Abstract
Six new species of Fusarium associated with soil and plant hosts from ecosystems of minimal anthropogenic disturbance in Australia are described. Fusarium coicis from Coix gasteenii, F. goolgardi from Xanthorrhoea glauca, F. mundagurra from soil and Mangifera indica, F. newnesense from soil, F. tjaetaba from Sorghum interjectum and F. tjaynera from soil, Triodia microstachya, Sorghum interjectum and Sorghum intrans. Morphology and phylogenetic analysis of EF-1α, RPB1 and RPB2 sequence data were used to delineate species boundaries. The new species were phylogenetically distributed in the Fusarium sambucinum, F. fujikuroi, and F. chlamydosporum species complexes, and two novel species complexes. These six new species have particular phylogeographic significance as not only do they provide further insight into the geographic patterns of Fusarium evolution but also challenge current phylogeographic hypotheses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fusarium is considered a ubiquitous fungal genus commonly isolated from the majority of bioclimatic regions and ecosystems (Summerell et al. 2010; Backhouse et al. 2001). Representatives cause devastating plant diseases (Nelson et al. 1983) and are major contributors to mycotoxin contamination of human and animal food supplies (Marasas et al. 1984; Desjardins 2006; Bryden 2012). Some species also cause diseases of humans and other animals (Boutati and Anaissie 1997; Mitchell and Attleber 1973). Consequently, Fusarium is one of the most studied fungal genera in ecosystems associated with anthropogenic activities.
Fusarium species also occur widely in natural ecosystems with considerable ecological plasticity, occurring as endophytes or latent plant pathogens and soil saprobes (Gordon and Martyn 1997; Burgess 1981; Walsh 2007). Despite the human and ecological importance of this genus, surveys in natural ecosystems of minimal anthropogenic disturbance have largely been undertaken in Australia with relatively few such surveys in other geographic regions of the world (Summerell et al. 2010).
A series of continental scale surveys of Fusarium in natural ecosystems of Australia have been conducted over the past 40 years, encompassing a wide range of bioclimatic regions, including tropical, arid, temperate and alpine bioregions (Summerell et al. 1993, 2010; Burgess and Summerell 1992; Sangalang et al. 1995b; Walsh et al. 2010). Biogeographical surveys in natural ecosystems of Australia have resulted in the discovery of novel species including F. nygamai (Burgess and Trimboli 1986), F. beomiforme (Nelson et al. 1987), F. babinda (Summerell et al. 1995), F. aywerte, F. nurragi (Benyon et al. 2000), F. gaditjirri (Phan et al. 2004), F. lyarnte, F. werrikimbe (Walsh et al. 2010) and F. burgessii (Laurence et al. 2011); as well as novel species complexes (Laurence et al. 2011); in addition to high levels of intraspecific diversity in plant pathogenic Fusarium species (Laurence et al. 2012, 2014). The fact that novel species have been discovered in each of these surveys suggests that the species diversity in Australia and indeed globally is yet to be fully determined.
Fusarium is a complex and diverse genus with a controversial taxonomic history (Summerell et al. 2010). The number of recognised species has fluctuated wildly from over 1,000 to as few as nine (Summerell and Leslie 2011), with current estimations ranging from 100 to 500 (Kirk et al. 2008; Leslie and Summerell 2006), depending on the species concept applied. Details of the historical taxonomic controversies have been comprehensively covered elsewhere (Leslie et al. 2001; Nelson 1991; Leslie and Summerell 2006) but many have resulted from the paucity and considerable plasticity of morphological characters. The key morphological markers for the genus are the shape of the macroconidia and microconidia, the mode of formation of microconidia and the presence or absence of chlamydospores in older cultures. The utility of morphology to describe the inherent diversity in Fusarium, however, is questionable as there is considerable plasticity of the characters between isolates within a species and the expression of these characters is also affected by environmental conditions (Leslie et al. 2001). DNA sequencing technology has enabled the taxonomic resolution of Fusarium based on multi-gene genealogies (Laurence et al. 2014; O’Donnell et al. 2000; O'Donnell et al. 1998b, 2004). Surveys of natural populations of Fusarium are helping to ensure that the intraspecific diversity is represented, improving not only the phylogenetic accuracy (Zwickl and Hillis 2002; Pollock et al. 2002; Hillis et al. 2003) but also taxonomic stability.
This study describes six novel morphospecies of Fusarium recovered from natural ecosystems of minimal anthropogenic disturbance throughout Australia. A bi-phasic approach of morphological description and DNA sequence analysis is adopted, consisting of a two-step phylogenetic analysis of deep and shallow node resolutions. An initial deep node phylogenetic analysis of the novel taxa is conducted using the largest and second largest subunits of RNA polymerase II (RPB1 and RPB2). These loci have been used previously to determine deep level Fusarium phylogeny (Laurence et al. 2011; O’Donnell et al. 2007; Gräfenhan et al. 2011). Phylogenetic resolution within species complexes is determined using the translation elongation factor 1-alpha (EF-1α) locus. The description and investigation of these species adds to existing knowledge on the phylogeny, taxonomy, phylogeography, ecology, and species evolution in the genus Fusarium.
Materials and methods
Origin of isolates examined
Six novel Fusarium morphospecies were recovered from three independent series of surveys conducted between 2003 and 2010 in natural ecosystems of minimal anthropogenic disturbance throughout Australia. Three novel Fusarium morphospecies species were recovered from Coix gasteenii, Triodia microstachya and Sorghum species in Litchfield National Park, Northern Territory in the first survey series (2003–2006). Two novel Fusarium morphospecies were recovered from soil samples obtained from Carnarvon Gorge National Park, Queensland and Rocky Cape National Park, Tasmania in the second survey series (2006–2007); and one novel Fusarium morphospecies associated with Xanthorrhoea glauca decline in Bungonia National Park, New South Wales in the third survey series (2010). The isolation, culture purification techniques and incubation conditions used were described by Burgess et al. (1994b).
Accession of types
The ex-type cultures were deposited in the Agricultural Research Service Culture Collection, Peoria, Illinois USA (NRRL number) with replicates maintained in the culture collection at the Royal Botanic Gardens Trust, Sydney, New South Wales, Australia (RBG and FRL numbers). The holotype for each species was deposited in the culture collection at the Royal Botanic Gardens Trust, Sydney, New South Wales, Australia.
Morphological characterisation
Morphological characteristics were examined after 12 days growth of cultures initiated from a single germinated macroconidium. Cultures were grown under 12 h light–dark (l/d) cycles with UV and daylight colour fluorescent lights at 24 °C. Morphological characters examined included the shape and size of macroconidia produced in sporodochia on Carnation Leaf Agar (CLA) (Fisher et al. 1982), the shape and mode of formation of microconidia on CLA and Spezieller Nährstoffarmer Agar (SNA) (Nirenberg 1976), the production of chlamydospores on CLA, and pigmentation of the agar on Potato Dextrose Agar (PDA). Descriptions of pigmentation colour were based on the Methuen Handbook of Colour (Kornerup 1978). The dimensions of the conidia were measured using a minimum of 30 conidia of each spore type.
