Abstract
An inventory of xylariaceous pyrenomycetes of Northern Thailand resulted in the discovery of a new monotypic genus, here named Rostrohypoxylon as well as two new species of Annulohypoxylon. These new taxa are introduced, fully described and compared with similar species in this paper. The new genus is recognized based on new combinations of anamorphic and teleomorphic characters. The status of these new taxa is supported by secondary metabolite profiling using high performance liquid chromatography, coupled with diode array detection and mass spectrometry (HPLC-DAD-MS).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The xylariaceous genus Annulohypoxylon was recently erected (Hsieh et al. 2005) to accommodate species formerly included in Hypoxylon sect. Annulata J.H. Mill. emend. Y.M. Ju & J.D. Rogers (Ju and Rogers 1996). The basis for the new genus was supported by molecular and morphological data. Annulohypoxylon was segregated from Hypoxylon Bull. by three key morphological features: a) carbonaceous stromata that become highly melanized at maturity; b) papillate ostioles surrounded by an annulate disc; c) ascospores with a perispore bearing a thickening on the same side as the germ slit. The characteristic disc is lacking in some taxa from Europe and New Zealand, and some species have an indehiscent perispore; this makes it difficult to locate the characteristic thickening of the perispore. However, the affinities of these “aberrant” species to Annulohypoxylon were also clearly demonstrated by molecular data, and supported by chemotaxonomic characters.
As in other xylariaceous genera referred to as “Hypoxyloideae” (e.g., the related Daldinia Ces. & De Not., Entonaema Möller and Hypoxylon Bull.), secondary metabolites are present in the stromata of Annulohypoxylon as subsurface granules yielding colored pigments in 10% KOH. Their chemotaxonomic importance has been evaluated by Quang et al. (2005a, b, 2006) and illustrated by Stadler and Fournier (2006).
During several field expeditions in the vicinity of the Mushroom Research Centre (MRC) in Northern Thailand from mid-May to mid-June 2005, several Annulohypoxylon spp. were collected, of which two species appeared to be new to science. Moreover, a taxon obviously related to Annulohypoxylon was encountered, for which the new genus Rostrohypoxylon is proposed. Based on morphological, cultural and chemotaxonomic data, these taxa are described and illustrated below. It is noteworthy that Annulohypoxylon was by far the best represented xylariaceous genus in good condition among the pyrenomycetes encountered during the above mentioned forays, which were conducted during the late dry season and early monsoon season. This period obviously favored drought-tolerant Xylariaceae with carbonaceous stromata, such as Annulohypoxylon.
Materials and methods
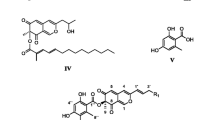
Measurements of asci and ascospores were made from slides mounted in water, the reaction of apical rings being tested by addition of a drop of Melzer’s reagent at the edge of the cover slip. Microscopic observations and photos were made and taken through a brightfield microscope. The dehiscence of the perispore was likewise tested by addition of a drop of 10% KOH to a water mount where free ascospores released from the asci had been first observed, for two reasons. First, the dehiscence of the perispore is often difficult to see while ascospores are still in the ascus, especially in some species where the perispores are hardly dehiscent. Second, unlike in Hypoxylon and Daldinia where perispores conspicuously dehisce by transverse breaking off, those in Annulohypoxylon dehisce by longitudinal splitting from one end and can be mistaken for immature hyaline ascospores. Adding KOH to a water mount allows microscopic determination of the perispore dehiscence with greater reliability. Colours refer to Rayner (1970), and therefore, also to the species descriptions provided by Ju and Rogers (1996) and Ju et al. (2004). Cultures were obtained from perithecial contents plated onto YMG agar as previously described by Stadler et al. (2004) and propagated in different culture media for secondary metabolite analyses as described by Stadler et al. (2008a, b). For HPLC profiling of stromatal methanol extracts, the methodology described by Stadler et al. (2008a) was also employed, using standards of the extrolites that were obtained previously (Bitzer et al. 2007, 2008; Quang et al. 2005a, b). The chemical structures of the secondary metabolites detected are depicted in Fig. 4. The HPLC-UV data presented in Figs. 5 and 8 were also verified by HPLC-MS analyses (data not shown).
Results
Chemotaxonomic studies
HPLC profiles of the species collected (and, if available, of their cultures), were recorded and compared with previously obtained data (e.g. Quang et al. 2005a; Bitzer et al. 2008) on Annulohypoxylon species and other Xylariaceae. The HPLC profiles of representative collections are depicted for comparison in Figs. 5 and 8.
Taxonomic part
Rostrohypoxylon J. Fourn. & M. Stadler, gen. nov.
Stromatal morphology of Rostrohypoxylon terebratum, from holotype specimen. Stromatal habit. b Pigments in KOH. c Rostrate ostioles and perforations seen from above. d,f Section through stroma, showing perithecia and perforations (arrows). e Stromatal surface in side view. g Section showing a perithecium (arrow) encased in carbonaceous tissue. Scale is indicated by bars. a 5 mm. b, c, d, e, f,g 1 mm
Asci (a) and ascospores (b, c) of Rostrohypoxylon terebratum, from holotype specimen, Fig. 2c clearly showing the ascospore germ slits. Scale bars: 10 µm
Rostrohypoxylon terebratum, ex type culture. a, b Cultures after 2 weeks on 9 cm agar slants. a Difco Oatmeal agar, showing characteristic red exsudates that are released from aerial mycelium b YMG medium. c–e Phase contrast micrographs (1000×) from YMG culture. c Tip of conidiophore, showing finely roughened conidiogenous cells and condidia. d Conidium arising laterally from undifferentiated hyphae. e Conidia. Scale is indicated by bars in c–e. c, d 10 µm; e 5 µm
MycoBank: MB512543
Etymology: from rostrum (= beak) for long-beaked ostiolar necks and Hypoxylon in reference to the affinities with this genus.
