Abstract
The present study was carried out to investigate the effect of individual drought, heat, and combined drought and heat stress on tomato plants. Combined stress resulted in the higher accumulation of Proline (101.9%), MDA (38.55%), H2O2 (101.19%), and lower levels of RWC (53.84%). Individual drought and heat stress decreased photosynthetic pigments like total chlorophyll content by (45.45%) and (25.35%), respectively, higher rates of pigment reduction (79.42%) were observed under combined drought and heat stress. Combined stress decreased PSII efficiency (Fv/Fm), quantum yield (ΦPSII), and photochemical efficiency (qp) and increased non-photochemical quenching (NPQ) levels. Moreover, the gas exchange parameters E, A, and Pn decreased by 5.36%, 36.45%, and 51.00%, respectively, in comparison to control plants. Antioxidant enzymes, SOD, APX, CAT, and GR showed a two- to threefold increase under combined drought and heat stress; however, the non-enzymatic antioxidants AsA and GSH displayed one–twofold increase under combined stress. Moreover, 2- to 2.5-fold decrease was observed in MDHAR and DHAR enzyme transcripts under combined stress conditions. The transcripts corresponding to AsA–GSH pathway enzymes SOD, APX, GR, DHAR, and MDHAR were up-regulated by 8- to 12-fold under combined drought and heat. Furthermore, DREB and LEA transcripts were up-regulated under drought and combined stress and down-regulated under drought stress. In the same manner, HSP70 and HSP90 transcripts were up-regulated under heat and combined stress; however, the transcription levels got down-regulated under drought stress. Additionally, rbcL and RCA transcripts were down-regulated especially under combined stress in comparison to individual drought and heat conditions. PSIP680 relative expression levels were up-regulated under drought stress; however, the transcripts were down-regulated under heat and combined stress. Taken together, the results suggest that the combined stress has a predominant effect over individual stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abiotic stresses prompted by numerous environmental factors could adversely affect plant growth and development (Mittler 2006; Zandalinas et al. 2017). In response to these abiotic stresses, innumerable physiological, biochemical, and molecular level modifications occur in crop plants (Aprile et al. 2013; Siddiqui et al. 2015; Zhou et al. 2015). The response of crop plants towards single stress has been the focus of several studies during the recent past (Chew and Halliday 2011; Hirayama and Shinozaki 2010; Siddiqui et al. 2015). However, most abiotic stresses are of concurrent occurrence; moreover, in field conditions, crops are continuously exposed to a combination of diverse abiotic stresses (Mittler 2006; Suzuki et al. 2014).
Drought and heat stress among various abiotic stresses are deliberated as two severe threats to crop production and sustainable agriculture worldwide (Awasthi et al. 2014; Boyer 1982; Lipiec et al. 2013). Drought stress as a magnitude of inadequate rainfall or underprovided soil moisture prompts numerous physiological, biochemical, and molecular responses in plants, which rigorously hamper plant growth and productivity (Seki et al. 2007; Vadez et al. 2011). Due to global warming, heat stress has gradually damaging effects on crop production and crops cultivated through summer are more liable to heat stress (Hall 2010). Usually, heat stress is associated with drought stress in field conditions (Ahuja et al. 2010), which makes it indispensable to study the response of plants to combined heat and drought stress. Tomato (Solanum lycopersicum L., formerly Lycopersicon esculentum Mill.) is one of the most significant and economically important vegetables grown worldwide for its fruits. Tomato is vulnerable to abiotic stresses due to which its productivity is decreasing day by day. To study the biochemical and physiological responses of tomatoes to heat and drought individually, many studies have been carried out. Although studies regarding the combination of drought and heat have been carried out in many plants like (Prasch and Sonnewald 2013; Rizhsky 2002), chickpea (Awasthi et al. 2014), wheat (Aprile et al. 2013; Grigorova et al. 2011), and tobacco (Rizhsky 2002).
The response of plants to the individual stresses could not be openly generalized from the plant's response to a combination of stresses (Prasch and Sonnewald 2013; Rivero et al. 2014; Rizhsky 2002). During its cultivation, tomato plants often come across the combination of drought and heat stress. Nevertheless, the consequence of the combined drought and heat stress on tomato and the association between the biochemical and physiological responses of tomatoes to single and combined stress remained unclear. A combination of drought and heat stress induces the expression of HSPs in wheat as compared to individual stress (Grigorova et al. 2011) and induces specific proteins in wild barley (Ashoub et al. 2015). Plants prime their physiological, biochemical, and molecular states to combat individual as well as combined stresses. There could be various responsive mechanisms operating in plants regarding these stresses (Prasch and Sonnewald 2013; Rivero et al. 2014; Suzuki et al. 2014). Drought stress leads to stomatal closure, as a consequence of limited CO2 diffusion into the leaf inhibition of photosynthesis is caused through unhinge between light reaction and Calvin–Benson cycle (Chaves et al. 2009). In contrast, heat stress limits plant photosynthesis mostly by disturbing biochemical reactions (Allakhverdiev et al. 2003; Havaux 1993). Higher photosynthetic rate and stomatal conductance during heat stress were observed in heat-tolerant wheat as compared to heat-sensitive cultivars where decreased rates of photosynthesis and stomatal conductance were observed (Sharma et al. 2015). A combination of drought and heat stress explicitly leads to the accumulation of photosynthetic products like sugars (Rizhsky et al. 2004). The most sensitive component to heat stress is Photosystem II (PSII) (Čajánek et al. 1998). An effectual and non-destructive procedure to quantify the photochemical efficiency of PSII is chlorophyll fluorescence and thus senses the harm of stress in PSII (Baker and Rosenqvist 2004). An estimation of the maximum quantum efficiency of PSII is provided by (Fv/Fm), which is mostly affected by heat stress (Sharma et al. 2012; Zhou et al. 2015).
The present study was undertaken to unfold the biochemical, physiological, and transcriptional responses of tomatoes under heat, drought and combined stress in terms of pigment content, RWC, proline, H2O2, chlorophyll fluorescence, photosynthesis, and antioxidants. We hypothesized that combined drought and heat stresses might cause a specific response on tomato plants than single stress, or similar response to single stress when one of the single stress played a predominant role. The study will help unfold the differences and relations existing between several physio–biochemical responses of Solanum lycopersicum to drought, heat, and combined stress that will deliver a resolution for tomato improvement programs under unstable climatic conditions.
Materials and methods
Experimental designs and stress treatments
Seeds of tomato (Solanum lycopersicum L.) were first treated with 70% ethanol for 1 min, washed with distilled water and then the seeds were surface sterilized with 4% sodium hypochlorite solution for 10 min, thoroughly washed three times with deionized water. After sterilization, the sowing of tomato seeds (ten seeds per pot) was carried out in 12-inch earthen parts containing a mixture of soil, vermiculite, and sand in the ratio of 2:2:1. Three plants per pot were maintained after thinning of seedlings. Full strength Hoagland’s nutrient solution (200 ml) for 15 days was used to water the plants every alternate day. Four groups of plants were made as per the experiment. The first group of plants, the control with no stress treatment was maintained at 25 °C, watered daily with full-strength Hoagland solution. The second group of plants was imposed with drought stress, watered with full-strength Hoagland solution for 7 days then the plants were withdrawn irrigation for 10 days until relative water content decreased to 60%. For the third group of plants, high temperature of 45 °C was imposed for 7 days and for the fourth group of plants, the plants were first imposed with severe drought stress conditions then exposing the plants to 45 °C temperature for 24 h; therefore, for the tomato plants, four experimental groups were established. Topmost fully grown young leaves were harvested and submerged in liquid nitrogen for biochemical, antioxidant, and gene expression analyses.