DNA sequencing and phylogenetic analysis
Isolates were grown on PDA for 5 days under dark incubation at 25 °C, after which the mycelium was harvested and the genomic DNA extracted using the FastDNA® Kit (Q-biogene Inc., Irvine, California, USA) according to the manufacturer’s instructions.
Portions of the DNA loci were amplified using primer sets and PCR conditions described in Carbone and Kohn (1999) for EF-1α, O’Donnell et al. (2010) for RPB1 and Reeb et al. (2004) for RPB2. All PCR were amplified in 25 μl reaction volumes containing PCR buffer (Promega Corporation, Madison, Wisconsin, USA), 2.5 mM of MgCl2 (Sigma-Aldrich Corporation, Louis, Missouri, USA), 1.25 units of GoTaq™ (Promega Corporation, Madison, Wisconsin, USA) and 0.25 mM each of dATP, dCTP, dGTP and dTTP (Promega).
The resulting PCR products were purified using ExoSAP-IT (USB Corporation Cleveland, Ohio, USA) following the manufacturer’s instructions and then electrophoresed to assess product integrity and estimate concentration. The PCR amplicons were sent to the Ramaciotti Centre for Gene Function Analysis at the University of New South Wales (Randwick, NSW, Australia) for DNA sequence determination by an ABI PRISM® 3700 DNA Analyser (Applied Biosystems Inc., Foster City, California, USA), using the same primers as for the PCR amplifications for each gene region, respectively. Both the forward and reverse strands were sequenced to minimise the presence of ambiguous nucleotides. All sequences from this study have been deposited in GenBank (Table 1).
Sequences from the current study were aligned with reference sequences obtained from GenBank using the multiple alignment program ClustalW (Version 1.83) plug-in (Thompson et al. 1997) in the software Geneious (Version 5.3.6) (Drummond et al. 2011). The alignment was edited manually within the sequence alignment editing program Geneious (Version 5.3.6) (Drummond et al. 2011) and all polymorphisms were confirmed by reexamining the electropherograms. Reference sequences for each set of analyses were selected on the basis of previously published phylogenetic relationships within the genus (O’Donnell et al. 1998a, 2007; O’Donnell 2000; Schroers et al. 2004; Baayen et al. 2001). Table 1 lists all the Fusarium strains used in the phylogenetic analyses.
Phylogenetic analyses were performed using PAUP 4.0b10 (Swofford 2002) on the combined RPB1 and RPB2 data set and individual EF-1α data set. Unweighted Maximum Parsimony (MP), Neighbour Joining (NJ) and Bayesian Likelihood (BL) analyses were performed on all data sets. The heuristic search option with 1,000 random addition sequences and tree bisection reconnection branch swapping was used to infer maximum parsimony. Gaps were treated as missing data. The Consistency Index (CI) and Retention Index (RI) were calculated to indicate the amount of homoplasy present. NJ analyses were also conducted on individual and combined datasets with trees generated using the HKY85 model (Hasegawa et al. 1985) with ties broken randomly. Base frequencies were estimated with among-site ratios assumed to be equal and the gamma distribution was also estimated. Clade stability was assessed in PAUP 4.0b10 (Swofford 2002) using 1,000 heuristic search bootstrap replications with random sequence addition. Trees were rooted using the outgroup method. Bayesian inference was used to estimate posterior probabilities for consensus nodes using MrBayes 3.1 (Huelsenbeck 2001) run with a 4,000,000-generation Monte Carlo Markov chain method with a burnin of 10,000 trees. JModeltest (Posada and Crandall 1998) was used to determine the most appropriate model for Bayesian analysis of the combined RPB1 and RPB2 and individual EF-1α data sets. Trees were visualised using FigTree v1.4 (Rambaut 2013). Appropriate outgroups for the intra-species complex EF-1α analyses were determined using the results from the RPB1 and RPB2 phylogenetic analyses.
Results
Phylogenetic analyses
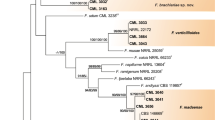
Phylogenetic analyses of the RPB1 and RPB2 resolved the phylogenetic positions of the six novel morphospecies in relation to the 15 currently recognised monophyletic species complexes in the genus Fusarium (Fig. 1). No major topological variations were detected between trees derived from MP, NJ and BL phylogenetic inferences (data not shown). The RPB1 and RPB2 data set consisted of 3074 nucleotides, of which 1230 were parsimony-informative (PIC). The MP analysis yielded 7 equally most-parsimonious trees (CI = 0.28, RI = 0.74) (Fig. 1).
One of 7 most-parsimonious trees inferred from the combined RPB1 and RPB2 data set indicating the phylogenetic relationship between the novel species Fusarium coicis, F. goolgardi, F. mundagurra, F. newnesense, F. tjaetaba and F. tjaynera and the major species complexes in the Fusarium genus. Branches with most parsimonious bootstrap partitions >70 % and Bayesian posterior probabilities >0.95 are indicated in bold. The number of Parsimony-Informative Characters (PIC), Retention Index (RI) and Consistency Index (CI) are indicated
The RPB1 and RPB2 phylogenetic analyses placed five of the novel Fusarium morphospecies in three Fusarium species complexes: the F. fujikuroi Species Complex (FFSC), F. chlamydosporum Species Complex (FCSC) and F. sambucinum Species Complex (FSAMSC) (Fig. 1). Fusarium newnesense sp. nov. did not cluster within any of the currently recognised complexes but formed an independent lineage closely related to the F. oxysporum Species Complex (FOSC), FFSC and F. nisikadoi Species Complex (FNSC).
The RPB1 and RPB2 phylogeny was used to select appropriate outgroup taxa for species level resolution using the EF-1α locus. It was determined that F. equiseti (NRRL20697), F. lacertarum (NRRL20423) and Fusarium sp. (NRRL5537) were appropriate outgroup taxa for resolving the phylogeny of F. goolgardi and F. tjaynera (Fig. 2). Reference taxa for separating these two novel species from close relatives were selected from the FCSC and FSAMSC. The EF-1α analyses did not result in any major topological variations between trees derived from MP, NJ and BL phylogenetic inferences (data not shown). The FCSC/FSAMSC EF-1α data set consisted of 557 nucleotides, of which 160 were PICs. The MP analysis yielded six equally most-parsimonious trees (CI = 0.64, RI = 0.84) (Fig. 2).