A totis generis Xylariaceis differt in stromatibus carbonaceis, ostolis compactis cum cerviculis prominentis praeditae, foraminis cylindricis profundis dispersis inter tumulis peritheciorum.
Stromata erumpent from bark, strongly carbonaceous, yielding pigments in 10% KOH, bearing stout ostiolar necks and deep cylindrical holes. Asci unitunicate, cylindrical, stipitate, lacking an apical apparatus. Ascospores brown, cylindrical with broadly rounded ends, one-celled, smooth, with a straight germ slit. Anamorph Nodulisporium-like (Sporothrix type to Virgariella type as defined in Ju and Rogers (1996).
Type species: Rostrohypoxylon terebratum.
Rostrohypoxylon terebratum J. Fourn. & M. Stadler, sp. nov.
MycoBank: MB512544
Etymology: from Latin: terebrare (= to bore) in reference to the perforated stromatal surface.
Stromata effuso-pulvinata, per corticem hospitis erupemtia, tumulis peritheciorum inconspicuis vel conspicuis, 1–1.7 mm crassis, textura carbonacea, crusta 100–120 µm crassa; externe nigra. Superficie confragosa, foraminis cylindricis profundis (180–280 µm diametro × 500–700 mm profundis) irregulatiter dispersis inter tumulis peritheciorum. Granulis inconspicuis pallide olivaceis in KOH dissolutis. Perithecia obovoidea, 0.6–1 mm alta × 0.5–0.6 mm diam. Ostiolis compactis, cerviculis prominentibus praeditae, 0.25–0.5 mm alti × 0.25–0.35 mm diametro ad basae, cum aperturis minutis, umbilicatis. Ostiola deffindentes ad apicis glomeratis conicis con cerviculis nigris praeditae.
Asci 80–90 µm longitudine tota × 4–5 µm crassi, partibus sporiferis 60–70 µm longitudine, stipitibus 20–30 µm longitudine, sine anulis apicalis. Ascosporae 6.6–8.5 × 3–4.2 µm, brunneae, unicellularae, oblongae, apicibus latis, rima germinativa recta inconspicua abbreviata praeditae; perisporium in KOH indehiscens; episporium leve.
Stromata effused-pulvinate, 9–42 mm long × 4–19 mm broad × 1–1.7 mm thick (excluding ostiolar necks), with inconspicuous to 1/3 exposed perithecial mounds, developing within bark, erumpent through the periderm, yielding a dilute Greenish Olivaceous (90) pigment in 10% KOH; surface dull black, strongly uneven owing to stout ostiolar necks and cylindrical holes 180–280 µm diam (arrows), 500–700 µm deep irregularly scattered between perithecial mounds, usually filled with olivaceous-yellow material; stromatal crust strongly carbonaceous, 100–120 µm thick; interperithecial tissue blackish, powdery, without visible colored granules, subperithecial tissue 0.15–0.7 mm thick, black.
Perithecia obovoid to flask-shaped, 0.6–1 mm high × 0.5–0.6 mm diam, completely encased in thick carbonaceous tissue.
Ostioles opening as minute, umbilicate pores at the broadly rounded top of stout, conical black necks 0.25–0.5 mm high × 0.25–0.35 mm diam at the base.
Asci unitunicate, cylindrical, eight-spored, fragile and readily deliquescing, 80–90 µm total length × 4–5 µm broad, the spore-bearing parts 60–70 µm long, the stipes 20–30 µm long, without apparent apical apparatus, not bluing in Melzer’s reagent. Paraphyses deliquescing, much longer than asci, hyaline, septate, 3 µm to 4 µm broad at the base, tapering above.
Ascospores 6.6–8.5 × 3–4.2 µm (M = 7.4 × 3.7 µm, n = 60), brown, one-celled, cylindrical with broadly rounded ends, with a faint straight germ slit 4/5 to almost spore length, lacking cellular appendages or gelatinous sheath; perispore not dehiscent in 10% KOH; epispore smooth
Anamorph: Sporothrix-like to Virgariella-like in culture
Cultural characteristics: Colonies on OA and YMG covering Petri dish in 8–10 days, at first whitish, becoming olivaceous green (90), felty, azonate, with diffuse margins. Reverse dark brick (60).on YMG, remaining uncolored on OA. Reddish exsudates are released from the aerial mycelia on OA after 2 wk. Melanized mycelia not containing conidiogenous structures, but rather comprising a network of hyphae of up to 4.5 µm diam, showing brownish incrustations, but not differentiating further. Sporulating regions only observed on YMG, arising first from the center of colonies, later scattered over entire surface of colony, olivaceous buff (89). Conidiogenous structure Sporothrix- to Virgariella-like as defined in Ju and Rogers (1996), yellowish, becoming finely roughened. Conidiophores up to 80 µm long, mostly simple occasionally branched. Conidiogenous cells hyaline, smooth, 13–25 × 3–3.5 µm. Conidia hyaline, smooth to finely roughened, subglobose to ellipsoid, 3.5–5.5 × 2.5–3 µm, normally arising from the tips of the conidiogenous cells,. but sometimes also arising from lateral parts of undifferentiated vegetative hyphae.
