Chaetosphaeria rivularia Réblová & J. Fourn., sp. nov.
Index Fungorum number: IF551489; Facesoffungi number: FoF00975; Figs. 76 and 77
Chaetosphaeria rivularia (holotype) a, b Ascomata c, d Vertical section of ascoma e, i Ascospores with indistinct middle septum (arrows indicate septum), e in Melzer’s reagent, i in India ink) f–h Asci j Ascal apex with an apical annulus k Paraphyses. Scale bars: a = 500 μm, b = 250 μm, c, d = 100 μm, e, i, j = 10 μm, f–h, k = 25 μm
Chaetosphaeria rivularia (holotype) a Conidiomata topped with masses of conidia in PCA culture (ESEM - Environmental Scanning Electron Microscope microscopy) b Palisade of branching conidiophores ending into a monophialide on PCA c, d Conidiogenous cells on PCA e, f Conidia on PCA g Microawns on PCA. Scale bars: a = 100 μm, b–f = 10 μm, g = 25 μm
Etymology: Rivularis (L), referring to rivulets, the natural habitat of the fungus.
Holotype: PRM 933847
Saprobic on decaying wood submerged in freshwater. Sexual morph: Ascomata non-stromatic, semi-immersed, scattered to gregarious, often with confluent walls, venter 200–250 μm diam., 250–330 μm high, subglobose to broadly conical, brown, glabrous, papillate, opening by a rounded pore. Ostiole central, periphysate. Peridium leathery to fragile, carbonaceous, two layered, 30–60 μm thick near apex and at the sides, up to 50–60 μm thick at the base; outer wall composed of brown, brick-like cells of textura prismatica, cells opaque in the upper part, pale brown at the base, towards the interior grading into several rows of thin-walled, hyaline, flattened cells. Hamathecium composed of abundant, persistent, septate, hyaline paraphyses ca. 3.5–4.5 μm wide, tapering to 2.5 − 3 μm, longer than the asci. Asci (72–)75–95(−98) × 7–8.5(−9) μm (\( \overline{x} \) ± SD = 86.7 ± 8.2 × 8 ± 0.6 μm), 8-spored, unitunicate, cylindrical-clavate, rounded, slightly tapering at the apex, short-pedicellate, with a distinct, non-amyloid apical annulus, ca. 1–1.3 μm high, 2–2.5 μm wide. Ascospores (15–)16 − 19 × 3–3.5 μm (\( \overline{x} \) ± SD = 16.8 ± 1.3× 3.4 ± 0.3 μm), ellipsoid to narrowly fusiform, slightly curved, 1-septate, septum indistinct, not constricted at the septum, hyaline to yellowish grey, smooth-walled, arranged obliquely uniseriate, often two-seriate only in the upper sporiferous part, with a hardly defined mucilaginous sheath swelling and diffusing in water. Asexual morph: Thozetella-like formed only in the axenic culture.
Culture characters: Colonies slow growing, reaching 10–15 mm diam. on PCA after 14 days at 25 °C. Colony circular, felty, zonate, brown-grey in the centre, brown towards the margin, reverse dark brown. Vegetative hyphae 2–3.5 μm wide, smooth, subhyaline or pale brown, thin-walled, septate, sparsely branched. Sporulation appears in a month. Synnemata scattered in circular zones around the centre and at the margin of the colony, composed of dark brown stalk bearing a slimy, glistening, white-grey mass of conidia. Stalk ca. 10–30 μm wide, composed of a brown, septate, sparsely branched conidiophores densely bunched and partly fused, ending in a monophialide, topped by a subglobose mass of conidia and sterile awn-shaped cells, so called microawns. Microawns produced from conidiophores and liberated with the conidia, straight to sigmoid or irregularly curved, basal part thin-walled, tapering towards the tip, thick-walled, pointed at the tip, 0–3-septate, hyaline, 65–130 μm long, 4–6.5 μm wide at the broadest point near the base, rigid, refractive. Conidiophores macronematous, up to 100 μm long, 2.5–3.5 μm wide, pale brown, septate, branched at intervals. Conidiogenous cells phialides, 16–35 × 2–2.4 μm (\( \overline{x} \) ± SD = 23.8 ± 7.5 × 4.5 ± 0.4 μm), integrated, terminal, cylindrical to clavate, tapering to ca. 1.5 μm, bearing a single conidiogenous locus in a minute, indistinct collarette. Conidia (9.5−)11 − 13 × 1.8 − 2.5 μm (\( \overline{x} \) ± SD = 12.2 ± 0.7 × 2.2 ± 0.2 μm), lunate to suballantoid, hyaline, aseptate, at each end slightly narrowed and provided with a single filiform setula 2.8 − 4.5(−5.5) μm long.
Material examined: FRANCE, Midi-Pyrénées, Ariège, Rimont, Grand Bois forest, Maury brook, 650 m a.s.l., submerged wood of Fagus sylvatica associated with Minutisphaera japonica, 17 November 2009, J. Fournier J.F. 09308 (PRM 933847, holotype); ex-type living culture CBS 127686; Ibid., 24 November 2009, J. Fournier J.F. 09316 (PRM 933847). Ibid., 8 December 2009, J. Fournier J.F. 09327.
Notes: Chaetosphaeria rivularia was repeatedly collected on wood submerged in freshwater in southern France. No conidiophores were observed on the host. The living culture derived from ascospore isolates from a fresh collection of C. rivularia yielded synnematous conidiophores with long, thick-walled and pointed microawns and setulose, aseptate Thozetella-like conidia. The relationship between Chaetosphaeria Tul. & C. Tul. and the synnematous dematiaceous hyphomycete Thozetella Kuntze was suggested by Paulus et al. (2004), based on the phylogenetic analysis of ITS rDNA sequence data. Thozetella comprises to date 19 species occurring primarily as saprobes on decaying wood, herbs, litter, palm fronds and rarely as endophytes. The only species so far reported from wood submerged in freshwater is T. nivea (Berk.) Kuntze, the type species (Sivichai et al. 2002). In our phylogenetic analysis based on ITS and nuc28S rDNA sequences of 82 members of the Chaetosphaeriales a close relationship of C. rivularia with other permanently asexual Thozetella species is well-supported.
Chaetosphaeria rivularia resembles C. ciliata, C. ovoidea and C. tortuosa (Holubová-Jechová 1973; Réblová et al. 2006; Réblová and Seifert 2008) in the size and shape of the ellipsoid to narrowly fusiform, often curved, septate ascospores and setulose conidia. The three latter species differ from C. rivularia by 3-septate ascospores and formation of the Menispora asexual morphs with mononematous conidiophores. The ascospores of C. rivularia are 1-septate, the middle septum is rather indistinct. Ascospores possess a mucilaginous sheath that is visible only in fresh material; the sheath diffuses in water and it is visible when observed in India ink. The asexual morph of C. rivularia is characterized by synnematous branching conidiophores, long, pointed, curved to sigmoid microawns that contain 1–3 septa, but sometimes appear as septate and aseptate conidia. It resembles T. gigantea in the morphology of microawns and conidia, but the latter species differs by longer conidia and longer setulae (Paulus et al. 2004).
Cordycipitaceae Kreisel ex G.H. Sung et al.
The family Cordycipitaceae was introduced together with Ophiocordycipitaceae as a result of multi-gene (LSU, SSU, RPB1, RPB2, β-tubulin, TEF1, and ATP6) phylogeny which rejected the monophyly of the family Clavicipitaceae and the genus Cordyceps (Sung et al. 2007a, b). Cordycipitaceae comprises four sexual morph genera − Ascopolyporus, Cordyceps, Hyperdermium, and Torrubiella. They are characterized by pale to brightly coloured species having either soft, fleshy stromata (e.g., C. militaris), or highly reduced, loosely organized hyphae or subiculum on the host (e.g., T. hemipterigena). Asexual morphs found in this family include Akanthomyces, Beauveria, Engyodontium, Evlachovaea, Gibellula, Isaria, Lecanicillium, Microhilum, and Simplicillium.
Beauveria is a cosmopolitan, soil-borne entomopathogenic fungus that infects a wide range of arthropod hosts (Rehner and Buckley 2005) and can also be found as an endophyte and saprobe (Vega et al. 2008). It is characterized by basally inflated conidiogenous cells that sympodially produce conidia on divergent denticles (De Hoog 1972). The characteristics of the conidia are the main morphological features used to distinguish between species of Beauveria, which are typically globose, cylindrical, ellipsoidal, reniform, or comma-shaped and are 1.5–5.5 μm (Rehner et al. 2011). The combined analysis of ITS rDNA and EF places both Beauveria gryllotalpidicola and B. loeiensis in the Cordycipitaceae nesting among other Beauveria species. Cordyceps loeiensis is closely related to Beauveria asiatica, found on Coleoptera and B. australis on Orthoptera (Acrididae), while B. gryllotalpidicola is in a subclade with B. sungii and B. malawiensis, both species found on Coleoptera and in the soil. The new species are introduced based on both morphological and molecular differences among other Beauveria species. The phylogenetic tree is presented in Fig. 78.
Phylogram inferred from ITS and TEF1 gene regions of Beauveria, generated with Maximum Parsimony and Bayesian Posterior probabilities. Maximum Parsimony bootstrap support values greater than 50 % are indicated above the node in black. Bayesian Posterior probabilities greater than 50 % are given beside the MP bootstrap values in blue
-
190.
Beauveria gryllotalpidicola Luangsa-ard, Ridkaew & Tasan., sp. nov.
Index Fungorum number: IF551323; Facesoffungi number: FoF00976; Fig. 79
Beauveria gryllotalpidicola (holotype) a Fleshy orange stromata emerging from the soil b Fungus on host c Semi-immersed perithecial heads emerging from the stroma d Perithecium e Asci and ascus caps e–g Part-spores h Conidiogenous structures bearing conidia i Conidia j, k Colony from above and below on PDA after 4 weeks. Scale bars: a–b, j–k = 10 mm, c = 1 mm, d = 100 μm, e–g = 10 μm, h–i = 5 μm
Etymology: The specific epithet refers to a mole dwelling fungus, ‘gryllotalpidae’ = from the family of the mole cricket Gryllotalpidae and ‘cola’ = dweller.
Holotype: BBH 23231
Parasitic in arthropods (Orthoptera, Gryllotalpidae). Sexual morph: Stromata simple to branched, clavate, fleshy, erect, protruding from the ground sometimes with one or several stromata loosely connected to the head, and to the tail of the host insect. Mycelia scarce, whitish covering the host, slightly rhizoid in the soil joining together to form a compact stipe upon emerging from the soil. Perithecial heads yellowish to reddish orange; perithecia narrowly ovoid, semi-immersed, 350–550 × 150–230 μm. Asci cylindrical, 390–430 × 5 μm, ascus tip 2.5 μm. Ascospores readily breaking into part-spores, 5–10 × 1 μm. Asexual morph: ‘Beauveria’ asexual state is readily produced in culture with flask-shaped conidiogenous cells and globose conidia, 2 × 2 μm diam.
Culture characters: Colonies on PDA are relatively fast-growing, reaching 10 mm diam. after 14 days at 25 °C, cottony, pale yellow in the center and white at the colony edges, sporulating, reverse pale yellow.
Material examined: THAILAND, Nakhon Ratchasima Province, Km.33 (Nong Pakchi), Khao Yai National Park, 10 July 2007, T. Laessøe, J.J. Luangsa-ard, R. Ridkaew, B. Thongnuch, P. Srikitikulchai (BBH 23231, holotype); ex-type living culture, BCC 26300.
Other specimen examined: on mole cricket, 1 December 2005, K. Tasanathai, W. Tongsridom, B. Thongnuch and R. Ridkaew, BBH15091.
-
191.
Beauveria loeiensis Luangsa-ard, Ridkaew & Tasan. sp. nov.
Index Fungorum number: IF551324; Facesoffungi number: FoF00977; Fig. 80
Beauveria loeiensis (holotype) a, b Fungus on host c Clusters of superficial perithecia on host d Perithecia e Phialides on host f Phialides on PDA g Mature ascus h Asci and asci cap i, j Part-spores k Colony on PDA after 20 days l Conidiogenous structures on the host m Conidiogenous structures on PDA. Scale bars: a–b, k = 10 mm, c = 2 mm, d = 200 μm, g = 50 μm, e–f, l–m = 10 μm
Etymology: named after the type locality.
Holotype: BBH 18836
Parasitic in arthropods (Orthoptera, Gryllacrididae). Sexual morph: Stromata on body and leg joints of adult Orthoptera several, scattered, simple or branched, cylindrical to enlarged at the apices, yellowish cream, 1–3 mm long, with perithecia in small clusters (20–30) at the apices. Perithecia narrowly ovoid with acute apices, 650–710 × 280–320 μm, superficial and crowded at the apex. Asci cylindrical, 370–450 × 5 μm, ascus caps 2.5 μm. Ascospores filiform, breaking into 32 part spores, 5–10 × 1 μm. Some of the specimens are accompanied with the Beauveria asexual morph. Asexual morph: Synnemata are scattered on the body and legs of the host, cream to yellowish brown, smooth-walled, 2–6 mm in length, the upper portion mealy with oblong-ellipsoid conidia, some concave to one side and flattened or convex to the other (2–4 × 1.2 − 1.5 μm), white; conidiogenous cells round or flask-shaped, producing conidia sympodially on divergent denticles, in whorls of two or more or singly, scattered along the hypha.
Culture characters: Colonies on PDA are relatively fast growing, reaching 10 mm diam. after 14 days at 25 °C; circular, dense, at first white and floccose, becoming cream to yellow cream in the center with age; conidiation starts as early as 12 h after inoculation. Conidiophores arise from the agar, white, smooth-walled, conidiogenous structures up to 12.5 μm long, cylindrical or narrowing at the tip producing ellipsoidal to cylindrical conidia (3.5 − 6 × 1.5 − 2 μm) with rounded ends.
Material examined: THAILAND, Phureua Mountain range, Loei Province, Chatchanat Farm, isolated on cricket on the leaf litter, 26 September 2006, K. Tasanathai, S. Mongkolsamrit, B. Thongnuch, R. Ridkaew, P. Srikitikulchai and C. Chuaseeharonnachai (BBH 18836, holotype); ex-type living culture, BCC 23107.
Other specimens examined: THAILAND, Loei Province, Chatchanat Farm, on Gryllacrididae, Orthoptera in forest habitat, (17° 27′ 17.99″N, 101° 21′ 47.99″E), BBH 18831 (BCC 23101), BBH 17765 (BCC 23102), BBH 17766 (BCC 23103), BBH 18834 (BCC 23105), BBH 18835 (BCC 23106) 26 September 2006, K. Tasanathai, S. Mongkolsamrit, B. Thongnuch, R. Ridkaew, P. Srikitikulchai, and C. Chuaseeharonnachai. BBH18832, (BCC23242), BBH18833, 26 September 2006, K. Tasanathai, S. Mongkolsamrit, B. Thongnuch, R. Ridkaew, P. Srikitikulchai, and C. Chuaseeharonnachai; Kaeng Krachan National Park, (12°54′24.00″N, 99°38′53.00″E), BBH 15059, (BCC 21108), 14 November 2005, K. Tasanathai, B. Thongnuch, R. Ridkaew.
Discosiaceae Maharachch. & K.D. Hyde
In their molecular data analyses, Senanayake et al. (2015) showed that Discosia sensu stricto groups as a distinct clade in Amphisphaeriales. Besides, Discosia, the clade comprises Adisciso, Discostroma, Sarcostroma and Seimatosporium (Fig. 81).
Phylogram generated from Maximum Parsimony (MP) analysis based on combined LSU and ITS sequence data of Seimatosporium species. Maximum parsimony bootstrap support values greater than 50 % are indicated above the nodes, and branches with Bayesian posterior probabilities greater than 0.95 are given in bold. The ex-type strains are in bold; the new isolates are in blue. The tree is rooted with Immersidiscosia eucalypti (AB593722). We used GenBank accession numbers of LSU sequence data instead of culture collection numbers as most of the strains only have accession numbers. The tree is based on species in (Barber et al. 2011)
Seimatosporium Corda, Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 3(13): 79 (1833)
Seimatosporium is characterized by fusiform or clavate or obovoid, (2−)3(−5)-septate, continuous or occasionally constricted, eguttulate, brown medium conidia (Sutton 1980; Nag Raj 1993; Maharachchikumbura et al. 2014; Norphanphoun et al. 2015). The species might have apical or/ and basal appendages or without any appendages (Sutton 1980; Nag Raj 1993).
-
192.
Seimatosporium sorbi Wijayawardene, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF551181; Facesoffungi number: FoF00669; Fig. 82
Etymology: Name after the host genus on which the taxon occurs.
Holotype: MFLU 15-0744
Saprobic on dead branches and stems of Sorbus torminalis L. Sexual morph: Undetermined. Asexual morph: Conidiomata 280–340 μm diam., 180–220 μm high, acervular, unilocular, subglobose, superficial, solitary, dark brown to black, apapillate ostiolate, dehiscent irregular. Conidiomata wall multi-layered, outer 20–30 μm wide, composed of brown, thin-walled cells of textura angularis, inner wall thin, hyaline. Conidiophores 8–20 × 1.5 − 3 μm, long, cylindrical, branched, hyaline to sub-hyaline, smooth-walled. Conidiogenous cells holoblastic, annellidic, cylindrical, simple, integrated, determinate, hyaline. Conidia 13–16.5 × 5.5 − 7.5 μm (\( \overline{x} \) = 14.95 × 6.47 μm, n = 20), clavate or obovoid, occasionally truncate base, straight, with 3-septate, septa brown to dark brown, slightly constricted at the septa, eguttulate, pale brown, with hyaline to sub-hyaline basal cell, smooth-walled, lacking appendages.
Culture characteristics: On PDA slow growing, white to light brown from above, pale brown from below, with sparse mycelium, flat, margin uneven, attaining a diam. of 2 cm in 7 days at 18 °C.
Material examined: ITALY, Province of Forlì-Cesena [FC], Fiumicello–Premilcuore, on dead leaf of Sorbus torminalis (Rosaceae), 8 May 2013, Erio Camporesi, IT 1233 (MFLU 15-0744, holotype); Ibid. (HKAS 88745, paratype), living cultures MFLUCC 14-0469, GUCC 65.
Notes: Our collection from Sorbus torminalis morphologically resembles Seimatosporium, and molecular analyses (Fig. 81) showed that it groups with Seimatosporium sensu stricto, hence it is compared with taxa in Sutton (1980) and Nag Raj (1993). Farr and Rossman (2015) reported S. cassiopes (Rostr.) B. Sutton (conidia 18.5 − 23 × 7.5 − 8.5 μm, fide Sutton 1980) and S. dacicum (Săvul. & Hulea) B. Sutton (conidia 12–15 × 4–6 μm, fide Sutton 1980) from Sorbus spp. Nevertheless, (Nag Raj 1993) listed both species under unexamined and excluded species. In molecular analyses, our taxon groups with S. vaccinii (Fuckel) B. Erikss. (conidia 13–18.5 × 4.5 − 5.5 μm, fide Sutton 1980). Our taxon is morphologically distinct from other species of Seimatosporium (Sutton 1980; Nag Raj 1993) and thus is introduced as a new species.
-
193.
Seimatosporium pseudorosarum Wijayawardene, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF551182; Facesoffungi number: FoF00670; Fig. 83
Etymology: Named as its morphological similarity to Seimatosporium rosarum.
Holotype: MFLU 15-0745
Saprobic or endophytic on living branches and stems of Rosa canina L. Sexual morph: Undetermined. Asexual morph: Conidiomata 200–250 μm diam., 100–150 μm high, acervular, unilocular, subglobose, superficial to subepidermal, solitary, black, ostiolate, apapillate. Conidiomata wall multi-layered, outer wall thick, composed of dark brown cells of textura angularis, inner wall thin, hyaline. Paraphyses 10–22 μm, filiform, cylindrical, aseptate, hyaline, smooth-walled. Conidiophores 6–25 × 2–4 μm, long, cylindrical, branched, hyaline, smooth-walled. Conidiogenous cells holoblastic, annellidic, simple, integrated, determinate, hyaline. Conidia 9–14 × 4–6 μm (\( \overline{x} \) = 11.31 × 6.06 μm, n = 20), ellipsoid to obovoid, obtuse apex and base, straight, 2-septate, with brown to dark brown septa, constricted at the septa, eguttulate, medium brown, with hyaline to sub-hyaline basal cell, smooth-walled, with or without basal appendage, if present appendages 9–30 μm, unbranched.
Culture characteristics: On PDA slow growing, white from above, pale brown from below, flat, margin even, attaining a diam. of 2 cm in 7 days at 18 °C.
Material examined: ITALY, Province of Rimini [RN], near Pennabilli–Rimini, on dead branch of Rosa canina (Rosaceae), 22 March 2014, Erio Camporesi, IT 1770 (MFLU 15-0745 holotype), living cultures MFLUCC 14–0466, GUCC 66.
Notes: Farr and Rossman (2015) reported Seimatosporium caudatum (Preuss) Shoemaker, S. discosioides (Ellis & Everh.) Shoemaker, S. lichenicola (Corda) Shoemaker & E. Müll., S. rosae Corda, S. rosarum (Henn.) B. Sutton and S. salicinum (Corda) Nag Raj from Rosa spp. Our collection morphologically resembles S. rosae, S. rosarum and S. salicinum as it has conidia with only basal appendages. However, S. pseudorosarum has only 2-septate conidia, while all other species have 2–3 or 3 septa. Hence, we introduce our species as a new species of Seimatosporium. The following taxonomic key can be used to distinguish S. pseudorosarum from other species occurring on Rosa spp.
-
1. Conidia with either apical or basal appendages ................. 2
-
1. Conidia lacking apical or basal appendages ................................................................... S. lichenicola
-
2. Conidia with appendages at both ends ................................................................... S. discosioides
-
2. Conidia with or lacking apical and/or basal appendages ................................................. 3
-
3. Conidia with only basal appendages .................................. 4
-
3. Conidia with or lacking basal or apical appendages ..................................... S. caudatum
-
4. Conidia only 2-septate ........................... S. pseudorosarum
-
4. Conidia with 2–3 septa ............................................. 5
-
5. Conidia with only 3 septa ...................................... S. rosae
-
5. Conidia with 2–3 septa ............................................. 6
-
6. Conidia 9.5 − 12 × 3–5 μm ................................. S. rosarum
-
6. Conidia 11–17 × 4–6 μm .......................... S. salicinum
Glomerellaceae Locq. ex Seifert & W. Gams
The family Glomerellaceae was invalidly published by Locquin (1984), validated in Zhang et al. (2006), and accepted as one of the three families of Glomerellales in Réblová et al. (2011). Glomerellaceae is a monotypic family characterized by the Glomerella sexual morph and a Colletotrichum asexual morph (Maharachchikumbura et al. 2015).
Colletotrichum Corda, in Sturm, Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 3(12): 41, tab. 21 (1831)
This genus has been placed in Glomerellaceae by Réblová et al. (2011) which has also been confirmed by the recent study of Maharachchikumbura et al. (2015). Maharachchikumbura et al. (2015) used Colletotrichum over its sexual name Glomerella. The most recent treatment of this genus is by Hyde et al. (2014) which was based on multi-gene phylogeny (Fig. 84).
Phylogram generated from parsimony analysis based on combined ITS, GADPH, CHS, ACT and β-tubulin sequence data of Colletotrichum. Parsimony bootstrap support values greater than 50 % and Bayesian posterior probabilities greater than 0.75 are indicated above or below the nodes. The ex-type strains are in bold; the new isolates are in blue. The tree is rooted with Monilochaetes infuscans CBS 869.96
-
194.
Colletotrichum aciculare Jayawardena, Tangthirasunun & K.D. Hyde, sp. nov.
Index Fungorum number: IF551298; Facesoffungi number: FoF: 00850; Fig. 85
Colletotrichum aciculare (holotype) a Herbarium specimen b Black acervuli on host c Section of the conidioma d Setae e, f Hyaline conidiogenous cells g Hyaline conidia h, j Germinating conidium i Appressoria k Upper view of the colony l Lower view of the colony. Scale bars: b = 200 μm, f = 50 μm, c = 20 μm, g, h, j = 15 μm, d, e = 10 μm, i = 5 μm
Etymology: Based on the acicular conidia.
Holotype: MFLU 13-0280
Saprobic on leaves. Sexual morph: Undetermined. Asexual morph: Conidiomata 200–450 μm (\( \overline{x} \) = 370 μm, n = 10) diam., solitary, an acervulus, black, globose, conidiophores and setae formed on a cushion of pale brown angular cells. Setae abundant, 109–215 μm long, dark brown to medium brown, paler towards the apex, smooth-walled to verruculose, straight or slightly curved, 1–4-septate, base cylindrical, conical with slightly inflated, 3–5 μm diam., apex slightly acute to roundish. Conidiophores septate, to 32 μm long, hyaline to pale brown, smooth–walled. Conidiogenous cells 17–26 × 3–4 μm (\( \overline{x} \) = 21 × 3 μm, n = 20), hyaline, smooth–walled, cylindrical to elongated ampulliform, opening 1–4 μm diam., collarette sometimes visible, periclinal thickening visible. Conidia 37–43 × 2–5 μm (\( \overline{x} \) = 40 × 4 μm, n = 40), hyaline, acicular, aseptate, curved, both sides gradually tapering towards the round to slightly acute apex and truncate base, guttulate, smooth-walled or verruculose, L/W ratio = 8.9. Appressoria 8–12 × 2–4 μm (\( \overline{x} \) = 12 × 3 μm, n = 10), solitary to aggregated, globose to subglobose, pale brown, smooth-walled.
Cultural characteristics: Colonies on PDA reaching 60 mm in 7 days at 28 °C, margin feathery, white aerial mycelium becoming pale yellow towards the edge from above, reverse peach coloured (4), becoming black with age.
Material examined: THAILAND, Chiang Rai, on a dead leaf, host unknown, 15 July 2012, Yee Than NTCL91-2 (MFLU 13-0280, holotype); ex-type living cultures, MFLUCC 12–0425, KUMCC, CFTCC.
Notes: Colletotrichum aciculare clusters in the truncatum species complex (Fig. 85), forming a separate branch with 80 % bootstrap support and 0.95 Bayesian posterior probability. This species differs from C. curcumae (Syd. & P. Syd.) E.J. Butler & Bisby by having dark brown to medium brown setae, becoming pale towards the apex, while setae of C. curcumae are dark brown up to the apex (Damm et al. 2009). The L/W ratio of C. aciculare conidia (8.9) is larger than the L/W ratio of C. curcumae conidia (4.2) (Damm et al. 2009).
-
195.
Colletotrichum fusiforme Jayawardena, Bhat, Tangthirasunun & K.D. Hyde, sp. nov.
Index Fungorum number: IF551299; Facesoffungi number: FoF00851; Fig. 86
Colletotrichum fusiforme (holotype) a Herbarium specimen b Black acervuli c Acute apex of the setae d Setae with conidiophores e Conidiophores f Hyaline conidia g Germinating conidium h Appressoria i Upper view of the colony j Lower view of the colony. Scale bars: b = 100 μm, c, d = 20 μm, e–g = 15 μm, h = 10 μm
Etymology: Based on the fusiform conidia.
Holotype: MFLU 13-0291
Saprobic on leaves. Sexual morph: Undetermined. Asexual morph: Conidiomata 70–140 μm (\( \overline{x} \) = 80 μm, n = 10) diam., solitary, an acervulus, black, globose- oval with pale yellow spore mass. Setae abundant, 95–225 μm long, dark brown to medium brown, smooth-walled, curved, 1–4-septate, base cylindrical, conical with slightly inflated, 3–5 μm diam., apex acute. Conidiophores septate, to 31 μm long, hyaline to pale brown, smooth–walled densely clustered. Conidiogenous cells 6–9 × 1–2 μm (\( \overline{x} \) = 7 × 2 μm, n = 20), reduced, hyaline, smooth–walled, cylindrical, collarette rarely observed, periclinal thickening not observed. Conidia 34–44 × 3–5 μm (\( \overline{x} \) = 35 × 4 μm, n = 40), hyaline, fusiform, aseptate, curved, base rounded and truncate with a slightly acute apex, guttulate, smooth-walled or verruculose, L/W ratio = 8.2. Appressoria 6–11 × 3–4 μm (\( \overline{x} \) = 10 × 3 μm, n = 10) solitary to aggregated, in short chains, oval, medium to dark brown, smooth-walled.
Cultural characteristics: Colonies on PDA reaching 55 mm in 7 days at 28 °C, margin undulate, white aerial mycelium becoming olivaceous green to dull green olivaceous green towards the edge from forward, reverse olivaceous green to dull green, concentric.
Material examined: THAILAND, Chiang Rai, on a dead leaf, host unknown, 14 July 2012, J. Bhat NTCL98, (MFLU 13-0291, holotype); ex-type living cultures, MFLUCC 12–0437, KUMCC, CFTCC.
Notes: Colletotrichum fusiforme clusters in the truncatum species complex (Fig. 86), forming a separate branch with 86 % bootstrap support and 0.96 Bayesian posterior probability. This species differs from its sister taxon C. truncatum (Schwein.) Andrus & W.D. Moore by having dark brown to medium brown setae, while the setae of C. truncatum are hyaline to pale brown. The L/W ratio of C. fusiforme conidia is larger (8.2) than that of C. truncatum (5.7). Conidia of C. fusiforme are curved, with a rounded and truncate base and slightly acute apex, while in C. truncatum conidia are strongly curved at the apex (Damm et al. 2009).
-
196.
Colletotrichum hymenocallidicola Chethana, Tangthirasunun, Jayawardena & K.D. Hyde, sp. nov.
Index Fungorum number: IF551304; Facesoffungi number: FoF: 00870; Fig. 87
Colletotrichum hymenocallidicola (holotype) a Specimen with conidiomata b Black acervuli on host c Light brown setae d, e Hyaline conidiogenous cells f Hyaline conidia g Germinating conidium h Upper view of the colony i Reverse view of the colony. Scale bars: b = 100 μm, c, f = 20 μm, d, e = 10 μm, g = 5 μm
Etymology: The specific epithet hymenocallidicola is named after the host Hymenocallis from which the taxon was collected.
Holotype: MFLU 13–0292
Pathogenic on leaves. Sexual morph: Undetermined. Asexual morph: Conidiomata 100–130 μm (\( \overline{\mathrm{x}} \) = 116 μm, n = 10) diam., solitary, an acervulus, black, oval. Setae 51–56 μm long, pale to dark brown, smooth-walled to verruculose, straight, 1–3-septate, base cylindrical, 2–4 μm diam., apex acute to roundish. Conidiogenous cells 10–20 × 3–5 μm (\( \overline{\mathrm{x}} \) = 13 × 4 μm, n = 20), enteroblastic, hyaline, smooth-walled, cylindrical to clavate, collarette not visible. Conidia 11–19 × 4–6 μm (\( \overline{\mathrm{x}} \) = 16 × 5 μm, n = 20), hyaline, cylindrical, aseptate, slightly curved, with rounded ends, guttulate, smooth-walled, L/W ratio 3.2. Appresoria not observed.
Cultural characteristics: Conidia germinating on water agar mostly from both ends. Colonies on PDA, edge entire, white to pale white, dense, cottony mycelium on the surface and reverse with pale white mycelium.
Material examined: THAILAND, Phang Nga, on a living leaf of Hymenocallis sp. (Amaryllidaceae), 3 August 2012, Narumon Tangthirasunun NTCL99 (MFLU 13–0292, holotype); ex-type living cultures, MFLUCC 12–0531, KUMCC 15–0076, CFTCC 15–0003.
Notes: Based on phylogenetic analyses and morphological comparison, our isolate belongs to Colletotrichum. Colletotrichum hymenocallidicola appears as a singleton species and forms a sister clade (Fig. 81) to C. brevisporum Noireung et al. (BCC 38876) with high bootstrap support (100 %) and Bayesian posterior probabilities (1.00). Colletotrichum brevisporum differs from C. hymenocallidicola in their smaller conidia (12–17 × 5–6 μm, L/W ratio 2.52) and fewer guttules. Original description of C. brevisporum does not contain setae characters (Noireung et al. 2012).
Halosphaeriaceae E. Müll. & Arx ex Kohlm.
The status and classification of the family was recently updated in Jones et al. (2015) and is not further discussed here (Fig. 88).
Bayesian phylogenetic tree showing the placement of Tinhaudeus formosanus based on a combined analysis of the 18S and 28S rRNA genes (GenBank accession nos. after species name, respectively) in BEASTv.1.7.2 (Drummond & Rambaut 2007) with the following analytical settings: GTR, estimated base frequency, gamma + invariant sites, number of gamma categories set at 4, a strict clock with estimated evolutionary rate and normal rate distribution, Coalescent: Constant Size as the speciation model, 10 million generations with parameters and trees sampled every 1000 generations. Convergence of the analyses was checked in Tracer v1.5 (Drummond & Rambaut 2007) and the effective sample size (ESS) of the parameter statistics >200 was ensured. The first 10 % of the trees were treated as burn-in and discarded. A summary tree was produced using TreeAnnotator v1.7.2 (Drummond & Rambaut 2007) and viewed and edited in FigTree v1.3.1 (Rambaut 2009). Bayesian posterior probabilities greater than 0.75 are near the nodes. The ex-type strains are in bold and the new isolates are in blue. The tree is rooted with Microascus trigonosporus AFTOL-ID 914 and Petriella setifera AFTOL-ID 956
-
197.
Tinhaudeus K.L. Pang, S.Y. Guo & E.B.G. Jones, gen. nov.
MycoBank number: MB 812936; Facesoffungi number: FoF00980
Etymology: ‘Tin Hau’, Cantonese for the sea goddess and ‘deus’ meaning ‘god’ in Latin.
Saprobic on decaying wood in mangroves. Sexual morph: Ascomata globose to subglobose, immersed, ostiolate, with long necks, coriaceous, light- to dark-coloured. Necks periphysate. Peridium one-layered, composed of elongate cells with large lumina of textura angularis. Catenophyses present, persistent. Asci 8-spored, clavate, with a long stalk, unitunicate, thin-walled, slightly thickened at the apex, persistent, developing at the base of the ascoma venter on a convex cushion of ascogenous cells. Ascospores ellipsoidal, 1-septate, not or slightly constricted at the septum, hyaline. Appendages bipolar, initially adpressed to the ascospore wall and extended over the mid-septum, unravelling immediately in sea water to form a long thin filament. Asexual morph: Undetermined.
Type species: Tinhaudeus formosanus K.L. Pang, S.Y. Guo & E.B.G. Jones
Notes: Ascospores of Tinhaudeus formosanus with bipolar unfurling appendages superficially link this species to a number of genera in the Halosphaeriaceae, but with the retraction of plasmalemma in T. formosanus, it particularly resembles Aniptodera, Phaeonectriella, Saagaromyces and Tirispora (Pang et al. 2003). Phaeonectriella species have dark-coloured ascospores and its appendages, if present, may be ephemeral (Hyde et al. 1999). In Tirispora (T. unicaudata E.B.G. Jones & Vrijmoed), asci are apically with a pore and ascospores are thick-walled with one appendage (Jones et al. 1994). Tinhaudeus formosanus is morphologically similar to Aniptodera mangrovei K.D. Hyde and A. salsuginosa Nakagiri & Tad. Ito, but the ascospores of T. formosanus are intermediate in size between the two Aniptodera species (Jones et al. 2009).
Tinhaudeus formosanus also closely resembles Saagaromyces, in particular, S. abonnis (Kohlm.) K.L. Pang & E.B.G. Jones, including their mangrove wood occurrence, asci with a slightly thickened apex and retraction of plasmalemma, but the asci, ascospores and appendages of the former species are smaller. Therefore, additional sequences from collections of S. abonnis made in Hong Kong and Taiwan were included in the phylogenetic analysis. Phylogenetically, two isolates of T. formosanus, collected from two different mangroves in Taiwan, are not related to Aniptodera (A. chesapeakensis Shearer & M.A. Mill.), Phaeonectriella (P. lignicola R.A. Eaton & E.B.G. Jones), Saagaromyces (S. ratnagiriensis (S.D. Patil & Borse) K.L. Pang & E.B.G. Jones) and Tirispora (T. unicaudata) (Fig. 2), but group with Sablecola chinensis E.B.G. Jones, K.L. Pang & Vrijmoed and Remispora maritima Linder, species with deliquescing asci and no unfurling ascospore appendages. Saagaromyces abonnis isolates formed a well-supported monophyletic clade with S. mangrovei Abdel-Wahab, Bahkali & E.B.G. Jones (Liu et al. 2015), but with no affinity with T. formosanus.
-
198.
Tinhaudeus formosanus K.L. Pang, S.Y. Guo & E.B.G. Jones, sp. nov.
MycoBank number: MB 812937; Facesoffungi number: FoF00981; Fig. 89
Holotype: F28727 (National Museum of Natural Science, Taiwan)
Etymology: In reference to Taiwan where the holotype was collected.
Saprobic on decaying mangrove wood. Sexual morph: Ascomata 211-(295)-442 × 114-(180)-263 μm (n = 6), light- to dark-coloured when mature, globose to subglobose, solitary or gregarious, immersed, coriaceous, ostiolate. Necks 104-(141)-174 μm long, 38-(64)-80 μm diam. (n = 2), periphysate. Peridium 7-(15)-31 μm (n = 6), composed of one layer of elongate cells with large lumina of textura angularis. Catenophyses present, persistent. Asci 133-(159)-181 × 32-(39)-49 μm (n = 15), clavate, unitunicate, 8-spored, thin-walled, persistent, with a long stalk, developing from inner wall of ascoma base, with a slightly thickened apex, plasmalemma retracted. Ascospores 26-(33)-38 × 10-(12)-14 μm (n = 61), ellipsoidal, hyaline, 1-septate, not or slightly constricted at the septum. Appendages bipolar, initially adpressed to the ascospore wall and extended over the mid-septum, unravelling immediately in sea water to form a long thin filament. Asexual morph: Undetermined.
Material examined: TAIWAN, Chunan, on a piece of decaying mangrove wood, 27 January 2011, Ka-Lai Pang, F28727 (National Museum of Natural Science, Taiwan, holotype); ex-type living culture, BCRC FU30496 (Fig. 90).
Pestalotiopsidaceae Maharachch. & K.D. Hyde
Senanayake et al. (2015) resurrected the order Amphisphaeriales based on molecular data and morphology and found that Pestalotiopsis and related genera were distinct from the families Bartaliniaceae and Discosiaceae. Hence, the new family Pestalotiopsidaceae is introduced for the genera Ciliochorella, Lepteutypa, Monochaetia, Neopestalotiopsis, Pestalotiopsis, Pseudopestalotiopsis and Seiridium.
-
199.
Pestalotiopsis subshorea Yong Wang bis, Y. Song, K. Geng & K.D. Hyde, sp. nov.
Index Fungorum number: IF551449; Facesoffungi number: FoF01045; Fig. 91
Etymology: The specific epithet is referring to Pestalotiopsis shorea, which is similar to this new taxon.
Holotype: HGUP d4118
Endophyte in leaves of Micelia hedyosperma Law. Sexual morph: Undetermined. Asexual morph: Conidiophores indistinct. Conidiogenous cells discrete, simple, filiform, thin-walled, hyaline. Conidia fusoid, straight to slightly curved, 4-septate, 18–24.5 × 5.5–7 μm (\( \overline{\mathrm{x}} \) = 22 × 6 μm), basal cell conic, hyaline or pale olivaceous, thin-walled and verruculose, 3.3–5.3 μm long (\( \overline{\mathrm{x}} \) = 4.5 μm), with three median cells, doliform to cylindrical, constricted at the septa, concolourous, olivaceous to brown, septa and periclinal walls darker than the rest of the cell, wall rugose, together 12–16 μm long (\( \overline{\mathrm{x}} \) = 14 μm) second cell from base 3.5–6 μm (\( \overline{\mathrm{x}} \) = 5 μm); third cell 4–6 μm (\( \overline{\mathrm{x}} \) = 4.5 μm); fourth cell 4–6 μm (\( \overline{\mathrm{x}} \) =5 μm); apical cell hyaline, conic to subcylindrical 3–5.3 μm long (\( \overline{\mathrm{x}} \) = 4 μm); with 2–3 tubular apical appendages, arising from the apex of the apical cell, 10–28 μm long (\( \overline{\mathrm{x}} \) = 17 μm), unequal; basal appendage present 2–5.5 μm (\( \overline{\mathrm{x}} \) = 3.5 μm), rarely absent.
Culture characteristics: Colonies on PDA reaching 7 cm diam. after 6 days at 25 °C, edge entire, whitish, with dense aerial mycelium on surface, fruiting bodies black; reverse of culture whitish.
Material examined: CHINA, Guizhou Province, Guiyang City, Xiaohe District, on living leaves of unidentified tree, Kun Geng, 25 September 2012 (HGUP d4118, holotype); ex-type culture, HGUP4118; CHINA, Guangxi Province, on living leaves of Micelia hedyosperma (Magnoliaceae), Jiguang Wei, 2011 (HGUP4279, paratype).
Notes: Phylogenetic analysis of three combined gene loci (ITS+β-tubulin+EF) show Pestalotiopsis subshorea (HGUP 4118 and HGUP 4279) forming a separate branch as a sister taxon to P. shorea (Fig. 90). Pestalotiopsis shorea was isolated from Shorea obtusa in Thailand (Song et al. 2014). Both species produce concolourous conidia and their conidial size overlap. The morphological distinction of P. subshorea and P. shorea is based on the length of apical and basal appendages. Apical appendages of P. subshorea are longer than P. shorea (3–5 μm long), but the basal appendage is shorter than P. shorea or lacking. Thus, we confirm the novelty of P. subshorea.
-
200.
Pestalotiopsis dracaenea Yong Wang bis, Y. Song, K. Geng & K.D. Hyde, sp. nov.
Index Fungorum number: IF551444; Facesoffungi number: FoF01045; Fig. 92
Etymology: The specific epithet refers to the host, Dracaena fragrans, from which the taxon was isolated.
Holotype: HGUPd 4037
Associated with diseased leaves of Dracaena fragrans (L.) Ker Gawl. Sexual morph: undetermined. Asexual morph: Conidiophores indistinct. Conidiogenous cells lageniform. Conidia fusoid to ellipsoid, straight to slightly curved, 4-septate, 18–24 × 6.5–8.5 μm (\( \overline{\mathrm{x}} \) = 20.5 × 7.5 μm), basal cell obconic, hyaline, thin-walled and verruculose, 3–4.5 μm long (\( \overline{\mathrm{x}} \) = 4 μm), with three median cells, subcylindrical to cylindrical, verruculose, slightly constricted at the septa, concolourous, brown, together 11.5–16 μm long (\( \overline{\mathrm{x}} \) = 13.5 μm) second cell from base 3.5–5.5 μm (\( \overline{\mathrm{x}} \) = 4.5 μm); third cell 4–5.5 μm (\( \overline{\mathrm{x}} \) = 5.5 μm); fourth cell 4–5.5 μm (\( \overline{\mathrm{x}} \) = 4.5 μm); apical cell hyaline, obconic to subcylindrical, 3–5.5 μm long (\( \overline{\mathrm{x}} \) = 4.4 μm); with 2–4 tubular appendages, 6.5–15.5 μm long (\( \overline{\mathrm{x}} \) = 11 μm), arising from the apex of the apical cell, unequal; basal appendage present, filiform, 3–7 μm long (\( \overline{\mathrm{x}} \) = 4.2 μm).
Culture characteristics: Colonies on PDA reaching 7 cm diam. after 7 days at 25 °C, edge crenate, whitish, with aerial mycelium on surface, with black, gregarious fruiting bodies; reverse of the culture white.
Material examined: CHINA, Hainan Province, Xinglong County, Tropical Botanical Garden, on living leaves of Dracaena fragrans (Asparagaceae), Kun Geng, 8 March 2012 (HGUP d4037, holotype); ex-type culture HGUP4037; CHINA, Hainan Province, Xinglong County, Tropical Botanic Garden, on living leaves of Dracaena fragrans, Kun Geng, 8 March 2012 (HGUP 4032, paratype).
Notes: We obtained two isolates of P. dracaenea (HGUP 4037 and HGUP 4032) which formed a relative independent branch with 100 % bootstrap value (Fig. 90), and had a close relationship with P. diploclisiae P. diploclisiae Maharachch., K.D. Hyde & Crous isolated on fruit of Diploclisia glaucescens and Psychotria tutcheri in Hong Kong, China and P. humus isolated on fruits of Llex cinerea in Hong Kong and soil in Papua New Guinea. Pestalotiopsis dracaenea differs from P. humus Maharachch., K.D. Hyde & Crous (18.5–22 × 5–7 μm) and P. diploclisiae (22–26.5 × 5–6.5 μm) by conidial morphology, viz. wider conidia (6.5–8.5 μm) and longer apical (6.5–15.5 μm long) and basal (3–7 μm) appendages for P. humus, but shorter apical appendages than P. diploclisiae (13–19 μm).
-
201.
Pestalotiopsis montellica (Sacc. & Voglino) Tak. Kobay., Trans. Mycol. Soc. Japan 15(4): 381 (1974)
Facesoffungi number: FoF01043; Fig. 93
= Pestalotia montellica Sacc. & Voglino, Atti Accad. Sci. Soc. Ven.-Trent. Sci. Nat. 9(2): 9 (1892)
Saprobic on dead plant material. Sexual morph: Undetermined. Asexual morph: Conidiomata pycnidial in culture on PDA, globose, scattered and confluent, semi-immersed, dark brown. Conidiophores indistinct. Conidiogenous cells lageniform to subcylindrical, colourless, smooth, proliferation once or twice. Conidia fusoid to ellipsoid, straight to slightly curved, 4-septate, 23–29 × 5.5–7 μm (\( \overline{\mathrm{x}} \) = 25 × 6.1 μm), basal cell obconic, colourless, thin- and verruculose, 5–6 μm long (\( \overline{\mathrm{x}} \) = 5.3 μm), with three median cells, subcylindrical, with thick verruculose walls, constricted at the septa, concolourous, pale brown, together 14–16.5 μm long (\( \overline{\mathrm{x}} \) = 15.2 μm) second cell from base 4.5–6.5 μm (\( \overline{\mathrm{x}} \) = 5.8 μm); third cell 4–6 μm (\( \overline{\mathrm{x}} \) = 5.2 μm); fourth cell 5–6 μm (\( \overline{\mathrm{x}} \) = 5.2 μm); apical cell colourless, obconic, acute at the apex, 4–5 μm long (\( \overline{\mathrm{x}} \) = 4.3 μm); with 4 tubular appendages, 14–20 μm long, one arising from the apex and rest arising from just above the septum separating upper median and apical cell; basal appendage present, 4–5 μm long (\( \overline{\mathrm{x}} \) = 4.5 μm).
Cultural characteristics: Colonies on PDA reaching 7 cm diam. after 7 days at 25 °C, edge lobate, whitish yellow, with dense, aerial mycelium on surface, with black, gregarious fruiting bodies; reverse of the colony whitish yellow.
Material examined: CHINA, Yunnan Province, on dead plant material, Wenping Wu (NN042849, MFLUCC 12-0279).
Notes: Pestalotiopsis montellica was described from the leaves of Quercus sp. in Italy and is well characterized and easily recognizable by the unique appendages attached to the apical cell. The arrangement of apical appendages in P. montellica is comparable with P. jesteri (Strobel et al. 2000). However, P. jesteri Strobel et al. differs from P. montellica by the presence of knobbed apical appendages. A megablast search of the NCBIs GenBank nucleotide sequence database using the sequences of P. montellica retrieves as closest hits P. jesteri (ITS: GenBank KM199380; Identities = 512/536(96 %), Gaps = 8/536(1 %); TUB GenBank KM199468; Identities = 403/444(91 %), Gaps = 7/444(1 %)). The attachment and arrangement of apical appendages at the apical cell are noticeably distinct in both species. Previously Maharachchikumbura et al. (2012) misidentified this isolate as a P. montellica.
Togniniaceae Réblová et al.
The family Togniniaceae comprises three genera, including Conidiotheca and Togninia (Mostert et al. 2006; Réblová and Mostert 2007; Maharachchikumbura et al. 2015). Phylogenetic analysis of 18S small subunit (SSU) rRNA was used to confirm that Togninia is the sexual morph of Phaeoacremonium (Mostert et al. 2003). Togniniaceae is a single family in the order the Togniniales (see recently updated in Maharachchikumbura et al. 2015) (Fig. 94).
The most parsimonious tree resulting from combined analysis of Actin and β- tubulin sequence data for 89 Phaeoacremonium species. The tree is rooted to Pleurostomophora richardsiae. Maximum parsimony bootstrap values ≥50 % are given at the nodes and ≥0.95 for Bayesian probability shown in bold branches. Isolates from this study are in bold. Type isolates are marked withT. Obsolete names or sexual morph name are in parentheses
-
202.
Phaeoacremonium tectonae Doilom & K.D. Hyde, sp. nov.
Index Fungorum number: IF551307; Facesoffungi number: FoF: 00880; Fig. 95
Phaeoacremonium tectonae (holotype) a Sixteen-day-old colony on MEA b Conidiophores with mycelia c Conidiophores with branched in the basal region d Conidiophore with conidia, gradually paler upwards e, i Type II phialides with conidia f Type I phialides with conidia and adjacent lateral phialides g Adelophialides with conidia h Type I phialides with conidia j Conidia k Conidiophores l-m. Conidiophores with conidia Notes: d–g in Lactophenol cotton blue. k–m. photos from Scanning electron microscope (SEM). Scale bars: b–d, k = 10 μm, e–j, l, m = 5 μm
Etymology: Name refers to the host genus Tectona on which the fungus was collected.
Holotype: MFLU 15-1392
Associated with heart rot and laurel wilt symptoms of Tectona grandis L.f.. Sexual morph: Undetermined. Asexual morph Structures on MEA: Mycelium 1–2.5 μm broad, consisting of branched, septate, single or in bundles, partly superficial, partly immersed, hyaline to pale brown, verruculose. Conidiophores (19–) 40–55 (−62) μm long, 1.5–2.7 diam. (\( \overline{\mathrm{x}} \) = 41 × 2 μm), branched in the basal region, arising from aerial or submerged hyphae, erect to slightly curved, up to 3-septate, mostly short, usually unbranched occasionally branched conidiophores, mostly slender, some swollen in the lower part, hyaline to pale brown, gradually paler upwards, smooth to verruculose. Phialides mostly monophialidic, smooth to verruculose; type I and type II phialides most common; type I phialides (1.5–) 3.5–6 (−13) high × (1.3–) 1.7–1.9 (−4) μm (\( \overline{\mathrm{x}} \) = 5 × 1.8 μm), most common, adelophialide, cylindrical, no basal septum; type II phialides (7–) 15–17 (−19) high × (1.5–) 2–2.3 (−3) μm (\( \overline{\mathrm{x}} \) = 15 × 2 μm), predominant, elongate-ampulliform attenuated at the base or subulate, constricted at the base, tapering towards the apex; type III phialides (30–) 48–58 (−62) high × (1.8–) 2.2–2.3 (−2.5) μm (\( \overline{\mathrm{x}} \) = 48 × 2.2 μm), subcylindrical, narrowing gradually to a long neck. Conidia (2–) 3.5–3.7 (−5) high × (0.9–) 1.5–1.7 (−2) μm (\( \overline{\mathrm{x}} \) ± S.D. = 3.5 ± 0.5 × 1.5 ± 0.4 μm, n = 30), hyaline, allantoid to oblong ellipsoidal occasionally reniform, smooth to verrucose.
