Abstract
Fusarium isolates were obtained from asymptomatic seeds of wild grasses collected in six regions of Brazil. Eleven phylogenetic species were identified among 41 isolates based on sequences of EF-1α. These are members of the F. fujikuroi (FFSC, n = 24), F. incarnatum-equiseti (FIESC, n = 13), and F. chlamydosporum (FCSC, n = 5) species complexes that encompass known plant pathogens, mycotoxigenic species, and endophytes. Phylogenetic analyses based on EF-1α, RPB2, and TUB revealed two new species, F. caapi and F. brachiariae, that belong to the African clade of the FFSC and share main morphological features of F. mundagurra and F. nygamai. Another encountered isolate formed a singleton phylogenetic lineage within the FIESC. This survey shows that naturally occurring and cultivated grasses not only harbor a high diversity of known species, which are pathogens of maize, sorghum, rice, and sugarcane, but also novel Fusarium species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Fusarium encompasses a variety of species that are pathogenic to plants, humans, and domestic animals and capable of producing bioactive secondary metabolites, including mycotoxins (Leslie and Summerell 2006; Kvas et al. 2009; Proctor et al. 2013). These fungi are also common endophytes of native and cultivated plants and especially grasses (Leslie and Summerell 2006; Kvas et al. 2009). The wide diversity of grass endophytes includes species from the following Fusarium species complexes: F. chlamydosporum (FCSC), F. fujikuroi (FFSC), F. incarnatum-equiseti (FIESC), F. oxysporum (FOSC), and F. sambucinum(FSAMSC)(Bentley et al. 2007; Nor Azliza et al. 2014; Kago et al. 2016; Laurence et al. 2016; Chehri et al. 2017).
Surveys of grass endophytes conducted in natural ecosystems have resulted in the discovery of new Fusarium species, especially those from the FFSC. Fusarium konzum was described from native grasses, mainly Andropogon and Sorghastrum species from prairies in Kansas, USA (Zeller et al. 2003). Fusarium gaditjirrii was reported from the grasses Heteropogon triticeus and Themeda triandra growing in the savannahs of Australia (Phan et al. 2004). Fusarium lyarnte, F. tjaetaba, and F. werrikimbe were described in association with Sorghum interjectum and Sorghum leiocladum, and F. coicis was isolated from Coix gasteenii(Walsh et al. 2010; Laurence et al. 2016). Known pathogenic and mycotoxigenic species also exist as endophytes in wild grasses. Fusarium sacchari, the main causal agent of pokkah boeng disease of sugarcane, was the predominant endophyte of Oryza australiensis in Australia (Petrovic et al. 2013). Fusarium verticillioides, F. proliferatum, F. andiyazi, and F. thapsinum are known producers of fumonisins and moniliformin and found in asymptomatic native grasses in the USA (Leslie et al. 2004). Fusarium fujikuroi causes bakanae disease in rice and was isolated from aquatic Echinochloa plants collected near rice fields (Carter et al. 2008). These results suggest that native or introduced grasses can act as inoculum reservoirs of known or unknown and potentially plant pathogenics and mycotoxigenic Fusarium species (Leslie et al. 2004).
Plants of Brachiaria (syn. Urochloa, “surinam grass”) and Panicum maximum (syn. Megathyrsus maximus, “guinea grass”) originate from Africa and Australia and are widely used as forage grasses in Brazil. Consequently, they are potentially invasive in natural grasslands. These grasses are also used for the recovery of degraded areas and prevention of erosive processes, in consortium with crops, such as maize and sorghum, and employed as source of straw in no-tillage agricultural systems (Timossi et al. 2007; Borghi et al. 2013a, b). There are few reports of endophytic Fusarium species associated with Brachiaria and Panicum, and reported identifications are usually limited to genus level (e.g., Mallmann et al. 2013; Kago et al. 2016; Teasdale et al. 2018). Since these forage grasses are frequently used in consortia with important crop plants, inventories were initiated to explore whether they may harbor plant pathogenic or mycotoxigenic Fusarium species. Endophytic Fusarium isolates from seeds of Brachiaria spp. and Panicum maximum collected in several geographic locations in Brazil were investigated using EF-1α and multilocus phylogenetic analyses coupled to morphological characterization. Known species from the FFSC, FIESC, and FCSC were found, together with two taxonomical novelties described herein.
