Abstract
After a short historical overview of past systematic studies on Cantharellus, discussing delimitation and species diversity of the genus as well as previous, morphology-based, infrageneric classifications, this paper presents the first molecularly-based infrageneric classification of this genus using a multigene phylogenetic approach (nucLSU, mitSSU, RPB2 and tef-1) on a dataset that covers approximately halve of the described chanterelles worldwide, including many type specimens. Six subgenera are recognized and the recognition of subgenus Afrocantharellus as a separate genus is not accepted. The taxonomic value of individual morphological features is discussed as challenged by this new multigene phylogeny which comprises five new sections, one new subgenus and many emendations for previously recognized infrageneric groups. The paper discusses the observed discrepancy in biodiversity of Cantharellus when comparing between studies that focus either on below- or above-ground presence. A preliminary biogeographic hypothesis suggests an ‘out of Africa’ Gondwanan origin as a result of vicariance and subsequent migrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Short historical overview of the study of Cantharellus
The name Cantharellus Adans.: Fr. is derived from the French word “chanterelle” for which we find the first published records in the middle of the 17th century (Bauhin and Cherler 1651). As pointed out by Eyssartier and Buyck (2000), the first use of the name “Chanterel” at the generic level was not by de Jussieu (1789) as often assumed, but by the French botanist Adanson (1763). His concept and genus name was later adopted - and as such sanctioned - by Fries (1821) with the current, latinized orthograph.
The generic concept of Cantharellus has evolved considerably over time. Persoon (1797) accepted Cantharellus and Craterellus Pers.:Fr. for a short time as two smaller, separate, but closely related genera, but then transferred both genera (Persoon 1801) to the much larger genus Merulius Fr. which grouped most fungi that had a veined hymenophore but were often morphologically very different in general habit (including e.g. Serpula (Pers.) Gray). Cantharellus, Craterellus and look-alikes constituted then together “Cantharellus”, one of four main subgroups recognized in Merulius. Fries (1838) interpreted Craterellus and Cantharellus again as separate genera but his generic concept was still much wider than today and Fries’ circumscription of Cantharellus applied to all fungi that morphologically resemble a chanterelle. Fries (1838) also introduced a systematical gap between craterelles and chanterelles, classifying the former in his “Auricularini”—which he later renamed “Thelephorei” (Fries 1874)—side by side with other fungi with a primarily smooth hymenophore, such as Stereum Hill ex Pers. or Thelephora Ehrh. ex Willd., for example.
During the second half of the 19th century, the introduction of microscopic features in fungal descriptions allowed for a more refined definition of Cantharellus. Léveillé (1843) followed Persoon’s classification when describing and illustrating for the first time the microscopic features of “Merulius cantharellus” with basidia bearing more than 4 sterigmata. Léveillé (1837) introduced the term ‘cystidium’ in mycology and noted their complete absence in Cantharellus, whereas he described the form of the spores as resembling “wheat grains”. Within the next 30–40 years, nearly all of the microscopically important features of Cantharellus and Craterellus were discovered: homomorphic trama (Forquignon 1886), related to ‘Agaricinae’ (Patouillard 1887), long basidia, similarities with Hygrophoraceae (Fayod 1889), stichic basidia (Juel 1898), evolutionary hypothesis based partly on absence-presence of clamps (Maire 1902). As such, at the dawn of the 20th century, all of the important “modern” features of Cantharellus had already been described, but progress on Cantharellaceae then stagnated for nearly a century. In fact, progress took a step back in Europe when, under the influence of some of the major identification works (e.g. Kühner and Romagnesi 1953), Craterellus was abandoned as a separate genus and included within Cantharellus at the rank of either section or subgenus.
Before the advent of molecular biology, Cantharellus and Craterellus were considered to be part of the “Aphyllophorales” in Friesian classifications, i.e. they were part of those fungi that lack true gills. This concept seemed justified for European taxa or, more in general, for the majority of northern hemisphere taxa and was never questioned, not even when numerous chanterelles with well-developed gill folds were described from subtropical and tropical parts of the world. With the arrival of molecular techniques, taxonomic progress on Cantharellus shifted from Europe to America. In general terms, molecular contributions towards a better understanding of Cantharellus and the cantharelloid clade can be situated at two levels.
A first series of contributions was directly interested in the genus Cantharellus and its delimitation from Craterellus, an interest that was probably triggered by the discovery that ITS sequences of Craterellus and Cantharellus differ significantly in length (Feibelman et al. 1994). Although Feibelman et al. (1997) demonstrated convincingly that Pseudocraterellus Corner and Craterellus did not belong in Cantharellus, it was Dahlman et al. (2000) who finally recombined some species of Cantharellus subgenus Leptocantharellus Corner into Craterellus, thereby circumscribing Cantharellus to its present day concept. With the natural concept of Cantharellus finally established, most of the recent descriptions of new, individual chanterelles continue nevertheless to be principally based on morphology (e.g. De Kesel et al. 2011; Tibuhwa et al. 2008; Tian et al. 2012; Wartchow et al. 2012a, b; Buyck and Randrianjohany 2013), although for some of the more important papers of the last 2–3 years, support from molecular data is now common practice, e.g. for chanterelles from the United States (Buyck et al. 2011; Buyck and Hofstetter 2011; Foltz et al. 2013), the Guyana shield (Wilson et al. 2012), East Africa (Buyck 2012; Buyck et al. 2013a; Tibuhwa et al. 2012) and Madagascar (Buyck et al. 2013b).
A second series of molecular papers focused on the frequent occurrence of convergence among different architectural forms of the fungal fruit body within the older concept of “Hymenomycetes”. Some of these papers contributed therefore indirectly to a better understanding of the relationships beween Cantharellus and some of the other groups within the ‘cantharelloid clade’. Since neither polyporoid (Hibbett and Donoghue 1995), cyphelloid (Bodensteiner et al. 2004), nor gasteroid fungi (Hibbett et al. 1997) have been demonstrated to exist within the cantharelloid clade, the first study that discussed in detail the modern delimitation of the cantharelloid clade was a paper that focused on the development of the hydnoid-clavarioid fruit body in hymenomycetes (Pine et al. 1999). In this paper, the phylogenetic importance of the stichobasidium was underlined when pointing out the close relationship between Hydnum L., Multiclavula R.H. Petersen, Craterellus and Cantharellus. In the past two decennia, a considerable number of papers dealt with the distribution of the resupinate architecture of the fungal fruit body (for discussions see Binder et al. 2005; Larsson 2007). These papers confirmed the placement of Botryobasidium, Ceratobasidiaceae and a polyphyletic Sistotrema within the cantharelloid clade, whereas continuing inventories of the neotropics have demonstrated the existence of resupinate forms in a morphologically suprisingly diverse Clavulina (Henkel et al. 2011; Uehling et al. 2012). In the most recently published paper that focused specifically on the delimitation of the cantharelloid clade, Moncalvo et al. (2007) confirmed the unexpected relationship with the heterobasidiomycetous Tulasnellales, already suggested by Hibbett and Binder (2002), and documented the extreme evolutionary rate heterogeneity in the nuclear ribosomal RNA genes of Tulasnella, Cantharellus and Craterellus. Consequently, Hibbett et al. (2007) proposed Cantharellales, with the provisional inclusion of Tulasnella, as the official nomenclatural rank for the cantharelloid clade.
Species diversity
The economically and commercially very important genus Cantharellus has drawn surprisingly little interest from taxonomists in the past. Part of the explanation resides very likely in the difficulty to recognize good species with the limited variation of the few morphological features available. Indeed, under the microscope, chanterelles exhibit a discouraging monotony: they have no cystidia (Léveillé 1837), their spores lack any type of ornamentation and vary little in size and form, and also hyphal endings in the pileipellis offer little variation in form and size, as well as—with rare exceptions—in their general orientation. The recognition of Cantharellus as a genus can be especially difficult in the tropics and some of the smaller species are nearly impossible to assign to genus in the field. Even careful microscopic examination may leave the morphologist in doubt, particularly in those rare cases where a Cantharellus has dull colors, predominantly unequal gills that do not fork, as well as regularly four-spored basidia (e.g. the Malagasy C. paucifurcatus) or even two-spored basidia (e.g. the African C. croceifolius). In this sense, Corner’s monograph (1966) which treated all fungi with a cantharelloid habit as a single artificial concept is a very understandable approach.
Given the limited micromorphological variation, it is comprehensible that Cantharellus was never easily distinguished from other, morphologically similar genera. After a revision of all 346 species names through detailed examination of available type specimens, Eyssartier (2001) retained merely 59 good and valid species in Cantharellus out of a total of 418 published names (346 specific and 72 infraspecific taxa, 35 of which were related to C. cibarius).
Apart from the long lasting confusion with species in its sister-genus Craterellus, many of the older Cantharellus names have been synonymized with such diverse genera as Guepinia (Auriculariales), Dacryopinax (Dacrymycetales), Arrhenia, Camarophyllus, Campanella, Cantharellula, Clitocybe, Cotylidia, Cuphophyllus, Cyphella, Gerronema, Haasiella, Hohenbuehelia, Hydropus, Hygrocybe, Hygrophorus, Leptoglossum, Marasmiellus, Marasmius, Merulius, Merismodes, Micromphale, Mniopetalum, Mycena, Omphalina, Rimbachia, Tetrapyrgos, Trogia (Agaricales), Afrocantharellus, Goossensia, Pterygellus, Pseudocraterellus (Cantharellales), Podoserpula (Atheliales), Hygrophoropsis, Paxillus (Boletales), Gomphus, Gloeocantharellus (Gomphales), Faerberia, Lentinus (Polyporales) and even Lactarius (Russulales).
Since the revision by Eyssartier (2001), already a total of 40 new species have been published in Cantharellus s.s., mainly from Mediterranean, subtropical and tropical areas (Arora and Dunham 2008; Blanco-Dios 2004, 2011; Buyck 2012; Buyck et al. 2010, 2011, 2013a, b; Buyck and Hofstetter 2011; Buyck and Randrianjohany 2013; Contu et al. 2009; De Kesel et al. 2011; Ducousso et al. 2004; Eyssartier et al. 2009; Foltz et al. 2013; Hermitte et al. 2005; Kumari et al. 2011; Olariaga and Salcedo 2007, 2008; Redhead 2012; Sasia et al. 2003; Shao et al. 2011; Tian et al. 2012; Tibuhwa et al. 2008; Wartchow et al. 2012a, b). Yet, a quick analysis of deposited sequences in the GenBank is enough to realize that a large number of sequenced collections represent undescribed species and many more species of Cantharellus are going to be described over the next years. In the senior author’s estimation, Cantharellus will account worldwide for at least some 200 to 300 species.