Culture characteristics: Pure culture was isolated by tissue transplanting technique. Colonies on MEA reaching 12–22 and 21–36 mm diam. after 8 and 16 days in the dark at 25 °C respectively (av = 17 and 26 mm for 8 and 16 days respectively n = 5), circular, flat, woollen tufts forming on older mycelium plugs, edge entire to undulate; after 8 days, white (3A1) above, in reverse yellowish white (3A2); after 16 days white (4A1) in the centre, greyish brown (6D3) to brownish grey (7E2) at the edge, sometimes colony like v-shaped greyish brown (6D3) to brownish grey (7E2) projections above, in reverse greyish brown (6 F3) to yellowish white (4A2) to mixed brownish grey (4E2).
Material examined: THAILAND, Chiang Rai Province, Mae Chan District, on heart rot symptoms of Tectona grandis (Lamiaceae), 23 July 2013, M. Doilom (MFLU 15-1392, holotype: dry culture), ex-type living culture, (MFLUCC 13-0707, MUCL); Chiang Rai Province, Muang District, Mae Khon Subdistrict, on laurel wilt symptom of T. grandis, 23 July 2013, M. Doilom, living culture, (MFLUCC 13-0708); Chiang Rai Province, Mae Suai District, Mae Lao garden, on laurel wilt symptoms of T. grandis, 5 July 2014, M. Doilom (living cultures, MFLUCC 13-0708, MFLUCC 14-1125, MFLUCC 14-1126, MFLUCC 14-1127, MFLUCC 14-1129, MFLUCC 14-1130, MFLUCC 14-1131).
Notes: Phaeoacremonium was established as a hyphomycete genus by Crous et al. (1996) with Pm. parasiticum as the type species. There are several related genera such as Acremonium, Exophiala and Phialophora. However, a clear classification of Phaeoacremonium and its relatives was provided in Crous et al. (1996) and Mostert et al. (2006). Species of Phaeoacremonium are vascular plant pathogens causing wilting and dieback of several woody plants, moreover species can also cause of human disease (Baddley et al. 2006; Damm et al. 2008; Gramaje et al. 2012).
Phaeoacremonium tectonae is introduced here as a new species based on molecular evidence and morphological features. Phaeoacremonium tectonae (MFLUCC 13-0707, MFLUCC 13-0708, MFLUCC 14-1125, MFLUCC 14-1126, MFLUCC 14-1127, MFLUCC 14-1129, MFLUCC 14-1130 and MFLUCC 14-1131) grouped near to, but separate from Pm. argentinense L. Mostert et al. CBS 777.83 (sexual morph Togninia argentinensis L. Mostert et al.) with 88 % MPBP and 0.99 PP support in the combined actin and β-tubulin phylogeny (Fig. 94). All Phaeoacremonium isolates in this study were from heart rot and laurel wilt symptoms in three locations in Chiang Rai Province, Thailand. Phaeoacremonium tectonae differs from Pm. argentinense in conidiophores as in Pm. tectonae (19–) 40–55 (−62) av. 41 μm they are longer than in Pm. argentinense (15–) 16–35 (−44) av. 24 μm. Type I and II phialide are the predominant phialide type of Pm. Tectonae, while Type II and III are the predominant phialide type of Pm. argentinense.
Valsaceae Tul. & C. Tul.
The family Valsaceae was introduced by Tulasne and Tulasne (1861) and later recognized as a family of Diaporthales by Barr (1978). Most species are important pathogenic taxa causing canker and dieback disease on plants, with severe commercial and ecological damage and significant losses worldwide (Adams et al. 2005; Fan et al. 2014, 2015). Previously, Valsaceae was restricted to five genera, i.e., Cytospora with asexual morphs in Valsa, Leucostoma, Valsella, and Valseutypella (Fries 1822; Saccardo 1884; Gvritishvili 1982; Spielman 1985; Adams et al. 2002, 2005; Castlebury et al. 2002). However, all sexual genera were synonymized under Valsa as a subgenus or species with no additional infrageneric rank (Adams et al. 2005). Cytospora (1818) is an older name than Valsa (1849) and the asexual morph is more common in nature; therefore, Valsa species were treated as synonyms of Cytospora (Adams et al. 2005; Fotouhifar et al. 2010; Fan et al. 2015). Cytospora usually produce asexual fruiting bodies that contain either a single or labyrinthine of locules, filamentous conidiophores (or clavate to elongate obovoid asci), and allantoid hyaline conidia (or allantoid hyaline ascospores) (Spielman 1983, 1985; Adams et al. 2005). In moist conditions, the conidia emerge from the fruiting bodies as yellow, orange and later red gelatinous tendrils (Adams et al. 2005, 2006). Cytospora comprises an estimated 110 species in (Kirk et al. 2008a, b), although 570 epithets are recorded in Index Fungorum (2015). Ex-type sequence data, are however, available for few species, thus identification to species level is difficult (Liu et al. 2015). Therefore, a systematic account of the genus Cytospora is needed which takes into account morphology and research into cryptic species and a phylogenetic analysis for Cytospora as in other pathogenic genera is needed (Adams et al. 2002; Fotouhifar et al. 2010; Hyde et al. 2010, 2014; Liu et al. 2015). Phylogenetic trees for Cytospora are presented in Figs. 96 and 97.
Phylogenetic tree based on an alignment of the sequences of the ITS regions of Cytospora, Leucostoma, and Valsa species, generated using the MP, ML and Bayesian posterior probabilities (PP) in PAUP. Numbers separated by a slash (or below and above branches) represent MP and ML bootstrap values >50 %. Thickened branches represent Bayesian posterior probabilities (PP) above 95 % are given at the nodes (MP/PP/ML). The tree is rooted in outgroup taxon Phomopsis vaccinii (ATCC 18451). The species obtained in this study are shown in bold
Maximum Parsimony (MP) majority rule consensus tree for the analyzed Cytospora isolates based on a combined dataset of ITS, LSU, β-tubulin and ACT sequence data. MP and ML bootstrap support values higher than 50 % and Bayesian posterior probabilities (PP) above 95 % are given at the nodes (MP/PP/ML). The tree is rooted with Phomopsis vaccinii (ATCC18451). The strain numbers are mentioned after the species names. New strains are in blue bold and ex-type strains are in black bold
-
203.
Cytospora parasitica C. Norphanphoun, Bulgakov & K.D. Hyde, sp. nov.
Index Fungorum number: IF551378; Facesoffungi Number: FoF01022; Figs. 98 and 99
Cytospora parasitica (holotype) a Stromatal habit in wood b Fruiting bodies on substrate c Surface view of fruiting bodies d Cross section of the stroma showing locules e Peridium f Apex of conidioma g–i Conidiogenous cell with attached conidia j–m Mature conidia n Germinating spore o–p Colonies on PDA (P from below). Scale bars: a = 2 mm, b = 1 mm, c = 500 μm, d = 400 μm, e = 50 μm, f = 200 μm, g = 50 μm, h = 20 μm, i = 10 μm, j–m = 5 μm, n = 30 μm
Etymology: The specific epithet parasitica reflects biological features of the fungus as parasite of trees.
Holotype: MFLU 14–0788
Aggressive necrotrophic pathogen on dying branches, causing canker of apple trees. Sexual morph: Undetermined. Asexual morph: Stromata multi-locules in a stroma, black, circular. Locules composed of numerous interconnecting chambers arranged radially or irregularly within a continuous mass of ectostromatic tissue, one conidiomata per multi-locule. Conidiomata 800–1000 μm diam. pycnidial, solitary, immersed, unilocular, dark brown, with an ostiole. Pycnidial wall 10–12 μm, brown, multi-layered, with 5–7 layers of brown, forming a textura angularis cell, with inner most layer thin, hyaline. Conidiophores reduced to conidiogenous cells. Conidiogenous cells blastic, phialidic, hyaline, smooth, formed from the inner most layer of pycnidium wall. Conidia (6–) 6.5–8 × 1.3–1.5(−2) μm (\( \overline{\mathrm{x}} \) = 7 × 1.75 μm, n = 30) unicellular, allantoid to slightly curved, hyaline, 1–celled, aseptate, smooth–walled.
Culture characteristics: Colonies on PDA growing, reaching 4.5 cm diam. after 11 days at 25 °C, later producing dense mycelium, circular, margin rough white, flat or effuse on the surface, without aerial mycelium.
Material examined: RUSSIA, Rostov Region, Shakhty City, near Grushevsky pond, grove of feral trees in gully, on dead and drying branches of Malus cf. domestica Borkh, 18 May 2014, T. Bulgakov (MFLU 14–0788, holotype); Ibid. (PDD, isotype); ex-type living culture, MFLUCC 14-1055, KUMCC.
Notes: Phylogenetic analyses (Figs. 96 and 97) and morphological comparison indicate that our isolate belongs in Cytospora, where it groups with Valsa malicola and its presumed asexual morph Cytospora schulzeri and are probably species complexes. The latter species was considered as an asexual form of Valsa malicola Z. Urb. and its presumed asexual morph Cytospora schulzeri Sacc. & P. Syd. and are probably species complexes. The latter species was considered as an asexual form of Valsa malicola. Cytospora parasitica has multi-loculate conidiomata with a single ostiole. This distinguishes it from Valsa malicola/Cytospora schulzeri which has multi-loculate conidiomata with 2–11 ostioles (Mehrabi et al. 2011). Cytospora parasitica also has larger conidia. Species identification is difficult, because Cytospora fruiting and vegetative structures, as well as spore size, vary greatly even in the isolates of the same species (Spielman 1985). However, in PDA, C. parasitica produces dense mycelium, with a circular, rough margin comprising white mycelium, but Valsa malicola and its asexual morph produces light greenish yellow to moderate to strong yellow, felty, slightly raised colonies, lacking growth zones (Mehrabi et al. 2011). Thus we introduce Cytospora parasitica as a new species.
-
204.
Cytospora tanaitica C. Norphanphoun, Bulgakov & K.D. Hyde, sp. nov.
Index Fungorum number: IF551377; Facesoffungi Number: FoF01021; Fig. 100
Cytospora tanaitica (holotype) a Stromatal habit in wood b Fruiting bodies on substrate c Surface of fruiting bodies d Cross section of the stroma showing locules, median longitudinal section show locules with independent walls, ostiolar necks converging into a discrete ostiole, and locules surrounded by stroma tissue e Peridium f Apex of conidiomata g Conidiogenous cells h Conidia i Germinating spore. Scale bars: a = 2 mm, b = 1 mm, c–d = 400 μm, e = 30 μm, f = 200 μm, g–h = 10 μm, i = 20 μm
Etymology: The specific epithet Tanaitica refers to the traditional ancient Greek name ‘Tanais’ of Don River and Lower Don region (modern Rostov region of Russia) in which the fungus was first collected.
Holotype: MFLU 14–0790
Pathogenic and saprobic on branches. Sexual morph: Undetermined. Asexual morph: Stromata 2–3-locule in a stroma, semi-immersed in bark, ovoid to elongate ovoid. Locule composed of numerous interconnecting chambers arranged radially or irregularly within a continuous mass of ectostromatic tissue, one conidiomata per one locule. Conidiomata 500–1000 μm diam. pycnidial, solitary, immersed, unilocular, dark brown, with an ostiole. Pycnidial wall 30–80 μm, consists with brown to dark brown, multi-layered of brown, forming a textura angularis cell, with inner most layer thin, hyaline. Conidiophore reduced to conidiogenous cells. Conidiogenous cells blastic, enteroblastic phialidic, hyaline, smooth, formed from the inner most layer of pycnidium wall. Conidia (3–) 3.5–4 × 0.6–0.7 (−1) μm (\( \overline{\mathrm{x}} \) = 3.4 × 0.7 μm, n = 30) unicellular, allantoid to subcylindrical, hyaline, 1–celled, aseptate, smooth–walled.
Culture characteristics: Colonies on PDA growing, reaching 7 cm diam. after 11 days at 25 °C, later produce dense mycelium, circular, rough margin gray to dark green, after 5 days, flat or effused on the surface, without aerial mycelium.
Material examined: RUSSIA, Rostov region, Shakhty City, Central Park, on dead branches of Betula pubescens Ehrh. var. glabrata Wahlenb. (syn. Betula borysthenica Klokov, Betulaceae) in culture, 5 May 2014, T. Bulgakov (MFLU 14–0790, holotype; isotype PDD); ex-type living culture, MFLUCC 14–1057, KUMCC.
Notes: Based on phylogenetic analyses and morphological comparison, our isolate belongs to the genus Cytospora in Valsaceae. Cytospora tanaitica has multi-loculate conidiomata with a single ostiole and shares common walls, and is similar to C. sacculus (Schwein.) Gvrit. on Juglans regia L. in conidia size (4.2 × 1 μm) (Fan et al. 2015). However phylogenetic analysis, using ITS sequence data (Fig. 96) show that Cytospora tanaitica can clearly be distinguished from C. gutnerae Gvrit., C. sacculus (Schwein.) Gvrit. and C. terebinthi Bres. Combined analysis of ITS, LSU, β-tubulin and ACT sequence data groups C. tanaitica in a separate clade from these taxa (Fig. 97).
Xylariaceae Tul. & C. Tul.
Xylariaceae (Xylariales, Ascomycota) constitutes one of the largest families of pyrenomycetous fungi, with 94 accepted genera (Maharachchikumbura et al. 2015). The majority of xylariaceous taxa are saprobes and endophytes, sometimes with known insect association, and only a small number of species are plant pathogens (Stadler et al. 2013; Pažoutová et al. 2013). Morphological characters of stromata, asci and ascospores are widely used to define the limits within the family and to segregate the genera. Ju and Rogers (1996) have traditionally classified Xylariaceae in two major sections based on asexual morphs known as nodulisporium-like and geniculosporium-like. Acanthodochium, Dematophora, Dicyma, Lindquistia, Periconiella, Virgariella, Xylocladium and Xylocoremium have recently been assigned to corresponding sexual morphs or to one of the larger groups, but the status of some genera with “diatrypaceous” libertella-like conidiogeneous structures remains unsettled (Stadler et al. 2013) (Fig. 101).
Phylogram generated from RAxML based on combined ITS and LSU sequenced data for taxa of Xylariaceae. Maximum likelihood bootstrap support values greater than 50 % are indicated above the nodes. Only ex-type and voucher strains are used and the new isolates are in blue. The tree is rooted with Sordaria fimicola
Annulohypoxylon Y.M. Ju, J.D. Rogers & H.M. Hsieh
Annulohypoxylon was introduced by was introduced for the former sect. Annulata sensu Ju and Rogers (1996) (which comprised species with papillate ostioles and such ones with annular disks). Currently 52 species and varieties are recorded in Index Fungorum. Liu et al. (2015) introduced a new species Annulohypoxylon thailandicum.
-
205.
Annulohypoxylon palmicola J.K Liu & K.D Hyde, sp. nov.
Index Fungorum number: IF551428; Facesoffungi number: FoF00984; Figs. 102 and 103
Etymology: Named after the palm host on which the fungus was collected.
Holotype: MFLU 15-0040
Saprobic on palms. Sexual morph Stromata effuse-pulvinate, 3.5–93 mm long, 2.5–22 mm broad, 0.95–1.5 mm high (excluding ostiolar necks), with inconspicuous to 1/3 exposed perithecial mounds, developing within bark, erumpent through the periderm, yielding a dull green pigment in 7 % KOH; surface dull black, strongly uneven owing to stout; stromatal crust strongly carbonaceous; interperithecial tissue blackish, powdery, without visible granules. Ascomata 0.6–0.95 mm high, 0.3–0.7 mm diam., obovoid to flask-shaped, completely encased in thick carbonaceous tissue. Ostioles conical, papillate, encircled with a flattened truncatum-type disc, 0.1–0.2 mm diam. Paraphyses not seen. Asci 74–170 × 4.5 − 7 μm (\( \overline{x} \) = 125 × 5.5 μm, n = 25), 8-spored, unitunicate, cylindrical, fragile and readily deliquescing, with long pedicel, up to 50 μm, without a visible apparent apical apparatus, not bluing in Melzer’s reagent. Ascospores 12–14.5 × 3.5 − 5 μm (\( \overline{x} \) = 13.5 × 4 μm, n = 60), fusiform, 1-septate, brown to dark brown, with a faint, curved, germ slit ca. 3/5th of the spore length, lacking cellular appendages or a gelatinous sheath; epispore smooth.
Material examined: THAILAND, Chiang Rai Province, Muang District, Khun Korn Waterfall, on dead sheath of Arenga westerhoutii Griff. (Arecaceae), 11 September 2010; Jian-Kui Liu, JKA 0037 (MFLU 15-0040, holotype), ex-type living culture, MFLUCC 11-0020.
Notes: This is a typical species of Annulohypoxylon in having pale brown fusoid ascospores and is most similar to A. leptascum, but differs by its slightly smaller ostiolar discs 0.1–0.2 vs 0.2–0.3 mm (Hsieh et al. 2005; Fournier et al. 2010). The phylogenetic analysis showed that the Annulohypoxylon species clustered together and formed a well-supported clade; within this clade, the new species A. palmicola clustered together with A. urceolatum (Rehm) Y.M. Ju, et al., and A. leptascum var. macrosporum and shows a close relationship with them?. These species share similar morphological characters, but form distinct species in the phylogenies (Fig. 101).
Biscogniauxia Kuntze, Revis. gen. pl. (Leipzig) 2: 398 (1891)
Biscogniauxia was resurrected and amended by Pouzar (1979, 1986) for a group of Xylariaceae with applanate dark stromata. The asexual morph varies from nodulisporium-like to periconiella-like (Callan and Rogers 1986; Jong and Rogers 1972; Ju and Rogers 1996). Ju et al. (1998) reviewed Biscogniauxia and recognized 49 taxa. Further species have since been introduced (Thienhirun et al. 2003; Tang et al. 2007; Okane et al. 2008; Ju and Rogers 2001).
-
206.
Biscogniauxia effusa Q.R. Li, J.C. Kang & K.D. Hyde, sp. nov.
Index Fungorum number: IF551308; Facesoffungi number: FoF: 00875; Fig. 104
Etymology: In reference to the effuse stromata.
Holotype: GZUH0122
Saprobic on dead bark. Sexual morph: Stromata more than 5 cm long and 3 cm diam., widely effuse, crust-like, flat or slightly undulate, black, bipartite, confluent, without raised margins, ascomatal mounds, with black ostioles. Ascomata 180–230 μm high, 320–450 μm diam. (\( \overline{\mathrm{x}} \) = 210 × 390 μm, n = 10), solitary, obovoid, black, carbonaceous, immersed, encasing a single ascoma, in vertical section, globose to subglobose, ostiolate. Peridium 40–60 μm wide, black. Paraphyses not seen. Asci spore-bearing part 170–200 × 12–14 μm (\( \overline{\mathrm{x}} \) = 190 × 13 μm, n = 30), 8-spored, unitunicate, cylindrical, stipes 25–30 μm long, apically rounded, with a J+, tubular, 7–8 μm high, 5–6 μm wide apical apparatus. Ascospores 25–31 × 8–10 μm (\( \overline{\mathrm{x}} \) = 28 × 9 μm, n = 30), uniseriate, light brown, unicellular, equilateral ellipsoidal, with broadly rounded ends, smooth-walled, with a full length, straight, germ slit. Asexual morph: Undetermined.
Culture characteristics: Colonies growing slowly on PDA, reaching 2 cm diam. after 2 weeks at 25 °C, brown, black in reverse side, cottony, flat, low, dense, with slightly wavy margin.
Material examined: CHINA, Hainan Province, Sanya City, Jianfengling National Nature Reserve, on dead bark of unknown plant, March, 2015, Q.R. Li (GZUH0122, holotype); ex-type living culture, GZUCC0122.
Notes: Biscogniauxia effusa has flattened stromata and is similar to B. atropunctata (Schwein.: Fr.) Pouzar. and B. weldenii (J.D. Rogers) Whalley & Læssøe in ascospore size (Ju et al. 1998). Biscogniauxia atropunctata differs in having 16–22 × 7.5–11 μm, ellipsoid ascospores, with straight germ slits, and shorter 150–170 μm asci with a J+, discoid, apical apparatus (Ju et al. 1998). Biscogniauxia weldenii differs in having 25–28 × 9.5–10.5 μm ascospores ornamented with striations and with an appendage (Ju et al. 1998). Biscogniauxia effusa groups with B. marginata (Fr.) Pouzar and B. arima F. San Martín et al. and forms a well-supported monophyletic clade in the Xylariaceae with 93 % bootstrap support.
Nemania Gray, Nat. Arr. Brit. Pl. (London) 1: 508, 516 (1821)
Nemania is characterized by more or less carbonaceous, dark brown to black stromata that do not release coloured pigments in 10 % KOH, with frequently pale brown ascospores, mostly with an inconspicuous germ-slit (Ju and Rogers 2002). The asexual morph is geniculosporium–like.
-
207.
Nemania fusoidispora Q.R. Li, J.C. Kang & K.D. Hyde, sp. nov.
Index Fungorum number: IF551309; Facesoffungi number: FoF: 00876; Fig. 105
Etymology: In reference to the fusoid ascospores.
Holotype: GZUH0098
Saprobic on dead bark. Sexual morph: Stromata pulvinate, wide spreading, following contours of wood, surface around ostioles mouse grey to dark mouse grey, 1.5 mm high, with inconspicuous ascomatal mounds, internally white between ascomata, carbonaceous, tissue, soft-textured, up to 0.2 mm wide. Ostioles coarsely papillate in discoid areas. Ascomata 0.5–0.6 mm diam., sphaerical to ellipsoid. Peridium 35–55 μm wide, black. Paraphyses 3–5 μm, hyaline, unbranched, septate. Asci 150–240 × 5.5–9.5 μm (\( \overline{\mathrm{x}} \) = 236 × 7.5 μm, n = 30), 8-spored, unitunicate, cylindrical, long-pedicellate, the spore-bearing part 80 μm, apically rounded with J+, inverted, hat-shaped, apical apparatus, 2 μm high, 1.5 μm broad. Ascospores 13–16 × 3–4 μm (\( \overline{\mathrm{x}} \) = 14.1 × 3.3 μm, n = 30), uniseriate to irregularly-biseriate, light brown, fusoid to inequilaterally ellipsoid, with acute ends, lacking a germ slit. Asexual morph: Undetermined.
Material examined: CHINA, Guiyang Province, Guiyang City, on dead bark of unknown plant, March, 2015, Q.R. Li (GZUH 0098, holotype).
Notes: Nemania fusoidispora is similar to N. illita (Schwein.) Pouzar in which ascospores are fusoid and lack a germ slit (Rogers et al. 2008; Ju and Rogers 2002) and differs from other species of Nemania. Nemania fusoidispora differs in having larger ascospores (13–16 × 3–4 μm) than N. illita (11–12 × 3–4 μm). Nemania fusoidispora groups with other Nemania species and forms a monophyletic clade with 87 % bootstrap support. The genus Nemania appears as a sister clade to both Brunneiperidium and Kretzschmaria.
Contributions to Basidiomycota
Agaricomycetes
Agaricaceae Chevall (Fig. 106).
Phylogram generated from Maximum likelihood (MEGA6) analysis based on ITS sequence data of Agaricus. Sequences used in this study have been sampled from previous studies to represent the ten known sections (Thongklang et al. 2014; Zhao et al. 2011; Wang et al. 2015) or newly generated from this work. Maximum likelihood bootstrap support values greater than 50 % and Bayesian posterior probabilities greater than 0.9 are indicated above or below the nodes (BS/PP), new species are annotated in blue. T type specimen
Agaricus L., Sp. pl. 2: 1171 (1753)
The genus Agaricus, the type of the family Agaricaceae contains many edible and medicinal species. Ten sections have been recognized within Agaricus based on molecular and morphological data (Parra 2008, 2013); Parra et al. 2014; Chen et al. 2015), and further lineages revealed by ITS sequences are potential new sections (Zhao et al. 2011). There are several morphological characters which are useful to separate those sections, such as the Schäffer’s and KOH reactions, odour, character of annulus and discolouring on bruising and cutting (Parra 2008, 2013). For example, members from sections Agaricus, Bivelares, Chitonioides, Sanguinolenti and Nigrobrunnescentes exhibit a context turning pink or reddening on exposure (Didukh et al. 2005; Challen et al. 2003; Parra 2008). Recently two new species have been described from China, with reddening discolouration (Zhao et al. 2015). Herein, four new species with two new species with red discolouring are described from Thailand.
-
208.
Agaricus pseudolangei K.D. Hyde & R.L. Zhao, sp. nov.
Index Fungorum number: IF551478; Facesoffungi number: FoF00985; Figs. 107 and 109a–d.
Etymology: refers to the similarity to Agaricus langei in morphology.
Holotype: BBH 19529
Pileus 6–8 cm diam., and 7–9 mm thick, hemisphaerical, convex, plano-convex, sometimes slightly depressed at the disc; surface heavily fibrillose, and breaking into scales towards the margin, light brown (6C3, 6B2). Lamellae free, crowded, lamellulae with 7 series, 4 mm broad, narrow to normal, white, dull red (8C3), brown, edge colour lighter than gill. Stipe 50–90 × 8–12 (base 14–16) mm, cylindrical to subbulbous, long clavate, smooth to silky, white. Annulus thick, rigid, pendent, white, entire, up to 12 mm broad. Odour mushroomy. Context firm, white, no staining on touching, strong scarlet staining on cutting. Macrochemical reaction: KOH reaction negative. Basidiospores 5.5–7 × 4.5–5.2 μm [\( \overline{\mathrm{x}} \) = 6.3 ± 0.5 × 5 ± 0.2, Q = 1.1–1.4, n = 20], ovoid to broad ellipsoid, brown, smooth. Basidia 21–26 × 7–9 μm, clavate, hyaline, smooth. Cheilocystidia present or disrupted in some basidiomata, 20–35 × 5–10 μm, ventricose-capitate, and some with an elongate neck, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis of hyphae of 7–15 μm diam., cylindrical, light brown, smooth. Annulus hyphae of 7–15 μm diam., cylindrical, hyaline, smooth.
Habitat and distribution: solitary or scattered in small group on soil with excrements next to a chicken coop. Known only from Thailand.
Material examined: THAILAND, Chiang Mai Province, Mae Taeng, Ban Mae Sae Village, on Highway 1095, near 50 km marker, N19°14. 599′ E98°39.456′, elevation 962 m., 3 June 2006, collector Ruilin Zhao, ZRL3012 (BBH 19529, holotype), (HMAS and SFSU, isotypes); Ibid., Ban Pha Deng Village, N 19°17.123′ E 98°44. 009′, elev. 900 m, 3 October 2005, collector Ruilin Zhao, ZRL2137 (BBH 19507, HMAS and SFSU); same location, 5 August 2005, collector Ruilin Zhao, ZRL2122 (BBH 19501, HMAS and SFSU).
Notes: The phylogenetic analysis (Fig. 106) shows that this new species is sister to the species described below (see entry 142 of the present paper), but did not allow us to classify these two red staining new species in any known sections containing species exhibiting such a trait (sections Agaricus, Bivelares, Brunneopicti, Chitonioides, Nigrobrunnescentes, and Sanguinolenti subsections Bohusia and Sylvatici). Samples ZRL3012 and ZRL2136 have been included into the phylogenetic analysis but not named in our previous study (Zhao et al. 2011), and the results showed they were clustered together and loosely related to A. bohusii Bon, which is a red discoloring species from section Sanguinolenti subsection Bohusia (Parra 2008). In present study these two species form a clade with same topology as before. Extensive sampling and DNA sequences from other genes will be necessary to know if this clade would belong to one of the sections listed above or represents a new section.
This species is distinct by its strong blood red discolouring on cutting and capitate cheilocystidia. Compared with the red staining species from the sections listed above, the new species is morphologically highly similar to A. langei (F.H. Møller) F.H. Møller of the section Agaricus because both have light brown scales on the pileus, a pilleipellis that remains undisrupted at the disc, and similar sized spores. However, A. pseudolangei has capitate cheilocystidia, while those of A. langei are pyriform. Agaricus pseudolangei has wide ellipsoid spores, Qm = 1.27, while A. langei has ellipsoid to elongate ellipsoid spores, Qm = 1.67. In addition, the distance between the two species in the tree confirms that they are not phylogenetically closely related. In section Sanguinolenti, A. depauperatus (F.H. Møller) Pilátis (also has light brown squamules on the pileus), however, this species has larger basidiospores (7–9.6 × 4–6 μm) than those of A. pseudolangei, and its pyriform cheilocystidia also differ from those of A. pseudolangei. This new species also similar to A. benesii (Pilát) Pilát and A. sylvaticus Schaeff which both exhibit a strong reddish discoloring on cutting and can have a light brown or even a pure white cap (Parra 2008), however these known species have clavate cheilocystidia with 1–2 septa at base which is completely different from the new species.
-
209.
Agaricus haematinus K.D. Hyde & R.L. Zhao, sp. nov.
Index Fungorum number: IF551479; Facesoffungi number: FoF00986; Figs. 108 and 109e–j
Etymology: “heamatinus” means blood red, which refers to the blood red discolouring of the context on cutting.
Holotype: BBH 19516
Pileus 4–7 cm diam., and 5–6 mm thick, convex with inrolled margin, or concave with flared margin with age, cuticle exceeding, covered by floccose scales, snow white. Lamellae free, crowded, lamellulae with 2–3 series, 4.5–6 mm broad, normal to slightly ventricose, dull red or reddish-brown, edge colour distinctly lighter than gills. Stipe 40–70 × 11–12 μm, cylindrical, taping to base, smooth above the annulus, floccose below the ring, but easy rubbed so appearing smooth in old fruiting bodies, white, small, hollow. Annulus fugacious, and often hanging along the pileus margin, middle or superior, torn. Context firm, white, no staining on touching, rubescence on cutting. Macrochemical reaction: KOH reaction negative. Basidiospores 5–7 × 4–5 μm [\( \overline{\mathrm{x}} \) = 5.9 ± 0.4 × 4.6 ± 0.4, Q = 1.1–1.5, n = 20], ovate with apiculus, broad ellipsoid, brown, reddish-brown, smooth. Basidia 17–20 × 7–8 μm, clavate, smooth, hyaline, 4-spored. Cheilocystidia 16–21 × 9–14 μm, broadly clavate, pyriform, some with 1–2 septa at the base, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis composed of hyphae of 7.5–10 μm diam., elongate cylindrical arranged in parallel, smooth, hyaline. Annulus consisted by slender hyphae of 2.5–5 μm diam., loose interwoven, hyaline, smooth, branched.
Habitat and distribution: scattered on soil with excrements next to a chicken coop. Known only from Thailand.
Material examined: THAILAND, Chiang Mai Province, Mae Taeng District, Ban Pha Deng Village, N 19°17.123′ E 98°44. 009′, elev. 900 m, 19 September 2005, collector Ruilin Zhao, ZRL2136 (BBH 19516, holotype), (HMAS and SFSU, isotypes); Ibid., 2 August 2005, collector Ruilin Zhao, ZR2109 (BBH 19489, SFSU).
The phylogenetic analysis (Fig. 106) shows that this new species forms a clade with the new species A. pseudolangei described above (see entry 141 of the present paper). As indicated in the notes on A. pseudolangei, further study will be necessary to know if this clade represent a new section or belong to a known section containing species exhibiting the red staining trait observed in these two species.
This new species is diagnosed by its pure white basidiomata and strong blood red discolouration on cutting. In the field, it quite resembles A. campestris L. in having a pure white pileus, the taping base of stipe, and the character of annulus. However it differs in having larger spores (6.7–8 × 4.6–5.3 μm, Kerrigan 1986; 6–8.5 × 4.6–6.7 μm, Parra 2008) and unchangeable context or which is slightly pink on exposure. Agaricus andrewii Freeman is another species having a pure white pileus and pinkish discoloration, however, it has larger spores (7.5–9.2 × 5.5–6 μm). Similarly A. benesii and A. sylvaticus can present the pure white caps sometimes, and septate cheilocystidia, but their lower side of annulus have cog-wheel scales.
-
210.
Agaricus atrodiscus L.J. Chen, Callac, R.L. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: IF551431; Facesoffungi number: FoF00987; Figs. 110a–d and 111
Etymology: atrodicus means black disc, and here refers to the dark disc of pileus surface.
Holotype: MFLU 12-1010.
Pileus 90–130 mm diam. and 9 mm thick, parabolic with truncated top when young, then hemispherical to plano-convex and finally applanate with age; surface dry, at first entirely covered with finely grey or dark grey fibrils, often densely arranged at the disc; sometimes with pileus expansion, the fibrils disrupted into tiny squamules radially distributed on a white background; margin decurved and shortly exceeding the lamellae. Lamellae free, crowded, lamellulae with more than 5 series, 7–8 mm broad, at first white, then pink, later pinkish brown and finally dark brown. Stipe 170–194 mm long, 12–21 (base to apex) mm wide, cylindrical and tapering towards the base, surface smooth, both above and below the annulus, silky, hollow, white, staining lightly yellowish when bruised. Annulus with two layers, membranous, pendent, superous, white, upper side smooth, lower side woolly, with flakes in a cogwheel arrangement, sometimes with a yellowish tinge. Odour phenolic. Context firm, white, colour unchanged when cut. Macrochemical reactions: KOH reaction positive orange. Schäffer’s reaction negative. Basidiospores 4.7–5.9 × 3–3.6 μm, [\( \overline{\mathrm{x}} \) = 5.2 ± 0.28 × 3.3 ± 0.16, Q = 1.44–1.78, n = 20], ellipsoid, smooth, brown, thick–walled. Basidia 14–17 × 5–7 μm, clavate to broadly clavate, hyaline, smooth, 4-spored. Cheilocystidia 10.5–26 × 6–11 μm, simple, pyriform, broadly clavate, or sphaeropedunculate, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis composed of hyphae of 5–10 μm wide, cylindrical, containing dark brown vacuolar pigments, smooth, often constricted at the septa; terminal element observed cylindrical to inflated, sometimes subglobose, up to 13–20 μm wide.
Habitat and distribution: scattered or gregarious on soil, under Bamboo. Known only from Thailand.
Material examined: THAILAND, Chiang Mai Prov., Mae Sa Waterfall, 14 September 2012, collector Jie Chen & Asanka Bandara, LD 2012185 (MFLU12-1010 holotype), (isotype, HMAS).
Notes: The positive KOH reaction and negative Schäffer’s reaction, yellowish discolouration when bruised and phenolic odour, place A. atrodiscus in Agaricus section Xanthodermatei. It is macroscopically remarkable by its relatively robust sporocarps, with grey fibrils or tiny squamules on the pileus surface and its tapering stipe. Microscopically, the spores have an average size of 5.2 × 3.3 μm, and the pileipellis hyphae contain dark brown vacuolar pigments. In addition to its tropical habitat, the consecutively dark grey coloured pileus containing vacuolar pigments are also found in A. endoxanthus Berk. & Broome, A. microvolvatulus Heinem., A. rotalis K.R. Peterson, Desjardin & Hemmes and A. xanthosarcus Heinem. & Gooss.-Font (Heinemann 1956; Kerrigan et al. 2005; Zhao et al. 2012). However, A. atrodiscus can be easily distinguished in the field from A. endoxanthus and A. rotalis by its robust sporocarps and its faint yellowish discolouration on stipe surface (Heinemann 1956; Zhao et al. 2012). The pileus diameter of A. microvolvatulus and A. xanthosarcus can reach to 10 cm, but they differ in having a distinctively bulbous stipe (Heinemann 1956; Thongklang et al. 2014). According to the phylogenetic analysis (Fig. 106), A. atrodiscus appears as an unbranched lineage arising near the common ancestor of the clade named Xan III by Thongklang et al. (2014), which is one of the three major clades constituting the section Xanthodermatei also in our tree (Fig. 106).
-
211.
Agaricus exilissimus L.J. Chen, Callac, R.L. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: IF551432; Facesoffungi number: FoF00988; Figs. 110e–f and 112
Etymology: exilissimus means very thin and refers to the very thin pileus and stipe.
Holotype: MFLU 12-0895.
Pileus 25–35 mm diam. and 1 mm thick, applanate and subumbonate at disc; surface dry, with dark brown fibrils congregated on the disc, tiny fibrillose squamules more or less concentrically arranged at elsewhere, except towards the margin, on a light brown background; margin crenulate, uplifted. Lamellae free, crowded, lamellulae with 5 series, 3 mm broad, ventricose, pink to brown. Stipe 30–40 × 2–3 (4.5 at base) mm, cylindrical with a subbulbous base, surface smooth, both above and below the annulus, silky, hollow, white, changing to yellowish when cut or bruised. Annulus superous, single, membranous, upward, white, upper side smooth, lower side fibrillose. Odour of ink. Context firm, white, without colour change when cut. Macrochemical reactions: KOH reaction yellow. Schäffer’s reaction negative. Basidiospores 5.3–6.2 × 3.2–3.8 μm, [\( \overline{\mathrm{x}} \) = 5.8 ± 0.25 × 3.5 ± 0.15, Q = 1.43–1.72, n = 20], ellipsoid, smooth, brown, thick-walled. Basidia 15–16 × 6.5–7 μm, broadly clavate, hyaline, smooth, 4-spored; Cheilocystidia 14–22 × 8.5–14 μm, simple, pyriform or sphaeropedunculate, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis composed of hyphae of 3.5–5 μm diam., cylindrical, with brown vacuolar pigments, smooth, rarely constricted at the septa.
Habitat and distribution: solitary on soil, in open area of forest. Known only from Thailand.
Material examined: THAILAND, Chiang Mai Prov., Mae La Noi Village, Mae Hong Song, highway 108, 26 June 2012, collector Jie Chen & Philippe Callac, LD 201254 (MFLU12- 0895, holotype).
Notes: The positive KOH reaction and negative Schäffer’s reaction, yellowish discolouration when bruised and ink odour, place A. exilissimus in Agaricus section Xanthodermatei. It can be easily recognized in the field by its small sized sporocarps with an ink odor, tiny brown fibrillose squamules on the pileus surface, and the slender stipe. Agaricus exilissimus is phylogenetically related to A. bisporiticus Nawaz et al., A. murinocephalus R.L. Zhao et al. and A. fuscopunctatus Thongklang et al. (Fig. 106). All these species have a pileus with brown, appressed squamules (Thongklang et al. 2014; Zhao et al. 2012). However, these three species have larger sporocarps than the new species with pileus diameters exceeding 50 mm when mature. Although such a criterion is not perfectly reliable, it could help to identify the new species. We note that the three species cited above plus A. pseudopratensis (Bohus) Wasser and A. microvolvatulus formed a clade in the tree of Fig. 106, although it was poorly supported. This agrees with the previous study of Thongklang et al. (2014). These authors showed that these five species shared two ITS XantII-specific markers: A and a deletion at positions 143 and 144, respectively (tcagc[A-]ybgyt) in the sequence alignment. The new species also possesses these two specific markers in agreement with them belonging in this group.
Amanitaceae R. Heim ex Pouzar
The family Amanitaceae is an important fungal family due to its ecological and economic importance (Yang 1997, 2005, 2015; Yang et al. 1999; Kernaghan 2005; Chen et al. 2013). The family probably comprises at least four genera: Amanita, Aspidella, Catatrama and Limacella (Moncalvo et al. 2002; Garnica et al. 2007; Justo et al. 2010; Vizzini et al. 2012; Wolfe et al. 2012), and Amanita is the most species-rich genus (Bas 2000; Tulloss 2005; Yang 2005).
Amanita Pers., Tent. disp. meth. fung. (Lipsiae): 65 (1797)
The genus Amanita (Agaricales, Basidiomycota) is a cosmopolitan genus, comprising about 500 described and accepted species worldwide (Bas 2000; Tulloss 2005; Yang 2005). This group of fungi can form ectomycorrhizal symbiosis with plants of more than ten families, such as Fagaceae, Myrtaceae and Pinaceae (Yang et al. 1999). Based on morphological and anatomical characters, Amanita could be divided into two subgenera, Amanita and Lepidella (E.J. Gilbert) Veselý (Corner and Bas 1962; Bas 1969), consisting of seven sections, Amanita, Caesareae Singer, Vaginatae (Fr.) Quél., Amidella (J.-E. Gilbert) Konrad & Maubl., Lepidella, Phalloideae (Fr.) Quél. and Validae (Fr.) Quél. (Yang 1997). Herein we described three new species of A. sect. Amanita supported by both morphological and molecular phylogenetic evidences. Sequence data used in this study are from previous studies (Weiβ et al. 1998; Zhang et al. 2004; Vargas et al. 2011; Wolfe et al. 2012; Sánchez-Ramírez et al. 2015) or are newly generated (Fig. 113).
Phylogram of Amanita sect. Amanita generated from Maximum likelihood (RAxML) analysis of LSU, rpb2 and tef1-α sequence data. Maximum likelihood bootstrap values greater than 50 % and Bayesian posterior probabilities over 0.90 are indicated above or below the nodes. The new species are indicated in blue, and the type collections of the species are in bold. The tree is rooted with two species of A. sect. Caesareae, namely A. caesarea and A. jacksonii
-
212.
Amanita melleialba Zhu L. Yang, Qing Cai & Yang Y. Cui, sp. nov.
Index Fungorum number: IF551331; Facesoffungi number: FoF00898; Figs. 113, 114, 115, 116 and 117
Etymology: melleialba, referring to the honey-coloured center and whitish margin of the pileus.
Holotype: HKAS 83446
Basidiomata small. Pileus 2.8–5 cm in diam., plano-convex to plane, center often slightly depressed, honey-coloured, yellowish (4A3–4) to yellow (3B5–6), becoming yellowish to whitish (3A2) toward margin, viscid when moist; margin tuberculate-striate (0.4–0.6R), non-appendiculate; volval remnants as subconical to granular, small warts up to 2 mm high, dirty white (5B2), cream-coloured to yellowish (4A2–3), randomly arranged, or densely placed at center; trama white (1A1), unchanging. Lamellae free, white (1A1), crowded; lamellar edges finely fimbriate, white (1A1), dirty white (5B2, 4B1–2) to cream-coloured (4A2–3); lamellulae truncate, plentiful, evenly distributed. Stipe 4–8 × 0.4–0.8 cm, subcylindric or slightly tapering upward, with apex slightly expanded, white (1A1) to cream-coloured (1A2), covered with white (1A1) floccose squamules above annulus, with white (1A1) floccose squamules to granules under annulus; context white, loosely stuffed to hollow in center; basal bulb subglobose to napiform, 0.8–1.2 cm in diam., white (1A1); volval remnants as white to cream-coloured (1A1) floccose squamules to granules, or forming a short limb. Annulus present, pendant from attachment 1.5–3 cm below apex of stipe, white (1A1) to cream-coloured (1A2), with a yellow floccose edge. Odor indistinct.
Lamellar trama bilateral. Mediostratum 30–60 μm wide, composed of abundant ellipsoid inflated cells (35–60 × 16–37 μm); filamentous hyphae abundant, 2–8 μm wide; vascular hyphae rare. Lateral stratum composed of abundant fusiform to ellipsoid inflated cells (22–65 × 9–20 μm), diverging at an angle of ca. 30–45° to the mediostratum; filamentous hyphae abundant, 2–6 (−9) μm wide; septa without clamps. Subhymenium 30–60 μm thick, with 2–3 layers of subglobose to ovoid or irregularly shaped cells, 9–17 × 7–15 μm. Basidia 30–50 × 9–14 μm, clavate, 4-spored; sterigemata 3–5 μm long; basal septa without clamps. Basidiospores [60/3/2] 7.5–9.5(−10) × (5.5–)6–7 μm [Q = (1.21–)1.29–1.58(−1.63), Qm = 1.41 ± 0.10], ellipsoid, sometimes broadly ellipsoid, inamyloid, colourless, hyaline, thin-walled, smooth; apiculus small. Lamellar edge appearing as sterile strip, composed of globose, subglobose to ellipsoid inflated cells (12–32 × 10–25 μm), single and terminal or in terminal chains of 2–3, thin-walled, colourless, hyaline; filamentous hyphae abundant, 2–4 μm wide, irregularly arranged or ± running parallel to lamellar edge. Pileipellis 100–150 μm; upper layer (50–70 μm thick) strongly gelatinized, composed of subradically to somewhat interwoven, thin-walled, colourless to nearly colourless, filamentous hyphae 3–5 μm wide; lower layer (50–80 μm thick) composed of radically and compactly arranged, filamentous hyphae 3–8 μm wide; vascular hyphae rare. Volval remnants on pileus composed of vertically to subvertically arranged elements; inflated cells very abundant to nearly dominant, globose to subglobose or ellipsoid to subfusiform (20–65 × 10–35 μm), in chains of 2–4, thin-walled, colourless to subcolourless; filamentous hyphae rare to fairly abundant, 3–6 μm wide, thin-walled, colourless to nearly colourless; vascular hyphae rare. Volval remnants on the stipe base composed of two layers of intergrading into each other. Outer layer composed of longitudinally to irregularly arranged elements: inflated cells abundant to very abundant, fusiform to ellipsoid, 20–45 × 10–30 μm, colourless to yellow brownish, thin- to slightly thick-walled, terminal or in chains of 2–3 and then terminal, becoming rare toward inner layer; filamentous hyphae very abundant to abundant; vascular hyphae rare. Inner layer somewhat gelatinized, filamentous hyphae very abundant to nearly dominant, mixed with scattered, long ellipsoid to clavate inflated cells, 65–150 × 15–20 μm. Stipe trama composed of longitudinally arranged, long clavate, terminal cells, 150–350 × 27–50 μm; filamentous hyphae scattered to fairly abundant, 2–9(−14) μm; vascular hyphae rare. Annulus slightly gelatinized, composed of subradially arranged elements; inflated cells very abundant, ellipsoid to long ellipsoid, 15–65 × 10–28 μm, colourless, thin-walled, becoming elongated toward lower surface; filamentous hyphae abundant, 2–5(−8) μm, colourless, thin-walled. Clamps absent in all parts of basidioma.
Habitat and distribution: Solitary or gregarious in subtropical forests dominated by Fagaceae. Known from southwestern and central China.
Material examined: CHINA, Hunan Province, Changsha City, Yuelu Mountain, Fagaceae (altitude ca. 260 m), 18 July 2014, S.C. Li 1 (HKAS 83216). Yunnan Province, Puer City, Caiyanghe Nature Reserve, Fagaceae (altitude 1300 m), 11 July 2014, G. Wu 1339 (HKAS 83446, holotype!); the same location, Fagaceae (altitude 1326 m), 11 July 2014, Xiao B. Liu 439 (HKAS 87085).
Notes: Amanita melleialba is characterized by its small basidiomata, ellipsoid basidiospores (7.5–9.5 × 6–7 μm), volval remnants on the pileus dominantly composed of abundant inflated cells in chains of 2–4, and occurrence in subtropical forests dominated by plants of Fagaceae.
Amanita melleialba is very similar to A. parvipantherina Zhu L. Yang, M. Weiss & Oberw., however, the latter possesses a brownish pileus with shorter marginal striations, larger basidiospores (8.5–11.5 × 6.5–8.5 μm) and is distributed in the mixed forests with Pinus yunnanensis (Yang et al. 2004; Yang 2005, 2015). Our molecular phylogenetic analysis also suggested that A. melleialba is a separate species and is different from A. parvipantherina (Fig. 113).
Amanita melleialba may be confused with A. elata (Massee) Corner & Bas, a species originally described from Singapore. However, A. elata has volval remnants on the pileus as small, scattered, irregularly shaped, floccose-membranous, flat patches, which are easily washed off by rain, and globose to subglobose basidiospores (Corner and Bas 1962; Yang 2015).
-
213.
Amanita pseudosychnopyramis Yang Y. Cui, Qing Cai & Zhu L. Yang, sp. nov.
Index Fungorum number: IF551334; Facesoffungi number: FoF00897; Figs. 113, 117, 118 and 119
Etymology: pseudosychnopyramis, named due to its similarity to A. sychnopyramis.
Holotype: HKAS 87999
Basidiomata small to medium-sized. Pileus 4–7 cm in diam., plano-convex to plane, often slightly depressed at center, at first yellow (3A4–8) over disk, then yellowish brown (3B2–8) to brownish (3C4–7) at center, becoming yellowish (3A2–3, 3B2–4) to dirty white (3B1–2) toward margin, viscid when moist; margin tuberculate-striate (0.25–0.3R), non-appendiculate; volval remnants as conical, subconical to pyramid, dirty white to grayish (3B1–2) to brownish grey (3A2–4; 3B2–3), randomly arranged, small warts; trama white (1A1), unchanging. Lamellae free, white (1A1), crowded; lamellulae truncate, plentiful, evenly distributed. Stipe 6–8.5 × 0.3–0.8 cm, subcylindric or slightly tapering upward, with apex slightly expanded, dirty white (3A1–3) to yellowish brown (4A2–3, much paler than 4B2–3), subglabrous or covered with white (1A1) floccose squamules; context white, hollow in center; basal bulb subglobose to long ellipsoid, 1–1.3 cm in diam., white (1A1) to dirty white (3A1–3), upper part covered with conical to subconical to granular grayish to brownish grey volval remnants arranged in several incomplete rings, usually forming a short limbate collar between the stipe and the bulb. Annulus present, superior, pendant from attachment 2–3 cm below apex of stipe, white (1A1), thin, membranous, fragile. Odour indistinct.
Lamellar trama bilateral. Mediostratum 40–75 μm wide, composed of abundant subglobose, ellipsoid to long ellipsoid inflated cells (35–110 × 25–50 μm); filamentous hyphae abundant, 2–8 μm wide; vascular hyphae rare. Lateral stratum composed of abundant ellipsoid to long ellipsoid inflated cells (25–70 × 7–17 μm), diverging at an angle of ca. 30–45° to the mediostratum; filamentous hyphae abundant, 3–5 μm wide; septa without clamps. Subhymenium 35–70 μm thick, with 2–3 layers of subglobose, ellipsoid or irregularly shaped cells, 7–30 × 12–25 μm. Basidia 36–47 × 10–13 μm, clavate, 4-spored, sometimes 2-spored; sterigemata 3–4 μm long; basal septa without clamps. Basidiospores [140/7/3] (7–)8–10(−11.5) × (6.5–)7–8.5(−10.5) μm [Q = 1.06–1.20(1.39), Qm = 1.13 ± 0.05], subglobose to broadly ellipsoid, inamyloid, colourless, hyaline, thin-walled, smooth; apiculus small. Lamellar edge appearing as sterile strip, composed of subglobose to ellipsoid inflated cells (9–25 × 7–15 μm), single and terminal or two in chain and then terminal, thin-walled, colourless, hyaline; filamentous hyphae abundant, 2–4 μm wide, irregularly arranged or ± running parallel to lamellar edge. Pileipellis 150–200 μm; upper layer (60–80 μm thick) strongly gelatinized, composed of radically to subradically, thin-walled, colourless to nearly colourless, filamentous hyphae 2–7 μm wide; lower layer (80–125 μm thick) composed of compactly arranged, filamentous hyphae 3–8 μm wide; vascular hyphae rare. Volval remnants on pileus composed of more or less vertically arranged elements; inflated cells very abundant to nearly dominant, subglobose to ellipsoid (18–70 × 15–50 μm), in chains of 2–3 and then terminal, thin- to slightly thick-walled, yellowish to brownish vacuolarly pigmented, sometimes nearly colourless; filamentous hyphae abundant, 3–11 μm wide, thin-walled, yellowish to brownish vacuolarly pigmented or nearly colourless; vascular hyphae rare. Volval remnants on the stipe base (outer surface of the limbate collar) composed of somewhat irregularly arranged elements; inflated cells very abundant, subglobose to ellipsoid (22–100 × 15–45 μm), thin- to slightly thick-walled, in chains of 2; filamentous hyphae abundant, 3–8 μm wide, colourless to nearly colourless, thin-walled; vascular hyphae rare; interior of limbate collar on the stipe base composed of very abundant to nearly dominant filamentous hyphae 3–8 μm wide, mixed with scattered inflated cells; inner surface of limbate collar gelatinized, composed of filamentous hyphae 3–6 μm wide. Stipe trama composed of longitudinally arranged, long clavate, terminal cells, 200–450 × 25–50 μm; filamentous hyphae scattered (in interior) to fairly abundant (on stipe surface), 5–18 μm wide; vascular hyphae rare. Annulus composed of loosely and irregularly arranged elements; inflated cells abundant, ellipsoid to long ellipsoid to clavate (28–95 × 15–35 μm), colourless to nearly colourless, thin-walled, single and terminal or in chains of 2–3 and then terminal; filamentous hyphae abundant to very abundant, 3–7(−10) μm wide, colourless to nearly colourless, thin-walled; vascular hyphae rare. Clamps absent in all parts of basidioma.
