Abstract
Sudden vegetation dieback (SVD) is defined as the loss and lack of recovery of smooth cordgrass (Spartina alterniflora) in salt marshes. A new species of a moderately pathogenic fungus called Fusarium palustre is consistently found in SVD sites, but greenhouse tests revealed that it is not capable of causing mortality of healthy plants. Similarly, root-knot nematodes (Meloidogyne spartinae) are also found in SVD sites, but their incidence in marshes affected by SVD is not known. To understand more about the ecology of F. palustre and M. spartinae, salt marshes along Connecticut’s Long Island Sound and Massachusetts’ Cape Cod that exhibited SVD and those that did not, were visited during the summers of 2007, 2008, and 2009. Belowground and aboveground tissues of smooth cordgrass plants from 18 marshes were removed, washed, and assayed for Fusarium spp. to determine if patterns between the incidence of the different species of Fusarium, their virulence on S. alterniflora, root-knot nematodes (M. spartinae), and the health of the marsh could be revealed. There were significantly more colonies of Fusarium growing from plants in SVD sites (6.1%) than in healthy marshes where no SVD was present (<1.0%). The incidence of Fusarium spp. from plants at the perimeter of the SVD site was not statistically different from asymptomatic plants 10–20 m from the SVD edge. The majority of isolates could be assigned to one of two species, F. palustre or another slightly pathogenic group called Fusarium cf. incarnatum (88% in 2007, 62% in 2008, and 96% in 2009). The ratio of F. palustre to F. cf. incarnatum was 6.7, 2.7, or 2.1 for 2007, 2008, or 2009, respectively. Greenhouse tests on healthy S. alterniflora revealed that isolates of F. palustre were more virulent than F. cf. incarnatum, regardless of whether they were recovered from plants in healthy marshes or in SVD sites. Root-knot nematodes were found sporadically and could not be associated with SVD. Factorial greenhouse experiments did not demonstrate any interaction between F. palustre and M. spartinae providing no experimental evidence that combining Fusarium and root-knot nematodes could cause mortality. The presence of Fusarium on S. alterniflora in healthy marshes also suggests an endophytic relationship that may subsequently function in the breakdown of tissue when plants are compromised.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the late 1990s, large areas of salt marshes along tidal creeks on the Atlantic and Gulf Coasts became barren, leaving areas of remnant peat (Alber et al. 2008; McKee et al. 2004; Smith 2009). In the absence of any known causes, the condition was initially termed sudden wetland dieback, then changed to sudden vegetation dieback (SVD), when it was concluded that only vegetation, primarily Spartina alterniflora, was affected (Alber et al. 2008). Examination of aerial photography suggested that many SVD sites in New England may have experienced dieback years before (Smith 2009). Many of the SVD sites in New England may have been coincident with the massive dieback of salt marshes along Louisiana’s coast in 2000, when over 150,000 ha suddenly died in less than 8 months (McKee et al. 2004). This condition was referred to as brown marsh to denote the rapid transition from living to dead tissue (McKee et al. 2004). Sudden vegetation dieback is still occurring in many sites along the Atlantic Coast and the causes are not clear.
Initially, observers implicated several possible factors causing SVD such as top down consumer control, drought, soil chemistry changes, wrack smothering, and fungal pathogens. A detailed examination of how these factors relate to SVD has been addressed (Alber et al. 2008). In southern marshes where SVD had occurred, heavy metal toxicity (Al, Mn, Fe) associated with drought and grazing by the periwinkle snail, Littoraria irrorata, was shown to affect plant growth (Silliman and Newell 2003; Silliman et al. 2005), but an extended drought has not been associated with SVD sites in the mid to northern Atlantic marshes (Alber et al. 2008) and the periwinkle snail, L. irrorata, is not found in New England. There are also reports of bare spots that are believed caused by the purple marsh crab, Sesarma reticulatum (Chrichton 1960; Holdredge et al. 2009). A study on Cape Cod found that densities of the S. reticulatum were strongly correlated with SVD sites (Holdredge et al. 2009). In both of these situations, herbivory has been shown to be ecologically important in expanding SVD sites and in restricting recovery from SVD, yet experimental evidence demonstrating causality is lacking at present.