Some of the older literature on chanterelles suffered from conservative species concepts that resulted most often in the description of new varieties and forms of European species or in the extension of their distributions to other continents. Previously considered to be widely distributed, C. cibarius is probably one of the most eloquent examples, and recent data seem to suggest its distribution might be limited to the northern parts of Europe (perhaps also to northern parts of Asia and North America, although modern studies are lacking there). Recent molecular studies of the C. cibarius-complex in the United States have resulted in the description of eight new species over the past 5 years (Arora and Dunham 2008; Buyck et al. 2010; Buyck and Hofstetter 2011; Foltz et al. 2013) and this seems only the beginning (Buyck unpubl.). Also in Europe, the publication of a new identification key for European chanterelles (Eyssartier and Buyck 2000) has triggered a renewed interest in Cantharellus which rapidly resulted in the description of six new, principally Mediterranean species in the C. cibarius complex, although all still exclusively based on morphological and ecological features (Blanco-Dios 2004, 2011; Hermitte et al. 2005; Sasia et al. 2003; Olariaga and Salcedo 2007, 2008; Contu et al. 2009). Surprisingly, molecular studies focusing on European Cantharellus are still lacking, but this shortcoming should be repaired soon.
Infrageneric classification
Molecular studies have confirmed the clear separation between Craterellus and Cantharellus, and also allowed for a more precise placement of Cantharellus within the broader context of a monophyletic “Cantharellales”, but they did not have the slightest impact on the infrageneric classification of Cantharellus itself. Nor did tropical inventories of the twentieth century have any substantial impact on the systematics of this genus until recently (Eyssartier and Buyck 2001b). Indeed, the infrageneric classification of Cantharellus has remained very rudimentary for a very long time (Table 1), and this notwithstanding the description, during the second half of the 20th century, of many tropical species with often surprising features.
Whereas all the recent new taxa from the temperate northern hemisphere conform well to the classical concept of Cantharellus, the situation in the tropics and southern hemisphere is quite different. During the 50’s and 60’s, Heinemann (1958, 1959, 1966) and Corner (1966) described and illustrated a number of very original chanterelles from Central Africa, respectively Malaysia. Many of these tropical chanterelles lack clamps for example, a fact that seems to have been ignored by many mycologists up to the present day. Many recent publications on chanterelles still present the presence of clamps as an important diagnostic feature to separate Cantharellus from Craterellus (Hansen and Knudsen 1997; Pegler et al. 1997; Watling and Turnbull 1998; Lindahl 2000; Pilz et al. 2003), something that seems justified only in a strictly temperate northern hemisphere context. Notwithstanding the impressive diversity of true Cantharellus in the paleotropics, most of the earlier recognized subdivisions at infrageneric level (see Table 1) concerned the opposition of true Cantharellus versus species that are now accepted in either Craterellus or Rimbachia (Redhead 1983). The considerable diversity in tropical Cantharellus resulted merely in a few small, mainly monotypic, infrageneric groups. These split-offs had no other reason than to allow for a more practical and easier identification: the monotypic subgenus Cutirellus (Corner 1966) for the then unique species with a trichoderm-like pileipellis, the equally monotypic section Congolenses (Heinemann 1958) for a single, African chanterelle with intensely blackening context, and section Tenues (Heinemann 1958) for some extremely small, tropical African species.
A detailed morphological revision of all available type material for Cantharellus worldwide finally resulted in the first argumented subdivision of Cantharellus species in six subgenera based on macro- and micromorphological features (Eyssartier and Buyck 2001a, b), proposing two diagnostic microcharacters as taxonomically most informative features for a subgeneric division : (1) absence/presence of clamps, a feature still widely believed to be informative solely to distinguish between Craterellus and Cantharellus, and (2): absence/presence of thick-walled, hyphal terminations on the cap surface.
The objective of the present paper is to test the latest morphology-based hypotheses for a subgeneric division of Cantharellus using a multigene phylogenetic approach (nucLSU, mitSSU, RPB2 and tef-1) on a dataset that covers approximately half of the described chanterelles worldwide and includes type specimens whenever possible.
Materials and methods
Molecular data
Taxon sampling, sequence data, new primers and dataset assembling
Nucleotide sequence data were generated for 82 fungal collections and four loci (Table 2). Taxon sampling consisted of 80 Cantharellus collections representing 46 species (not accounting for additional subspecies and five provisional ID’s labeled as ‘aff’ or ‘cf’) and two outgroup taxa, Craterellus tubaeformis and Hydnum cf. repandum, following Moncalvo et al. (2007). Our sampling is representative of all subgenera proposed in the latest infrageneric classification of the genus (Eyssartier and Buyck 2001a, b). DNA was isolated from fresh material stored in cetyl-trimethyl-ammonium-bromide buffer or from dried fruit bodies. Data produced in this study were as described in Hofstetter et al. (2002) for ribosomal genes: part of the nuclear large subunit (nucLSU) and part of the mitochondrial small subunit (mitSSU). The primers used for amplification and sequencing of part of the transcription elongation factor 1-alpha (tef-1) gene were those published by Morehouse et al. (2003), with the exception of a few species for which these primers did not work. For those species, the primer pair tef-1Fcanth: 5′- AGCATGGGTDCTYGACAAG -3′ and tef-1Rcanth: 5′- CCAATYTTRTAYACATCYTGGAG -3′ was designed. New primers were also designed to amplify region 5–7 of the second largest subunit of the RNA polymerase II (RPB2) within Cantharellales (RPB2-5FCanth: 5′-GAAWGCTATTCCGRAAGCTCAC-3′and RPB2-7cRCanth: 5′-ATGCCCAGAATCATRCTTGGRTG-3′). These newly designed RPB2 primers were used together or alternatively with the primers designed by Liu et al. (1999) for this region (fRPB2-5F [or 6F] and fRPB2-7cR). As these newly designed RPB2 primers were not well adapted to sequencing conditions, PCR products were cloned using pSTBlue-1 AccepTor VectorTM Kit (Novagen). Sequencing used the M13F and M13R vector primers and reagents and conditions of the BibDye®Terminator v3.1 Cycle sequencing Kit and an automated capillary sequencer ABI 3700 DNA analyzer (Perkin Elmer, Applied Biosystems, Foster City, CA, USA).
A total of 213 sequences were newly produced for this study: 74 nucLSU, 65 mitSSU, 73 RPB2 and 1 tef-1 (Table 2). We also used published sequences already available in Genbank (Table 2) from previous studies (Buyck et al. 2011, 2013a; Buyck and Hofstetter 2011; Moncalvo et al. 2007; Schoch et al. 2012). Sequences were assembled and edited using the software package Sequencher 3.0 (Gene Codes Corp., USA).
The combined alignment of these four genes totaled 6,674 characters (nucLSU: 1,447 characters; mitSSU: 3,163 characters; RPB2: 1,200 characters; tef-1: 864 characters). After removal of introns and unalignable regions, the final data set contained 3,201 characters (mitSSU: 66 taxa, 488 characters; nucLSU: 79 taxa, 1,107 characters; RPB2: 78 taxa, 977 characters; tef-1: 70 taxa, 629 characters).
Phylogenetic analyses
To test for topological incongruence among loci we used the program compat3 (available at www.lutzonilab.net/downloads). For each individual locus of the combined data set, 500 bootstrap replicates (Felsenstein 1985) were generated with RAxML 7.2.7 (Stamatakis 2006; Stamatakis et al. 2008) and all pairwise comparisons between the four loci were performed with compat3. A conflict between two loci was assumed when a clade was supported as monophyletic with bootstrap support (BS) ≥70 % in one tree, but supported as non-monophyletic in another (Mason-Gamer and Kellogg 1996).
Phylogenetic searches were carried out implementing Maximum Likelihood (ML) and Bayesian analyses. All analyses were run on the computer cluster of the Nano + Bio Center (University of Kaiserslautern, Germany). For the ML analyses the combined data set was partitioned into eight partitions: mitSSU, nucLSU, RPB2 1st position, RPB2 2nd position, RPB2 3rd position, tef-1 1st position, tef-1 2nd position, and tef-1 3rd position. The ML analyses were carried out using RAxML 7.2.1 (Stamatakis 2006), implementing a GTR model of nucleotide substitution (Rodríguez et al. 1990) with a gamma shape distribution and a proportion of invariable sites, and searching for the most likely tree with 500 heuristic replicates. Bootstrap frequencies (Felsenstein 1985; Stamatakis et al. 2008) were estimated with 500 replicates. For the Bayesian analyses, the combined data sets were partitioned as described above. Three independent runs with 10,000,000 generations, and four independent chains each were started with MrBayes 3.1.2 (Ronquist and Huelsenbeck 2003) for each data set, sampling every 500th tree. The burn-in fraction of sampled trees was estimated both by eye with ln-likelihood plots and using AWTY (Nylander et al. 2008). Only nodes that received posterior probabilities (PP) ≥ 0.95 and ML-bootstrap support values (MLBS) ≥ 70 % were interpreted as significantly supported.
Morphological data
This paper is principally based on material collected by the senior author over the past 20 years. All cited specimens are deposited at the mycological herbarium of the Paris’ Natural History Museum (PC) unless otherwise stated. All sequenced specimens were microscopically examined in ammoniacal Congo red after a short pre-heating in KOH, at a magnification of ×1,000. Drawings and measurements of individual elements were made for every specimen at a magnification of ×2,400 using a camera lucida. Part of these observations is summarized in Fig. 2. Species concepts are based on the re-examination of all existing type material for Cantharellus (Eyssartier 2001).
Results and discussion
Phylogenetic analyses
Our ML approach for combinability detected significant conflicts within 3 of the 6 possible pairwise comparisons of single locus bootstrap analyses. NucLSU conflicts with RPB2 and tef-1 for the placement of one of the two collections of Cantharellus platyphyllus subsp. bojeriensis (nr. 458), that is supported as sister species of C. symoensii by BS analysis of the nucLSU (MLBS = 80 %) while RPB2 and tef-1 single gene BS analyses both support it as monophyletic with C. platyphyllus subsp. bojeriensis (nr. 459) and C. platyphyllus (respectively with MLBS = 92 % and MLBS = 98 %). BS analysis of nucLSU supports the monophyly of C. subpruinosus with C. tenuithrix (MLBS = 84 %) while RPB2 supports its monophyly with C. cibarius (MLBS = 90 %). Finally RPB2 and tef-1 BS analyses conflict for the placement of C. densifolius that clusters with significant support with C. albidolutescens (nr. 457) based on RPB2 (MLBS = 85 %) but clusters with the other C. albidolutescens (nr. 456) based on tef-1 (MLBS = 100 %). Each of these three conflicts takes place among very closely related species based on morphology and might consequently result from saturation at the 3rd position of protein-coding genes. Therefore we ignored them and combined the data.
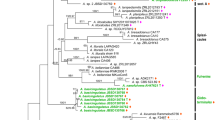
Combining 4 loci for 82 taxa, the best ML tree estimated with RAxML (ln likelihood = −19908.132327) is shown in Fig. 1. Bootstrap proportions (MLBS) and bayesian posterior probabilities (PP) are shown along the branches. The three independent runs of MrBayes plateaued at the same likelihood levels, and after discarding the first 5.000.000 generations as burnin (50 %), the last 10,000 trees of each run were used to calculate the posterior probabilities for internal branches.