Habitat and distribution: Solitary or gregarious in southern subtropical forests of Fagaceae. Distributed in southweatern and southern China.
Material examined: CHINA, Guangdong Province, Fengkai County, Heishiding, Fagaceae (altitude 500 m), 20 March 2012, F. Li 66 (HKAS 78417); the same location, Fagaceae (altitude 320 m), 16 March 2014, F. Li 1593 (HKAS 82293); Yunnan Province, Jinghong City, Dadugang Township, Fagaceae (altitude ca. 1000 m), 30 June 2014, K. Zhao 467 (HKAS 87999, holotype!).
Notes: Amanita pseudosychnopyramis is characterized by its conical to pyramid grey to brownish grey volval remnants on the pileus, a basal bulb with a short limbate collar and its subglobose to broadly ellipsoid basidiospores (8.5–10 × 7.5–8.5 μm). It is associated with subtropical forests dominated by Fagaceae.
Amanita pseudosychnopyramis resembles A. sychnopyramis f. sychnopyramis and f. subannulata, by its small to medium-sized basidiomata, characterized by a brownish to brown pileus with grey to brownish grey, conical to pyramid volval remnants. However, A. sychnopyramis f. sychnopyramis and f. subannulata has usually a bulb with conical to granular volval remnants arranged in incomplete rings, smaller basidia and smaller basidiospores (Corner and Bas 1962; Hongo 1971; Yang 1997). Amanita pseudosychnopyramis is also similar to A. parvipantherina, and A. subparvipantherina by its small to medium-sized basidiomata, characterized by a brownish to brown pileus with grey to brownish grey, conical to pyramid volval remnants. Amanita parvipantherina possesses a subglobose basal bulb, with floccose to granular volval remnants which usually do not form a limbate volva on the base of the stipe and broadly ellipsoid to ellipsoid basidiospores (8.5–11.5 × 6.5–8.5 μm) (Yang et al. 2004; Yang 2005, 2015). Furthermore, it occurs in mixed forests with pine trees. Amanita subparvipantherina has a short limbate collar volval remnants on the stipe base, broadly ellipsoid to ellipsoid basidiospores (9–11.5 × 6.5–8.5 μm), and volval remnants on the pileus with more abundant filamentous hyphae. In our molecular analyses, A. pseudosychnopyramis is supported to be a distinct species and is distantly related to A. parvipantherina, A. subparvipantherina, A. sychnopyramis f. sychnopyramis and f. subannulata (Fig. 113).
-
214.
Amanita subparvipantherina Zhu L. Yang, Qing Cai & Yang Y. Cui, sp. nov.
Index Fungorum number: IF551335; Facesoffungi number: FoF00899; Figs. 113, 120, 121, 122 and 123
Etymology: “subparvipantherina”, named due to its similarity to A. parvipantherina.
Holotype: HKAS 56986
Basidiomata medium-sized. Pileus 5–7 cm in diam., at first hemispherical, then convex to plano-convex, center often slightly umbonate, yellowish brown (4A4–5, 4B4–7) to brown (4C5–8) or dark brown (4D6–8, 4E6–8), becoming yellowish (4A2–4) to brownish (4B3–4, 4C3–4) toward margin, viscid when moist; margin tuberculate-striate (0.2–0.3R), non-appendiculate; volval remnants as conical, subconical to granular, dirty white to grayish (3B1–2) to brownish (3A2–4; 3B2–3), randomly arranged, small warts; trama white (1A1), unchanging. Lamellae free, white (1A1), crowded; lamellulae truncate, plentiful, evenly distributed. Stipe 11–16.8 × 0.8–2 cm, subcylindric or slightly tapering upward, with apex slightly expanded, whitish (1A1), with yellowish brown (3B2–4) tinge, often glabrous to subglabrous, rarely covered with white (1A1) floccose squamules; context white, hollow in center; basal bulb subglobose to ovate to napiform, 2–3 cm in diam., white (1A1), upper part covered with subconical to granular greyish to brownish volval remnants, often forming a short limbate collar on the stipe base. Annulus present, superior to median, pendant from attachment 2–4 cm below apex of stipe, white (1A1), thin, membranous, fragile. Odour indistinct.
Lamellar trama bilateral. Mediostratum 25–40 μm wide, composed of abundant fusiform, ellipsoid to long ellipsoid inflated cells (20–200 × 10–55 μm); filamentous hyphae abundant, 3–7 μm wide; vascular hyphae rare. Lateral stratum composed of abundant fusiform to ellipsoid inflated cells (20–45 × 10–20 μm), diverging at an angle of ca. 30–45° to the mediostratum; filamentous hyphae abundant, 2–6 μm wide; septa without clamps. Subhymenium 25–40 μm thick, with 2–3 layers of ellipsoid or irregularly shaped cells, 10–28 × 7–10 μm. Basidia 30–40 × 9–13 μm, clavate, 4-spored, rarely 2-spored; sterigemata 3–5 μm long; basal septa without clamps. Basidiospores [140/7/5] (8–)9–11.5(−13) × (5.5–)6.5–8(−9.5) μm [Q = (1.20–)1.28–1.5(−1.69), Qm = 1.38 ± 0.11], broadly ellipsoid to ellipsoid, rarely elongate, inamyloid, colourless, hyaline, thin-walled, smooth; apiculus small. Lamellar edge appearing as sterile strip, composed of inflated cells, mixed with abundant thin-walled, yellow brownish filamentous hyphae. Pileipellis 100–200 μm; upper layer (50–100 μm thick) strongly gelatinized, composed of radically to subradically, thin-walled, colourless to nearly colourless, filamentous hyphae 2–6 μm wide; lower layer (50–110 μm thick) composed of compactly arranged, filamentous hyphae 3–8 μm wide; vascular hyphae rare. Volval remnants on pileus composed of somewhat irregularly arranged elements; inflated cells fairly abundant to abundant, fusiform to ellipsoid to subglobose (25–50 × 15–40 μm), single and terminal or in chains of 2–3 and then terminal, thin-walled, colourless to yellowish vacuolarly pigmented; filamentous hyphae very abundant, 2–10 μm wide, thin-walled, nearly colourless to yellowish vacuolarly pigmented; vascular hyphae rare. Interior of limbate collar on the stipe base composed of longitudinally to somewhat irregularly arranged elements; inflated cells fairly abundant, fusiform, ellipsoid, broadly clavate to pyriform (30–75 × 15–45 μm), becoming more abundant toward the outer surface of limbate collar, colourless to nearly colourless, thin-walled to slightly thick-walled, single, sometimes two in chains; filamentous hyphae abundant to very abundant, 2–10 μm wide, colourless to nearly colourless, thin-walled; vascular hyphae rare. Outer surface of limbate collar similar to the interior but with more abundant inflated cells; inner surface of limbate collar gelatinized, composed of 3–8 μm wide filamentous hyphae. Stipe trama composed of primarily of longitudinally arranged, long clavate, terminal cells 210–350 × 25–45 μm; filamentous hyphae scattered (in interior), to fairly abundant (on stipe surface), 6–16 μm wide; vascular hyphae rare. Annulus composed of loosely and subradially arranged elements; inflated cells fairly abundant, clavate, ellipsoid to long ellipsoid (35–65 × 15–25 μm), colourless to nearly colourless, thin-walled, single and terminal, rarely two in chains and then terminal; filamentous hyphae abundant to very abundant, 2.5–6 μm wide, colourless to nearly colourless, thin-walled; vascular hyphae rare. Clamps absent in all parts of basidioma.
Habitat and distribution: Solitary or gregarious in subtropical forests composed of Pinus, Quercus, Keteleeria and Rhododendron. Common in southwestern China.
Material examined: CHINA, Yunnan Province, Binchuan County, Jizu Mountain, Pinus mixed with Quercus and Rhododendron (altitude 2000–2100 m), 11 August 2011, Q. Cai 657 (HKAS 70252); the same location, 9 August 2014, Q. Cai 1288 (HKAS 83757); Chuxiong City, Zixi Mountain, Pinus and Quercus (altitude 2000 m), 17 June 2008, Z. W. Ge 2035 (HKAS 54231); Jingdong County, Ailao Mountain, Pinus and Quercus (altitude 2500 m), 15 July 2008, L. P. Tang 333 (HKAS 54564); Longyang District, Haitangwa Village, Pinus and Quercus (altitude 2400 m), 12 August 2011, Y.J. Hao 485 (HKAS 71594); Lushui County, Luzhang Town, Xiapianma Village, Pinus (altitude 2000 m), 7 August 2010, Q. Cai 291 (HKAS 67853); Tengchong County, Guanpojiao Village, Pinus, Quercus, Keteleeria and Rhododendron (altitude 1800 m), L. P. Tang 860 (HKAS 56817); Yongping County, Shangzhai Village, Pinus and Quercus (altitude 1991 m), 30 July 2009, L. P. Tang 1029 (HKAS 56986, holotype!).
Notes: Amanita subparvipantherina is distinguished by its slender basidioma with a long stipe, broadly ellipsoid to ellipsoid basidiospores (9–11.5 × 6.5–8 μm), volval remnants on the pileus and the limbate collar on the stipe base, composed of abundant to very abundant filamentous hyphae, mixed with fairly abundant inflated cells.
Amanita subparvipantherina is similar to A. parvipantherina. However, A. parvipantherina has floccose to granular volval remnants on the upper bulb, and volval remnants on the pileus with more abundant to nearly dominant inflated cells (Yang et al. 2004; Yang 2005, 2015).
Amanita subparvipantherina is also somewhat similar to A. sychnopyramis f. subannulata. However, the latter taxon has volval remnants on the pileus, with more abundant to nearly dominant inflated cells, and globose to subglobose basidiospores (6.5–8.5 × 6–8 μm) with Q = 1.03–1.16 (Hongo 1971; Yang 1997, 2005, 2015).
Amanita subparvipantherina may be confused with A. subglobosa Zhu L. Yang, a species described from southwestern China, in their brownish pileus with white to yellow-brown conical volval remnants on the pileus. However, A. subglobosa has a larger basidioma, a distinctly globose to subglobose bulb on the base of the stipe, volval remnants on the pileus with more abundant inflated cells and a common presence of clamps (Yang 1997, 2005). Our molecular analyses also indicate that A. subparvipantherina is a distinct species and it is probably related to A. parvipantherina, A. mellaialba and A. altipes, but their relationships lack significant statistical supports (Fig. 113).
Entolomataceae Kotlaba & Pouzar
The family Entolomataceae is a family of Agaricales provided with spores that are pink in mass and have unique spore wall ornamentations, being formed by local thickenings in the spore wall, the epicorium (Clémençon et al. 2004). According to some recent phylogenetic studies (Moncalvo et al. 2002; Matheny et al. 2006) the family is monophyletic and sister to the Lyophyllaceae and includes the agaricoid genera Clitopilus (Fr. ex Rabenh.) P. Kumm. with spores showing longitudinal ribs, Entoloma Fr. ex P. Kumm. with angular spores and Rhodocybe Maire with bumpy spores, plus gasteroid genera like Richoniella Costantin & L.M. Dufour and Rhodogaster E. Horak. Other analyses (Baroni et al. 2011) did not confirm the monophyly of Entolomataceae. Based on a multigene analysis, Kluting et al. (2014) segregated from Rhodocybe the genera Clitocella Kluting et al., Clitopilopsis Maire and Rhodophana Kühner (Fig. 124).
Bayesian phylogram (MrBayes 3.2) of Entoloma subgen. Nolanea based on ITS sequences. Representative taxa from subgenus Claudopus were used as outgroups. Bayesian posterior probabilities (BPP) above 0.70 and RAxML bootstrap values (MLB) above 50 % are shown. Thickened lines indicate both BPP ≥ 0.95 and MLB ≥ 90 %
Entoloma P. Kumm., Führ. Pilzk. (Zerbst): 97 (1871)
The genus Entoloma encompasses about 1000 taxa (Kirk et al. 2008a, b) distributed throughout the world, from (sub)arctic (Noordeloos and Morozova 2010) and temperate (e.g., Largent 1994; Noordeloos 1988b, 1992, 2004) to tropical regions (Baroni et al. 2008; Manimohan et al. 2006; Romagnesi 1941; Romagnesi and Gilles 1979).
Several authors recognized segregate genera within Entoloma (e.g., Horak 1976, 1978; Largent 1994; Aime et al. 2010), while others (Romagnesi 1941; Noordeloos 1992, 2004) treat it as a unique genus separable into different subgenera. The recent study by Co-David et al. (2009) showed that Entoloma sensu lato is monophyletic with the inclusion of Rhodogaster and Richoniella, the sequestrate taxa of the Entolomataceae. In his monograph on European Entoloma species Noordeloos (2004) reported about 380 taxa, but that number is likely to rise.
-
215.
Entoloma calabrum Battistin, Marsico, Vizzini, Vila & Ercole, sp. nov.
Index Fungorum number: IF551433; Facesoffungi number: FoF01046; Figs. 125 and 126
Etymology: the Latin epithet calabrum refers to the Italian region (Calabria) where the species has been first collected.
Holotype: MCVE 28566
Colour notations in the macroscopic descriptions refer to the Munsell Soil-Color Charts (1994).
Habit mycenoid. Pileus 19–30 mm broad, at first conical-campanulate to convex, then applanate with or without a prominent, obtuse umbo, yellow (Mu 10YR 8/6, 8/8), reddish yellow (Mu 7.5YR 6/8, 7/6, 7/8); surface dry, mat, fibrillose, tomentose or pubescent in some specimens, not or hardly hygrophanous; margin at first inflexed than straight, wavy, shortly translucently striate. Lamellae moderately crowded, adnexed to sinuate-emarginate, arcuate, up to 6–7 mm broad, very pale brown (Mu 10YR 8/3, 8/4) with many lamellulae. Stipe 50–60 × 2–3 mm, central, cylindrical or slightly compressed, equal or tapering at the apex, (sub)concolourous with the pileus, almost completely covered with white fibrils and a white tomentum. Context subconcolourous with pileus; odour not distinctive and taste mild. Spores 6.9–10.4 × 5.1–8.4 μm, on average 9 × 6.4 μm, Q = 1.16–1.81 (Qm = 1.41), almost exclusively heterodiametrical, 5–7 angled in side view. Basidia 35–47 × 11.7–15 μm, clavate, 4-spored, clamped. Sclerobasidia absent. Hymenial cystidia absent. There are some cylindroid, more or less flexuose, thin-walled, sterile hyaline elements scattered among basidia, which scarcely protrude beyond the hymenium. Caulocystidia up to 180 μm long, 5–11.7 μm wide, abundant, cylindrical, straight or curved, flexuose, some coralloid, septate, hyaline. Hymenophoral trama regular in the mediostratum, a bit less in the outer layer, made up of cylindroid or subfusiform hyphae 94–300 × 3.3–23 μm (n = 22; mean: 172 × 12 μm). Pileipellis a cutis of cylindrical, radially arranged hyphae up to 16.7 μm wide. Pigment very finely encrusting and sometimes also parietal. Clamp-connections present.
Habitat and known distribution: Gregarious on grassy soil. So far known only from southern Italy.
Material examined: ITALY, Calabria, Cosenza, municipality of San Pietro in Guarano, locality Serra Vaccaro, about 39°20′ N 16°24′ E on acid soil in a grassy clearing of a Fagus sylvatica stand near Cytisus scoparius and Astragalus calabrus, 8 October 2010, leg. O. Marsico (MCVE 28566, holotype); same loc., 20 October 2010, leg. O. Marsico (TO AV201010a; same loc., 27 October 201 (TO AV271010a)).
Notes: Both Bayesian and Maximum likelihood analyses produced the same topology; therefore, only the Bayesian tree with both BPP (Bayesian Posterior Probabilities) and MLB (Maximum Likelihood Bootstrap) values is shown (Fig. 124). In the ITS sequence analysis the sequence of Entoloma calabrum clustered within the Nolanea major clade, in a clade consisting of E. infula, E. clandestinum, E. conicum, E. alboumbonatum and E. aff. minutum.
Macroscopically Entoloma calabrum is a distinct taxon on account of its sometimes zonate, umbonate, strongly fibrillose, almost villose pileus, the brown stipe covered with abundant white fibrils and a white tomentum, the cream or cream-brownish lamellae, and the odourless and mild context.
Microscopically it is characterised by the presence of clamp-connections, heterodiametrical spores, pigment mainly finely encrusting but also parietal, absence of hymenial cystidia, and presence of caulocystidia.
A combination of morphological features such as size, habit, overall colours and the structure of the hymenophoral trama and pileipellis placed it in the subgenus Nolanea (Fr.: Fr.) Noordel. (typified by Agaricus hirtipes Schumach.) as circumscribed by Noordeloos (1980, 1992, 2004). This placement is supported by the ITS phylogenetic analysis (Fig. 124) and by the fact that LSU query sequence produced the following matches: 99 % max. identity with Nolanea sericea (accessions GQ289191, AF223171, AY207197 and AF223170) and N. conica (accession AF2613170).
According to Noordeloos’ taxonomic arrangement (1992, 2004) and with regard to the five sections included in Nolanea, i.e., Mammosa (Romagn.) Noordel., Cosmeoexonema (Largent & Thiers) Noordel., Endochromonema (Largent & Thiers) Noordel., Fernandae Noordel. and Canosericei Noordel., we think that on a strictly morphological basis E. calabrum should be placed within section Endochromonema subsection Endochromonema (Largent & Thiers) Noordel., especially on account of the heterodiametrical spores and the presence of an intraparietal pigment.
Consulting the iconography of Noordeloos’ monograph (1992, 2004), Entoloma calabrum looks vaguely like E. pallescens (P. Karst.) Noordel., E. lanuginosipes Noordel. and E. cetratum (Fr.) M.M. Moser, which belong to section Endochromonema subsection Endochromonema, but the former differs especially by the presence of a subzonate and strongly fibrillose, almost villose pileus and 4-spored basidia.
The species gravitating around Entoloma sericeum Quél., some of them recently described (Vila et al. 2013), such as E. minutisporum (Vila & Llimona) J. Carbó et al., E. conicosericeum Vila et al., E. atrosericeum (Kühner) Noordel., E. cinereoopacum (Noordel.) Vila et al., E. vindobonense Noordel. & Hauskn., E. nitens (Velen.) Noordel., E. juncinum (Kühner & Romagn.) Noordel., E. brunneosericeum Noordel. et al., E. llimonae Vila et al., differ from E. calabrum especially in the iso- or subisodiametrical spores and the abundant encrusting epiparietal pigment.
Entoloma hebes (Romagn.) Trimbach and closely related taxa like E. hirtipes (Schumach.) M.M. Moser, E. fuscohebes Vila et al., E. psammophilohebes Vila & J. Fernández, E. tenellum (J. Favre) Noordel., E. kristiansenii Noordel., E. tibiicystidiatum Arnolds & Noordel. and E. pseudofavrei Noordel. & Vila can be distinguished from E. calabrum on the basis of the occurrence of distinct cheilocystidia (Noordeloos 2004; Vila et al. 2013).
No extra-European known species fits our new taxon (Hesler 1967; Horak 1973, 1976, 1978, 1980, 1982, 2008; Romagnesi and Gilles 1979; Pegler 1983, 1997; Noordeloos 1988a, 2008; Largent 1994; Wölfel and Noordeloos 2001; Manimohan et al. 2006; Gates and Noordeloos 2007; Noordeloos and Gates 2009, 2012; Noordeloos and Hausknecht 2007; Noordeloos and Morozova 2010; Henkel et al. 2014). According to the ITS sequence data analysis (Fig. 1), the most closely related species are Entoloma conicum (Sacc.) Hesler and E. infula (Fr.) Noordel. two taxa sometimes with subzonate pileus. E. conicum (Peck) Hesler, described from North America (= E. alboumbonatum Hesler; = E. subquadratum Hesler fide Noordeloos (1988b, 2008), who studied type collections, Horak (1976) and Largent (1994), differs from E. calabrum mainly in having a subfarinaceous odour and taste, isodiametrical, and quadrate to 5-angled spores, Qm = 1.2 (Hesler 1967; Noordeloos 1988b).
E. infula is distinguished by its subglabrous pileus and stipe surfaces, presence of intracellular and encrusting pigment in the pileipellis and its smaller spores (Noordeloos 1987, 1988a, 1992, 2004, 2012; Noordeloos and Hausknecht 1993).
Finally, E. minutum (P. Karst.) Noordel. is characterised by a strongly translucently striate pileus, often with a slight depression in the centre, subisodiametrical spores, and growth in moist grasslands, on damp places with Alnus, Fraxinus, Betula and Salix (Noordeloos 1992, 2004).
Hygrophoraceae Lotsy
The importance of the Hygrophoraceae for lichenization in the Basidiomycota was only recently recognized, with the inclusion of Dictyonema and relatives in this family (Lawrey et al. 2009). Until about ten years ago, Dictyonema was considered to contain only five, widespread species in a single genus (Parmasto 1978), but the clade is now known to comprise five genera (Acantholichen, Cora, Corella, Cyphellostereum, Dictyonema) and several hundred species (Dal-Forno et al. 2013; Lücking et al. 2013, 2014). In particular, the genus Cora is hyperdiverse, with 114 species recognized based on molecular phylogeny and over 400 species predicted (Lücking et al. 2014). While Cora is essentially neotropical, Dictyonema sensu stricto is found in all tropical areas and hence should have a similarly high number of species. Based on recent phylogenetic studies, further two species in the clade are here described as new (Fig. 127).
Best-scoring maximum likelihood tree with bootstrap support values computed with RAxML 8.2.0 of selected species of Cora (Hygrophoraceae), including the new species C. barbulata. GenBank numbers and voucher information are indicated in the tree (see Lücking et al. 2014 for full tree)
-
216.
Cora barbulata Lücking, Dal-Forno & Lawrey, sp. nov.
Index Fungorum number: IF551501; Facesoffungi number: FoF01049; Fig. 128a–c
Etymology: Referring to the beard-like concentric rings of irregular tomentum on the upper lobe surface.
Holotype: R. Lücking R18 (CR).
Diagnosis: Differing from the morphologically similar Cora aspera in the crenulate, undulate lobe margins and the large, broad hymenophore patches.
Thallus epiphytic on stems and branches of shrubs, foliose, up to 15 cm across, composed of 1–5(−10) semicircular lobes per thallus; lobes 2–7 cm wide and 1–5 cm long, often branched and with short radial branching sutures, marginally distinctly crenulate (with short secondary branches) and undulate, light bluish to greenish or brownish grey with slight concentric colour zonation when fresh, with thin but distinct, involute, white to light grey margins, becoming white to grey in the herbarium. Upper surface smooth, glabrous except for a few concentric lines of short, irregular, white trichomes; trichomes 0.2–0.3 mm long and 7–15 μm thick at the base, composed of agglutinated hyphae; involute margin glabrous; lower surface ecorticate, finely felty-arachnoid (representing the exposed medulla), light grey when fresh and becoming white in the herbarium. Thallus in section 200–300 μm thick, with upper cortex, photobiont layer, and medulla; upper cortex formed by a 20–50 μm thick layer of rather loosely packed, irregularly arranged to nearly periclinal, 4–5 μm thick hyphae supported by an indistinct, 20–30 μm high ‘medullary’ layer of spaced groups of densely packed, anticlinal, 3–5 μm thick hyphae; photobiont layer 50–120 μm thick, composed of clusters of short, coiled cyanobacterial filaments wrapped in a dense, paraplectenchymatous hyphal sheath formed by jigsaw puzzle-shaped cells, clusters 20–30 μm diam., individual photobiont cells 11–13 μm broad and 5–8 μm long, dark blue-green to lighter green in upper portions, penetrated by tubular fungal hyphae; heterocytes sparse, hyaline to pale yellow, 9–11 μm wide and 5–6 μm long; cells of hyphal sheath wavy in lateral outline, 3–4 μm thick; medulla 50–100 μm thick, composed of loosely woven, irregularly arranged to more or less periclinal hyphae 4–5 μm thick; clamp connections not observed. Hymenophore developed as angular-rounded, resupinate patches irregularly dispersed on the underside, patches 5–15 mm diam., with pale orange-yellow, smooth surface and felty, involute margins; hymenophore in section 50–100 μm thick, composed of a paraplectenchymatous layer resting on loose, 4–6 μm thick, generative medullary hyphae and supporting the hymenium; hymenium composed of numerous, palisade-like basidioles and scattered basidia; basidioles 25–35 × 5–6 μm; basidia 25–40 × 6–7 μm, 4-sterigmate; basidiospores not observed. Chemistry: No substances detected by TLC.
Material examined: COSTA RICA, San José, Los Santos Forest Reserve, km 90 on road (ruta 2) from Cartago to San Isidro, access road to towers on summit; 83° 45′ W, 09° 34′ N, 3400–3450 m; upper montane cloud forest and subalpine paramo zone, disturbed low paramo shrub with Chusquea, on stems and twigs of unidentified shrubs; 12 September 2007, R. Lücking 21007 (CR holotype; F isotype); same locality; 21 May 2012, M. Dal-Forno 1710, 1712 (GMUF paratypes); COSTA RICA, Cartago: Tapantí-Cerro de la Muerte National Park, km 90 on road (ruta 2) from Cartago to San Isidro, roadside forest remnants; 83° 45′ W, 09° 34′ N, 3350–3400 m; upper montane cloud forest zone, disturbed low oak forest, on stems and twigs of unidentified shrubs; 21 May 2012, M. Dal-Forno 1713, 1714 (GMUF paratypes).
Distribution and ecology: Known from several collections as shrub epiphyte in the upper montane cloud forest and paramo zone in the Cordillera de Talamanca in Costa Rica.
Notes: Cora barbulata is morphologically similar to C. aspera Wilk et al. (Lücking et al. 2013) in the epiphytic growth habit, the rather large thallus composed of several lobes, and the concentric lines of irregular tomentum. The two species are, however, only distantly related, each falling into one of the two major Cora clades (Lücking et al. 2014). A closer relative of C. barbulata is the terrestrial C. arachnoidea J. E. Hern. & Lücking (Fig. 128a–c), which is grey-brown when fresh and uniformly thinly tomentose on the upper surface (Lücking et al. 2013). Cora barbulata can be distinguished from C. aspera mainly by the coarsely crenulate, undulate lobe margins and the different hymenophore, forming large, irregularly dispersed patches on the underside.
-
217.
Dictyonema gomezianum Lücking, Dal-Forno & Lawrey, sp. nov.
Index Fungorum number: IF551502; Facesoffungi number: FoF01050; Fig. 131d–f
Etymology: Dedicated to the late Dr. Luis Diego Gómez, prominent Costa Rican botanist, naturalist, and conservationist and long-time director of Las Cruces Biological Station.
Holotype: R. Lücking 18053 (CR).
Diagnosis: Differing from the morphologically similar and related Dictyonema metallicum in the narrower cyanobacterial filaments with more or less paraplectenchymatous hyphal sheath and the very narrow associated hyphae.
Thallus epiphytic on tree trunks and overgrowing nearby bryophytes, appressed filamentous, continuous, up to 5 cm across, forming a strongly compressed mat of horizontal, densely interwoven, dark aeruginous fibrils almost completely embedded in a gelatinous matrix with metallic shimmer, except for a broad, white, opaque, byssoid prothallus; thallus in section 50–100 μm thick, composed of an irregular photobiont layer and an irregular medulla or hypothallus. Photobiont Rhizonema, in a layer composed of numerous cyanobacterial filaments wrapped in a closed hyphal sheath formed by small, paraplectenchymatous or indistinctly jigsaw puzzle-shaped cells; cyanobacterial filaments composed of 7–9 μm wide and 3–5 μm high, dark aeruginous cells penetrated by tubular fungal hyphae; heterocytes sparse, yellowish, 6–10 μm wide and 2–4 μm high; cells of hyphal sheath angular to slightly wavy in lateral outline, 1.5–2 μm thick; hyphae associated with hyphal sheath straight, 2–3 μm thick, lacking clamp connections; compacted prothallus mostly formed by densely arranged empty hyphal sheaths admixed with straight hyphae. Hymenophore not observed. Secondary chemistry: no substances detected by TLC.
Material examined: COSTA RICA, Puntarenas, Las Cruces Biological Station near San Vito de Coto Brus; 82° 58′ W, 08° 47′ N, 1200 m; lower montane rain forest zone, on ridge beyond Río Java, on trunk of tree in disturbed primary forest; October 2004, R. Lücking 18053 (CR holotype; F isotype).
Distribution and ecology: Known from lower montane rain forest in southern Costa Rica in the broader southern Central American Choco region.
Notes: Dictyonema gomezianum is similar and closely related to the recently described D. metallicum Lücking et al. (2013). Both share the strongly appressed, filamentous thallus in which the horizontally oriented fibrils are embedded in a gelatinous matrix that gives the thallus a strong metallic shimmer. While the phylogenetic distance between D. metallicum and its sister species, D. gomezianum, is considerable (Dal-Forno et al., in prep.), the morphological differences are minor: D. metallicum has a thinner thallus with indistinct medulla, the cyanobacterial filaments are broader (likely influenced by the fungus which produces a sheath with more distinctly puzzle-shaped cells), and particularly the associated fungal hyphae are thicker (4–6 μm).
Inocybaceae Jülich
The family Inocybaceae is a monophyletic lineage within Agaricales. It is species rich and has a worldwide distribution. The species are small to medium sized with a brown spore deposit, and most species form ectomycorrhiza with a broad range of host trees and shrubs. Besides Inocybe the family today include Tubariomyces (Alvarado et al. 2010) and Auritella (Matheny and Bougher 2006).
Inocybe (Fr.) Fr. Monogr. Hymenomyc. Suec. (Upsaliae) 2(2): 346 (1863)
As currently circumscribed, Inocybe is a morphologically and genetically diverse genus. Molecular phylogenetic analyses suggest the genus can be divided in the four major lineages, Inocybe s.s, Pseudosperma, Inosperma and Mallocybe (Larsson et al. 2009; Ryberg et al. 2010), and the family Inocybaceae to be composed of at least seven evolutionary lineages (Matheny 2009; Matheny et al. 2009). However, the proposed taxonomical rearrangements have not yet been fully implemented.
The species in subgenus Mallocybe are frequently encountered in arctic/alpine, subalpine and boreal habitats (Cripps et al. 2010; Jacobsson and Larsson 2012). Many of the species are associated and forming ectomycorrhiza with dwarf and shrub Salix species. Morphology, ecology and comparison of sequence data, including ITS sequences of type specimens, support the recognition of a new species in subgenus Mallocybe from the northern boreal and subalpine regions of Scandinavia, associated with Salix phyllicifolia on more calcareous ground (Fig. 129).
-
218.
Inocybe granulosa Jacobsson & E. Larss., sp. nov.
MycoBank number: MB 812048; Facesoffungi number: FoF00989; Figs. 130 and 131.
Etymology: Refers to the granular small scaly appearance of the stipe and pileus.
Holotype: SWEDEN, Jämtland, Berg, close to Lake Storsjön, 4 km N Svenstavik, 1 September 2009, E. Larsson 138-09 (Herbarium GB).
Pileus 10–32 mm diam., at first convex with an incurved margin, then expanded, when mature flat or frequently with somewhat depressed centre, not umbonate, at first pale yellowish, later becoming yellowish brown at least in the centre, covered by numerous small, floccose scales. The scales are often pointed and upraised at least in the centre, at first yellowish brown, sometimes but not always becoming dark brown with age. The scales often loosen and disappear at least partly in mature basidiomata. Lamellae normally distant, at first pale beige to yellowish, gradually rather dark greyish brown with a paler edge. Stipe 15–35 mm long, 2–4 (5) mm wide, equal or somewhat thicker towards the base, pale yellowish brown with a white base, with granular to floccose brownish scales similar to those on the cap. Veil beige sometimes forming a thin and evanescent annulus. Flesh pale, whitish without distinct smell, taste mild. Basidia clavate, 35–45 × 8–11 μm, 4-spored, sometimes with brownish necropigments. Spores (8–)9–12 × 5–7(−7.5) μm, Q = 1.4–1.8, mostly ellipsoid and slightly phaseoliform in profile. Cheilocystidia numerous, more or less septate with a broadly clavate to almost globose end cells, 12–24 μm broad, thin walled, some with brownish necropigments, Fig. 3. Pleurocystidia absent. Clamp-connections present.
Habitat: On sandy roadsides or gravelly ground, often near mines or industrial sites, likely associated with Salix phyllicifolia but probably also with other Salix species. Likely favoured by calcareous ground.
Distribution: Found in upper boreal and subalpine zones in the western part of the provinces of Jämtland and Härjedalen, central Sweden and in adjacent parts of central Norway. Rather common and often abundant in its special habitat in central Scandinavia but so far not known outside this area.
Material examined: SWEDEN, Jämtland, Åre, Handöl, sandy road-side with Salix, 10 August 1984, S. Jacobsson 84030 (GB); Jämtland, Berg, N Svenstavik, resting-place, among Salix phyllicifolia, 1 September 2009, E. Larssson 138-09 (GB, holotype) (Isotypus TUR-A); the same locality 26 August 2011, S. Jacobsson 11007 (GB); Jämtland, Åre, Undersåker, Nulltjärnsgården, road-side with Salix, 27 August 2011, S. Jacobsson 11008 (GB); Härjedalen, Tännäs, Bruksvallarna, roadside with Salix phyllicifolia 2 July 2012, S. Jacobsson 12002 (GB). NORWAY, Hedmark, Folldal, on clinkers close to the mine, 23 August 2012, S. Jacobsson 12016 (GB); Oppdal, Dovre, Hjerkinn, roadside with Salix phyllicifolia, 20 August 2012, S. Jacobsson 12017 (GB).
Notes: Members of subgenus Mallocybe frequently occur in boreal, subalpine and arctic/alpine regions (Kühner 1988; Cripps et al. 2010). Several of these species may have more or less distinct scales on the pileus surface and some of them may possibly be mistaken for Inocybe granulosa. The most similar species in the literature is I. squarrosoannulata (originally described as I. dulcamara f. squamosoannulata by Favre 1955) encountered in alpine environments growing with dwarf Salix. It is depicted with distinct scales both on the pileus surface and the stipe (Bon 1997; Ferrari 2006), but the scales are coarser than in I. granulosa and arranged in garlands, somewhat similar to I. terrigena (Fr.) Kuyper. Comparison of ITS sequence data of the holotype (Fig. 1) show that I, squarrosoannulata and I. granulosa are distinct species.
Another species about the same size and colour as I. granulosa and with similar habitats and ecology is I. malenconii Heim. However, the pileus is more finely scaly and the scales are absent or rare on the stipe. In micromorphology I. malenconii is easily separated from I. granulosa by having a narrowly cylindrical spore shape, more reminiscent of those of I. lacera (Fr.) P. Kumm. Inocybe malenconii was described from France (Heim 1931) and is widely distributed. It has been recorded several times in central Sweden and the Nordic countries, often found along roadsides (Jacobsson and Larsson 2012).
Inocybe granulosa is readily identified in the field and commonly encountered in a restricted part of central Scandinavia, but so far not known outside this region. Despite similarity in macro-morphology with I. malenconii and I. squarrosoannulata the phylogenetic analysis (Fig. 1) indicate I. granulosa to be more closely related to I. agardhii (Lund) P.D. Orton, I. substraminipes Kűhn. and I. fulvipes Kűhn., all growing associated with Salix.
Boletales
Boletaceae Chevall.
The family Boletaceae is characterized by species developing their spores in a tubular hymenophore on the underside of the pileus, although some species (e.g., those in the genus Phylloporus Quél.) show highly anastomosed lamellae and others exhibit a hypogeous behavior. Boletaceae species produce fleshy basidiomes, sometimes quite large, with a central stipe. The spore print colours vary from olivaceous-tobacco-brown to yellow, yellowish or vinaceous. In many species, flesh after wound or cut turns blue, as a result of the oxidation of pulvinic acid derivatives.
Singer (1986) included 26 genera in this family. Molecular phylogenetic studies of the 2000s have revised the concept of the family: Binder and Hibbett (2006) recognized 38 genera. Some changes in classification have moved some genera out of the Boletaceae; nevertheless, it remains a large family with many genera. According to the Kirk et al. 2008a, b, 35 genera are recognized; in the overall work of Wu et al. (2014), seven major clades at subfamily level and 59 generic lineages were uncovered, including 4 new subfamilies and 22 new potential generic clades (Fig. 132).
Bayesian phylogram (MrBayes 3.2) of Xerocomellus species based on ITS sequence data. Xerocomellus rubellus and X. communis were used as outgroups. Bayesian posterior probabilities (BPP) above 0.90 and RAxML bootstrap values (MLB) above 50 % are shown. Thickened lines indicate both BPP ≥ 0.95 and MLB ≥ 70 %. Newly sequenced collections are in bold
Xerocomellus Šutara, Czech Mycol. 60(1): 44 (2008)
The genus Xerocomellus was described by J. Šutara (2008) with X. chrysenteron (Bull.) Šutara as the type species. This genus, segregated from Xerocomus, included species characterized by a pileipellis arranged from the early stage in a characteristic palisadoderm of incrusted hyphae, and a spore surface which is never bacillate, but smooth or with specialized apex (“truncature”) and/or longitudinally striate. As originally circumscribed, the genus included X. armeniacus (Quel.) Šutara, X. engelii (Hlavaček) Šutara (= Xerocomus communis (Bull.) Bon s. auct), X. fennicus (Harmaja) Šutara, X. marekii (Šutara & Skala) Šutara, X. porosporus (Imler ex Moreno & Bon) Šutara, X. pruinatus (Fr.) Šutara, X. ripariellus (Redeuilh) Šutara and X. rubellus (Krombh.) Šutara. Xerocomus cisalpinus (Simonini et al.) Klofac was included in the genus by Klofac (2011). In 2014, Xerocomus dryophilus was also included in this genus as X. dryophilus (Thiers) N. Siegel et al. (Frank 2014). Vizzini (2015) established for Boletus rubellus Krombh. (= X. rubellus (Krombh.) Šutara) the new genus Hortiboletus Simonini et al. and for Xerocomus armeniacus, as well as for the close Xerocomus persicolor H. Engel et al., the new genus Rheubarbariboletus Vizzini et al..
-
219.
Xerocomellus sarnarii Simonini, Vizzini & Eberhardt, sp. nov.
Index Fungorum number: IF551434; Facesoffungi number: FoF01047; Figs. 133 and 134
Xerocomellus sarnarii. Basidiomes. a to h Coll. MCVE 28572, MCVE 28579, MCVE 28574, MCVE 28576, MCVE 28573, MCVE 28571, MCVE 28577 (holotype), MCVE 28578, respectively, from Sardinia, Calangianus (OT), basidiomes in all stages of development, under Quercus suber, in granitic acid soil i Coll. MCVE 18361, from M. Argentario (GR), Tuscany, Centre Italy, mature basidiome under Quercus ilex, in calcareous soil j Coll. MCVE 17797, from Sardinia, Cala Gonone (NU), mature basidiome under Quercus ilex, in calcareous soil. Scale bars: 10 mm
Xerocomellus sarnarii Microscopic features a Spores, in L4, coll MCVE 28572 b Spores, in L4, coll. MCVE 28576, the arrows indicate the specialized apex (small “truncature”) c Pileipellis, in L4 + ammoniacal Congo red, coll. MCVE 28572 d Basidia, in L4, coll. MCVE 28572 e Pleurocystidia, in L4, in interferential phase contrast, coll. MCVE 28572 f Cheilocystidia, in L4, with interferential phase contrast, coll. GS 10151. Scale bars: = 10 μm
Etymology: dedicated to our friend Mauro Sarnari, who found this bolete at Monte Argentario.
Holotype: MCVE 28577
Colour terminology and alphanumeric codes are those of Kornerup and Wanscher (1978) and Seguy (1936).
Pileus 35–90 mm broad, fleshy, convex to pulvinate, rarely almost plane, also slightly depressed at the centre but conserving pulvinate shape; surface dry, tomentose-velutinous, soon cracking into scabs independently on weather conditions, with a subpellis very weakly and not always pink-reddish coloured, being this colour rarely visible in the fissures, sometimes only in the bites of the snails; surface variable in colour: in very young basidiomes with uncracked pileus dark brown with olive shade (6–7/F6–F4, 6/F8–F7), with fading patches (5/D5–D3), cracking into scabs and discolouring to ochre-beige with olive shade (4–5/D6–D3), passing through transition colours (5–6/D7–F4). Dull cream-whitish (3/4A2) cracks appear beneath the pileipellis layer in dry weather.
Tubes up to 5–10 mm long, separable, depressed to sinuate around the stipe, sometimes slightly decurrent, as high as the context of the pileus or even more; initially bright yellow with olive shades (1/2A7/8, 3A/B7/8), later dull greenish yellow (2C/D7/8, 3/4C/D6), bluing then darkening to blackish when bruised. Pores angular, uneven, in mature basidiomes 0.7–2 mm diam., concolourous to the tubes, slowly bluing (S351, 352, 366, 367) then darkening to blackish when bruised. Stipe 30–90 × 5–25 mm, cylindrical, slender, curved, somewhat tapering towards the base or also slightly widened. Surface dry, smooth to ingrown fibrillose, chrome yellow in the upper part, in the lower part and towards the base or partially or completely, sometimes abruptly, red, dark purple red or even blackish-red coloured (11–12/D8–F4). Context soft in the pileus, fibrillose and brittle in the stipe; in the pileus pale yellow (3A3) soon fading to dirty whitish when exposed (29/30A2), below the pileipellis sometimes showing a fine red line, often inconspicuous or really absent; in the stipe vivid yellow (3A4/5) or yellow with dull reddish tinges that are concolourous to the cortex (8 to 11C/D7/8), bluing (S446 to 448) in the whole pileus and in the stipe upper part when cut, with the dark purple red areas in the stipe base darkening, the stipe context at the extreme base dull ochre-brownish (S336 to 339); smell not distinctive, typical of the Xerocomellus, somewhat like wet iron; taste not distinctive, slightly sour. Spore-print: brownish with some olive tinges. Macrochemical reactions: weak fleeting-amyloid reaction in stipe context and hymenophoral trama of dry specimens. Spores (13.5) 13.8–15.1 (15.7) × (5.5) 5.5–6.1 (6.6) μm, Q = 2.51 ± 0.08, V = 256 ± 41 μm3 (n = 330/10/10), subfusiform, slender, with a weakly developed but distinct supra-apicular depression, spore wall up to 0.5 μm thick, intensely honey-coloured and with one or two guttules when mature, with a specialized spore apex (“truncature” faint and not always visible under the optical MS). Basidia 25–36 × 10–14 μm, inconspicuous, clavate, hyaline to yellowish, mainly 4-spored, not rarely 2-spored. Pleurocystidia scarce, 35–52 × 6–11 μm, fusiform or ventricose-fusiform, sometimes with a obtuse-rounded apex, resembling those of Psathyrella barlae (Bres.) A.H. Sm., with hyaline to slightly yellowish content. Cheilocystidia similar, but smaller, 22–32 × 4–10. In some of the specimens collected it was observed an abundance of pseudocystidia with a yellowish, refringent, oily content, reacting to dark blue with Cresyl blue. Pileipellis a palisadoderm strongly reminiscent of the pileipellis of X. chrysenteron, with long parallel chains of elements, terminal elements rarely wider than the basal ones, mostly cylindrical and somewhat tapering towards the apex, obtusely triangular, mitriform, also acorn-shaped to exceptionally subsphaerical, rarely papillate, (36.5) 36.2–38.2 (39.2) × (13.1) 13.3–15.9 (16.1) μm, Q = 2.62 ± 0.22 (n = 99/3/3), terminal and subterminal elements moderately to heavily incrusted, only exceptionally smooth. Stipitipellis consisting of loosely interwoven, 3–6 μm broad hyphae with hyaline to yellowish content. In the upper part of the stipe is present a well-developed caulohymenium with spore-forming caulobasidia and caulocystidia. Stipe texture formed of parallel running, hyaline, 2.5–5 μm broad hyphae; no “pruinatus-hyphae” in the lower part of the stipe are present (Ladurner and Pöder 2000). No clamp-connections observed.
Microchemical reactions: with the exception of the amyloid reacting hyphae in the stipe, no particular reproducible macrochemical reactions were observed.
Habitat and known distribution: X. sarnarii is a rare species, so far known only from Italy (Sardinia and Tuscany) and France (see notes), in Mediterranean areas under Quercus suber and Quercus ilex. This taxon seems to prefer climatically moderate zones and undisturbed habitats. The main fruiting period is late autumn (November).
Material examined: ITALY, Sardinia, Province of Olbia-Tempio Pausania, Common of Calangianus, along road SP 38 “Via S. Antonio”, loc. Monti di La Jescia, “Cava Merzari”, 40°56′21″N, 9°12′35″E, 425 m a.s.l., on granitic soil, under Quercus suber, mixed with Xerocomus cisalpinus, 10 November 2011, leg. G. Simonini (MCVE 28577, holotype), (GS 10025, isotype); same locality and date of the typus, MCVE 28571, MCVE 28572, MCVE 28573, MCVE 28576, MCVE 28578, MCVE 28579; Sardinia, NU, Dorgali, Cala Gonone, “Via dei Lecci”, 40°16′25″ N, 9°36′37″ E, 230 m a.s.l., on calcareous soil, under Quercus ilex, 7 November 1994, leg. G. Redeuilh (MCVE 17797); Tuscany, GR, Monte Argentario, “Via Acquedotto Leopoldino”, 42°24′53″N, 11°10′1″E, 185 m a.s.l., on calcareous soil, under Quercus ilex, 1 November 1998, leg. M. Sarnari (MCVE 18361).
Additional material examined. Xerocomellus cisalpinus: ITALY, Sardinia, Province of Olbia-Tempio Pausania, Common of Calangianus, along road SP 38 “Via S. Antonio”, loc. Monti di La Jescia, “Cava Merzari”, 40°56′21″N, 9°12′35″E, 425 m a.s.l., on granitic soil, under Quercus suber, mixed with Xerocomellus sarnarii, 10 November 2011, leg. G. Simonini (GS10020). Xerocomellus porosporus: ITALY, Latium, Manziana (RM), 320 m a.s.l., under Quercus cerris in acid soil, 20 June 2009, legit. M. Gelardi and V. Migliozzi (MG197a).
Notes: Both Bayesian and Maximum likelihood analyses produced the same topology; therefore, only the Bayesian tree with both BPP and MLB values is shown (Fig. 132). In the ITS sequence analysis the 7 sequenced collections of X. sarnarii form a well-supported clade / sarnarii, (BPP = 1, MLB = 97 %), together with two environmental sequences (uncultured fungus clone OTU175 KM247624 from ectomycorhiza of Quercus ilex, Pézilla-de-Conflent, southern France, and uncultured Xerocomus HE601897 from ectomycorrhizal mantle of Quercus ilex, Sardinia, Italy). As the sequences of this clade show a high ITS sequence homology (pairwise % identity value = 99.4), X. sarnarii seems present also in France. The X. sarnarii clade is sister (BPP = 99, MLB = 78 %) to X. chrysenteron.
As with the other Xerocomellus species, X. sarnarii is difficult to be recognized in the field based only on its macromorphological features.
At a first glance, the brownish cracked pileus with the pallid context showing through, recalls X. porosporus, but the vivid purple-red stipe base is disconcerting. In young specimens, or specimens in which the pileus surface does not crack clearly, the confusion with X. redeuilhii nom. prov. (Taylor et al. 2012 = X. dryophilus sensu Simonini 1994, and indicated in Fig. 1 as X. aff. dryophilus) is possible, due to the two-coloured stipe cortex, chrome yellow in the upper part and abruptly dark red in the lower part, very close to that of X. redeuilhii. (Fig. 1a–j); moreover, some collections of X. redeuilhii may also have occasionally a brown pileus. The spores of the two boletes are however, clearly different mainly in Q ratio: Q = 2.1–2.4 for X. redeuilhii and Q = 2.4–2.6 for X. sarnarii. In its most typical appearance, because of its obvious cracked pileus surface, X. sarnarii is somewhat similar to X. chrysenteron (Bull.) Šutara, X. cisalpinus (Simonini et al.) Klofac, and X. porosporus (Imler ex Bon & G. Moreno) Šutara (= Boletus marekii Šutara et Skála, unpublished data); moreover, all these boletes have a similar pileipellis structure, with long chains of parallel, incrusted elements (Ladurner and Simonini 2003). Nevertheless, X. sarnarii can easily be distinguished from these species based on its spore features: the smooth spores with the characteristic specialized apex, showing a hardly detectable, small “truncation” are typical for X. sarnarii, and are different from both those of X. chrysenteron (without any truncation), X. cisalpinus (with no truncation and faintly but clearly longitudinally striate) and X. porosporus (smooth, but with a much more evident truncation) (Ladurner and Simonini 2003; Šutara 2008). In addition, the spores of X. sarnarii are significantly broader (5.5–6.1 μm) than those of both X. chrysenteron (4.7–5.4 μm) and X. cisalpinus (4.6–5 μm). However, X. porosporus has spores as broad (5.6–6.4 μm) as X. sarnarii (Supplementary Table 4).
Xerocomellus truncatus (Singer et al.) Klofac (2011), based on Xerocomus truncatus Singer et al. (1960), is a North American taxon that shares with X. sarnarii the general appearance, with the tendency of the pileus surface to crack and the stipe having also red areas. It differs for “the (pileus) cracks pinkish-purple or rarely with the pallid flesh showing through”, while the context among pileus cracks of X. sarnarii is, oppositely, whitish pallid and very rarely pinkish; the context “in the central portion of the stipe red”, while in X. sarnarii the red colour is concentrated in the lower half or the bottom; the spores “with a truncate-applanate apex which is thicker-walled at the two apical angles and often with markedly thinned wall between these angles, there also frequently depressed to umbilicate-depressed, a minority with the apex merely rounded”, while in X. sarnarii the spores apex is only faintly truncate, and in the most of the cases the truncation is not clearly perceptible at the optical MS. In the later illustration by Snell and Dick (1970, pl. 79 Fig. 5), the truncature is really wide and sharply angled in the spore adaxial profile, with an estimable width on the drawing = 3 μm; the spores profile of X. sarnarii, is quite different, with a truncation that is hardly perceptible, having an estimable width = 1 μm.