One of six most-parsimonious trees inferred from the EF-1α data set indicating species resolution of Fusarium goolgardi sp. nov. and F. tjaynera sp. nov. Branches with most parsimonious bootstrap partitions >70% and Bayesian posterior probabilities >0.95 are indicated in bold. The number of Parsimony-Informative Characters (PIC), Retention Index (RI) and Consistency Index (CI) are indicated.
Fusarium goolgardi clustered in the FSAMSC and was closely related to the described species F. langsethiae, F. palustre, F. sporotrichoides and the undescribed Fusarium species NRRL36351. Fusarium tjaynera formed a sister relationship to F. aywerte (Figs. 1 and 2). Although the closest relatives to F. aywerte and F. tjaynera were in the FCSC there were high levels of divergence (170 PICs from the combined RPB1 and RPP2 data set) suggesting that F. aywerte and F. tjaynera should be considered a separate monophyletic species complex/lineage, hereby designated Fusarium aywerte Species Complex (FASC).
Taxa in the Fusarium redolens Species Complex (FRSC) were determined to be appropriate outgroups for resolving the phylogeny of F. coicis, F. mundagurra, F. newnesense and F. tjaetaba (Fig. 3). Reference taxa for separating F. coicis, F. mundagurra, F. newnesense and F. tjaetaba were selected from the FFSC and FOSC. The FOSC/FFSC EF-1α data set consisted of 585 nucleotides, of which 142 were PICs. The MP analysis yielded over 5,000 equally most-parsimonious trees (CI = 0.64, RI = 0.85) (Fig. 1).
One of ≥5,000 most-parsimonious trees inferred from the EF-1α data set indicating species resolution of Fusarium coicis sp. nov., Fusarium mundagurra sp. nov., F. newnesense sp. nov., and F. tjaetaba sp. nov.. Branches with most parsimonious bootstrap partitions >70 % and Bayesian posterior probabilities >0.95 are indicated in bold. The number of Parsimony-Informative Characters (PIC), Retention Index (RI) and Consistency Index (CI) are indicated
Fusarium coicis, F. mundagurra and F. tjaetaba clustered within the African Clade of the FFSC (O’Donnell et al. 1998a) (Fig. 1). Fusarium coicis was closely related to the undescribed species NRRL25615 (origin: Nigeria) and NRRL26793 (origin: Sudan), differing by 12 and 18 EF-1α PICs, respectively. Fusarium mundagurra was closely related to the undescribed species NRRL25221 (origin: Zimbabwe) and F. sacchari, differing by 6 and 29 EF-1α PICs, respectively. Fusarium tjaetaba was closely related to F. brevicatenulatum NRRL25446 (origin: Madagascar) differing by 19 EF-1α PICs.
Fusarium newnesense did not cluster within the established Fusarium species complexes and formed an independent lineage that was basal to the FOSC, FFSC and near F. commune. The F. newnesense lineage resolved into two phylogenetic species separated by 3 EF-1α mutations and correlated to geographic origin. The closest relative was the undescribed species NRRL25184 (origin: Germany) and was separated by 3 EF-1α PICs.
Taxonomy
Fusarium coicis R.M. Johanssen, J.L. Walsh, M.H. Laurence, L.W. Burgess, E.C.Y. Liew & T. Petrovic, sp. nov. -Figs. 4–8 MB812304
Etymology: Refers to the host, Coix gasteenii, from which this species was isolated.
Description Colonies on PDA with powdery to floccose aerial mycelium, white to pale pink in colour, darkening with age. Pigment on reverse side is greyish-rose to dark violet. Sporodochia small, cream in colour, forming on carnation leaf pieces on CLA, difficult to observe in some isolates. Macroconidia very long, slender, parallel dorso-ventral sides, tapering apical cells and barely notched basal cells, 4–10 septate, usually 7 septate, 55–123 μm (\( \overline{x} \) = 85 μm) long × 4–6 μm (\( \overline{x} \) = 5 μm) wide. Microconidia forming in false heads and short to long chains, arising from monophialides and occasionally polyphialides, oval to clavate in shape, 8–23 μm (\( \overline{x} \) = 14 μm) long, 0–3 septate. Chlamydospores absent.
Specimen examined Type Strain (living and dried) NRRL66233, RBG5368, FRL19329 and additional strains FRL19328, FRL19333, FRL19335, FRL19336 isolated from Coix gasteenii, Mareeba, Queensland; 16°59′S, 145°19′E 10/06/2005. Additional strains FRL19330, FRL19331, FRL19332, FRL19334, FRL19337 isolated from Coix gasteenii Lakefield National Park, Queensland; 14°36′S, 143°56′E.
Fusarium goolgardi D.M. Robinson, M.H. Laurence, E.C.Y. Liew & B.A. Summerell sp. nov.-Figs. 9–18, MB812305
Morphological characters of F. goolgardi. 9-10. Macroconidia (arrow in Fig. 10 indicates basal notch). 11. Microconidia, aseptate and with 1, 2 and 3 septa. 12-14. Sympodial hyphal branching (Figs. 12-13 with attached microconidia, Fig. 14 without microconidia). 15. Monophialide with attached microconidium (arrow indicates monophialide). 16. Polyphialide with attached microconidia. 17-18. Chlamydospores. Bars: Figs. 9-12, 14= 20 µm, Figs. 13, 15-18 = 10 µm
Etymology: Goolgardi is the Australian Aboriginal Dharug name for Xanthorrhoea, the genus from which this fungus was isolated.
Description Colonies on PDA powdery, velvety to floccose with abundant dense aerial mycelium, orange white, pale orange, greyish orange, brownish orange in colour. Pigment on reverse side brown, brownish-orange, reddish-orange, greyish-orange, yellowish-brown. Sporodochia pale orange to orange white forming on carnation leaf pieces in CLA and on agar surface. Macroconidia long, slender, slightly curved, parallel dorso-ventral sides, hooked apical cells, distinctly notched basal cells, 3 to 5 septate, mostly 3 septate, 23–66 μm (\( \overline{x} \) = 40 μm) long × 3–11 μm (\( \overline{x} \) = 5 μm) wide. Microconidia produced on monophialides and polyphialides proliferating sympodially creating a zig-zag or spiral appearance. Microconidia straight or curved, oval 0–3 septate, mainly 1 septate, 10-31 μm (\( \overline{x} \) = 17 μm) long × 3–5 μm (\( \overline{x} \) = 4 μm) wide. Chlamydospores produced sporadically in most cultures after 2 weeks in the dark at 23 °C on CLA, hyaline, smooth walled, formed in chains, terminal and intercalary, globose, subglobose, cylindrical to subcylindrical, 3–18 μm (\( \overline{x} \) = 8 μm) long × 4–10 μm (\( \overline{x} \) = 7 μm) wide.