The hypothesis that SVD was caused by a plant pathogen was first investigated in Louisiana, where researchers isolated potential fungal pathogens and tested them for pathogenicity on healthy S. alterniflora plants (Useman and Schneider 2005). Fungi that belong to the genus Fusarium were found to incite leaf spot and stem rots; however, there was no evidence that the pathogen(s) alone could cause plant mortality. A similar survey was conducted in SVD sites along the Atlantic Coast (Elmer and Marra 2011) where over 190 isolates of Fusarium were collected from Connecticut, Delaware, Georgia, Maine, Massachusetts, New York, and Virginia. Over 85% of the isolates fell into two undescribed morphospecies that were found in all sites. These morphospecies differed from the ones found in Louisiana. Sequencing three genes from a subset of isolates revealed that one of these morphospecies was closely related and represented a new species that was later named Fusarium palustre (from the Latin palus, referring to the marsh habitat; Elmer and Marra 2011). The other morphospecies was more diverse and probably contained many species within a complex of Fusarium species known as the Fusarium incarnatum–Fusarium equiseti species complex (O’Donnell et al. 2009). These species were labeled as F. cf. incarnatum. Pathogenicity tests in the greenhouse on wound-inoculated S. alterniflora stems and seedling roots revealed that isolates of F. palustre were more virulent than F. cf. incarnatum, but no Fusarium isolate was able to cause mortality on healthy plants. F. palustre may be an endophyte that increases in SVD sites due to other stressors that may be present.
Endophytes like Fusarium are common in many terrestrial monocots like asparagus (LaMondia and Elmer 1989), maize (Schneider and Pendery 1983), and winter wheat (Sieber et al. 1988). Certain species function as opportunistic pathogens that exploit their host tissue and increase in numbers once defense mechanisms have been compromised by a stress, such as drought, salinity, poor nutrition, or insects. It is still unclear if a stress factor similar to that on terrestrial plants may exist in S. alterniflora that could lead to SVD. In addition, root-knot nematodes (Meloidogyne spartinae) are pathogens that are specific to S. alterniflora (LaMondia and Elmer 2009). They have been associated with SVD sites in Connecticut and Massachusetts, and may impose an additional plant stress (LaMondia and Elmer 2009).
If Fusarium spp. and root-knot nematodes increase in density on S. alterniflora following a stress event, it would be reasonable to expect a higher incidence of these organisms on S. alterniflora in SVD sites as opposed to marshes where SVD is absent. In addition, relatively healthy appearing plants in an SVD site that are proximal (10–20 m) to plants on an SVD perimeter may have similar levels of Fusarium colonization as the declining plants provided the entire marsh experienced the same stress event. The objectives were to sample S. alterniflora plants in healthy marshes and marshes where SVD has occurred in Connecticut and Massachusetts for relative incidence of Fusarium spp., to identify them, and to determine the pathogenicity of a subset of isolates on S. alterniflora. Roots were also examined for galls produced by the root-knot nematodes, M. spartinae. Factorial greenhouse experiments were conducted to investigate potential interactions between F. palustre and M. spartinae on S. alterniflora.
Materials and Methods
Salt marshes in Connecticut and Massachusetts (both SVD sites and healthy sites) were visited in 2007, 2008, and 2009 (Table 1). All of the SVD sites were located along creek banks in low marsh areas that were dominated by S. alterniflora. Three declining plants near the perimeter of each SVD site (SVD edge) were sampled. These plants were often stunted and thin, but occasionally appeared robust and healthy. Evidence of herbivory was rare, but noted when it was observed. In six marshes, three additional plants were removed from the same site, but approximately 10–20 m away from barren peat (SVD area). These plants were sampled to determine if Fusarium were present on plants proximal to an SVD site, but not on its perimeter. Three additional plants were removed from a neighboring marsh that was within 50–300 yards from the SVD site, but where no signs of SVD were present (healthy area). Many times the healthy marsh was adjacent to the SVD site, but demarcated by a forest or a road.