Relative water content, malanaldehyde, proline concentration, and H2O2 content
Leaf samples were used for RWC assay according to the method described previously (Barrs and Weatherley 1962). The fresh weight (W1), turgid weight (W2), and dry weight (W3) of leaves were measured, and the RWC was calculated as follows:
MDA content was analyzed as described by Gao et al. (2011). Leaf samples (0.5 g) were homogenized in 10 ml 10% (w/v) trichloroacetic acid (TCA) solution on ice. The homogenate was centrifuged at 4000xg for 10 min at 4 °C, and then the supernatant was collected. 2 ml 0.6% thiobarbituric acid (TBA) was added to 2 ml aliquot of the supernatant. The mixture was kept in the boiling water for 15 min and then rapidly cooled in an ice bath. After centrifuged at 4000 rpm for 10 min, the absorbance of the supernatant at 532 and 600 nm was measured. The concentration of MDA was calculated according to its extinction coefficient of 155 mM/cm.
Proline (Pro) content was determined according to the protocol described previously by Bates et al. (1973). Leaves (0.5 g) were used to extract the proline, homogenized in 3% sulfosalicylic acid, and the supernatant was mixed with an equal volume of glacial acetic acid and acidic ninhydrin for the reaction. Following heating under 100 °C for 30 min, a volume of 5 ml toluene was added to the mixture. The absorbance of the supernatant was measured at 520 nm using a UV–vis spectrometer (Schmdzu Japan, 1800) and the standard curves which were made using l-proline in the same way.
Hydrogen peroxide levels were determined as described by Murshed et al. (2008). Frozen leaves from tomato plants (0.25 g) were homogenized in an ice bath with 1 ml 0.1% (w: v) TCA. The homogenate was centrifuged at 12,000×g for 15 min at 4 °C. Aliquots of 100 μl from each tube were placed in 96-well plates and 50 μl of 10 mM potassium phosphate buffer (pH 7.0) and 100 μl of 1 M KI was added to each well. Commercial H2O2 was used to generate a standard curve. The plate was briefly vortexed, incubated at room temperature for 30 min and the absorbance readings were taken at 390 nm in a microplate reader. The content of H2O2 was determined using the standard curve.
Photosynthetic pigments, chlorophyll fluorescence, and photosynthesis
The DMSO extraction method of Hiscox and Israelstam (1979) was used for the estimation of photosynthetic pigments. Fresh leaf sample 0.5 mg was cut into small pieces (1 cm × 1 cm) and put in a glass test tube containing 10 ml DMSO and 3-ml double-distilled water. For almost an hour, the tubes were placed in an oven at 65 °C for extraction of chlorophyll pigments. Optical density was measured at 480, 510, 645, and 663 nrn on the Beckman DU 640B Spectrophotometer. Maclachlan and Zalik (1963) and Duxbury and Yentsch (1956) formulae were used for calculations of the values. Units were represented as mg g−1 fw. Chlorophyll fluorescence measurements were performed through Pulse Modulation Fluorometer (PAM 2500; Germany). Randomly selected tomato plants and dark adapted for approximately 10 min, before determining initial fluorescence (Fo), actual photochemical efficiency of PSII (Φ PSII), maximal fluorescence (Fm), and non-photochemical quenching (NPQ), and photochemical quenching (qP), at 1200 μmol m–2 s–1PAR. To obtain the minimal fluorescence of the light-adapted state, (Fo′) 3 s of far-red light was applied after the removal of actinic light (AL) source. Under AL (λ = 665 nm), the steady-state fluorescence (Fs) was determined. The relative effective quantum yield of photochemical energy conversion at steady-state photosynthesis was calculated as yield = (Fm′ − Fs)/Fm′, where Fs and Fm′ are the fluorescence at steady-state photosynthesis and maximum fluorescence in the light, respectively. Next, qP, Φ PSII, and NPQ were calculated as (Fm′ − Fs)/(Fm′ − Fo′), (Fm′ − Fs)/Fm′, and (Fm − Fm′)/Fm, respectively (Baker and Rosenqvist 2004). Photosynthetic parameters like stomatal conductance (gs), carbon dioxide assimilation (A), transpiration rate (E), and net photosynthetic rate (Pn), were carried out with the help of IRGA (LI-COR, USA), and measured in the morning between 7:00 and 9:30.
Enzyme extraction and assays
For enzyme extraction and assay, fresh leaf sample (1 g) from 2-month-old tomato plants were collected and homogenized in a buffer solution 100 mM Tris–HCL (pH 7.5) in presence of DTT (5 mM), 1.0 mM EDTA, MgCl2 (10 mM), PVP (1.5%),5.0 mM magnesium acetate, and aprotinin (1 μg/ml). To obtain the supernatant for enzyme assays, the extract was centrifuged for 15 min at 10,000×g. For extracting APX, ascorbate (2 mM) was added to the buffer (Guo et al. 2006).
The activity of superoxide dismutase (SOD; EC1.15.1.1) was measured by the ability of the enzyme to inhibit the light-dependent reduction of nitro blue tetrazolium chloride (NBT). The mixture was read at 560 nm and the amount of enzyme required to produce a 50% inhibition in the photo-reduction rate of NBT was defined as one unit of SOD activity calculated as enzyme units (EU) per mg of protein. The activity, in EU mg−1 of protein, of the catalase (CAT; EC 1.11.1.6) was measured according to Guo et al. (2006) by reduction of hydrogen peroxide and recording the change in the absorbance of the mixture at 240 nm for 3 min. Ascorbate peroxidase (APX; EC 1.11.1.11) activity, in EU mg−1 of protein, was assayed using the protocol by Guo et al. (2006), following a reduction in absorbance of the mixture containing hydrogen peroxide and ascorbic acid at 290 nm for 3 min. The glutathione reductase (GR; EC 1.6.4.2) activity was determined by assessing the decreased absorbance of the reaction mixture containing GSSG and NADPH at 340 nm for 3 min, using the method described by Foyer and Halliwell (1976), and the activity was measured as EU mg−1 of protein. Monodehydroascorbate reductase (MDHAR; EC1.6.5.4) activity was estimated as suggested by Hossain et al. (2009). The change in absorbance of both the reaction mixtures was determined spectrophotometrically at 340 nm for 3 min, with the activity expressed as EU mg–1 protein. Dehydroascorbate reductase (DHAR; EC 1.8.5.1) was estimated by replicating the method of Foyer (1989) by reading the absorbance at 265 nm for 3 min. Protein contents in the enzyme extracts were determined using Coomassie brilliant blue G-250 (Bradford 1976).
Non-enzymatic antioxidants
Fresh leaves (0.8 g) were homogenized in 3 ml ice-cold metaphosphoric acid (5%) containing 1 mM EDTA, the homogenate was centrifuged for 10 min at 10,000×g. For the assay of total ascorbate and reduced ascorbate, 400 μl of supernatant was distributed in two sterilized Eppendorf tubes. 200 μl of 10% TCA was added to each tube and vortexed before centrifugation, 10 μl of NaOH solution was added to it. 200 μl of 150 mM of NaH2PO4 was added to 200 μl of the supernatant, along with 200 μl of water. 100 μl of 10 mM DDT, 200 μl of buffer was added to another 200 μl of supernatant and mixed thoroughly. To each tube, 100 μl of 0.5% N-ethylmaleimide was then added, vortex mixed, and incubated for 30 min at room temperature. To each tube was then added 400 μl of 44% H3PO4, 4 μl of 4% bipyridyl, 200 μl of 3% FeCl3, and 400 μl of 10% TCA, vortex mixed, and the samples wereincubated for 60 min at 33 °C. The supernatant was then used for ascorbate analysis (Hossain et al. 2009), and the absorbance was recorded at 525 nm on UV–vis spectrophotometer.