ML best tree inferred from the analysis of four loci for 82 specimens. Branches that received both bootstrap support (MLBS) ≥ 70 and Bayesian posterior probabilities (PP) ≥ 95 % are in bold; branches supported by either bootstrap or PP are in gray. Both values (MLBS/PP) are reported along the branches. Names of African-Malagasy chanterelles are in red font, Malayian chanterelles are in lilac and North American and European chanterelles are in green and blue respectively. Outgroups are in black lettering. Numbers on the right refer to the recognized clades as discussed in the Results section
Commented tree topology
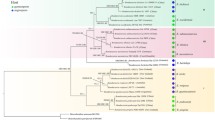
The following paragraphs depict and discuss the six major clades that are here obtained and accepted at subgeneric levell (see Fig. 1). The morphological characters of the sampled species referred to below are mapped on the phylogeny (Fig. 2).
Mapping of various morphological characters for the studied taxa as revealed by original descriptions or recent examination of type specimens by either Bart Buyck (BB) or Guillaume Eyssartier (GE). Taxa are presented as they appear in the phylogeny, with horizontal lines and numbers on the right referring to the clades discussed in the Results section. From left to right, columns of mapped characters correspond to: MACROSCOPIC FEATURES: maximum cap size, squamulose aspect of the cap surface, presence of concentrical veination-anastomosing in between the radial veins or gill-folds, forking of the radial veins or gill-folds, height of the hymenophore, compact (white) or fistulose (black) stipe interior; MICROSCOPIC FEATURES: filamentous (white) or cellular (black) subhymenium type, maximum basidium length (not accounting for extreme values), number of sterigmata for majority of basidia, absence (white) or presence (black) of clamp connections, mean length/width ratio for spores, thickening of hyphal endings on the cap surface, with signs baring one-ended thickenings corresponding to species that have occasional thickened cell walls for terminal cells only. Unless specified otherwise above, black, gray and white dots correspond to strong, weak or absent respectively for character states; COLORS: discoloration of the context (black, blue, bright yellow, rusty), main overall color of the fruit body (lilac, bluish purple, red-pink, yellow, brown), color of the spore print (ochre-yellow, cream, pale pinkish, white)
Clade 1 (subgenus Cantharellus)
This clade (MLBS = 88 %; PP = 0.99) is defined by the presence of the type species of the genus, C. cibarius, and thus corresponds to subgenus Cantharellus. All of its constituant species have abundant clamps and, with the exception of C. quercophilus and to a lesser degree also C. tenuithrix, all of the sequenced species in this subclade possess distinctly thick-walled hyphal extremities in the pileipellis. Both features were considered the main characters allowing for the morphological subdivision of the genus (Eyssartier and Buyck 2001a, b). This clade is entirely composed of northern hemisphere taxa. Most of the species are yellow, but this color is sometimes mixed with greenish, brownish, vinaceous to lilac-purple pigments. Some species have a squamulose cap, although the squamulae are less developed and more appressed compared to the majority of species in clade 2. Clade 1 consists of variously supported subclades. The well-supported subclade 1c (MLBS = 85 %, PP = 0.99) contains C. cibarius and most of its northern temperate look-alikes. The smooth chanterelle, C. lateritius, constitutes subclade 1b. Amethyst chanterelles constitute subclade 1a in which the European C. amethysteus is sister to the Malayian C. subamethysteus, a species that clusters with maximum support (MLBS = 100 %, PP = 1.0) with the North American C. lewisii. The monophyly of 1a + 1b received only significant support in Bayesian analysis (MLBS = 65 %, PP = 0.95). As both the/amethysteus and/lateritius clades include known representatives from the tropics, contrary to clade 1c, these have here been accepted as individual entities.
Clade 2 (subgenus Rubrinus)
The strongly supported Clade 2 (MLBS = 91 %, PP = 1.00) is sister to the previous clade with significant support (MLBS = 80 %, PP = 0.97). Clade 2 is here proposed as separate subgenus (instead of section Isabellinus Eyssart. & Buyck, type species C. isabellinus) because of strong geographical and morphological differences with its sister clade: it is for the moment exclusively composed of tropical species of African or Malagasy origin and all species completely lack clamps in their tissues. Clade 2 is subdivided in two highly supported subclades: subclade 2a (MLBS = 99 %, PP = 1.0) for predominantly medium-sized to large, yellowish-brownish, often strongly squamulose chanterelles, and subclade 2b (MLBS = 94, PP = 1.00) for very small to medium-sized, smooth, yellowish orange to red species, corresponding to section Heinemannianus Eyssart. & Buyck.
In subclade 2a the Malagasy, eucalypt-associated C. sp.ined is clearly separated from the other species nested in that subclade (MLBS = 100 %, PP = 1.00). Near maximum support (MLBS = 99 %, PP = 1.00) is recovered for the monophyly of the African C. tomentosus, C. isabellinus var. parvisporus, C. addaiensis and the Malagasy C. paucifurcatus. Resolved as sister to these species, however without support, the African C. miomboensis occupies an isolated position. Several species in this subclade possess very small basidia (Fig. 2). One of those, the very small C. addaiensis (= C. floridulus sensu auct., see Buyck 2012), differs from the other, closely related taxa in its bright red color and thin-walled hyphal extremities. Two other terminal relationships received maximal support: the monophyly of C. albidolutescens with C. densifolius and the monophyly of C. aff tanzanicus with C. tanzanicus. Both species groups are suggested to be monophyletic but this relationship is only supported by Bayesian analysis (MLBS = 50 %, PP = 0.99).
Subclade 2b contains C. heinemannianus, type species of the hitherto monotypic section Heinemannianus, previously placed in subgenus Cantharellus (Eyssartier and Buyck 2001a, b). All of its members lack the squamulose surface of pileus and stipe, most lack also distinctly thick-walled hyphal endings, while half of the species are very small (1–2[3] cm diam.). In this subclade, C. heinemannianus and C. ambohitantelyensis are resolved, but not supported, as sister species and are clearly separated from a strongly supported subclade (MLBS = 84 %, PP = 1.00) including C. sebosus (a relatively small, yellow cibarius look-a-like from the Uapaca woodlands in Madagascar (MLBS = 100 %, PP = 1.00) and a well resolved and strongly supported (MLBS = 97 %, PP = 1.00) species group including C. gracilis and a monophyletic Malagasy C. ibityensis and African C. humidicolus (MLBS = 100 %, PP = 1.00).
Clade 3 (subgenus Cinnabarinus)
This well-supported clade (MLBS = 81 %, PP = 1.00) contains predominantly small species that are mostly orange, pink or red, while some have also purplish tints. All possess abundant clamps and nearly all have thin-walled hyphal ends at the cap surface (Fig. 2). Cantharellus afrocibarius, a medium-sized to large, fleshy, yellow-capped and often caespitose species that grows in the miombo woodlands and resembles C.cibarius, is the odd exception in this group. The monophyly of the Malagasy species pair C. aff. decolorans (eucalypt-associated) + C. decolorans received maximum support (MLBS = 100 %, PP = 1.00). The remainder of the species form a sisterclade only resolved with Bayesian support as monophyletic (MLBS < 50 %, PP = 0.98). Within the latter subclade, only the terminal relationships are supported: the African C. conspicuus + C. fistulosus (MLBS = 88 %, PP = 0.99) and the North American C. texensis + C. cinnabarinus (MLBS = 99 %, PP = 1.00).
Clade 4 (subgenus Parvocantharellus)
This clade is suggested to be monophyletic and sister, however without support, to clade 3. All species nested in this clade possess abundant clamps and have predominantly thin-walled hyphal ends at the cap surface.
Morphologically and phylogenetically distinct from the other species of clade 4, the Malagasy C. subcyanoxanthus and a morphologically similar African specimen constitute subclade 4c (MLBS = 100, PP = 1.00). Both specimens belong to the C. cyanoxanthus species complex (see Buyck 2012), exclusively known from Madagascar and tropical Africa, with species possessing very strong, blue-violet-lilac to vinaceous colors on the fruit bodies and a yellowing context (particularly in the stipe base). Rather than leaving this clade orphan because of the lack of bootstrap support, we accept it provisionally as part of a larger clade 4 as resolved by the analyses.
Two more subgroups are resolved and significantly supported as monophyletic within this clade (4a and 4b; MLBS = 74 %, PP = 1.00) and with strongly supported internal relationships (MLBS = 87–100 %). Subclade 4a is composed of several collections of a single species (taken sensu lato) possessing a strongly blackening context and reacting strongly with most macrochemical reagents: the Malagasy, eucalypt-associated C. aff. congolensis and the African C. congolensis, type species of the monospecific subgenus Afrogomphus Eyssart. & Buyck. Subclade 4b comprises several, small to very small taxa that have a northern hemisphere distribution and range in color from yellow-orange to brown: the North American species pair C. tabernensis + C. appalachiensis is sister to a subclade in which the North American C. minor is again sister to the European species pair C. pseudominimus + C. romagnesianus, the type species of subgenus Parvocantharellus, name here adopted for this clade.
Clade 5 (subgenus Pseudocantharellus)
This monospecific clade consists of two specimens of the recently described Malagasy C. subincarnatus subsp. rubrosalmoneus (MLBS = 100 %, PP = 1.00), a taxon that is morphologically very close to the very rare Central African type variety and the nearly identical C. rhodophyllus Heinem. (see Buyck et al. 2013b). Cantharellus ruber Heinem., type species of subgenus Pseudocantharellus Eyssart. & Buyck, is a species occurring in the Zambezian woodlands with discoloring fruit bodies and nearly identical morphological features. It should, therefore, be very closely related. We were unable to obtain good sequences from our C. ruber collections, but a preliminary analysis of partial LSU data for this species (Tibuhwa et al. 2012), suggest indeed a close relationship to our Malagasy collections of C. subincarnatus. We therefore identified clade 5 as subgenus Pseudocantharellus until additional sequences of C. ruber allow for a well supported placement.
The reasons for this clade being genetically so different from the rest of the genus are for the moment impossible to understand as C. subincarnatus and allies are morphologically completely ‘normal’ chanterelles. Notwithstanding the lack of distinctly thick-walled cells in their pileipellis, Eyssartier and Buyck (2001a, b) had even placed C. subincarnatus in the core-group of the genus (subgenus Cantharellus section Cantharellus, corresponding to clade 1 in our phylogeny) because of the typical cibarius-like habit and abundant clamps.
Clade 6 (subgenus Afrocantharellus)
This highly supported clade in our phylogeny (MLBS = 100 %, PP = 1.00), corresponds to subgenus Afrocantharellus Eyssart. & Buyck (type species: C. symoensii). Its sister position to the rest of the genus obtained only weak support from Bayesian inference (PP = 0.96). All species have a high proportion of four-spored basidia, lack clamp connections and have thin-walled hyphal extremities at the cap surface. All taxa are tropical, with a distribution that is presently limited to Africa + Madagascar and Malaysia.