Materials and methods
Fungal isolates
Seeds collected in full maturity were disinfested in 70% alcohol for 30 s and hypochlorite 2% for 2 min and washed in sterile water and dried on filter paper. Around 100 seeds from each sample were macerated in a crucible; the small fragments were transferred to five Petri plates (6 cm diam.) containing 2% malt extract agar (20 g of malt extract L−1; HiMedia Laboratories, Mumbai, India) and incubated at 25 °C for 4–7 days with 12 h light/12 h dark cycle. Individual conidia from selected Fusarium colonies were subcultured using a micromanipulator. The isolates are deposited in the Coleção Micológica de Lavras (CML), Departamento de Fitopatologia, Universidade Federal de Lavras, Minas Gerais, Brazil (http://www.dfp.ufla.br/cml/) (Table 1). Types were deposited at Herbarium UB, Universidade de Brasília.
PCR, sequencing, and phylogenetic analyses
Isolates were grown in malt extract broth for 4 days under agitation of 100 rpm on an orbital shaker at room temperature. DNA was extracted from mycelia using the Wizard® Genomic DNA Kit (Promega Corporation, Madison, WI, USA), according to the manufacturer’s instructions. DNA concentration was estimated using NanoDrop 2000 (Thermo Fisher Scientific Inc., Waltham, USA). Fragments of the translation elongation factor 1-alpha (EF-1α) gene were amplified with primer pair EF-1/EF-2 or EF-3/EF-22 (O’Donnell et al. 1998, 2008) for all isolates, following the cycle conditions described by O’Donnell et al. (2008). Portions of the RNA polymerase second largest subunit (RPB2, using primer pair 5F2/7cR, Sung et al. 2007; Liu et al. 1999) and beta-tubulin (TUB, T1/T2, O’Donnell and Cigelnik 1997) genes were amplified for selected FFSC isolates according to O’Donnell et al. (2008) and O’Donnell and Cigelnik (1997). PCR reactions were performed with GoTaq® Colorless Master Mix (Promega, Madison, USA), purified with Wizard® SV Gel and PCR Clean-up System kit (Promega). Sequencing of PCR products was done with primers used for amplifications. SeqAssem program was used to assemble and edit the sequences (Hepperle 2004), which were deposited in GenBank (Table 1). Alignments were generated using CLUSTALW as implemented in the MEGAX software (Kumar et al. 2018). DNA sequences from selected Fusarium species were downloaded from GenBank and added to the alignments (Supplementary Table 1). The alignments were deposited in TreeBASE (www.treebase.org; study number: S26843). Aligned sequences consisted of 97 parsimony-informativepositions/450 bp(FFSC) and 110/522 (FIESC and FCSC) for EF-1α, 110/794 for RPB2, and 76/528 for TUB. Phylogenetic analyses were performed for each gene partition and for the concatenated dataset of FFSC isolates. Maximum parsimony (MP) and maximum likelihood (ML) methods were performed in MEGAX software with 1000 bootstrap replications. Bayesian inference (BI) analyses were performed with using MrBayes 3.2.7 (Ronquist et al. 2012) with two independent analyses run for 1,000,000 generations, sampled every 500 generations, after discarding 25% of initial trees. The best-fitted models of nucleotide substitution used in the ML and BI analyses, estimated using jModelTest (Darriba et al. 2012), were K2 + G for EF-1α, RPB2, and TUB(FFSC) and GTR + G for EF-1α (FIESC and FCSC).
Morphological characterization
The isolates were characterized morphologically according to Leslie and Summerell (2006) and Zeller et al. (2003). The morphological characteristics of micro- and macroconidia such as shape and size (25–30 measurements per isolate), arrangement of conidiogenous cells, and presence or absence of conidial chains and chlamydospores were examined after growing the isolates on synthetic nutrient-poor agar with carnation leaf pieces for 10 to 14 days at 20 °C. The colony growth radius was assessed after 4 days and mycelium and colony color (surface and reverse) at 25 °C on potato dextrose agar (PDA, Merck, Darmstadt, Germany) in the dark.
Mating type and sexual compatibility test
The mating types of the isolates representing the new species were determined by PCR, using the protocols described by Steenkamp et al. (2000). Crosses were conducted between isolates from opposite mating types as described by Klittich and Leslie (1988). The isolates were tested as both female and male parent. Crosses were also carried out between the isolates of the new species and the tester isolates of other biological species from the FFSC (Supplementary Table 2).