Glutathione pool was estimated according to Hossain et al. (2009). The concentration of GSH and GSSG was calculated from the standard curves obtained from the known concentrations of GSH and GSSG. At 4 °C, a slurry of fresh leaf material (0.5 g) was prepared by homogenizing them in 2 ml of 5% sulphosalicylic acid. From the supernatant obtained through centrifugation at 10,000×g for 10 min, an aliquot of 0.5 ml was taken in a sterilized Eppendorf tube, 40 μl of DTNB and 0.6 ml of reaction buffer was added to the tube. After 2 min, absorbance was read at 412 nm to determine GSH concentration. 2 μl of GR and 50 μl of NADPH were added to determine total glutathione in the sample. Oxidized glutathione was determined by subtracting the reduced glutathione pool from the total glutathione pool. The reaction was allowed to run for 30 min at 25 °C. The change in absorbance at 412 nm on the UV–VIS spectrophotometer (Model DU 640, Beckman, USA) was recorded. Values are corrected for the absorbance of supernatant and DTNB.
Expression analysis of antioxidant and stress-responsive genes
RNA extraction and cDNA synthesis
Total RNA from tomato leaves was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). RNA was quantified by spectrophotometric analysis and the quality was evaluated through agarose gel electrophoresis. First-strand complementary DNA (cDNA) synthesis was carried out using the SuperScript cDNA Synthesis Kit (Invitrogen).
Quantitative real-time PCR
Quantitative real-time PCR was carried out using Light Cycler (Roche) with Light Cycler Fast Start DNA Master SYBR Green kit (Roche). Amplification of antioxidants (SOD, APX, GR, DHAR, and MDHAR), stress-related genes, DREB, LEA, HSP70, HSP90) and photosynthesis-related genes (PSIID2, PSIP680, PsaH, rbcS, rbcL, and RCA was carried out according to the manufacturer’s protocol. 20 μl reaction mixture contains 1.5 μl cDNA, 0.3 μl of primer (forward and reverse) 12.5 μl SYBR Premix, and 5.4 μl dH2O. All genes were tested in triplicate with appropriate primers along with tubulin used as an internal control (Table 1). The gene expression data were calculated comparative to β-tubulin, and Ct values of the used target genes were normalized using the Ct values of tubulin. The levels of mRNA were also normalized with tubulin and its value was expressed relative to that of the control, which was given an arbitrary value 1 (Liu et al. 2012). The relative differential gene expression was measured according to the equation 2−ΔΔCt (Livak and Schmittgen 2001). Data were derived from three experimental replicates.
Statistical analysis
All experiments were conducted in five replications. SPSS 16.0 (IBM statistics) was used to analyze the data which were expressed as mean ± standard error (SE). Significance differences were determined by one-way analysis of variance (ANOVA) with Duncan’s multiple range test as a post hoc test (p < 0.05).
Results
Proline concentration and MDA concentration
In response to individual and combined stresses, endogenous proline levels were observed in tomato leaves (Fig. 1a). Basal proline levels in stressed tomato plants almost doubled the levels observed in control plants. Furthermore, the concentration of proline significantly elevated in response to individual stresses. In addition to this, combined stress prompted to maximum proline concentrations. Under drought stress conditions, 65.83% increase in proline content was observed; however, in comparison to control under heat stress conditions, only 48.30% was observed in tomato leaves (Fig. 1a). Besides, substantial increments of proline build-up (101.9%) were observed in tomato plants subjected to stress combination.
In response to heat, drought, and combined stresses, the oxidative damage in terms of MDA concentration was studied in tomato plants (Fig. 1b). In comparison to control, elevated levels of MDA were observed in tomato seedlings subjected to both individual and combined stress conditions. 8.25% increase in MDA content was observed in tomato seedlings under drought stress conditions. Contrary to drought stress, 5.35% increase in MDA concentrations was observed under heat stress; however, much higher levels of MDA 38.55% accumulated under combined heat and drought conditions.
Leaf relative water content (RWC) and H2O2 concentration
In tomato seedlings, RWC was measured under different stress conditions. Under individual as well as under stress combination, RWC levels showed a significant decrease under stress conditions as compared to control. Drought stress decreased RWC levels by 32.59%, heat stress by 25.25%, and combined heat and drought had an additive effect on RWC content in tomato seedlings. RWC levels under stress combination decrease to about 53.84% (Fig. 1c). Tomato plants subjected to individual and stress combinations resulted in elevated H2O2 levels. Higher H2O2 levels (109.79%) were observed under combined drought and heat stress. Almost similar H2O2 levels were observed under individual and drought stress conditions (Fig. 1d).
Photosynthetic pigment, chlorophyll fluorescence-, and leaf gas exchange-related parameters
Compared to control, a decrease of about 45.45% was observed in total chl under drought stress. Under heat stress, a decrease of about 25.35% was observed; however, a sharp decrease of about 79.42% was observed under combined drought and heat stress. Almost a similar trend was observed in the case of chl a and chl b, respectively, under individual and combined stress. Carotenoid levels also decreased under all the stress treatments, higher levels of decrease were observed under combined drought and heat stress (58.69%) followed by drought stress (52.17%) (Fig. 2a).
Compared to control, all the parameters related to photosynthesis showed a sharp decline under all the stress treatments. A decline of about 12.5% and 6.94% in the case of PSII efficiency (Fv/Fm) was observed under individual drought and heat stress conditions, respectively; however, combined stress resulted in much more reduction (41.66%) of Fm/Fm. Combined stress resulted in the sharp decrease of PSII quantum yield (ΦPSII) and photochemical efficiency (qp), the decrease was less as compared to the individual stress. Under drought stress alone, the ΦPSII and qp decreased to an extent of 19.04% and 34.37%, respectively. Additionally, under heat stress conditions, the reduction was about 42.85% and 39.06%, respectively. Moreover, under all the stress conditions, an increase in non- photochemical quenching (NPQ) was observed. Drought stress increased NPQ by 44.44%, heat stress by 64.28%, and combined stress by about 73.68% (Fig. 2b).
The parameters related to gas exchange E, A, and Pn decreased by 45.45%, 4.06%, and 19.46%, respectively, under conditions of drought; however, under heat stress, the decrease observed was about 49.09%, 5.91%, and 40.65%, respectively. Combined drought and heat stress further depreciated the gas exchange parameters by 5.36%, 36.45%, and 51.00% in comparison to control plants (Figs. 3a, c, d). Higher levels of stomatal conductance (gs) were observed under stress combinations followed by individual drought and heat conditions. Under heat stress conditions, the stomatal conductance decreased by 4.26% and the same decreased by 4.53% under drought stress; however, stomatal conductance was much exacerbated when plants were exposed to plants combined heat and drought stress (Fig. 3b).