Specimen examined Type Strain (living and dried) NRRL66250, RBG5411 and additional strains RBG5412 and RBG5418 isolated from Xanthorrhoea glauca Bungonia State Conservation Area, NSW, Australia, 34°48′S 150° 0′E on January 2010 by D.M Robinson and M.H. Laurence.
Fusarium mundagurra M.H. Laurence, E.C.Y. Liew, L.A. Shuttleworth & L.W. Burgess sp. nov.-
Morphological characters of Fusarium mundagurra. 19. Macroconidia. 20-25. Microconidia. 21. Produced in false heads on short monophialide. 22. Produced in false heads on long monophialide. 23. Produced in false heads on polyphialide. 24. Produced in sliding chains on monophialide. 25. Produced in sliding chain on polyphialide (arrow indicates the larger terminal microconidium). 26. Chlamydospores. All bars: 20 µm
Etymology: Mundagurra is named for the rainbow serpent that created Carnarvon Gorge in the Australian Aboriginal Dreamtime. Carnarvon Gorge is the geographic origin of the isolate first recognised as belonging to this species.
Description Colonies on PDA incubated in the dark for 2 weeks at 23 °C. Mycelia abundant, floccose, velvety to powdery, sometimes touching lid of agar plate, powdery pale violet, greyish-violet, greyish-blue, dull blue, greyish magenta and white. Reverse greyish blue, dark blue, blackish blue and greyish yellow. Sporodochia white, formed extensively on carnation leaf pieces of CLA. Macroconidia long, straight to falcate, dorso-ventral sides, blunt apical cells, foot-shaped basal cells, hyaline, 3 to 5 septate, mostly 3 septate, 20.5-51.5 μm (\( \overline{x} \) = 31.2 μm) long × 3.2-5.2 μm (\( \overline{x} \) = 4 μm) wide. Microconidia formed abundantly on long and short monophialides and polyphialides, hyaline, 4.2-68.7 μm (\( \overline{x} \) = 23 μm) long, microconidia formed abundantly in sliding chains 2–20 (\( \overline{x} \) = 10) in number, and less abundantly in false heads. Microconidia oval to obovate, 0 to 1 septate, 6.2-18.9 μm (\( \overline{x} \) = 11 μm) long × 2.7-4 μm (\( \overline{x} \) = 3.2 μm) wide. Terminal microconidium on sliding chains often larger than the sub-terminal microconidia in the chain. Chlamydospores formed abundantly under 12 h l/d cycles within 2 weeks at 23 °C, hyaline, smooth walled, formed in chains, terminal and intercalary, globose, subglobose, cylindrical to subcylindrical, 3.7-17.2 μm (\( \overline{x} \) = 9.3 μm) long × 4.9-16.6 μm (\( \overline{x} \) = 8.7 μm) wide.
Specimen examined Type Strain (living and dried) NRRL66235, RBG5717 isolated from soil Carnarvon Gorge National Park, Queensland, Australia, 25° 02’S 148° 11’E on October 2008 by S.M. Dunstan and M.H. Laurence. Additional strains RBG5599 from Northern Territory, Australia and RBG94 Queensland, Australia.
Fusarium newnesense M.H. Laurence & B.A. Summerell sp. nov. -Figs. 27–38, MB812307
Morphological characters of F. newnesense. 27-28. Macroconidia (arrow in Fig. 28 shows basal notch). 29-31. Microconidia. 29. Oval to obovate, straight and curved. 30. Pyriform. 31. Napiform. 32. Pyriform and napiform microconidia on monophialide and polyphialide. 33. Oval to obovoid microconidia in false heads. 34. Oval to obovoid microconidia in disordered chains and false heads. 33. On short monophialide. 35. On long polyphialide. 36. Microconidia on sliding/disordered chain. 37-38. Chlamydospores. Bars: Figs. 27-28, 32-35 =20 µm, Figs. 29-31, 37-38 =10 µm
Etymology: Refers to Newnes Plateau State Forest, the location where this species was first collected.
Description Colonies on PDA in dark for 2 weeks, 23 °C. Abundant velvety, floccose to powdery aerial mycelium, top surface violet white, yellowish white to white in colour, reverse beaver, blonde, coffee, dull yellow and olive brown. Macroconidia, slender, straight to falcate, slightly hooked apical cells and foot shaped basal cells, 2–4 septate, mostly 3 septate, 24.9-54 μm (\( \overline{x} \) = 41.2 μm) long × 3.1-5.5 μm (\( \overline{x} \) = 4.5 μm) wide. Sporodochia when produced are orange. Microconidia, two forms, pyriform to napiform and oval. Pyriform to napiform aseptate, 5.2-12.2 μm (\( \overline{x} \) = 7.6 μm) long × 3.2-8.7 μm wide (\( \overline{x} \) = 5.5 μm) arising terminally on long and short monophialides and polyphialides, oval straight or curved 2.6-24.7 μm (\( \overline{x} \) = 12.2 μm) long × 2.7-14.7 μm wide (\( \overline{x} \) = 4 μm), aseptate to 3 septate, mostly aseptate, arising in disordered chains 1–17 in number (\( \overline{x} \) = 6) or in false heads, on the end of long and short monophialides and polyphialides 1.7-46.4 μm (\( \overline{x} \) = 16.5 μm) long. Chlamydospores formed abundantly in isolates RBG610 and seldom in RBG614 and RBG5444 within 2 weeks at 23 °C under 12 h l/d cycles, hyaline, smooth walled, single, in pairs and in short chains, mostly single, terminal and intercalary, globose, subglobose, cylindrical to subcylindrical, 4.4-18.1 μm (\( \overline{x} \) = 9.9 μm) long × 4.5-15.2 μm (\( \overline{x} \) = 8.4 μm) wide.
Specimen examined Type Strain (living and dried) NRRL66241, RBG610 and additional strains RBG614 from soil Newnes State Forest, New South Wales, Australia 33°20′S, 150°15′E, March 1992 by B.A. Summerell. Additional strains RBG5444 from soil Rocky Cape National Park, Tasmania, Australia 40° 52′S 145° 31′E on March 2009 by B.H. Laurence and M.H. Laurence and RBG6847 from soil Grampians National Park, Victoria, Australia 37° 28′S 142° 25′E on May 2007 by E.L. Laurence and M.H. Laurence.
Fusarium tjaetaba T.T.H. Vu, J.L. Walsh, M.H. Laurence, L.W. Burgess, E.C.Y. Liew, & B.A. Summerell, sp. nov. -Figs. 39–43, MB812308
Etymology: Refers to Tjaetaba Falls, Litchfield National Park, the geographic origin of this species.