Results
Phylogenetic analyses
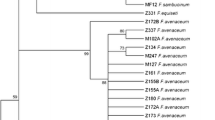
Phylogenetic analysis based on the EF-1α gene showed that 23 isolates belong to the FFSC (Fig. 1). Sixteen isolates were identified as F. thapsinum (n = 5), F. madaense (n = 4), F. verticillioides (n = 3), F. proliferatum (n = 3), and F. fujikuroi (n = 1). Two monophyletic groups, composed by two and five isolates from this study, did not correspond to any known species within the FFSC (Figs. 1 and 2) and are here described as F. caapi and F. brachiariae. EF-1α sequences from three rice root endophytes collected in Kenya and one strain from a clinical environment in Brazil grouped within the F. caapi clade (Fig. 1). Phylogenetic analyses of RPB2, TUB(Supplementary Figs. 1 and 2), and the combined dataset (EF-1α, RPB2 and TUB) (Fig. 2) confirmed the exclusive monophyly of both new species and their placement in the African clade of the FFSC. Fusarium caapi is closely related to F. mundagurra and Fusarium sp. NRRL 25221, while F. brachiariae is only distantly related to F. denticulatum, F. thapsinum, and F. nygamai(Fig. 2).
Maximum parsimony phylogenetic tree inferred from partial EF-1α sequences showing the phylogenetic relatedness of Fusarium species associated with Brachiaria spp. with other species of the Fusarium fujikuroi species complex. Bootstrap values ≥ 70% (MP and maximum likelihood) and posterior probability ≥ 95% (Bayesian inference) are shown at the internodes. Ex-type strains are indicated with T and ex-epitype strains with ET. Isolates of F. caapi with blue asterisks were obtained from rice root in Kenya and with red asterisks from a clinical indoor environment in Brazil. Fusarium inflexum (NRRL 20433) and F. oxysporum (NRRL 22902) were used as outgroup
Maximum parsimony phylogenetic tree inferred from partial EF-1α, TUB, and RPB2 sequences showing the phylogenetic relatedness of Fusarium species associated with Brachiaria spp. with other species of the Fusarium fujikuroi species complex. Bootstrap values ≥ 70% (MP and maximum likelihood) and posterior probability ≥ 95% (Bayesian inference) are shown at the internodes. Ex-type strains are indicated with T and ex-epitype strains with ET. Fusarium inflexum (NRRL 20433) and F. oxysporum (NRRL 22902) were used as outgroup
The remaining 18 isolates were identified as members of the FIESC or FCSC (Fig. 3). Three species were placed in the FIESC: F. hainanense (n = 7), F. lacertarum (n = 3), and F. duofalcatisporum (n = 2). The isolate CML 4092 formed a clade with F. arcuatisporum and FIESC 30 and could represent a new phylogenetic species. Fusarium chlamydosporum was the only species identified within the FCSC (Fig. 3).
Maximum parsimony phylogenetic tree inferred from partial EF-1α sequences showing the phylogenetic relatedness of Fusarium species associated with Brachiaria spp. with other species of the Fusarium incarnatum-equiseti and F. chlamydosporum species complexes. Bootstrap values ≥ 70% (MP and maximum likelihood) and posterior probability ≥ 95% (Bayesian inference) are shown at the internodes. Ex-type strains are indicated with T and ex-neotype strains with NT. Fusarium brachygibbosum (NRRL 34033) and F. concolor (NRRL 13459) were used as outgroup
Mating type identification and sexual stage induction
Two isolates of F. caapi were identified as MAT-1 and three as MAT-2. Both isolates of F. brachiariae were identified as MAT-1(Table 1). Perithecia were not formed in crosses between isolates of F. caapi or between isolates from both new species and tester strains from nine known biological species within the FFSC.
Taxonomy
Fusarium brachiariae M. M. Costa, M. P. Melo, F. S. Carmo & L. H. Pfenning, sp. nov. Fig. 4
Morphological characters of the asexual stage of Fusarium brachiariae. a Monophialides. b Polyphialide. c Microconidia in short chains. d 0-septate microconidia. e Sporodochia formed on carnation leaf on SNA. f 3–5 septate macroconidia. g Chlamydospores in chains. h–i Reverse and obverse of PDA colony incubated for 14 days at 25 °C. Bars: a–d = 20 μm and f–g = 30 μm
MycoBank: MB 836892
Etymology: Refers to Brachiaria, the genus, from which this fungus was isolated.
Typification: BRAZIL. Mato Grosso do Sul: Campo Grande, a dried culture on SNA of a strain isolated from Brachiaria decumbens seed, 2012, M. P. Melo (holotype UB 24188). Ex-type culture CML 3032. GenBank accession numbers: EF-1α = MT901348; TUB = MT901321; and RPB2 = MT901314.