Photosynthesis- and gas exchange-related parameters influenced by combined heat and drought stress. a Transpiration rate (E), b stomatal conductance (gs), c CO2 assimilation rate (A), and d net photosynthesis (Pn) in tomato. Data presented are the means ± SE (n = 5) and significant difference calculated at P ≤ 0.05
Activity of antioxidant enzymes (AsA–GSH) pathway
The enzymes corresponding to ASA–GSH pathway were studied under individual and combined stress conditions in tomato plants, differential responses observed under different treatments are listed in Figs. 4 and 5. The activity of SOD displayed a higher increase 2.5-fold under combined stress; however, the enzymatic activity demonstrated under individual and drought stress were almost the same (twofold) in comparison to control plants (Fig. 4a). Similar to SOD, APX, CAT, and GR activities augmented under individual and combined stress. Higher APX activities (threefold) were observed under drought stress, followed by heat stress (2.5-fold); however, stress combination showed lesser enzyme activities (twofold) in comparison to control. Higher catalase activity was observed under combined stress conditions in comparison to individual stress. Almost twofold enzyme activity was observed in tomato plants exposed to stress combination and drought alone; however, under heat stress, there was no significant increase observed in CAT activity (Fig. 4c). Moreover, tomato plants displayed an enhancement in GR activity under individual and combined stress conditions. Highest GR activity almost twofold was observed under drought stress, followed by combined drought and heat. A slight increase in GR activity was observed under heat stress concerning control (Fig. 5b). Additional to this, DHAR and MDHAR showed a declined trend under all the stress conditions. Both MDHAR and DHAR showed a decrease of about twofold under stress combination; however, under individual stress, no sharp decrease was observed in comparison to control (Figs. 4d and 5a). Compared to control, ASA levels increased about threefold and twofold under heat stress; however, no significant increase was observed under drought stress. In addition to this, AsA/DHA ratio showed an increase of about 1.5-fold and onefold under individual drought and combined stress, respectively (Figs. 5c, d, 6a). Tomato plants accumulated higher GSH and GSSG under heat stress and stress combination; however, under drought stress conditions, no significant accumulation in GSH and GSSG levels was observed (Fig. 6b, c). Additionally, stress conditions whether individual or combined decreased GSH/GSSG ratio in tomato plants. Stress combination decreased GSH/GSSG ratio to about fourfold; however, individual heat and drought resulted in 2- and 2.5-fold decrease in GSH/GSSG ratio, respectively, in comparison to control (Fig. 6d).
Transcriptional expression of antioxidant-, stress-, and photosynthesis-related genes
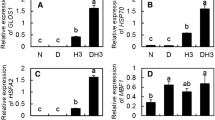
The transcripts corresponding to AsA–GSH pathways were up-regulated under all the stress conditions. Six–eightfold increase was observed in SOD transcript levels under drought stress conditions, seven- to ninefold under heat stress and 8- to 12-fold under combined drought and heat. Similar to SOD, APX, GR, DHAR, and MDHAR transcripts were up-regulated under all the stress conditions. Higher transcripts of the antioxidant genes were observed under combined stress followed by individual heat and drought stress respectively (Fig. 7a).
Transcriptional expression of ROS detoxifying genes, stress-related genes and photosynthesis-related genes in tomato under individual drought, heat, and combined drought and heat. a Antioxidant gene expression, b stress-responsive gene expression, and c photosynthesis-related genes showing different expression profiles
Furthermore, the transcription levels of stress-related genes DREB, LEA, HSP70, and HSP90 were also studied under different stress conditions. A remarkable up-regulation in relative accumulation of DREB and LEA transcripts were observed under combined stress and individual drought stress conditions. Contrary to this the transcripts were down-regulated under heat stress conditions (Fig. 7b). Relative expression levels of HSP70 and HSP90 showed 6 to eightfold up-regulation under heat stress, and seven- to eightfold under combined stress, however, the transcripts down-regulated under drought stress conditions (Fig. 7b). Additionally, the transcripts corresponding to photosynthesis were also studied under different stress conditions. PSIID2, PsaH, and rbcS transcripts got remarkably up-regulated under drought stress, however, the same gene transcript expression levels got down-regulated under combined stress and individual heat stress. rbcL and RCA transcripts were down-regulated especially under combined stress in comparison to individual drought and heat conditions. PSIP680 relative expression levels were up-regulated under drought stress; however, the transcripts were down-regulated under heat and combined stress conditions (Fig. 7c).
Discussion
Plants being sessile are constantly exposed to several abiotic stresses comprising drought, heat or different stress combinations that tempt several metabolic disparities leading to oxidative damage due to ROS production and accumulation. ROS buildup in plants triggers organelle integrity, oxidation of cellular components, and even can lead to cell death (Nath et al. 2016; Raja et al. 2017; Suzuki et al. 2012). During the present study, increased levels of H2O2 and MDA were observed in tomato plants under individual drought, heat, and combined stress, suggesting that the level of oxidative damage is directly related to the predisposition of tomato plants to combined drought and heat stress (Zandalinas et al. 2017). Plants subjected to different stress conditions whether individual or combined resulted in the accumulation of reactive oxygen species (ROS) which degrade polyunsaturated lipids, thereby, forming MDA and being the end product of lipid peroxidation in biomembranes, its content is directly proportional to the extent of lipid peroxidation and membrane injury (Ayala et al. 2014; García-Gómez et al. 2017) (Fig. 1b and d).
Proline in plants functions as an osmoprotectant and allows them to tolerate stress (Akram et al. 2017; Alzahrani et al. 2018). Higher levels of proline accumulation in plants occur under stress conditions. In this study, we evaluated the contents of proline and found that tomato plants accumulated lesser proline content under heat stress in comparison to drought and combined stress (Fig. 1a). Recently it was demonstrated that the plants under heat stress accumulate sucrose instead of proline as an osmoprotectant to ameliorate the toxicity of proline to cells (Mittler 2006; Rizhsky et al. 2004). However, drought and combined stress showed a higher buildup of proline, which is in line with the findings of (Cvikrová et al. 2013) who demonstrated that the higher levels of proline are involved in plant protection against combined drought and heat stress. Several recent studies also support our findings that proline accumulation occurs in plants exposed to stress conditions (Claussen 2005; Kaur and Asthir 2015; Moreno-Galván et al. 2020) because of its property to stabilize subcellular structures, scavenging free radicals and buffer cellular redox potential (Dar et al. 2016; Hazman et al. 2015; Nurdiani et al. 2018). Under different stress conditions, plants maintain their physiological balance through higher RWC values particularly under higher rates of transpiration normally. In plants, RWC is reflected as an indicator of drought resistance (Aref et al. 2016). Related to protoplasmic permeability; plants with lower RWC content are believed to be more sensitive to drought. During the present study, plants subjected to different stress conditions displayed decreased RWC values suggesting the sensitivity of tomato plants towards stress (Fig. 1c). Upreti et al. (2000) also reported decreased RWC in pea plants under drought stress. Maintenance of higher RWC in tomato cultivars was established as an indicator of combined stress resistance (Zandalinas et al. 2017). Moreover, low leaf perimeter values, dissection index, and the higher RWC also contribute to improved drought tolerance through lower evaporation and more preserved cell membrane integrity (Petrov et al. 2018).
Chlorophyll pigment in plants is directly involved in various metabolic processes, under stressful environments; chlorophyll measurements are deliberated to be an imperious tool to evaluate the effect on plants. Chlorophyll reduction directly hampers plant productivity reduces growth and tolerance (Joshi and Swami 2009; Verma and Singh 2006; Kalaji et al. 2018). During the present study, a significant reduction was observed in different plant pigments under individual and combined stress conditions in tomato plants, which may be related to chloroplast damage (Fig. 2a). Reduction in chlorophyll content was also observed in wheat (Li et al. 2007), maize (Anjum et al. 2016), and ryegrass (Digrado et al. 2017) under combined stress. Carotenoids prevent chlorophyll photo-oxidation through their antioxidant potential, Being defensive, carotenoids are reported to be more inclined to damage under several environmental pressures which often upshots cellular destruction, including pigment dilapidation (Sharma and Tripathi 2008).