Cantharellales
Cantharellus Adans. ex Fr., Syst. mycol. (Lundae) 1: 316 (1821)
Madagascar is extremely rich in chanterelles (Buyck 2008, 2012, 2014, Buyck et al. 2014, 2015; Buyck and Randrianjohany 2013; Liu et al. 2015) with many species showing very close relationships to mainland African taxa. In the following section we describe four new chanterelles that are associated with imported eucalypts in Madagascar, but are clearly sister-species to other African or Malagasy chanterelles (Fig. 135).
Phylogram generated from maximum likelihood analysis based on tef-1 locus for 85 taxa. Branches that received bootstrap support ≥70 and BPP ≥95 % are in bold (ML/BPP). Only BS values are reported along the branches except for the branch in bold grey which is supported only by Bayesian analysis. The type strains are in bold and the new species are in blue. Sequence data used in this study have been taken from (Buyck et al. 2015)
-
220.
Cantharellus eucalyptorum Buyck, Randrianjohany & V. Hofstetter, sp. nov.
MycoBank: MB 813239; Facesoffungi number: FoF00990; Figs. 136 and 140
Etymology: refers to its association with Eucalyptus robusta.
Diagnosis: Differs from the Malagasy C. tricolor principally by the much paler gill folds, and genetically by its closer relationship to C. tanzanicus.
Holotype: MADAGASCAR, Central Highlands, 25 km before reaching Antananarivo when returning from Andasibe, collected under E. robusta in hills along the RN2 before reaching the village of Ambanitsena, 6 February 2006, Buyck & V. Hofstetter 06.159 (PC0085037).
Cap mostly 25-45(−60) mm diam., regular or with uneven, waving or undulating lobes, when young with strongly incurved margin becoming even or uplifted when old with a sunken center to strongly infundibiliformous; surface when young tomentose-granulose to pubescent, with age usually fragmenting in larger, appressed and concentrically arranged squamules, when wet smooth to velvety, colours varying from cream or ochre (3A2 to 4A3-5) to pale grayish brown (5C4) or even dull gray in the center. Hymenophore decurrent and not abruptly delimited from the sterile stipe surface, formed of well-developed gill folds, 2-4(6) mm high, starting out as very pale cream or off-white (at the most 3-4A2), but then developing pale yellowish tints (3-4A4) with age, forkings not abundant except closer to the cap margin, either completely smooth or frequently anastomosed in between gill folds. Stipe mostly distinctly shorter than the cap diam., more rarely quite slender and exceeding the cap diam., (3-)5-10 mm diam., and mostly (15)20-30(45) mm long, subcylindrical or slightly narrowing downwards, mostly off-white to creamish yellow and somewhat paler than the hymenophore, in the upper half often developing appressed squamae deposited in circular girdings, solid. Flesh firm and thick beneath the cap center, whitish, but showing distinct yellowing when cut or handled, particularly in the lower stipe half. Taste mild. Smell faint and not very typical. Spore print probably pale yellowish, insufficient for a good appreciation.
Spores ellipsoid to narrowly ellipsoid, often slightly constricted in the central part and reniformous, measuring (7.5)7.8-8.33-8.8(9.4) × (4.2)4.5-4.91-5.3(5.4) μm, Q = (1.5)1.6-1.71-1.8(1.9), neither amyloid nor cyanophilous, smooth, containing mostly a distinct, large oil drop. Basidia quite long and slender, mostly 50-70(−85) × 5–7 μm, clavate, (2)4-5-spored. Cystidia absent. Subhymenial cells often long and slender, but often also wider than the basal part of the basidia. Lamellar trama comprised of predominantly narrow and slender elements, sometimes as thin as 1 μm diam., some being inflated towards the septa on one side. Pileipellis with thick-walled (up to 1 μm), flexuous hyphal extremities, ca 4–9 μm wide, that often adhere together forming tufts or short, coiled, rhizoid-like structures, falling easily apart into smaller fragments when squashed, very frequently giving the impression that cells narrow abruptly near the septa and only stick together in the more central part of the septum; most cells slender and narrowly cylindrical; the terminal cell mostly 30–60 μm long, more often slightly inflated, clavate, fusoid or irregularly undulate in outline, near the tip obtuse-rounded or frequently constricted and papillate to subcapitate. Clamp connections absent from all tissues.
Material examined: MADAGASCAR, Central Highlands, Arivonimamo, under eucalypts, on lateritic soil at 1400 m alt., 30 January 1997, Buyck, Eyssartier & Moreau leg., in Buyck 97.078 (PC0084828), ibid., 30 January 1996, Buyck 96.543 (PC0084830); near Andasibe, under Eucalyptus, 12 February 1997, Buyck, Eyssartier & Moreau leg., in Buyck 97.406 (PC0084829); 25 km before reaching Antananarivo when returning from Andasibe, bought from vendors along the RN2 before reaching the village of Ambanitsena, 28 February 2000, Buyck 00.1828 (PC0085036); ibid. 4 February 2006, Buyck & V. Hofstetter V. 06.148 (PC0084127), 06.149 (PC0084128); ibid., collected under eucalypts, 6 February 2006, Buyck & V. Hofstetter 06.153 (PC0084112), 06.159 (PC0085037), 06.165 (PC0084789).
Notes: Cantharellus eucalyptorum is very closely related to the mainland African C. tanzanicus and we hesitated at first to describe it as a different species since our phylogeny (Fig. 135) shows no support to distinguish the two taxa, mainly due to the fact that C. tanzanicus is still known from a single collection. When taking a closer look at the sequences, the Malagasy specimens represent without any doubt a clearly different species. The 92 % BS that supports the distinction between a subclade formed by three specimens of C. eucalyptorum versus the grouping of the remaining two specimens together with C. tanzanicus, is based on a single mutation (Fig. 135). However, the latter species differs from all six Malagasy specimens by four apomorphies in the coding part of the tef-1 sequences, and two additional mutations in the introns, which is far more than the number of apomorphies that support for example in subgenus Cantharellus the distinction between the North American C. tenuithrix, C. flavus and C. phasmatis (Foltz et al. 2013).
Morphological characters that may help to distinguish C. eucalyptorum from C. tanzanicus are the slightly more voluminous spores, more pronounced filamentous subhymenium and somewhat longer basidia of the Malagasy species. Such differences, however, would need to be confirmed by comparing more collections. Judging from their general appearance, the specimens depicted and described in Härkönen et al. (2003, as C. isabellinus), most likely correspond to our C. tanzanicus and they share superposable spore sizes with the holotype of the latter [7.5–9 × 3.5–4.5 versus (7.3)7.9–8.4–8.9(9.2) × (3.7)3.8–4.1–4.4(4.6) μm], which suggests that the smaller and more cylindrical spores of C. tanzanicus might represent a reliable distinction. Spore measurements of other collections for C. eucalyptorum confirm those obtained for the holotype:
-
97.078/ (6.75)7-7.71-8.5(9) × (4.25)4.5-4.94-5.5(6) μm Q = (1.3)1.4-1.57-1.7(1.8)
-
96.543/ 7–7.37-7.7(8) × (4.5)4.8-5.17-5.5(6) Q = (1.3)1.5-1.67-1.7
-
96.550/ (6.5)7.3-83-8.7(9.5) × (4)4.5-5.00-5.5(6) Q = (1.1)1.4-1.62-1.8(2.1)
-
Holotype/ (7.5)7.8-8.33-8.8(9.4) × (4.2)4.5-4.91-5.3(5.4) μm, Q = (1.5)1.6-1.71-1.8(1.9)
This fleshy and very variable chanterelle is locally abundant under E. robusta plantations on the Central Plateau of Madagascar and is frequently offered for sale along roads and in market places. It is often sold as ‘girolle’, the common name in France for C. cibarius Fr.:Fr., of which it has the general habit, but not the yellow colour. It differs from this European species most markedly in the white, but distinctly yellowing flesh, particularly so in the stipe and, of course, in the grayish brown, fibrillose to cottony surface of the cap and to a lesser degree, also of the stipe. This type of surface is typical for many species in subgenus Rubrinus sect. Isabellinus (see Buyck et al. 2014), but can be quite variable in its appearance and development. This variability contributes a lot to the sometimes important differences in general aspect of these species. Typically smooth and continuous when young, at least in the cap center, this type of surface tissue frequently breaks up at maturity into concentric rings or into appressed to sometimes even dressed squamules that are more or less concentrically arranged. A similar pattern may be observed on the stipe surface with darker squamules in more or less horizontal arrangements circling the stipe, although the squamae are usually much less pronounced compared to the cap.
The cap colour can vary from pale yellowish to quite dark brown or locally almost blackish gray when fresh and humid, but turns to an isabelline or dirty white or creamish colour when dry. The hymenophore is typically composed of gill-like folds that can be mixed with smaller lamellulae or forked to varying degrees; in some cases more strongly anastomosing, veined types of hymenophore can be observed. The colour of the hymenophore is typically very pale, sometimes nearly white when young, but then develops pale yellowish tinges with age, which distinguishes this species from C. albidolutescens, another very similar, usually somewhat paler chanterelle with intensively yellowing context.
-
221.
Cantharellus nigrescens Buyck, Randrianjohany & V. Hofstetter, sp. nov.
MycoBank: MB 813240; Facesoffungi number: FoF00991; Figs. 137 and 140
Etymology: the name refers to the rapidly blackening basidiomata
Diagnosis: Can be distinguished from C. congolensis by its more robust stature and frequently fasciculate growth, as well as by its association with Eucalyptus robusta.
Holotype: MADAGASCAR, Talatanivolonondry, in E. robusta plantations along the road to Anzojorobe, 10 February 2006, Buyck & V. Hofstetter 06.197 (PC0084076).
Cap up to 15 cm or more, when young very pale, cream to pale yellowish brown or even whitish when remaining covered by dead leaves or other detritus, with appressed, grayish-brownish squamules which become less visible when the cap is expanding, rapidly turning to reddish brown or blackish brown, and finally black. Hymenophore strongly decurrent and well delimited, irregularly and strongly veined-sinuate as well as anastomosing as to almost forming pores, up to 3 mm high, with obtuse edges, pale grayish or isabelline, then turning mouse gray and finally blackish gray to black in an unregular pattern. Stipe short and robust, mostly 20–30 × 10–20 mm, subcylindrical or slightly narrowing upwards or even ventricose when young, dirty cream or even pure white when young, quickly staining brown or gray and finally blackening with handling or age, smooth, compact and firm. Flesh very firm and thick beneath the cap center, white to whitish with almost some greenish tinges toward the stipe base, rapidly turning to a dirty brownish gray and finally black when cut. Taste mild to even slightly bitter. Smell weak but typical, of apricot. Spore print with distinct yellowish tinges.
Spores ellipsoid, (6)6.5-6.98-7.5(8.1) × (3.7)4-4.35-4.7(4.8) μm, Q = (1.4)1.5-1.61-1.8(1.9), smooth, filled with one to numerous oily inclusions. Basidia mostly 50-65(74) × 7-8(−9) μm, subcylindrical to weakly clavulate, often sinuate, (3-)5-6-spored; basidiola slender, subcylindrical, sinuate, becoming tardily clavate. Subhymenium filamentous, of slender, strongly septate, hyphal cells, 2-3(4) μm wide like the basidium base. Cystidia none. Pileipellis a loose cutis of ramifying, thin-walled hyphal extremities, measuring mostly 5-8(12) μm diam., terminal cells subcylindrical and hardly differentiated from the subapical ones, but often slightly constricted subterminally and subcapitate, all containing a brown diffuse pigment; no incrusting pigments observed at the surface but present in lower tissues. Clamp connections present everywhere.
Material examined: MADAGASCAR, Central Highlands, Andasibe, along road side with planted eucalypts, 17 February 1997, Buyck, Eyssartier & Moreau 97.547 (PC0084821), near Antananarivo, from E. robusta plantations, 6 February 2006, Buyck & V. Hofstetter 06.166 (PC0084979), 06.176 (PC0084078), Talatanivolonondry, in E. robusta plantations along the road to Anzojorobe, 10 February 2006, Buyck & V. Hofstetter 06.197 (PC0084076 holotype), 06.203 (PC0084980).
Notes: This chanterelle is one of the more common and abundantly fruiting species found in the E. robusta plantations of Madagascar’s Central Highlands. On some occasions, it can literally cover the soil but it is not easily noticed because the pale to dirty or grayish brown cap colour is so similar to the fallen, dry eucalypt leaves lying around it. It is often found in important clusters, frequently more or less aggregated or in very tight groups sitting on mycelial mats. Microscopic examination of specimens collected from different places on the Central Plateau show a remarkable homogeneity among these specimens.
During the first years of our fungal inventory of Madagascar, we never saw it for sale, but more recently it is commonly found for sale around and in the capital Antananarivo, perhaps as a result of our collecting trips where people observed us filling baskets of this species. There appears to be no particular reason why local people did not eat this species other than the unappealing aspect and almost instant blackening tissues when cooked. In mainland Africa, the similar C. congolensis is a considered a mediocre edible (Beeli 1928; Buyck 1994; Härkönen et al. 2003). We have prepared and consumed it on several occasions without any further complications. Nevertheless, these blackening species appear to be chemically somewhat different from most other chanterelles as they exhibit remarkable macrochemical reactions: first greenish, then brick red and finally dirty brown with potassium, dark gray with silver nitrate, slowly dark olive green with iron sulfate and green with Guaiac (Eyssartier 2001).
Our phylogeny shows it to be very closely related to mainland African collections from miombo woodland that are traditionally identified as C. congolensis Beeli (e.g., Buyck et al. 2000), which is a species originally described from equatorial rain forest. Preliminary sequence data reveal that the blackening chanterelles represent more than just a single species with a large distribution in the whole of tropical Africa, and C. nigrescens is not conspecific with the Central African species of the original description (Buyck & Hofstetter unpubl.).
Cantharellus avellaneus Pat. is another Malagasy chanterelle possessing a mouse gray hymenophore, but is definitely a different taxon because of the very elongated spores (see discussion in Buyck et al. 2015). Additional spore measurements for other collections of C. nigrescens give very similar results compared to those made for the holotype:
-
06.203/ 7–7.65-8.25(9) × (4)4.5-4.75-5 μm Q = 1.44-1.62-2
-
06.166/ (6.2)6.6-7.15-7.7(8.1) × (3.7)3.8-4.16-4.5(5) μm, Q = (1.5)1.6-1.72-1.8(2)
-
97.547/ (6)6.5-7.01-7.5(8) × 4–4.31-4.75(5) μm Q = 1.4-1.63-1.88
-
Holotype/ (6)6.5-6.98-7.5(8.1) × (3.7)4-4.35-4.7(4.8) μm, Q = (1.4)1.5-1.61-1.8(1.9)
-
222.
Cantharellus tricolor Buyck, Randrianjohany & V. Hofstetter, sp. nov.
MycoBank: MB 813241; Facesoffungi number: FoF00992; Figs. 138 and 140
Etymology: the name refers to the three contrasting colours resulting from the brown cap, white stipe and bright yellow hymenophore.
Diagnosis: Differs from C. eucalyptorum in its more bright yellow colour of the hymenophore, particularly when young, and its closer genetic relationship to C. densifolius.
Holotype: MADAGASCAR, Central Highlands, near Ranomafana, collected under E. robusta along the road, 18 February 2006, Buyck & V. Hofstetter 06.247 (PC0085151).
Cap mostly 25-45(−60) mm diam., regular or with uneven, waving or undulating lobes, when young with strongly incurved margin becoming even or uplifted when old with a sunken center to strongly infundibiliformous; surface when young mostly entirely cottony-tomentose to pubescent, with age usually disrupting in larger, appressed and concentrically arranged squamules, when wet smooth and sometimes almost velvety, colours varying from coffee-with-milk or the colour of milk chocolate, to more reddish or grayish hazelnut or cinnamon brown (5C3-4, 5B3, 4B2-3). Hymenophore decurrent, of well-developed, gill folds, 2-4(6) mm high, quite thin and often fissured over their entire height, a bright chrome to yellowish orange when young (more intensely yellow than 2-3A7), forkings not abundant and mostly close to the cap margin, varying from completely smooth to heavily anastomosed in between gills. Stipe mostly and sometimes distinctly shorter than the cap diam., more rarely quite slender and exceeding the cap diam., 8–17 mm diam., up to 30 (04) mm long, subcylindrical or slightly narrowing downwards, mostly white, but sometimes also tinged with chrome yellow or a diluted yellowish to cream over part or all of its surface, except for the extreme base which remains generally pure white, often developing similar squamae as the cap, but lower and solid. Flesh firm and thick beneath the cap center, white but yellowing near the surface or when handled. Taste mild. Smell faint and untypical. Spore print distinctly yellowish.
Spores ellipsoid, reniform to almost oblong or even cylindrical, l(7.3)8-8.31-8.7 × (4.2)4.5-4.90-5.3(5.4) μm, Q = (1.3)1.5-1.71-1.9(2.1), smooth, filled with one large, oily inclusion. Basidia 50-60(65) × 7–9 μm, subcylindrical to weakly clavulate, (4)5-6-spored and with 5–8 μm long sterigmata (longer for 2–3 spored basidia and also rather frequently deformed); basidiola not particularly irregular in form. Subhymenium filamentous, with cells of similar diam. as the basidium base. Cystidia none, however some specimens are completely sterile forming aborted basidia, resembling very irregular, cystidia-like cells in the hymenium. Pileipellis a loose (tricho)cutis of ramifying, thick-walled hyphal extremities, densely septate with up to 10 consecutive cells, sparsely branching, measuring mostly 4-6(9) μm diam. toward the cap margin often somewhat wider, terminal cells generally subcylindrical and regular, sometimes locally inflated or subapically constricted; pigment inclusions very apparent inside cells, strongly refringent. Clamp connections absent from all tissues.
Material examined: MADAGASCAR, Central Highlands, 24 km before reaching Antananarivo when returning from Andasibe, bought from vendors along the RN2 before reaching the village of Ambanitsena, 28 February 2000, Buyck 00.1827 (PC0085149); ibid., collected in Eucalypt plantations on lateritic soil, 4 February 2006, Buyck & V. Hofstetter 06.147 (PC0084110), ibid., 7 February 2000, Buyck & V. Hofstetter 06.179 (PC0084129), 06.180 (PC0084130), 06.219 (PC0084827); near Ranomafana, collected under E. robusta 18 February 2006, Buyck & V. Hofstetter 06.247 (PC0085151); near Antananarivo, along RN7 direction Antsirabe, bought from vendors, 25 January 2008, Buyck & V. Hofstetter 08.159 (PC0085148), 08.163 (PC0085150); near Ambila Lemaitso, in littoral forest with Uapaca littoralis, Sarcolaena and Leptolaena spp. and Asteropeia multiflora on deep sandy soil, 29 June 2011, Buyck & V. Hofstetter 11.041(PC0085572).
Notes: This chanterelle is clearly a cryptic species that cannot be unequivocally distinguished from its twin C. eucalyptorum with morphological features unless specimens with very typical colour are compared. Under the microscope, C. tricolor has usually somewhat more densely septate hyphal extremities, as well as overall shorter basidia compared to C. eucalyptorum, but with more collections such differences may not hold up. Notwithstanding the morphological similarities, our phylogeny (Fig. 135) reveals that both species are not sister to each other, and that each one is more closely related to a different African mainland chanterelle: viz. the African C. tanzanicus is sister to C. eucalyptorum, whereas C. tricolor is closer to the African C. densifolius and the Malagasy C. albidolutescens as well as to another, still undescribed Malagasy chanterelle, both of which have a much paler, practically white hymenophore.
The two chanterelles grow side by side in exactly the same localities and are sold in mixtures, often after having been washed, which makes both species even more alike. On several occasions, we have tried without success to sort all specimens to the correct species when buying mixtures from merchants. We have observed this species also once in endemic vegetation, growing in the deep sandy soils of the littoral forest where we noted Uapaca littoralis, various Sarcolaena and Leptolaena spp. as well as Asteropeia multiflora as possible host trees. Nevertheless, we would not dare, in retrospect, to confirm with absolute certainty the complete absence of any Eucalyptus specimen in the surroundings. This east coast collection had a much paler cap than usual, also a more slender stipe, but shared the intensely yellow gill folds. Spore measurements of other collections of this species revealed a greater variability than for the other species here described:
-
00.1827/ (8.1)8.5-9.14-9.8 (10.4) × (4.2)4.5-4.86-5.2(5.4) μm, Q = (1.6)1.7-1.89-2.1(2.3)
-
Holotype/ 06.246 (7.3)8-8.31-8.7 × (4.2)4.5-4.90-5.3(5.4) μm, Q = (1.3)1.5-1.71-1.9(2.1)
-
06.180/ (6.9)7.4-7.85-8.3(8.5) × (4.2)4.6-4.98-5.4(5.6) μm, Q = (1.3)1.4-1.59-1.7(2)
-
223.
Cantharellus variabilicolor Buyck, Randrianjohany & V. Hofstetter, sp. nov.
MycoBank: MB 813242; Facesoffungi number: FoF00993; Figs. 139 and 140
General habit of the species a, b Cantharellus eucalyptorum, different aspects of the holotype collection, c, e, g Cantharellus variabilicolor aspects of colour variation: orange form (BB 06.174), yellow form (BB 06.175), pink form (BB 08.291), d, f Cantharellus tricolor, field aspect of east coast form (d, BB 11.041); field aspect of highland form (f, holotype); h Cantharellus nigrescens (BB 06.166). (photos B. Buyck)
Diagnosis: Differs from the endemic Cantharellus decolorans and from the other reddish species in subgenus Cinnabarinus in its much more variable colour and its association with E. robusta in Madagascar.
Holotype: MADAGASCAR, Central Highlands, along the road 15 km before reaching Ranomafana, 31 January 2008, under Eucalyptus robusta, Buyck & V. Hofstetter 08.290 (PC0084806).
Cap small. 10–20 mm diam., thin, rapidly depressed in the center and finally often strongly infundibuliformous, mostly irregularly undulate, flexuous or even lobed but not striate near the margin; the surface dull, smooth, either pale pinkish red to light lilac pink or orange to almost deep yellowish in colour, hygrophanous and much paler when dry. Hymenophore strongly decurrent, composed of well differentiated gill folds, relatively thick, up to 2 mm high, forking and anastomosing in different degrees, concolourous or slightly paler than the cap. Stipe 10–20 × 1.5–2 mm, subcylindrical or slightly narrowing downwards, smooth or nearly so and concolourous with the cap, solid then narrowly fistulose. Flesh relatively thick in the cap center, but rapidly narrowing outward, concolourous and hygrophanous. Taste mild. Smell typical. Spore print very pale cream to off-white, insufficient for a correct appreciation of the colour, but a priori not yellowish.
Spores shortly ellipsoid to ellipsoid or slightly reniformous, measuring (7.1)7.6-8.08-8.6(9.2) × (4.2)4.5-4.81-5.2(5.4) μm, Q = (1.5)1.6-1.68-1.8(2), smooth, uni- to pluriguttulate. Basidia slender, poorly to distinctly clavate, (50)60-75(80) × (8.5)9-12 μm, (3)4-5(6)-spored; basidiola longtime subcylindrical. Cystidia none. Subhymenium filamentous but strongly septate, composed of rather short, inflated cells that are hardly wider than the basidium base. Pileipellis a thin and loose cutis of thin- to slightly thick-walled, fragile and easily collapsing hyphae measuring (5)7-14(17) μm diam., terminal cells subcylindrical to slightly fusiformous or clavulate. Clamp connections everywhere.
-
1826: (7)7.5-8.14-9 × 5–5.59-6(6.5) Q = 1.33-1.46-1.56(1.75)
-
1825: (7.1)7.3-7.70-8(8.3) × (5)5.1-5.39-5.7(5.8) Q = 1.3-1.43-1.5(1.6)
-
holotype: (7.1)7.6-8.08-8.6(9.2) × (4.2)4.5-4.81-5.2(5.4) μm, Q = (1.5)1.6-1.68-1.8(2)
Material examined: MADAGASCAR, Central Highlands, 25 km southeast of Antananarivo, along RN2 to Antsirabe, bought from road stall before reaching the village of Ambanitsena, 28 February 2000, Buyck & Randrianjohany 00.1826 (PC 0085152), 00.1825 (PC 0085153); along RN2 bought from road stall and said to have been collected under E. robusta, 4 February 2006, Buyck & V. Hofstetter 06.145 (PC0084111), 06.146 (PC0084757); ibid., collected in young E. robusta plantation, 6 February 2006, Buyck & V. Hofstetter 06.151 (PC0124634), 06.168 (PC0124633); close to Ambohimasoa along the RN7 from Ambositra to Fianarantsoa, 1420 m alt., 29 January 2008, under E. robusta, Buyck & V. Hofstetter 08.243 (PC0084733), 08.260 (PC0084808), 08.261 (PC0085154); along the road 15 km before reaching Ranomafana, 31 January 2008, under Eucalyptus robusta, Buyck & V. Hofstetter 08.290 (PC0084806), 08.291 (PC0084807).
Notes: The most remarkable feature of this species is the variation in its overall colour which is frequently not pink but more lilac or sometimes even entirely yellowish orange (Fig. 140). The different colour forms of this species grow mixed in the same localities. The pinkish red colour forms are undistinguishable from another Malagasy species, C. decolorans Eyssart. & Buyck and our phylogeny (Fig. 135) resolves and supports it as the sister species of C. variabilicolor. (BS = 88 %).
Our species differs from C. decolorans principally in its ecology, as it is found exclusively under introduced Eucalyptus robusta on the Central Plateau, whereas the latter chanterelle is only found growing with endemic species of the genus Uapaca (U. densifolia or U. ferruginea), mostly near mountain crests. Contrary to C. decolorans, which is too rare to be of any commercial value, C. variabilicolor is one of the chanterelles that is frequently offered for sale in road stalls and on market places during the rainy season It belongs in Cantharellus subg. Cinnabarinus Buyck & V. Hofstetter.
Athough C. variabilicolor is similar in the field to other small pinkish red species, such as the North American C. cinnabarinus and C. texensis, or the European C. friesii, there is no doubt that the Australian C. concinnus is quite its closest match outside Madagascar and both species are near identical in their overall morphology. Yet, on the basis of RPB2 sequences (Buyck & V. Hofstetter unpubl.), C. concinnus is genetically more distant, being closely related to the New Caledonian C. garnieri Ducousso & Eyssart. and C. wellingtonensis McNabb described from New Zealand. We were unfortunately unable to obtain tef-1 sequences for these species.
Corticiales
The order Corticiales contains a group of corticioid basidiomycetes with diverse ecology and trophic modes. Ghobad-Nejhad et al. (2010) made a first family arrangement for the order, and recognized three monophyletic families in Corticiales, viz., Corticiaceae, Punctulariaceae, and Vuilleminiaceae. In the recent study by Ghobad-Nejhad and Duhem (2014), a new genus Dendrominia Ghobad-Nejhad & Duhem was introduced in Corticiales. Dendrominia takes a basal position in Corticiales and is sister to the other three families mentioned above. A new family Dendrominiaceae is described to settle this genus (Fig. 141).
Phylograms generated from Bayesian analyses a Tree from LSU dataset b Tree from combined ITS-LSU dataset. Phylograms show the position of Dendrominiaceae fam. nov., Punctulariopsis cremeoalbida comb. nov., and P. efibulata comb. nov. (in blue). Bayesian Posterior Probabilities are indicated above or below the nodes. Both trees are rooted with Gloeophyllum sepiarium. Details of Vuilleminiaceae clade follow (Ghobad-Nejhad & Duhem 2014); the clade is collapsed here for conciseness
Cortinariaceae R. Heim
The limits of the family Cortinariaceae remain unknown, although most of the species are currently included in the genus Cortinarius. Many genera formerly placed in Cortinariaceae e.g., Phaeocollybia, Hebeloma, Galerina, and others have been moved to other families in the Agaricales. However, the sequestrate genera, Thaxterogaster, Quadrispora, Protoglossum and Hymenogaster p.p., as well as Cuphocybe, Rapacea and Rozites, once thought to be genera in the Cortinariaceae, are currently included in the genus Cortinarius (Peintner et al. 2001, 2002). Species in this family range from agaricoid to sequestrate, many have well-developed veils, and they typically have brown, ornamented basidiospores.
Cortinarius (Pers.) Gray, Nat. Arr. Brit. Pl. (London) 1: 627 (1821)
Cortinarius is the largest genus of Agaricales with a cosmopolitan distribution and over 2000 described species. The species are important ectomycorrhizal fungi and are associated with different trees and shrubs, belonging to the families Fagaceae, Salicaceae, Caesalpiniaceae, Cistaceae, Dipterocarpaceae, Myrtaceae, Rhamnaceae, Rosaceae and Pinaceae, as well as some herbaceous plants in the Cyperaceae and Polygonaceae. Revealing the true diversity of species using only morphological and ecological characteristics has proven to be a difficult if not impossible task. The use of DNA sequence data has made it possible to elucidate phylogenetic relationships within the genus, show patterns of speciation, and uncover convergent and cryptic species. None the less there are many species still that remain to be undescribed.
-
224.
Cortinarius alboamarescens Kytöv., Niskanen & Liimat. sp. nov.
Index Fungorum number: IF551436; Facesoffungi number: FoF00996; Figs. 143a and 144a
Etymology: The name refers to the white colour of the basidiomata and the bitter taste.
Holotype: T. Niskanen 04-850, H6031311 (H).
Pileus 10–30 mm, conical to hemispherical, then low conical to low convex, white to very pale ochraceous white, viscid to somewhat glutinous, with hygrophanous streaks. Lamellae almost crowded, adnexed, very pale brown. Stipe 35–60 mm long, 3–5 mm thick at apex, 4–7 mm at base, somewhat clavate, often slightly rooting at base, white, viscid. Universal veil viscid. Context very pale yellow, marbled hygrophanous. Odor of flesh sweetish. Taste bitter at least of the pileus surface. Basidia 4-spored. Basidiospores 5.2–6.3 × 4.1–5 μm (av. = 5.8 × 4.5 μm, 160 spores, 7 specimens), Q = 1.17–1.40 (av. = 1.29), ovoidly subglobose, finely to fairly finely verrucose, somewhat dextrinoid. Lamellar trama hyphae smooth, sometimes finely granulose, pale yellowish to yellowish in Melzer’s reagent. Pileipellis hyphae in the surface layer narrow, 2–4 μm wide, hyaline, embedded in mucus. Hyphae under the surface layer 5–15 μm wide, pale yellow in Melzer’s reagent, not encrusted. ITS sequence (GenBank KR011136, holotype) distinct from the other known members of the section Vibratiles, and differs from them in the ITS region by more than 10 substitutions and indel positions.
Ecology and distribution. In coniferous forests, mostly in low-herb depressions of Picea forests, more seldom in pure Pinus heaths, one collection from Fagus forest, sometimes on calcareous ground. Often solitary or only a few basidiomata in each location. Producing basidiomata in late summer and autumn. Fairly rare to occasional, known from Northern and Central Europe.
Material examined: DENMARK, Nordjylland, Sindal, Tolne, small Fagussylvatica forest, 12 September 1998, leg. I. Kytövuori 98-1889 (H). GenBank ITS: KR011134. FINLAND, Varsinais-Suomi, Lohja, Esker of Lohja, outdoor recreation area by the road 36, dry pine (Pinus sylvestris) heath forest on sandy soil with lime dust effect, 19 September 2004, leg. I. Kytövuori & T. Niskanen 04-867, H6029887 (H). GenBank ITS: KR011137. Varsinais-Suomi, Vihti, Vihtijärvi, dryish pine forest with some Picea and Betula, 12 October 2003, leg. H. Tuovila 03-227 (H). Uusimaa, Helsinki, Herttoniemi, Kivinokka, mesic to grass-herb Picea forest, a shallow depression, 17 August 1994, leg. I. Kytövuori 94-061 (H); Malminkartano, 15 August 1979, J. Issakainen (H); Oulunkylän urheilupuisto, mesic to grass-herb Picea forest, 22 September 2000, leg. I. Kytövuori (H). Uusimaa, Inkoo, Kalasatama, mesic to grass-herb Picea forest, 3 September 2004, leg. I. Kytövuori (H). Uusimaa, Porvoo, W side of the lake Venjärvi, mesic to damp, mossy, grass-herb spruce forest (Picea abies) with some Populus tremula, Betula and Pinus sylvestris, 17 September 2004, leg. K. Liimatainen & T. Niskanen 04-850, H6031311 (holotype, H), (isotype, NY); Stensböle, Natura area, Picea abies dominated mesic grass-herb forest with Betula, old wooded pasture land, 24 September 2012, leg. T. von Bonsdorff (H). Etelä-Savo, Leivonmäki, Selänpohja, Syysniemi, slightly paludified spruce forest with some hardwood trees and bushes, 22 August 1993, leg. I. Kytövuori 93-454 (H). Etelä-Häme, Lahti, Pesäkallio, spruce forest, 30 September 2002, leg. T. von Bonsdorff & I. Kytövuori (H). Etelä-Häme, Valkeakoski, Mälkiäinen, Kariniemi, on mossy ground in rich, mixed forest, 8 September 1990, K. Syrjänen (OULU). Etelä-Häme, Virrat, Pohjaslahti, Monoskylä, Hennilä, moist spruce forest, swampy depression, 19 October 1996, leg. I. Kytövuori 96-1338 (H). Laatokan Karjala, Parikkala, Saari, congregation camp center at the lake, below the church, dry pine heath forest on fine sand, some Betula, 18 September 2009, leg. I. Kytövuori 09-1290 (H). Laatokan Karjala, Uukuniemi, Tarnala, mesic conifer forest, on the sawdust track, 11 September 2006, leg. I. Kytövuori 06-716 (H). Pohjois-Häme, Jyväskylä, Vesanka, Sivulanmäki NW, nature reserve, old Picea forest, 31 August 2014, leg. T. Jaakkonen (H). Pohjois-Häme, Konginkangas, Kivetty, mesic Picea forest, 9 September 2002, leg. H. Tuovila 02-026 (H). FRANCE, Haute-Savoie, pessière de Quintal, 24 October 1990, leg. P. Moënne-Loccoz 1761 (PC). SWEDEN, Medelpad, Ånge, Alby church yard, conifer dominated forest (Pinus, Picea) on calcareous soil, 28 August 2003, leg. I. Kytövuori, K. Liimatainen & T. Niskanen 03-823, H7018118 (H). GenBank ITS: KR011135. Medelpad, Liden, Sundsjöåsen Nature Reserve, mesic to grass-herb Picea forest, 13 September 2014, leg. I. Kytövuori (H). Härjedalen, Tännäs, Ramundberget, half-open pasture with thickets of Betula, Alnusincana and Populustremula, 18 August 2006, leg. M. Toivonen & I. Kytövuori 06-189 (H). Jämtland, Bräcke, Bodtjärnbäcken, spruce forest with some hardwood trees and bushes, 4 September 1997, I. Kytövuori 97-735 (H). Pite Lappmark, Arvidsjaur, Grundsel, dryish conifer forest with Picea, Pinus and some Betula, some moist depressions, 8 September 1997, leg. I. Kytövuori 97-970 (H).
Notes: Cortinarius alboamarescens is a small, white species with very small, almost subglobose spores. The viscid pileus and stipe, and the bitter taste place it in the /Vibratiles and the placement is confirmed in the phylogenetic analysis (Fig. 142). Thus far, C. alboamarescens is the only known white species of the section that occurs in coniferous forests. Cortinarius leucophanes P. Karst. and C. lustratus Fr. Sensu Jeppesen et al. of the /Leucophanes are also white and occur in coniferous forests, but their basidiomata are somewhat larger, the taste is mild or farinaceous, and the spores are somewhat larger and ellipsoid.
Phylogram generated from Maximum likelihood (RAxML) analysis based on ITS sequence data. Maximum likelihood bootstrap support values greater than 50 % are indicated above or below the nodes, new species are in blue and species for which obtained sequences are based on type material have names in bold. The tree is rooted with Hebeloma spp. Asterisk = the holotype of C. collinitus var. olympianus
-
225.
Cortinarius brunneoalbus Ammirati, Liimat. & Niskanen, sp. nov.
syn. C. collinitus var. olympianus A.H. Sm., Lloydia 7(3): 175 (1944).
Index Fungorum number: IF551437; Facesoffungi number: FoF00997; Figs. 143c and 144c
Cortinarius species a Cortinarius alboamarescens 04-850, H6031311 (holotype, H) b Cortinarius ochroamarus 04-761, H6030006 (holotype, H) c Cortinarius brunneoalbus 09-075 (holotype, H) d Cortinarius seidlii 09-132 (holotype, H) e Cortinarius putorius 07-411 (holotype, H). Scale bars: a–e = 10 mm. Photographs by K. Liimatainen
Cortinarius species a Spores of a Cortinarius alboamarescens 04-850, H6031311 (holotype, H) b Cortinarius ochroamarus 04-761, H6030006 (holotype, H) c Cortinarius putorius 07-411 (holotype, H) d Cortinarius brunneoalbus 09-075 (holotype, H) e Cortinarius seidlii 09-132 (holotype, H), in Melzer’s reagent. Scale bars: a–e = 10 μm. Drawings by T. Niskanen and I. Kytövuori
Etymology: The name refers to the colours of the basidiomata.
Holotype: T. Niskanen 09-075 (H).
Pileus 30–65 mm, at first hemispherical, then low convex to plane, margin somewhat plicate-striate, ochraceous brown to dull yellowish brown, strongly glutinous, hygrophanous. Lamellae medium spaced, emarginated, at first very pale brown, later pale brown. Stipe 50–100 mm long, 7–13 mm thick at apex, 8–15 mm at base, cylindrical or very slightly and evenly enlarged downward, white, glutinous. Universal veil glutinous, colourless. Context white to very pale yellow. Odour in the context at the base of the stipe very faintly honey-like. Basidia 4-spored. Basidiospores 13–15(−16) × 7.5–8.5(−9) μm (av. = 14.2 × 8.5 μm, 40 spores, 2 specimens), Q = 1.65–1.8(−1.9) (av. = 1.75), citriform, finely to moderately, densely verrucose, somewhat dextrinoid. Lamellar trama hyphae smooth, somewhat yellow in Melzer’s reagent. Pileipellis. Hyphae in the surface layer 2.5–7 μm wide, hyaline, without clamp connections, embedded in mucus. Hyphae under the surface layer 3–15 μm wide, pigmented, brownish yellow in Melzer’s reagent, somewhat spot-like encrusted. ITS sequence (GenBank KR011128, holotype) distinct from the other known members of the section Defibulati and differs from them it in the ITS region by more than 10 substitutions and indel positions.
Ecology and distribution. In mossy, mesic coniferous forests (Abies, Tsuga). Producing basidiomata in autumn. To date only known from U.S.A., Washington.
Material examined: USA, Washington, Clallam, Olympic Hot Springs, Olympic National Park, gregarious under fir, 30 September 1941, leg. A.H. Smith 17437, MICH10337 (MICH, holotype of C. collinitus var. olympianus). GenBank ITS: KR011129. Snohomish County, Barlow Pass, Mount Baker-Snoqualmie national forest, mixed coniferous forest with Tsuga heterophylla and Abies amabilis, 10 October 2009, leg. J.F. Ammirati, K. Liimatainen & T. Niskanen 09-075, (holotype, H), (isotype, NY).
Notes: In our phylogenetic analysis Cortinarius brunneoalbus is placed in the /Defibulati, a sister group of/Myxacium (Fig. 1). Its placement in the /Defibulati is supported by a combination of morphological characteristics: medium to large basidiomata, glutinous pileus and stipe, large, amygdaloid to citriform spores, and the absence of clamp connections. Cortinarius brunneoalbus is most reminiscent of C. mucifluus Fr., which belongs to the same section and also has a white stipe. Cortinarius mucifluus, however, does not occur in the Pacific North West and has relatively narrower spores (12–16(−17) × 7–8.5(−9) μm). Western North American C. vanduzerensis A.H. Sm. & Trappe and C. seidlii Ammirati, Niskanen & Liimat. differ in having a purplish stipe. Sometimes the stipe of C. seidlii is almost white but then it can be distinguished from C. brunneoalbus by its broader spores, Qav. = 1.58.
-
226.
Cortinarius ochroamarus Niskanen, Kytöv. & Liimat., sp. nov.
Index Fungorum number: IF551438; Facesoffungi number: FoF00998; Figs. 143b and 144b
Etymology: The name refers to the ochraceous colour of the pileus and the bitter taste.
Holotype: T. Niskanen 04-761, H6030006 (H).
Pileus 20–60 mm, hemispherical, then low convex, ochraceous yellow to yellow, sometime more yellowish red brown when moist, viscid, with hygrophanous streaks. Lamellae almost crowded, adnexed to emarginated, very pale brown, later pale brown. Stipe 40–80 mm long, 4–8 mm thick at apex, 6–13 mm at base, somewhat clavate, white, almost dry. Universal veil viscid. Context in pileus and upper part of the stipe pale yellow, white to pale yellow at the base of the stipe marbled hygrophanous. Odor of flesh sweetish, pleasant. Taste very bitter at least of the pileus surface. Basidia 4-spored. Basidiospores 7–8.4 × 4.5–5.4 μm (av. = 7.7 × 5 μm, 120 spores, 3 specimens), Q = 1.44–1.67 (av. = 1.54), amygdaloid-ellipsoid, finely to fairly strongly verrucose, most strongly at apex, fairly weakly dextrinoid. Lamellar trama hyphae smooth, often somewhat granulose, yellow in Melzer’s reagent. Pileipellis. Hyphae in the surface layer narrow, 2–4 μm wide, hyaline, embedded in mucus. Hyphae under the surface layer 5–15 μm wide, pale yellow in Melzer’s reagent, not encrusted. ITS sequence (GenBank KR011132, holotype) distinct from the other known members of the section Vibratiles, and differing from them it in the ITS region by more than 10 substitutions and indel positions.
Ecology and distribution. In herb-rich, mossy Picea abies-dominated forests on calcareous ground. Producing basidiomata in late summer and autumn. Rare to occasional, known from Northern Europe.
Material examined: FINLAND, Uusimaa, Espoo, Luukkaa outdoor recreation area, N of Hauklampi, nature reserve area, mesic, partly grass-herb spruce forest (Picea abies) with some Populus tremula, Betula and Pinus sylvestris, 9 September 2004, leg. K. Liimatainen & T. Niskanen 04-761, H6030006 (holotype, H), (isotype, NY). Varsinais-Suomi, Karjaa, Mustio, Kohagen, herb-rich Picea abies forest with some Corylus avellana, Quercus robur, Betula and Populus tremula, or under Populus tremula, 2 September 2008, leg. K. Liimatainen & T. Niskanen 08-045, H6001939 (H). SWEDEN, Jämtland, Östersund, Böle, Fillstabäcken, damp to submesic coniferous forest (Picea, Pinus) on calcareous ground, 2 September 2003, leg. I. Kytövuori, K. Liimatainen & T. Niskanen 03-1065, H7018099 (H).
Notes: Cortinarius ochroamarus belongs to /Vibratiles (Fig. 142). Typical for the species of this clade are viscid to glutinous pileus, almost dry to glutinous stipe, and bitter taste. Cortinarius ochroamarus is best recognized by the combination of ochraceous yellow pileus, almost dry stipe, amygdaloid-ellipsoid spores, and habitat on calcareous soils. The species is most similar to C. vibratilis (Fr.: Fr.) Fr. sensu Moser and of Kytövuori & Niskanen, but C. vibratilis has a saturated apricot yellow to yellowish red brown pileus, somewhat narrower spores, 7–8 × 4.3–4.8 μm, and it occurs on more acid soils. Large basidiomata of C. ochroamarus can even resemble species of /Multiformes, but those have a clavate to bulbous stipe base, mild taste, and larger spores, over 8 μm long.
-
227.
Cortinarius putorius Niskanen, Liimat. & Ammirati sp. nov.
Index Fungorum number: IF551439; Facesoffungi number: FoF00999; Figs. 143e and 144e
Etymology: The name refers to the unpleasant smell of the basidiomata.
Holotype: T. Niskanen 07-411 (H).
Pileus 30–90 mm, hemispherical, then low convex to plane, purple when young, later very pale purple to almost whitish, viscid. Lamellae medium spaced, adnexed to emarginated, at first purple, later strong brown. Stipe 50–130 mm long, 6–10 mm thick at apex, 6–13 mm at base, cylindrical to somewhat clavate, pale purple when young, later very pale brown to whitish silky fibrillose. Universal veil white, thin, forming some complete and/or incomplete girdles on the stipe. Basal mycelium white. Context at first purple later strong yellowish brown. Odor of lamellae unpleasant. Basidia 4-spored. Basidiospores 8.8–9.5(−10) × 5–5.7 μm (av. = 9.2 × 5.4 μm, 40 spores, 2 specimens), Q = (1.55–)1.6–1.8(−1.85) (av. = 1.71), amygdaloid to somewhat ellipsoid, finely and densely verrucose, almost indextrinoid to somewhat dextrinoid. Lamellar trama hyphae smooth, somewhat yellow in Melzer’s reagent. Cheilocystidia 35–45 × 7–13 μm, cylindrical to weakly lageniform. Pileipellis. Hyphae in the surface layer 3–7 μm wide, in gelatinous substance, hyaline, with granulose contents. Hyphae under the surface layer 5–10 μm wide, hyaline, not encrusted. ITS sequence (GenBankKR011124, holotype) distinct from the other members of /Camphorati, and differing from them in the ITS region by more than 10 substitutions and indel positions.
Ecology and distribution. In mesic coniferous forests (Tsuga, Abies, Pseudotsuga, Picea). Producing basidiomata in autumn. Rare, to date known from Washington and California in western North America.
Material examined: USA, California, Humboldt Co., Redwood National Park, coniferous forest, leg. J. Olsson & K. Liimatainen, T. Niskanen 12-230 (H). Washington, Olympic peninsula, Olympic National Park, surroundings of Sol Duc camp ground, coniferous forest (at least Tsuga heterophylla), 6 October 2007, leg. K. Liimatainen & T. Niskanen 07-411 (holotype, H), (isotype, NY).
Notes: Cortinarius putorius belongs to /Camphorati (Fig. 142). The group is characterized by the purple to almost white basidiomata, unpleasant smell, and distinct cheilocystidia. The closely related C. camphoratus (Fr.) Fr., which also occurs in the coniferous forests of the Pacific North West, has less purple basidiomata, the universal veil is at first pale bluish violet, and the spores are larger (9–)9.5–10.5 × (5.5–)6–6.5 μm.
-
228.
Cortinarius seidlii Ammirati, Niskanen & Liimat., sp. nov.
Index Fungorum number: IF551440; Facesoffungi number: FoF01002; Figs. 143d and 144d
Etymology: Named after Michelle T. Seidl.
Holotype: T. Niskanen 09-132 (H).
Pileus 40–80 mm, at first hemispherical, then low convex to plane with a low and broad umbo and with margin plane to uplifted, strongly radially wrinkled, dirty ochraceous olive brown to brown, center dark brown but ochraceous when dried, edge at least at first whitish or pale, strongly glutinous, hygrophanous. Lamellae medium spaced, emarginated, at first very pale brown, later pale brown, sometimes with a purple tinge. Stipe 80–150 mm long, 7–15 mm thick at apex, 10–21 mm at the broadest portion, cylindrical to narrowly ventricose, base tapered to rounded, white, and glutinous. Universal veil glutinous, very pale purple to pale purple, sometimes almost white. Context in pileus brown, in stipe apex whitish to very pale brown, in base of the stipe pale yellow brown. Odor in the context of the stipe base indistinct, faintly iodoform-like or sweet. Lamellar trama hyphae smooth, somewhat yellow in Melzer’s reagent. Basidia 4-spored. Basidiospores (12–)12.5–14.5(−15) × (7.5–)8–9 μm (\( \overline{\mathrm{x}} \) = 13.5 × 8.5 μm, Q = 1.5–1.7(−1.8), n = 40), broadly citriform, moderately, densely verrucose, almost indextrinoid to somewhat dextrinoid. Pileipellis. Hyphae in the surface layer 3–6 μm wide, hyaline, without clamp connections, embedded in mucus. Hyphae under the surface layer 5–17 μm wide, pigmented, brownish yellow in Melzer’s reagent, finely to coarsely encrusted. ITS sequence (GenBank KR011125, holotype) distinct from the other known members of the section Defibulati and differs from them it in the ITS region by more than 10 substitutions and indel positions.
Habitat and distribution: In mesic, mossy coniferous forests (Pseudotsuga, Tsuga, Abies). Producing basidiomata from late summer to late autumn. Common in Pacific North West of North America, known from USA. Oregon to Canada British Columbia.
Material examined: USA, Oregon, Clackamas Co., Bull Run Watershed, fairly mesic, mixed coniferous forest (Tsuga, Pseudotsuga, Abies), 8 September 1995, L. Norvell, M.T. Seidl 4166 (WTU) (as C. vanduzerensis). Washington, Olympic Peninsula, Sol Duc camp ground, coniferous forest (Tsuga heterophylla, Pseudotsuga), 25 October 2009, K. Liimatainen & T. Niskanen 09-132 ((holotype, H), isotype, NY). Snohomish Co., Boardman Lake Trail, coniferous forest (mostly Tsuga heterophylla), 5 October 2009, J.F. Ammirati & T. Niskanen 09-063 (H); Mount Rainier National Park, Cougar Rock Campground, Tsuga, Abies, Pseudotsuga, 18 October 1995, J.F. Ammirati 11591 (WTU) (as C. mucifluus). Olympic Peninsula, Big Quilcene Trail, Abies, Tsuga, Pseudotsuga, deep humus, 11 October 1995, J.F. Ammirati 11543 (WTU). CANADA, British Columbia, Tsuga heterophylla ectomycorrhizal root tip, GenBank ITS: FJ152500 (as C. sp.). Mt. Washington, Trail to Divers Lake, 12 September 2001, O. Ceska OC44, F17116 (UBC) (as C. cf. pseudosalor). Port Renfrew, near the 2nd Bridge on Way to Fairy Lake, 24 August 2003, O. Ceska OC131, F17203 (UBC) (as C. stillatitius). Queen Charlotte Islands, Moresby Island, West side of Rose Inlet, Boschniakia Point, 5 September 2005, O. Ceska et al., F15962 (UBC), (as C. cf. vanduzerensis). Roberts Creek, Mt. Elphinstone Provincial Park, Upper Area #1, 22 August 2000, P. Kroeger, PK5331, F16402 (UBC), (as C. vanduzerensis); 3 September 2000, P. Kroeger, PK5336, F16403, (as C. vanduzerensis); area #2, in old growth Pseudotsuga, 4 September 2000, K. Goodwin & P. Kroeger, PK3374, F15805 (UBC), (as C. cf. vanduzerensis); area #3, 5 November 2000, K. Goodwin & P. Kroeger, PK3396, F15827 (UBC), (as C. cf. vanduzerensis). USA, Oregon, HJ Andrews Experimental Forest, Pseudotsuga menziesii, OSC 1064174 (OSC), (as C. sp.).
Notes: Cortinarius seidlii is a typical member of /Defibulati (Fig. 142). Members in this clade are characterized by the medium to large basidiomata, glutinous pileus and stipe, large, amygdaloid to citriform spores, and the absence of clamp connections. Cortinarius seidlii is characterized by the dirty ochraceous olive brown to brown pileus with dark brown center, very pale purple to pale purple, glutinous universal veil, and broadly citriform spores. Originally the species was identified as C. vanduzerensis A.H. Sm. & Trappe, but the study of the type specimen of C. vanduzerensis (MICH10434, GenBank ITS: KR011130, holotype) revealed that it is a different species. The pileus of C. vanduzerensis is chestnut black with chestnut brown margin and the spores are narrower 7–8(−9) μm wide. The species is currently only known for sure from the type locality, Tillamook Co, Oregon. Cortinarius stillatitius Fr. is also very similar to C. vanduzerensis, but it has a strong honey-like smell in the context of the base of the stipe and it does not occur in Pacific North West. Cortinarius brunneoalbus, which occurs in the same area, can be distinguished from C. seidlii by the narrower spores, Q = 1.75, and the white stipe. ITS sequence (GenBank KR011125, holotype) distinct from the other known members of the section Defibulati and differing from them in the ITS region by more than 10 substitutions and indel positions.