Colonies on PDA reaching 3.4 cm at 25 °C in 4 days. Aerial mycelium floccose. Colony color cream, reverse white with violet color. Odor absent. On SNA, microconidia hyaline, oval to clavate, 0 to 1 septate, mostly 0 septate: 2–7(− 11) × 1.5–3 μm, 1-septate: 5–14 × 1.8–4.5 μm, produced in false heads and in short chains, arising from mono- and polyphialides with up to three openings, phialides 12.5–30.5 × 2–3.5 μm in size, formed on simple or branched conidiophores. Sporodochia on SNA abundant, forming within 10 days on carnation leaf pieces and occasionally on the agar surface, producing orange conidial masses. Macroconidia relatively slender with a significant curvature, with evident apical and basal cells, 3–4(− 6) septate, 3-septate: 21–48 × 2.5–5 μm, 4-septate: 30–43 × 2.5–5 μm, 5-septate: 45–48 × 2.5–5 μm, 6-septate: 45–65 × 2.5–5 μm. Chlamydospores formed abundantly within 1 week, singly or in chains, thin-walled, terminal or intercalary, globose, subglobose, cylindrical to subcylindrical, 3.5–20.2 × 5–15.5 μm.
Host: Brachiaria decumbens
Known distribution: Brazil
Other specimen examined. BRAZIL, Mato Grosso do Sul: Campo Grande, from seeds of Brachiaria decumbens, 2016, M. P. Melo (CML 3163).
Fusarium caapi M. M. Costa, M. P. Melo, F. S. Carmo & L. H. Pfenning, sp. nov. Fig. 5
Morphological characters of the asexual stage of Fusarium caapi. a Monophialides on conidiophores. b Microconidia in short chains. c 0-septate microconidia. d Polyphialide. e Sporodochia formed on carnation leaf on SNA. f Sporodochia. g 3–6 septate macroconidia. h Chlamydospores. i–j Reverse and obverse of PDA colony incubated for 14 days at 25 °C. Bars: a–c = 20 μm, d = 10 μm, and f–h = 30 μm
MycoBank: MB 836897
Etymology: caapi meaning grass or slender leaf in tupi guarani, an extinct language of the Tupinambá Brazilian Indians.
Typification: BRAZIL. São Paulo: Guaíra, a dried culture on SNA of a strain isolated from Brachiaria brizantha seed, 2016, F. S. Carmo. (holotype UB 24189). Ex-type culture CML 3657. GenBank accession numbers: EF-1α = MT901350; TUB = MT901323; and RPB2 = MT901316.
Colonies on PDA reaching 3.8 cm at 25 °C in 4 days. Aerial mycelium floccose, with a powdery appearance. Colony color cream, reverse white to gray. Odor absent. On SNA, microconidia hyaline, oval to obovate, 0 to 1 septate, mostly 0 septate. 0-septate: 2–5–(11) × 1.5–3 μm, 1-septate: 7.5–12.5 × 2.5–5.5 μm, produced in false heads or short chains arising from mono- and polyphialides, phialides 17.5–32.5 × 2–5 μm in size, formed on simple or branched conidiophores. Sporodochia abundant, forming within 10 days on carnation leaf pieces and occasionally on the agar surface, producing orange conidial masses. Macroconidia straight to slightly curved, relatively slender and thin walled, with hardly evident apical and basal cells, 3–5(− 6) septate, mostly 3-septate: 3-septate: 20–47.5 × 2.5–5 μm, 4-septate: 35–50 × 2.5–5 μm, 5-septate and 6-septate: 45–52.5 × 2.5–5. Chlamydospores produced abundantly within 1 week, in chains, thin-walled, terminal or intercalary, globose, subglobose, cylindrical to subcylindrical, 4.5–22.2 × 5–18.5 μm.
Host: Brachiaria brizantha, Oryza sativa and indoor air in a clinical environment
Known distribution: Brazil and Kenya
Other specimens examined. BRAZIL, São Paulo: Guaíra, from seeds of Brachiaria brizantha, 2016, F. S. Carmo (CML 3660, CML 3658, CML 3659); Minas Gerais: Montes Claros, from seeds of Brachiaria brizantha, 2017, F. S. Rocha (CML 3881).