In plants, under stressful environments, chlorophyll fluorescence is an unconventional method generally used to enumerate stress tolerance and acclimation responses (Choi et al. 2016). The effect of drought stress on the photosynthetic efficiency and, photosynthetic electron transport, the variation in chlorophyll fluorescence parameters may be the result of inhibition of electron transport or damage acceptor side of PSII. Under drought stress, the values of FV/F0 decreased, while NPQ significantly increased in tomato seedlings. Reduction in FV/F0, an indicator of PSII structure and function, and an increase in dissipated energy as heat and fluorescence may be due to the accumulation of inactive PSII reaction centers and a lower quantum yield of PSII photochemistry in plants (Stirbet et al. 2018). Chlorophyll fluorescence analysis revealed that the drought stress caused the impaired electron transfer to PSII reaction center due to the variations in energy absorption, trapping, electron transport, and dissipation per cross section, which results in reduction of photosynthetic efficiency of PSII (Stirbet et al. 2018; Khatri and Rathore 2019). According to Živčák et al. (2013), drought stress gradually decreased PSII electron transport and non-photochemical quenching increased in wheat leaves which supports the roles of alternative electron sinks (either from PSII or PSI) and cyclic electron flow for photoprotection of PSII and PSI, which also generates ATP needed for countering the drought stress conditions. Moreover, the drought stress moderately decreased absorption and electron transport rate as well as the number of functional reaction centers in crop plants (Brestič and Živčák 2013). Under individual drought and heat stress, Fv/Fm and primary photochemical processes of PSII were not much affected (Zhou et al. 2015). Notably, during the present study, Fv/Fm decreased under individual and combined stress; however, the decrease was much severe under combined stress. The findings are consistent with that of Dreesen et al. (2012) and Rollins et al. (2013). Furthermore, a momentous decrease was noticed in qp and ΦPSII, in addition to this, an increase was observed in NPQ under individual and combined stress conditions (Fig. 2a and b). The results suggest that drought stress limits chloroplast CO2 supply by causing stomatal closure. Lower Fv/Fm indicated severe damage to PSII under combined stress conditions (Ort and Baker 2002). Stress-induced photoinhibition under drought stress is remunerated by improved heat dissipation through NPQ. From primary to secondary acceptor, electron transport at PSII acceptor side is blocked that severely decreases the ratio Fv/Fm (Suzuki et al. 2014; Zandalinas et al. 2017). Likewise, notable distractions in PSII function under combined heat and drought stress were reported in L. japonicas plants (Sainz et al. 2010). Compared to individual stresses combined heat and drought stress led to enhanced reduction in photochemical efficiency of PS-II in F. arundinacea and Lolium perenne (Jiang and Huang 2001).
Among several physiological traits, photosynthesis is considered to be more sensitive to heat and drought stress. Stomatal closure is the limiting factor that reduces CO2 availability under drought stress (Chaves 2004; Chaves et al. 2003); however, non-stomatal traits such as Rubisco activity and electron transport capacity inhibit photosynthesis under heat stress (Salvucci and Crafts-Brandner 2004b, c; Way and Oren 2010). While studying photosynthetic performance under individual and combined stress, we found decreased stomatal conductance, transpiration rate, CO2 assimilation, and the photosynthetic rate decreased heat, drought, and combined drought and heat stress, with more detrimental effect under combined stress. Our findings advocate that acclimation of plants to combined stress significantly depends upon the maintenance of photosynthetic activity, and together heat and drought prompted limitations to act concurrently to impede photosynthesis under the combined stress condition in the field.
Photosynthetic responses of plants towards individual and combined stress are species dependent. Drought stress resulted in decreased leaf water potential, stomatal conductance, (Fv/Fm), and maximum quantum efficiency of photosystem II (PSII); however, the photosynthetic rate increased under high temperature. Further, these physiological activities were much aggravated when plants were subjected to high temperatures and drought in combination (Arend et al. 2013). Similar physiological responses to heat, drought, and their combination were also found in the case of tobacco plants (Demirevska et al. 2010). Decreased transpiration rate (E) CO2 assimilation rate (A), stomatal conductance (gs) and net photosynthesis (Pn), under individual drought, heat and combined drought and heat were found during the present study, are in consistence with those of Anjum et al. (2016); Suzuki et al. (2014); Zandalinas et al. (2017); Zhou et al. (2017) (Fig. 3a–d). Plant productivity under drought stress is considerably affected, which is mainly associated with the decrease of photosynthetic activity (Dąbrowski et al. 2019). Inhibition of photosynthesis under drought stress mostly occurs via stomatal closure and subsequent decrease in assimilation transport (Chaves et al. 2009). Contrary to this, PN under heat stress is affected through biochemical reactions of photosynthesis (Havaux 1993; Sharma et al. 2015). Lower rates of gs significantly inhibit PN under combined drought and heat stress conditions than the individual drought and heat treatment. Osmotic stress reduces PSII activity, decreases the effective quantum yield of PSII, and causes degradation of D1 protein leading to the inactivation of PSII reaction center (Batra et al. 2014; Asrar et al. 2017). Under conditions of heat stress, no significant reduction was observed in photosynthetic activity of tobacco plants; however, 80% reduction in photosynthetic activity was observed under individual drought and combined heat and drought stress (Rizhsky 2002). Awasthi et al. (2014) in Cicer arietinum reported enhanced activity of RuBisCO; moreover, the same plants displayed reduced enzyme activities under drought and combined stress. Enhanced ROS production under combined stress in Festuca arundinacea resulted in reduced photochemical efficiency of photosystem II (PS-II) (Jiang and Huang 2001) and photosynthetic activity in Populus yunnanensis (Li et al. 2014). However, enhanced photorespiration under heat stress is believed to be the main cause behind the reduced activity of RuBisCO, PS-II activity and photosynthesis (Prasad et al. 2008; Salvucci and Crafts-Brandner 2004a; Yang et al. 2006) (Fig. 3a–d).
Chlorophyll fluorescence related parameters can be used as specific contrivances to test the response of adverse environmental conditions, such as drought (Krasteva et al. 2013), NaCl (Hniličková et al. 2017), temperature (Martinazzo et al. 2012, Feng et al. 2014), ultraviolet radiation (Šprtová et al. 2000, Faseela and Puthur 2018), and the indirect assessment of their impact on plants. The measurement of photochemical processes by chlorophyll fluorescence gives us a clear idea of the intensity of the stress encountered by the plants (Murchie and Lawson 2013).
Antioxidants and the AsA-GSH cycle
In plants, ROS are released as the byproducts of aerobic metabolism; however, environmental stresses prompt ROS production. Plants survive under these environmental insults by modulating their antioxidant metabolism (Raja et al. 2017). Superoxide dismutase (SOD) is considered to be the first line of defense to safeguard plants against environmental fluctuations. Ascorbate glutathione pathway in plants operate in various cellular compartments plays a key role in ROS detoxification, because of its enzymatic and non-enzymatic antioxidant components. AsA-GSH pathway comprises of four enzymatic components (APX, MDHAR, DHAR, and GR) and two antioxidants (AsA and GSH) (Pandey et al. 2015; Raja et al. 2017). Reduction of H2O2 to water through APX in the cycle initially yields an unstable product in the form of MDHA, dismutated to AsA and DHA, reduced by GSH to GSSG by DHAR. Primarily, NADPH produced from photosynthesis is used by GR to regenerate GSH from oxidized GSSG (Gill and Tuteja 2010; Pandey et al. 2015).