Habitat: Stromata grow on dead bark (so far only found on Lithocarpus).
Known distribution: Northern Thailand.
Material examined: Thailand: Chiang Mai Province, Mae Teang District, Bahn Pha Deng, Mushroom Research Centre, N 19° 01′ 615″ E 98° 41′ 884″, 900 m, on bark of Lithocarpus sp., 6 Jun. 2005, J. Fournier JF-TH 06-04 (MFLU holotype of Rostrohypoxylon terebratum, extype cultures BCC and CBS 119137; Genbank Acc. No for DNA sequences: DQ631943, DQ840069, DQ631954, DQ840097, cf. Tang et al. 2009); Chiang Mai Province, Mae Teang District, track to Tung Joaw Village, N 19°807″ E 98° 389″, 1,350 m, on bark of Lithocarpus sp., 7 Jun. 2005, J. Fournier JF-TH 24-02 (MFLU 08-0521).
Notes: Owing to its erumpent effused stromata featuring stout, strongly protruding ostiolar necks, the present fungus first recalls a member of Diatrypaceae, such as Eutypa or Diatrype. A more exhaustive study shows that, unlike in Diatrypaceae the stroma is strongly carbonaceous and yields pigments in 10% KOH, ostiolar necks are smooth and broadly rounded at the top, asci are cylindrical and ascospores are brown, not allantoid and have a germ slit.
Based on stromatal shape, ascospore morphology and unitunicate asci in a paraphysate hamathecium, and despite the lack of apical apparatus, R. terebratum appears best placed in the Xylariaceae. The presence of an apical apparatus, usually bluing in iodine reagents, is considered a key character for the Xylariaceae, but exceptions are known for several genera and species currently accepted in this family, including Leprieuria Læssøe, J.D. Rogers & Whalley, Obolarina Pouzar, Phylacia Lév., Poroleprieuria M.C. González et al.,, Pyrenomyxa Morgan, Rhopalostroma D. Hawksw., Theissenia Maubl., Thamnomyces Ehrenb., Wawelia Namysl., and some species in Anthostomella Sacc. and Hypoxylon (Rogers 1994; Ju and Rogers 1996; Stadler et al. 2005). Anamorphic structures in stromata and cultures have not yet been observed in many species of the Xylariaceae; others are known to sporulate only sporadically.
The strongly carbonaceous stromata yielding pigments in KOH points toward close affinities with Annulohypoxylon (Hsieh et al. 2005), which primarily differs in having low papillate ostioles encircled with a disc and ellipsoid-inequilateral ascospores. Some Thamnomyces spp. resemble R. terebratum in featuring flask-shaped, separate perithecia in a carbonaceous stroma, asci lacking an apical ring and similar ascospores with indehiscent perispore. Thamnomyces is strikingly different in that its stromata are wiry vs. effused, and ascospores reniform to ellipsoid-inequilateral vs. cylindrical.
Arguably, the carbonaceous, erumpent stroma with conical ostioles also recall Entoleuca Syd. sensu Rogers and Ju (1996). However, no Geniculosporium-like anamorph, which is produced readily by all Entoleuca cultures we so far studied, was observed. Furthermore, molecular phylogenetic and chemotaxonomic data (see “Discussion”) preclude its inclusion in the xylarioid Xylariaceae in any case. As pointed out to us by Yu-Ming Ju (pers. comm.). Hypoxylon tormentosum Ces. shows certain morphological similarities to R. terebratum. However, the types of this name were studied by Ju and Rogers (1996), who found it depauperate and neither observed asci nor the characteristic stromatal surface. They could not even safely determine whether or not the stromata are bipartite. We therefore refrain from speculations on the possible synonymy of this taxon.
The deep cylindrical holes penetrating the stroma are not known from any other xylariaceous genus. Their function, if they have any, remains unclear. One could suppose they are associated with anamorphic structures, but this needs further observations.
Both hitherto obtained records of R. terebratum were encountered in dry, sun-exposed locations, on branches not in contact with the soil, at the end of the dry and hot season. This indicates that R. terebratum, like several other xylariaceous genera with carbonaceous stromata (e.g., Annulohypoxylon, Biscogniauxia, Camillea), is xerophilic. Further collections are needed to confirm its ecological requirements and its possible host specificity for Lithocarpus.