This clade contains two fully resolved and supported subclades: the first (6a: MLBS = 97 %, PP = 0.98) places C. splendens sister with high support to C. cuticulatus + C. cerinoalbus, two very closely related Malayan species. Cantharellus cuticulatus had been set apart from the rest of the genus in the monotypic section Cutirellus Corner because of the trichodermal structure of the pileipellis, a feature shared with the African C. splendens. The second subclade (6b: MLBS = 100 %, PP = 1.00) is comprised of taxa lacking the trichodermal structure in the pileipellis. The African C. platyphyllus and its Malagasy subspecies bojeriensis, both remarkable because of their sometimes distinctly bluish context, are clearly different from the African C. symoensii, which lacks this bluish context and has different spores (Fig. 2).
General discussion
Phylogenetics versus morphology
When one realizes the limited extent of morphological variation in the genus, especially at the microscopic level (Fig. 2), it is surprising to see that our multigene analysis (Fig. 1) corresponds in several respects rather well to the latest morphological classification proposed by Eyssartier and Buyck (2001a, b). This classification introduced for the first time as main criteria for an infrageneric division the presence/absence of clamps in combination with the presence/absence of thick-walled hyphal extremities in the pileipellis and, as secondary characters, some general macroscopic features (size, context discoloration, general coloration, etc.…) as taxonomic informative characters for the recognition of six major subdivisions in the genus. In the following paragraphs we will discuss the taxonomic value of these individual morphological features as challenged by our multigene phylogeny.
-
Clamp connections. − Our phylogeny clearly confirms that, just as in sister-genus Craterellus, the genus Cantharellus is divided in a number of strongly supported groups of species that either lack or possess distinct clamps at virtually all septa. As this feature is perfectly stable within each of the six main clades that result from our phylogenetic analyses, its importance as a taxonomically informative character is hereby confirmed. Interestingly, clamps are absent in those clades that lack northern hemisphere representatives (clades 2 and 6), with the exception of the monospecific clade 5 for C. subincarnatus.
-
Hyphal terminations. – The presence of thick-walled hyphal extremities in the pileipellis was considered to be of great importance (Eyssartier and Buyck 2001a, b) and to be characteristic for subgenus Cantharellus sensu Eyssart. & Buyck (corresponding to clades 1 + 2), although with some exceptions, e.g. C. cyanoxanthus, C. friesii, C. subincarnatus which were placed in subgenus Cantharellus on the basis of their general morphology, notwithstanding the fact that these species had hardly thickened terminal cells. Our phylogenetic analyses now place these species in different subgenera which might give the impression that this feature is more waterproof than previously thought. However, some additional, more recently described species with irregularly thickened to thin-walled hyphal endings have since been suggested as close relatives to C. cibarius in analyses based on the protein coding gene tef-1 (Buyck et al. 2010; 2013a, 2013a, b; Buyck and Hofstetter 2011) and these are now indeed confirmed as good species of subgenus Cantharellus: e.g. C. quercophilus, C. tenuithrix. Also the monotypic section Heinemannianus (clade 2b) has recently been extended to embrace a number of species with thin-walled hyphal extremities, leaving C. heinemannianus as sole thick-walled species in this section, although its sister-species, C. ambohitantelyensis, does have some dispersed, slightly thickened hyphae at the cap surface. According to Eyssartier (2001), the thickness of the hyphal endings in C. heinemannianus varies among collections.
On the other hand, our analyses now show that thick-walled extremities also occur outside clades 1 + 2, viz. in clade 3, where C. cinnabarinus has distinctly thickened hyphal terminations (see Buyck et al. 2011), but in this case the thickening of the cell wall is more irregular, more variable and often restricted to the last one or two terminal cells, not to five or more subterminal cells as is often the case in clades 1 and 2. The closely related C. friesii was also described by Eyssartier (2001) as having only slightly thickened, ‘refringent’ hyphae, and we have observed similar refringent walls in C. decolorans and C. afrocibarius. Absence of distinctly thick-walled hyphal endings remains a good feature for clades 4, 5 and 6.
We can therefore conclude that the presence of distinctly thick-walled (>1 μm) hyphal endings in the cap is less clear-cut than previously suggested, but remains a valuable indication for the systematic position of a chanterelle. Example given, Fig. 2 clearly shows that a chanterelle with distinctly thickened hyphal endings in the northern hemisphere (clamps always present), belongs in subgenus Cantharellus (clade 1) when it is yellowish-brownish or has violet tinges, or belongs in subgenus Cinnabarinus (clade 3) when it is reddish-orange. If one collects such a species in the southern hemisphere (necessarily without clamps if it has thickened cell-walls), then it belongs in subgenus Rubrinus sect. Isabellinus (clade 2a) if it is yellowish-brownish, or in subgenus Rubrinus sect. Heinemannianus (clade 2b) if it is orange-red.
Finally, the form of the terminal cell of hyphal endings at the cap surface is not informative for the placement of a species, but can certainly be a useful feature for identification. Most chanterelles have more or less cylindrical terminal cells, but in some cases the terminal cells are often clavate (e.g. C. cinnabarinus, C. tabernensis) or either strongly undulate or repetively constricted near the apex (e.g. C. sebosus, C. tomentosus, C. paucifurcatus).
-
General size. − The general size of the basidiomata has been used in past morphology-based classifications for the delimitation of some infrageneric groups (e.g. sect. Tenues, subgenus Parvocantharellus – see Table 1). From a practical point of view, we can group species in categories with respect to the cap diameter: very small (mostly ca 1 cm diam), small (2–4 cm diam.), medium-sized (4–10 cm diam.), large (10–20 cm diam.) and very large (20–30 cm diam.). One the one hand, our phylogeny suggests indeed that size matters as small to very small species tend to group together within clades 4b (sect. Flavobrunnei), 3 (subg. Cinnabarinus) and 2b (sect. Heinemannianus) for example. On the other hand, conspicuously small taxa are absent from clades 1, 5 and 6.
-
Stipe. − The stipe of chanterelles is generally believed to be solid and firm as opposed to the hollow stipe that perforates the cap in Craterellus. Nevertheless, several of the very small chanterelles have a fistulose, although not perforate stipe (e.g. C. fistulosus, C. minor).
-
Hymenophore configuration. − The configuration of the hymenophore has been used previously to delimit clade 6 (subgenus Afrocantharellus) in which some of the African species have the appearance of a Cuphophyllus (Hygrophoraceae) because of their exceptionally well-developed gill-folds (Eyssartier and Buyck 2001a, b). Our phylogeny gives maximum support to this subgenus which is in our dataset now extended to encompass also two species from Malaysia (Eyssartier et al. 2009). These Malayan species have nevertheless much less well-developed gill-folds and, for example, C. cuticulatus had been placed in subgenus Cantharellus by Eyssartier and Buyck (2001a, b).
At the other extreme of this morphological range, clade 1 (subgenus Cantharellus) is the only clade that includes a species with a constantly (nearly) smooth hymenophore in our phylogeny (C. lateritius, clade 1b). This subgenus exhibits undoubtedly the weakest overall hymenophore development between species. Apart from C. lateritius and the Chinese C. vaginatus, which also belongs in clade 1 (Shao et al. 2011), the hymenophore of the other species ranges from low gill-like folds to a hardly perceptible veination that is usually intensily anastomosing and abundantly forked.
In the other clades, the hymenophore configuration is more variable, with several species having well-developed gill-folds, in some cases accompanied by a near absence of both furcations and insterstitial anastomoses. Although there are no species other than C. lateritius in our sampling that have a constantly ‘smooth’ hymenophore, occasional specimens of C. sebosus or C. ambohitantelyensis (both in sect. Heinemannianus, clade 2), come quite close to C. lateritius in terms of (absence of) hymenophore development (see Buyck et al. 2013b).
Our analyses suggest that hymenophore development may show clear tendencies in some major clades, but is not taxonomically informative. Hymenophore development can even be extremely variable within a single species or even within a single collection.
-
Hymenium composition. − The constituant elements of the hymenium are in theory limited to basidia in various stages of their development since distinct types of cystidia are reputedly absent throughout the genus. Nevertheless, in several species we observed cells that were unlikely to develop into mature basidia because of their (sometimes much larger) size and different form. Therefore, leptocystidia taken in the broadest sense, i.e. thin-walled, sterile cells that are not going to develop into basidia, do occur in the hymenium of chanterelles. In our opinion, such cells represent aborted basidia and their presence seems not taxonomically informative. We have found unexpectedly high numbers of such leptocystidia in some African species, e.g. certain specimens of C. splendens, C. heinemannianus or C. humidicolus. (Fig. 3).
-
Basidia. − Cantharellus is reputed for producing very long and narrow basidia and this character is often claimed to be a generic feature. Yet, there is considerable variation in the size of basidia (Fig. 2) and this character seems to have some phylogenetic signal. Whereas most Cantharellus have basidia that are on average ca. 80 μm long, the African chanterelles of clade 2a, for example, include several species with extremely short basidia (some basidia measuring only 25 μm), up to five times shorter compared to species having the longest basidia (up to 120–140 μm) in the genus. The latter are mostly found in clade 1, especially sections Sublaeves and Amethystini, as well as many of the chanterelles that have a poorly developed hymenophore (apart from C. lateritius, also for example C. afrocibarius, C. sebosus, C. solidus, etc.…). Length of basidia is not correlated with the size of the fruit bodies and one of the smallest European chanterelles, C. pseudominimus, has particularly long basidia.
The number of spores per basidium is most frequently five to six (publications citing regularly two- to four-spored basidia for Cantharellus are generally based on inaccurate observations: e.g. Buyck 1994; Eyi Ndong et al. 2011). There exist nevertheless exceptions to this rule. In clade 4b, the north American C. appalachiensis may produce predominantly seven to eight-spored basidia (Buyck et al. 2010; Shao et al. 2011), whereas in the African clade 2a there is a clear tendency towards basidia with less spores. In subgenus Afrocantharellus, several taxa show a remarkably high percentage of four-spored basidia, but these are always mixed with five and six-spored basidia. Exclusively two-spored basidia have so far only been confirmed for two tropical African species of still uncertain systematic position (not represented in our phylogeny): the recently described C. solidus (De Kesel et al. 2011) and the unsufficiently known C. croceifolius as confirmed by the holotype examination (Eyssartier 2001).
-
Spores. – Size and form of spores vary too little to be relevant for the systematic position of the species but may nevertheless be important for the identification of certain species. Example given: they constitute the single distinctive character to distinguish between frequent lookalikes of C. platyphyllus and C. symoensii (clade 6).
Spore print color varies from white to yellow or pinkish salmon. Yet, good spore deposits that are sufficient for a precise appreciation of spore color are more difficult to obtain for chanterelles: unlike most gilled hymenomycetes, they form long-lived fruit bodies that start to produce spores from the very first primordial stages and continue to do so over much longer periods but apparently at a much slower pace (Reijnders 1963; Eyssartier 2001). As a consequence, information on spore print color in descriptions of new taxa is rare, whereas a good color evaluation of the obtained spore print remains difficult.
Presently available data on spore print color for known chanterelles (see Fig. 2) does not allow for a sufficient appreciation of the evolution of spore print color in Cantharellus, but our analyses do not seem to suggest that different subgenera may be characterized by differences in spore print color.
In some instances, spore print color has been used to differentiate between some very closely related species, e.g. in the C. cibarius complex (Petersen 1969; Foltz et al. 2013).