Description Colonies on PDA with powdery aerial mycelium, pale pink to orange in colour. Pigment on reverse side pale orange to violet or dark blue. Sporodochia orange in colour, forming on CLA within 10 d on carnation leaf pieces and occasionally on the agar surface. Macroconidia long, slender, falcate, parallel dorso-ventral sides, hooked apical cells and distinctly notched basal cells, 3–7 septate, mostly 4 septate, 40–60 μm (\( \overline{x} \) = 50 μm) long × 3–4 μm (\( \overline{x} \) = 3 μm) wide. Microconidia forming abundantly in false heads and sometimes in short chains, arising from monophialides and polyphialides, pyriform 5–19 μm (\( \overline{x} \) = 9 μm) long, 0–1 septate or oval 5–25 μm (\( \overline{x} \) = 10 μm) long, 0–1 septate in shape. Chlamydospores formed within 11 d on SNA in some isolates, varying in abundance in different isolates, produced in chains on aerial hyphae, terminal or intercalary, round 4–7.9 μm (\( \overline{x} \) = 6.2 μm) diameter to cylindrical 6.8 –12.9 μm (\( \overline{x} \) = 9.5 μm) × 4.2-7.4 μm (\( \overline{x} \) = 4.8 μm), smooth walled, and hyaline.
Specimens examined Type Strain (living and dried) NRRL66243, RBG5361 and FRL14350, additional strains FRL14348, FRL 14349, FRL14351, FRL14352, FRL14354, FRL14355, FRL14356, FRL14357 and FRL14358 isolated from Sorghum interjectum; Litchfield National Park, Northern Territory, Australia; 13°14′S, 130°42′E in July 2009 by J.L. Walsh.
Fusarium tjaynera J.L. Walsh, M.H. Laurence, L.W. Burgess, E.C.Y. Liew, & B.A. Summerell, sp. nov. -Figs. 44–48, MB812309
Etymology: Refers to Tjaynera Falls, Litchfield National Park, the geographic origin of isolates first recognised as belonging to this species.
Description Colonies on PDA with abundant dense aerial mycelium, white to greyish-rose in colour becoming darker as the culture ages. Pigment on reverse side is red to burgundy, sometimes concentric. Orange sporodochial masses are produced in most isolates. Sporodochia large, orange in colour, forming on CLA within 10 d on carnation leaf pieces and on the agar surface. Macroconidia very long, slender, falcate, parallel dorso-ventral sides, tapering apical cells and distinctly notched basal cells, 4–7 septate, usually 5 septate, 60–95 μm (\( \overline{x} \) = 78 μm) long × 4–5 μm (\( \overline{x} \) = 4 μm) wide. Microconidia forming singly or in false heads, arising from monophialides and polyphialides, oval in shape, 8–20 μm (\( \overline{x} \) = 13 μm) long, 0–1 septate, may be absent or very sparse in some isolates, but more abundant on SNA than CLA. Chlamydospores absent.
Specimens examined Type Strain (living and dried) NRRL66246, RBG5367 and additional strains FRL14482, FRL14483, FRL14484, FRL14485, FRL19308, FRL19309, FRL19310, FRL19311, FRL19312, FRL19313, FRL19314, FRL19315, FRL19316, FRL19318, FRL19319, FRL19320, FRL19321, FRL19322, FRL19323 FRL19325, FRL19326 isolated from Triodia microstachya Litchfield National Park, Northern Territory, Australia; 13°13′S, 130°44′E in 2009 by J.L. Walsh. Additional strains FRL14486, FRL14487 isolated from Sorghum intrans Litchfield National Park, Northern Territory; 13°15′S, 130°45′E, FRL19317 isolated from Sorghum interjectum; Litchfield National Park, Northern Territory; 13°14′S, 130°42′E and FRL11240, FRL11241 isolated from soil Kununurra, Western Australia; 15°46′S, 128°44′E in 2009 by J.L. Walsh.
Discussion
The current study identified and described six novel Fusarium species from Australian natural ecosystems of minimal anthropogenic disturbance. These novel species were phylogenetically distributed throughout the Fusarium genus in the FSAMSC, FFSC, FCSC and at least one novel lineage (FASC).
Morphological affinities
Morphologically F. coicis is similar to Fusarium species producing microconidia in medium to long chains, including F. andiyazi, F. fractiflexum, F. fujikuroi, F. gaditjirri, F. globosum, F. miscanthi, F. napiforme, F. nisikadoi, F. phyllophilum, F. proliferatum, F. pseudonygamai, F. thapsinum, and F. verticillioides. Fusarium coicis can be differentiated from the majority of these species by its very long macroconidia and the presence of long chains of large oval-clavate microconidia, which may be up to 3 septate. The macroconidia of F. nisikadoi are within the range reported for F. coicis; however, on average F. coicis produces longer macroconidia than F. niskadoi (Nirenberg and Aoki 1997). Fusarium coicis can be differentiated from F. nisikadoi and F. miscanthi morphologically by the colour of the sporodochia that are cream in F. coicis and orange in F. niskadoi and F. miscanthi. These latter species are also reported to produce pyriform microconidia, which have not been observed in F. coicis (Gams et al. 1999; Nirenberg and Aoki 1997).
Fusarium goolgardi has morphological affinities with F. acuminatum, F. armeniacum, F.avenaceum, F. langsethiae, F. sambucinum, F. sporotrichoides and F. venenatum, sharing the shape of the macroconidia, typical of species in the FSAMSC. However, F. goolgardi can be differentiated from these and all other Fusarium species by the sympodially branching monophialides that produce the microconidia.
Morphologically F. mundagurra is similar to Fusarium species that produce microconidia in short to medium length chains, including F. brevicatenulatum, F. fractiflexum, F. globosum, F. lactis, F. napiforme, F. nygamai, F. phyllophilum, F. proliferatum, F. pseudocircinatum, and F. pseudonygamai. Fusarium mundagurra; however, is differentiated from F. fractiflexum, F. globosum, F. lactis, F. phyllophilum, F. proliferatum, F. pseudocircinatum and F. pseudonygamai in the production of chlamydospores. In addition, F. mundagurra is differentiated from F. brevicatenulatum in that it does not produce pyriform microconidia. Fusarium mundagurra is morphologically difficult to distinguish from F. nygamai and can only be reliably separated on the basis of DNA sequence comparison.