Enhanced CAT and SOD activities under individual and combined stress during the present study has also been reported by other workers (Anjum et al. 2016; Suzuki et al. 2014; Zandalinas et al. 2017; Zhou et al. 2017). Conversion of superoxide anion (O2.−) into O2 and H2O2 is enhanced through the action of SOD. H2O2 still being toxic is converted into H2O and oxygen by CAT (Carvalho et al. 2015). Removal of H2O2 under combined drought and heat by APX and CAT is considered to be one of the vital processes for the tolerance of plants (Koussevitzky et al. 2008). During the present study, a significant increase was observed in APX activity under drought, heat and combined stress in tomato plants (Fig. 4b), proposing an effectual H2O2 scavenging capacity under these stresses. However, this enzyme activity under high temperatures could be inadequate to scavenge the surplus of H2O2 when the activity of CAT is not initiated, triggering oxidative damage. Additionally using AsA as an electron donor, APX dismutase H2O2 (Foyer and Noctor 2011). During the present study, activities of DHAR, MDHAR, GSH/GSSG ratio showed a considerable decline under all stress conditions (individual or combined). Synthesis of GSH and AsA through the initiation of DHAR and MDHAR enzymes consequently delivers a resolute supply of GSSG to GR and AsA to APX (Li et al. 2016). Higher MDHAR and DHAR activities, advocate that upon H2O2 reduction to H2O by APX, the radicals produced were transported promptly back to AsA via MDHAR or by unprompted disparity processes (Ahammed et al. 2013). During the transformation of GSSG to GSH, a considerable part is played by GSH in upholding GSH/GSSG ratio. For electron transport under stress conditions, higher GR activities help plants to uphold NADP+ pool and also assists them to safeguard photosynthetic apparatus from photo-oxidation (Hayat et al. 2010). It is also useful to maintain the GSH/GSSG ratio for the normal functioning of the cells (Yuan et al. 2013). Plasma membrane oxidation under stressful environments is prevented by antioxidants like ascorbate and glutathione which often serve as redox buffering agents under these conditions (Pandey et al. 2015). Furthermore, AsA and GSH serve as electron donors to APX and GPX (Batth et al. 2017). In response to combined stress, the tomato plants displayed higher leaf AsA and DHA and lower AsA/DHA ratio, signifying a strong stress pressure (Fig. 5c and d). The maintenance of a promising GSH/GSSG ratio is a prerequisite for cellular redox regulation which involves precise modulation of the GSH cycle. In this manner, at the disbursement of NADPH, GR activity could efficiently reutilize GSH (Foyer and Noctor 2011). Concerning control, tomato plants under individual and combined stress exhibited a decrease in GSH/GSSG ratio advocates an impairment in GSH recycling. As reported earlier that GSSG status maintenance under stress could be a magnitude of ROS accumulation (Foyer and Noctor 2011). Our findings are in agreement with this proclamation since MDA significantly increased in tomato plants under combined stress (Fig. 1b). Additionally, the initiation of GR activity detected in plants under stress combination (Fig. 5b) might be inadequate to retain a proper GSH/GSSH ratio, leading to impaired ROS detoxification (Arbona et al. 2008). Differences were also observed in ROS detoxification genes induced under these stress conditions indicating stress reliant mechanism of ROS detoxification. CAT and GPX were observed to be induced under drought stress, thioredoxin peroxidase (TPX) and cytosolic APX were induced under heat stress. Conversely, under combined stress, genes encoding copper–zinc superoxide dismutase (CuZnSOD), GPX, alternative oxidase (AOX), glutathione S transferase (GST) and glutathione reductase (GR) were observed to be induced explicitly (Panday et al. 2015; Zandalinas et al. 2017; Zhou et al. 2017).
During the present study, differential, transcriptional expression profiles were identified in tomato plants under individual and combined stress conditions. Transcriptional expression levels of different antioxidant genes, stress-related genes, and photosynthetic genes revealed that environmental perturbations induced the expression of different groups of genes. Enhanced transcript expression levels were observed in antioxidant-related genes (Fig. 7a). In Hordeum vulgare and maize seedling, similar results of increased expression were reported under drought stress conditions (Harb et al. 2015). Under abiotic stress conditions, antioxidant-related gene expression is reported to be up-regulated (Teixeira et al. 2006). Transcriptional expression of SOD, CAT, APX, and GR increased under individual drought, heat and combined heat and drought in citrus plants (Zandalinas et al. 2017). Contrary to heat or drought alone, combined stress caused the induction of several different stress-responsive transcripts. With numerous significant functions, LEA proteins are reported to occur in various organisms that safeguard the clumping of proteins because of desiccation or osmotic stress encouraged through diverse environmental conditions (Hundertmark and Hincha 2008).
In response to drought, heat and combined drought and heat stress abundant transcript profiles of the LEA gene were observed in tomato plants (Fig. 7b). Involved in stress tolerance, DREB transcriptional factors control several downstream stress-responsive genes. Dehydration in plants induces the expression of DREB1 and DREB2 (Sakuma et al. 2002). Similarly, DREB2A and DREB2B transcriptional expressions were detected under high osmotic stress and salt instead of cold stress (Nakashima et al. 2000). In Pennisetum glaucum, PgDREB2A gene transcriptional expression was induced under drought stress (Agarwal et al. 2007). In tobacco plants, Rizhsky (2002) also found the increased transcriptional profiles of LEA7 and dehydrins in response to individual and combined stress The induction of heat shock proteins (HSPs), under combined heat and drought stress is the common response established by plants in field conditions. Combined drought and heat stress in tomato led to the induction of enzymes involved in ROS detoxification ROS, HSPs, and the enzymes related to glycolysis and photosynthesis (Rampino et al., 2012; Johnson et al., 2014). In addition to these HSPs, genes like dehydrins, LEA, enzymes involved in the biosynthesis of anthocyanin and pentose pathway were also induced under combined stress (Rizhsky et al., 2004). The increased transcript expression of HSP70 and HSP90 during the present study is consistent with those of Rizhsky (2002) who reported the induction of small HSPs, HSP70, HSP90, and HSP100 in N.tabacum plants under combined and individual stress. Under combined stress and heat alone, chaperones are the largest class of proteins commonly regulated. However, under combined drought and heat, hydrolases are commonly regulated genes (Rizhsky et al. 2004).
Plants exposed to stress showed suppression of many photosynthetic genes. Expression of PSIID2 was induced under drought and combined stress; however, PsaH, rbcS, and rbcL genes showed increased transcripts under drought and heat stress. Transcripts relating to PSIP680 and RCA were down-regulated under heat and combined stress. Differential expression profiles of photosynthetic-related genes during the present study suggest that photosynthesis is more prone to stress conditions. Similar findings were reported by several workers (Johnson et al. 2014; Rampino et al. 2012; Rizhsky 2002; Rizhsky et al. 2004).
Conclusion
Our study suggests that the combined drought and heat stress negatively affects the tomato plants, the accumulation of different osmolytes, antioxidants and increased transcriptional levels of antioxidant and stress-related genes provide tolerance to tomato species under these stressful conditions. Moreover, studies focusing on single abiotic stress do not represent the particular response of plants to a combination of different stresses, likely affecting crops growing in the field. Further, the study will reveal the alteration and association among the physiological response of tomato plants to drought, heat and combined stress, and be important for the selection and breeding of tolerant tomato cultivars under single and combined stress.
Availability of data and material
Data will be available on request to corresponding or first author.