-
Subhymenium. – The subhymenium is rarely given much attention in descriptions of chanterelles, but the senior author distinguishes two main types of subhymenia: (1) ‘filamentose’ subhymenia (subhymenial cells have a similar diameter as the basidium base and are often long and cylindrical), and (2) a more ‘cellular’ type of subhymenium (with subhymenial cells that are considerably more inflated and wider than any part of the basidium itself). Both types occur in small or large species, and in species with or without clamp connections, but the cellular type is much less common in the genus (Fig. 2). Both types can occur in closely related species: in clade 2 (subgenus Rubrinus), for example, the two closely related species that have the smallest basidia in the genus, C. tomentosus and C. addaiensis, have different types of subhymenia (filamentose in the former, cellular in the latter), even between the extremely close C. tanzanicus and C. aff. tanzanicus, the latter differs from the former by its strongly filamentose subhymenium. In clade 6b (subgenus Afrocantharellus sect. Cutirellus), the Malayan C. cerinoalbus and C. cuticulatus have a cellular subhymenium, whereas the African C. splendens has not. On the other hand, in species with very long basidia (>80 μm) we never observed a cellular subhymenium.
-
Pileipellis structure. − The structure of the pileipellis is generally a cutis or trichocutis, exceptionally a trichoderm regularly constituted of vertically oriented hyphal endings only known so far from two species in section Cutirellus of subgenus Afrocantharellus (clade 6).
Another structural character of the pileipellis is the disruption of the upper layer into squamae that are more or less concentrically arranged. This feature is especially well-developed (although much less than in some Craterellus) in many yellowish brown species within Clade 2a (subgenus Rubrinus section Isabellinus), but occasional squamulose species are also found in other subgenera, e.g. C. conspicuus (clade 3) or/amethysteus subclade (clade 1).
One or two genera?
The question whether subgenus Afrocantharellus deserves recognition as a separate genus as recently proposed by Tibuhwa et al. (2012) has already been discussed by Buyck et al. (2013a). In fact, apart from the more developed gill folds for some of the African species, nothing distinguishes Afrocantharellus from other chanterelles. As discussed above, the Malaysian species in subgenus Afrocantharellus have much less developed gill folds. None of the morphological arguments invoked by Tibuhwa et al. (l.c.) to justify the distinction of two separate genera, is supported by factual data. Species in subgenus Afrocantharellus are neither more variegated, nor are the gill folds more widely spaced than in some other groups of the genus. Tibuhwa et al. (2012) interpret Afrocantharellus as ‘mostly lacking clamps’ as opposed to the rest of the genus where clamps are “usually present”. The present phylogeny clearly shows that the complete absence of clamps is a feature that characterizes the large majority of all chanterelles in Africa: it is a diagnostic feature for both subgenus Rubrinus and subgenus Afrocantharellus, representing together more than 70 % of the African chanterelles. The presence of A. fistulosus, the only species with clamps in Afrocantharellus sensu Tibuhwa et al. is one of several identification problems in the latter paper. Both the phylogenies presented by Tibuhwa et al. and our-selves use sequence data (presumably) obtained from the type specimen. Yet, whereas our sequence data place this species in subgenus Cinnabarinus where it is morphologically similar to closely related species, the phylogenetic analyses by Tibuhwa et al. place C. fistulosus with full support in Afrocantharellus, a group of morphologically completely different taxa. The presumed A. fistulosus-sequences produced by Tibuhwa et al. (l.c.) are indeed clearly related to the other species in Afrocantharellus, but can impossibly correspond to the specimen on which the original description was based.
As discussed above, Afrocantharellus is sister to the rest of the genus with very weak support (MLBS = 59 %, PP = 0.96) and lacks morphological characters to support its segregation from Cantharellus. We think it is therefore unwarranted to split the genus at this time as we may deal here with a problem of unsufficient sampling in the southern hemisphere. Interestingly, the nucLSU analysis that was recently published by Wilson et al. (2012) places the South American C. guyanensis as sister to African representatives of Afrocantharellus, but when introducing these same nucLSU sequences in our nucLSU dataset (not shown), the resulting consensus tree places C. guyanensis as sister to [Afrocantharellus + the rest of Cantharellus], which would imply that, if one accepts Tibuhwa’s viewpoint, C. guyanensis represents in this case yet another genus for a species that is, morphologically speaking, a completely ‘normal’ chanterelle.
Preliminary observations on the evolutionary history of Cantharellus
When looking at our phylogeny (Fig. 1), there is a striking tendency of clades to be of predominantly northern or southern hemisphere composition. Whereas some of the major clades entirely lack northern hemisphere taxa, the reverse is not true: all clades contain at least a few tropical representatives. The sister relationship between major (sub)clades of restricted geographical distribution suggests ancient separation events followed by evolution in isolation. Interestingly, as one of the most basal clades in Homobasidiomycetes (Binder et al. 2005), Cantharellales might have been present well before the breakup of Gondwana, whereas the ectomycorrhizal nutritional mode that characterizes the whole family Cantharellaceae (as well as Clavulinaceae, Hydnaceae and part of Sistotrema) may indeed be responsible for very limited long distance dispersal, if any at all (see discussion below). A vicariance-migration scenario seems, therefore, the most logical explanation since it is independent of far-fetched hypotheses that have to account for long distance dispersal events of these ectomycorrhizal symbionts. Transatlantic presence (Western Europe, USA) of C. cibarius and C. amethysteus has recently been proven inaccurate for example (Buyck et al. 2011; Buyck and Hofstetter 2011; Foltz et al. 2013), and also the islands of oceanic origin in the Mascarenes and Comores have never been colonized by African or Malagasy ECM fungi and their hosts notwithstanding millions of years of history. In the case of Cantharellus, there is another element that argues against long distance dispersal: Cantharellus is known to produce predominantly uninucleate spores (Horton 2006) which would virtually exclude successful establishment by long distance dispersal. Not only would two spores of compatible mating type need to arrive within mm distance of each other in order to allow primary mycelia to form a secondary mycelium, they also need to arrive in near contact with fine roots or germinating seeds of a compatible host. However, the fact that most of the tropical African chanterelles lack clamps with some showing a clear tendency to a reduced number of spores - unlike the North American species examined by Horton (l.c.)—calls for caution before generalizing this for the whole genus.
The distribution of afibulate clades
Africa and Madagascar are not only characterized by a very high species diversity, but also by a very high percentage of chanterelles that lack clamp connections (clade 2 and 6), unlike on other continents. With the exception of the few afibulate species described by Eyssartier et al. (2009) from Malaysia (all placed by our phylogeny in clade 2 = subgenus Afrocantharellus), the sole other afibulate chanterelle from outside Africa was described from New Zealand: C. elsae (G. Stev.) Horak. This apparently rare species, collected under Nothofagus, but able to grow with Kunzea/Leptospermum (Myrtaceae), is an entirely pinkish chanterelle with widely spaced gills. It lacks clamp connections and does have predominantly 5–6 spored basidia (Eyssartier 2001), contrary to what is mentioned in the original description and the redescription of the type by McNabb (1971), both of which report 4-spored basidia. Cantharellus elsae therefore is most likely a good species of Cantharellus and should not belong in Craterellus as suggested by Petersen and Mueller (1992), a recombination motivated at the time probably precisely because of the lack of clamp connections. The fact that it was first published as a Hygrophorus is a possible indication that it is very similar to species in subgenus Afrocantharellus.
Our phylogeny does not include species from either Australia or South America, but published descriptions of chanterelles from South America and Australia all indicate the complete absence of species without clamps, a typical feature of the two dominant subgenera in Africa and Madagascar (Rubrinus and Afrocantharellus). The same is true for chanterelles collected in New Caledonia (Ducousso et al. 2004; Buyck unpubl.) and the Indian Himalaya (Deepika et al. 2011), where all collected species so far have clamps and share morphological similarities with northern temperate taxa.
The discrepancy between studies focusing on below- and above-ground presence
The very high species diversity in African and Madagascan chanterelles stands in sharp contrast to the poor representation of Cantharellus in the rest of the southern hemisphere, at least in the case of Australia and South-America. This discrepancy is not an artifact due to differences in the fungal inventory. The intensely studied ectomycorrhizal forests of the Guyana shield in tropical America are rich in Clavulinaceae, but poor in Cantharellaceae, and particularly so in species of Cantharellus.
The few studies on African below ground ECM fungal communities do not reflect this high above-ground species diversity of the genus and principally report on the diversity of Clavulinaceae (Ba et al. 2012; Diédhiou et al. 2010; Tedersoo et al. 2011; Jairus et al. 2011), the latter remaining nevertheless less diverse compared to South America (Wilson et al. 2012; Smith et al. 2013; Moyersoen 2006, 2012). Studies on below-ground ECM fungi in worldwide dipterocarp forests reviewed by Brearley (2012) also report a poor below-ground presence of Cantharellaceae, although the latter publication contains several serious inaccuracies with respect to Cantharellaceae (the six taxa of/Cantharellaceae associated with Vateriopsis in the Seychelles should in fact read as a single/Clavulinaceae (Tedersoo et al. 2007), the 22/Clavulinaceae associated with the southamerican Parakaraima should read as 2/Clavulinaceae, and the four/Cantharellaceae and three/Clavulinaceae from Thai dipterocarp forests (Poshri et al. 2012) are in reality three/Cantharellaceae (all Craterellus) and two/Clavulinaceae). Judging from described taxa, Cantharellus is nevertheless much more diverse in Asia when compared to e.g. South-America (Wartchow et al. 2012a) or Australia (Eyssartier and Buyck 2001a). Preliminary data for Asia, but based on fruit bodies, indicate that chanterelles are quite more common and diverse in dipterocarp dominated forests and exhibit close affinities with the African species (Corner 1966; Eyssartier et al. 2009; Buyck unpubl.). Yet, nearly all Asian Cantharellaceae detected on root-tips correspond to Craterellus and not Cantharellus.
This high discrepancie between above- and below ground diversity is very likely related to two aspects of the unusual ITS of Cantharellus. Indeed, the exceptional length of the ITS of most Cantharellus may bias PCR reactions that are known to favor the amplification of short fragments over longer ones (Ihrmark et al. 2012; Quist and Chapela 2001; Sagerström et al. 1997; Suzuki and Giovannoni 1996), whereas the hypervariability of the ITS in Cantharellus may be responsible for mismatches with the applied primers. In any case, our phylogeny shows Cantharellus to be clearly more diverse in tropical ecosystems compared to temperate ecosystems and, in this particular case, contradicts the hypothesis of Tedersoo and Nara (2010), based on studies of below-ground diversity, according to which ectomycorrhizal groups are less diverse in tropical ecosystems compared to temperate ones.
Host associations of early Cantharellus
Whereas the clampless C. elsae may shed some doubt as to whether or not some of the more ancient Cantharellus were associated with Nothofagus, remarkably few chanterelles are associated with this host tree genus. Of the three reported chanterelles that do so, both C. elsae and the New Caledonian C. garnierii appear to be host generalists (Ducousso et al. 2004), whereas the very small C. nothofagorum R. H. Petersen & Mueller is only known from the type collection in Argentina. Based on morphology, both latter chanterelles would seem to fit in subgenus Cinnabarinus or Parvocantharellus.