Fusarium newnesense has morphological affinities with species in both the FOSC and FFSC. Fusarium newnesense resembles F. oxysporum (FOSC) and F. foetens (FOSC) in the production of oval microconidia in false heads on short monophialides. However, F. newnesense is differentiated from F. oxysporum and F. foetens by the production of pyriform microconidia and short disordered chains of oval microconidia. Fusarium newnesense is also morphologically similar to species in the FFSC that produce pyriform microconidia, including F. anthophilum, F. brevicatenulatum, F. dlaminii, F. globosum, F. konzum, F. miscanthi, F. nisikadoi and F. proliferatum. However, it is differentiated from these species by the production of chlamydospores. Furthermore, F. newnesense only produces short disordered chains of microconidia compared to the long chains of microconidia formed by F. globosum, F. miscanthi, F. nisikadoi and F. proliferatum. Fusarium newnesense is also differentiated from the two species outside of the FFSC that produce pyriform microconidia, F. sporotrichioides and F. tricinctum, by the macroconidial shape as F. newnesense produces macroconidia typical of species in the FFSC and FOSC, being mostly slender, thin-walled and slightly falcate. In contrast, F. sporotrichioides and F. tricinctum produce macroconidia that are falcate to almost lunate.
Morphologically F. tjaetaba is most similar to F. anthophilum, F. brevicatenulatum, and F. konzum, but differs from them by the production of pyriform microconidia in short chains and false heads from both monophialides and polyphialides, as well as the presence of chlamydospores. It should be cautioned, however, that chlamydospores are not formed by all isolates of F. tjaetaba and the ability to produce chlamydospores may be lost with repeated sub-culturing of isolates within this species.
Fusarium tjaynera morphologically resembles F. aywerte, a species first described by Sangalang et al. (1995a) as a subspecies of F. avenaceum. Fusarium aywerte was later promoted to species rank by Benyon et al. (2000). Isolates of F. tjaynera are morphologically similar to F. aywerte, but are distinguished by the production of microconidia and red pigmentation on PDA. Fusarium aywerte does not produce microconidia and produces peach or pale orange to orange-white pigmentation on PDA (Sangalang 1992).The two species are distinguished on basis of the shape of the basal and apical cells of the macroconidium. In F. tjaynera the basal cell is distinctly notched, whereas F. longipes has a very distinctive exaggerated elongate foot shape (Leslie and Summerell 2006). Both species have tapering apical cells; however the apical cell of F. tjaynera is not as exaggerated as the elongate whip-like apical cell of F. longipes (Burgess et al. 1994a; Leslie and Summerell 2006).
Phylogeography
Surveys of natural ecosystems are not only important for uncovering Fusarium species diversity but also for detecting phylogeographical signals which are likely to be influenced by anthropogenic disturbance (Summerell et al. 2010). In the current study we recovered species in the FSAMSC, FFSC, FCSC and at least one novel lineage (FASC). The phylogeographical relationships within each species complex raise interesting hypotheses on the distribution and evolution of this genus.
Multi-gene genealogies of the FFSC have generated phylogeographical hypotheses and debates within the Fusarium community. A landmark phylogenetic study of the FFSC uncovered three major clades (O’Donnell et al. 1998a). The authors presented a phylogeographical hypothesis to explain the origin of these clearly demarcated clades, correlating them to three major geographical regions; ‘American’, ‘African’ and ‘Asian’. Although a number of exceptions have been noted (Kvas et al. 2009; Walsh et al. 2010), the distribution of FFSC species generally fit the ‘American’, ‘African’ and ‘Asian’ hypothesis, while allowing for the anthropogenic movement of species. Adding to the exceptions, the three novel FFSC species recovered from natural ecosystems in the current study all belong to the ‘African’ clade. Fusarium coicis has a restricted geographic and host distribution in Australia and has been thus far only recovered in association with the extremely rare grass, Coix gasteenii. This grass is known to occur naturally only in small populations occurring in Lakefield National Park, Queensland, Australia (Simon 1989). The closest phylogenetic relatives to F. coicis are the undescribed Fusarium species NRRL25615 (host: Oryza sativa seed) and NRRL26793 (host: Striga hermonthica) that were both isolated from Africa. Significant EF-1α sequence divergence was detected between these relatives, suggesting a long evolutionary separation from F. coicis. The pattern for F. tjaetaba is similar with high levels of sequence divergence from its closest relative in the ‘African’ clade, F. brevicatenulatum NRRL25446 (host: Striga asiatica), isolated from Madagascar. Fusarium tjaetaba also has a very narrow known host range, isolated only from Sorghum interjectum, in spite of extensive sampling of other grass species from the same locality, including Heteropogon triticeus, Pseudopogonatherum irritans, Sehima nervosum, Sorghum intrans and Triodia microstachya. The closest relative of F. mundagurra is the undescribed Fusarium sp. NRRL25221 (host: Zea mays) that was isolated from Zimbabwe, Africa. Once again there were high levels of EF-1α sequence divergence suggesting a long period of separation. Although F. mundagurra was recovered from both soil and mango, a cultivated species not endemic to Australia, the isolation frequency from mango was low. The recovery of F. mundagurra from mango may reflect an opportunistic rather than a close host association. The close phylogenetic relationships between the novel Australian species and African species in the FFSC supports the post Gondwanaland radiation of the FFSC in the late Miocene as proposed by O’Donnell et al. (2013), perhaps in association with hosts endemic to Australia, as in the case of F. coicis and F. tjaetaba. However, we believe that further survey work that compares Fusarium communities in natural ecosystems between Africa, Australia and South America is required to shed further light on the phylogeography of species in the FFSC.
Fusarium goolgardi belongs to the FSAMSC, a trichothecene producing clade, with its closest relative being Fusarium sp. NRRL36351 that was recovered from stored peanuts (Arachis hypogaea) in Lisboa, Portugal. The closest described species are F. langsethiae (origin: Europe), F. palustre (origin: USA) and F. sibiricum (origin: Russia). It is interesting that F. goolgardi was associated with Xanthorrhoea decline that was symptomatically similar to the dieback associated with F. palustre in the USA (Elmer and Marra 2011; Elmer et al. 2012). Trichothecenes are involved in plant pathogenesis (Proctor et al. 2002; Desjardins and Hohn 1997) and given that F. goolgardi clusters in a trichothecene producing clade it would be valuable to determine if it produces trichochenes, which may have a role in Xanthorrhoea decline at the Bungonia National Park site.
Fusarium newnesense is an interesting species morphologically, sharing characters with both the FOSC and FFSC, suggesting close ancestry. However, phylogenetically the deep nodes are conflicted, with RPB1 placing F. newnesense as basal to the FOSC and RPB2 as basal to the FFSC (data not shown). The combined RPB1/RPB2 phylogeny places this lineage as an intermediate, within the FOSC and basal to the FFSC. These observations may indicate that this species is the ancestor to these important Fusarium species complexes. Further characterisation of this species may provide interesting insights into the evolution of pathogenicity and mycotoxin production in Fusarium.