-
229.
Dendrominiaceae Ghobad-Nejhad, fam. nov.
MycoBank number: MB812413; Facesoffungi number: FoF01003
Etymology: In reference to the type genus Dendrominia.
Type genus: Dendrominia Ghobad-Nejhad & Duhem, Mycol. Progr.: 10.1007/s11557-012-0881-3 [7] (2013)
Saprobic on decorticated or barked wood. Basidiome effused, resupinate, ceraceous to crustaceous. Hyphal system monomitic, hyphae with or without clamps, encrusted with fine crystals. Basidia large cylindrical to clavate, flexuose, arising from inflated basidioles (probasidia). Dendrohyphidia present. Basidiospores lunate to allantoid, walls smooth.
The family Dendrominiaceae currently contains the genus Dendrominia with three species: D. dryina (Pers.) Ghobad-Nejhad & Duhem, D. ericae (Duhem) Ghobad-Nejhad & Duhem, and D. maculata (H.S. Jacks. & P.A. Lemke) Ghobad-Nejhad & Duhem.
Punctulariaceae Donk, Persoonia 3(2): 287 (1964)
Punctulariaceae is a small family in Corticiales and accounts for corticioid taxa with simple resupinate basidiomes growing on angiosperm wood. All species possess dendrohyphidia and have ellipsoid spores negative in Melzer’s reagent. The family contains the three genera Dendrocorticium M.J. Larsen & Gilb., Punctularia Pat., and Punctulariopsis.
Punctulariopsis Ghobad-Nejhad, Taxon 59(5): 1529 (2010)
The genus Punctulariopsis was established by Ghobad-Nejhad et al. (2010) to accommodate P. obducens (Hjortstam & Ryvarden) Ghobad-Nejhad, and P. subglobispora (Hallenb. & Hjortstam) Ghobad-Nejhad (both species were first described in the genus Vuilleminia, but shown to represent an independent genus based on morphology and DNA sequence data). During our survey on Corticiales taxa, the phylogenetic affinity of two Corticium species namely C. cremeoalbidum and C. efibulatum (Fig. 141) was examined for the first time. Phylogenetic analyses of ITS and LSU sequences (Fig. 141), show that both C. cremeoalbidum and C. efibulatum well reside in the genus Punctulariopsis, and their morphological characters also match the concept of the genus (see below). Therefore, two new combinations are proposed in the following.
-
230.
Punctulariopsis cremeoalbida (M.J. Larsen & Nakasone) Ghobad-Nejhad, comb. nov.
MycoBank number: MB812414; Fig. 145
≡ Laeticorticium cremeoalbidum M.J. Larsen & Nakasone, Mycologia 76(3): 528 (1984).
= Corticium cremeoalbidum (M.J. Larsen & Nakasone) M.J. Larsen, in Nakasone, Mycol. Mem. 15: 59 (1990).
-
231.
Punctulariopsis efibulata (M.J. Larsen & Nakasone) Ghobad-Nejhad, comb. nov.
MycoBank number: MB812415; Fig. 145
≡ Laeticorticium efibulatum M.J. Larsen & Nakasone, Mycologia 76(3): 530 (1984).
= Corticium efibulatum (M.J. Larsen & Nakasone) M.J. Larsen, in Nakasone, Mycol. Mem. 15: 60 (1990).
Notes: Punctulariopsis cremeoalbida and P. efibulata were originally described by Larsen and Nakasone (1984) for two corticioid species with effused basidiomes growing on Vitis (the former), and on Vaccinium (the latter) in North America. The light-coloured, thin, adnate basidiomes of both species, their little-branched dendrohyphidia, large and broadly ellipsoid spores with blunt apiculus, and their growth on hardwood match well with the concept of Punctulariopsis (see Ghobad-Nejhad et al. 2010). Both species are apparently known only from their type locality in the USA. With the two species combined here in Punctulariopsis, the number of species in this genus grows to four. Punctulariopsis efibulata is currently the only species in the genus lacking clamps on its hyphae.
Material examined: Laeticorticium cremeoalbidum: USA, Florida, Gannet Pond Quelet, Tall Timbers-Leon County, on Vitis, 29 July 1977, Burdsall 9616 (CFMR, holotype). Laeticorticium efibulatum: USA, Mississippi, Harison Expt. Forest, Rd. H2, Desoto National Forest, Harrison County, on Vaccinium, 26 February 1976, Burdsall 8824 (CFMR, holotype).
Order Hymenochaetales
Hymenochaetaceae Donk
The family Hymenochaetaceae is one of the most important families in Basidiomycota because it accommodates species with medicinal properties (Dai et al. 2009; Dai 2010). Around 480 species have been recorded in the family.
Hymenochaete Lév., Annls Sci. Nat., Bot., sér. 3 5: 150 (1846)
Hymenochaete is a large wood-inhabiting genus with more than 120 species He and Dai (2012). This genus was charactered by its corticioid hymenophore within the Hymenochaetaceae, until Wagner and Fischer (2002) combined lamellate and poroid species from Cyclomyces Kunze ex Fr. Later, three more poroid species of Cyclomyces were addressed in Hymenochaete (He and Dai 2012; Gomes-Silva et al. 2012). Two new poroid species of Hymenochaete are described in this study (Fig. 146).
-
232.
Hymenochaete micropora L.W. Zhou & Y.C. Dai, sp. nov.
Index Fungorum number: IF551490; Facesoffungi number: FoF01004; Fig. 147
Etymology: referring to small pores distinguishing from other species of Hymenochaete.
Holotype: BJFC006546
Basidiocarps annual, pileate, sometimes attached by a lateral tapering base, imbricate, corky and without odour or taste when fresh, hard corky and brittle when dry. Pilei dimidiate to usually fan-shaped, projecting up to 3 cm, 4 cm wide and 2 mm thick at base. Pileal surface yellowish brown to reddish brown, narrowly concentrically zoned in different shades, tomentose to velutinate; margin acute, convex when dry. Pore surface rust-brown to greyish brown; sterile margin distinct, yellowish brown, up to 1 mm wide; pores circular to angular, 9–11 per mm; dissepiments thin, entire. Context reddish brown, up to 1 mm thick, duplex, towards the tomentum separated by one black line, lower part hard corky, upper tomentum soft corky. Tubes honey-yellow, paler than pores, up to 1 mm long. Hyphal system monomitic; generative hyphae with simple septa; tissue darkening and slightly swelling in KOH. Contextual hyphae in the lower dense context yellowish, thick-walled with a wide lumen, unbranched, interwoven, 2.5–4 μm in diam; hyphae in the black line dark brown, distinctly thick-walled with a narrow lumen, strongly agglutinated; hyphae in the upper tomentum yellow to brown, thick-walled with a wide to narrow lumen, unbranched, regularly arranged, 3–4.5 μm diam. Tramal hyphae varying from pale yellowish and slightly thick-walled to brown and thick-walled with a wide lumen, occasionally branched close to a septum, frequently simple septate, straight, parallel along the tubes, 2–3.5 μm diam. Setae frequent, distinctly subulate, arising from trama, most part embedded in hymenium, slightly curved at base, dark brown, thick-walled, 10–28 × 4–8 μm; cystidia and cystidioles absent; basidia more or less barrel-shaped, with four sterigmata and a simple septum at the base, 7–12 × 4–6 μm; basidioles in shape similar to basidia, distinctly smaller. Basidiospores ellipsoid, hyaline, thin-walled, smooth, IKI–, CB–, (2.5–)2.6–3(−3.1) × (1.5–)1.6–2(−2.1) μm, L = 2.81 μm, W = 1.8 μm, Q = 1.56 (n = 30/1).
Material examined: CHINA, Yunnan Province, Tengchong County, Gaoligong Mountain, on fallen angiosperm trunk, 24 October 2009, Cui 8057 (BJFC006546, holotype; IFP019137, isotype).
Notes: Hymenochaete micropora has similar basidiospores to H. porioides T. Wagner & M. Fisch. (2.5–3.5 × 1.5–2 μm, Ryvarden 2004; 2.5–3.1 × 1.5–1.9 μm, measured from LWZ 20140719–11). However, the small pores make H. micropora distinguished from all other known species of Hymenochaete. In addition, the duplex context of H. micropora is separated by one black line, while H. porioides has two black lines in context. In ITS-based phylogeny (Fig. 146), the clade formed by two H. micropora was also distinct from that of H. porioides.
-
233.
Hymenochaete subporioides L.W. Zhou & Y.C. Dai, sp. nov.
Index Fungorum number: IF551491; Facesoffungi number: FoF01005; Fig. 148
Etymology: referring to the similarity to Hymenochaete porioides.
Holotype: BJFC011058
Basidiocarps annual, pileate, sometimes attached by a lateral tapering base, imbricate, corky and without odour or taste when fresh, hard corky and brittle when dry. Pilei dimidiate to usually fan-shaped, projecting up to 2.5 cm, 3 cm wide and 4 mm thick at base. Pileal surface cinnamon-buff, yellowish brown to brown, narrowly concentrically zoned in different shades, tomentose to velutinate; margin acute, convex when dry. Pore surface yellowish brown to dark brown; sterile margin distinct, yellowish brown, up to 1 mm wide; pores circular to angular, 6–8 per mm; dissepiments thin, entire. Context orange-brown, up to 3 mm thick, duplex, towards the tomentum separated by two black lines, lower and medium parts hard corky, upper tomentum soft corky. Tubes honey-yellow, paler than pores, up to 1 mm long. Hyphal system monomitic; generative hyphae with simple septa; tissue darkening and slightly swelling in KOH. Contextual hyphae in the lower dense context yellow, thick-walled with a wide lumen, unbranched, loosely interwoven, 3–5 μm in diam; hyphae in the two black lines dark brown, distinctly thick-walled with a narrow lumen, strongly agglutinated; hyphae between the two black lines yellow to brown, unbranched, parallel along the black lines, 3–4.5 μm in diam; hyphae in the upper tomentum brown, thick-walled with a wide lumen, unbranched, regularly arranged, 3–4.5 μm diam. Tramal hyphae varying from pale yellowish and slightly thick-walled to brown and thick-walled with a wide lumen, rarely branched, straight, parallel along the tubes, 2.5–4 μm diam. Setae frequent, distinctly subulate, arising from trama, most part embedded in hymenium, slightly curved at base, dark brown, thick-walled, 22–43 × 4–8 μm; cystidia and cystidioles absent; basidia more or less barrel-shaped, with four sterigmata and a simple septum at the base, 9–15 × 4–6 μm; basidioles in shape similar to basidia, distinctly smaller. Basidiospores oblong-ellipsoid to ellipsoid, hyaline, thin-walled, smooth, IKI–, CB–, (3.4–)3.7–4.3(−4.4) × (1.8–)1.9–2.4(−2.5) μm, L = 3.92 μm, W = 2.18 μm, Q = 1.8 (n = 30/1).
Material examined: CHINA, Zhejiang Province, Yongjia County, Longwantan Forest Park, on angiosperm stump, 21 August 2011, Cui 10163 (BJFC011058, holotype), (IFP019138, isotype).
Notes: Hymenochaete subporioides resembles H. porioides by its pore size (H. porioides: 7–9 per mm, Ryvarden 2004; 6–8 per mm, measured from LWZ 20140719-11) and duplex context separated by two black lines, but microscopically H. subporioides could be easily differentiated by its larger basidiospores (H. porioides: 2.5–3.5 × 1.5–2 μm, Ryvarden 2004; 2.5–3.1 × 1.5–1.9 μm, measured from LWZ 20140719-11). The ITS-based phylogeny (Fig. 146) also supports H. subporioides was separated from H. porioides.
-
234.
Neoantrodiellaceae Y.C. Dai, B.K. Cui, Jia J. Chen & H.S. Yuan, fam. nov.
Index Fungorum number: IF551497; Facesoffungi number: FoF01011
Habitat: grows on gymnosperm wood, including dead trees, fallen trunks and rotten wood, causes a white rot.
Basidiocarps annual to perennial, resupinate, effused-reflexed or pileate with smooth to poroid hymenophores, soft corky to hard corky, white, cream or pink. Hyphal system monomitic to dimitic; generative hyphae bearing clamp connections; skeletal hyphae thick-walled, cyanophilous; cystidia present in most species; basidiospores cylindrical, allantoid or ellipsoid, thin-walled, hyaline, smooth, indextrinoid, acyanophilous.
Type genus: Neoantrodiella Y.C. Dai, B.K. Cui, Jia J. Chen & H.S. Yuan
Notes: The family Neoantrodiellaceae is established to accommodate the genera Neoantrodiella Y.C. Dai et al., Cyanotrama Ghob.-Nejh. & Y.C. Dai, Poriodontia Parmasto and Fibricium J. Erikss, in the order Hymenochaetales, Agaricomycetes based on its distinct lineage in the phylogenetic analysis (Figs. 149 & 150). The combined phylogeny of ITS, nLSU, RPB2 and mtSSU gene data demonstrate that Neoantrodiella show no affinity to the other orders of Agaricomycetes (Fig. 149). The sampled specimens of Neoantrodiella clustered in the Hymenochaetales clade and formed a well-supported lineage. According to the ITS and nLSU rDNA-based phylogeny (Fig. 150), Neoantrodiellaceae (including Neoantrodiella, Cyanotrama, Poriodontia and Fibricium) grouped close to Hyphodontia in the Kneiffella family, but formed a distinct clade with moderate support (73% MP and 0.93 BPPs) within the order Hymenochaetales. Previously, Neoantrodiella (including Antrodiella gypsea and A. thujae) was included in Phanerochaetaceae, Polyporales (Hattori and Ryvarden 1994; Kirk et al. 2008; Dai and Yuan 2007; Dai 2012); Cyanotrama was included in Hymenochaetales without existed family (Ghobad-Nejhad and Dai 2010), Fibricium was included in Corticiaceae, Corticiales (Jülich 1974); and Poriodontia was included in Schizoporaceae, Hymenochaetales (Kirk et al. 2008). However, recent studies (Ghobad-Nejhad and Dai 2010; Miettinen and Larsson 2011; Yuan 2014) and our current phylogenetic analyses showed these four genera are included in an unknown family. Thus, the new family Neoantrodiellaceae is established. Cyanotrama is similar to Neoantrodiella in morphology, so Neoantrodiella gypsea and N. thujae were combined as Cyanotrama gypsea (Yasuda) Miettinen and C. thujae (Y.C. Dai & H.S. Yuan) Miettinen & Y.C. Dai (Miettinen 2011). However, these two genera formed two well-supported lineages in the ITS and nLSU rDNA-based phylogeny, respectively (Fig. 150). Antrodiella Ryvarden & I. Johans. was described by Ryvarden and Johansen (1980) to comprise the so-called Polyporus semisupinus Berk. & M.A. Curtis complex (Ryvarden 1991). At present there are about 50 species in Antrodiella, either transferred from other genera or described as new species in the genus (Dai 2012; Miettinen et al. 2012; Ryvarden and Melo 2014; Yuan 2014). However, molecular studies showed that species of Antrodiella are polyphyletic and most species in the genus, including the type species belong to Polyporales (Miettinen et al. 2012; Yuan 2014). But in our study, Antrodiella gypsea and A. thujae phylogenetically are distinctly distant from species in the Polyporales (Fig. 150), and both species belong to Hymenochaetales. It is necessary to set up the new genus and family to accommodate them. So the above new genus, family and combinations are proposed.
Maximum Parsimony strict consensus tree illustrating the phylogeny of Neoantridieelaceae, and related species in Agaricomycetes based on ITS + nLSU + RPB2 + mtSSU sequence data. Branches are labelled with parsimony bootstrap proportions (before slanting line) higher than 50 % and Bayesian posterior probabilities (after slanting line) more than 0.95
Maximum Parsimony strict consensus tree illustrating the phylogeny of the new family Neoantridieelaceae, and more representative available families in Hymenochaetales, based on the combined ITS + nLSU sequence datasets. Branches are labelled with parsimony bootstrap proportions (before slanting line) higher than 50 % and Bayesian posterior probabilities (after slanting line) more than 0.95
In the current study, we resolve the placement of Fibricium, Neoantrodiella, Cyanotrama and Poriodontia. The combined ITS, nLSU, RPB2 and mtSSU sequence dataset was used for order placement (see note for order). The combined ITS and nLSU sequence dataset was used for family placement (see note for family). The new sequences were generated for this study (Table 1), and other reference sequences were obtained from GenBank (Ghobad-Nejhad and Dai 2010; Miettinen and Larsson 2011).
-
235.
Neoantrodiella Y.C. Dai, B.K. Cui, Jia J. Chen & H.S. Yuan, gen. nov.
Index Fungorum number: IF551498; Facesoffungi number: FoF01012
Etymology: Neoantrodiella (Lat.): referring to resembling Antrodiella.
Basidiocarps annual to perennial, resupinate, effused-reflexed or pileate with poroid hymenophores, corky to hard corky, white to cream. Hyphal system dimitic; generative hyphae bearing clamp connections; skeletal hyphae thick-walled, cyanophilous; cystidia present or absent; basidiospores allantoid or ellipsoid, thin-walled, hyaline, smooth, indextrinoid, acyanophilous.
Type species: Neoantrodiella gypsea (Yasuda) Y.C. Dai, B.K. Cui, Jia J. Chen & H.S. Yuan
-
236.
Neoantrodiella gypsea (Yasuda) Y.C. Dai, B.K. Cui, Jia J. Chen & H.S. Yuan, comb. nov.
Index Fungorum number: IF551499; Facesoffungi number: FoF01013
Basionym: Polystictus gypseus Yasuda, Bot. Mag., Tokyo 32: 249 (1918).
= Antrodiella gypsea (Yasuda) T. Hatt. & Ryvarden, Mycotaxon 50: 35 (1994).
-
237.
Neoantrodiella thujae (Y.C. Dai & H.S. Yuan) Y.C. Dai, B.K. Cui, Jia J. Chen & H.S. Yuan, comb. nov.
Index Fungorum number: IF551500; Facesoffungi number: FoF01014
Basionym: Antrodiella thujae Y.C. Dai & H.S. Yuan, Cryptog. Mycol. 28: 179 (2007).
Basidiocarps perennial, resupinate, effused-reflexed or pileate with poroid hymenophores, corky, white, cream. Hyphal system dimitic; generative hyphae bearing clamp connections; skeletal hyphae thick-walled, cyanophilous; cystidia present; basidiospores allantoid or ellipsoid, thin-walled, hyaline, smooth, indextrinoid, acyanophilous (Fig. 151).
Schizoporaceae
-
238.
Xylodon ramicida Spirin & Miettinen, sp. nov.
MycoBank number: MB 813990; Facesoffungi number: FoF01006; Fig. 152
Holotype: Spirin 7664
Etymology: Branch killer, derived from ramus (Lat.), branch
Basidiocarps annual, resupinate, arid, covering several cm. Sterile margin first white, floccose, up to 1 mm wide, absent in older basidiocarps. Hymenial surface pale ochraceous, odontoid; spines solitary or fused together, rather regularly arranged, up to 0.6 mm long, (3)4–5 per mm, with sharpened fimbriate apices. Hyphal structure monomitic, hyphae clamped, faintly cyanophilous. Subicular hyphae densely and mostly irregularly arranged, some in parallel bundles, with thickened walls, (2.8)3.1–3.8(4.3) μm (n = 40/2). Tramal hyphae subparallel, thin- to slightly thick-walled, in subhymenium short-celled, (2)2.4–3(3.4) μm (n = 40/2). Cystidia of hymenial origin, variable in shape and size, moniliform or capitate to bottle-shaped, (13)19.2–31.3(34.8) × (3)3.1–4(4.7) μm (n = 20/2), accidentally encrusted by small crystalline aggregations. Basidia suburniform, 4-spored, 18–23.6 × 3.6–4.6 μm, with thickened wall at basal part. Basidiospores thin-walled, cylindrical, often tapering to distal end, (6)6.1–7.8(8.1) × (2.4)2.5–3(3.1) μm, L = 6.66, W = 2.74, Q = 2.41–2.48 (n = 90/3), ventral side flat or indistinctly convex, very rarely slightly concave, occasionally with several oil drops. Oily matter scanty or absent.
Notes: Morphology and DNA data place X. ramicida in the vicinity of X. quercinus (Pers.) Gray, the type species of Xylodon (Fig. 1). ITS sequence difference is only 5–7 bp. They have similar macroscopic characters and hyphal structure, but basidiospores of X. quercinus are wider, thick cylindrical, and often with a depressed ventral side (Fig. 2), (5.6)5.7–7.5(7.6) × (2.7)2.8–3.3(3.4) μm, L = 6.53, W = 3.07, Q = 2.06–2.18 (n = 90/3). Moreover, tissues of X. quercinus exude abundant oily matter in Cotton Blue. Ecology and distribution areas of these species are different as well. Xylodon quercinus is found in Europe and Siberia, and it inhabits thick, dead and usually fallen branches of angiosperm trees, mostly of Quercus spp. It has not been reported from China, Japan, or Russian Far East (Langer 1994; Dai 2011). Xylodon ramicida is an East Asian species. It attacks living or dying but still attached branches of gymnosperms (mostly Picea ajanensis). A few records of X. ramicida come from living stems of Pinus pumila, the shrub-like pine species, thus indicating its possible pathogenic facilities. Xylodon quercinus has been reported from North America, but these data should be rechecked (Langer 1994).
Material examined: Xylodon quercinus: FINLAND, Uusimaa: Vantaa, Tammisto Nat Res., Quercus robur, 1 June 2013 Niemelä 9040 (H). Uusimaa, Helsinki, Patola, Salix caprea (?), 2 November 2011 Miettinen 15050.1 (H), INSD KT361632. RUSSIA, Krasnoyarsk Reg.: Turukhansk Dist. Mirnoe, Alnus sibirica, 19 August 2013 Kotiranta 26316 (H). Nizhny Novgorod Reg.: Lukoyanov Dist., Razino, Q. robur, 8 September 2011 Spirin 4482 (H). Xylodon ramicida. RUSSIA. Khabarovsk Reg.: Solnechnyi Dist., Igdomi, Abies nephrolepis (fallen log), 6 August 2011 Spirin 3851 (H); Verkhnebureinskii Dist., Kyvyty, Picea ajanensis (attached branches), 17 August 2014 Spirin 7390,7469 (H), Hegdy, P. ajanensis (attached branches), 18 August 2014 Spirin 7482, 7505, 7518 (H), 22 August 2014 Spirin 7797 (H), A. nephrolepis (attached branch), 18 August 2014 Spirin 7536 (H), Pinus pumila (living stem), 22 August 2014 Spirin 7856 (H), Dublikan Nat Res., P. ajanensis (attached branches), 19–21 August 2014 Spirin 7583, 7635, 7664 (H, holotype, INSD KT361634), 7715, 7724, 7753 (H), 23 August 2014 Spirin 7904 (H), P. pumila (living stem), 21 August 2014 Spirin 7764 (H), Sidorka, Picea obovata (attached branch), 24. VIII.2014 Spirin 7961 (H).
Polyporales
Polyporaceae Fr. ex Corda
-
239.
Colospora Miettinen & Spirin, gen. nov.
MycoBank number: MB813992; Facesoffungi number: FoF00994
Etymology: Colus (Lat.), distaff, refers to the shape of spores.
Basidiocarps resupinate, minutely odontioid, corticioid on dead wood. Hyphal system dimitic throughout, clamps present, short-branched dendrohyphidia common, spines sterile towards the tip. Basidiospores large, thin-walled, biapiculate.
Type species: Colospora andalasii Miettinen & Spirin
Other species: Colospora citrispora (Boidin & Lanquetin) Miettinen comb. nov.; Basionym Epithele citrispora Boidin & Lanquetin, Mycotaxon 16: 467, (1983); MycoBank number MB 813996.
Notes: This genus contains two Epithele-like fungi with a dimitic hyphal structure and biapiculate spores. The type species of Epithele (E. typhae) and Skeletohydnum (S. nikau), the two existing genus names for Epithele-like fungi, are not particularly closely related (Fig. 1). Epithele typhae (Pers.) Pat. is a monomitic species with smooth, fusiform spores. Skeletohydnum nikau (G. Cunn.) Jülich also has smooth, fusiform spores, which are thick-walled, and skeletal hyphae are restricted in its spines making the basidiocarps more fragile than in Colospora (Boidin and Lanquetin 1983; Nakasone 2013). Colospora belongs to the core polyporoid clade (Polyporaceae) of the Polyporales (Binder et al. 2013). Closest relatives are found among the polypore genera Porogramme, Tinctoporellus, Theleporus and the corticioid genus Erythromyces (Fig. 2). Those genera contain species with smaller, thin-walled spores. Of these, the light-coloured Theleporus spp. are most similar morphologically, but their spores and hymenial cells are of much smaller dimensions. Also Diplomitoporus venezuelicus Ryvarden & Iturraga is closely related to Colospora. The species is morphologically similar to Theleporus spp. (Ryvarden and Johansen 1980; Zhou and Dai 2012). Ryvarden and Iturriaga (2003) did not report dendrohyphidia or branching skeletal hyphae in the type of D. venezuelicus, but we confirm that these characters are present.
The best placement of D. venezuelicus is for the time being in Theleporus venezuelicus (Ryvarden & Iturraga) Miettinen, comb. nov.; basionym Diplomitoporus venezuelicus Ryvarden & Iturraga, Mycologia 95:1069, 2003; MycoBank number: MB 813995 (Fig. 153).
-
240.
Colospora andalasii Miettinen & Spirin, sp. nov.
MycoBank number: MB 813994; Facesoffungi number: FoF00995; Figs. 154 and 155
Etymology: After Andalas University, the leading botanical research institution in Sumatra.
Holotype: Miettinen 13096
Basidiocarps annual, resupinate, leathery, up to 15 cm in widest dimension, up to 0.3 mm thick. Margin compact, sterile. Hymenial surface pale cream coloured, odontoid, in older parts with vinaceous stains; spines (sterile hyphal pegs) solitary, regularly arranged, up to 200–300 × 40–60 μm, 5–7 per mm, with sharp apices. Subiculum (thickness excluding the spines) 150–250 μm. Hyphal structure dimitic (amphimitic), generative hyphae clamped. Skeletal hyphae dominating in all parts of basidiocarps, irregularly arranged in subiculum, (1.8)2–2.8(3.4) μm (n = 34/1), subparallel in spine trama, (2)2.6–2.9(3.2) μm (n = 20/1), branched and tapering but sparingly, thick-walled, with a capillary, rather indistinct lumen (one sixth of hyphal diam. or less), acyanophilous, faintly yellowish or hyaline in Melzer’s reagent. Generative hyphae thin-walled, 1.8–2.8 μm. Coarse crystals abundant in subiculum, up to 20 μm in widest dimension, mostly of square or rhomboidal shape; also fine sand-like encrustation. Hymenium covers spines only close to their base and does not extend close to the tip. Dendrohyphidia present in hymenium, usually not projecting, 2.5–4 μm in diam., with short blunt branches, collapsing easily. Basidia utriform, 4-spored, 30–40 × 9–10 μm, with oil drops inside, sterigmata subulate, up to 12 × 2 μm. Basidiospores slightly thick-walled, finely ornamented (covered by minute warts), biapiculate, apical parts distinctly tapering and refractive, with numerous oil drops inside, faintly cyanophilous, showing small amyloid patches in apices, 14.7–18.8 × (5.7)6–7.3(7.8), L = 16.99 μm, W = 6.65 μm, Q = 2.56 (n = 32/1).
Material examined: Colospora andalasii: INDONESIA, Sumatera Barat, Padang, Limau Manis, dry fallen angiosperm branch, 15 July 2008 Miettinen 13096 (ANDA holotype, H), INSD KT361629. Epithele alba: BRAZIL, São Paulo, Campinas, Moji-Guaçu, 29 January 1987 Ryvarden 24517 (H ex O). Epithele subfusispora: BRAZIL, São Paulo, Campinas, Moji-Guaçu, 23 January 1987 Ryvarden 24554 (H ex O). Epithele typhae: ESTONIA, Valgamaa, Otepää, Valkjärva, Carex acutiformis, 10 September 2012 Kotiranta 25248 (H). Erythromyces crocicreas; INDONESIA, Sumatera Barat, Padang, Limau Manis, dicot log, 16 July 2008 Miettinen 13157 (ANDA, H), INSD KT361630. Skeletohydnum nikau: NEW ZEALAND, Auckland, Waitakere Ranges, Rhopalostylis sapida (fallen twig), 24 February 1984 Buchanan 84/016 (H ex O). Theleporus venezuelicus: VENEZUELA, Estado Bolivar, Sifontes, Tumeremo, 17 November 1994 Ryvarden 35205 (O, holotype), INSD KT361631.
Notes: Colospora andalasii is very similar morphologically to Epithele citrispora described from Gabon. The main separating character is the ornamentation of spores–clearly visible in C. andalasii, but not reported for E. citrispora. Boidin and Gilles (1998) produced an ITS sequence of a paratype of E. citripora. Even if we do not have control over the quality of the E. citrispora sequence, the ITS difference between the two seems convincingly large (>10 %) to exclude conspecificity.
A few Epithele species have ornamented spores: E. alba (Viegas) Boidin et al., E. interrupta Bres. and E. subfusispora (Burds. & Nakasone) Hjortstam & Ryvarden (Hjortstam and Ryvarden 2005). Their hyphal structure is different from that one of Colospora, considered as monomitic (Hjortstam and Ryvarden 2005) or dimitic with ‘microbinding hyphae’ (Nakasone 2013).
Russulales
Russula Pers., Observ. mycol. (Lipsiae) 1: 100 (1796) (Fig. 156)
Phylogram generated from Maximum Parsimony (MP) analysis based on sequence data of ITS for 58 Russula and two outgroup sequences (Albatrellus flettii and Gloeocystidiellum aculeatum). Sequences used in this study have been sampled from a previous study (Miller and Buyck 2002; Eberhardt 2002; Li et al. 2015b) or newly generated for R. guangxiensis and R. hakkae. Significant supported bootstrap values (≥70 %) are shown above the branches. Thickened clades represent posterior probabilities (PP ≥ 0.95) from Bayesian inference
Russula subsection Roseinae Singer ex Sarnari is a member of subgenus Incrustatula Romagn., together with subsections Amethystinae (Romagn.) Bon, Chamaeleontinae Singer, and Lilaceinae (Melzer & Zvára) Jul. Schaeff., all of which have primordial hyphae in pileipellis (Romagnesi 1985, 1996). Members of Roseinae can be distinguished from those of the other subsections of subgenus of Incrustatula by brightly orange, pink to reddish tinged pileus, a whitish spore print, and a persistent bright reddening in dried fruit bodies. The concept of subsection Roseinae was established by Singer based on Russula, then corresponded by Romagnesi, and emended by Sarnari (Singer 1986; Romagnesi 1967, 1985; Sarnari 1998). North American mycologists also made contributions in recognizing the species diversity of Roseinae (Adamčík and Buyck 2012). Although 21 species and 3 varieties of Russula have been described from China, only one variety R. minulula var. minor Z.S. Bi can be placed in subsection Roseinae (Singer 1935; Chiu 1945; Ying 1983; Bi and Li 1986; Zang and Yuan 1999; Wen and Ying 2001; Song et al. 2007; Wang et al. 2009; Li et al. 2011, 2012, 2013a, b, 2015a, b).
Southern China is a region characterized by subtropical to tropical climates, rather diverse rainforests and evergreen broad-leaved forests. Russula species form associations with diverse higher plants in these forests as ectomycorrhizal symbionts. Fruit bodies of some edible Russula species such as R. cf. griseocarnosa X.H. Wang et al. are often collected, sold and consumed as precious food of highly nutritious values for parturients in this area. However, not all of the Russula taxa are gathered, because some of them are regarded as “poisonous mushrooms” by indigenous people. After some fungus forays in southern China, newly collected specimens were examined, and two “suspected poisonous” Russula taxa unknown to science were found. These new species are described and illustrated using morphological and phylogenetic evidence. Comparisons with the other closely related and similar Russula taxa are also made.
-
241.
Russula guangxiensis G.J. Li, H.A. Wen & R.L. Zhao, sp. nov.
Index Fungorum number: IF551492; Facesoffungi number: FoF01007; Fig. 157
Etymology: refers to the type locality.
Holotype: CHINA. Guangxi Zhuang Autonomous Region, Wuzhou City, Tengxian County, Xiangqi Township, Xiangqi Village, 21 August 2013, Xin-Hua Chen 35 (HMAS 267867).
Basidiomata small to medium sized. Pileus 30–65 mm diam., first hemispheric to plano-convex, then expanding to applanate, finally slightly depressed in center to concave, smooth, viscid when wet, slightly glabrous when young, peeling 1/3–1/2 from the edge, sometimes desquamated in small patches, margin sometimes undulate, not striate, rarely cracked; pale pinkish red tinged with Begonia Rose (I1b) to Geranium Pink (I3d), intermixed with Strawberry Pink (I5d) in center, Hermosa Pink (I1f), Shrimp Pink (I5f), or even White (LIII) towards the margin. Lamellae adnate, equal to sub equal, 2–4 mm in height, 15–21 pieces per cm at the edge, rarely forked near the stipe and pileal edge, often interveined, White when fresh, slowly turning Orange Cinnamon (XXIX13″) to Mikado Brown (XXIX13″i) when injured, lamellulae absent. Stipe central, 6–9 × 0.8–1.5 cm, subcylindrical, surface dry, smooth, rarely rugulose longitudinally, dull, sometimes slightly attenuate upwards or downwards, white, a tinge of when injured and dry, first stuffed, becoming hollow at last. Context 2–4 mm at the center of the pileus, white, fragile, odour indistinct, taste mild. Spore print White (RomagnesiIa).
Basidiospores(Fig.) [200/10/10] 5.9–6.9 (−7.2) × 4.9–6.1 μm, Q = (1.05–) 1.08–1.23 (−1.26), (Q = 1.16 ± 0.05), hyaline, subglobose to broadly ellipsoid; ornamentation composed of amyloid conical warts that are mostly isolated or rarely linked, not forming network, warts 0.7–1 μm in height; suprahilar are distinct and amyloid. Basidia 38–47 × 7–10 μm, mostly with four sterigmata 3–5 μm long, hyaline, sometimes yellowish in KOH, subclavate to clavate, rarely subcylindrical. Pleuroystidia scattered, 55–63 × 7–12 μm, projecting 10–20 μm beyond the basidia, subfusoid to subcylindrical, sometimes clavate to subclavate, apices round to subacute, often appendiculate to subcapitate, thin–walled, contents granular, crystal, refractive, weakly grey in SV. Cheilocystidia same as pleurocystidia. Subhymenium a cellular layer 15–30 μm thick composed of inflated cells 6–17 μm diam., hyaline, sometimes pale yellowish in KOH. Pileipellis orthochromatic in cresyl blue, composed of epipellis and subpellis; epipellis a dense, hymeniderm 50–60 μm thick, composed of thin-walled, cylindrical hyaline hyphae 3–6 μm wide; terminal cells 15–30 × 5–10 μm, septate, clavate, apex mostly obviously inflated; subpellis less dense, a pseudoparenchymatic region, 40–60 μm thick, composed of irregular inflated sphaerocysts 20–30 μm diam., interweaved hyaline hyphae 2–6 μm wide; primordial hyphae cylindrical, septate, 3–6 μm diam., with heteromorphous-opalescent inclusions and acid-resistant incrustations, apex obtuse; pileocystidia not observed. Stipitipellis a cutis, composed of filamentous hyphae 3–6 μm diam., interweaved with inflated cells 10–15 μm diam., hyaline, some hyphae yellowish to pale ochre in KOH; caulocystidia absent. Trama composed of sphaerocytes 30–90 μm diam. Clamp connections and lacticiferous hyphae absent from all tissues.
Habitat and distribution: Single or scattered in broad-leaved forests (dominated by Lithocarpus spp.).
Additional specimens examined: CHINA, Guangxi Zhuang Autonomous Region, Wuzhou City, Tengxian County, Xiangqi Township, Xiangqi Village, 21 August 2013, Xin-Hua Chen 57 (HMAS267863); ibid., Xin-Hua Chen 29 (HMAS267831); ibid., Xin-Hua Chen 19 (HMAS267869); ibid., Xin-Hua Chen 24 (HMAS267829); ibid., Xin-Hua Chen 41 (HMAS267833); ibid., Xin-Hua Chen 25 (HMAS267868); ibid., Xin-Hua Chen 28 (HMAS267832); ibid., Xin-Hua Chen 7 (HMAS267866); ibid., Xin-Hua Chen 14 (HMAS267836).
Notes: The combination of white spore print, mild taste, bright red pileus, pileipellis with primordial hyphae but without pileocystidia, pseudoparenchymatic subpellis, and the topology of phylogenetic analysis of ITS sequences clearly place R. guangxiensis within subgenus Incrustatula Romagn. Subsection Roseinae Singer ex Sarnari. Two new taxa, R. guangxiensis and R. hakkae, clustered together with strong support (BS 92 %, PP 1.00) with most members of subsection between the two new taxa through a fairly good support (BS 80 %, PP 1.00). The two Roseinae involved in this study. Our phylogenetic analysis also indicated a close relationship new taxa have context without distinctive odour, amyloid and distinct basidiospores uprahilar area, and habitat of Lithocarpus forest. However, R. hakkae has an acrid tasted context, larger basidiospores with dense ornamentation up to 1.2 μm in height, and a palisade epipellis.
Other species of subsection Roseinae originally described from Europe can be distinguished from R. guangxiensis as follows. Russula lepidicolor Romagn. has a low basidiospore ornamentation composed of hemispherical warts up to 0.4 μm high which is often linked by fine lines as short to long ridges, and wider basidia (9–12 μm) (Romagnesi 1967; Sarnari 2005). Russula minutula Velen. has a small, velutinous pileus 17–30 mm, a context with a strong but short-lived odour of rosemary, and a quite rarely little amyloid basidiospores uprahilar area (Romagnesi 1967; Sarnari 2005). Russula velutipes Velen. has a pileus with copper brown or apricot orange shade, a bulbous stipe, and a low basidiospore ornamentation composed of hemispherical warts up to 0.4 μm high are which often linked by fine lines as short to long ridges.
Type specimens of several members of subsection Roseinae which were described from America has been studied recently (Adamčík and Buyck 2012). Among good members of subsection Roseinae, R. albida Peck can be distinguished from R. guangxiensis by a white to slightly yellow tinged pileus which has a narrowly tuberculose-striatepileus margin, and a basidiospore ornamentation composed of spines which form an almost complete reticulum, connected by frequent line connections; R. nigrescentipes Peck differs in a stipe becoming blackish by handling or bruising, basidiospore ornamentation composed of warts connected by frequent line connections, and short basidia (22–30 μm); R. rimosa Murrill can be distinguished by shortly ellipsoid to ellipsoid basidispores which have dense, low ornamentations (0.2–0.5 μm) with numerous line connections, and pileipellis with pileocystidia SV-insensitive contents (Adamčík and Buyck 2012).
-
242.
Russula hakkae G.J. Li, H. A. Wen & R.L. Zhao, sp. nov.
Index Fungorum number: IF551493; Facesoffungi number: FoF01048; Fig. 158.
Etymology: refers to the hakka people living in the type locality.
Holotype: CHINA. Guangdong Province, Meizhou City, Meixian County, Longwen Township, 9 August 2013, Xin-Hua Chen 34 (HMAS 267765).
Basidiomata small to large sized. Pileus 40–100 mm diam., first hemispheric, then flat hemispheric to applanate, plano-convex to convex when mature, smooth, viscid when wet, slightly glabrous when young, peeling 1/4–1/2 from the edge, sometimes desquamated in small patches; brightly tinged with Spectrum Red (I1), Peach Red (I5b) to Scarlet (I5), intermixed with pink tinge of Rose Doree (I3b) to Strawberry Pink (I5d) in center, Eosine Pink (I1d), Hermosa Pink (I1f) to Hydrangea Pink (XXVII5″f) towards margin. Lamellae adnate, equal, 2–6 mm high, 17–22 pieces per cm at the edge, rarely forked near the stipe and pileal edge, often interveined, white when fresh, lamellulae absent. Stipe central to subcentral, 5–7 × 0.8–2 cm, subcylindrical, slightly tapering towards the apex and base, smooth, sometimes rugulose longitudinally, mostly pinkish tinged with Eosine Pink (I1d) to La France Pink (I3f), partly longitudinally fade to White (LIII), a tinge of Pale Yellow–Orange (III15f) when injured, old and dry, first unchanging, spongy to hollow when mature. Context 2–5 mm thick from the lamellae attachment to the stipe, White, unchanging or becoming Sanford’s Brown (II11k) when injured, fragile, odour indistinct, taste acrid. Spore print White (Romagnesi Ia).
Basidiospores [200/10/10] (6–) 6.7–8.1 (−8.8) × (5.5–) 5.8–6.9 (−7.5) μm, Q = (1.06–) 1.13–1.27 (−1.31), (Q = 1.17 ± 0.06), hyaline, subglobose to broadly ellipsoid, rarely ellipsoid; ornamentation composed of dense, amyloid warts that are 0.9–1.2 μm high; suprahilar are distinct and amyloid. Basidia 40–50 × 10–15 μm, mostly with four sterigmata3–7 μm long, hyaline, sometimes yellowish in KOH, subclavate to clavate, rarely cylindrical. Pleuroystidia scattered, 55–77 × 8–12 μm, distinctly projecting 15–25 μm beyond the basidia, subfusoid to subcylindrical, sometimes clavate to subclavate, apices round to subacute, often appendiculate to subcapitate, thin–walled, contents granular to crystal, weakly grey in SV. Cheilocystidia same as pleurocystidia. Subhymenium a cellular layer 20–30 μm thick composed of inflated cells 10–25 μm diam., hyaline, sometimes pale yellowish in KOH. Pileipellis orthochromatic in cresyl blue, composed of epipellis and subpellis; epipellis a palisade 25–50 μm thick, composed of thin-walled, dense, ascending to erect, cylindrical hyaline hyphae 3–8 μm wide arising from a gelatinous matrix; terminal cells 6–10 μm wide, septate, clavate to cylindrical, apex obtuse; subapical cells often irregular, inflated, branched, up to 30 μm diam., subpellis composed of inflated cells same as the subapical cells, forming a pseudoparenchymatic region, interweaved hyaline hyphae 2–6 μm wide; primordial hyphae septate, protruding, with heteromorphous-opalescent inclusions and acid-resistant incrustations, 3–7 μm wide, apex obtuse; pileocystidia not observed. Stipitipellis a cutis, composed of filamentous hyphae 3–6 μm diam., interweaved with inflated cells 10–25 μm diam., hyaline, some hyphae yellowish to pale ochre in KOH; caulocystidia absent. Trama composed of sphaerocytes 35–100 μm diam. Clamp connections and lacticiferous hyphae absent from all tissues.
Habitat and distribution: Solitary to scattered in broad leaved forest dominated by Lithocarpus spp.
Additional specimens examined: CHINA, Guangdong Province, Meizhou City, Meixian County, Longwen Township, 9 August 2013, Xin-Hua Chen 9 (HMAS 267862); ibid., Xin-Hua Chen 4 (HMAS 267870); ibid., Xin-Hua Chen 45 (HMAS 267767); ibid., Xin-Hua Chen 9 (HMAS 267862); ibid., Xin-Hua Chen 2 (HMAS 267865); ibid., Xin-Hua Chen 34 (HMAS 267765); ibid., Xin-Hua Chen 37 (HMAS 267768); ibid., Xin-Hua Chen 6 (HMAS 267861); ibid., Xin-Hua Chen 15 (HMAS267864); ibid., Xin-Hua Chen 25 (HMAS 267769).
Notes: Russula hakkae can be easily distinguished from most of the other taxa of subsection Roseinae by its stabile pinkish tinge of stipe, acrid context taste, and very dense basidiospore ornamentation up to 1.2 μm. There are only a few members of subsection Roseinae which have pinkish tinged stipe. These taxa can be distinguished from R. guangxiensis as follows. Russula peckii Singer [as “undescribed species” in subsection Roseinae in Adamčík and Buyck (2012)] has a crenulate lamellar edge, a mild context taste, an ixotrichoderm pileus epicutis, and a habitat of coniferous forest (Peck 1907; Singer 1935). Russula pseudopeckii Fatto has a pileus edge with a few long lamellulae, a fruity to nondescript context odour, a cream spore print, and a basidiospore ornamentation composed of low warts (0.4–0.6 μm). Russula rubellipes Fatto has a mild context taste, a cream spore print, and some warts of basidiospore ornamentations forming a variable number of connectives (Fatto 1998).
Tremellomycetes–Tremellales
The class Tremellomycetes currently includes the orders Cystofilobasidiales, Filobasidiales, Holtermanniales and Tremellales (Boekhout et al. 2011; Wuczkowski et al. 2011; Millanes et al. 2011). The circumscription of these orders and the families within the Tremellomycetes is very much based on morphology and culture characteristics, and few molecular based investigations have tested these concepts. Tremella itself is highly paraphyletic, and the taxonomy and classification of filamentous and yeast-forming groups have developed in parallel (Chen 1998; Fell et al. 2000; Scorzetti et al. 2002). We expect the classification of the Tremellales to change rather drastically in the future. Some filamentous tremellalean groups were distinguished by Chen (1998), and Millanes et al. (2011) identified several groups of mainly lichenicolous species. A phylogenetic tree of Tremellales is presented in Fig. 159, to place the three newly described lichenicolous Tremella species in a phylogenetic context.
Phylogram generated from maximum likelihood analysis (implemented in RAxMLGUI 1.3) based on combined LSU and ITS sequence data. Maximum likelihood bootstrap values ≥ 70 % are indicated over the branches. Newly described species are in blue, and are represented by the type specimen (T). The tree is rooted with Tetragoniomyces uliginosus (Millanes et al. 2011)
-
243.
Tremella dirinariae Diederich, Millanes & Wedin, sp. nov.
Index Fungorum number: IF551494; Facesoffungi number: FoF01008; Fig. 160.
Tremella dirinariae (NY, holotype) a Black basidiomata on thallus of Dirinaria aegialita b Hymenium with probasidium (arrow shows basal clamp), and old basidia and epibasidia (not stained) c–j Mature 1-septate basidia k–n Basidiospores o–q Haustoria. All microscopical photos in Phloxin B using DIC optics. Scale bars: a = 200 μm, b = 20 μm, c–q = 5 μm (scale bar in q). Photographs by P. Diederich
Etymology: In reference to the host Dirinaria.
Holotype: Harris 37673 (NY).
Lichenicolous on the thallus of Dirinaria aegialita, not gall-inducing, not causing any visible damage to the host. Known only from the type locality in Florida. Sexual morph: Basidiomata initially as black swellings on the host thallus, breaking through the cortex, then black, pulvinate, often slightly taller than broad, strongly gelatinous, not gall-inducing, surface smooth to rugose, roundish to sometimes irregular in form, mainly 0.25–0.35(−1) mm diam., up to 0.3 mm tall. Context hyphae thick-walled, 2.2–3.5 μm diam., clamp connections not observed; haustorial branches present, mother cells subsphaerical, 3.5–4.5 μm long, 2.5–4 μm wide, haustorial filament 0.5–1 μm diam. Hymenium hyaline, containing numerous probasidia; hyphidia absent; probasidial initials clavate, proliferations occurring through the basal clamp. Basidia, when mature, 2-celled, with one transverse or oblique, exceptionally longitudinal septum, slightly constricted at the septum, (15–)17–24 × 6.5–10(−12) μm (excl. epibasidia), Q = (1.5–)2–3.5, often with an attenuated stalk-like base of variable length (explaining the rather large variability of basidial size); epibasidia subcylindrical, up to 50 μm long (only old collapsed epibasidia measured), 2.5–4 μm diam. Basidiospores ellipsoid to subsphaerical, with a distinct apiculus, 6–8 × 5.5–6.5 μm, Q = 1–1.3. Asexual morph: Undetermined.
Material examined: USA, Florida, Seminole Co., Little Big Econlockhatchee State Forest, along Florida Trail from entrance on Co. Rd. 426, 3.3 mi NE of Co. Rd. 419 in Oviedo, 28°41′ N, 81°10′ W, Sabal-Quercus virginiana-hardwood swamp, on Liquidambar, on Dirinaria aegialita, 10 January 1996, R. C. Harris 37673 (NY, holotype), (herb. Diederich, isotype).
Notes: Amongst the lichenicolous Tremella species with basidiomata developing on the host thallus and with 1-septate basidia, the new species is distinguished from most by the frequently stalked basidia and by the black basidiomata that are often slightly taller than broad. An undescribed species on Leptogium s. lat. studied by Diederich (1996, as ‘Tremella sp. 6’) has much shorter basidia, 11–15 × 8.5–10 μm, and pale brown basidiomata. Another yet undescribed species on Anaptychia (‘Tremella sp. 5’) is distinguished by larger blackish basidiomata and slightly shorter and distinctly broader basidia, 15–19 × 10–15 μm. Within the species with not or rarely stalked basidia with mainly transverse or rarely oblique septa and distinct, not gall-inducing basidiomata, Tremella santessonii Diederich on Usnea is distinguished by dark reddish brown and flatter basidiomata, T. phaeographidis Diederich et al. on Phaeographis by larger basidiomata, 0.4–0.7 mm diam., and T. psoromicola Diederich on Psoroma by a hymenium composed of a dense layer of cylindrical, septate, little ramified hyphidia intermixed with basidia (Diederich 1996). The species is not closely related to other lichenicolous Tremella species growing on Caliciales (e.g., Tremella christiansenii and T. phaeophysciae), but rather groups with species growing on Parmeliaceae, within a clade designated as clade III by Millanes et al. (2011). This relationship is not recovered with support, however, in our phylogenetic analyses. Dirinaria belongs to Caliciaceae (Wedin et al. 2002; Helms et al. 2003; Miadlikowska et al. 2014) and the hosts of T. christiansenii and T. phaeophysciae belong to the Physciaceae.
-
244.
Tremella graphidis Diederich, Millanes, Wedin & Common, sp. nov.
Index Fungorum number: IF551495; Facesoffungi number: FoF01009; Fig. 161
Tremella graphidis (BR, holotype) a, b Brownish basidiomata parasitizing the hymenium of Graphis assimilis (top left of a visibly non-infected part of host apothecia). c Hymenium with basidia d Context hyphae e, f Probasidia (basal clamp visible in d) g–j Mature 1-septate basidia k–m Basidiospores n–o Haustoria. All microscopical photos in Phloxin B using DIC optics. Scale bars: a–b = 200 μm, c–d = 10 μm, e–o = 5 μm (scale bar in o). Photographs by P. Diederich
Etymology: In reference to the host Graphis.
Holotype: Common 9434B (BR).