Our data do not suggest that conifers have played an important role in the early history of Cantharellus. In our sampling, conifers are known host trees for a few individual terminal taxa in subgenus Cantharellus, and potentially (although data on precise host associations are lacking) in subgenus Parvocantharellus. Our dataset also suggests that the northern hemisphere species in these subgenera may have evolved from tropical representatives, but unfortunately our sampling is too restricted and lacks confidence for some of the basal relationships. In the case of subgenus Cantharellus, there is a clear need for more sampling and sequencing of both the/lateritius and/amethysteus clades (see Buyck and Hofstetter 2011; De Kesel et al. 2011) to document the link between tropical and temperate taxa in this subgenus. In particular the/lateritius clade seems to have a worldwide distribution with the exception of Europe where it may have become extinct during recent glaciation periods. Branch lengths leading to terminal taxa are on average shorter in clade 1, composed of northern hemisphere taxa, compared to its sister-clade (clade 2), composed of African and Malagasy species. These longer terminal branches are a general phenomenon for tropical taxa in our phylogeny. As African chanterelles have been collected and studied for many decennia and have been fairly well sampled in our phylogeny, the absence of a “broom” effect for terminal taxa in our tree (pointing to a recent radiation) seems therefore to reflect a reality. This suggests that these tropical taxa may have been subjected for a very long time to more or less stable, environmental conditions. In contrast, the shorter branch lengths for the northern temperate species could point to a more recent origin for these taxa and the reduced sampling almost certainly hides terminal “broom” effects due to more recent diversifications (possibly coinciding with the Pinaceae-Fagales migrations). The C. cibarius complex, accounting in the United States alone approximately 15 different species (Buyck unpubl.), is a good example.
Our phylogeny suggests also a possible secondary colonization of the southern hemisphere by northern hemisphere taxa in clade 3 (subgenus Cinnabarinus): C. friesii is resolved, although without support, as basal to a number of Malayian and African species. Also here more sampling and sequencing is needed.
Although our sampling does not contain Australian chanterelles, the phylogeny suggests that Myrtaceae were equally not associated with early Cantharellus as the morphology of the few Australian species suggests affinities to northern temperate species. The only eucalypt-associated taxa in our phylogeny (C. aff. congolensis, C. aff. decolorans, C. sp. ined. (eucalyptus) and C. aff. tanzanicus) were collected in exotic plantations in Madagascar and seem to be the result of a recent host shift (Buyck unpubl.).
A provisional biogeographic working hypothesis
As a provisional, biogeographic working hypothesis, we suggest that the earliest Cantharellus may not have been in contact with the most southern parts of Gondwana when Nothofagus was common there (although there still is the enigmatic C. elsae), but that its present distribution is the result of an ‘out of Africa’ history when the genus was associated with ancestors of the extant Phyllantaceae, Caesalpiniaceae, and particularly Dipterocarpaceae and Sarcolaenaceae, using the Indian plate to arrive in Asia where very similar and phylogenetically close taxa are indeed associated with extant dipterocarps (Eyssartier et al. 2009), to then disperse east and west in the northern hemisphere where they may have radiated migrating together with Pinaceae and Fagales in the more temperate climates of the Eocene-Oligocene transition as already suggested for other ectomycorrhizal fungal groups (Bruns et al. 1998). Whether the few chanterelles in neotropical lowland (where Clavulinaceae are much more diverse), such as C. guyanensis, are the result of an even older vicariance, as already suggested for their host trees (Moyersoen 2006), remains to be investigated. Yet, the sister-relationship between some African Inocybe and those recovered from root tips of neotropical dipterocarps (Moyersoen 2012) is consistent with our preliminary analysis of existing LSU data for C. guyanensis that puts this neotropical chanterelle sister to the rest of the genus (not shown). The general vicariance and migration hypothesis for Cantharellus parallels therefore the evolutionary hypothesis for their host trees (Moyersoen 2006). The latter hypothesis was recently supported by Dutta et al. (2011), who concluded, based on fossil resin chemistry and palynological data from 53 Myr old sediments in Western India, that Dipterocarpaceae must have originated in Gondwana and migrated from India into the SE Asian region from the middle Eocene onwards. The palynological assemblage accompanying these 53Myr old dipterocarps suggested a warm, humid, tropical climate that still characterizes extant dipterocarps (Dutta et al. 2011). The early history of Cantharellus therefore seems completely different from that suggested for Hysterangiales, another ancient group of Agaricomycetidae (Hosaka et al. 2008), where basal clades appear to have been associated primarily with Myrtaceae. Hysterangiales are also very diverse on those continents where Cantharellus is poorly represented (i.e. South America and Australia). Early Hysterangiales thus seem to have been associated with sclerophyllous vegetation in the southern part of Gondwana, whereas the early Cantharellus of Western Gondwanaland may well have been primarily associated with lowland rain forests as suggested by their presence in all of the important rain forest blocks where Dipterocarpaceae (and related Sarcolaenaceae in the case of Madagascar) are still present or dominant.
Conclusion
Proposal for a new infrageneric arrangement
Cantharellus Adans.: Fr.
Fruit bodies growing individual or in groups, rarely clustered at the base, long-lived compared to other gilled fungi, gymnocarpic, growing on soil, rarely fruiting on dead wood. Cap of very variable size (30 cm to <1 cm diam.), smooth to strongly squamulose, of very variable color, with parietal to vacuolar pigments of the bicyclic carotenoid type (particularly ß-carotène et ketonic derivatives). Hymenophore varying from nearly smooth, over veined to distinctly lamellate, with gills mostly distinctly forked in varying degrees or polydymous, concolorous or not to the rest of the fruit body; hymenium (always?) accrescent. Stipe central or excentric, not lateral, smooth to strongly squamulose, solid, although in some smaller species often rapidly fistulose, but without perforating the cap center as in Craterellus, concolorous or not with cap and/or hymenophore. Context very variable in thickness, in many species characteristically discoloring and yellowing, browning, blueing or blackening, either entirely or in certain parts of the fruit body. Odor mostly distinctly fruity (apricots). Taste slightly acrid to mild. Spore print white to yellow or salmon pink. Pileipellis poorly differentiated, a cutis or trichocutis, rarely a trichoderm, hyphal terminations thin- to thick-walled. Basidia (always ?) stichic, very variable in length between species, bearing mostly (2–4)5–6(7–8) sterigmata, exceptionally regularly 2- or 4-spored. Cystidia of a particular type not differentiated, but leptocystidia (in the broadest sense) and aborted basidia sometimes present. Spores ovoid, ellipsoid, reniform to shortly cilindrical, inamyloid, not dextrinoid, not cyanophilous, smooth, thin-walled. Clamp connections absent or present. Ectomycorrhizal, neither lichenicolous nor saprotroph. Type species: Cantharellus cibarius Fr.: Fr.
-
Subgenus Cantharellus emend. Buyck & V. Hofstetter: Fruit bodies fleshy, medium sized to large; hymenophore veined with blunt, mostly strongly forking-anastomosing ridges, rarely smooth or nearly so; cap and stipe usually smooth, sometimes with appressed squamae; hyphal endings mostly thick-walled; clamp connections abundant everywhere. Automatic type: C. cibarius Fr.:Fr.
-
Section Cantharellus: Fruit bodies yellow to whitish, or with brownish, greenish or pinkish lilac tones. Automatic type: C. cibarius Fr.:Fr.
-
Section Amethystini Buyck & V. Hofstetter sect. nov. — MycoBank MB 806113; Fruit bodies developing clear lilac-purple colors on either hymenophore or cap and sometimes also on stipe; cap often with appressed squamae. Type: C. subamethysteus Eyssart. & Stubbe
-
Section Sublaeves Buyck & V. Hofstetter sect. nov. — MycoBank MB 806114; Fruit bodies yellow to orange, hymenophore with poorly developed veins to smooth. Type: C. lateritius (Berk.) Singer
-
-
Subgenus Rubrinus Eyssart. & Buyck emend. Buyck & V. Hofstetter: Fruit bodies variably fleshy, large to very small; hymenophore very variable, from nearly smooth to producing well-developed gill-folds, forking-anastomosing or not; cap and stipe smooth to strongly squamulose; hyphal endings thick- to thin-walled; clamp connections absent. Type: C. floridulus Heinem.
-
Sect. Isabellinus Eyssart. & Buyck: Fruit bodies fleshy, medium-sized to large, off-white, yellowish to grayish brown, exceptionally red; cap and also stipe often strongly squamulose from disruption of the outer layer, forming more or less dressed squamae; hymenophore usually producing well-developed gill-folds, with furcations and anastomoses often poorly developed to absent; hyphal endings mostly thick-walled, basidia sometimes very small. Type: C. isabellinus Heinem.
-
Sect. Heinemannianus Eyssart. & Buyck emend. Buyck & V. Hofstetter: Fruit bodies medium-sized to very small and yellow, orange, pink or red; hymenophore variable, nearly smooth, veined or with more or less well-developed gill folds. Hyphal endings thin- (mostly) to thick-walled. Type: C. heinemannianus Eyssart. & Buyck
-
-
Subgenus Parvocantharellus Eyssart. & Buyck emend. Buyck & V. Hofstetter: Fruit bodies thick- or thin-fleshed, medium-sized to very small and then often with a slender stipe that may become fistulose, yellowish to brownish, sometimes with lilac-purple stains, context yellowing, rarely strongly blackening. Hyphal endings usually thin-walled, sometimes slightly thickened. Clamp connections abundant everywhere. Type: C. romagnesianus Eyssart. & Buyck
-
Sect. Flavobrunnei Buyck & V. Hofstetter sect. nov. — MycoBank MB 806115; Fruit bodies (very) small to hardly medium-sized, usually thin-fleshed and with a long stipe, yellowish to brownish. Type species: C. romagnesianus Eyssart. & Buyck
-
Sect. Congolenses Heinem. Fruit bodies medium-sized, fleshy, strongly and intensily blackening. Type: C. congolensis Beeli.
-
Sect. Cyanomaculati Buyck & V. Hofstetter sect. nov. — MycoBank MB 806116; Fruit bodies medium-sized, fleshy, yellow but in places distinctly lilac-purple to almost bluish, context slightly yellowing. Type species: C. subcyanoxanthus Buyck, Randrianjohany & Eyssart.
-
-
Subgenus Cinnabarinus Buyck & V. Hofstetter subgen. nov. — MycoBank MB 806117; Fruit bodies fleshy or not, mostly quite small, yellow, orange, pink or red, sometimes with lilac-purple or brownish tones, particularly in the cap center, smooth or with appressed squamules; hymenophore strongly veined to lamellate; thick-walled hyphal endings either absent or rare and then mostly limited to terminal cells; clamp connections abundant everywhere. Type species: C. cinnabarinus (Schwein.) Schwein.