Biogeographically F. newnesense was recovered from three locations in Australia: Tasmania, Victoria and New South Wales. Phylogenetic analyses of the EF-1α region showed clustering that corresponded to geography, suggesting allopatric divergence. The broad geographic range in natural ecosystems and polymorphisms suggest that F. newnesense may be endemic to Australia. The closest relative is the undescribed Fusarium sp. NRRL25184 that was isolated as an endophyte of grape vines in Europe. As discussed, it is difficult to draw phylogeographic conclusions based on isolates from heavily modified ecosystems, especially in a crop, such as grapes, which has experienced significant global movement of germplasm.
Fusarium tjaynera clusters with F. aywerte in a well-supported and differentiated clade basal to the FCSC. Fusarium aywerte has only been recovered from endemic spinifex grasses in central Australia. Similarly, F. tjaynera is primarily associated with the spinifex species Triodia microstachya. No species in the FASC have been recovered from international surveys, suggesting that the FASC is endemic to Australia, perhaps evolving with endemic flora.
The current study highlights the importance of mycogeographic surveys in natural ecosystems of minimal anthropogenic disturbance for providing base line information for rigorous studies on the phylogeny, taxonomy, phylogeography, pathology and ecology of Fusarium (Burgess 2014). This base line research can be used to infer the origin and evolutionary relationships in this genus. Further continental scale surveys are justified in Australian natural ecosystems as novel species continue to be recovered from biogeographic areas and plant species that have not previously been studied. Furthermore substantive progress in understanding the phylogeography of Fusarium will depend on rigorous phylogeographic surveys of Fusarium in soil and plants in natural ecosystems in other regions of the world, particularly South America, which is largely unsurveyed.
References
Baayen RP, O’Donnell K, Breeuwsma S, Geiser DM, Waalwijk C (2001) Molecular relationships of fungi within the Fusarium redolens F. hostae clade. Phytopathology 91(11):1037–1044
Backhouse D, Burgess LW, Summerell BA (2001) Biogeography of Fusarium. In: Summerell BA, Leslie JF, Backhouse D, Bryden W, Burgess LW (eds) Fusarium. The American Phytopathology Society, Minnesota, pp 122–137
Benyon F, Burgess L, Sharp P (2000) Molecular genetic investigations and reclassification of Fusarium species in sections Fusarium and Roseum. Mycol Res 104(10):1164–1174
Boutati EI, Anaissie EJ (1997) Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood 90(3):999–1008
Bryden WL (2012) Mycotoxin contamination of the feed supply chain: implications for animal productivity and feed security. Anim Feed Sci Technol 173(1–2):134–158. doi:10.1016/j.anifeedsci.2011.12.014
Burgess LW (1981) General ecology of fusaria. Fusarium: diseases, biology and taxonomy. Pennsylvania State University Press, Pennsylvania
Burgess LW (2014) 2011 McAlpine memorial lecture - a love affair with Fusarium. Australas Plant Pathol 43(4):359–368. doi:10.1007/s13313-013-0261-8
Burgess LW, Liddell CM, Summerell BA (1994a) Laboratory manual for Fusarium research. Laboratory manual for Fusarium research, 3rd edn. University of Sydney, Sydney
Burgess LW, Summerell BA (1992) Mycogeography of Fusarium - survey of Fusarium species in subtropical and semiarid grassland soils from Queensland, Australia. Mycol Res 96:780–784
Burgess LW, Summerell BA, Bullock S, Gott KP, Backhouse D (1994b) Laboratory manual for Fusarium research, 3rd edn. The University of Sydney, Sydney
Burgess LW, Trimboli D (1986) Characterization and distribution of Fusarium nygamai, sp. nov. Mycologia 78(2):223–229
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91(3):553–556
Desjardins AE (2006) Fusarium mycotoxins : Chemistry, genetics and biology. APS Press, St. Paul
Desjardins AE, Hohn TM (1997) Mycotoxins in plant pathogenesis. Mol Plant-Microbe Interact 10(2):147–152. doi:10.1094/mpmi.1997.10.2.147
Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Duran C, Field M, Heled J, Kearse M, Markowitz S, Moir R, Stones-Havas S, Sturrock S, Thierer T, Wilson A Geneious v5.4 (2011) Geneious v5.4
Elmer WH, LaMondia JA, Caruso FL (2012) Association between Fusarium spp. on Spartina alterniflora and dieback sites in Connecticut and Massachusetts. Estuar Coasts 35(2):436–444. doi:10.1007/s12237-011-9448-9
Elmer WH, Marra RE (2011) New species of Fusarium associated with dieback of Spartina alterniflora in Atlantic salt marshes. Mycologia 103(4):806–819. doi:10.3852/10-155
Fisher NL, Burgess LW, Toussoun TA, Nelson PE (1982) Carnation leaves as a substrate and for preserving cultures of Fusarium species. Phytopathology 72(1):151–153
Gams W, Klamer M, O’Donnell K (1999) Fusarium miscanthi sp. nov. from Miscanthus litter. Mycologia 91:263–268
Gordon TR, Martyn RD (1997) The evolutionary biology of Fusarium oxysporum. Annu Rev Phytopathol 35:111–128
Gräfenhan T, Schroers HJ, Nirenberg HI, Seifert KA (2011) An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Stud Mycol 68:79–113
Hasegawa M, Kishino H, Yano TA (1985) Dating of the human ape splitting by a molecular clock of mitochondrial-DNA. J Mol Evol 22(2):160–174
Hillis DM, Pollock DD, McGuire JA, Zwickl DJ (2003) Is sparse taxon sampling a problem for phylogenetic inference? Syst Biol 52(1):124–126
Huelsenbeck JP (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Kirk PM, Ainsworth GC, Bisby GR, International CAB (2008) Ainsworth & Bisby’s dictionary of the fungi. CABI, Wallingford
Kornerup A (1978) Methuen handbook of colour, 3rd edn. Methuen London Ltd, London
Kvas M, Marasas WFO, Wingfield BD, Wingfield MJ, Steenkamp ET (2009) Diversity and evolution of Fusarium species in the Gibberella fujikuroi complex. Fungal Divers 34:1–21
Laurence M, Summerell B, Burgess L, Liew E (2011) Fusarium burgessii sp. nov. representing a novel lineage in the genus Fusarium. Fungal Divers 49(1):101–112
Laurence MH, Burgess LW, Summerell BA, Liew ECY (2012) High levels of diversity in Fusarium oxysporum from non-cultivated ecosystems in Australia. Fungal Biol 116(2):289–297
Laurence MH, Summerell BA, Burgess LW, Liew ECY (2014) Genealogical concordance phylogenetic species recognition in the Fusarium oxysporum species complex. Fungal Biol 118(4):374–384. doi:10.1016/j.funbio.2014.02.002