References
Agarwal P, Agarwal PK, Nair S, Sopory S, Reddy M (2007) Stress-inducible DREB2A transcription factor from Pennisetum glaucum is a phosphoprotein and its phosphorylation negatively regulates its DNA-binding activity. Mol Genet Genom 277:189–198
Ahammed GJ, Choudhary SP, Chen S, Xia X, Shi K, Zhou Y, Yu J (2013) Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J Exp Bot 64:199–213. https://doi.org/10.1093/jxb/ers323
Ahuja I, de Vos RCH, Bones AM, Hall RD (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15:664–674. https://doi.org/10.1016/j.tplants.2010.08.002
Akram NA, Iqbal M, Muhammad A, Ashraf M, Al-Qurainy F, Shafiq S (2017) Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 255:163–174. https://doi.org/10.1007/s00709-017-1140-x
Allakhverdiev SI et al (2003) Glycinebetaine protects the D1/D2/Cytb559 complex of photosystem II against photo-induced and heat-induced inactivation. J Plant Physiol 160:41–49. https://doi.org/10.1078/0176-1617-00845
Alzahrani Y, Kuşvuran A, Alharby HF, Kuşvuran S, Rady MM (2018) The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol Environ Saf 154:187–196. https://doi.org/10.1016/j.ecoenv.2018.02.057
Anjum SA, Tanveer M, Hussain S, Ashraf U, Khan I, Wangggggg L (2016) Alteration in growth, leaf gas exchange, and photosynthetic pigments of maize plants under combined cadmium and arsenic stress. Water Air Soil Pollut. https://doi.org/10.1007/s11270-016-3187-2
Aprile A et al (2013) Different stress responsive strategies to drought and heat in two durum wheat cultivars with contrasting water use efficiency. BMC Genom 14:821. https://doi.org/10.1186/1471-2164-14-821
Arbona V, Hossain Z, López-Climent MF, Pérez-Clemente RM, Gómez-Cadenas A (2008) Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol Plant 132:452–466. https://doi.org/10.1111/j.1399-3054.2007.01029.x
Aref IM, Khan PR, Khan S, El-Atta H, Ahmed AI, Iqbal M (2016) Modulation of antioxidant enzymes in Juniperus procera needles in relation to habitat environment and dieback incidence. Trees 30:1669–1681. https://doi.org/10.1007/s00468-016-1399-0
Arend M, Brem A, Kuster TM, Günthardt-Goerg MS (2013) Seasonal photosynthetic responses of European oaks to drought and elevated daytime temperature. Plant Biol 15:169–176. https://doi.org/10.1111/j.1438-8677.2012.00625.x
Ashoub A, Baeumlisberger M, Neupaertl M, Karas M, Brüggemann W (2015) Characterization of common and distinctive adjustments of wild barley leaf proteome under drought acclimation, heat stress and their combination. Plant Mol Biol 87:459–471. https://doi.org/10.1007/s11103-015-0291-4
Asrar H, Hussain T, Hadi SMS, Gul B, Nielsen BL, Khan MA (2017) Salinity induced changes in light harvesting and carbon assimilating complexes of Desmostachya bipinnata (L.) Staph. Environ Exp Bot 135:86–95
Awasthi R, Kaushal N, Vadez V, Turner NC, Berger J, Siddique KHM, Nayyar H (2014) Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct Plant Biol 41:1148. https://doi.org/10.1071/fp13340
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med Cell Longev 2014:1–31. https://doi.org/10.1155/2014/360438
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413. https://doi.org/10.1071/bi9620413
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/bf00018060
Batra NG, Sharma V, Kumari N (2014) Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiate. J Plant Interact 9:712–721
Batth R, Singh K, Kumari S, Mustafiz A (2017) Transcript profiling reveals the presence of abiotic stress and developmental stage specific ascorbate oxidase genes in plants. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00198
Boyer JS (1982) Plant productivity and environment. Science 218:443–448. https://doi.org/10.1126/science.218.4571.443
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Brestic M, Zivcak M (2013) PSII fluorescence techniques for measurement of drought and high temperature stress signal in crop plants: protocols and applications. Molecular stress physiology of plants. Springer, India, pp 87–131
Čajánek M, Štroch M, Lachetová I, Kalina J, Spunda V (1998) Characterization of the photosystem II inactivation of heat-stressed barley leaves as monitored by the various parameters of chlorophyll a fluorescence and delayed fluorescence. J Photochem Photobiol B 47:39–45. https://doi.org/10.1016/s1011-1344(98)00197-3
Carvalho LSC, Vidigal PC, Amancio S (2015) Oxidative stress homeostasis in grapevine (Vitis vinifera L.). Front Environ Sci. https://doi.org/10.3389/fenvs.2015.00020
Chaves MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55:2365–2384. https://doi.org/10.1093/jxb/erh269
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239. https://doi.org/10.1071/fp02076
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560. https://doi.org/10.1093/aob/mcn125
Chew YH, Halliday KJ (2011) A stress-free walk from Arabidopsis to crops. Curr Opin Biotechnol 22:281–286. https://doi.org/10.1016/j.copbio.2010.11.011
Choi HG, Moon BY, Kang NJ (2016) Correlation between strawberry (Fragaria ananassa Duch.) productivity and photosynthesis-related parameters under various growth conditions. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01607
Claussen W (2005) Proline as a measure of stress in tomato plants. Plant Sci 168:241–248. https://doi.org/10.1016/j.plantsci.2004.07.039
Cvikrová M, Gemperlová L, Martincová O, Vanková R (2013) Effect of drought and combined drought and heat stress on polyamine metabolism in proline-over-producing tobacco plants. Plant Physiol Biochem 73:7–15. https://doi.org/10.1016/j.plaphy.2013.08.005
Dąbrowski P et al (2019) Exploration of chlorophyll a fluorescence and plant gas exchange parameters as indicators of drought tolerance in perennial ryegrass. Sensors 19:2736
Dar MI, Naikoo MI, Rehman F, Naushin F, Khan FA (2016) Proline accumulation in plants: roles in stress tolerance and plant development. Springer India. https://doi.org/10.1007/978-81-322-2616-1_9
Demirevska K, Simova-Stoilova L, Fedina I, Georgieva K, Kunert K (2010) Response of oryzacystatin I transformed tobacco plants to drought heat and light stress. J Agron Crop Sci 196:90–99. https://doi.org/10.1111/j.1439-037x.2009.00396.x
Digrado A et al (2017) Long-term measurements of chlorophyll a fluorescence using the JIP-test show that combined abiotic stresses influence the photosynthetic performance of the perennial ryegrass (Lolium perenne) in a managed temperate grassland. Physiol Plant 161:355–371
Dreesen FE, De Boeck HJ, Janssens IA, Nijs I (2012) Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ Exp Bot 79:21–30. https://doi.org/10.1016/j.envexpbot.2012.01.005
Duxbury AC, Yentsch CS (1956) Plankton pigment nomographs. J Air Pollut Contr Assoc 16:145–150
Faseela P, Puthur JT (2018) The imprints of the high light and UV-B stresses in Oryza sativa L. ‘Kanchana’ seedlings are differentially modulated. J Photochem Photobiol B Biol 178:551–559
Feng B, Liu P, Li G, Dong ST, Wang FH, Kong LA, Zhang JW (2014) Effect of heat stress on the photosynthetic characteristics in flag leaves at the grain-filling stage of different heat-resistant winter wheat varieties. J Agron Crop Sci 200:143–155
Foyer C (1989) Responses of photosynthesis and the xanthophyll and ascorbate-glutathione cycles to changes in irradiance, photoinhibition and recovery. Plant Physiol Biochem 27:751–760
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25. https://doi.org/10.1007/bf00386001