Discussion
In this study, eleven phylogenetic species from three Fusarium species complexes, F. fujikuroi, F. incarnatum-equiseti, and F. chlamydosporum, were isolated from apparently healthy seeds of Brachiaria spp. and Panicum maximum collected in different geographic regions of Brazil. Most FCSC and FIESC isolates were obtained from Rio Grande do Sul state in southern Brazil, and two FIESC isolates were encountered from seeds collected in the Central-West region of the country (Goiás and Mato Grosso states). In contrast, only one FFSC isolate was obtained from the South Region, eight from the Central-West, and 14 from the Southeast Region of Brazil. While FCSC and FIESC were predominant in Panicum maximum, FFSC isolates were only found in association with Brachiaria spp. Despite the observed variations in the species complex composition and in the number of isolates, the small sampling size prevents the establishment of more clear relationships between host species and/or location and the prevalence of particular Fusarium species. Larger inventories are thus required for resolving grass host range and geographic distribution especially of the here newly described Fusarium species.
Phylogenetic analyses of three gene regions supported the identification of two new species from the FFSC. Fusarium brachiariae and F. caapi belong to the African clade, and Africa is the geographical origin of Brachiaria species. This is consistent with the phylogeographic hypothesis that species in the FFSC are distributed into three major clades (African, American, and Asian), representing the origin of Fusarium species and their plant hosts (O’Donnell et al. 1998). Fusarium caapi is phylogenetically close to F. mundagurra, which was described from soil and Mangifera indica in Australia (Laurence et al. 2016). The phylogenetic affinities of F. brachiariae to other species within the FFSC are less clearly defined. Three endophytic strains from rice root in Kenya (Pili et al. 2016) and one strain from hospital indoor air in Brazil (Moretti et al. 2018) were identified as F. caapi. It is thus possible that F. caapi was introduced in Brazil via propagating material of Brachiaria brought from Africa and is now distributed in the environment. Fusarium caapi and F. brachiariae are likely associated with other substrates and broadly distributed in Brazil.
Fusarium caapi and F. brachiariae differ in shape and size of the macroconidia. Macroconidia of F. brachiariae are larger and more elongated and have more prominent apical cells than in F. caapi. Colonies of F. brachiariae isolates were white or violet on PDA medium, whereas colonies of F. caapi have a cream color. Both species are morphologically similar to F. mundagurra and F. nygamai that produce conidia in short chains arising from polyphialides and abundant chlamydospores (Burgess and Trimboli 1986; Laurence et al. 2016). Chlamydospore production is restricted to few species of the FFSC, among them F. acutatum, F. mundagurra, F. napiforme, F. nygamai, F. udum, and F. xylarioides, all belonging to the African clade (Leslie and Summerell 2006; Laurence et al. 2016; Pfenning et al. 2019). No fertile crosses were obtained between F. caapi isolates. Likewise, it was not possible to cross the F. brachiariae isolates, as both have the same mating type. On the other hand, F. caapi and F. brachiariae did not cross with the tester isolates of any other FFSC species used in this work, confirming the reproductive isolation of the known mating populations. It remains unclear if the sexual stage of F. brachiariae and F. caapi exists in nature.
Other members of the FFSC isolated from Brachiaria in this survey, i.e., F. fujikuroi, F. madaense, F. proliferatum, F. thapsinum, and F. verticillioides, are important pathogens of maize, rice, sugarcane, and millet worldwide, and some are producers of fumonisins and moniliformin (Leslie et al. 2004, 2005; Costa et al. 2019; Ezekiel et al. 2020; Nicolli et al. 2020). Fusarium madaense has been isolated in Brazil as an endophyte in maize, rice, sorghum, finger millet, and pearl millet and as food contaminants in African countries (Ezekiel et al. 2020; Nicolli et al. 2020; Costa et al. unpublished). Fusarium fujikuroi, F. verticillioides, F. proliferatum, and F. thapsinum have been isolated as endophytes of native grasses in different countries (Leslie et al. 2004; Carter et al. 2008; Nor Azliza et al. 2014).
Species from FCSC and FIESC are endophytes or opportunistic pathogens of cultivated plants (Leslie and Summerell 2006). Members of both complexes are frequently associated with native and wild grasses (Bentley et al. 2007; Nor Azliza et al. 2014; Elmer et al. 2016) and commonly isolated from wheat, maize, rice, barley, and oat (Villani et al. 2016; Moreira et al. 2020). FIESC species can produce a dozen of mycotoxins, mainly trichothecenes and zearalenone (Villani et al. 2016; O’Donnell et al. 2018), while members from the FCSC may produce B-trichothecenes (O’Donnell et al. 2013). Although these complexes are rarely associated with cattle poisoning, their presence in wild Brachiaria and Panicum suggests the possibility of mycotoxin contamination when these grasses are used for animal feed (Botha et al. 2014).