-
Subgenus Afrocantharellus Eyssart. & Buyck: Fruit bodies mostly brightly colored, yellow, orange to red, sometimes with grayish to bluish hues, often with stipe, cap and hymenophore of different color, fleshy, medium-sized to large; hymenophore with (sometimes very) well-developed gill folds, interstitial veination-anastomoses absent or present; hyphal extremities thin-walled, clamp connections absent. Type species: C. symoensii Heinem.
-
Sect. Afrocantharellus Buyck & V. Hofstetter sect. nov. — MycoBank MB 806118; Pileipellis without a tendency towards trichodermal structure, gill folds very well developed. Type species: C. symoensii Heinem.
-
Sect. Cutirellus Corner. Pileipellis structure often trichodermal, gill folds not always very well developed. Type species: C. cuticulatus Corner
-
Subgenus Pseudocantharellus Eyssart. & Buyck emend. Buyck & V. Hofstetter: Fruit bodies medium-sized to large, fleshy, pink, pinkish orange to reddish pink or red. Hyphal endings thin-walled. Clamp connections abundant everywhere. Type species: C. ruber Heinem.
-
References
Adanson M (1763) Familles des plantes. Chez Vincent, Imprimeur-Libraire de Mgr le Comte de Provence, Paris
Arora D, Dunham SM (2008) A new, commercially valuable chanterelle species, Cantharellus californicus sp. nov., associated with live Oak in California, USA. Econ Bot 62:376–391
Ba AM, Duponnois R, Moyersoen B, Diédhiou AG (2012) Ectomycorrhizal symbiosis of tropical African trees. Mycorrhiza 22:1–29
Bauhin J, Cherler JH (1651) Historia plantarum universalis, nova et absolutissima cum consensu et dissensu circa eas. […]. Tomus III. Ebroduni
Binder M, Hibbett DL, Larsson K-H, Larsson E, Langer E, Langer G (2005) The phylogenetic distribution of resupinate forms in the homobasidiomycetes. Syst Biodivers 3:113–157
Blanco-Dios JB (2004) Notas sobre la familia Cantharellaceae en el noroeste de la Península Ibérica (I). Cantharellus romagnesianus Eyssartier et Buyck, novedad para el catálogo micológico ibérico, y Cantharellus cibarius Fr.: Fr. var. gallaecicus var. nov. Bol Soc Micol Madrid 28:181–185
Blanco-Dios JB (2011) Notas sobre la familia Cantharellaceae en el noroeste de la península ibérica (III): Cantharellus lourizanianus y C. romagnesianus var. parvisporus, dos nuevos taxones del subgénero Parvocantharellus, y Craterellus lutescens f. citrinosulphureus, f. nov. Tarrelos 13:7–15
Bodensteiner P, Binder M, Moncalvo J-M, Agerer R, Hibbett DS (2004) Phylogenetic relationships of cyphelloid homobasidiomycetes. Mol Phylogenet Evol 33:501–515
Brearley FQ (2012) Ectomycorrhizal assocations of the Dipterocarpaceae. Biotropica 44(5):637–648
Bruns TD, Szaro TM, Gardes M, Cullings KW, Pan JJ, Taylor JJ, Horton TR, Kretzer A, Garbelotto M, Li Y (1998) A sequence database for the identification of ectomycorrhizal basidiomycetes by phylogenetic analysis. Mol Ecol 7:257–272
Buyck B (1994) Ubwoba: les champignons comestibles de l’ouest du Burundi. Admin Gén Coop Dévelopm 34:1–123. Publ Agric, Bruxelles
Buyck B (2012) One neo- and four epitypifications for Cantharellus species from tropical African savannah woodlands. Cryptog Mycolog 33:11–17
Buyck B, Hofstetter V (2011) The contribution of tef-1 sequences for species delimitation in the Cantharellus cibarius complex in the southeastern USA. Fungal Divers 49:35–46
Buyck B, Randrianjohany E (2013) Cantharellus eyssartierii sp. nov. (Cantharellales, Basidiomycota) from monospecific Uapaca ferruginea stands near Ranomafana (eastern escarpment, Madagascar). Cryptog Mycolog 34(1):29–34
Buyck B, Lewis DP, Eyssartier G, Hofstetter V (2010) Cantharellus quercophilus sp.nov. and its comparison to other small, yellow or brown American chanterelles. Cryptog Mycolog 31(1):17–33
Buyck B, Cruaud C, Couloux A, Hofstetter V (2011) Cantharellus texensis sp. nov. from Texas, a Southern lookalike of C. cinnabarinus revealed by tef-1 sequence data. Mycologia 103:1037–1046
Buyck B, Kauff F, Cruaud C, Hofstetter V (2013a) Molecular evidence for novel Cantharellus (Cantharellales, Basidiomycota) from tropical African miombo woodland and a key to all tropical African chanterelles. Fungal Divers 58:281–298
Buyck B, Kauff F, Randrianjohany E, Hofstetter V (2013b) Sequence data reveal a high diversity of endemic Cantharellus associated with native vegetation in Madagascar. Mycologia (in print)
Contu M, Vizzini A, Carbone M, Setti L (2009) Identity and neotypification of Craterellus cinereus and description of Cantharellus atrofuscus sp. nov. Mycotaxon 110:139–149
Corner EJH (1966) A monograph of Cantharelloid fungi. Ann Bot Mem 2:255
Dahlman M, Danell E, Spatafora JW (2000) Molecular systematics of Craterellus: Cladistic analysis of nuclear LSU rDNA sequence data. Mycol Res 104:388–394
de Jussieu AL (1789) Genera plantarum: secundum ordines naturales disposita, juxta methodum in Horto Regio Parisiensi exaratam
De Kesel A, Yorou NS, Buyck B (2011) Cantharellus solidus, a new species from Benin (West-Africa) with a smooth hymenium. Cryptog Mycolog 32(3):277–283
Deepika K, Reddy MS, Upadhyay RC (2011) Unpubl studies on the diversity and nutritional value of Cantharellaceae of Western Himalayas, India. Electronic Theses & Dissertations (unpubl., http://hdl.handle.net/10266/1664)
Diédhiou AG, Selosse M-A, Galiana A, Diabaté M, Dreyfus B, Bâ AM, Miana de Faria S, Béna G (2010) Multi-host ectomycorrhizal fungi are predominant in a Guinean tropical rainforest and shared between canopy trees and seedlings. Environ Microbiol 12(8):2219–2232
Ducousso M, Contesto C, Cossegal M, Prin Y (2004) Cantharellus garnierii sp. nov., une nouvelle chanterelle des maquis miniers nickélifères de Nouvelle-Calédonie. Cryptog Mycolog 25(2):135–145
Dutta S, Tripathi SM, Mallick M, Mathews RP, Greenwood PF, Rao MR, Summons RE (2011) Eocene out-of-India dispersal of Asian dipterocarps. Rev Paleobot Palyno 166:63–68
Eyi Ndong HE, Degreef J, De Kesel A (2011) Champignons comestibles des forêts denses d’Afrique Centrale. Taxonomie et identification. ABC Taxa 10, 253pp. Brussels
Eyssartier G (2001) Vers une monographie du genre Cantharellus Adans.:Fr. 259 p. Dissertation, National History Museum Paris
Eyssartier G, Buyck B (2000) Le genre Cantharellus en Europe. Nomenclature et taxinomie. Bull Soc Mycol Fr 116(2):91–137
Eyssartier G, Buyck B (2001a) Notes on the Australian species described in the genus Cantharellus. Aust Syst Bot 14(3):587–598
Eyssartier G, Buyck B (2001b) Novitates. Note nomenclaturale et systématique sur le genre Cantharellus. Doc Mycol 31(121):55–56
Eyssartier G, Stubbe D, Walleyn R, Verbeken A (2009) New records of Cantharellus species (Basidiomycota, Cantharellaceae) from Malaysian dipterocarp rainforest. Fungal Divers 36:57–67
Fayod MV (1889) Prodrome d’une histoire naturelle des Agaricinés. Ann Sci Nat 7e série IX:181–411, 1 pl
Feibelman TP, Bayman P, Cibula WG (1994) Length variation in the internal transcribed spacer of ribosomal DNA in Chanterelles. Mycol Res 98:614–618
Feibelman TP, Doudrick RL, Cibula WG, Bennett JW (1997) Phylogenetic relationships within the Cantharellaceae inferred from sequence analysis of the nuclear large subunit rDNA. Mycol Res 101(2):1423–1430
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. J Mol Evol 39:783–791
Foltz MJ, Perez KE, Volk TJ (2013) Molecular phylogeny and morphology reveal three new species of Cantharellus within 20 m of one another in western Wisconsin, USA. Mycologia 105(2):447–461
Forquignon L (1886) Les champignons supérieurs: physiologie, organographie, classification, détermination du genre. Doin, Paris, 231pp
Fries EM (1821–1832) Systema mycologicum sistens fungorum ordines, genera et species, hucusque cognitas, quas ad norman methodi naturalis determinavit, disposuit atque descripsit Elias Fries. Lund (Berlin), Greifswald (vol. 3: Ernesti Mauritii), 3 vols
Fries EM (1838) Epicrisis systematis mycologici, seu synopsis Hymenomycetum. Typographia Academica, Uppsala
Fries EM (1874) Hymenomycetes europaei epicriseos systematis mycologici editio altera. Ed. Berling, Uppsala
Hansen L, Knudsen H (1997) Nordic macromycetes, vol 3. (Hetero., Aphylloph., Gastero.). Nordsvamp, Copenhagen, ISBN 87-983961-1-0, 444 pp
Heinemann P (1958) Champignons récoltés au Congo Belge par Madame Gossens-Fontana. III. Cantharellineae. Bulletin du jardin botanique de l‘État Bruxelles 28:385–438.