Lichenicolous in the hymenium of Graphis species, incl. G. assimilis, G. caesiella, G. cupei and G. cf. desquamescens, not gall-inducing, distinctly enlarging the width of the host ascomata and gradually replacing the host hymenium by the basidiomata. Known only from Florida. Sexual morph: Basidiomata developing within the host hymenium, strongly enlarging the width of the initially narrowly lirelliform host apothecia, pale pinkish to brown, strongly gelatinous, surface rather smooth, elongate, up to 2(−3) × 0.25 mm (i.e., the same form and size as the broadened disk of the host apothecia). Context hyphae thin-walled, 1.5–2.5 μm diam., clamp connections not observed; haustorial branches present, mother cells subsphaerical, 2.5–3.5 μm long, 1.5–2.5 μm wide, haustorial filament 1.5–4 μm long, 0.5–1 μm thick. Hymenium hyaline, containing numerous probasidia; hyphidia absent; probasidial initials narrowly clavate cylindrical, proliferations occurring through the basal clamp. Basidia, when mature, narrowly and elongate cylindrical, 2-celled, with one transverse septum in the upper third or quarter, not or slightly constricted at the septum, 30–38 × 4–5(−5.5) μm (excl. epibasidia), Q = 6–8.5, without a stalk-like base; epibasidia subcylindrical, reaching at least 40 μm in length, probably 2–3 μm diam. (only old collapsed epibasidia observed). Basidiospores broadly ellipsoid, with a distinct apiculus, 6.3–6.8 × 5.3–5.8 μm, Q = 1.1–1.2. Asexual morph: Undetermined.
Material examined: USA, Florida, Collier Co., Fakahatchee Strand State Preserve, trail north of Boardwalk, 25°56.51′ N, 81°28.16′ W, on Graphis assimilis and G. caesiella, 11 November 2011, R. Common 9434B (BR, holotype), (S, herb. Diederich, isotypes); ibid., on G. cf. desquamescens, R. Common 9443F (herb. Diederich); ibid., trail from Gate 7, 25°58.78′ N, 81°24.61′ W, on Graphis sp., R. Common 9370F (herb. Diederich); ibid., on G. caesiella, R. Common 9370H (herb. Diederich); near lake by Ranger Station, 25°57.25′ N, 81°21.80′ W, on G. caesiella, R. Common 9393H (herb. Diederich); ibid., Janes Scenic Drive, 25°58.74′ N, 81°22.26′ W, on G. cupei, 2014, R. Common 9736D, 9755J, 9763B, 9788C (herb. Diederich).
Notes: Tremella graphidis is a very distinct species, distinguished from all hitherto known lichenicolous Tremella species by the particularly long and narrow basidia, with a single transverse septum in the upper third or quarter. It mostly resembles Biatoropsis usnearum Räsänen s. lat., an assemblage of closely related species belonging to the non-monophyletic genus Tremella s. lat. (Millanes et al. 2014), and which has 1–3-septate, clavate to subcylindrical basidia, 20–44 × 3–6.5 μm, with usually missing basal clamps, and basidiospores 4.5–8 × 4–7.5 μm. All intrahymenial Tremella species described by Diederich (1996) have broader basidia.
Tremella graphidis is rather common in the hymenium of Graphis species in Fakahatchee Strand State Preserve in Florida, and resembles macroscopically Tremella phaeographinae Diederich & Aptroot, a species probably confined to Phaeographis s. lat., which is also common in Florida. The original material of T. phaeographinae was described from specimens of Phaeographina, a genus no longer recognized. The host of the holotype (Aptroot 26229, B) may belong in Platygramme, whilst the host of a paratype (Buck 22971, NY) is Platygramme pachnodes (det. Lendemer & Tripp). The host of a further specimen (Harris 36016, NY) is Leiorreuma explicans (det. Lendemer & Tripp). Both Leiorreuma and Platygramme belong to the Phaeographis clade, which might represent a single large genus Phaeographis (Rivas Plata et al. 2013), but none of the known host species has yet been included in a phylogenetic analysis. R. Harris and H. Sipman are acknowledged for information on the hosts identity. Tremella phaeographinae is macroscopically rather similar to T. graphidis, with basidiomata occurring on the host thallus and hymenium, but differs microscopically by very different, usually 3-celled basidia, often with a mixture of transverse and longitudinal septa. Tremella phaeographinae is here for the first time included in a phylogenetic analysis. Our results show that the Tremella species on Graphidaceae for which DNA sequences are available (i.e., T. diploschistina, T. graphidis and T. phaeographinae), do not group together. Tremella phaeographinae forms a well-supported clade with T. ramalinae, another Tremella species that combine both transverse and longitudinal septa within the same basidium (Diederich 1996).
Sequenced specimens of Tremella phaeographinae examined: USA, Florida, Hillsborough Co., Hillsborough River State Park, Florida Trail, 28°08.94′ N, 82°14.10′ W, on Phaeographis, 15 September 2011, R. Common 9481B (S, herb. Diederich); sequence ‘a’ in Fig. 159; ibid., 28°08.90′ N, 82°14.01′ W, on Phaeographis, 26 October 2011, R. Common 9249C (S, herb. Diederich); sequence ‘b’ in Fig. 159.
-
245.
Tremella pyrenulae Diederich, Millanes, Wedin & Common, sp. nov.
Index Fungorum number: IF551496; Facesoffungi number: FoF01010; Fig. 162.
Tremella pyrenulae (BR, holotype) a, b Brown to pinkish basidiomata on thallus of Pyrenula ochraceoflavens c Hymenium with numerous basidia d–i, Basidiospores j–l Mature 1-septate basidia with epibasidia m–r Basidia. All microscopical photos in Phloxin B using DIC optics. Scale bars: a–b = 200 μm, c = 10 μm, d–r = 5 μm (scale bar in r). Photographs by P. Diederich
Etymology: In reference to the host Pyrenula.
Holotype: Common 9170B (BR).
Lichenicolous on the thallus of Pyrenula ochraceoflavens, not gall-inducing, not causing any visible damage to the host. Known only from Florida. Sexual morph: Basidiomata pink to pale brown, pulvinate, strongly gelatinous, surface rather smooth, roundish to slightly elongate, up to 0.5 × 0.4 mm, up to 0.3 mm tall, when mature with a constricted base. Context hyphae thin-walled, 2–3 μm diam., clamp connections not observed; haustorial branches not observed. Hymenium hyaline, containing numerous probasidia; hyphidia absent; probasidial initials ellipsoid to rarely clavate, proliferations occurring through the basal clamp. Basidia, when mature, 2-celled, with one longitudinal septum, slightly constricted at the septum, 13–17(−19) × 9.5–13 μm (excl. epibasidia), Q = 1–1.6(−2), generally without attenuated stalk-like base; epibasidia subcylindrical, at least 45 μm long, 3–6 μm diam. Basidiospores ellipsoid to subsphaerical, with a distinct apiculus, 7–8 × 6.5–7.5 μm, Q = 1–1.1. Asexual morph: Undetermined.
Material examined (all on Pyrenula ochraceoflavens): USA, Florida: Hernando Co., Baypoint Park at end of CR 50/550, 28°32.166′ N, 82°39.04′ W, 25 August 2011, R. Common 9170B (BR, holotype), (S, herb. Diederich, isotypes); ibid., R. Common 9165B (BR, herb. Diederich); Collier Co., Fakahatchee Strand State Park, Janes Scenic Drive, 25°58′45″ N, 81°22′15″ W, 2014, R. Common 9870Q (herb. Diederich); ibid., picnic area off US 41, 25°55′53″ N, 81°26′39″ W, 2014, R. Common 9705 K (herb. Diederich); Pinellas Co., Caladasi Island State Park, shrubs near beach, 28°02′ N, 82°49′ W, 2013, R. Common 9616A (herb. Diederich).
Notes: This species is distinguished from many lichenicolous Tremella species by the pale non-gall-inducing basidiomata developing over the host thallus, and by the non-stalked basidia with one longitudinal septum. Tremella phaeographidis Diederich et al. has smaller basidiospores, 5.5–7.5 × 5–6 μm, longer and narrower basidia (Q = c. 2) and larger basidiomata, 0.4–0.7 mm. Tremella microcarpa Diederich and T. coccocarpiae Diederich have smaller basidiomata, rarely exceeding 0.2 mm diam., the former smaller basidia, 9.5–12.5 × 7–11 μm, the latter smaller basidiospores, 5.5 × 4–4.5 μm. Tremella macroceratis Diederich & Hafellner and T. papuana Diederich have narrower basidiospores, 3.5–5.5 μm broad. Tremella montis-wilhelmii Diederich and the very similar T. normandinae Diederich are both distinguished by basidia with a larger l/w ratio (Q = 1.5–2) (12–17 × 7–9 μm in T. montis-wilhelmii, and 14.5–21 × 8.5–11.5 μm in T. normandinae), and T. montis-wilhelmii furthermore by slightly smaller basidiospores, 6–7 × 5–6 μm. In our phylogeny, Tremella pyrenulae and T. graphidis form a monophyletic group. Neither the morphology, nor the host preference, however, explain this relationship. This is not surprising since previous studies have shown that morphological characters in Tremella are highly variable in closely related species, and that host switches have probably been frequent in the evolution of the group (Millanes et al. 2011, 2014).
Contributions to Zygomycota
Mucorales
Basal lineages of terrestrial fungi are traditionally summarized to the Zygomycota in a colloquial sense (Voigt 2012). The conquest of land as earliest fungi facilitated the invention of aplanosporic mitospores which do not rely on water for dispersal. Based on the potential to form zygospores during conjugation of two yoke-shaped gametangia it is referred to a phylogenetically coherent group named the Zygomycota for zygosporic fungi, even though these basal fungal clades lack phylogenetic resolution at the molecular level (James et al. 2006). Molecular phylogenetic analyses revealed the dispersal of this phylum into four subphyla (Hibbett et al. 2007; Hoffmann et al. 2011) and one phylum (Humber 2012).
Absidia proposed by (Van Tieghem 1876), classifies in the subphylum Mucoromycotina, order Mucorales, family Cunninghamellaceae (Voigt 2012). It includes mesophilic species morphologically characterized by producing sporangiophores arising from stolons and apophysate sporangia with deliquescent walls. A septum is frequently observed below the sporangium (Benny 2009). Zygospores enveloped by appendages are produced by sexual reproduction after conjugation of heterothallic gametangia (Hoffmann et al. 2007).
Species of Absidia are commonly isolated from soil and animal dung (Richardson 2009) and the last species proposed to this genus was A. idahoensis Hesselt., M.K. Mahoney & S.W. Peterson, isolated from honey bees in the state of Idaho, USA (Hesseltine et al. 1990). In 2001, A. graminea L.S. Loh was described in Malaysian (Loh et al. 2001), but it was considered invalid for not having a holotype deposited in a Culture Collection (http://www.indexfungorum.org; accessed 25 June 2015).
During a study on the Mucorales from semi-arid regions in Brazil, an Absidia specimen that differs morphologically and genetically from the other species of the genus was isolated and is being described as new to science. Another Absidia specimen was isolated from soil of Dokdo Island in Korea, which differs morphologically and phylogenetically from any other species within the genus. Furthermore, a new species of Gongronella was described from forest soil in the Jeonnam Province in Korea. At the generic level Gongronella is related to Absidia within the Cunninghamellaceae (Voigt 2012) (Fig. 163).
a Phylogram generated from Bayesian analysis based on LSU sequence data. Bayesian posterior probabilities (PP) greater than 0.90 are indicated above or below the nodes. The new isolates are in blue. The tree is rooted with Mortierella parvispora. b Phylogram generated from Bayesian analysis based on 5.8S rDNA sequence data. Bayesian posterior probabilities (PP) greater than 0.90 are indicated above or below the nodes. The new isolates are in blue. The tree is rooted with Actinomucor elegans
-
246.
Absidia caatinguensis D.X. Lima & A.L. Santiago, sp. nov.
Index Fungorum number: IF551223; Facesoffungi number: FoF01015; Fig. 164
Absidia caatinguensis (holotype) a Colony surface b Four sporangiophores in a whorl c Two sporangiophores in a whorl with occasional swellings d, e Branched sporangiophores f Simple sporangiophore with a bell-shaped apophysis under the sporangium g Simple sporangiophore with columella and a single projection h Sporangiospores
Etymology: caatinguensis. Referring to biome where the species was first isolated.
Holotype: URM 7156
Colony initially white and becoming brownish gray (MP 15C2), covering the Petri dish (9 cm diam.) for four days and reaching the plate lid (1.5 cm height) in some points in MEA; colony reverse wavy zonate, initially cream to buff (MP 12H3) then becoming blackish brown in older cultures due to pigment production in the culture medium. Odour none; rhizoids branched and stolons 5–12 μm diam., smooth-walled, hyaline to light gray and irregularly septate. Sporangiophores 40–150(−210) × 2.5–5 μm, erect, smooth and hyaline, growing along the stolons and terminally, showing occasionally swellings; solitary to 2–6 (−7) in a whorl and these often branched again (up to five times, mostly after 6 days of incubation), often with a septum below the sporangium, rarely showing two septa. Sporangia pyriform (15–)17.5–27.5 μm diam., multi-spored and smooth-walled, apophysate, sometimes with a bell shaped apophysis. Columellae hemispheric, sometimes subglobose, 10–20 μm diam., smooth-walled; collar present sometimes. One projection is usually present on upper surface of columellae up to 5.75 μm in length, some are bulbous at distal end up to 2.5 μm in width. Sporangiospores 5–7.5 × 2.5–3.7 μm, smooth, regular in size and shape, cylindrical, slightly constricted at the central portion. Chlamydospores absent. Zygospores not observed. Probably heterothallic.
Media, temperature tests and mating experiments: On MEA. At 15 °C–slow growth (7 cm in 192 h). At 20 °C–Good growth (9 cm in 96 h) and good sporulation. At 25 °C–Good growth with better sporulation than at 20 °C (9 cm in 96 h). A brown pigment is produced in media. At 31 °C–slower growth than at 25 °C (9 cm in 120 h) and good sporulation. At 35 °C–Lack of growth and sporulation. The growth of A. caatinguensis on PDA was similar to the growth on MEA at 25 °C, but the brown pigment was not produced. At 31 °C–the growth was slower in PDA than in MEA (9 cm in 120 h) with low sporulation. Influence of light: not detected.
Material examined: BRAZIL, Pernambuco, Buíque, Catimbau National Park (8°31′55.8″S, 37°15′34.2″W), Soil. 2013, D.X. Lima. Holotype (URM 7156); living culture deposited at Jena Microbial Resource Collection (University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany) (JMRC: SF:012107).
Habitat: soil
Notes: Absidia caatinguens is morphologically similar to A. fusca, but differs primarily in its pattern of sporangiophores branching, by the size of sporangiospores and the pigment production in the culture medium (Fig. 164). As A. fusca, some strains of A. caatinguensis produce branched sporangiophores and a brown pigment that begins to appear by 20th day. However, sporangiophores of A. caatinguensis are much more branched than those of A. fusca, and the pigment is produced at temperatures higher than 15 °C d. Absidia caatiguensis sporangiospores are greater than those of A. fusca (3.3–4.5 × 1.8–3.5) (Hesseltine and Ellis 1964). Additionally, A. fusca colonies show a smooth colony reverse, different from the wavy zonate reverse observed in the new species. Our molecular analyzes (LSU rDNA and 5.8S rDNA) showed that A. caatinguensis is genetically different from the other species of the genus (Fig. 163a, b).
Phylogenetic tree for Absidia koreana EML-IFS45-1 and EML-IFS45-2 and related species based on Maximum likelihood (ML) analysis of multigenes including 18S and 28S rDNA, elongation factor (EF-1α), and actin (Actin-1). Sequences of Umbelopsis nana and U. isabellina were used as outgroups. Bootstrap support values >50 % are indicated at the nodes. The bar indicates the number of substitutions per position
-
247.
Absidia koreana H.B. Lee, H.W. Lee & T.T.T. Nguyen, sp. nov.
MycoBank number: MB 813805; Faces of fungi number: FoF 01015; Fig. 166
Morphology of Absidia koreana (EML-IFS45-1, holotype). a colony in synthetic mucor agar. b simple sporangiophore with young sporangium. c mature sporangia with sporangial net wall and sporangiospores. d attachment of sporangiophores. e columella with collarette and septum (yellow arrow). f sporangiospores. Scale bars: b, c, f = 10 μm, d = 50 μm, e = 20 μm
Etymology: koreana. Referring to the country which from the species was first isolated (Korea, Dokdo island).
Holotype: CNUFC-EML-IFS45-1, deposited at the Environmental Microbiology Laboratory Herbarium, Chonnam National University, Gwangju, Korea.
Colony exhibits rapid growth on SMA, attaining a diameter of 62–65 mm after 4 days at 25 °C. The initial color of the colonies is white, which later changed to grayish white or smoky gray, and the colonies cover almost the entire surface of the agar in the petri dishes within 5 days. The colony reverse is white and irregularly zonate. Although the mycelial growth on SMA is sparse, the sporulation is excellent. Sporangia 19.33–23.64 μm × 21.06–26.35 μm in diam., and are globose to slightly elliptical, having sporangial net wall. Sporangiophores are 3.84–4.60 μm wide and arise as 1–6 sporangiophores (average 2–4) per whorl from a single point in the stolons. They show occasional branching, with the presence of a septum approximately 17.71–23.53 μm in length below the apophysis. Collar appears around the base of each columella. Sporangiospores are short-cylindrical or cylindrical, and measure 2.15–2.35 μm wide × 3.54–4.48 long. Columellae are 10.90–16.96 μm × 11.46–18.89 μm diam., and typically globose with a collarette. Zygospores are not observed in this medium. Colonies exhibit slower growth on PDA than on SMA, attaining 39.5–41 mm in diam. after 4 days at 25 °C, which is about 15 mm higher than that attained in 3 days. The colonies exhibit slower growth on PDA than on SMA, attaining 39.5–41 mm in diam., and 1.5 mm in height after 4 days at 25 °C. At 25 °C, the colonies appear dark brown in the center, with a lighter margin. The colony reverse is white, with the wavy zonation much more pronounced than that shown by colonies grown on the SMA medium. Sporangiophores are 3.69–4.68 μm wide, erect, and arise as a single sporangiophore or in groups of 2–3 from the same location on the stolon. They exhibit occasional branching with a septum consistently present 14.38–19.80 μm below the apophysis. Sporangia measure 18.78–27.59 μm × 19.80–29.57 μm in diam., and are globose to slightly elliptical. Sporangiospores measure 1.73–1.98 μm wide × 2.07–4.28 μm long, and are cylindrical. Columellae measure 10.64–12.98 μm × 15.60–16.83 μm, and appear globose, with a small collarette. Chlamydospores and vesiculate sporangiophores were observed at 32 °C.
On SDA, Colonies grow more rapidly than on SMA and PDA, attaining 73–75 mm in diam. after 4 days at 25 °C. The initial color of the colonies at 25 °C is grayish white, which later changed to grayish brown. The colony reverse is grayish white, with wavy zonation. Sporangiophores are 2.17–4.28 μm wide, erect, and arise either singly or in groups of 2–4 sporangiophores from the same location on the stolon. They exhibit occasional branching, and always have a septum under the sporangium. Sporangia measure 24.97–42.21 μm × 23.24–35.94 μm in diam., and are globose to slightly elliptical in appearance. Sporangiospores are cylindrical and measure 2.0–2.57 μm wide × 3.25–4.15 μm long. No chlamydospores or zygospores are detected in this medium. Although the SDA medium exhibit good mycelial growth, a small number of sporangia are produced. A temperature from 27 °C to 32 °C is found to be optimal for the growth of Absidia, as shown in the colonies observed on PDA, SMA, and SDA. The fungus shows restricted growth at 37 °C.
Material examined: REPUBLIC OF KOREA, Division of Food Technology, Biotechnology & Agrochemistry, College of Agriculture & Life Sciences, Chonnam National University, Gwangju 61186, Korea, from soil in Dokdo island in the East Sea of Korea; KOSPFGC 1143 (ex-type) at Culture Collection of National Institute of Biological Resources (NIBR), Incheon, Korea, and isotype EML-IFS45-2 preserved as glycerol stock at −80 °C in the CNUFC.
The isolate was observed to grow over a wide range of temperatures with varying growth rates on PDA, SMA, and SDA. The average growth rates of EML-IFS45-1 on PDA, SMA, and SDA were 13 mm, 21 mm, and 27 mm per day, respectively. Optimal growth was observed at 27 °C, slow growth was observed at 18 °C, and extremely slow growth at 37 °C (Fig. 168).
NJ phylogenetic tree inferred from the individual datasets of rDNA ITS sequences from Gongronella koreana sp. nov. EML-TS2Bp and EML-TS2Bp-1 and related species. Sequences of Gongronella lacrispora was used as outgroups. Numbers at the nodes indicate the bootstrap values (>50 %) from 1000 replications. The bar indicates the number of substitutions per position
Maximum likelihood phylogenetic tree inferred from the combined dataset of 18S rDNA, 28S rDNA, elongation factor (EF-1α), and actin (Actin-1) gene sequences from Gongronella koreana sp. nov. EML-TS2Bp and EML-TS2Bp-1 and related species. Sequences of Umbelopsis nana and U. isabellina were used as outgroups. Numbers at the nodes indicate the bootstrap values (>50 %) from 1000 replications. The bar indicates the number of substitutions per position
-
248.
Gongronella koreana H.B. Lee & T.T.T. Nguyen, sp. nov.
MycoBank number: MB 811445; Faces of fungi number: FoF 01015; Fig. 169
Morphology of Gongronella koreana. a, d colony in potato dextrose agar. b, e colony in synthetic mucor agar c, f colony in malt extract agar after 7 days at 25 °C. a-c colony representing the obverse plate view. d-f colony representing the reverse plate view. g sporangiophore with short columella, apophysis, and collarette (yellow arrow). h, i simple sporangiophore with young sporangium. j development of sporangia and sporangial septum (red arrow). k mature sporangia and sporangiospores. Scale bars: g–j = 20 μm, k = 10 μm
Etymology: koreana. Referring to the country which from the species was first isolated (Korea, Jeonnam Province).
Holotype: EML-TS2Bp was maintained permanently in a metabolically inactive state at the Environmental Microbiology Laboratory Herbarium, Chonnam National University, Gwangju, Korea. CNUFC- EML-TS2Bp, as dried fungal mass from culture (PDA), August 2013, by H. B. Lee, in the Chonnam National University Fungal Collection (CNUFC), Gwangju, Korea.
Colonies exhibit slow growth on SMA, attaining 21–25 mm in diam. after 7 days at 25 °C. All colonies are cotton white in color. The colony reverse is also white with an irregular margin. Although the growth of mycelia on SMA is sparse, the sporulation observed is extensive. Sporangiophores grow to a width of 2.5–2.8 μm and a variable length surfacing directly from the aerial mycelia, erect, many-branched, and always displaying a septum clearly below the apophysis, separating the sporangium from the sporangiophore (Fig. 169g). Sporangia measure 12.3–15.5 × 12.4–15.6 μm in diam., and are globose, light yellow to chestnut yellow, and are multispored. The sporangial wall is thin and purple. Sporangiospores are released after the sporangial wall is deliquesced at maturity. The sporangiospores measure 1.7–2.1 × 2.1–2.8 μm, and are mostly bean-shaped. Columellae 1.2–2.3 × 2.6–3.3 μm in size, hemispherical with a collarette. Apophyses are 5.4–6.5 × 5.9–7.1 μm in size, and typically pyriform (Fig. 169g). Chlamydospores are present on the aerial mycelia. Zygospores are not observed. Rhizoids are not well developed.
On PDA, colonies grow more rapidly than on SMA, reaching 31.5–33 mm in diam. after 7 days at 25 °C. The initial color of the colonies at 25 °C is light white, which later turned to cotton white. The color of the colony reverse is light-colored to white. The center of the colony appeared moist. Sporangiophores are 2.8–3.0 μm wide, erect, but slightly curved inwardly, and arising from the aerial mycelia, either singly or with up to six branches. Sporangia measure 17.5–20.6 × 17.0–21.0 μm in diam., and are mostly globose and multispored. Sporangiospores are subglobose to ellipsoidal or bean-shaped, became hollow inwardly and measure 1.3–1.6 × 2.7–3.2 μm. Apophyses measure 8.7–10.0 × 5.7–8.2 μm, and display a subglobose to pyriform shape. Columellae are hemispherical and globose and measure 2.7–3.3 × 3.9–5 μm, and a large collarette is usually present. Mycelial development and chlamydospore formation is well defined on PDA medium. Zygospores are not observed on this medium.
Colonies on MEA attain a diameter of 27.0–28.5 mm. after 7 days at 25 °C. The color of the colonies is creamy-white. The colony reverse color is milky-white. Sporangiophores are 2.4–2.7 μm wide, erect, displaying either a single branch or 2–4 branches. Sporangia measure 13.2–15.6 × 13.7–16.0 μmindiam., and are typically globose. Sporangiospores measure 1.4–1.6 × 2.6–3.2 μm, and are subglobose to ellipsoidal, or bean-shaped. Apophyses are pyriform, and measure 4.4–7.5 × 5.4–8.1 μm. Zygospores are not observed on this medium. Mycelial development is notably better than that on SMA medium; however, a smaller number of sporangia are produced on MEA medium.
Material examined: REPUBLIC OF KOREA, Division of Food Technology, Biotechnology & Agrochemistry, College of Agriculture and Life Sciences, Chonnam National University, Gwangju 500–757, Korea, from forest soil in the Jeonnam Province in Korea; KOSPFGC 1268 (ex-type) at the Culture Collection of National Institute of Biological Resources (NIBR), Incheon,, Korea, and also additional ex-types EML-TS2Bp-1 to −3 preserved as glycerol stock at −80 °C in the CNUFC.
The isolate was observed to grow in a wide range of temperatures with varying growth rates on PDA, SMA, and MEA media. The average growth rates of the EML-TS2Bp isolate on PDA, SMA, and MEA were 5.5 mm, 3.5 mm, and 4.5 mm per day, respectively. The optimal growth temperature was found to be 27 °C, and slow growth was observed at 18 °C. No growth was observed at 37 °C.
Mortierellales
Mortierella is currently a member of the family Mortierellaceae, order Mortierellales, subphylum Mortierellomycotina (Hibbett et al. 2007; Hoffmann et al. 2011), now approximately 85 species (Kirk et al. 2008a, b).
They are ubiquitously saprotrophic or parasitic microfungi, usually can be isolated from soil, leaf letter or animal dung (Gams 1977; Hoffmann et al. 2011). Their colonies appear zonate and rosette-like, some species produce garlic-like odor. Young mycelia are coenocytic and produce irregular septa when age. Sporangiophores are simple or with various branches terminating with sporangia and swelling at the base. Sporangia are spherical, multi-, few- or uni–spored, forming only a rudimentary columella or lacking it altogether. Some species produce chlamydospores or stylospores. Zygospores are naked, or surrounded by a hyphal sheat and with unequal suspensors (Zycha et al. 1969; Gams 1977; Degawa and Seiji 1997; Weber and Webster 2007; Wagner et al. 2013). Identification is usually based on morphological characters. In a survey of Zygomycetes in Taiwan, soil and leaf letter specimens were collected form forests, national parks, roadsides and school campus. Among the Mortierella species collected, two undescribed species of Mortierella were isolated. Because they do not correspond to any described species, thus, are proposed here as two new species (Fig. 170).
NJ phylogenetic tree based on a MEGA 5.0 analysis (Tamura et al. 2011) using ITS rDNA sequences of Mortierella species with Umbelopsis isabellina as the out group taxon. The bootstrap values are based on 1500 replicates
-
249.
Mortierella pisiformis H.M. Ho, S.F. Wei & K. Voigt, sp. nov.
MycoBank: MB 811959; Facesoffungi number: FoF01016; Figs. 171 and 172
Mortierella pisiformis (TEFA5) a Photograph of fresh culture taken with a stereomicroscope; b–d, f, l, m; e, g, SEM. a Habit on CMA b Sporangiophore with branch c Columellae (arrow) and collar (arrow head) Spores e Spores f Chlamydospores g Chlamydospores. Scales bars: a = 300 μm, b = 100 μm, c, f = 20 μm, d, g = 10 μm, e = 5 μm
Etymology: From the Latin pisiformis, meaning pea-shaped, in reference to the shape of spores.
Holotype: TNMF 28604
Colonies grown on PDA at 25 °C for 7 days, reaching 9 cm in diameter, white, with abundant aerial hyphae, turf cottony. On CMA, aerial hyphae dispersed, creeping on the agar surface. Sporangiophores developing from creeping aerial hyphae, simple or with 1–2 branches arising from the lower portion near base, 643–874.5 μm long, 10–12.5 μm wide near base, tapering upward, becoming 2.5–5 μm wide near sporangium, basal rhizoid absent or with one simple rhizoid. Sporangia globose, multisporous, hyaline, 25.5–33 μm in diam., wall smooth, after deliquescence leaving indistinct columellae and trace of collar. Spores ellipsoid, pea-shaped, hyaline, surface smooth, 7.5–10 × 12.5–15 μm. Chlamydospores globose, 15–23 μm in diam., submerged in agar medium or on the agar surface, usually on short hyphae terminal, sometimes in chains. Zygospores not observed. Odour faint.
Material examined: TAIWAN, Taichung City, Dongshi Forest Garden, from leaf litter, December 2013, by S.F. Wei, TEFA4 (TNMF 28604, holotype), BCRC FU30283, living culture deposited at Jena Microbial Resource Collection (University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany) (JMRC: SF:012101).
Other specimen: Taichung City, Dongshi Dist., from leaf litter, Decembe 2013, S-F Wei. TEFA5 = BCRC FU30284
Notes: According to Gams (1977), this fungus belongs to Section Hygrophila, and is similar to M. Beljakovae Mil’ko, M. parazychae W. Gams and M. zychae Linnem. by having the following characters in common: multisporous sporangia; sporangiophores longer than 120 μm, basitonous ramification and with chlamydospores aggregated in rows or clusters. However, this fungus can be differentiated from the other three by spore shape, which is pea-shaped, ellipsoid in the former and is more or less globose in M. beljokovae, ellipsoid to cylindrical in the other two species. Molecular data revealed that our two isolates are clustered in on clade and can be separated from the other species of Mortierella in phylogenetic tree of ITS region. We thus identified this fungus as a new species.
-
250.
Mortierella formosana S.F. Wei, H.M. Ho & K. Voigt, sp. nov.
MycoBank: MB812482; Facesoffungi number: FoF01017; Figs. 173 and 174
Mortierella formosana (PUSTF9A) a Photograph of fresh culture taken with a stereomicroscope; b, c, e, LM; d, f, SEM a Habit on CMA b Sporangiophore with branch c Rudimentary columella (arrow) d Columella (arrow) and collar (arrow head) e spores f spores. Scales bars: b, c = 20 μm, e = 10 μm, d, f = 5 μm
Etymology: Referring to Taiwan where the fungus was collected.
Holotype: TNMF 28605
Colonies growing on PDA at 25 °C for 7 days reaching 9 cm in diam., white, zonnate, rosette, with abundant aerial hyphae. Sporangiophores on CMA, vertically arising from aerial hyphae, numerously along the sporangiophores, usually simple, sometimes producing a branch from the upper portion of sporangiophore near the middle, (57-)106-116(−227.5) μm long, 4–5 μm wide near base, tapering upward reaching 2.5–4 μm wide near sporangium, basal rhizoid indistinct. Sporangia globose, multisporous, hyaline, 11.5–25.5 μm in diam., wall smooth, after deliquescence, leaving collar and in distinct columella. Spores globose, wall smooth, hyaline, 6.5–7.5 μm in diam. Chlamydospores and zygospores not observed. Odour faint.
Material examined: TAIWAN, Pingtung County, Pingtung University of Science and Technology, from soil, July 2014, by S.F. Wei, PUSTF9A (TNMF 28605, holotype), BCRC FU30355, living culture deposited at Jena Microbial Resource Collection (University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany) (JMRC:SF:012102).
Other specimen: National Pingtung University of Science and Technology, PingtungCounty, from soil, July 2014, by S-F Wei, PUSTF9C.
Notes: According to Gams (1977), this fungus belongs to Section Mortierella, and similar to M. polycephala Coemans by having the following characters in common: multisporous sporangia; sporangiophores having branches arising above the middle of the sporangiophore and with smooth walled spores. However, they can be differentiated by the spore characters which are globose and 6.5–7.5 μm in diam. in our isolates and globose or oval and 10–12 μm in the latter. Molecular data revealed that our two isolates are clustered in one clad and can be separated from the other species of Mortierella in phylogenetic tree based on ITS sequenced data. We thus identified this fungus as a new species.
Contributions to Neocallimastigomycota
Neocallimastigales
Anaerobic fungi were formerly classified among the chytridiomycete fungi (Li et al. 1990), later designated to their own phylum Neocallimastigomycota (Hibbett et al. 2007) and subsequently summarized as Chytridiomycota for flagellate fungi (Voigt 2012). Since their recognition as Fungi by Orpin (1974), eight genera of obligately anaerobic fungi, currently classified in the class Neocallimastigomycetes are described (Barr et al. 1989; Breton et al. 1990; Callaghan et al. 2015; Dagar et al. 2015; Gold et al. 1988; Griffith et al. 2010; Gruninger et al. 2014; Orpin 1975; Ozkose et al. 2001).
The first of these was the genus Neocallimastix, originally named as a flagellate protozoan Callimastix frontalis by Braune (1913), renamed by Vavra and Joyon (1966) as Neocallimastix frontalis, and eventually recongised as a fungus by Orpin (1975). It was formally named by Heath et al. (1983) as Neocallimastix frontalis, within the new family Neocallimastigaceae within the chytrid order Spizellomycetales (Fig. 175).
Within genus Neocallimastix, three other species have since been named: N. patriciarum (Orpin and Munn 1986), N. hurleyensis (Webb and Theodorou 1991) and N. variabilis (Ho et al. 1993). However, Wubah et al. (1991) undertook a direct comparison of the type cultures of N. frontalis and N. patriciarum and found them to be morphologically and culturally indistinguishable. Ho and Barr (1995) later synonymised these two species and also N. variabilis as N. frontalis, and also cast doubt on the distinctiveness of N. hurleyensis. The morphological variability of these fungi in culture and the paucity of morphological traits (Gruninger et al. 2014) makes it difficult to assess the validity of this decision. Type material exists only for Orpin’s N. particiarum (IMI 295997 at RBG Kew) and also for N. hurleyensis (IMI 344175 at RGB Kew) and isolate CX, Orpin’s original culture from which the N. patriciarum type material was derived also remains viable, having been cryopreserved in liquid nitrogen at the Rowett Institute (Aberdeen, Scotland).
The genus Piromyces was first observed as flagellate zoospores in rumen fluid by Liebetanz in (1910) and can probably be assumed as ‘the oldest’ known genus of anaerobic fungi, even if at that time it was described as a protozoan: Piromonas communis. Orpin (1977) much later adopted this name for his isolate correctly characterized as a fungus, which was the reason to rename this organism as Piromyces (Gold et al. 1988). The emended description of Piromyces communis was published by Barr et al. (1989) and currently seven other species have been described in the genus including P. mae (Li et al. 1990), P. dumbonica (Li et al. 1990), P. rhizinflata (Breton et al. 1991), P. spiralis (Ho et al. 1993c), P. minutus (Ho et al. 1993b), P. citronii (Gaillard-Martinie et al. 1995), P. polycephalus (Chen and Hseu 2002) and P. cryptodigmaticus (Kirk 2012). This genus thus covers the highest number of species and represents also the most studied anaerobic fungus, especially from the enzymological point of view (Harhangi et al. 2002, 2003a, b, c; Steenbakkers et al. 2001, 2002a, b, 2003, 2008). The recent pyrosequencing analysis of feces samples from 30 animal species has moreover revealed the Piromyces as the most abundant genus being encountered in 28 different animals and representing 36 % of all obtained sequences (Liggenstoffer et al. 2010). Comparing with other anaerobic fungi this genus is also the most heterogenous with some of its member grouping close to Neocallimastix strains and next to Orpinomyces strains (Hausner et al. 2000; Fliegerová et al. 2004) and therefore sometimes wrongly classified (Fliegerová et al. 2010). This heterogenity however can indicate the presence of new hidden Piromyces species.
As part of collaborative, to achieve a stable taxonomy and to name the novel clades that exist within this phylum, we and others (Griffith et al. 2010) are in the process of collecting and distributing reference materials and cultures to diverse laboratories engaged in research on these fungi. Here we provide DNA barcode data for Orpin’s isolate CX (GenBank ID: KR920744) and also provide a description for a second species, Neocallimastix cameroonii, which forms a distinct clade within the genus Neocallimastix based on morphological examination (Fig. 176) and phylogenetic analysis of 28S rRNA gene sequence (Fig. 175). No ITS sequence data is provided since within anaerobic fungi there is substantial (up to 13 %) intragenomic variation (Callaghan et al. 2015), which complicates direct sequencing of PCR products and the unambiguous assignment of reference sequences.
Anaerobic fungi were isolated from the faeces of Cameroon sheep (a rare breed of domesticated sheep originally from West Africa), which are kept at Wildpark Poing, Munich, Germany (48.17 N, 11.83 W) and fed on a mixture of grass forage and silage. Faeces were collected on 15th September 2013 and stored frozen during transfer to Aberystwyth, where isolation was undertaken using the methods described by Callaghan et al. (2015). Individual thalli were excised from roll tubes. Three cultures (CaDo3a, CaDo3b, CaDo3d) were isolated into axenic culture with wheat straw as carbon source, and later cryopreserved in liquid nitrogen.
Thalli of CaDo3a were consistently monocentric (Fig. 176c, f), with rhizoids radiating from a single sporangium. Mature sporangia were mostly ovoid to spherical (30–50 μm diameter). Zoospores (6–9 μm diameter) were observed in most cultures after 3d growth on wheatstraw at 39 °C (Fig. 176c) and these consistently bore multiple (9–15) flagella (Fig. 176a, b) 15–25 μm long. The rhizoidal system emanating from the base of the sporangium was often observed to be distinctly bifurcated (Fig. 176d, f).
-
251.
Neocallimastix cameroonii G.W. Griff., Dollhofer, Veronika & T. Callaghan sp. nov.
Index Fungorum number: IF551212; Facesoffungi number: FoF01018; Fig. 176
Etymology: In reference to the breed of sheep from which it was isolated.
Holotype: ABS CaDo3a
An obligately anaerobic fungus isolated from the faeces of Cameroon sheep (Ovis aries) kept at Wildpark Poing (Germany). The fungus forms a determinate monocentric thallus and spherical to ovoid sporangia. The rhizoidal system emanating from the base of the sporangium is often bifurcated at base of sporangium. Sporangia ovoid to spherical (30–50 μm), non-papillate. Zoospores formed abundantly, spherical (6–9 μm diam.) with multiple (9–15) flagella 15–25 μm long.
Material examined: The reference culture is lodged in biorepositories at: Aberystwyth University (ABS CaDo3a, holotype); Royal Botanic Gardens, Kew, London (K(M), isotype); and University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany (Jena Microbial Resource Collection JMRC:SF:012157, isotype).
The D1/D2 region of the 28S large ribosomal subunit (LSU) was amplified using the primers NL1 (GCATATCAATAAGCGGAGGAAAAG) and NL4 (GGTCCGTGTTTCAAGACGG; ca. 750 bp amplicon), as described by Dagar et al. (2011). The sequences for all three isolates was identical and that for isolate CaDo3a is deposited at GenBank (ID: KR920745). Phylogenetic reconstruction of the two genera of anaerobic fungi with multiflagellate zoospores (Neocallimastix and Orpinomyces) with the reference isolate for the genus Anaeromyces as outgroup recovered CaDo3a and several unpublished sequences from India in a distinct clade with high bootstrap support (Fig. 177).
Phylogenetic analyses based on maximum likelihood statistics, Tamura-Nei substitution model and nearest–neighbor Interchange (NNI) ML heuristic method using MEGA 5.2 (Tamura et al. 2011). The bootstrap values are based on 1000 replicates
-
252.
Piromyces irregularis Fliegerová, K. Voigt & P.M. Kirk, sp. nov.
Index Fungorum number: IF550065; Facesoffungi number: FoF01019; Fig. 178
Morphology of Piromyces irregularis (KF9) a Globose zoospores released from matured sporangium (lugol stained culture, 1000×) b Biflagellate lamp-bulb zoospore (DAPI stained culture, 1000×) c Multiflagellate ovoid zoospore (DAPI stained culture, 1000×) d Sporangia of variable shapes (lugol stained culture, 200×) e Irregular horned sporangium (lugol stained culture, 400×) f Irregular branched sporangium (lugol stained culture, 400×) g Nuclei in sporangium (left) and in apex of branched sporangial stalk (right) (DAPI stained culture, 400×) h Nuclei present in sporangium and sporangiophore (DAPI stained culture, 400×) i Nuclei in sporangium separated by septum from sporangiophore (DAPI stained culture, 400×)
Etymology: Referring to irregularily shaped sporangia.
Holotype: JMRC SF:011205
Colonies tiny (Ø 3 mm) with irregular edges on agar plates and fragile mycelium in liquid M10 medium (Caldwell and Bryant 1966) enriched by 25 % (V/V) rumen fluid with either glucose or cellobiose (both 4 g/l) as carbon source. Thallus with highly branched rhizoidal system without evidence of a subsporangial swelling formed by fine rhizoids with a multitude of tiny lateral stolons. Sporangiophores of varying length and shape, from short to long, narrow to very wide, simple, as well as flexuous or branched. . Sporangia consistently produced, highly variable in both shape and size, globose, ellipsoidal, elongated, pestle-shaped, triangular, pyriform or rounded rectangular, often also bifurcated, horned and with irregular shape, both open to the sporangiophore or with a basal septum, nuclei present both in the sporangium and the sporangiophore. Zoospores when freshly released from sporangium globose, free floating zoospores ovoid to somewhat lamp-bulb shaped, mononucleate, with variable flagellation, ranging from uniflagellate to quadriflagellate or multiflagellateMaterial examined: CZECH REPUBLIC, Institute of Animal Physiology and Genetics, Czech Academy of Sciences, v.v.i., Videnska 1083, 14220 Prague, Czech Republic, from rumen fluid of slaughtered cow, July 2009, by K. Fliegerova, KF 9 (holotype Jena Microbial Resource Collection (University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany) JMRC SF:011205).
Nuclei may remain in the zoospore cyst, which has developed into single sporangium and sporangiophore; rhizoids are anucleate. The accompanying illustrations display also a more complex development when some sporangiophores are not terminated by sporangia, they do, however, contain nuclei. Both types of development occurs together in one culture. Barr et al. (1989) described this as two types of thallus development, type I as endogenous and monocentric, type II as exogenous and monocentric. However, the rhizoid system is always in both cases anucleate and in our opinion, therefore, it represents only the endogenous and monocentric development.
Notes: The species thus exhibits all types of morphological variations described by Barr et al. (1989) for P. communis, however analysis of ITS1-5.8S-ITS2 rDNA revealed sequence divergence sufficient to justify the description of a new species of Piromyces (Fig. 177). The new species introduces a new character: zoospores of all previously described species of Piromyces are uni- or bi-flagellate, and when polyflagellate, there are no more then 4 flagella.