All Fusarium isolates were recovered from seeds, suggesting that inflorescences could be the sites of infection by airborne conidia. Maize, sorghum, rice, and sugarcane are often planted near or in consortium with Brachiaria and Panicum grasses and could serve as inoculum sources, or the other way around. Inflorescences act as sites of infection for F. culmorum, F. graminearum, and F. verticillioides inducing ear rot disease in maize (Duncan and Howard 2010; Oldenburg and Ellner 2015). Systemic colonization of aerial tissues is another way, by which endophytic Fusaria could reach the seeds of grasses in the field, as described for F. verticillioides in maize (Duncan and Howard 2010).
This is the first survey of Fusarium species associated with Brachiaria spp. and Panicum maximum in Brazil, in which known pathogens and potential mycotoxin producers were recovered from these important plants of agronomic and livestock use. Even though these grass species are not native to Brazil, they are present throughout the territory and part of the country’s natural vegetation.
Over the years, the interest in investigating Fusarium species associated with asymptomatic native or introduced plants has increased (Leslie et al. 2004; Phan et al. 2004; Walsh et al. 2010; Laurence et al. 2016). Our results from a relatively small sampling reinforce the understanding that Fusarium species known as plant pathogens are also endophytes in noncrop hosts (Phan et al. 2004). Furthermore, this survey confirms that wild grasses are sources of unknown fungi and harbor a high diversity of Fusarium species (Walsh et al. 2010). Thus, an even greater diversity of Fusarium species may be recovered from grasslands and from other unexplored ecosystems in Brazil where Brachiaria, Panicum, and other seeded forages occur along with native or naturally occurring species.
Data availability
Biological reference material is deposited and available in official collections and DNA sequences and alignments at GenBank and TreeBASE, respectively.
References
Bentley AR, Petrovic T, Griffiths SP, Burgess LW, Summerell BA (2007) Crop pathogens and other Fusarium species associated with Austrostipa aristiglumis. Australasian Plant Pathol 36:434–438
Borghi E, Crusciol CAC, Mateus GP, Nascente AS, Martins PO (2013a) Intercropping time of corn and palisadegrass or guineagrass affection grain yield and forage production. Crop Sci 53:629–636
Borghi E, Crusciol CAC, Nascente AS, Sousa VV, Martins PO, Mateus GP, Costa C (2013b) Sorghum grain yield, forage biomass production and revenue as affected by intercropping time. Eur J Agron 51:130–139
Botha CJ, Truter M, Jacobs A (2014)Fusarium species isolated from Pennisetum clandestinum collected during outbreaks of kikuyu poisoning in cattle in South Africa: research communication. Onderstepoort J Vet Res 81:1–8
Burgess LW, Trimboli D (1986) Characterization and distribution of Fusarium nygamai, sp. nov. Mycologia 78:223–229
Carter LLA, Leslie JF, Webster RK (2008) Population structure of Fusarium fujikuroi from California rice and water grass. Phytopathology 98:992–998
Chehri K, Hajeb S, Maassoumi SM (2017) Morphological and molecular identification and PCR amplification to determine the toxigenic potential of Fusarium graminearum species complex (FGSC) isolated from wild grasses in Iran. J Agric Sci Technol 19:1617–1629
Costa MM, Melo MP, Guimarães EA, Veiga CMO, Costa SS, Sandin FC, Moreira GM, Pfenning LH (2019) Identification and pathogenicity of Fusarium species associated with pokkah boeng of sugarcane in Brazil. Plant Path 68:1350–1360
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Duncan KE, Howard RJ (2010) Biology of maize kernel infection by Fusarium verticillioides. Mol Plant-Microbe Interact 23:6–16
Elmer WH, Robert E, Marra RE, Li H, Li B (2016) Incidence of Fusarium spp. on the invasive Spartina alterniflora on Chongming Island, Shanghai, China. Biol Invasions 18:2221–2227
Ezekiel CN, Kraak B, Sandoval-Denis M, Sulyok M, Oyedele OA, Ayeni KI, Makinde OM, Akinyemi OM, Krska R, Crous PW, Houbraken J (2020) Diversity and toxigenicity of fungi and description of Fusarium madaense sp. nov. from cereals, legumes and soils in north-central Nigeria. MycoKeys 67:95–124