Plants and the associated peat were dug to a depth of approximately 30 cm and transported to the greenhouse, washed in tap water, and were examined for signs of leaf spots and stem rots. Roots were examined for galls of the root-knot nematode (M. spartinae). From each plant, 20 pieces (0.5 × 0.5 cm) of aboveground tissue (stems and leaf pieces) and 20 pieces of belowground tissue (roots and crowns) from each plant were washed again in tap water, surface disinfested in 10% bleach (0.053% NaHCLO2), rinsed in tap water, and placed on peptone PCNB agar that is selective for species of Fusarium (Leslie and Summerell 2006). Plates were incubated at room temperature and, after 5–8 days, colonies were sub-cultured to carnation leaf agar (CLA; Leslie and Summerell 2006). These plates were held for 7–10 days at room temperature (20–25°C) under cool-white and black-light fluorescent lights set for a 12 h photoperiod. Cultures were examined under an Olympus microscope (Olympus, Center Valley, PA) BX41 at ×100 and ×400 magnification. Colonies found to be Fusarium spp. were again sub-cultured to new CLA plates by transferring a single spore and incubating as described before. Cultures were incubated under the same conditions described above and then used for identification (Elmer and Marra 2011; Leslie and Summerell 2006). All or a subset of cultures of each species/site were stored on silica gel (Windels et al. 1988). In 2009, more healthy marshes were sampled. Isolates from previously sampled SVD sites were not saved in 2009. The total number of Fusarium colonies/total number of pieces plated from each plant was recorded and expressed as the percent colonization of that plant. Data from replicate plants were used in analysis of variance (ANOVA) and means were separated using the Kruskal–Wallis test at P = 0.05.
Pathogenicity tests were conducted with a subset of Fusarium isolates. Each isolate was cultured on quarter-strength potato dextrose agar (PDA; 9.75 g Difco PDA plus 15 g agar/L) for 5 days under the culture conditions described above. Inoculations were done on 9-month-old healthy S. alterniflora plants that were produced from seeds removed from a healthy marsh in Madison, Connecticut, with no known history of SVD. Freshly collected seeds were disinfested in 0.053% Na hypochlorite (10% household bleach) for 1 min to eliminate any surface contamination by Fusarium spp. Seeds were immediately rinsed in tap water and germinated in wet sand. Seedlings were fertilized with 20–10–20 soluble fertilizer approximately once a month. Inoculations were done by making a small puncture with a sterile needle in the two largest stems below the first node on each plant. Wounding was done to place additional stress on the plant. Isolates were cultured on quarter-strength PDA from which colonized agar plugs (4 mm in diameter) were placed over the wound. Inoculation sites were wrapped in Parafilm (American National Co., Chicago, IL) to prevent dehydration. Stems inoculated with sterile agar plugs served as controls. After 1 month, the length of the lesion was measured to the nearest millimeter and compared with controls that were stab-inoculated with sterile PDA plugs. Two plants were inoculated per isolate on different stems, and the experiment was repeated the next year on a different set of isolates. Isolates of Fusarium solani that were found in previous studies (Elmer and Marra 2011) to be avirulent were included in the first trial as nonpathogenic controls. Lesion length from each species or morphospecies was analyzed by the Kruskal–Wallis one-way ANOVA at P < 0.05.