References
Abbott SP, Currah RS (1997) The Hevellaceae: systematic revision and occurrence in northern and northwestern North America. Mycotaxon 62:1–125
Abdel-Aziz FA, Abdel-Wahab MA (2010) Lolia aquaticagen. et sp. nov. (Lindgomycetaceae, Pleosporales), a new coelomycete from freshwater habitats in Egypt. Mycotaxon 114(1):33–42
Abdel-Wahab MA, Pang KL, Nagahama T, Abdel-Aziz FA, Jones EBG (2010) Phylogenetic evaluation of anamorphic species of Cirrenalia and Cumulopsora with the description of eight new genera and four new species. Mycol Prog 9:537–558
Abdel-Wahab MA, Abdel-Aziz FA, Mohamed SS, Abdel-Aziz AE (2011) Annulatascus nilensis sp. nov., a new freshwaterascomycete from the River Nile, Egypt. IMA Fungus 2:1–6
Adamčík S, Buyck B (2012) Type-studies in American Russula (Russulales, Basidio-mycota): in and out subsection Roseinae. Nova Hedwigia 94(3–4):413–428
Adams GC, Surve-Iyer RS, Iezzoni AF (2002) Ribosomal DNA sequence divergence and group I introns within the Leucostoma species L. cinctum, L. persoonii, and L. parapersoonii sp. nov., ascomycetes that cause Cytospora canker of fruit trees. Mycologia 94:947–967
Adams GC, Wingfield MJ, Common R, Roux J (2005) Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Stud Mycol 52:1–144
Adams GC, Roux J, Wingfield MJ (2006) Cytospora species (Ascomycota, Diaporthales, Valsaceae): introduced and native pathogens of trees in South Africa. Australas Plant Pathol 35:521–548
Aime MC, Largent DL, Henkel TW, Baroni TJ (2010) The Entolomataceae of the Pakaraima Mountains of Guyana IV: new species of Calliderma, Paraeccilia and Trichopilus. Mycologia 102:633–649
Alvarado P, Manjón JL, Matheny PB, Esteve-Raventós F (2010) Tubariomyces, a new genus of Inocybaceae from the Mediterra- nean region. Mycologia 102:1389–1397
Angeles Vinuesa MDL, Sanches-Puelles JS, Tibell L (2001) Intraspecific variation in Mycocalicium subtile (Mycocaliciaceae) elucidated by morphology and the sequences of the ITS1-5.8SITS2 region of rDNA. Mycol Res 105(3):323–330
Ariyawansa HA, Camporesi E, Thambugala KM, Mapook A, Kang JC, Alias SA, Chukeatirote E, Thines M, Mckenzie EHC, Hyde KD (2014a) Confusion surrounding Didymosphaeria — phylogenetic and morphological evidence suggest Didymosphaeriaceae is not a distinct family. Phytotaxa 176(1):102–119
Ariyawansa HA, Tanaka K, Thambugala KM, Phookamsak R, Tian Q, Camporesi E, Hongsanan S, Monkai J, Wanasinghe DN, Chukeatirote E, Kang JC, Xu JC, McKenzie EHC, Jones EBG, Hyde KD (2014b) A molecular phylogenetic reappraisal of the Didymosphaeriaceae (= Montagnulaceae). Fungal Divers 68:69–104
Ariyawansa HA, Hawksworth DL, Hyde KD, Jones EBG, Maharachchikumbura SSN, Manamgoda DS, Thambugala KM, Udayanga D, Camporesi E, Daranagama A, Jayawardena R, Liu JK, McKenzie EHC, Phookamsak R, Senanayake IC, Shivas RG, Tian Q, Xu JC (2014c) Epitypification and neotypification: guidelines with appropriate and inappropriate examples. Fungal Divers 69(1):57–91
Ariyawansa HA, Thambugala KM, Manamgoda DS, Jayawardena R, Camporesi E, Boonmee S, Hyde KD (2015) Towards a natural classification and backbone tree for Pleosporaceae. Fungal Divers 71(1):85–139
Aveskamp MM, de Gruyter J, Woudenberg JHC, Verkley GJM, Crous PW (2010) Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol 65:1–60
Baddley JW, Mostert L, Summerbell RC, Moser SA (2006) Phaeoacremonium parasiticum Infections Confirmed by β-Tubulin Sequence Analysis of Case Isolates. Clin Microbiol 44(6):2207–2211
Barber PA, Crous PW, Groenewald JZ, Pascoe IG, Keane P (2011) Reassessing Vermisporium (Amphisphaeriaceae), a genus of foliar pathogens of eucalypts. Persoonia 27:90–118
Baroni TJ, Cantrell SA, Perdomo-Sánchez OP, Lodge DJ (2008) New species of Pouzarella (Entolomataceae, Agaricales) from the Dominican Republic and Jamaica. N Am Fungi 3:241–260
Baroni TJ, Hofstetter V, Largent DL, Vilgalys R (2011) Entocybe is proposed as a new genus in the Entolomataceae (Agaricomycetes, Basidiomycota) based on morphological and molecular evidence. N Am Fungi 6(12):1–19
Barr ME (1978) The Diaporthales in North America with emphasis on Gnomonia and its segregates. Mycol Mem 7:1–232
Barr ME (1979) A classification of Loculoascomycetes. Mycologia 71:935–957
Barr ME (1983) Muriform ascospores in class Ascomycetes. Mycotaxon 18:149–157
Barr ME (1984) Herpotrichia and its segregates. Mycotaxon 20:1–38
Barr ME (1987) Prodromus to class loculoascomycetes. University of Massachusetts, Amherst
Barr ME (1992a) Additions to and notes on the Phaeosphaeriaceae (Pleosporales, Loculoascomycetes). Mycotaxon 43:371–400
Barr ME (1992b) Notes on the Lophiostomataceae (Pleosporales). Mycotaxon 45:191–221
Barr ME (1996) Planistromellaceae, a new family in the Dothideales. Mycotaxon 60:433–442
Barr ME, Huhndorf SM (2001) Loculoascomycetes. In: Esser K, Lemke PA (eds) The Mycota VII systematics and evolution part A. Springer Press, London, pp 283–305
Barr DJS, Kudo H, Jakober KD, Cheng KJ (1989) Morphology and development of rumen fungi: Neocallimastix sp., Piromyces communis, and Orpinomyces bovis gen. nov., sp. nov. Can J Bot 67:2815–2824
Bas C (1969) Morphology and subdivision of Amanita and a monograph of its section Lepidella. Persoonia 5:285–579
Bas C (2000) A broader view on Amanita. Boll Gruppo Micol G Bresadota 43:9–12
Beeli M (1928) Contribution à l’étude de la Flore Mycologique du Congo. Fungi Goossensiani, VI. Bull Soc R Bot Belg 61:78–100
Benny GL (2009) Zygomycetes. Published on the internet at www.zygomycetes.org. Accessed 17 July 2010
Berkeley MJ (1874) Notices of North American fungi. Grevillea 3(26):49–64
Berkeley MJ, Curtis MA (1874) Myxosporium nitidum. Grevillea 3:13
Bi ZS, Li TH (1986) A preliminary note on Russula species from Guangdong, with a new species and a new variety. Guihaia 6(3):193–199 (in Chinese)
Binder M, Hibbett DS (2006) Molecular systematics and biological diversification of Boletales. Mycologia 98(6):971–981
Binder M, Justo A, Riley R, Salamov A, Lopez-Giraldez F, Sjökvist E, Copeland A, Foster B, Sun H, Larsson E, Larsson KH, Townsend J, Grigoriev IV, Hibbett DS (2013) Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 105(6):1350–1373
Boekhout T, Fonseca A, Sampaio JP, Bandoni RJ, Kwon-Chung KJ (2011) Discussion of teleomorphic and anamorphic basidiomycetous yeasts. In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts: a taxonomic study. Elsevier, London, pp 1339–1372
Boidin J, Gilles G (1980) Contribution à l'étude des genres Dendrocorticium, Dendrodontia et Dentocorticium (Basidiomycotina) = Contribution to the study of Dendrocorticium, Dendrodontia et Dentocorticium (Basidiomycotina). Elsevier, Paris, FRANCE Cryptogamie. Mycol 19(3):181–202
Boidin J, Lanquetin P (1983) Basidiomycetes Aphyllophorales epitheloides etales. Mycotaxon 16:461-499
Boise J (1985) An amended description of Trematosphaeria. Mycologia 77(2):230–237
Bon M (1997) Clé monographique du genre Inocybe (Fr.)Fr. Documents Mycologiques 27(105):1–51
Boonmee S, Ko-Ko TW, Chukeatirote E, Hyde KD, Chen H, Cai L, McKenzie EHC, Jones EBG, Kodsueb R, Hassan BA (2012) Two new Kirschsteiniothelia species with Dendryphiopsis anamorphs cluster in Kirschsteiniotheliaceae fam. nov. Mycologia 104:698–714
Braune R (1913) Untersuchungen über die im Wiederkäuermagen vorkommenden Protozoen. Arch Protistenkd 32:111–170
Breton A, Bernalier A, Dusser M, Fonty G, Gaillard-Martinie B, Guillot J (1990) Anaeromyces mucronatus nov. gen., nov. sp. A new strictly anaerobic rumen fungus with polycentric thallus. FEMS Microbiol Lett 58:177–182
Breton A, Dusser M, Gaillard-Martinie B, Guillot J, Millet L, Prensier G (1991) Piromyces rhizinflata nov. sp., a strictly anaerobic fungus from feces of the saharan as–a morphological, metabolic and ultrastructural study. FEMS Microbiol Lett 82:1–8
Buyck B (1994) Ubwoba: les champignons comestibles de l’ouest du Burundi, vol 34, Adm Gén Coop Dév. Publ Agric, Bruxelles, pp 1–123
Buyck B (2008) Wild edible mushrooms in Madagascar: an evolving enigma. Econ Bot 62:509–520
Buyck B (2012) Observations on some enigmatic Cantharellus (Cantharellales, Basidiomycota) with lilac-violaceous tints from Africa and Madagascar. Cryptogam Mycol 33(2):167–179
Buyck B (2014) Exploring the diversity of “smooth chanterelles” (Cantharellus, Cantharellales). Cryptogam Mycol 35:23–40
Buyck B, Randrianjohany E (2013) Cantharellus eyssartieri sp.nov. (Cantharellales, Basidiomycota) from monospecific Uapaca ferruginea stands near Ranomafana (eastern escarpment, Madagascar). Cryptogam Mycol 34(4):29–34
Buyck B, Eyssartier G, Kivaisi A (2000) Addition to the inventory of the genus Cantharellus (Basidiomycotina, Cantharellaceae) in Tanzania. Nova Hedwigia 71(3/4):491–502
Buyck B, Kauff F, Eyssartier G, Couloux A, Hofstetter V (2014) A multigene world phylogeny for Cantharellus. Fungal Divers 64:101–121
Buyck B, Kauff F, Randrianjohany E, Hofstetter V (2015) Sequence data reveal a high diversity of Cantharellus associated with endemic vegetation in Madagascar. Fungal Divers 70(1):189–208
Cáceres MES, Aptroot A, Parnmen S, Lücking R (2014) Remarkable diversity of the lichen family Graphidaceae in the Amazon rain forest of Rondônia, Brazil. Phytotaxa 189:87–136
Cai L, Hyde KD (2007) Ascorhombisporaaquatica gen. et sp. nov. from a freshwater habitat in China, and its phylogenetic placement based on molecular data. Cryptogam Mycol 28(4):291–300
Cai L, Zhang KQ, McKenzie EHC, Hyde KD (2003) Freshwater fungi from bamboo and wood submerged in the Liput River in the Philippines. Fungal Divers 13:1–12
Caldwell DR, Bryant MP (1966) Medium without rumen fluid for non-selective enumeration and isolation of rumen bacteria. Appl Microbiol 14:794–801
Callaghan TM, Podmirseg SM, Hohlweck D, Edwards JE, Puniya AK, Dagar SS, Griffith GW (2015) Buwchfawromyces eastonii gen. nov., sp. nov.: a new anaerobic fungus (Neocallimastigomycota) isolated from buffalo faeces. MycoKeys 9:11–28
Callan BE, Rogers JD (1986) Cultural characters and anamorphs of Biscogniauxia (=Nummularia) marginata, B. dennisii, and B. repanda. Can J Bot 64:842–847
Campbell J, Shearer CA (2001) Annulatascaceae pruned. In: Abstracts, annual meeting of Mycological Society ofAmerica (MSA): joint meeting with APS and SON, Salt Lake City, Utah, 25–29 Aug. S105 p
Campbell J, Shearer CA (2004) Annulusmagnus and Ascitendus, two new genera in the Annulatascaceae. Mycologia 96:822–833
Castlebury LA, Rossman AY, Jaklitsch WJ, Vasilyeva LN (2002) A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94:1017–1031
Cesati V, De Notaris G (1863) Schema di classificazione degle sferiacei italici aschigeri piu’ o meno appartenenti al genere Sphaeria nell’antico significato attribuitoglide Persono. Comment Soc Crittog Ital 1(4):177–420
Challen MP, Kerrigan RW, Callac P (2003) A phylogenetic reconstruction and emendation of Agaricus section Duploannulatae. Mycologia 95:61–73
Chen CJ (1998) Morphological and molecular studies in the genus Tremella. Bibl Mycol 174:1–225
Chen YC, Hseu RS (2002) Piromyces polycephalus (Neocallimastigaceae), a new rumen fungus. Nova Hedwigia 75:409–414
Chen CY, Hsieh WH (2004) Astrosphaeriella from Taiwan, including two new species. Bot Bull Acad Sin 45:171–178
Chen ZH, Zhang P, Zhang ZG (2013) Investigation and analysis of 102 mushroom poisoning cases in southern China from 1994 to 2012. Fungal Divers 64(1):123–131
Chen J, Zhao RL, Parra LA, Guelly AK, Kesel AD, Rapior S, Hyde KD, Chukeatirote E, Callac P (2015) Agaricus section Brunneopicti: a phylogenetic reconstruction with descriptions of four new taxa. Phytotaxa 192:145–168
Chiu WF (1945) The Russulaceae of Yunnan. Lloydia 8:31–59
Clémençon H, Emmett V, Emmett E (2004) Cytology and plectology of the Hymenomycetes. Bibl Mycol 199. Cramer, Germany 199 viii+488 pp
Clements FE, Shear CL (1931) Genera of fungi, 2nd edn. H.W. Wilson Company, New York
Co-David D, Langeveld D, Noordeloos ME (2009) Molecular phylogeny and spore evolution of Entolomataceae. Persoonia 23:147–176
Cooke MC, Plowright CB (1879) British Sphaeriacei. Grevillea 7:77–89
Corbaz R (1957) Recherches sur le genre Didymella Sacc. Phytopathol Z 28(4):375–414
Corner EJH, Bas C (1962) The genus Amanita in Singapore and Malaya. Persoonia 2(3):241–304
Cripps C, Larsson E, Horak E (2010) Subgenus Mallocybe (Inocybe) in the Rocky Mountain alpine zone with molecular reference to European Arctic-alpine material. N Am Fungi 5(5):97–126
Crivelli PG (1983) Über die heterogene Ascomycetengattung Pleospora Rabh.: vorschlag für eine Aufteilung. Dissertation ETH Nr. 7318, Zürich, Germany
Crous PW, Gams W, Wingfield MJ, Van Wyk PS (1996) Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia 88(5):786–796
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Phillips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253
Crous PW, Wingfield MJ, Schumacher RK, Summerell BA, Giraldo A, Gené J, Guarro J, Wanasinghe DN, Hyde KD, Camporesi E (2014) Fungal Planet description sheets: 281–319. Persoonia 33(1):212–289
Crous PW, Carris LM, Giraldo A, Groenewald JZ, Hawksworth DL, Hernández-Restrepo M, Jaklitsch WM, Lebrun M-H, Schumacher RK, Stielow B, van der Linde EJ, Vilcāne J, Voglmayr H, Wood AR (2015a) The Genera of Fungi - fixing the application of the type species of generic names–G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus 6(1):163–198
Crous PW, Wingfield MJ, Guarro J, Hernández-Restrepo M, Sutton DA, Acharya K, Barber PA, Boekhout T, Dimitrov RA, Dueñas M, Dutta AK, Gené J, Gouliamova DE, Groenewald M, Lombard L, Morozova OV, Sarkar J, Smith MT, Stchigel AM, Wiederhold NP, Alexandrova AV, Antelmi I, Armengol J, Barnes I, Cano-Lira JF, Castañeda Ruiz RF, Contu M, Courtecuisse PR, da Silveira AL, Decock CA, de Goes A, Edathodu J, Ercole E, Firmino AC, Fourie A, Fournier J, Furtado EL, Geering ADW, Gershenzon J, Giraldo A, Gramaje D, Hammerbacher A, He X-L, Haryadi D, Khemmuk W, Kovalenko AE, Krawczynski R, Laich F, Lechat C, Lopes UP, Madrid H, Malysheva EF, Marin-Felix Y, Martin MP, Mostert L, Nigro F, Pereira OL, Picillo B, Pinho DB, Popov ES, Rodas Peláez CA, Rooney-Latham S, Sandoval-Denis M, Shivas RG, Silva V, Stoilova-Disheva MM, Telleria MT, Ullah C, Unsicker SB, van der Merwe NA, Vizzini A, Wagner H-G, Wong PTW, Wood AR, Groenewald JZ (2015b) Fungal planet Description Sheets: 320–370. Persoonia 34:167–266
da Cunha CK (2014) Pithomyces species (Montagnulaceae) from clinical specimens: identification and antifungal susceptibility profiles. Med Mycol 52:748–757
Dagar SS, Kumar S, Mudgil P, Singh R, Puniya AK (2011) D1/D2 domain of large-subunit ribosomal DNA for differentiation of Orpinomyces spp. Appl Environ Microbiol 77:6722–6725
Dagar SS, Kumar S, Griffith GW, Edwards JE, Callaghan TM, Singh R, Nagpal AK, Puniya AK (2015) A new anaerobic fungus (Oontomyces anksri gen. nov., sp. nov.) from the digestive tract of the Indian camel (Camelus dromedarius). Fungal Biol 119(8):731–737
Dai YC (2010) Hymenochaetaceae (Basidiomycota) in China. Fungal Divers 45:131–343
Dai YC (2011) A revised checklist of corticioid and hydnoid fungi in China for 2010. Mycoscience 52:69–79
Dai YC (2012) Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 53:49–80
Dai YC, Cui BK, Yuan HS (2007a) Notes on polypores from Gansu and Qinghai Province, Northwest China. Cryptogam Mycol 28:177–187
Dai YC, Cui BK, Yuan HS, Li BD (2007b) Pathogenic wood-decaying fungi in China. For Pathol 37:105–120
Dai YC, Yang ZL, Cui BK, Yu CJ, Zhou LW (2009) Species diversity and utilization of medicinal mushrooms and fungi in China (Review). Int J Med Mushrooms 11:287–302
Dal-Forno M, Lawrey JD, Sikaroodi M, Bhattarai S, Gillevet PM, Sulzbacher M, Lücking R (2013) Starting from scratch, evolution of the lichen thallus in the basidiolichen Dictyonema (Agaricales, Hygrophoraceae). Fungal Biol 117:584–598
Damm U, Mostert L, Crous PW, Fourie PH (2008) Novel Phaeoacremonium species associated with necrotic wood of Prunus trees. Persoonia 20:87–102
Damm U, Woudenberg JHC, Cannon, PF, Crous PW (2009) Colletotrichum species with curved conidia from herbaceous hosts. Fungal Diversity 39:45–87
Day MJ, Gibas CFEC, Fujimura KE, Egger KN, Currah RS (2006) Monodictys arctica, a new hyphomycetes from the roots of Saxifraga oppositifolia collected in the Canadian High Arctic. Mycotaxon 98:261–272
De Gruyter JD, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW (2009) Molecular phylogeny of Phoma and allied anamorph genera: Towards a reclassification of the Phoma complex. Mycol Res 113:508–519
De Gruyter JD, Woudenberg JHC, Aveskamp AA, Verkley GJM, Groenewald JZ, Crous PW (2012) Redisposition of Phoma-like anamorphs in Pleosporales. Stud Mycol 75:1–36
De Hoog GS (1972) The genera Beauveria, Isaria, Tritirachium, and Acrodontium, gen. nov. Stud Mycol 1:1–41
Degawa Y, Seiji T (1997) Zygospore formation in Mortierella capitata. Mycoscience 38(4):387–394
Didukh M, Vilgalys R, Wasser SP, Isikhuemhen OS, Nevo E (2005) Notes on Agaricus section Duploannulati using molecular and morphological data. Mycol Res 109:729–740
Diederich P (1996) The lichenicolous heterobasidiomycetes. Bibl Lichenol 61:1–198
Dingley JM (1962) Pithomyces chartarum, its occurrence morphology, and taxonomy. N Z J Agric Res 1–2(5):49–61
Dissing H (1966) The genus Helvella in Europe, with special emphasis on the species found in Norden. Dansk Bot Ark 25:1–172
Doidge EM (1942) South African Microthyriaceae. Bothalia 4:273–344
Doilom M, Liu JK, Jaklitsch WM, Ariyawansa H, Wijayawardene NN, Chukeatirote E, Zhang M, Roux J, McKenzie EHC, Geml J, Voglmayr H, Hyde KD (2013) An outline of the family Cucurbitariaceae. Sydowia 65(1):167–192
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214
Eberhardt U (2002) Molecular kinship analyses of the agaricoid Russulaceae: correspondence with mycorrhizal anatomy and sporocarp features in the genus Russula. Mycol Prog 1(2):201–223
Ellis MB (1960) Dematiaceous hyphomycetes I. Mycol Pap 76:1–36
Ellis MB (1971) Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew 44–45
Ellis JB, Everhart BM (1892) The North American Pyrenomycetes. Newfield, N.J. :Ellis & Everhart, pp 209–210
Eriksson OE (1981) The families of bitunicate ascomycetes. Opera Bot 60:1–220
Eriksson OE, Yue J-z (1990) Notes on bambusicolous pyrenomycetes. Mycotaxon 38(1–10):201–220
Ertz D, Diederich P, Lawrey JD, Berger F, Freebury CE, Coppins B et al. (2015) Phylogenetic insights resolve Dacampiaceae (Pleosporales) as polyphyletic: Didymocyrtis (Pleosporales, Phaeosphaeriaceae) with Phoma-like anamorphs resurrected and segregated from Polycoccum (Trypetheliales, Polycoccaceae fam. nov.). Fungal Divers 1–37. doi:10.1007/s13225-015-0345-6
Esfandiari E (1947) Beiträge zur Iranischen Pilzflora. Sydowia 1(4–6):161–168
Eyssartier G (2001) Vers une monographie du genre Cantharellus Adans. :Fr. [doctoral dissertation]. Paris, National History Museum p 259
Fan X, Liang YM, Ma R, Tian CM (2014) Morphological and phylogenetic studies of Cytospora (Valsaceae, Diaporthales) isolates from Chinese scholar tree, with description of a new species. Mycoscience 55:252–259
Fan X, Hyde KD, Liu M, Liang Y, Tian C (2015) Cytospora species associated with walnut canker disease in China, with description of a new species C. gigalocus. Fungal Biol 119:310–319
Farr DF, Rossman AY (2015) Fungal Databases, Systematic Mycology and Microbiology Laboratory, ARS, USDA. Retrieved January 11, 2015, from http://nt.ars-grin.gov/fungaldatabases/
Fatto RM (1998) Notes on four little red Russulas. Mycotaxon 68:193–204
Favre J (1955) Les champignons supérious de la zone alpine du Parc National Suisse. Lüdin AG, Liestal
Fell JW, Boekhout T, Fonseca A, Scorzetti G, Statzell-Tallman A (2000) Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int J Syst Evol Microbiol 50:1351–1371
Ferrari E (2006) Inocybe alpine e subalpine. Fungi Non Delinineatii, Pars. Edizioni Candusso, Alassio, pp 34–36
Fischer H (1944) Untersuchungen über Massariamacrospora (Desm.) Sacc., ihre Nebenfruchtform Coryneummacrosporum Berk. Und Asterosporium Hoffmanni Kze. Phytopathol Z 14:512–517
Flagey C (1896) Catalogue des lichens de l’Algérie. In: Battandier JA, Trabut L (eds), Flore de l’Algérie 2, pp 1–140
Fliegerová K, Hodrová B, Voigt K (2004) Classical and molecular approaches as a powerful tool for the characterization of rumen polycentric fungi. Folia Microbiol 49:157–164
Fliegerová K, Mrázek J, Hoffman K, Zábranská J, Voigt K (2010) Diversity of anaerobic fungi within cow manure determined by ITS1 analysis. Folia Microbiol 55:319–325
Foltz MJ, Perez KE, Volk TJ (2013) Molecular phylogeny and morphology reveal three new species of Cantharellus within 20 m of one another in western Wisconsin, USA. Mycologia 105(2):447–461
Fotouhifar KB, Hedjaroude GA, Leuchtmann A (2010) ITS rDNAphylogeny of Iranian strains of Cytospora and associated teleomorphs. Mycologia 102:1369–1382
Fournier J, Stadler M, Hyde KD, Duong ML (2010) The new genus Rostrohypoxylon and two new Annulohypoxylon species from Northern Thailand. Fungal Divers 40(1):23–36
Frank JL (2014) Index Fungorum no. 179, Nomenclatural novelties, Xerocomellus dryophylus and X. rainisii, ISSN 2049-2375
Fries EM (1822) Systema Mycologicum II(1):14
Gaillard-Martinie B, Breton A, Dusser M, Julliand V (1995) Piromyces citronii sp. nov., a strictly anaerobic fungus from the equine cecum- a morpological, metabolic, and ultrastructural study. FEMS Microbiol Lett 130:321–326
Galloway DJ (2015) Contributions to a history of New Zealand lichenology 5. James Murray (1923–1961). Phytotaxa 198:1–67
Gams W (1977) A key to the species of Mortierella. Persoonia 9:381–391
Garnica S, Weiss M, Walther G, Oberwinkler F (2007) Reconstructing the evolution of agarics from nuclear gene sequences and basidiospore ultrastructure. Mycol Prog 111:1019–1029
Gates GM, Noordeloos ME (2007) Preliminary studies in the genus Entoloma in Tasmania–I. Persoonia 19:157–226
Ghobad-Nejhad M, Dai YC (2010) Diplomitoporus rimosus is found in Asia and belongs to the Hymenochaetales. Mycologia 102(6):1510–1517
Ghobad-Nejhad M, Duhem B (2014) Novelties in the Corticiales: Vuilleminia nilsii sp. nov. andDendrominia gen. nov. (Basidiomycota). Mycol Prog 13:1–11
Ghobad-Nejhad M, Nilsson RH, Hallenberg N (2010) Phylogeny and taxonomy of the genus Vuilleminia (Basidiomycota) based on molecular and morphological evidence, with new insights into Corticiales. Taxon 59:1519–1534
Gold JJ, Heath IB, Bauchop T (1988) Ultrastructural description of a new chytrid genus of caecum anaerobe, Caecomyces equi gen. nov. Biosytems 125:311–415
Gomes-Silva AC, Baltazar JM, Gibertoni TB (2012) Coltricia and Hymenochaete (Hymenochaetaceae) from the Amazonia and the Atlantic Forest, Brazil: One new combination and new records. J Torrey Bot Soc 139:428–436
Gramaje D, Agustí-Brisach C, Pérez-Sierra A, Moralejo E, Olmo D, Mostert L, Damm U, Armengol J (2012) Fungal trunk pathogens associated with wood decay of almond trees on Mallorca (Spain). Persoonia 28:1–13
Griffith GW, Baker S, Fliegerova K, Liggenstoffer A, van der Giezen M, Voigt K, Beakes G (2010) Anaerobic fungi: Neocallimastigomycota. IMA Fungus 1:181–185
Gruninger RJ, Puniya AK, Callaghan TM, Edwards JE, Youssef N, Dagar SS, Fliegerova K, Griffith GW, Forster R, Tsang A (2014) Anaerobic fungi (phylum Neocallimastigomycota): advances in understanding their taxonomy, life cycle, ecology, role and biotechnological potential. FEMS Microbiol Ecol. doi:10.1111/1574-6941.12383
Guatimosim E, Firmino AL, Bezerra JZ, Pereira OL, Barreto RW, Crous PW (2015) Towards a phylogenetic reappraisal of Parmulariaceae and Asterinaceae (Dothideomycetes). Molecular Phylogeny and Evolution of Fungi. Persoonia 35:230–241
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Gvritishvili MN (1982) The fungal genus Cytospora in the USSR. Izdatelstve Sabchota Sakarstvelo, Tbilisi
Hall T (2004) BioEdit. Ibis Therapeutics, Carlsbad, CA, 92008, USA. (http://www.mbio.ncsu.edu/BioEdit/bioedit.html) (18 Mar 2005)
Harhangi HR, Steenbakker PJM, Akhmanova AS, Jetten MSM, van der Drift C, Op den Camp HJM (2002) A highly expressed family 1 β-glucosidase with transglycosylation capacity from the anaerobic fungus Piromyces sp. E2. Biochim Biophys Acta Gene Struct Expr 1574:293–303
Harhangi HR, Akhmanova AS, Emmens R, van der Drift C, de Laat WTAM, van Dijken JP, Jetten MSM, Pronk JT, Op den Camp HJM (2003a) Xylose metabolism in the anaerobic fungus Piromyces sp. strain E2 follows the bacterial pathway. Arch Microbiol 180:134–141
Harhangi HR, Akhmanova AS, Steenbakkers PJM, Jetten MSM, van der Drift C, Op den Camp HJM (2003b) Genomic DNA analysis of genes encoding (hemi-) cellulolytic enzymes of anaerobic fungus Piromyces sp. E2. Gene 314:73–80
Harhangi HR, Freelove ACJ, Ubhayasekera W, van Dinther M, Steenbakkers PJM, Akhmanova AS, van der Drift C, Jetten MSM, Mowbray SL, Gilbert HJ, Op den Camp HJM (2003c) Ce6A, a major exoglucanase from the cellulosome of the anaerobic fungi Piromyces sp.E2 and Piromyces equi. Biochim Biophys Acta Gene Struct Expr 1628:30–39
Härkönen M, Niemelä T, Mwasumbi L (2003) Tanzanian mushrooms. Edible, harmful and other fungi. Norrlinia 10:1–200
Hattori T, Ryvarden L (1994) Type studies in the Polyporaceae. 25. Species described from Japan by R. Imazeki & A. Yasuda. Mycotaxon 50:27–46
Hausner G, Inglis GD, Yanke LJ, Kawchuk LM, McAllister TA (2000) Analysis of restriction fragment length polymorphisms in the ribosomal DNA of a selection of anaerobic chytrids. Can J Bot 78:917–927
Hawksworth DL (1985) A redisposition of the species referred to the ascomycete genus Microthelia. Bull Br Mus Bot Ser 14:43–181
He SH, Dai YC (2012) Taxonomy and phylogeny of Hymenochaete and allied genera of Hymenochaetaceae (Basidiomycota) in China. Fungal Divers 56:77–93
Heath IB, Bauchop T, Skipp RA (1983) Assignment of the rumen anaerobe Neocallimastix frontalis to the Spizellomycetales (Chytridiomycetes) on the basis of its polyflagellate zoospore ultrastructure. Can J Bot Rev Can Bot 61:295–307
Heim R (1931) Le Genre Inocybe. Encyclopédie Mycologique. Tome 1, Paris 1:1–429
Heinemann P (1956) Champignons récoltés au Congo belge par Mme M. Goossens-Fontana, II. Agaricus Fr. s.s. Bull Jard Bot Brux 26:1–127
Helms G, Friedl T, Rambold G (2003) Phylogenetic relationships of the Physciaceae inferred from rDNA sequence data and selected phenotypic characters. Mycologia 95:1078–1099
Henkel TW, Aime MC, Largent DL, Baroni TJ (2014) The Entolomataceae of the Pakaraima Mountains of Guyana 6: ten new species and a new combination in Nolanea. Mycotaxon 129(1):119–148
Hesler LR (1967) Entoloma in southeastern North America. Beih Nova Hedwig 23:1–245
Hesseltine CW, Ellis JJ (1964) The genus Absidia: Gongronella and cylindrical-spored species of Absidia. Mycologia 56:568–601
Hesseltine CW, Mahoney MK, Peterson SW (1990) A new species of Absidia from an alkali bee brood chamber. Mycologia 82(4):523–526
Hjortstam K, Ryvarden L (2005) Notes on the genus Epithele (Basidiomycotina, Aphyllophorales) from South America. Synopsis Fungorum 20:23–32
Hibbett DS, Binder M, Bischoff JF, BlackwellM CPF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, Lumbs HT, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Kõljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schüßler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao YJ, Zhang N (2007) A higher-level phylogenetic classification of the fungi. Mycol Res 111:509–547
Hirayama K, Tanaka K (2011) Taxonomic revision of Lophiostoma and Lophiotrema based on reevaluation of morphological characters and molecular analyses. Mycoscience 52:401–412
Hirayama K, Tanaka K, Raja HA, Miller AN, Shearer CA (2010) A molecular phylogenetic assessment of Massarina ingoldiana sensu lato. Mycologia 102:729–746
Ho YW, Barr DJS (1995) Classification of anaerobic gut fungi from herbivores with emphasis on rumen fungi from Malaysia. Mycologia 87:655–677
Ho YW, Barr DJS, Abdullah N, Jalaludin S, Kudo H (1993a) Neocallimastix variabilis, a new species of anaerobic fungus from the rumen of cattle. Mycotaxon 46:241–258
Ho YW, Barr DJS, Abdullah N, Jalaludin S, Kudo H (1993b) Piromyces spiralis, a new species of anaerobic fungus from the rumen of goat. Mycotaxon 48:59–68
Ho YW, Barr DJS, Abdullah N, Jalaludin S, Kudo H (1993c) A new species of Piromyces from the rumen of deer in Malaysia. Mycotaxon 47:285–293
Hoffmann K, Discher S, Voigt K (2007) Revision of the genus Absidia (Mucorales, Zygomycetes) based on physiological, phylogenetic, and morphological characters; thermotolerant Absidia spp. form a coherent group, Mycocladiaceae fam. nov. Mycol Res 111:1169–1183
Hoffmann K, Voigt K, Kirk PM (2011) Mortierellomycotina subphyl. Nov. Based on multi-gene genealogies. Mycotaxon 155:353–363
Hofmann TA (2010) Plant parasitic Asterinaceae and Microthyriaceae from the Neotropics (Panama). PhD thesis, The Faculty of Biological Sciences at the J.W. Goethe-University Frankfurt am in, Germany, p 408
Hofmann TA, Piepenbring M (2006) New records and host plants of fly-speck fungi from Panama. Fungal Divers 22:55–70
Holm L (1957) Etudes taxonomiques sur les pléosporacées. Symb Bot Upsal 14:1–188
Holm L (1961) Taxonomical notes on Ascomycetes. IV. Notes of Nodulosphaeria Rbh. Sven Bot Tidskr 55:63–80
Holubová-Jechová V (1973) Lignicolous hyphomycetes from Czechoslovakia. 4. Menispora. Folia Geobot Phytotax 8:317–336
Hongo T (1971) Notulae mycologicae (10). Mem Fac Liberal Arts Shiga Univ, Pt 2. Nat Sci 21:62–68
Hongsanan S, Chomnunti P, Crous PW, Chukeatirote E, Hyde KD (2014a) Introducing Chaetothyriothecium, a new genus of Microthyriales. Phytotaxa 161(2):157–164
Hongsanan S, Li YM, Liu JK, Hofmann T, Piepenbring M, Bhat JD, Boonmee S, Doilom M, Singtripop C, Tian Q, Mapook A, Zeng XY, Bahkali AH, Xu JC, Mortimer PE, Wu HX, Yang JB, Hyde KD (2014b) Revision of genera in Asterinales. Fungal Divers 68(1):1–68
Horak E (1973) Fungi Agaricini Novazelandiae. Entoloma (Fr.) and related genera. Beih Nova Hedwig 43:1–86
Horak E (1976) On cuboid-spored species of Entoloma (Agaricales). Sydowia 28:171–236
Horak E (1978) Entoloma in South America. I. Sydowia 30:40–111
Horak E (1980) Entoloma (Agaricales) in Indomalaya and Australasia. Beih Nova Hedwig 65:1–352
Horak E (1982) Entoloma in South America. II. Sydowia 35:75–99
Horak E (2008) The fungi of New Zealand. Vol. 5: Agaricales of New Zealand 1. Pluteaceae–Entolomataceae. Fungal Diversity Research Series, vol. 19. Fungal Diversity Press, Hong Kong, p 305
Hosagoudar VB (2012) Asterinales of India. Mycosphere 2(5):617–852
Hosagoudar VB, Goos RD (1991) Meliolaceae of South India. X. Mycotaxon 42:125–147
Hsieh HM, Ju YM, Rogers JD (2005) Molecular phylogeny of Hypoxylon and closely related genera. Mycologia 97(4):844–886
Huelsenbeck J, Ronquist PF (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Huhndorf SM, Crane JL, Shearer CA (1990) Studies in Leptosphaeria. Transfer of L. massarioides to Massariosphaeria. Mycotaxon 37:203–210
Humber RA (2012) Entomophthoromycota: a new phylum and reclassification for entomophthoroid fungi. Mycotaxon 120:477–492
Hwang J, Zhao Q, Yang ZL, Wang Z, Townsend JP (2015) Solving the ecological puzzle of mycorrhizal associations using data from annotated collections and environmental samples—an example of saddle fungi. Environ Microbiol Rep. doi:10.1111/1758-2229.12303
Hyde KD, Frohlich J (1998) Fungi from palms XXXVII. the genus Astrosphaeriella, including ten new species. Sydowia 50(1):81–132
Hyde KD, Eriksson OE, Yue JZ (1996) Roussoella, an ascomycete genus of uncertain relationships with a Cytoplea anamorph. Mycol Res 100(12):1522–1528
Hyde KD, Goh TK, Steinke TD (1998) Fungi on submerged wood in the Palmiet River, Durban, South Africa. S Afr J Bot 64(3):151–162
Hyde KD, Ho WH, Tsui CKM (1999) The genera Aniptodera, Halosarpheia, Nais and Phaeonectriella from freshwater habitats. Mycoscience 40:165–183
Hyde KD, Abd-Elsalam K, Cai L (2010) Morphology: still essential in a molecular world. Mycotaxon 114:439–451
Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake I, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, Hoog SD, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yan JY, Yacharoen S, Zhang M (2013) Families of Dothideomycetes. Fungal Divers 63:1–313
Hyde KD, Nilsson RH, Alias SA, Ariyawansa HA, Blair JE, Cai L, de Cock AWAM, Dissanayake AJ, Glockling SL, Goonasekara ID, Gorczak M, Hahn M, Jayawardena RS, van Kan JAL, Laurence MH, Lévesque CA, Li XH, Liu JK, Maharachchikumbura SSN, Manamgoda DS, Martin FN, McKenzie EHC, McTaggart AR, Mortimer PE, Nair PVR, Pawłowska J, Rintoul TL, Shivas RG, Spies CFJ, Summerell BA, Taylor PWJ, Terhem RB, Udayanga D, Vaghefi N, Walther G, Wilk M, Wrzosek M, Xu JC, Yan JY, Zhou N (2014) One stop shop:backbones trees for important phytopathogenic genera: I. Fungal Divers 67:21–125
Index Fungorum (2015) http://www.indexfungorum.org/Names/Names.asp. Accessed on June 2015
Ingold CT (1942) Aquatic Hyphomycetes of decaying alder leaves. Trans Br Mycol Soc 25:339–417
Jacobsson S, Larsson E (2012) Inocybe (Fr.) Fr. In: Knudsen H, Vesterholt J (eds) Funga Nordica. Agaricoid, boletoid, cyphelloid and gasteroid genera. Nordsvamp, Copenhagen, pp 981–1021
James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, Cox CJ, Celio G, Gueidan C, Fraker E, Miadlikowska J, Lumbsch HT, Rauhut A, Reeb V, Arnold AE, Amtoft A, Stajich JE, Hosaka K, Sung GH, Johnson D, O’Rourke B, Crockett M, Binder M, Curtis JM, Slot JC, Wang Z, Wilson AW, Schüler A, Longcore JE, O’Donnell K, Mozley-Standridge S, Porter D, Letcher PM, Powell MJ, Taylor JW, White MM, Griffith GW, Davies DR, Humber RA, Morton JB, Sugiyama J, Rossman AY, Rogers JD, Pfister DH, Hewitt D, Hansen K, Hambleton S, Shoemaker RA, Kohlmeyer J, Volkmann-Kohlmeyer B, Spotts RA, Serdani M, Crous PW, Hughes KW, Matsuura K, Langer E, Langer G, Untereiner WA, Lücking R, Büdel B, Geiser DM, Aptroot A, Diederich P, Schmitt I, Schultz M, Yahr R, Hibbett DS, Lutzoni F, McLaughlin DJ, Spatafora JW, Vilgalys R (2006) Reconstructing the early evolution of the fungi using a six-gene phylogeny. Nature 443:818–822. doi:10.1038/nature05110
Jayasiri SC, Hyde KD, Abd-Elsalam KA, Abdel-Wahab MA, Ariyawansa HA, Bhat J, Buyck B, Dai YC, Ertz D, Hidayat I, Jeewon R, Jones EBG, Karunarathna SC, Kirk P, Lei C, Liu JK, Maharachchikumbura SSN, McKenzie E, Ghobad-Nejhad M, Nilsson H, Pang KL, Phookamsak R, Rollins AW, Romero AI, Stephenson S, Suetrong S, Tsui CKM, Vizzini A, Wen TC, De Silva NI, Promputtha I, Kang JC (2015) The faces of fungi database: fungal names linked with morphology, molecular and human attributes. Fungal Divers (In press)
Jeffers WF (1940) Studies on Caryospora putaminum. Mycologia 32(4):550–566
Jones EBG, Vrijmoed LLP, Read SJ, Moss ST (1994) Tirispora, a new ascomycetous genus in the Halosphaeriales. Can J Bot 72:1373–1378
Jones EBG, Sakayaroj J, Suetrong S, Somrithipol S, Pang KL (2009) Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers 35:1–187
Jones EBG, Suetrong S, Cheng WH, Rungjindamai N, Sakayaroj J, Boonyuen N, Somrithipol S, Abdel-Wahab MA, Pang KL (2014) An additional fungal lineage in the Hypocreomycetidae (Falcocladium species) and the taxonomic re-evaluation of Chaetosphaeria chaetosa and Swampomyces species, based on morphology, ecology and phylogeny. Cryptogam Mycol 35:119–138
Jones EBG, Suetrong S, Sakayaroj J, Bahkali AH, Abdel-Wahab MA, Boekhout T, Pang KL (2015) Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Divers 73:1–72
Jong SC, Rogers JD (1972) Illustrations and descriptions of conidial states of some Hypoxylon species. Wash State Agric Exp Sta Bull 71:51
Ju YM, Rogers JD (1996) A revision of the genus Hypoxylon, vol 20, Mycol Mem. APS Press, St. Paul, p 365
Ju YM, Rogers JD (2001) New and interesting Biscogniauxia taxa, with a key to the world species. Mycol Res 105(09):1123–1133
Ju YM, Rogers JD (2002) The genus Nemania (Xylariaceae). Nova Hedwigia 74(1–2):75–120
Ju YM, Rogers JD, Martin FS, Granmo A (1998) The genus Biscogniauxia. Mycotaxon 66:1–98
Jülich W (1974) The genera of the Hyphodermoideae (Corticiaceae). Persoonia 8:59–97
Justo A, Morgenstern I, Hallen-Adams HE, Hibbett DS (2010) Convergent evolution of sequestrate forms in Amanita under Mediterranean climate conditions. Mycologia 102(3):675–688
Kaiser WJ, Viruega JR, Peever TL, Trapero A (2008) First report of ascochyta blight outbreak of pea caused by Ascochytapisi in Spain. Plant Dis 92:1365
Kernaghan G (2005) Mycorrhizal diversity: Cause and effect? Pedobiologia 49(6):511–520
Kerrigan RW (1986) Agaricales of California, vol 6, Agaricaceae. Mad River Press, Eureka, pp 1–62
Kerrigan RW, Callac P, Guinberteau J, Challen MP, Parra LA (2005) Agaricus section Xanthodermatei: a phylogenetic reconstruction with commentary on taxa. Mycologia 97:1292–1315
Khashnobish A, Shearer CA (1996) Phylogenetic relationships in some Leptosphaeria and Phaeosphaeriaspecies. Mycol Res 100:1355–1363
Kirk PM (2012) Nomenclatural novelties. Index Fungorum 1:1
Kirk PM, Cannon PF, David JC, Stalpers JA (2001) Dictionary of the fungi, 9th edn. CABI, Wallingford
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008a) Dictionary of the fungi. CABI Publishing, UK
Kirk PM, Cannon PF, David JC, Minter DW, Stalpers JA (2008b) Ainsworth and bisby’s dictionary of the fungi, 10th edn. CAB International Press, Wallingford
Klofac W (2011) Rotfußröhrlinge (Gattung Xerocomellus) in aktueller Sicht. Öst Z Pilzk 20:35–43
Kluting KL, Baroni TJ, Bergemann SE (2014) Toward a stable classification of genera within the Entolomataceae: a phylogenetic re-evaluation of the Rhodocybe-Clitopilus clade. Mycologia 106(6):1127–1142
Knudsen K, Kocourková J, Nordin A (2014) Conspicuous similarity hides diversity in the Acarospora badiofusca group (Acarosporaceae). Bryologist 117:319–328
Kohlmeyer J, Kohlmeyer E (1979) Marine mycology. The higher fungi. Academic, New York
Kohlmeyer J, Volkmann-Kohlmeyer B (1990) Revision of marine species of Didymosphaeria (Ascomycotina). Mycol Res 94:685–690
Kohlmeyer J, Volkmann-Kohlmeyer B (1993) Atrotorquata and Loratospora: new ascomycete genera on Juncus roemerianus. Syst Ascomycet 12:7–22
Kohlmeyer J, Volkmann-Kohlmeyer B (2000) Fungi on Juncus roemerianus 14. Three New Coelomycetes, including Floricola, anam.-gen. nov. Bot Mar 43(4):385–392
Kornerup A, Wanscher JH (1978) Methuen handbook of colour, 3rd edn. Methuen, London
Kühner R (1988) Diagnoses de quelques nouveaux Inocybes récoltés en zone alpine de la Vanoise (Alpes francaises). Doc Mycol 19(74):1–27
Kurniawati E, Zhang H, Chukeatirote E, Sulistyowati L, Moslem MA, Hyde KD (2010) Diversity of freshwater ascomycetes in freshwater bodies at Amphoe Mae Chan, Chiang Rai. Cryptogam Mycol 31(3):323–331
Ladurner H, Pöder R (2000) A new hyphal type found in Xerocomus pruinatus. Öst Z Pilzk 9:11–15
Ladurner H, Simonini G (2003) Xerocomus. Fungi Europaei, vol 8. Ediz. Candusso, Alassio, p 528
Landerose F, Korf RP (2012) Nomenclatural notes 13. An incorrect neotype designation and provision for a lectotype and an epitype for Helvella fusca. Mycotaxon 119:431–438
Largent DL (1994) Entolomatoid fungi of the Pacific Northwest and Alaska. Mad River Press, USA, p 516
Larsen MJ, Nakasone KK (1984) Additional New Taxa of Laeticorticium (Aphyllophorales, Corticiaceae). Mycologia 76:528–532
Larsson E, Ryberg M, Moreau P-A, Mathiesen AD, Jacobsson S (2009) Taxonomy and evolutionary relationships within species of section Rimosae (Inocybe) based on ITS, LSU, and mtSSU sequence data. Persoonia 23:86–98
Lawrey JD, Lücking R, Sipman HJM, Chaves JL, Redhead SA, Bungartz F, Sikaroodi M, Gillevet PM (2009) High concentration of basidiolichens in a single family of agaricoid mushrooms (Basidiomycota: Agaricales: Hygrophoraceae). Mycol Res 113:1154–1171
Leuchtmann A (1984) Über Phaeosphaeria Miyake und andere bitunicate Ascomyceten mit mehrfach querseptierten Ascosporen. Sydowia 37:75–194
Leveillé PJ (1845) De la péritonite puerpérale (Doctoral dissertation)
Li J, Heath IB, Bauchop T (1990) Piromyces mae and Piromyces dumbonica, two new species of uniflagellate anaerobic chytridiomycete fungi from the hindgut of the horse and elephant. Can J Bot 68:1021–1033
Li GJ, Li SF, Wen HA (2011) Russulazhejiangensis sp. nov.from East China. Cryptogam Mycol 32(2):127–133
Li GJ, Li SF, Liu XZ, Wen HA (2012) Russulajilinensis sp. nov. (Russulaceae) from northeast China. Mycotaxon 120:9–58
Li GJ, Zhao D, Li SF, Yang HJ, Liu XZ (2013a) Russula changbaiensis sp. nov. from northeast China. Mycotaxon 124(1):269–278
Li GJ, Zhao Q, Zhao D, Yue SF, Li SF, Wen HA, Liu XZ (2013b) Russula atroaerugineaand R. sichuanensisspp. nov. from southwest China. Mycotaxon 124(1):173–188
Li GJ, Zhao D, Li SF, Wen HA (2015a) Russula chiui and R. pseudopectinatoides, two new species from southwestern China supported by morphological and molecular evidence. Mycol Prog 14:33
Li YK, Zhang X, Yuan Y, Cao Z, Liang JF (2015b) Morphological and molecular evidence for a new species of Russula (Russulaceae) from southern China. Phytotaxa 202(2):94–102
Liebetanz E (1910) Die parasitischen Protozoen des Wiederkauermagens. Arch Prot 19:19–90
Liew ECY, Aptroot A, Hyde KD (2000) Phylogenetic significance of the pseudoparaphyses in Loculoascomycete taxonomy. Mol Phylogenet Evol 16:392–402
Liggenstoffer AS, Youseff NH, Couger MB, Elshaded MS (2010) Phylogenetic diversity and community structure of anaerobic gut fungi (phylum Neocallimastigomycota) in ruminant and non-ruminant herbivores. ISME J 4:1225–1235
Liu JK, Phookamsak R, Jones EBG, Zhang Y, KoKo TW, Hu HL, Boonmee S, Doilom M, Chukeatirote E, Bahkali AH, Wang Y, Hyde KD (2011) Astrosphaeriella is polyphyletic with species in Fissuroma gen. nov., and Neoastrosphaeriella gen. nov. Fungal Divers 51(1):135–154
Liu F, Hu D-H, Cai L (2012a) Conlarium duplumascospora gen. et. sp. nov. and Jobellisia guangdongensis sp. nov. from freshwater habitats in China. Mycologia 104:1178–1186
Liu JK, Phookamsak R, Doilom M, Wikee S, Li YM, Ariyawansha H, Boonmee S, Chomnunti P, Dai DQ, Bhat JD, Romero AI, Zhuang WY, Monkai J, Jones EBG, Chukeatirote E, KoKo TW, Zhao YC, Wang Y, Hyde KD (2012b) Towards a natural classification of Botryosphaeriales. Fungal Divers 57:149–210
Liu JK, Phookamsak R, Dai DQ, Tanaka K, Jones EBG, Xu JC, Chukeatirote E, Hyde KD (2014) Roussoellaceae, a new pleosporalean family to accommodate the genera Neoroussoellagen. nov., Roussoella and Roussoellopsis. Phytotaxa 181(1):1–33
Liu JK, Hyde KD, Jones EBG, Ariyawansa HA, Bhat DJ, Boonmee S, Maharachchikumbura SSN, McKenzie EHC, Phookamsak R, Phukhamsakda C, Shenoy BD, Abdel-Wahab MA, Buyck B, Chen J, Chethana KWT, Singtripop C, Dai DQ, Dai YC, Daranagama DA, Dissanayake AJ, Doilom M, D’souza MJ, Fan XL, Goonasekara ID, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathna SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Peršoh D, Pinruan U, Senanayake IC, Somrithipol S, Suetrong S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga D, Wijayawardene NN, Wanasinghe DN, Wisitrassameewong K, Zeng XY, Abdel-Aziz FA, Adamčík S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Lewis D, Li XH, Liu XZ, Liu ZY, Matsumura M, Mortimer PE, Rambold G, Randrianjohany E, Sato G, Sri-Indrasutdhi V, Tian CM, Verbeken A, von Brackel W, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E (2015) Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers 72:1–197
Locquin M (1984) Mycologie Générale et Structurale. Masson, Paris
Loh LS, Nawawi A, Kuthubutheen AJ (2001) Mucoraceous fungi from Malaysia. Institute of Biological Sciences, University of Malaya, pp 1–122
Lücking R, Dal-Forno M, Lawrey JD, Bungartz F, Holgado Rojas ME, Hernández JEM, Marcelli MP, Moncada B, Morales EA, Nelsen MP, Paz E, Salcedo L, Spielmann AA, Wilk K, Will-Wolf S, Yánez A (2013) Ten new species of lichenized Basidiomycota in the genera Dictyonema and Cora (Agaricales: Hygrophoraceae), with a key to all accepted genera and species in the Dictyonema clade. Phytotaxa 139:1–38
Lücking R, Dal-Forno M, Sikaroodi M, Gillevet PM, Bungartz F, Moncada B, Yánez A, Chaves JL, Coca LF, Lawrey JD (2014) A single macrolichen constitutes hundreds of unrecognized species. PNAS 111:11091–11096
Lumbsch HT, Huhndorf SM (2010) Myconet Volume 14. Part One. Outline of Ascomycota--2009. Fieldiana Life Earth Sci 1:1–60
Lumbsch HT, Lücking R (2015) Lecanoromycetes. In: Jaklitsch, Baral, Lücking, Lumbsch (eds) Engler’s syllabus of plant families, part 1/2: ascomycota, 13th edn. Borntraeger, Stuttgart
Lumbsch HT, Ahti T, Altermann S, Amo De Paz G, Aptroot A, Arup U, Bárcenas Peña A, Bawingan PA, Benatti MN, Betancourt L, Björk CR, Boonpragob K, Brand M, Bungartz F, Cáceres MES, Candan M, Chaves JL, Clerc P, Common R, Coppins BJ, Crespo A, Dal Forno M, Divakar PK, Duya MV, Elix JA, Elvebakk A, Fankhauser JD, Farkas E, Ferraro LI, Fischer E, Galloway DJ, Gaya E, Giralt M, Goward T, Grube M, Hafellner J, Hernández JEM, Herrera Campos MA, Kalb K, Kärnefelt I, Kantvilas G, Killmann D, Kirika P, Knudsen K, Komposch H, Kondratyuk S, Lawrey JD, Mangold A, Marcelli MP, McCune B, Messuti MI, Michlig A, Miranda González R, Moncada B, Naikatini A, Nelsen MP, Øvstedal DO, Palice Z, Papong K, Parnmen S, Pérez-Ortega S, Printzen C, Rico VJ, Rivas Plata E, Robayo J, Rosabal D, Ruprecht U, Salazar Allen N, Sancho L, Santos De Jesus L, Santos Vieira T, Schultz M, Seaward MRD, Sérusiaux E, Schmitt I, Sipman HJM, Sohrabi M, Søchting U, Zeuthen Søgaard M, Sparrius LB, Spielmann A, Spribille T, Sutjaritturakan J, Thammathaworn A, Thell A, Thor G, Thüs H, Timdal E, Truong C, Türk R, Umaña Tenorio L, Upreti DK, Van Den Boom P, Vivas Rebuelta M, Wedin M, Will-Wolf S, Wirth V, Wirtz N, Yahr R, Yeshitela K, Ziemmeck F, Wheeler T, Lücking R (2011) One hundred new species of lichenized fungi: a signature of undiscovered global diversity. Phytotaxa 18:1–127
Luo J, Yin J, Cai L, Zhang KQ, Hyde KD (2004) Freshwater fungi in Lake Dianchi, a heavily polluted lake in Yunnan, China. Fungal Divers 16(1):93–112
Luttrell ES (1973) Loculoascomycetes. In: Ainsworth GC, Sparrow FK, Sussman AS (eds) The fungi, An advanced treatise. Academic, New York, pp 135–219
Magnusson AH (1924) A monograph of the Scandinavian species of the genus Acarospora. Göteborgs Kungl Vetensk Samhälles Handl ser 4(28):1–150
Magnusson AH (1929) A monograph of the genus Acarospora. Kungl Svenska Vetenskapsakad Handl ser 3(7):1–400
Maharachchikumbura SSN, Guo LD, Cai L et al (2012) A multi-locus backbonetree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Divers 56:95–129
Maharachchikumbura SSN, Hyde KD, Groenewald JZ, Xu JJ, Crous PW (2014) Pestalotiopsis revisited. Stud Mycol 79:121–186
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Huang S-K, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’souza MJ, Goonasekara ID, Hongsanan S, Jayawardena RS, Kirk PM, Konta S, Liu J-K, Liu Z-Y, Norphanphoun C, Shenoy BD, Xiao Y, Bahkali AH, Kang J, Somrothipol S, Suetrong S, Wen T, Xu J (2015) Towards a natural classification and backbone tree for Sodariomycetes. Fungal Divers 72:199–301