Leslie JF, Summerell BA (2006) The Fusarium Laboratory manual, 1st edn. Blackwell Publishing, Iowa
Leslie JF, Zeller KA, Summerell BA (2001) Icebergs and species in populations of Fusarium. Physiol Mol Plant Pathol 59(3):107–117
Marasas WFO, Nelson PE, Toussoun TA (1984) Toxigenic Fusarium species, identity and mycotoxicology. Pennsylvania State University Press, University Park, USA
Mitchell JS, Attleber MH (1973) Fusarium Keratomycosis in horse. Vet Med Small AnimClin 68(11):1257–1260
Nelson PE (1991) History of Fusarium systematics. Phytopathology 81(9):1045–1048
Nelson PE, Toussoun TA, Burgess LW (1987) Characterization of Fusarium beomiforme sp. nov. Mycologia 79(6):884–889. doi:10.2307/3807690
Nelson PE, Toussoun TA, Marasas WFO (1983) Fusarium species : An illustrated manual for identification. Pennsylvania State University Press, University Park
Nirenberg H (1976) Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Parey
Nirenberg H, Aoki T (1997) Fusarium nisikadoi, a new species from Japan. Mycoscience 38:329–333
O’Donnell K (2000) Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 92(5):919–938
O’Donnell K, Cigelnik E, Nirenberg HI (1998a) Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90(3):465–493
O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC (1998b) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Acad Sci U S A 95(5):2044–2049
O’Donnell K, Nirenberg HI, Aoki T, Cigelnik E (2000) A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetically distinct species. Mycoscience 41(1):61–78
O’Donnell K, Sarver BAJ, Brandt M, Chang DC, Noble-Wang J, Park BJ, Sutton DA, Benjamin L, Lindsley M, Padhye A, Geiser DM, Ward TJ (2007) Phylogenetic diversity and microsphere array-based genotyping of human pathogenic fusaria, including isolates from the multistate contact lens - Associated US Keratitis outbreaks of 2005 and 2006. J Clin Microbiol 45(7):2235–2248
O’Donnell K, Sutton DA, Rinaldi MG, Sarver BA, Balajee SA, Schroers HJ, Summerbell RC, Robert VA, Crous PW, Zhang N, Aoki T, Jung K, Park J, Lee YH, Kang S, Park B, Geiser DM (2010) Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J Clin Microbiol 48(10):3708–3718
O’Donnell K, Ward TJ, Geiser DM, Kistler HC, Aoki T (2004) Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet Biol 41(6):600–623
O’Donnell K, Rooney AP, Proctor RH, Brown DW, McCormick SP, Ward TJ, Frandsen RJN, Lysøe E, Rehner SA, Aoki T, Robert VARG, Crous PW, Groenewald JZ, Kang S, Geiser DM (2013) Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet Biol 52:20–31. doi:10.1016/j.fgb.2012.12.004
Phan HT, Burgess LW, Summerell BA, Bullock S, Liew ECY, Smith-White JL, Clarkson JR (2004) Gibberella gaditjirrii (Fusarium gaditjirri) sp. nov., a new species from tropical Grasslands in Australia. Stud Mycol 50:261–272
Pollock DD, Zwickl DJ, McGuire JA, Hillis DM (2002) Increased taxon sampling is advantageous for phylogenetic inference. Syst Biol 51(4):664–671
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14(9):817–818
Proctor RH, Desjardins AE, McCormick SP, Plattner RD, Alexander NJ, Brown DW (2002) Genetic analysis of the role of trichothecene and fumonisin mycotoxins in the virulence of Fusarium. In: Logrieco A, Bailey JA, Corazza L, Cooke BM (eds) Mycotoxins in plant disease. Springer, Netherlands, pp 691–698. doi:10.1007/978-94-010-0001-7_12
Rambaut A (2013) FigTree (http://tree.bio.ed.ac.uk/software/figtree/). http://tree.bio.ed.ac.uk/software/figtree/. Accessed 6.2.2014
Reeb V, Lutzoni F, Roux C (2004) Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol Phylogenet Evol 32(3):1036–1060
Sangalang A, Summerell B, Burgess L, Backhouse D (1995a) Taxonomy of Fusarium: characterisation of Fusarium avenaceum subsp. aywerte and Fusarium avenaceum subsp. nurragi. Mycol Res 99(3):287–290
Sangalang AE (1992) Ecology and Taxonomy of Fusarium Species. PhD, The University of Sydney, Sydney
Sangalang AE, Burgess LW, Backhouse D, Duff J, Wurst M (1995b) Mycogeography of Fusarium species in soils from tropical, arid and Mediterranean Regions of Australia. Mycol Res 99:523–528
Schroers HJ, Baayen RP, Meffert JP, de Gruyter J, Hooftman M, O’Donnell K (2004) Fusarium foetens, a new species pathogenic to begonia elatior hybrids (Begonia X hiemalis) and the sister taxon of the Fusarium oxysporum species complex. Mycologia 96(2):393–406
Simon BK (1989) A new species of Coix L. (Poaceae) from Australia. Austrobaileya 3:1–5
Summerell BA, Laurence MH, Liew ECY, Leslie JF (2010) Biogeography and phylogeography of Fusarium: a review. Fungal Divers 44(1):1–11
Summerell BA, Leslie JF (2011) Fifty years of Fusarium: how could nine species have ever been enough? Fungal Divers 50(1):135–144. doi:10.1007/s13225-011-0132-y
Summerell BA, Rugg CA, Burgess LW (1993) Mycogeography of Fusarium - survey of Fusarium species associated with forest and woodland communities in north. Queensland, Australia. Mycol Res 97:1015–1019
Summerell BA, Rugg CA, Burgess LW (1995) Characterization of Fusarium babinda sp. nov. Mycol Res 99:1345–1348
Swofford DL (2002) PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). vol Version 4. Sinauer Associates, Sunderland,Massachusetts
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Walsh J, Laurence M, Liew E, Sangalang A, Burgess L, Summerell B, Petrovic T (2010) Fusarium: two endophytic novel species from tropical grasses of northern Australia. Fungal Divers 44(1):149–159
Walsh JL (2007) Fusarium species associated with savanna ecosystems in Australia : Taxonomy, ecology and pathogenicity. University of Sydney, Sydney
Zwickl DJ, Hillis DM (2002) Increased taxon sampling greatly reduces phylogenetic error. Syst Biol 51(4):588–598
Acknowledgments
We would like to thank Emma Laurence and Dr Victoria Ludowici for preparation of the figures and table and Dr Ian Cowie and Dr John Clarkson for advice and assistance with field work and plant taxonomy. We also thank Dr Peter Wilson for his advice on botanical nomenclature. The authors would also like to thank The University of Sydney and The Royal Botanic Gardens and Domain Trust for their continued support
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laurence, M.H., Walsh, J.L., Shuttleworth, L.A. et al. Six novel species of Fusarium from natural ecosystems in Australia. Fungal Diversity 77, 349–366 (2016). https://doi.org/10.1007/s13225-015-0337-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-015-0337-6