The potential interaction between M. spartinae and F. palustre on the health of S. alterniflora was investigated in factorial greenhouse experiments in 2008 and 2009. S. alterniflora seedlings were placed into plastic cone-like pots (3.5-cm-diameter) in 90 cm3 of a 1:1 mix of pasteurized field soil (72% sand, 23% silt, and 5% clay) and Sunshine #3 potting mix (Sun Gro Horticulture, Bellevue, WA). After 6 weeks of establishment in 2008 or 12 weeks in 2009, plants were inoculated with water alone or a suspension of 3,000 eggs and juveniles of M. spartinae in two 0.75-cm-diameter holes 2 cm deep per container. Two weeks later (2008) or 1 day later (2009), plants were drenched with water alone or a suspension of 8 × 106 colony-forming units of F. palustre per container in 10 ml. Plants were maintained in the greenhouse for 8 weeks. Plants were watered as needed with tap water and were watered once per week with 10,000 ppm NaCl (5 ml per container). After 8 weeks, roots were removed from the soil mix and roots, shoots, and stolons weighed. Roots were rated for disease using a scale of 0 to 10 where 0 = no disease, 1 = 10% of the surface with discolored tissue, and 10 = 100% affected. In both years, plants were evaluated in the second week of August. Two people made independent ratings that were averaged. Data were analyzed by ANOVA and rating data were transformed by the square root of X + 1 prior to analysis.
Results
In all 3 years of the study, there were significantly more colonies of Fusarium growing from plants in SVD sites than in healthy marshes where no SVD was present (Fig. 1). The mean colonization of plant pieces sampled from SVD sites was 6.6% in 2007, 5.4% in 2008, and 3.5% in 2009. The relative incidences ranged from 0% to 30%. Over 80% of the isolates in all areas was found colonizing the aboveground tissue compared to roots (data not shown). In 2007 and 2008, the incidence of Fusarium spp. from plants at the perimeter of the SVD site was compared with asymptomatic plants 10–20 m from the SVD edge. In an SVD site, there were no differences in the incidence of Fusarium colonization between symptomatic plants and the adjacent asymptomatic plants in either year (Fig. 1).
The incidence of Fusarium colonies growing from Spartina alterniflora sampled from salt marshes where sudden vegetation dieback (SVD) had occurred or from healthy marshes where no SVD was observed. In 2007 and 2008, samples removed from SVD sites were taken along the perimeter of the dieback (SVD Edge) and from plants in the vicinity of the dieback (SVD Area)
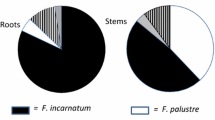
In all 3 years, the majority of isolates could be assigned to either F. palustre or F. cf. incarnatum (88% in 2007, 62% in 2008, and 96% in 2009; Tables 2, 3, and 4). A greater number of F. palustre isolates were observed than F. cf. incarnatum. The ratio of F. palustre to F. cf. incarnatum was 6.7, 2.7, or 2.1 for 2007, 2008, or 2009, respectively. This declining ratio probably reflects the increasing number of non-SVD sites sampled in 2008 and 2009 where the incidence of F. palustre was lower than on plants from SVD sites. The cool wet summer of 2009 may have reduced stress in the marsh and lowered the incidence of Fusarium spp. Root galls, consistent with those caused by M. spartinae, were occasionally noted in SVD sites and in one site where no SVD was observed. When galls were detected, they were usually associated with only one plant and sometimes in very low densities, obscuring any pattern from being detected.
A subset of different isolates from each Fusarium species was tested for pathogenicity on healthy S. alterniflora by wound-inoculating stems with agar plugs that were colonized by the test isolate. Although there was considerable variation in how each isolate behaved, in general, F. palustre was significantly more virulent than the F. cf. incarnatum group in both repetitions of the study (Table 5). The amount of stem rot and discoloration within the stems varied from 0.3 to 3.7 cm for F. palustre. In the first set of tests, F. cf. incarnatum produced reactions not statistically different from the known nonpathogenic species (F. solani) or the control, but in the second set of tests, the isolates of F. cf. incarnatum produced larger lesions and were significantly more virulent than the control.
To determine whether or not the pathogenicity of an isolate was associated with whether the site exhibited SVD, a test between isolates of F. palustre and F. cf. incarnatum was performed comparing the mean lesion length from S. alterniflora collected from seven SVD sites to lesion length from plants collected from six sites without SVD (Fig. 2). Pathogenicity of either species was not associated with site, suggesting that isolates of Fusarium from SVD sites were probably similar to those from healthy marshes.