Makhija U, Adawadkar B (2007) Trans-septate species of Acanthothecis and Fissurina from India. Lichenologist 39:165–185
Manimohan P, Noordeloos ME, Dhanya AM (2006) Studies on the genus Entoloma (Basidiomycetes, Agaricales) in Kerala State, India. Persoonia 19:45–94
Matheny PB (2009) A phylogenetic classification of the Inocybaceae. McIlvainea 18(1):11–21
Matheny PB, Bougher NL (2006) The new genus Auritella from Africa and Australia (Inocybaceae, Agaricales): molecular sys- tematics, taxonomy and historical biogeography. Mycol Progr 5:2–17
Matheny PB, Curtis JM, Hofstetter V, Aime MC, Moncalvo JM, Ge Z-W, Yang Z-L, Slot JC, Ammirati JF, Baroni TJ, Bougher NL, Hughes KW, Lodge DJ, Kerrigan RW, Seidl MT, Aanen DK, DeNitis M, Daniele GM, Desjardin DE, Kropp BR, Norvell LR, Hibbett DS (2006) Majorclades of Agaricales: a multi-locus phylogenetic over view. Mycologia 98(6):984–997
Matheny PB, Aime MC, Bougher NL, Buyck B, Desjardin DE, Horak E, Kropp BR, Lodge DJ, Soytong K, Trappe JM, Hibbett DS (2009) Out of the Palaeotropics? Historical biogeography and diversification of the cosmopolitan ectomycorrhizal mushroom family Inocybaceae. J Biogeogr 36:577–592
Mehrabi M, Mohammadi Goltapeh E, Fotouhifar KB (2011) Studies on Cytospora canker disease of apple trees in Semirom region of Iran. J Agric Technol 7(4):967–982
Miadlikowska J, Kauff F, Högnabba F, Oliver JC, Molnár K, Fraker E, Gaya E, Hafellner H, Hofstetter V, Gueidan C et al (2014) A multigene phylogenetic synthesis for the class Lecanoromycetes (Ascomycota): 1307 fungi representing 1139 infrageneric taxa, 312 genera and 66 families. Mol Phylogenet Evol 79:132–168
Miettinen O (2011) Taxonomy and phylogeny of white-rot polypores: case studies in Hymenochaetales and Polyporalres (Basidiomycota). Yliopistopaino, Helsinki, Finland
Miettinen O, Larsson KH (2011) Sidera, a new genus in Hymenochaetales with poroid and hydnoid species. Mycol Prog 10:131–141
Miettinen O, Larsson E, Sjokvist E, Larsson KH (2012) Comprehensive taxon sampling reveals unaccounted diversity and morphological plasticity in a group of dimitic polypores (Polyporales, Basidiomycota). Cladistics 28:251–270
Millanes AM, Westberg M, Wedin M, Diederich P (2012) Tremella diploschistina (Tremellomycetes, Basidiomycota, Fungi) a new lichenicolous species growing on Diploschistes. Lichenologist 44:321–332
Millanes AM, Truong C, Westberg M, Diederich P, Wedin M (2014) Host switching promotes diversity in host-specialized mycoparasitic fungi: uncoupled evolution in the Biatoropsis-Usnea system. Evolution 68:1576–1593. doi:10.1111/evo.12374
Miller SL, Buyck B (2002) Molecular phylogeny of the genus Russula in Europe with a comparison of modern infrageneric classifications. Mycol Res 106(3):259–276
Miller MA, Holder MT, Vos R, Midford PE, Liebowitz T, Chan L, Hoover P, Warnow T (2009) The CIPRES Portals. http://www.phylo.org/sub_sections/portal
Moncada B (2012) El Género Sticta (Schreb.) Ach. en Colombia: Taxonomía, Ecogeografía e Importancia. Doctoral Thesis, Universidad Nacional de Colombia. http://www.bdigital.unal.edu.co/11296/1/190844.2012.pdf
Moncada B, Lücking R, Betancourt-Macuase L (2013) Phylogeny of the Lobariaceae (lichenized Ascomycota: Peltigerales), with a reappraisal of the genus Lobariella. Lichenologist 45:203–263
Moncada B, Lücking R, Suárez A (2014a) Molecular phylogeny of the genus Sticta (lichenized Ascomycota: Lobariaceae) in Colombia. Fung Divers 64:205–231
Moncada B, Reidy B, Lücking R (2014b) A phylogenetic revision of Hawaiian Pseudocyphellaria sensu lato (lichenized Ascomycota: Lobariaceae) reveals eight new species and a high degree of inferred endemism. Bryologist 117:119–160
Moncalvo JM, Vilgalys R, Redhead SA, Johnson JE, James TY, Aime MC, Hofstetter V, Verduin SJW, Larsson E, Baroni TJ, Thorn RG, Jacobsson S, Clémencon H, Miller OK Jr (2002) One hundred and seventeen clades of euagarics. Mol Phylogenet Evol 23(3):357–400
Montagne JFC (1834) Notice sur les plantes cryptogames récemment découvertes en France contenant aussi l’indication précis des localités de quelques espèces les plus rares de la flore française. Annales des Sciences Naturelles Botanique, Sér 2 1:295–307
Morin L, Shivas RG, Piper MC, Tan YP (2010) Austropleospora osteospermi gen. et sp. nov. and its host specificity and distribution on Chrysanthemoides monilifera ssp. rotundata in Australia. Fungal Divers 40(1):65–74
Mostert L, Crous PW, Groenewald JZ, Gams W, Summerbell RC (2003) Togninia (Calosphaeriales) is confirmed as teleomorph of Phaeoacremonium by means of morphology, sexual compatibility, and DNA phylogeny. Mycologia 95(4):646–659
Mostert L, Groenewald JZ, Summerbell RC, Gams W, Crous PW (2006) Taxonomy and pathology of Togninia (Diaporthales) and its Phaeoacremonium anamorphs. Stud Mycol 54:1–115
Mouzouras R, Jones EBG (1985) Monodictys pelagica, the anamorph of Nereiospora cristata (Halopshaeriaceae). Can J Bot 63:2444–2447
Mugambi GK, Huhndorf SM (2009a) Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostomataceae recircumscribed (Plesporomycetidae, Dothideomycetes, Ascomycota). Stud Mycol 64:103–121
Mugambi GK, Huhndorf SM (2009b) Parallel evolution of hysterothecial ascomata in ascolocularous fungi (Ascomycota, Fungi). Syst Biodivers 7:453–464
Müller E (1950) Die schweizerischen Arten der Gattung Leptosphaeria und ihrer Verwandten. Sydowia 4:185–319
Müller E, von Arx JA (1962) Die Gattungen der didymosporen Pyrenomyceten. Beiträge zur. Kryptogamenflora der Schweiz 11(2):1–922
Muñiza D, Hladun NL (2007) Mycocalicium llimonae, a new species from the Iberian Peninsula. Lichenologist 39:205–210
Munk A (1953) The system of the pyrenomycetes. A contribution to a natural classification of the group Sphaeriales sensu Lindau. Dansk Bot Ark 15:1–163
Munsell C (1994) Munsell soil color charts. Macbeth Division of Kollmorgen Instruments Corporation, New Windsor
Nag Raj TR (1993) Coelomycetous anamorphs with appendage-bearing conidia. Mycologue Publications, Waterloo
Nakasone KK (2013) Taxonomy of Epithele (Polyporales, Basidiomycota). SYDOWIA 65(1):59–112
Nees of Esenbeck CG (1816) Ichneumonides Adsciti, Nature of Research in Genera et Familias Divisi: magazine Society friends to Berlin. 7(1813):243–277
Nguyen NH, Landeros F, Garibay-Orijel R, Hansen K, Vellinga EC (2013) The Helvella lacunosa species complex in western North America: cryptic species, misapplied names and parasites. Mycologia 105:1275–1286
Noireung P, Phoulivong S, Liu F, Cai L, McKenzie EHC, Chukeatirote E, Jones EBG, Bahkali AH, Hyde KD (2012) Novel species of Colletotrichum revealed by morphology and molecular analysis. Cryptogam Mycol 33(3):350
Noordeloos ME (1980) Entoloma subgenus Nolanea in the Netherlands and adjacent regions with a reconnaissance of its remaining taxa in Europe. Persoonia 10:427–534
Noordeloos ME (1987) Entoloma (Agaricales) in Europe. Beih Nova Hedwig 91:1–419
Noordeloos ME (1988a) Entolomataceae. In: Bas (ed) Flora Agaricina Neerlandica; Critical monographs on families of agarics and boleti occuring in the Netherlands, Vol 1. A. A. Balkema, Rotterdam, pp 77–177
Noordeloos ME (1988b) The species described by L.R. Hesler, A.H. Smith & S.J. Mazzer: type-species and comments. Cryptogamic studies, vol 2. Gustav Fisher Verlag, Stuttgart, p 164
Noordeloos ME (1992) Entoloma s.l. Fungi Europaei, vol 5. Giovanna Biella, Saronno, p 760
Noordeloos ME (2004) Entoloma s.l. Fungi Europaei, vol 5a. Edizione Candusso, Italy, p 617
Noordeloos ME (2008) Entoloma in North America 2: the species described by C.H. Peck–type studies and comments. Öst Z Pilzk 17:87–152
Noordeloos ME (2012) Entoloma (Fr.) P. Kumm. In: Knudsen H, Vesterholt J (eds) Funga Nordica: agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera2, 2nd edn. Nordsvamp, Copenhagen, pp 517–576
Noordeloos ME, Gates GM (2009) Preliminary studies in the genus Entoloma in Tasmania II. Cryptogam Mycol 30:107–140
Noordeloos ME, Gates GM (2012) The Entolomataceae of Tasmania. Fungal diversity research series, vol 22. Springer, Dordrecht, p 400
Noordeloos ME, Hausknecht A (1993) Die Gattung Entoloma in Ostösterreich. Öst Z Pilzk 2:45–96
Noordeloos ME, Hausknecht A (2007) The genus Entoloma (Basidiomycetes, Agaricales) of the Mascarenes and Seychelles. Fungal Divers 27:111–144
Noordeloos ME, Morozova OV (2010) New and noteworthy Entoloma species from the Primorsky Territory, Russian Far East. Mycotaxon 112:231–255
Norphanphoun C, Maharachchikumbura SSN, Daranagama A, Bulgakov TS, Bhat DJ, Bahkali AH, Hyde KD (2015) Towards a backbone tree for Seimatosporium, with S. physocarpi sp. nov. Mycosphere 6(3):385–400
Okane I, Srikitikulchai P, Toyama K, Læssøe T, Somsak S, Nigel H-J, Akira N, Wanchern P, Ken-ichiro S (2008) Study of endophytic Xylariaceae in Thailand: diversity and taxonomy inferred from rDNA sequence analyses with saprobes forming fruit bodies in the field. Mycoscience 49:359–372
Orpin CG (1974) Rumen flagellates Callimastix frontalis and Monas communis - Zoospores of phycomycete fungi. J Appl Bacteriol 37:R9–R10
Orpin CG (1975) Studies on the rumen flagellate Neocallimastix frontalis. J Gen Microbiol 91:249–262
Orpin CG (1977) The rumen flagellate Piromonas communis: its life - history and invasion of plant material in the rumen. J Gen Microbiol 99:107–117
Orpin CG, Munn EA (1986) Neocallimastix patriciarum Sp. Nov., a New Member of the Neocallimasticaceae Inhabiting the Rumen of Sheep. Trans Br Mycol Soc 86:178–181
Ozkose E, Thomas BJ, Davies DR, Griffith GW, Theodorou MK (2001) Cyllamyces aberensis gen.nov sp.nov. a new anaerobic gut fungus with branched sporangiophores isolated from cattle. Can J Bot 79:666–673
Pang KL, Vrijmoed LLP, Kong RYC, Jones EBG (2003) Polyphyly of Halosarpheia (Halosphaeriales, Ascomycota): implications on the use of unfurling ascospore appendage as a systematic character. Nova Hedw 77:1–18
Parmasto E (1978) The genus Dictyonema (‘Thelephorolichenes’). Nova Hedwigia 29:99–144
Parnmen S, Lücking R, Lumbsch HT (2012) Phylogenetic classification at generic level in the absence of distinct phylogenetic patterns of phenotypical variation: A case study in Graphidaceae (Ascomycota). PLoS ONE 7(12):e51392
Parra LA (2008) Agaricus L. Allopsalliota, Nauta & Bas. Part I. Fungi Europaei. Edizioni Candusso, Alassio
Parra LA (2013) Agaricus L. Allopsalliota, Nauta & Bas. Part 2. Fungi Europaei. Edizioni Candusso, Alassio
Parra LA, Muñoz G, Callac P (2014) Agaricus caballeroi sp. nov., una nueva especie de la sección Nigrobrunnescentes recolectada en España. Micologia e Vegetazione Mediterranea 29:21–38
Paulus B, Gadek P, Hyde KD (2004) Phylogenetic and morphological assessment of five new species of Thozetella from an Australian rainforest. Mycologia 96:1074–1087
Pažoutová S, Follert S, Bitzer J, Keck M, Surup F, Šrůtka P et al (2013) A new endophytic insect-associated Daldinia species, recognised from a comparison of secondary metabolite profiles and molecular phylogeny. Fungal Divers 60(1):107–123
Peck CH (1907) New York species of Russula. Bull New York State Mus Nat Hist 116:67–98
Pegler DN (1983) Agaric flora of the Lesser Antilles. Kew Bull Additional Series 9:1–668
Pegler DN (1997) The agarics of São Paulo, Brazil. Royal Botanic Garden Kew, London, p 70
Peintner U, Bougher NL, Castellano MA, Moncalvo JM, Moser MM, Trappe JM, Vilgalys R (2001) Multiple origins of sequestrate fungi related to Cortinarius (Cortinariaceae). Am J Bot 88(12):2168–2179
Peintner U, Horak E, Moser M, Vilgalys R (2002) Phylogeny of Rozites, Cuphocybe and Rapacea inferred from ITS and LSU rDNA sequences. Mycologia 94(4):620–629
Petrak F (1940) Beiträge zur Pilzflora der Umgebung von Wien. Annales Mycologici 38:339–386
Phillips AJL, Alves A, Pennycook JPR, Ramaley A, Akulov A, Crous PW (2008) Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21:29–55
Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76:51–167
Phookamsak R, Liu JK, Chukeatirote E, McKenzie EHC, Hyde KD (2013) Phylogeny and morphology of Leptosphaerulina saccharicola sp. nov. and Pleosphaerulina oryzae and relationships with Pithomyces. Cryptogam Mycol 34(4):303–319
Phookamsak R, Liu JK, McKenzie EHC, Manamgoda DS, Ariyawansa HA, Thambugala KM, Dai DQ, Camporesi E, Chukeatirote E, Wijayawardene NN, Bahkali AH, Mortimer PEXJC, Hyde KD (2014) Revision of Phaeosphaeriaceae. Fungal Divers 68:159–238
Phookamsak R, Manamgoda DS, Li WJ, Dai DQ, Singtripop C, Hyde KD (2015) Poaceascoma helicoides gen et sp. nov., a new genus with scolecospores in Lentitheciaceae Cryptogamie. Mycologie 36(2):1–12
Poengsungnoen V, Manoch L, Mongkolsuk P, Boonpragob K, Parnmen S, Lücking R, Tehler A, Lumbsch HT (2014) Phylogenetic analysis reveals two morphologically unique new species in the genera Astrochapsa and Nitidochapsa (lichenized Ascomycota: Graphidaceae). Phytotaxa 189:268–291
Pouzar Z (1979) Notes on taxonomy and nomenclature of Nummularia (Pyrenomycetes). Česká Mykologie 33:207–219
Pouzar Z (1986) A key and conspectus of Central European species of Biscogniauxia and Obolarina (Pyrenomycetes). Česká Mykologie 40:1–10
Pratibha J, Prabhugaonkar A (2015) Multi-gene phylogeny of Pithomyces with the sexual morph of P. flavus Berk. & Broome. Phytotaxa 218(1):084–090
Quaedvlieg W, Verkley GJM, Shin H-D, Barretto RW, Alfenas AC, Swart WJ, Groenewald JZ, Crous PW (2013) Sizing up Septoria. Stud Mycol 75:307–390
Rabenhorst (1858) Herb myc, ed. 2 no. 725 (in sched)
Raja H, Campbell J, Shearer CA (2003) Freshwater ascomycetes:Cyanoannulus petersenii, a new genus and speciesfrom submerged wood. Mycotaxon 88:1–17
Raja HA, Ferrer A, Miller AN, Shearer CA (2010) Freshwater Ascomycetes: Wicklowia aquatica, a new genus and species in the Pleosporales from Florida and Costa Rica. Mycoscience 51(3):208–214
Raja HA, Oberlies NH, El-Elimat T, Miller AN, Zelski SE, Shearer CA (2013) Lindgomyces angustiascus, (Lindgomycetaceae, Pleosporales, Dothideomycetes), a new lignicolous species from freshwater habitats in the USA. Mycoscience 54(5):353–361
Ramaley RW (1999) Three species of Microthyrium from Nonila. Mycotaxon 70:8
Rambaut A (2009) FigTree v1.3.1. Department of Zoology, University of Oxford, Oxford. http://tree.bio.ed.ac.uk/software/figtree/. Accessed 21 Dec 2009
Rao VG, Reddy KA (1981) Two new hyphomycetes. Indian J Bot 4(1):108–114
Réblová M, Mostert L (2007) Romellia is congeneric with Togninia, and description of Conidiotheca gen. nov. for one species of this genus with polysporous asci. Mycol Res 111:299–307
Réblová M, Seifert KA (2008) A new species of Chaetosphaeria with Menispora ciliata and phialophora-like anamorphs. Fungal Divers 29:99–105
Réblová M, Seifert KA, White GP (2006) Chaetosphaeria tortuosa, the newly discovered teleomorph of Menispora tortuosa, with a key to known Menispora species. Mycol Res 110:104–109
Réblová M, Gams W, Seifert KA (2011) Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Stud Mycol 68:163–191
Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-a sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97(1):84–98
Rehner SA, Minnis D, Sung G-H, Luangsa-ard JJ, DeVotto L, Humber RA (2011) Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 103(5):1055–1073
Reiss MLC (1854) Neue Kernpilze. Hedwigia 1:23–28
Richardson M (2009) The ecology of the Zygomycetes and its impact on environmental exposure. Clin Microbiol Infect 15:2–9
Rikkinen J (2003) Chaenothecopsis nigripunctata, a remarkable new species of resinicolous Mycocaliciaceae from western North America. Mycologia 95:98–103
Rivas Plata E, Lücking R, Sipman HJM, Mangold A, Kalb K, Lumbsch HT (2010) A world-wide key to the thelotremoid Graphidaceae, excluding the Ocellularia-Myriotrema-Stegobolus clade. Lichenologist 42:139–185
Rivas Plata E, Lücking R, Lumbsch HT (2012) A new classification for the family Graphidaceae (Ascomycota: Lecanoromycetes: Ostropales). Fung Divers 52:107–121
Rivas Plata E, Parnmen S, Staiger B, Mangold A, Frisch A, Weerakoon G, Hernández JEM, Cáceres MES, Kalb K, Sipman HJM, Common RS, Nelsen MP, Lücking R, Lumbsch HT (2013) A molecular phylogeny of Graphidaceae (Ascomycota, Lecanoromycetes, Ostropales) including 428 species. MycoKeys 6:55–94. doi:10.3897/mycokeys.6.3482
Rogers JD, Miller AN, Vasilyeva LN (2008) Pyrenomycetes of the Great Smoky Mountains National Park. VI. Kretzschmaria, Nemania, Rosellinia and Xylaria (Xylariaceae). Fungal Divers 29:107–116
Romagnesi H (1941) Les Rhodophylles de Madagascar. Prodr. Fl. Myc. Madagascar, vol. 3
Romagnesi H (1985) Les Russules d’Europe et d’Afrique du Nord. Reprint. J. Cramer
Romagnesi H (1996) Les russues d’Europe et d’Afrique du nord. Bordas, Paris
Romagnesi H, Gilles G (1979) Les Rhodophylles des fôrets côtières du Gabon et de la Côte d’ Ivoire. Beih Nova Hedwig 59:1–649
Ryberg M, Larsson E, Jacobsson S (2010) An evolutionary perspective on morphology and ecological characters in the mushroom family Inocybaceae (Agaricomycotina, Fungi). Mol Phylogenet Evol 55:431–442
Ryvarden L (1991) Genera of polypores. Nomenclature and taxonomy. Syn Fungorum 5:1–363
Ryvarden L (2004) Neotropical polypores 1. Introduction, Ganodermataceae and Hymenochaetaceae. Synopsis Fungorum 19:1–228
Ryvarden L, Iturriaga T (2003) Studies in neotropical polypores 10. New polypores from Venezuela. Mycologia 95(6):1066–1077
Ryvarden L, Johansen I (1980) A preliminary polypore flora of East Africa. Fungiflora, Oslo
Ryvarden L, Melo I (2014) Poroid fungi of Europe. Syn Fungorum 31:1–455
Saccardo PA (1878) Fungi Italici autographice delineati a Prof. P.A. Saccardo. Patavii 1878. - Fascicoli V.-VIII. Michelia 1(3):326–350
Saccardo PA (1883) Fungi Gallici ser. II. Michelia 2:57
Saccardo PA (1884) Sylloge Fungorum. Typis Seminarii, Italy (in Latin)
Sánchez-Ramírez S, Tulloss RE, Amalfi M, Moncalvo J-M, Carine M (2015) Palaeotropical origins, boreotropical distribution and increased rates of diversification in a clade of edible ectomycorrhizal mushrooms (Amanita section Caesareae). J Biogeogr 42(2):351–363
Sarnari M (1998) Monographia illustrata del genere Russula in Europa. Tomo 1. Associazone Micologica Bresadola. Fondazione Centro Studi Micologici, Vicenca
Sarnari M (2005) Monografia illustrate de genere Russula in Europa. Tomo Secondo. AMB, Centro Studi Micologici, Trento
Schatz S (1984) The life history, developmental morphology, and taxonomy of Lautitia danica gen. nov., comb. nov. Can J Bot 62:28–32
Scheinpflug H (1958) Untersuchungen uber die Gattung Didymosphaeria Fuckel und eingige verwandte Gattungen. Ber Sch Bot Ges 68:325–385
Schmidt A (1970) Anatomisch-taxonomische Untersuchungen an europa$ischen Arten der Flechtenfamilie Caliciaceae. Mitteilung zur Staatsinstitut fuXr Aligemeine Botanik, Hamburg 13:111–166
Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, de Gruyter J, de Hoog GS, Dixon LJ, Grube M, Gueidan C, Harada Y, Hatakeyama S, Hirayama K, Hosoya T, Huhndorf SM, Hyde KD, Jones EBG, Kohlmeyer J, Kruys Å, Li YM, Lücking R, Lumbsch HT, Marvanová L, Mbatchou JS, McVay AH, Miller AN, Mugambi GK, Muggia L, Nelsen MP, Nelson P, Owensby CA, Phillips AJL, Phongpaichit S, Pointing SB, Pujade-Renaud V, Raja HA, Rivas Plata E, Robbertse B, Ruibal C, Sakayaroj J, Sano T, Selbmann L, Shearer CA, Shirouzu T, Slippers B, Suetrong S, Tanaka K, Volkmann-Kohlmeyer B, Wingfield MJ, Wood AR, Woudenberg JHC, Yonezawa H, Zhang Y, Spatafora JW (2009) A class–wide phylogenetic assessment of Dothideomycetes. Stud Mycol 64:1–15
Scorzetti G, Fell JW, Fonseca A, Statzell-Tallman A (2002) Systematics ofbasidiomycetous yeasts, a comparison of large subunit D1/D2 and internaltranscribed spacer rDNA regions. FEMS Yeast Res 2:495–517
Seguy E (1936) Code universel des couleurs. P. Lechevalier, Paris
Senanayake IC, Maharachchikumbura SSN, Hyde KD, Bhat JD, Jones EBG, McKenzie EHC, Dai DQ, Daranagama DA, Dayarathne MC, Goonasekara ID, Konta S, Li WJ, Shang QJ, Stadler M, Wijayawardene NN, Xiao YP, Norphanphoun C, Li Q, Liu XZ, Bahkali AH, Kang JC, Wang Y, Wen TC, Wendt L, Xu JC, Camporesi E (2015) Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers 73(1):1–85. doi:10.1007/s13225-015-0340-y
Shearer CA (1993) A new species of Kirschsteiniothelia (Pleosporales) with an unusual fissitunicate ascus. Mycologia 85(6):963–969
Shoemaker RA (1984) Canadian and some extralimital Nodulosphaeria and Entodesmium species. Can J Bot 62:2730–2753
Shoemaker RA, Babcock CE (1989) Phaeosphaeria. Can J Bot 67:1500–1599
Silvestro D, Michalak I (2011) RaxmlGUI: A graphical front-end for RAxML. Org Divers Evol 12:335–337. doi:10.1007/s13127-011-0056-0
Simmons EG (1986) Alternaria themes and variations (22–26). Pleospora/ Stemphylium and Lewia / Alternaria Mycotaxon 25:287–308
Simonini G (1994) Boletus dryophilus Thiers, specie nuova per l’Europa. Riv Micol 37(3):205–219
Singer R (1935) Supplement ezumeinermono graphie der gutting Russula. Ann Mycol 33:297–352
Singer R (1986) Theagaricales in modern taxonomy, 4th edn. Koeltz Scientific Books, Koenigstein, p 981
Singtripop C, Camporesi E, Ariyawansa HA, Wanasinghe DN, Boonmee S, Mortime PE, Xu JC, Hyde KD (2015) Keissleriella dactylidis, sp. nov., from Dactylis sp. and its phylogenetic placement. Science Asia (in press)
Sivanesan A (1984) The bitunicate ascomycetes and their anamorphs. J. Cramer, Vaduz
Sivichai S, Jones EBG, Hywel-Jones N (2002) Fungal colonisation of wood in a freshwater stream at Tad Ta Phu, Khao Yai National Park, Thailand. Fungal Divers 10:113–129
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol Rev 21:90–106
Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ (2004) Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96:83–101
Snell WH, Dick EA (1970) The Boleti of north-eastern north America. J Cramer, Lehre, p 115
Snell WH, Singer R, Dick EA (1960) [1959] Notes on boletales. XI. Mycologia 51(4):564–577
Sohrabi M, Lücking R, Lumbsch HT (2014) One hundred and seventy five new species of Graphidaceae. Phytotaxa 189:5–6
Song B, Li TH, Wu XL, Li JJ, Shen YH, Lin QY (2007) Known species of Russula from China and their distribution. J Fungal Res 5(1):20–42 (in Chinese)
Song Y, Tangthirasunun N, Maharachchikumbura SSN, Jiang YL, Xu JJ, Hyde KD, Wang Y (2014) Novel Pestalotiopsis species from Thailandpoint to the rich undiscovered diversityof this chemically creative genus. Cryptogam Mycol 35(2):139–149
Spielman LJ (1983) Taxonomy and biology of Valsa species on Hardwoods in North America, with special reference to species on maples. Cornell University, Ithaca
Spielman LJ (1985) A monograph of Valsa on hardwoods in North America. Can J Bot 63:1355–1387
Spooner BM, Kirk PM (1982) Taxonomic notes on Excipularia and Scolicosporium. Trans Br Mycol Soc 78:247–257
Stadler M, Kuhnert E, Peršoh D, Fournier J (2013) The Xylariaceae as model example for a unified nomenclature following the “One Fungus-One Name” (1F1N) concept. Mycology 4(1):5–21
Staiger B (2002) Die Flechtenfamilie Graphidaceae. Studien in Richtung einer natürlicheren Gliederung. Bibl Lichenol 85:1–526
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi:10.1093/bioinformatics/btl446
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–777
Steenbakkers PJM, Li XL, Ximenes EA, Arts JG, Chen H, Ljungdahl LG, Op Den Camp HJM (2001) Noncatalytic docking domains of cellulosomes of anaerobic fungi. J Bacteriol 183:5325–5333
Steenbakkers PJM, Freelove A, van Cranenbroek B, Sweegers BMC, Harhangi HR, Vogels GD, Hazlewood GP, Gilbert HL, Op Den Camp HJM (2002a) The major component of the cellulosomes of anaerobic fungi from the genus Piromyces is a family 48 glycoside hydrolase. DNA Seq 13:313–320
Steenbakkers PJM, Ubhayasekera W, Goossen HJAM, van Lierop EMHM, van der Drift C, Vogels GD, Mowbray SL, Op Den Camp HJM (2002b) An intron-containing glycoside hydrolase family 9 cellulase gene encodes the dominant 90 kDa component of the cellulosome of the anaerobic fungus Piromyces sp. strain E2. Biochem J 365:193–204
Steenbakkers PJM, Harhangi HR, Bosscher MW, van der Hooft MMC, Keltjens JT, van der Drift C, Vogels GD, Op Den Camp HJM (2003) β-glucosidase in cellulosome of the anaerobic fungus Piromyces sp. E2 is a family 3 glycoside hydrolase. Biochem J 379:963–970
Steenbakkers PJM, Irving JA, Harhangi HR, Swinkels WJC, Akhmanova A, Dijkerman R, Jetten MSM, van der Drift C, Whisstock JC, Op Den Camp HJM (2008) A serpin in the cellulosome of the anaerobic fungus Piromyces sp. Strain E2. Mycol Res 112:999–1006
Strobel G, Li JY, Ford E, Worapong J, Gary IB, Hess WM (2000) Pestalotiopsisjester sp. nov., anendophyte from Fragraeabodenii Wernh., acommon plantinthe southern high lands of Papua NewGuinea. Mycotaxon 76:257–266
Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann Kohlmeyer B, Sakayaroj J, Phongpaichit S, Tanaka K, Hirayama K, Jones EBG (2009) Molecular systematics of the marine Dothideomycetes. Stud Mycol 64:155–173
Sung G-H, Hywel-Jones NL, Sung J-M, Luangsa-ard JJ, Shrestha B, Spatafora JW (2007a) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol 57:5–66
Sung G-H, Sung J-M, Hywel-Jones NL, Spatafora JW (2007b) A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol Phylogenet Evol 44(3):1204–1223
Šutara J (2008) Xerocomus s.l. in the light of the present state of knowledge. Czech Mycol 60(1):29–62
Sutton BC (1980) The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. Common Wealth Mycological Institute, Kew
Swofford DL (2002) PAUP: phylogenetic analysis using parsimony,version 4.0b10. Illinois Natural History Survey, Champion, Ill
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 28(10):2731–2739
Tanaka K, Harada Y (2004) Pleosporales in Japan (4). The genus Massariosphaeria. Mycoscience 45(2):96–105
Tanaka K, Hirayama K, Yonezawa H, Hatakeyama S, Harada Y, Sano T, Shirouzu T, Hosoya T (2009) Molecular taxonomy of bambusicolous fungi: Tetraplosphaeriaceae, a new pleosporalean family with Tetraploa-like anamorphs. Stud Mycol 64:175–209
Tang AMC, Jeewon R, Hyde KD (2007) Phylogenetic relationships of Nemania plumbea sp. nov. and related taxa based on ribosomal ITS and RPB2 sequences. Mycol Res 111:392–402
Taylor AFS, Douhan GW, Hills AE, Simonini G, Binder M, Eberhardt U (2012) A molecular analysis of European and North American taxa within the Xerocomus chrysenteron complex and the description of X. redeuilhii Taylor, Eberhardt & Simonini sp. nov. Mycological Society of America, 2012 Meeting Yale University, New Haven, Connecticut, July 15–18 2012, Conference Paper, 60p
Thambugala KM, Singtripop C, Chunfang Y, Mckenzie EH, Liu ZY, Chukeatirote E, Hyde KD (2014) Towards a natural classification of Dothideomycetes 7: The genera Allosoma, Austropleospora, Dangeardiella, Griggsia and Karschia (Dothideomycetes incertae sedis). Phytotaxa 181(1):34–46
Thambugala KM, Hyde KD, Tanaka K, Tian Q, Wanasinghe DN, Ariyawansa H, Boonmee S, Camporesi E, Hashimoto A, Hirayama K, Jayasiri SC, Schumacher RK, Promputtha I, Liu ZY (2015) Towards a natural classification and backbone tree for Lophiostomataceae, Floricolaceae, and Amorosiaceae fam. nov. Fungal Divers (in press)
Thienhirun S, Rodtong S, Phukhawan N, Suwannasai N (2003) Xylariaceous fungi in Phu Hin Rongkra National Park, Thailand. Proceedings of the 2nd International Conference on Medical Mushroom. Pattaya, p 505–508
Thongklang N, Nawaz R, Khalid AN et al (2014) Morphological and molecular characterization of three Agaricus species from tropical Asia (Pakistan, Thailand) reveals a new group in section Xanthodermatei. Mycologia 106:1220–1232. doi:10.3852/14-076
Tian Q, Hyde KD, Liu JK, Wanasinghe DN, Boonmee S., Senanayak IC, Luo ZL, Ariyawansa H, Li WJ, Thambugala KM, Jones EBG, Bhat DJ, Bahkali AH, Chomnunti P, Mortimer PE, Xu JC, Campesori E (2015) Phylogenetic relationships and morphological reappraisal of Melanommataceae. Fungal Divers (in press)
Tibell L, Titov A (1995) Species of chaenothecopsis and mycocalicium (Caliciales) on exudate. The Bryologist 98(4):550–560
Trakunyingcharoen T, Lombard L, Groenewald JZ, Cheewangkoon R, Toanun C, Alfenas AC, Crous PW (2014) Mycoparasitic species of Sphaerellopsis, and allied lichenicolous and other genera. IMA Fungus 5(2):391–414
Tsui CKM, Berbee ML (2006) Phylogenetic relationships and convergence of helicosporous fungi inferred from ribosomal DNA sequences. Mol Phylog Evol 39:587–597
Tulasne LR, Tulasne C (1861) Selecta Fungorum Carpologia. Erysiphei. 1
Tulloss RE (2005) Amanita-distribution in the Americas, with comparison to eastern and southern Asia and notes on spore character variation with latitude and ecology. Mycotaxon 93:189–231
Vainio EA (1890) E!tude sur la classification naturelle et la morphologie des lichens du Bre!sil. Acta Societas pro Fauna et Flora Fennica 7(1):i–xxix, 1–247(2):1–256
Van Tieghem P (1876) Troisième mémoire sur les Mucorinées. Annales des Sciences Naturelles VI 4:312–399
Vargas N, Bernal A, Sarria V, Franco-Molano A, Restrepo S (2011) Amatoxin and phallotoxin composition in species of the genus Amanita in Colombia: a taxonomic perspective. Toxicon 58(6–7):583–590
Vavra J, Joyon L (1966) Étude sur la morphologie, le cycle évolutif et la position systématique de Callimastix cyclopis Weissenberg 1912. Protistologica 2:5–15
Vega FE, Posada F, Aime MC, Pava-Ripoll M, Infante F, Rehner SA (2008) Entomopathogenic fungal endophytes. Biol Control 46:72–82
Vila J, Carbó J, Caballero F, Català S, Llimona X, Noordeloos ME (2013) A first approach to the study of the genus Entoloma subgenus Nolanea s.l. using molecular and morphological data. Fungi non Delineati LXVI (Studies on Entoloma). Edizioni Candusso, Alassio, p 150
Vizzini A (2015) Index Fungorum no. 244, Nomenclatural novelties, Hortiboletus, Rheubarbariboletus, ISSN 2049-2375
Vizzini A, Contu M, Ercole E, Voyron S (2012) Rivalutazione e delimitazione del genere Aspidella (Agaricales, Amanitaceae), nuovamente separato da Amanita. Micol Vegetazione Mediterr 27(2):75–90
Voglmayr H (2004) Spirosphaera cupreorufescens sp. nov., a rare aeroaquatic fungus. Stud Mycol 50:221–228
Voigt K (2012) Chytridiomycota. In: Frey W (ed) Syllabus of plant families–A. Engler’s Syllabus der Pflanzenfamilien. Part 1/1: Blue-green algae, Myxomycetes and Myxomycete-like organisms, Phytoparasitic protists, Heterotrophic Heterokontobionta and Fungi p.p. Borntraeger Verlag, Stuttgart, pp 106–129, ISBN 978-3-443-01061-4
Wagner T, Fischer M (2002) Classification and phylogenetic relationships of Hymenochaete and allied genera of the Hymenochaetales, inferred from rDNA sequence data and nuclear behaviour of vegetative mycelium. Mycol Prog 94:93–104
Wagner L, Stielow B, Hoffmann K, Petkovits T, Papp T, Vágvölgyi C, de Hoog GS, Verkley, Voigt K (2013) A comprehensive molecular phylogeny of the Mortierellales (Mortierellomycotina) based on nuclear ribosomal DNA, Persoonia. Mol Phylogeny Evol Fungi 30:77–93
Wanansinghe DN, Jones EBG, Camporesi E, Boonmee S, Ariyawansa HA, Wijayawardene NN, Hyde KD (2014) An Exciting Novel Member of Lentitheciaceae in Italy from Clematis Vitalba. Cryptogam Mycol 35(4):323–337
Wanasinghe DN, Jones EBG, Camporesi E, Mortimer PE, Xu JC, Bahkali AH, Hyde KD (2015) The genus Murispora (in press)
Wang HK, Aptroot A, Crous PW, Hyde KD, Jeewon R (2007) The polyphyletic nature of Pleosporales: an example from Massariosphaeria based on rDNA and RBP2 gene phylogenies. Mycol Res 111:1268–1276
Wang XH, Yang ZL, Li YC, Knudsen H, Liu PG (2009) Russula griseocarnosa sp. nov. (Russulaceae, Russulales), a commercially important edible mushroom in tropical China: mycorrhiza, phylogenetic position, and taxonomy. Nova Hedwigia 88:269–282
Wang ZR, Parra LA, Callac P, Zhou JL, Fu WJ, Dui SH, Hyde KD, Zhao RL (2015) Edible species of Agaricus (Agaricaceae) from Xinjiang Province (Western China). Phytotaxa 202(3):185–197
Webb J, Theodorou MK (1991) Neocallimastix hurleyensis sp. nov., an anaerobic fungus from the ovine rumen. Can J Bot Rev Can Bot 69:1220–1224
Weber NS (1972) The genus Helvella in Michigan. Mich Bot 11:147–201
Weber R, Webster J (2007) Introduction to fungi. New York, Cambridge
Wedin M, Baloch E, Grube M (2002) Parsimony analyses of mtSSU and nITS rDNA sequences reveal the natural relationships of the lichen families Physciaceae and Caliciaceae. Taxon 51:655–660
Weiβ M, Yang ZL, Oberwinkler F (1998) Molecular Phylogenetic studies in the genus Amanita. Can J Bot 76:1170–1179
Wen HA, Ying JZ (2001) Study on the genus Russula Pers. from China II.Two new taxa from Yunnan and Guizhou. Mycosystema 20(2):153–155
Westberg M, Millanes A, Knudsen K, Wedin M (2015) Phylogeny of Acarosporaceae (Lecanoromycetes, Ascomycota, Fungi) and the evolution of carbonized ascomata. Fungal Divers. doi:10.1007/s13225-015-0325-x
Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL, Boonmee S, Braun U, Dai DQ, D’souza MJ, Diederich P, Dissanayake A, Doilom M, Hongsanan S, Jones EBG, Groenewald JZ, Jayawardena R, Lawrey JD, Liu J-K, Luecking R, Madrid H, Manamgoda DS, Muggia L, Nelsen MP, Phookamsak R, Suetrong S, Tanaka K, Thambugala KM, Wanasinghe DN, Wikee S, Zhang Y, Aptroot A, Ariyawansa HA, Bahkali AH, Bhat DJ, Gueidan C, Chomnunti P, De Hoog GS, Knudsen K, Li W-J, McKenzie EHC, Miller AN, Phillips AJL, Piatek M, Raja HA, Shivas RS, Slippers B, Taylor JE, Tian Q, Wang Y, Woudenberg JHC, Cai L, Jaklitsch WM, Hyde KD (2014) Naming and outline of Dothideomycetes-2014 including proposals for the protection or suppression of generic names. Fungal Divers 69:1–55
Winter G (1885) Pilze–Ascomyceten. In: GL Rabenhorst’s Kryptogamen–Flora von Deutschland, Oesterreich und der Schweiz. 1:65–528
Wolfe BE, Tulloss RE, Pringle A (2012) The irreversible loss of a decomposition pathway marks the single origin of an ectomycorrhizal symbiosis. PLoS ONE 7(7):e39597
Wölfel G, Noordeloos ME (2001) Neue oder bemerkenswerte Entoloma–Arten der Kanarischen Inseln. Öst Z Pilzk 10:185–200
Wong SW, Hyde KD, Jones EBG (1998) Annulatascaceae, a new ascomycete family from the tropics. Syst Asc 16:17–25
Woudenberg JHC, Aveskamp MM, de Gruyter J, Spiers AG, Crous PW (2009) Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia 22:56
Woudenberg JHC, Groenewald JZ, Binder M, Crous PW (2013) Alternaria redefined. Stud Mycol 75:171–212
Wu X, Schoch CL, Boonmee S, Bahkali AH, Chomnunti P, Hyde KD (2011) A reappraisal of Microthyriaceae. Fungal Divers 51(1):189–248
Wu G, Feng B, Xu JP, Zhu XT, Li YC, Zeng NK, Hosen I, Yang ZL (2014) Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers 69(1):93–115
Wubah DA, Fuller MS, Akin DE (1991) Studies on Caecomyces communis: morphology and development. Mycologia 83:303–310
Wuczkowski M, Passoth V, Turchetti B, Andersson AC, Olstorpe M, Laitila A, Theelen B, van Broock M, Buzzini P, Prillinger H, Sterflinger K, Schnurer J, Boekhout T, Libkind D (2011) Description of Holtermanniella takashimae sp. nov., Holtermanniella gen. nov. and proposal of the order Holtermanniales to accommodate Tremellomycetous yeasts of the Holtermannia clade. Int J Syst Evol Microbiol 61:680–689. doi:10.1099/ijs.0.019737-0
Yang ZL (1997) Die Amanita-Arten von Südwestchina. Biblioth Mycol 170:1–240
Yang ZL (2005) Flora fungorum sinicorum, vol 27, Amanitaceae. Science Press, Beijing (in Chinese)
Yang ZL (2015) Atlas of the Chinese species of Amanitaceae. Science Press, Beijing (in Chinese)
Yang ZL, Weiβ M, Kottke L, Oberwinkler F, Nehls U, Guttenberger M, Hampp R (1999) Amanita. In: Cairney JWG, Chambers SM (eds) Ectomycorrhyzal fungi: key genera in profile. Springer, New York, 201–230p
Yang ZL, Weiβ M, Oberwinkler F (2004) New species of Amanita from the eastern Himalaya and adjacent regions. Mycologia 96(3):636–646
Ying JZ (1983) A study on Russulavirdi-rubrolimbata sp. nov. and its related species of subsection virescentinas. Mycosystema 2(1):34–37
Yuan HS (2014) Molecular phylogenetic evaluation of Antrodiella and morphologically allied genera in China. Mycol Prog 13:353–364
Zang M, Yuan MS (1999) Contribution to the knowledge of new basidiomycetous taxa from China. Acta Botanica Yunnanica 21(1):37–42
Zelski SE, Raja HA, Miller AN, Shearer CA (2011) Chaetorostrum quincemilensis, gen. et sp. nov., a new freshwater ascomycete and its Taeniolella-like anamorph from Peru. Mycosphere 2:593–600
Zhang LF, Yang JB, Yang ZL (2004) Molecular phylogeny of eastern Asian species of Amanita (Agaricales, Basidiomycota): taxonomic and biogeographic implications. Fungal Divers 17:219–238
Zhang N, Castlebury LA, Miller AN, Huhndorf SM, Schoch CL, Seifert KA, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung GH (2006) An overview of the systematics of the Sordariomycetes based on four-gene phylogeny. Mycologia 98:1077–1088
Zhang K, Ma LG, Zhang XG (2009a) New species and records of Shrungabeeja from southern China. Mycologia 101(4):573–578
Zhang Y, Fournier J, Crous PW, Pointing SB, Hyde KD (2009b) Phylogenetic and morphological assessment of two new species of Amniculicola and their allies (Pleosporales). Persoonia 23:48–54
Zhang Y, Schoch CL, Fournier J, Crous PW, De Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Hyde KD (2009c) Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud Mycol 64:85–102
Zhang Y, Wang HK, Fournier J, Crous PW, Jeewon R, Pointing SB, Hyde KD (2009d) Towards a phylogenetic clarification of Lophiostoma / Massarina and morphologically similar genera in the Pleosporales. Fungal Divers 57:149–210
Zhang H, Jones GEB, Zhou DQ, Bahkali AH, Hyde KD (2011) Checklist of freshwater fungi in Thailand. Cryptogam Mycol 32(2):199–217
Zhang H, Hyde KD, Mckenzie EHC, Bahkali AH, Zhou D (2012a) Sequence data reveals phylogenetic affinities of Acrocalymma aquatica sp. nov., Aquasubmersa mircensis gen. et sp. nov. and Clohesyomyces aquaticus (Freshwater Coelomycetes). Cryptogam Mycol 33:333–346
Zhang Y, Crous PW, Schoch CL, Hyde KD (2012b) Pleosporales. Fungal Divers 52:1–225
Zhang H, Hyde KD, Abdel-Wahab MA, Abdel-Aziz FA, Ariyawansa HA, KoKo TW, Zhao RL, Alias SA, Bahkali AH, Zhou DQ (2013a) A modern concept for Helicascus with a Pleurophomopsis-like asexual state. Sydowia 65(1):147–166
Zhang Y, Fournier J, Phookamsak R, Bakhali AH, Hyde KD (2013b) Halotthiaceae fam. nov. (Pleosporales) accommodates the new genus Phaeoseptum and several other aquatic genera. Mycologia 40:107–110
Zhang Y, Yang Y, Zhang X, Cui B, He S, Fournier J (2014) Helicascus gallicus sp. nov., a new freshwater pleosporalean ascomycete from France. Phytotaxa 183(3):183–192
Zhao RL, Karunarathna S, Raspé O, Parra LA, Guinberteau J, Moinard M, Kesel AD, Barroso G, Courtecuisse R, Hyde KD, Guelly AK, Desjardin DE, Callac P (2011) Major clades in tropical Agaricus. Fungal Divers 51:279–296
Zhao RL, Desjardin DE, Callac P, Parra LA, Guinberteau J, Soythong K, Karunarathna SC, Zhang Y, Hyde KD (2012) Two species of Agaricus sect. Xanthodermatei from Thailand. Mycotaxon 122:187–195. doi:10.5248/122.187
Zhou LW, Dai YC (2012) Wood-inhabiting fungi in southern China 5. New species of Theleporus and Grammothele (Polyporales, Basidiomycota). Mycologia 104(4):915–924
Zycha H, Siepmann R, Linnemann G (1969) Mucorales. J. Cramer, Lehre, pp 1–355
Acknowledgments
The authors extend their sincere appreciations to the Deanship of Scientific Research at King Saud University for its funding this Prolific Research Group (PRG-1436-09). This research was also supported by Featured microbial resources and diversity investigation in Southwest Karst area (2014FY120100). Kevin D. Hyde thanks the Chinese Academy of Sciences, project number 2013T2S0030, for the award of Visiting Professorship for Senior International Scientists at Kunming Institute of Botany. K.D. Hyde thanks the Thailand Research Fund grant – Taxonomy, Phylogeny and biochemistry of Thai Basidiomycetes (BRG 5580009). This study was financially supported by the Joint Funds of the National Natural Science Foundation of China and Yunnan Provincial Government (Grant No. U1302263).Y.P. Xiao and T.C. Wen are grateful to The National Natural Science Foundation of China (No. 31460012 & No. 31200016). R.L. Zhao is grateful to The National Natural Science Foundation of China (No. 31000013, 31360014 and 31470152). J.C. Kang is grateful to the Agricultural Science and Technology Foundation of Guizhou Province (Nos. NY[2013]3042), the International Collaboration Plan of Guizhou Province (No. G [2012]7006) and the Innovation Team Construction for Science and Technology of Guizhou Province (No. [2012]4007) from the Science and Technology Department of Guizhou Province, China. Vizzini and co-workers are grateful to their friends Guy Redeuilh† and Mauro Sarnari for having supported their study with the collection of the CalaGonone (NU) and Monte Argentario (GR), which for first cleared us the distinctive characters of this species of Xerocomellus. Special thanks also to Stefano Restocchi for his aid in performing microscopic features investigation. They thank Kanyawim Kirtikara and Lily Eurwilaichitr at BIOTEC for their continued interest and support. A. Millanes and M. Wedin acknowledge a grant from the Spanish Ministry of Economy and Competitiveness (CGL2012–40123), M. Wedin and M. Westberg acknowledge support from The Swedish Taxonomy Initiative (SvenskaArtprojektet, administered by the Swedish Species Information Centre/ArtDatabanken), and M. Wedin gratefully received support from the Swedish Research Council (grant VR 621-2012-3990). New species described by R. Lücking and co-authors (J. D. Lawrey, H. T. Lumbsch) resulted from the NSF projects DEB 0841405 (PI J. Lawrey; Co-PIs: R. Lücking, P. Gillevet), DEB-1354884 (PI H. T. Lumbsch; Co-PI: R. Lücking), DEB-1025861 (PI H. T. Lumbsch; Co-PI: R. Lücking) and DEB-0715660 (PI R. Lücking). JJ. Luangsa-ard, K. Tasanathai, A. Khonsanit, D. Thanakitpipattana, S. Somrithipol was supported by the project “Raising the quality and standards of microorganism data for use in biotechnology” grant number P-12-01829. They thank the Department of National Parks for supporting taxonomic and diversity studies. They thank Prof. YodathaiThebtaranonth for permission to collect in a new area in Loei Province. U. Pinruan, S. Sommai, S. Nuankaew, S. Suetrong was supported by the project collection and identification of additional fungi for research and corporation projects of BIOTEC, Grants P-12-01294. D.X. Lima, C.A. Fragoso de Souza, C.M. de Souza-Motta, A.L.C.M. de Azevedo Santiago were supported from the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) for a post-graduate scholarship to Diogo X. Lima. They also thank Conselho Nacional de DesenvolvimentoCientífico e Tecnológico for financial support through the projects: ‘Mucoromycotina in upland forests from the semi-arid of Pernambuco’ (CNPq - 458391/2014-0), ‘Mycological diagnosis in native and disturbed areas in the Catimbau National Park, Pernambuco, Brazil’ (552083/2011-9/CNPQ) and Programa de PesquisaemBiodiversidade do Semiárido - PPBIO (457498/2012-9/CNPq). K. Voigt was supported by the JSMC at the University Jena and the Leibniz Institute for Natural Product Research and Infection Biology–HKI Jena and the Deutsche Forschungsgemeinschaft (DFG) within CRC/TR 124 FungiNet: project Z1. Tuula Niskanen, Kare Liimatainen, and Joe Ammirati are grateful to Oskar Öflunds Stiftelse, the Daniel E. Stuntz Memorial Foundation, and Finnish Cultural Foundation. Qing Cai, Yang Y. Cui and Zhu L. Yang are very indebted to Drs. Flavius Popa, TolgorBau and Fang Li for providing them materials and images of Amanita, and to the Joint Funds of the National Natural Science Foundation of China and Yunnan Provincial Government (Grant No. U1302263). M. Ghobad-Nejhad acknowledges the support received from Iran National Science Foundation (INSF), project number 93001054. Huang Zhang thanks for the Scientific Foundation of Kunming University of Science and Technology (Project ID: 14118899). Buyck & V. Hofstetterare grateful to the staff members of CNRE in Antananarivo for logistic support and field assistance. Their field work in Madagascar was financed in part through grants from the National Geographic Society (grants 6365-98, 7921-05) and in more recent years by the ATM-projects “Past and present biodiversity” and “Emergences” of the Muséum national d’histoirenaturelle (Dirs. Ph. Janvier and S. Peigné). For sequencing, this study received support from the ATM-projects “Barcode of life” (Dirs. L. Legall and S. Samadi) and “Emergences” (Dir. S. Peigné) of the Muséum national d’histoirenaturelle. Z.L. Luo and H.Y. Su are grateful to The National Natural Science Foundation of China (No.31460015). Ellen Larsson acknowledge the Swedish Taxonomy Initiative, ArtDatabanken, SLU Uppsala (grant dha152/2011). Erio Camporesi thanks Giancarlo Lombardi, Sergio Montanari and Gigi Stagioni for their invaluable help in the identifying host plants. S. Maharachchikumbura thanks the Featured microbial resources and diversity investigation in Southwest Karst area (2014FY120100). K.L. Pang would like to thank the Ministry of Science and Technology, Taiwan, for financial support (NSC101-2621-B-019-001-MY3). R. Phookamsak (PHD/0090/2551), Chayanard Phukhamsakda (PHD/0020/2557) and Mingkwan Doilom (PHD./0072/2553) acknowledge the The Royal Golden Jubilee Ph.D. Program under the Thailand Research Fund. Saranyaphat Boonmee would like to thank TRF/BIOTEC Special Program which supported this work (BRT R_251181, BRT R_253012). Ausana Mapook is grateful to Research and Researchers for Industries (RRI) PHD57I0012 under the Thailand Research Fund for providing financial support. T.T.T. Nguyen, H.W. Lee and H.B. Lee were supported by the Graduate Program for the Undiscovered Taxa of Korea(NIBR201524202) and by the Project on Survey and Discovery of Indigenous Fungal Species of Korea funded by NIBR of the Ministry of Environment (MOE), Republic of Korea. T.C. Wen and X.Y. Zeng are grateful to the Modernization of Traditional Chinese Medicine Program of Guizhou Province (No. [2012]5008) and the Science Foundation of Guizhou University (No. 201309). K. Tanaka would like to thank the Japan Society for the Promotion of Science (JSPS, 25440199) and Hirosaki University Grant for Exploratory Research by Young Scientists and Newly‒appointed Scientists for financial support. Hiran Ariyawansa is grateful to A.D Ariyawansa, D.M.K Ariyawansa, Ruwini Ariyawansa and Amila Gunasekara for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 2
(DOC 31 kb)
Supplementary Table 3
(DOC 35 kb)
Supplementary Table 4
(DOC 28 kb)
Rights and permissions
About this article
Cite this article
Ariyawansa, H.A., Hyde, K.D., Jayasiri, S.C. et al. Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 75, 27–274 (2015). https://doi.org/10.1007/s13225-015-0346-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-015-0346-5