Hepperle D (2004) SeqAssem©. Win32–version. A sequence analysis tool contig assembler and trace data visualization tool for molecular sequences. Available at: http://www.sequentix.de
Kago L, Njuguna J, Njarui DMG, Ghimire SR (2016) Fungal endophyte communities of Brachiaria grass (Brachiaria spp.) in Kenya. In: Njarui DMG, Gichangi EM, Ghimire SR, Muinga RW. (eds). Climate smart Brachiaria grasses for improving livestock production in East Africa – Kenya experience. Proceedings of the workshop held in Naivasha, Kenya, 14–15 September. Nairobi, Kenya. 271 p
Klittich CJR, Leslie JF (1988) Nitrate reduction mutants of Fusarium moniliforme (Gibberella fujikuroi). Genetics 118:417–423
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Kvas M, Marasas WFO, Wingfield BD, Wingfield MJ, Steenkamp ET (2009) Diversity and evolution of Fusarium species in the Gibberella fujikuroi complex. Fungal Divers 34:1–21
Laurence MH, Walsh JL, Shuttleworth LA, Robinson DM, Johansen RM, Petrovic T, Vu TTH, Burgess LW, Summerell BA, Liew ECY (2016) Six novel species of Fusarium from natural ecosystems in Australia. Fungal Divers 77:349–366
Leslie JF, Summerell BA (2006) The Fusarium laboratory manual. Blackwell, Sydney 388p
Leslie JF, Zeller KA, Logrieco A, Mulè G, Moretti A, Ritiene A (2004) Species diversity of and toxin production by Gibberella fujikuroi species complex strains isolated from native prairie grasses in Kansas. Appl Environ Microbiol 70:2254–2262
Leslie JF, Zeller KA, Lamprecht SC, Rheeder JP, Marasas WFO (2005) Toxicity, pathogenicity, and genetic differentiation of five species of Fusarium from sorghum and millet. Phytopathology 95:275–283
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16:1799–1808
Mallmann G, Verzignassi JR, Fernandes CD, Santos JM, Vechiato MH, Inácio CA, Batista MV, Queiroz CA (2013) Fungos e nematoides associados a sementes de forrageiras tropicais. Summa Phytopathol 39:201–203
Moreira GM, Nicolli CP, Gomes LB, Ogoshi C, Scheuermann KK, Silva-Lobo VL, Schurt DA, Ritieni A, Moretti A, Pfenning LH, Del Ponte EM (2020) Nationwide survey reveals high diversity of Fusarium species and related mycotoxins in Brazilian rice: 2014 and 2015 harvests. Food Control 113:107171
Moretti ML, Busso-Lopes AF, Tararam CA, Moraes R, Muraosa Y, Mikami Y, Gonoi T, Taguchi H, Lyra L, Reichert-Lima F, Trabasso P, Hoog GS, Al-Hatmi AMS, Schreiber AZ, Kamei K (2018) Airborne transmission of invasive fusariosis in patients with hematologic malignancies. PLoS One 13:e0196426
Nicolli CP, Haidukowski M, Susca A, Gomes LB, Logrieco AF, Stea G, Del Ponte EM, Moretti A, Pfenning LH (2020)Fusarium fujikuroi species complex in Brazilian rice: unveiling increased phylogenetic diversity and toxigenic potential. Int J Food Microbiol 330:108667
Nor Azliza IN, Hafizi R, Nurhazrati M, Salleh B (2014) Production of major mycotoxins by Fusarium species isolated from wild grasses in peninsular Malaysia. Sains Malaysiana 43:89–94
O’Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol 7:103–116
O’Donnell K, Cigelnik E, Nirenberg HI (1998) Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90:465–493
O’Donnell K, Sutton DA, Fothergill A, McCarthy D, Rinaldi MG, Brandt ME, Zhang N, Geiser DM (2008) Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J Clin Microbiol 46:2477–2490
O’Donnell K, Rooney AP, Proctor RH, Brown DW, McCormick SP, Ward TJ, Frandsen RJ, Lysoe E, Rehner SA, Aoki T, Robert VA, Crous PW, Groenewald JZ, Kang S, Geiser DM (2013) Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fung Gen Biol 52:20–31
O’Donnell K, McCormick SP, Busman M, Proctor RH, Ward TJ, Doehring G, Geiser DM, Alberts JF, Rheeder JP (2018) Marasas et al. 1984 “Toxigenic Fusarium species: identity and mycotoxicology” revisited. Mycologia 110:1058–1080
Oldenburg E, Ellner F (2015) Distribution of disease symptoms and mycotoxins in maize ears infected by Fusarium culmorum and Fusarium graminearum. Mycotoxin Res 31:117–126