The interaction between M. spartinae and F. palustre on the health of S. alterniflora was investigated in factorial greenhouse experiments in 2008 and 2009. Plants inoculated with F. palustre exhibited reduced stolon weight and increased root disease ratings in 2008 (Table 6). There were no significant effects of M. spartinae infection, and there was no interaction between the two pathogens. There were significant effects of M. spartinae and F. palustre on the health of S. alterniflora in the 2009 experiment (Table 7). In both years, M. spartinae root galls were only found on plants inoculated with the nematode.
Discussion
SVD is an Atlantic and Gulf Coast phenomenon that was reported in 1999, but may have occurred before that. A key signature of SVD is the death of rhizomes. We found that Fusarium spp. were recovered in significantly higher frequency in S. alterniflora in SVD sites than in plants from marshes where SVD did not appear. Eighty percent of the isolates (n = 240) collected over the 3 years was placed into one of two species (F. palustre, n = 155, and F. cf. incarnatum, n = 38). Isolates of F. palustre were significantly more virulent on stems than F. cf. incarnatum, which confirmed earlier studies (Elmer and Marra 2011). We have no reason to suspect Fusarium to be a causal factor in SVD. However, the pattern of observing higher incidences of Fusarium in SVD sites than in healthy marshes suggests that Fusarium may function in the secondary destruction of S. alterniflora. Since there were no differences in the incidence of Fusarium colonization between symptomatic plants and asymptomatic plants sampled from an SVD site, it is unlikely that Fusarium infection is a primary factor in causing SVD. Another reason to discount Fusarium as a primary factor causing SVD is that the fungus was isolated preferentially from the aboveground tissue and not from the roots or rhizomes. For the pathogen to cause mortality, it would need to infect and destroy the belowground organs, and thus, would be recovered from these tissues in greater numbers than the aboveground tissue. We found that the root-knot nematode, M. spartinae, was found sporadically on roots in SVD sites and in some sites where there was no SVD. Given that M. spartinae is an obligate parasite, its parasitic relationship with S. alterniflora may be more complex than that with Fusarium. Therefore, single plant samplings probably provided an incomplete picture. F. palustre reduced stolon growth and increased root disease ratings in one of two greenhouse experiments. These effects were seen in 2008 when the plants inoculated were younger (and smaller) than in 2009. Nematode infection reduced root and stolon weights in 2009, but results were not significant. We did not observe any interactions between M. spartinae and F. palustre in either year, regardless of whether plants were inoculated with both pathogens simultaneously or whether nematodes were inoculated 2 weeks prior to the Fusarium. This differs from results on terrestrial plants. Wilt on tobacco, caused by Fusarium oxysporum, was increased by co-infection with Meloidogyne, but wilt development was greater when nematodes were inoculated weeks prior to inoculation with Fusarium compared to simultaneous infection (Porter and Powell 1967).
Disease was suspected to be the cause of dieback of Spartina townsendii Agg. in the UK in the 1950s, where over 200 ha was completely denuded (Goodman 1959; Goodman et al. 1959; Goodman and Williams 1961). Although more than 20 fungal species were isolated from dead plants onto nonselective media, Fusarium was not reported. Attempts to transmit the dieback from unhealthy plants to healthy plants also failed. They concluded that a parasite was not involved (Goodman et al. 1959). Our current understanding of endophytic Fusarium spp. would suggest that if Fusarium was present, Goodman and colleagues probably failed to recover the fungus since dead plants were examined instead of declining plants. Furthermore, more aggressive saprophytic organisms may have colonized the dead tissue making it very difficult to observe Fusarium. In contrast, Useman and Schneider (2005) reported that declining S. alterniflora plants on the perimeter of SVD sites in Louisiana had symptoms of black leaf spots and internal stem rots that were incited by Fusarium proliferatum, Fusarium fujikuroi, and an undescribed species of Fusarium, all belonging to the Gibberella fujikuroi species complex (Elmer, unpublished; O’Donnell et al. 2000). Our survey in Connecticut and Massachusetts found different species of Fusarium than those found in Louisiana. It is not uncommon for the Fusarium species structure on a host to change with climate and geography (Burgess et al. 1981; Elmer et al. 1997; Francis and Burgess 1975; Kommedahl and Windels 1981).