Chemical structures of some chemotaxonomically significant secondary metabolites detected in this study (BNT = Bi-Naphthalene Tetrol = 4,4′-Dihydroxy-5,5′-dimethoxy-1,1′-binaphthyl). See further Stadler and Fournier (2006)
Chemotaxonomic data from HPLC profiling also revealed significant differences of R. terebratum as compared to the species of Annulohypoxylon and many other Xylariaceae that were hitherto examined by us. The stromatal HPLC profile of both extant specimens (see Fig. 5 for the holotype) revealed various unknown compounds that did not correspond with any other known components of Annulohypoxylon, hence it can be assumed with certainty that the weak greenish stromatal pigments observed are not caused by daldinone A, truncatone, or azaphilones of the cohaerin/multiformin type. BNT, a rather ubiquitous component in the stromata of Annulohypoxylon, was only detected in traces and would have been overlooked if mass spectrometric detection and a standard had not been available to confirm its occurrence. Still, its occurrence provided corroborating evidence to regard the new genus as a member of the hypoxyloid Xylariaceae. The culture of R. terebratum also showed a rather specific HPLC profile. Besides some apparently specific compounds, 5-methylmellein was present in traces. Such dihydroisocoumarins were already detected in many species of the Xylariaceae by Whalley and Edwards (1995). Recently, Bitzer et al. (2008) refined these results for a larger number of taxa, with representative strains being available in public collections. They also showed that 5-methylmellein itself is not a genus specific marker metabolite but the compound occurs in many species of Annulohypoxylon, Biscogniauxia, Camillea, Lopadostoma, and Hypoxylon. While even being present in some of the Annulohypoxylon spp. treated here, 5-methylmellein was so far not found in Entoleuca and other xylarioid Xylariaceae with Geniculosporium-like anamorphs. It was also detected in the cleistocarpous Pyrenomyxa, but not in Daldinia and its immediate allies (i.e. Entonaema, Phylacia).
Rostrohypoxylon terebratum, HPLC-UV chromatograms (210 nm) of the stromatal methanol extract of holotype specimen (above) and ethyl acetate extract of submerged YMG culture after 168 h of fermentation (below). In both cases, the UV-vis spectra of some of the major, yet unknown components (a–d in the stromatal extract and e–h in the extract from YMG culture) were included. In the stromatal extract, the binaphthalene BNT (Fig. 4) was detected in traces, overlaid by one of the unknown major components, as indicated
As another significant difference to Annulohypoxylon spp., the Rostrohypoxylon cultures were apparently devoid of isosclerones, a second marker metabolite class, found by Bitzer et al. (2008) in several species of Annulohypoxylon. Even though the specific compounds of R. terebratum remain to be isolated and identified, the above chemotaxonomic results agree well with the proposed status of Rostrohypoxylon as being derived from Annulohypoxylon, or that it constitutes a separate, yet unknown lineage of the hypoxyloid Xylariaceae that evolved in parallel to Annulohypoxylon from biscogniauxioid forms. The molecular study by Tang et al. (2009) supports this hypothesis (see “Discussion”)
Annulohypoxylon bahnphadengense J. Fournier & M. Stadler, sp. nov.
(Fig. 6)
Annulohypoxylon bahnphadengense, from holotype specimen. a Vertical section of a stroma. b Stromatal habit. c Pigments in KOH. D Conidiophore from natural substrate, in 3% KOH. e Ascus in water. f Stromatal surface with ostiolar discs. g Ascus tip in Melzer‘s reagent. h Ascospore in 10% KOH with dehiscent perispores showing the dorsal thickening (arrow) Scale is indicated by bars. a, b, f 1 mm. C, D: 100 µm, e, g, h 10 µm
MycoBank: MB512545
Etymology: In reference to Bahn Pha Deng (Thailand), the locality where the fungus was collected.
Stromata effuso-applanata, tumulis peritheciorum inconspicuis vel conspicuis, 0.7–1.1 mm crassa, textura carbonacea; externe atrovinosa vel nigra, con granulis brunneis vel pallide olivaceis in KOH dissolutis. Perithecia globosa vel obovoidea, 0.5–0.65 mm alta × 0.5–0.6 mm diam. Ostiola papillata ab disco truncatum simili 0.25–0.3 mm diam.
Asci 100–130 µm longitudine tota, partibus sporiferis 60–70 µm longitudine × 4.5–5.5 µm crassi, stipitibus 37–60 µm longitudine, annulo apicali in liquore iodato Melzeri cyanescente, discoideo, 0.8–1 µm alto × 1.5–2 µm lato. Ascosporae 6.5–8.4 × 3–3.6 µm, brunneolae, unicellulares, ellipsoideo-inequilaterales vel equilaterales, apicibus angustatis vel latis, rima germinativa recta inconspicua longa praeditae; perisporium in KOH dehiscens; episporium leve.
Stromata effused-applanate, 6–70 mm long × 4–18 mm broad × 0.7–1.1 mm thick, with inconspicuous to 1/3 exposed perithecial mounds, hard-textured; surface dull black to shiny black, with a Dark Vinaceous (82) outermost tomentose layer progressively worn off at upper half but remaining between perithecial mounds; interperithecial tissue blackish-brown without visible colored granules. Dull olivaceous granules can be detected by microscopic examination in water, yielding Isabelline (65) to Grayish Sepia (106) pigments in 10% KOH; subperithecial tissue inconspicuous to 0.4 mm thick, woody, blackish.
Perithecia spherical to obovoid, 0.5–0.65 mm high × 0.5–0.6 mm diam, encased in carbonaceous tissue. Ostioles conical-papillate, encircled with a flattened truncatum-type disc 0.25–0.3 mm diam.
Asci cylindrical, eight-spored, short-stipitate, 100–130 µm total length, the spore-bearing parts 60–70 µm long × 4.5–5.5 µm broad, the stipes 37–60 µm long, with apical ring bluing in Melzer’s reagent, discoid, 0.8–1 µm high × 1.5–2 µm broad.