Heinemann P (1959) Cantharellineae. Fl Icon Champ Congo 8:153–165, pl. 26–28
Heinemann P (1966) Cantharellineae du Katanga. Bull Jard Bot État Bruxelles 36:335–352
Henkel TW, Aime MC, Uehling JK, Smith ME (2011) New species and distribution records for Clavulina (Cantharellales, Basidiomycota) from the Guiana Shield. Mycologia 103:883–894
Hermitte JC, Eyssartier G, Poumarat S (2005) Cantharellus lilacinopruinatus sp. nov., une nouvelle chanterelle termophile. Bull Féd Assoc Mycol Medit 28:27–32
Hibbett DS, Binder M (2002) Evolution of complex fruiting-body morphologies in homobasidiomycetes. Proc R Soc Lond B 269:1963–1969
Hibbett DS, Donoghue MJ (1995) Progress toward a phylogenetic classification of the Polyporaceae through parsimony analysis of mitochondrial ribosomal DNA sequences. Can J Bot 73(suppl 1):853–861
Hibbett DS, Pine EM, Langer E, Donoghue MNJ (1997) Evolution of gilled mushrooms and puffballs inferred from ribosomal DNA sequences. PNAS 94:12002–12006
Hibbett DS, Binder M, Bischfoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Luecking R, Thorsten Lumbsch H, Lutzoni F, Matheny PB, Mclaughlin DJ, Powell MJ, Redhead S, Schoch C, Spatafora JW, Stalpers JA, Vilgalys R et al (2007) A higher-level phylogenetic classification of the Fungi. Mycol Res 111:509–547
Hofstetter V, Clémençon H, Vilgalys R, Moncalvo JM (2002) Phylogenetic analyses of the Lyophylleae Lyophyllaceae (Agaricales, Basidiomycetes) based on nuclear and mitochondrial rDNA sequences. Mycol Res 106(9):1043–1059
Horton TR (2006) The number of nuclei in basidiospores of 63 species of ectomycorrhizal Homobasidiomycetes. Mycologia 98(2):233–238
Hosaka K, Castellano MA, Spatafora JW (2008) Biogeography of Hysterangiales (Phallomycetidae, Basidiomycota). Mycol Res 112(4):448–462
Ihrmark K, Boedeker ITM, Cruz-Martinez K, Friberg H, Kubartova A, Schenck J, Strid Y, Stenlid J, Brandstroem-Durling M, Clemmensen KE, Lindahl BD (2012) New primers to amplify the fungal ITS2 region—evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol 82:666–677
Jairus T, Mpumba R, Chinoya S, Tedersoo L (2011) Invasion potential and host shifts of Australian and African ectomycorrhizal fungi in mixed eucalypt plantations. New Phytol 192:179–187
Juel HO (1898) Die Kerntheilungen in den Basidien und die Phylogenie der Basidiomyceten. Jahrb Wiss Bot 32:361–388, pl. 4
Kühner R, Romagnesi H (1953) Flore analytique des champignons supérieurs. Masson, Paris
Kumari D, Upadhyay RC, Reddy MS (2011) Cantharellus pseudoformosus, a new species associated with Cedrus deodara from India. Mycoscience 52:147–151
Larsson KH (2007) Rethinking the classification of corticioid fungi. Mycol Res 111:1040–1063
Léveillé JH (1837) Sur l’hyménium des champignons. Ann Sci Nat Sér Bot 8:321–338
Léveillé JH (1843) Observations sur quelques champignons de la Flore des environs de Paris. Ann Sci Nat Sér Bot 1:213–231
Lindahl B (2000) Mycological research news. Mycol Res 104(4):385–387
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol 16(12):1799–1808
Maire R (1902) Recherches cytologiques et taxonomiques sur les Basidiomycetes. Bull Soc Mycol France (supplement):1–212
Mason-Gamer R, Kellogg E (1996) Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Syst Biol 45:524–545
McNabb RFR (1971) Some new and revised taxa of New Zealand Basidiomycetes (fungi). N Z J Bot 9(2):355–370
Moncalvo J-M, Nilsson RH, Koster B, Dunham SM, Bernauer T, Matheny PB, McLenon T, Margaritescu S, Weiß M, Garnica S, Danell E, Langer G, Langer E, Larsson E, Larsson K-H, Vilgalys R (2007 for 2006) The cantharelloid clade: dealing with incongruent gene trees and phylogenetic reconstruction methods. Mycologia 98(6):937–948
Morehouse EA, James TY, Ganley ARD, Vilgalys R, Berger L, Murphy PJ, Longcore JE (2003) Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol Ecol 12:395–403
Moyersoen B (2006) Pakaraimaea dipterocarpacea is ectomycorrhizal, indicating an ancient Gondwanaland origin for the ectomycorrhizal habit in Dipterocarpaceae. New Phytol 172:753–762
Moyersoen B (2012) Dispersion, an important radiation mechanism for ectomycorrhizal fungi in neotropical lowland forests? In: Sudarshana P, Nageswara-Rao M, Soneji JR (eds) Tropical forests, pp 93–116. ISBN: 978-953-51-0255-7
Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL (2008) AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24:581–583
Olariaga I, Salcedo I (2007) Cantharellus gallaecicus (Blanco-Dios) Olariaga, comb. & stat. nov. (Cantharellaceae). Anal Jard Bot Madrid 64(2):221–222
Olariaga I, Salcedo I (2008) Cantharellus ilicis sp. nov., a new species from the Mediterranean basin collected in evergreen Quercus forests. Rev Catalana Micol 30:107–116
Patouillard NT (1887) Les Hyménomycètes d'Europe. Anatomie générale et classification des champignons supérieurs. 166pp., 4 tabs. Paris; Klincksieck
Pegler DN, Roberts PJ, Spooner BM (1997) British chanterelles and tooth fungi. Royal Botanic Gardens, Kew
Persoon CH (1797) Commentatio de fungis clavaeformibus sistens specierum hus usque notarum descriptiones cum differentiis specificis, nec non auctorum synonymis. Accedunt tab. IV, colore fucatae. Lipsiae. Apud Petr. Philipp. Wolf
Persoon CH (1801) Synopsis methodica fungorum. Sistens enumerationem omnium huc usque detectarum specierum, cum brevibus descriptionibus nec non synonymis et observationibus selectis. […] Cum tabulis aeneis. Gottingae apud Henricum Dietrich
Petersen RH (1969) Notes on Cantharelloid fungi—II. Some new taxa, and notes on Pseudocraterellus. Persoonia 5:211–223
Petersen RH, Mueller G (1992) New South American taxa of Cantharellus, C. nothofagorum, C. xanthoscyphus and C. lateritius var. colombianus. Bol Soc Argent Bot 28(1–4):195–200
Pilz D, Norvell L, Danell E, Molina R (2003) Ecology and management of commercially harvested chanterelle mushrooms. Gen. Tech. Rep. PNW-GTR-576. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Portland, 83 p
Pine EM, Hibbett DS, Donoghue MJ (1999) Phylogenetic relationships of cantharelloid and clavarioid Homobasidiomycetes based on mitochondrial and nuclear rDNA sequences. Mycologia 91:944–963
Poshri C, Põlme S, Taylor AFS, Kõljalg U, Suwannasai N, Tedersoo L (2012) Diversity and community composition of ectomycorrhizal fungi in a dry deciduous dipterocarp forest in Thailand. Biodivers Conserv 21:2287–2298
Quist D, Chapela IH (2001) Transgenic DNA introgressed into traditional maize landraces in Oaxaca, Mexico. Nature 414:541–543
Redhead SA (1983) Arrhenia and Rimbachia, expanded generic concepts, and a reevaluation of Leptoglossum with emphasis on muscicolous North American taxa. Can J Bot 62:865–892
Redhead S (2012) Nomenclatural novelties. Index Fung 5:1. ISSN 2049–2375
Reijnders AFM (1963) Les problèmes du développement des carpophores des Agaricales et quelques groupes voisins. N. V. Dijkstra’s drukkerij v. h. Boekdrukkerij Gebroeders Hoitsema, Groningen
Rodríguez F, Oliver JL, Marín A, Medína JR (1990) The general stochastic model of nucleotide substitution. J Theor Biol 142:485–501
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Sagerström CG, Sun BI, Sive HL (1997) Subtractive cloning: past, present, and future. Ann Rev Biochem 66:751–783
Sasia RF, Pérez-de-Gregorio MA, Eyssartier G (2003) Cantharellus parviluteus, une nouvelle espèce décrite de la Péninsule Ibérique. Bull Soc Mycol France 119(3–4):261–266
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque A, Chen W, Fungal Barcoding Consortium (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. PNAS USA 109(16):6241–6246
Shao S-C, Tian X-F, Liu P-G (2011) Cantharellus in Southwestern China: a new species and a new record. Mycotaxon 116:437–446
Smith ME, Henkel TW, Uehling JK, Fremier AK, Clarke HD, Vilgalys R (2013) The ectomycorrhizal fungal community in a Neotropical forest dominated by the endemic dipterocarp Pakaraimaea dipterocarpacea. Plos One 8(1):e55160
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–771
Suzuki MT, Giovannoni SJ (1996) Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol 62:625–630
Tedersoo L, Nara K (2010) General latitudinal gradient is reversed in ectomycorrhizal fungi. New Phytol 185:351–354
Tedersoo L, Suvi T, Beaver K, Kõljalg U (2007) Ectomycorrhizal fungi of the Seychelles: diversity patterns and host shifts from the native Vateriopsis seychellarum (Dipterocarpaceae) and Intsia bijuga (Caesalpiniaceae) to the introduced Eucalyptus robusta (Myrtaceae), but not Pinus caribea (Pinaceae). New Phytol 175:321–333
Tedersoo L, Bahram M, Jairus T, Bechem E, Chinoya S et al (2011) Spatial structure and the effects of host and soil environments on communities of ectomycorrhizal fungi in wooded savannas and rain forests of Continental Africa and Madagascar. Mol Ecol 20:3071–3080
Tian XF, Buyck B, Shao SC, Liu PG, Fang Y (2012) Cantharellus zangii, a new subalpine basidiomycete from southwestern China. Mycotaxon 120:99–103
Tibuhwa DD, Buyck B, Kivaisi AK, Tibell L (2008) Cantharellus fistulosus sp. nov. from Tanzania. Cryptog Mycolog 29:129–135
Tibuhwa DD, Savic J, Tibell L, Kivaisi A (2012) Afrocantharellus gen. stat. nov. is part of a rich diversity of African Cantharellaceae. IMA Fungus 3(1):25–38
Uehling JK, Henkel TW, Aime MC, Smith ME (2012) New species of Clavulina with effused or resupinate basidiomata from the Guiana Shield. Mycologia 104:547–556
Wartchow F, Buyck B, Maia LC (2012a) Cantharellus aurantioconspicuus (Cantharellales), a new species from Pernambuco, Brazil. Nova Hedwig 94:129–137
Wartchow F, Santos JC, Fonseca MDP (2012b) Cantharellus amazonensis, a new species from Amazon. Mycosphere 3(4):414–418
Watling R, Turnbull E (1998) Cantharellaceae, Gomphaceae and amyloid-spored and xeruloid members of Tricholomataceae (excl. Mycena). British Fungus Flora, Agarics and Boleti, vol. 8. Royal Botanic Garden, Edinburgh
Wilson AW, Aime MC, Dierks J, Mueller GM, Henkel TW (2012) Cantharellaceae of Guyana I: new species, combinations and distribution records of Craterellus and a synopsis of known taxa. Mycologia 104(6):1466–1477
Acknowledgments
We are grateful to the curators of the herbaria AD, BR, DAR, K, MICH, MPU, PMR and TENN for access to type specimens. Part of the sequencing for this study was performed by A. Couloux at the Genoscope or “Consortium National de Recherche en Génomique” near Paris (France) as part of the agreement n°2005/67 on the project “Macrophylogeny of life” between the Genoscope and the “service de systématique moléculaire” (CNRS IFR 101) of the Muséum national d’histoire naturelle. For sequencing, this study also received continuing support from the ATM-project “Barcode of life” of the Muséum national d’histoire naturelle (Dirs. L. Legall and S. Samadi).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buyck, B., Kauff, F., Eyssartier, G. et al. A multilocus phylogeny for worldwide Cantharellus (Cantharellales, Agaricomycetidae). Fungal Diversity 64, 101–121 (2014). https://doi.org/10.1007/s13225-013-0272-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-013-0272-3