Numerous terrestrial plants are asymptomatically colonized by Fusarium endophytes (LaMondia and Elmer 1989; Schneider and Pendery 1983; Sieber et al. 1988), so it is not surprising to add S. alterniflora to the list. Endophytic colonization allows fungi the first opportunity to exploit the tissue upon senescence and increases the competitive saprophytic ability once the tissue dies. The dominant species in this study, F. palustre, appeared to be more prevalent than F. cf. incarnatum. A previous survey from SVD sites along the Atlantic Coast found surprisingly close genetic relatedness among isolates of F. palustre in every marsh (Elmer and Marra 2011). Certain endophytic species may function as opportunistic pathogens that exploit their host tissue once defense mechanisms have been compromised by a stress such a drought, salinity, poor nutrition, or insects. At the present, the stressors that lead to SVD are not clear. The underlying factors associated with SVD may differ by region. In the Gulf region, heavy metal toxicity associated with drought was demonstrated to selectively kill S. alterniflora and was implicated as a primary factor that was causal to SVD (Brown Marsh; McKee et al. 2004). Silliman and Newell (2003) found a close association between grazing from L. irrorata and plants in southern US marshes where SVD was observed. Conversely, in the north Atlantic, drought was not believed to be associated with SVD sites (Alber et al. 2008). Similarly, herbivory by marsh crabs (S. reticulatum) has been associated with SVD sites in New England marshes (Holdredge et al. 2009; Smith 2009), but experimental data demonstrating causality have not been demonstrated.
While the difficulties of experimentally manipulating biotic and/or abiotic agents in salt marshes hinder attempts to resolve the etiologies of SVD, it is imperative that a successful completion of Koch’s Postulates between an agent(s) and SVD be demonstrated in order to advance any management or restoration attempts. In the current study, pathogenicity tests demonstrated pathogenicity, but symptoms of SVD, mainly death of the rhizomes, was not observed.
References
Alber, M., E.M. Swenson, S.C. Adamowicz, and I.A. Mendelssohn. 2008. Salt marsh dieback: an overview of recent events in the U.S. Estuarine. Coastal and Shelf Science 80: 1–11.
Burgess, L.W., R.L. Dodman, W. Pont, and P. Mayer. 1981. Fusarium diseases of wheat, maize and grain sorghum in eastern Australia. In Fusarium: Diseases, Biology, and Taxonomy, ed. P.E. Nelson, T.A. Toussoun, and R.J. Cook, 64–76. University Park: Pennsylvania State University Press.
Chrichton, O.W. 1960. The marsh crab. Estuarine Bulletin 5: 3–10.
Elmer, W.H., and R.E. Marra. 2011. New species of Fusarium associated with dieback of Spartina alterniflora in Atlantic salt marshes. Mycologia 103: 806–819.
Elmer, W.H., B.A. Summerell, L.W. Burgess, D. Backhouse, and A.A. Abubaker. 1997. Fusarium species associated with asparagus crowns and soil in Australia and New Zealand. Australasian Plant Pathology 28: 255–261.
Francis, R.G., and L.W. Burgess. 1975. Surveys of Fusaria and other fungi associated with stalk rot of maize in Eastern Australia. Australian Journal of Agricultural Research 26: 801–807.
Goodman, P.J. 1959. The possible role of pathogenic fungi in the ‘die-back’ of Spartina townsendii Agg. Transactions of the British Mycological Society 42: 409–415.
Goodman, P.J., and W.T. Williams. 1961. Investigations into ‘die-back’ in Spartina townsendii Agg. Journal of Ecology 49: 391–398.