Ascospores 6.5–8.4 × 3–3.6 µm (M = 7.5 × 3.5 µm, n = 90), medium brown, one-celled, ellipsoid-inequilateral to nearly equilateral with broadly to narrowly rounded ends, uniseriate in the ascus, with a faint straight germ slit spore-length on the more convex side; perispore dehiscent in 10% KOH but not readily, with a thickening on the more convex side; epispore smooth.
Anamorph on natural substrate (JF-TH 07-03): Olivaceous (48), downy, on bark around young stromata. Conidiogenous structure showing a Nodulisporium-like branching pattern as defined in Ju and Rogers (1996), with erect conidiophores up to 320 µm high, brown to pale olivaceous brown, finely roughened. Conidiogenous cells pale brown, smooth to finely roughened, 10–18 × 2.5–3.5 µm. Conidia subhyaline, smooth, ellipsoid, 4–5 × 2.5–3 µm.
Known distribution: Northern Thailand.
Habitat: Stromata on dead bark or wood.
Material examined: Thailand: Chiang Mai Province, Mae Teang District, Bahn Pha Deng, Mushroom Research Centre, N 19° 01′ 615″ E 98° 41′ 884″, 900 m, on wood, 29 May 2005, J. Fournier JF-TH 29-02 (MFLU-holotype); same location, on bark, 7 Jun. 2005, JF-TH 07-03 (MFU08-1552 ); same location, on bark, 8 Jun. 2005, JF-TH 08-01 (MFU08-1523).
Notes: Annulohypoxylon bahnphadengense clearly belongs to the genus Annulohypoxylon based on stromatal morphology (carbonaceous stroma with conic papillate ostioles encircled with a discoid ring, ascospores with perispores bearing a thickening on the same side as the germ slit) and typical secondary metabolites yielding pigments in 10% KOH. It is characterized by its stromatal surface becoming shiny by fading of the Dark Vinaceous outermost layer, small ostiolar discs, isabelline to greyish sepia KOH-extractable pigments and small ascospores.
Although its mature stromata display a more or less shiny surface like in A. nitens (Ces.) Y.M. Ju, J.D. Rogers & H.M. Hsieh and A. purpureonitens (Ju & Rogers) Y.M. Ju, J.D. Rogers & H.M. Hsieh, A. bahnphadengense is different in having truncatum-type ostiolar discs sensu Ju and Rogers (1996), while they are of bovei-type in the two above species. Assessing the morphological type of ostiolar discs in H. bahnphadengense proved difficult in absence of clear observations on their dehiscence at early stage. Although they somewhat recall the bovei-type in gross morphology, they are herein referred to the truncatum-type based on their slightly notched rims. In addition, it yields brownish pigments in KOH while these pigments are Greenish Olivaceous (90) and Vinaceous Purple (101), respectively, in the aforementioned relatives.
The stromatal HPLC profile (Fig. 8) reveals additional differences to the above taxa as exclusively binaphthalene derivatives were detected in the stromata of the specimens examined. A series of such derivatives was detected in the specimens examined. By contrast, A. purpureonitens was found to yield only BNT as major component, while different compounds of the daldinone/truncatone type were previously encountered in A. nitens (cf. Quang et al. 2005a).
In the key provided by Ju and Rogers (1996), the present fungus keys out to A. moriforme (Henn.)Y.M. Ju, J.D. Rogers & H.M. Hsieh, with which it is likely to be closely related. Judging from three collections, it differs mainly from A. moriforme in having effused-applanate stromata with purplish-brown tone on surface vs effused-pulvinate to glomerate with olivaceous-brown tone, and Isabelline to Grayish Sepia pigments in KOH vs Greenish Olivaceous to Dull Green. Moreover, it has slightly smaller and more slender ascospores 6.6–8.4 × 3–3.6 µm than typical A. moriforme collected at the same place [8.5–9(−9.5) × 3.8–4.5 µm]. In addition, its stromatal HPLC profiles revealed that the colours of its stromatal pigments are mainly due to the presence of naphthalene derivatives. Truncatone was only detected in minor quantities and daldinone A was not detected at all, but both compounds are present in fairly large amounts in A. moriforme.
Annulohypoxylon bahnphadengense should be likewise compared with A. elevatidiscum Y.M. Ju, J.D. Rogers & H.M. Hsieh, a recently described species (Ju et al. 2004, as Hypoxylon) that is morphologically similar but differs in having convex ostiolar discs raised above the rims and Greenish Olivaceous pigments in KOH. We have not yet studied the latter species by HPLC, but the colour of stromatal pigments suggests that either daldinone A or truncatone, which are widespread in all Annulohypoxylon spp. that show this characteristic colour, are probably contained in its stromata as well.
A culture was obtained from the holotype specimen but unfortunately it was soon lost due to contamination by mites carrying moulds. We failed to observe anamorphic structures, but a fermentation of the strain in YMG medium revealed 5-methylmellein as in other members of Annulohypoxylon that are deemed related to the new species.
Annulohypoxylon maeteangense J. Fourn. & M. Stadler, sp. nov.
(Fig. 7)
Annulohypoxylon maeteangense , from holotype specimen. a Vertical section of a stroma. b Stromatal habit. c Pigments in KOH. d Conidiophore from natural substrate, in 3% KOH. e Ascus in water. f Stromatal surface with ostiolar discs. g Ascus tip in Melzer‘s reagent. H. Ascospores in 10% KOH with dehiscent perispore showing the dorsal thickening (arrow). Scale is indicated by bars. a, f 1 mm. b 5 mm. c, d 100 µm. e, g, h 10 µm
MycoBank: MB512546
Etymology: from the Mae Teang district in Chiang Mai Province where the collections were made.