Petrovic T, Burgess LW, Cowie I, Warren RA, Harvey PR (2013) Diversity and fertility of Fusarium sacchari from wild rice (Oryza australiensis) in northern Australia, and pathogenicity tests witch wild rice, rice, sorghum and maize. Eur J Plant Pathol 136:773–788
Pfenning LH, Melo MP, Costa MM, Reis A, Cabral CS, Lima CS, Abreu LM, Costa SS (2019)Fusarium udum revisited: a common, but poorly understood member of the Fusarium fujikuroi species complex. Mycol Prog 18:107–117
Phan HT, Burgess LW, Summerell BA, Bullock S, Liew EC, Smith-White JL, Clarkson JR (2004) Gibberella gaditjirrii (Fusarium gaditjirrii) sp. nov., a new species from tropical grasses in Australia. Stud Mycol 50:261–272
Pili NN, França SC, Kyndt T, Makumba BA, Skilton R, Höfte M, Mibey RK, Gheysen G (2016) Analysis of fungal endophytes associated with rice roots from irrigated and upland ecosystems in Kenya. Plant Soil 405:371–380
Proctor RH, Van Hove F, Susca A, Stea G, Busman M, Lee T, Ward TJ (2013) Birth, death and horizontal transfer of the fumonisin biosynthetic gene cluster during the evolutionary diversification of Fusarium. Mol Microbiol 90:290–306
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Steenkamp ET, Wingfield BD, Coutinho TA, Zeller KA, Wingfield MJ, Marasas WFO, Leslie JF (2000)PCR–based identification of MAT–1 and MAT–2 in the Gibberella fujikuroi species complex. Appl Environ Microbiol 66:4378–4382
Sung GH, Sung JM, Hywel-Jones NL, Spatafora JW (2007) A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstrap approach. Mol Phylogen Evol 44:1204–1223
Teasdale SE, Caradus JR, Johnson LJ (2018) Fungal endophyte diversity from tropical forage grass Brachiaria. Plant Ecol Div 11:611–624
Timossi PC, Durigan JC, Leite GJ (2007) Formação de palhada por braquiárias para adoção do sistema plantio direto. Bragantia 66:617–622
Villani A, Moretti A, Saeger SD, Han Z, Mavungu JDD, Soares CMG, Proctor RH, Venâncio A, Lima N, Stea G, Paciolla C, Logrieco AF, Susca A (2016) A polyphasic approach for characterization of a collection of cereal isolates of the Fusarium incarnatum-equiseti species complex. Int J Food Microbiol 234:24–35
Walsh JL, Laurence MH, Liew ECY, Sagalang AE, Burgess LW, Summerell BA, Petrovic T (2010)Fusarium: two endophytic novel species from tropical grasses of northern Australia. Fungal Divers 44:149–159
Zeller KA, Summerell BA, Bullock S, Leslie JF (2003)Gibberella konza (Fusarium konzum) sp. nov. from prairie grasses, a new species in the Gibberella fujikuroi species complex. Mycologia 95:943–954
Acknowledgments
Thanks are due to Edson Luis Rezende for the technical assistance.
Funding
Part of this research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (Proc. 406335/2013-5). The first and second authors acknowledge a fellowship given by CNPq (Proc. 140818/2020-0 and Proc. 156738/2011-2). LHP acknowledges a grant given by CNPq (Proc. 311888/20178).
Author information
Authors and Affiliations
Contributions
Marileide M. Costa and Ludwig H. Pfenning designed the project, supervised its execution, wrote the first draft of the manuscript, and prepared the taxonomic descriptions. Maruzanete P. Melo, Filipe S. Carmo, Elaine A. Guimarães, and Fernando S. Rocha contributed with collection of material, isolation, identification, and preservation of fungal species and the elaboration of the discussion. Gláucia M. Moreira, Sarah S. Costa, and Lucas M. Abreu contributed with sequence analysis, preparation of phylogenetic trees, and overall analysis of the data. All authors commented on previous versions of the manuscript and approved the final version. Therefore, they had full access to all the data obtained in this study and take responsibility for the integrity and security of the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Editorial Responsibility: Hans-Josef Schroers
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Costa, M.M., Melo, M.P., Carmo, F.S. et al. Fusarium species from tropical grasses in Brazil and description of two new taxa. Mycol Progress 20, 61–72 (2021). https://doi.org/10.1007/s11557-020-01658-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-020-01658-5