Goodman, P.J., E.M. Braybrooks, and J.M. Lambert. 1959. Investigations into ‘die-back’ in Spartina townsendii Agg.: the present status of Spartina townsendii in Britain. Journal of Ecology 47: 651–677.
Holdredge, C., M.D. Bertness, and A.H. Altieri. 2009. Role of crab herbivory in die-off of New England salt marshes. Conservation Biology 23: 672–679.
Kommedahl, T., and C.E. Windels. 1981. Root-, stalk- and ear-infecting Fusarium species on corn in the USA. In Fusarium, Biology, and Taxonomy, ed. P.E. Nelson, T.A. Toussoun, and R.J. Cook, 94–103. University Park: Pennsylvania State University Press.
LaMondia, J.A., and W.H. Elmer. 1989. Pathogenicity and vegetative compatibility among isolates of Fusarium oxysporum and F. moniliforme colonizing asparagus. Canadian Journal of Botany 67: 2420–2424.
LaMondia, J.A., and W.H. Elmer. 2009. Ecological relationships between Meloidogyne spartinae and salt marsh grasses in Connecticut. Journal of Nematology 40: 217–220.
Leslie, J.F., and B.A. Summerell. 2006. The Fusarium laboratory manual. Ames: Blackwell Publishing.
McKee, K.L., I.A. Mendelssohn, and M.D. Materne. 2004. Acute salt marsh dieback in the Mississippi River deltaic plain: a drought-induced phenomenon? Global Ecology and Biogeography 13: 65–73.
O’Donnell, K., H.I. Nirenberg, T. Aoki, and E. Cigelnik. 2000. A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetically distinct species. Mycoscience 41: 61–78.
O’Donnell, K., D.A. Sutton, M.G. Rinaldi, C. Gueidan, P.W. Crous, and D.M. Geiser. 2009. Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum–F. equiseti and F. chlamydosporum species complexes within the United States. Journal of Clinical Microbiology 47: 3851–3861.
Porter, D.M., and N.T. Powell. 1967. Influence of certain Meloidogyne species on Fusarium wilt development in flue-cured tobacco. Phytopathology 57: 282–285.
Schneider, R.W., and W.E. Pendery. 1983. Stalk rot of corn: mechanism of predisposition by an early season water stress. Phytopathology 73: 863–871.
Sieber, T., T.K. Riesen, E. Muller, and P.M. Fried. 1988. Endophytic fungi in four winter wheat cultivars (Triticum aestivum L.) differing in resistant against Stagonospora modorum (Beck.) Cast. & Germ. = Septoria nodorum (Berk.) Berk. Journal of Phytopathology 122: 289–306.
Silliman, B.R., and S.Y. Newell. 2003. Fungal farming in a snail. PNAS 100: 15643–15648.
Silliman, B.R., J. van de Koppel, M.D. Bertness, L.E. Stanton, and I.A. Mendelssohn. 2005. Drought, snails, and large-scale die-off of southern U.S. salt marshes. Science 310: 1803–1806.
Smith, S.S. 2009. Multi-decadal changes in salt marshes of Cape Cod, MA: photographic analyses of vegetation loss, species shifts, and geomorphic change. Northeastern Naturalist 16: 183–208.
Useman, S., Schneider, R.W. (2005) The possible role of plant pathogens in Louisana’s brown marsh syndrome. Proceedings of the 14th Biennial Coastal Zone Conference, New Orleans, LA.
Windels, C.E., P.M. Burnes, and T. Kommedahl. 1988. Five-year preservation of Fusarium species on silica gel and soil. Phytopathology 78: 107–109.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elmer, W.H., LaMondia, J.A. & Caruso, F.L. Association Between Fusarium spp. on Spartina alterniflora and Dieback Sites in Connecticut and Massachusetts. Estuaries and Coasts 35, 436–444 (2012). https://doi.org/10.1007/s12237-011-9448-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-011-9448-9