Stromata effuso-applanata, tumulis peritheciorum conspicuis, 0.6–0.75 mm crassa, textura carbonacea; externe olivacea tomentosa vel nigra, granulis inconspicuis viridis in KOH dissolutis. Perithecia globosa, 0.35–0.55 mm diam. Ostiola papillata con discis Hypoxylo, truncato simili 0.15–0.25 mm diam.
Asci 95–115 µm longitudine tota, partibus sporiferis 45–62 µm longitudine × 4–4.5 µm crassi, stipitibus 50–65 µm longitudine, annulo apicali in liquore iodato Melzeri cyanescente, discoideo, 0.8–1 µm alto × 1.5–1.8 µm lato. Ascosporae 6.5–8.5 × 3–3.5 µm, brunneolae, unicellulares, ellipsoideo-inequilaterales, apicibus angustatis vel latis, rima germinativa recta inconspicua longa praeditae; perisporium in KOH dehiscens; episporium leve.
Stromata irregularly effused-applanate, 5–60 mm long × 2–28 mm broad × 0.6–0.75 mm thick, with conspicuous perithecial mounds, often nearly rosellinioid at the effused margins, carbonaceous and hard-textured; surface brown to dull black with an Olivaceous (48) tone, matt, coated with a long-persistent tomentum of coiled, pale brown to brown, smooth to granulose hyphae 2 µm to 3 µm broad mixed with remnants of conidiophores and dull olivaceous-brown granules; interperithecial tissue entirely carbonaceous, without visible colored granules, yielding a Dull Green (70) pigment in 10% KOH; subperithecial tissue entirely inconspicuous to 0.2 mm thick, woody, black.
Perithecia spherical, 0.35–0.55 mm diam, encased in thick carbonaceous tissue.
Ostioles broadly conical-papillate, shiny black, encircled with a slightly concave truncatum-type disc 0.15–0.25 mm diam, sometimes overlain with white material.
Asci cylindrical, eight-spored, long-stipitate, 95–115 µm total length, the spore-bearing parts 45–62 µm long × 4–4.5 µm broad, the stipes 50–65 µm long, with apical ring blueing in Melzer’s reagent, discoid, 0.8–1 µm high × 1.5–1.8 µm broad.
Ascospores 6.5–8.5 × 3–3.5 µm (M = 7.3 × 3.3, n = 90), medium brown, one-celled, ellipsoid-inequilateral with narrowly to broadly rounded ends, with a faint, straight germ slit spore-length on the more convex side; perispore dehiscent in 10% KOH, with a thickening on the more convex side; epispore smooth.
Anamorph on natural substrate (JF-TH 26-01): Olivaceous (48), downy, on bark around young stromata. Conidiogenous structure Nodulisporium-like with erect conidiophores 120–300 µm high, brown to pale olivaceous brown, septate finely roughened. Conidiogenous cells pale olivaceous brown, smooth, 12–18 × 2.5–3 µm. Conidia pale brown to subhyaline, smooth, ellipsoid, 4–5 × 3–3.5 µm.
Anamorph in culture: Colonies on Difco OA covering Petri dish in 9 days, at first white, becoming Hazel (88), floccose, azonate, with diffuse margins, with scattered black patches; reverse Dull Green (70). Sporulating regions scattered over entire surface of colony, Olivaceous (48) to Olivaceous Buff (89). Conidiogenous structure Nodulisporium-like, yellowish to pale brown, roughened. Conidiogenous cells hyaline, smooth, 10–20 × 2.5–3 mm. Conidia hyaline, smooth to finely roughened, ellipsoid, 3.5–6 × 2.5–3 mm.
Notes: Annulohypoxylon maeteangense is another member of the genus Annulohypoxylon (Hsieh et al. 2005), as revealed from its carbonaceous stromata yielding pigments in 10% KOH, papillate ostioles encircled with a disc and the presence of a thickening on the more convex side of the perispores.
Among other species with greenish KOH-extractable pigments and similar ascospore size range that can be confused with the present taxon, A. moriforme (Henn.) Y.M. Ju, J.D. Rogers & H.M. Hsieh differs in having larger perithecia 0.4–0.8 mm diam and ostiolar discs 0.2–0.4 mm diam and frequently pulvinate to glomerate stromata, and A. microcarpum (Penz. & Sacc.) Y.M. Ju, J.D. Rogers & H.M. Hsieh differs in having smaller perithecia 0.15–0.2 mm diam. and ostiolar discs 0.1 mm diam.
Annulohypoxylon squamulosum (Y.M. Ju, J.D. Rogers & H.M. Hsieh) Y.M. Ju, J.D. Rogers & H.M. Hsieh is likewise similar in ascospore size range, perithecial diameter and ostiolar discs diameter, but differs primarily in having a persistent reticulately cracked surface and slightly papillate ostioles with bovei-type discs. The type of this species [Taiwan, Taiwan Prov., I-lan Co., Fu-shan, on dead wood, 19 Aug 2001, Y.-M. Ju & H.-M. Hsieh 90081905 (HAST-holotype of H. squamulosum)] additionally revealed large amounts of daldinone A and traces of BNT and truncatone. It has a similar HPLC profile as the present fungus, which, however, contained no daldinone A but some unidentified compounds with characteristic truncatone-like UV-Vis spectra (Fig. 8).
Externally, A. maeteangense is well characterized by effused-applanate stromata with a matt, olivaceous-brown and tomentose surface strongly contrasting with the small black discs with broadly conical ostioles. The brown tomentum is progressively worn off with age and in mature stromata the ostiolar discs become less contrasted.
Known distribution: Northern Thailand.
Habitat: Stromata on dead bark.
Specimens examined: THAILAND: Chiang Mai Province, Mae Teang District, Bahn Pha Deng, Mushroom Research Centre, N 19° 01′ 615″ E 98° 41′ 884″, 900 m, on a corticated branch, 5 Jun. 2005, J. Fournier JF-TH 05-01 (MFLU-holotype); same location, 26 May 2005, J. Fournier JF-TH 26-01 (BCC culture CBS 123835); same location, 5 Jun. 2005, J. Fournier JF-TH 05-02. (MFU08-1525).
Discussion
While the erection of new genera and species is based mainly on morphological and other phenetic data, in comparison with the definition of accepted Xylariaceae genera and species, we also wish to discuss the current situation with respect to the molecular phylogeny of Xylariaceae. All materials described here were found in Thailand, but the only available molecular study on hypoxyloid Xylariaceae (Suwannasai et al. 2005) unfortunately did not include any reference sequences derived from other studies. In any case, the authors did not accept Annulohypoxylon, even though the genus had already been erected at the time of publication. They used 5.8S/ITS nrDNA and only compared the species they exclusively found in Thailand with one another, except for two reference specimens from Taiwan. From their results, they found it difficult to establish a phylogeny that would justify the segregation of the species included in Hypoxylon sensu Ju and Rogers (1996). The new taxa they reported all belong to Hyxpoxylon sensu Hsieh et al. (2005) and do therefore not correspond to the fungi we have described and illustrated. In addition, Suwannasai et al. (2005) have summarised morphological data of six species of Hypoxylon sect. Annulata (i.e., Annulohypoxylon), including two taxa named A. cf. archeri and A. atroroseum, in a table. We have so far been unable to obtain the described specimens for comparison. Nonetheless, from the data presented in this table, it is not likely that one of their specimens corresponds to the new taxa described here. A concurrent preliminary molecular phylogenetic study based on similarity analyses of various different genes (Tang et al. 2009, “Xylariaceae sp. 1”), in which the holotype material of the new genus was included, clearly revealed that R. terebratum is nested inside the hypoxyloid Xylariaceae, with close affinities to the representatives of Annulohypoxylon. It is therefore likely that the inclusion of Rostrohypoxylon in molecular phylogenies using additional representatives of Annulohypoxylon would render the latter genus paraphyletic, as previously observed with other groups of the Xylariaceae (e.g. Daldinia vs. Hypoxylon; see Hsieh et al. 2005). However, we agree here with the authors of the latter study. They cited Brummit (2002), who convincingly explained that paraphyletic groups are inevitable in the Linnaean hierarchical classification system, to defend why they did not merge Daldinia with Hypoxylon.
A case could be made to integrate Rostrohypoxylon in Annulohypoxylon, but this would afford emending the latter, which is even now sometimes difficult to discriminate from Hypoxylon, based on morphological methods. A fungus like Rostrohypoxylon would not even match easily the concept of Hypoxylon sensu Ju and Rogers (1996), but could have been easily accommodated in the broad, outdated concept of Hypoxylon sensu Miller (1961). We think it is unwise to use molecular phylogenetic data to turn back the clock of fungal taxonomy by five decades, ignoring evidence that was meanwhile obtained from highly conclusive phenotype-based, descriptive taxonomy. Actually, several recent molecular phylogenetic studies (Peláez et al. 2008; Tang et al. 2009, and references cited therein) have shown in unison that the hypoxyloid and xylarioid Xylariaceae constitute two very distinct phylogenetic lineages. In scope of such data, it might be a better option to consider rearrangements at the suprageneric level, after a conclusive decision has been reached on the status of the higher taxa of the Sordariomycetes and the Xylariales. At this time, we fear that excessive lumping of genera that are well-defined by morphological traits may lead to further confusion and ultimately disguise the true diversity of the Xylariaceae.
Chemotaxonomic data proved to be informative as a means of “taxonomic quality control” at various different levels (Stadler and Hellwig 2005) in other taxa of Xylariaceae and may be useful in other families (Zhang et al. 2009). For Rostrohypoxylon, the limited evidence so far obtained on two specimens and a single culture appears to be insignificant, but this must be regarded in a broader context.
When a large number of hypoxyloid Xylariaceae was studied for the occurrence of characteristic metabolites, the results did not disagree with molecular data, thus proving phylogenetically informative (Bitzer et al. 2008; Stadler et al. 2008a). A comparison of such data revealed significant deviations of Rostrohypoxylon to Annulohypoxylon and other accepted genera. We admit that this evidence is so far mainly based on lack of characteristic extrolites and remains to be validated by identification of the characteristic unknown compounds that occur in Rostrohypoxylon and comparison of their potential biogenetic pathways.
References
Bitzer J, Koepcke B, Stadler M, Hellwig V, Ju Y-M, Seip S, Henkel T (2007) Accelerated dereplication of natural products, supported by reference libraries. Chimia 61:332–338
Bitzer J, Læssøe T, Fournier J, Kummer V, Decock C, Tichy H-V, Piepenbring M, Peršoh D, Stadler M (2008) Affinities of Phylacia and the daldinoid Xylariaceae, inferred from chemotypes of cultures and ribosomal DNA sequences. Mycol Res 112:251–270
Brummit RK (2002) How to chop up a tree. Taxon 51:31–41
Hsieh HM, Ju Y-M, Rogers JD (2005) Molecular phylogeny of Hypoxylon and closely related genera. Mycologia 97:844–865
Ju Y-M, Rogers JD (1996) A revision of the genus Hypoxylon. Mycologia Memoir No. 20. APS, St. Paul
Ju Y-M, Rogers JD, Hsieh HM (2004) New Hypoxylon species and notes on some names associated with or related to Hypoxylon. Mycologia 96:154–161
Miller JH (1961) A monograph of the world species of Hypoxylon. University of Georgia Press, Athens
Peláez F, González V, Platas G, Sánchez-Ballesteros J, Rubio V (2008) Molecular phylogenetic studies within the family Xylariaceae based on ribosomal DNA sequences. Fungal Divers 31:111–134
Quang DN, Hashimoto T, Nomura Y, Wollweber H, Hellwig V, Fournier J, Stadler M, Asakawa Y (2005a) Cohaerins A and B, azaphilones from the fungus Hypoxylon cohaerens, and comparison of HPLC-based metabolite profiles in Hypoxylon sect. Annulata. Phytochemistry 65:797–809
Quang DN, Hashimoto T, Stadler M, Radulovic N, Asakawa Y (2005b) Antimicrobial azaphilones from the fungus Hypoxylon multiforme. Planta Med 71:1058–1072
Quang DN, Stadler M, Fournier J, Tomita A, Hashimoto T (2006) Cohaerins C-F, four azaphilones from the xylariaceous fungus Annulohypoxylon cohaerens. Tetrahedron 62:6349–6354
Rayner RW (1970) A mycological colour chart. Commonwealth Mycological Institute, Kew
Rogers JD (1994) Problem genera and family interfaces in the Eupyrenomycetes. In: Hawksworth DL (ed) Ascomycete systematics: problems and perspectives in the nineties. Plenum, New York, pp 321–331
Rogers JD, Ju Y-M (1996) Entoleuca mammata comb. nov. for Hypoxylon mammatum and the genus Entoleuca. Mycotaxon 59:441–448
Stadler M, Hellwig V (2005) Chemotaxonomy of the Xylariaceae and remarkable bioactive compounds from Xylariales and their associated asexual stages. Recent Research Developments in Phytochemistry 9:41–93
Stadler M, Fournier J (2006) Pigment chemistry, taxonomy and phylogeny of the Hypoxyloideae (Xylariaceae). Revista Iberoamericana de Micologia 23:160–170
Stadler M, Læssøe T, Simpson JA, Wollweber H (2004) A survey of Daldinia species with large ascospores. Mycol Res 108:1025–1041
Stadler M, Læssøe T, Vasilyeva L (2005) The genus Pyrenomyxa and its affinities to other cleistocarpous Hypoxyloideae as inferred from morphological and chemical traits. Mycologia 97:1129–1139
Stadler M, Fournier J, Læssøe T, Lechat C, Tichy H-V, Piepenbring M (2008a) Recognition of hypoxyloid and xylarioid Entonaema species from a comparison of holomorphic morphology, HPLC profiles, and ribosomal DNA sequences. Mycological Progress 7:53–73
Stadler M, Fournier J, Beltrán-Tejera E, Granmo A (2008b) The “red Hypoxylons” of the Northern Hemisphere. In: Glawe DA, Ammirati JF (eds) A festschrift in honor of Professor Jack D. Rogers. North American Fungi 3: 73–125
Suwannasai N, Rodtong S, Thienhirun S, Whalley AJS (2005) New species and phylogenetic relationships of Hypoxylon species found in Thailand inferred from the internal transcribed spacer regions of ribosomal DNA sequences. Mycotaxon 94:303–324
Tang AMC, Jeewon R, Hyde KD (2009) A re-evaluation of the evolutionary relationships within the Xylariaceae based on ribosomal and protein-coding gene sequences. Fungal Divers 34:155–153
Whalley AJS, Edwards RL (1995) Secondary metabolites and systematic arrangement within the Xylariaceae. Can J Bot 73:S802–810
Zhang Y, Wang HK, Fournier J, Crous PW, Jeewon R, Pointing SB, Hyde KD (2009) Towards a phylogenetic clarification of Lophiostoma/Massarina and morphologically similar genera in the Pleosporales. Fungal Divers 38:225–251
Acknowledgements
We greatly acknowledge the help of Dr. Yu-Ming Ju (Academica Sinica, Taipei). We also thank the curators of HAST, S, and LPS, who also kindly provided specimens and Beata Schmieschek (InterMed Discovery GmbH) for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fournier, J., Stadler, M., Hyde, K.D. et al. The new genus Rostrohypoxylon and two new Annulohypoxylon species from Northern Thailand. Fungal Diversity 40, 23–36 (2010). https://doi.org/10.1007/s13225-010-0026-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-010-0026-4