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18. https://doi.org/10.1104/pp.110.167569
Gao S, Yuan L, Zhai H, Liu C, He S, Liu Q (2011) Transgenic sweetpotato plants expressing an LOS5 gene are tolerant to salt stress. Plant Cell Tissue Organ Cult (PCTOC) 107:205–213. https://doi.org/10.1007/s11240-011-9971-1
García-Gómez C, Obrador A, González D, Babín M, Fernández MD (2017) Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci Total Environ 589:11–24. https://doi.org/10.1016/j.scitotenv.2017.02.153
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Grigorova B, Vaseva II, Demirevska K, Feller U (2011) Expression of selected heat shock proteins after individually applied and combined drought and heat stress. Acta Physiol Plant 33:2041–2049. https://doi.org/10.1007/s11738-011-0733-9
Guo Z, Ou W, Lu S, Zhong Q (2006) Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol Biochem 44:828–836. https://doi.org/10.1016/j.plaphy.2006.10.024
Hall AE (2010) Breeding for Heat Tolerance. Wiley. doi: 10.1002/9780470650011.ch5
Harb A, Awad D, Samarah N (2015) Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. J Plant Interact 10:109–116. https://doi.org/10.1080/17429145.2015.1033023
Havaux M (1993) Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant Cell Environ 16:461–467. https://doi.org/10.1111/j.1365-3040.1993.tb00893.x
Hayat S, Hasan SA, Yusuf M, Hayat Q, Ahmad A (2010) Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiate. Environ Exp Bot 69:105–112. https://doi.org/10.1016/j.envexpbot.2010.03.004
Hazman M, Hause B, Eiche E, Nick P, Riemann M (2015) Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J Exp Bot 66:3339–3352. https://doi.org/10.1093/jxb/erv142
Hirayama T, Shinozaki K (2010) Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J 61:1041–1052. https://doi.org/10.1111/j.1365-313x.2010.04124.x
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334. https://doi.org/10.1139/b79-163
Hniličková H, Hnilička F, Martinkova J, Kraus K (2017) Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant Soil Environ 63:362–367
Hossain Z, López-Climent MF, Arbona V, Pérez-Clemente RM, Gómez-Cadenas A (2009) Modulation of the antioxidant system in citrus under waterlogging and subsequent drainage. J Plant Physiol 166:1391–1404. https://doi.org/10.1016/j.jplph.2009.02.012
Hundertmark M, Hincha DK (2008) LEA (Late Embryogenesis Abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom 9:118. https://doi.org/10.1186/1471-2164-9-118
Jiang Y, Huang B (2001) Physiological responses to heat stress alone or in combination with drought: a comparison between tall fescue and perennial ryegrass. HortScience 36:682–686. https://doi.org/10.21273/hortsci.36.4.682
Johnson SM, Lim F-L, Finkler A, Fromm H, Slabas AR, Knight MR (2014) Transcriptomic analysis of Sorghum bicolor responding to combined heat and drought stress. BMC Genom 15:456. https://doi.org/10.1186/1471-2164-15-456
Joshi P, Swami A (2009) Air pollution induced changes in the photosynthetic pigments of selected plant species. J Environ Biol 30:295–298
Kalaji HM et al (2018) Prompt chlorophyll fluorescence as a tool for crop phenotyping: an example of barley landraces exposed to various abiotic stress factors. Photosynthetica 56:953–961
Kaur G, Asthir B (2015) Proline: a key player in plant abiotic stress tolerance. Biol Plant 59:609–619. https://doi.org/10.1007/s10535-015-0549-3
Khatri K, Rathore MS (2019) Photosystem photochemistry, prompt and delayed fluorescence, photosynthetic responses and electron flow in tobacco under drought and salt stress. Photosynthetica 57:61–74
Koussevitzky S et al (2008) Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J Biol Chem 283:34197–34203. https://doi.org/10.1074/jbc.m806337200
Krasteva V et al (2013) Drought induced damages of photosynthesis in bean and plantain plants analyzed in vivo by chlorophyll a fluorescence Bulg. J Plant Physiol 19:39–44
Li C-X, Feng S-L, Shao Y, Jiang L-N, Lu X-Y, Hou X-L (2007) Effects of arsenic on seed germination and physiological activities of wheat seedlings. J Environ Sci 19:725–732. https://doi.org/10.1016/s1001-0742(07)60121-1
Li X et al (2014) Comparative physiological and proteomic analyses of poplar (Populus yunnanensis) plantlets exposed to high temperature and drought. PLoS ONE 9:e107605. https://doi.org/10.1371/journal.pone.0107605
Li M et al (2016) Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00615
Lipiec J, Doussan C, Nosalewicz A, Kondracka K (2013) Effect of drought and heat stresses on plant growth and yield: a review. Int Agrophys 27:463–477. https://doi.org/10.2478/intag-2013-0017
Liu S, Chen C, Chen G, Cao B, Chen Q, Lei J (2012) RNA-sequencing tag profiling of the placenta and pericarp of pungent pepper provides robust candidates contributing to capsaicinoid biosynthesis. Plant Cell Tissue Organ Cult (PCTOC) 110:111–121. https://doi.org/10.1007/s11240-012-0135-8
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Maclachlan S, Zalik S (1963) Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can J Bot 41:1053–1062
Martinazzo EG, Ramm A, Bacarin MA (2012) The chlorophyll a fluorescence as an indicator of the temperature stress in the leaves of Prunus persica. Braz J Plant Physiol 24:237–246
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19. https://doi.org/10.1016/j.tplants.2005.11.002
Moreno-Galván AE et al (2020) Proline accumulation and glutathione reductase activity induced by drought-tolerant rhizobacteria as potential mechanisms to alleviate drought stress in Guinea grass. Appl Soil Ecol 147:103367
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64:3983–3998
Murshed R, Lopez-Lauri F, Keller C, Monnet F, Sallanon H (2008) Acclimation to drought stress enhances oxidative stress tolerance in Solanum lycopersicum L. fruits. Plant Stress 2:145–151
Nakashima K, Shinwari ZK, Sakuma Y, Seki M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K (2000) Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins involved in dehydration-and high-salinity-responsive gene expression. Plant Mol Biol 42:657–665
Nath M, Bhatt D, Prasad R, Gill SS, Anjum NA, Tuteja N (2016) Reactive oxygen species generation-scavenging and signaling during plant-arbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01574
Nurdiani D, Widyajayantie D, Nugroho S (2018) OsSCE1 encoding SUMO E2-conjugating enzyme involves in drought stress response of Oryza sativa. Rice Sci 25:73–81. https://doi.org/10.1016/j.rsci.2017.11.002
Ort DR, Baker NR (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5:193–198. https://doi.org/10.1016/s1369-5266(02)00259-5
Pandey P, Singh J, Achary VMM, Reddy MK (2015) Redox homeostasis via gene families of ascorbate-glutathione pathway. Front Environ Sci. https://doi.org/10.3389/fenvs.2015.00025
Petrov P, Petrova A, Dimitrov I, Tashev T, Olsovska K, Brestic M, Misheva S (2018) Relationships between leaf morpho-anatomy, water status and cell membrane stability in leaves of wheat seedlings subjected to severe soil drought. J Agron Crop Sci 204:219–227
Prasad PVV, Staggenborg SA, Ristic Z, Ahuja LR, Reddy VR, Saseendran SA, Yu Q (2008) Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. Am Soc Agron Crop Sci. https://doi.org/10.2134/advagricsystmodel1.c11
Prasch CM, Sonnewald U (2013) Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol 162:1849–1866. https://doi.org/10.1104/pp.113.221044
Raja V, Majeed U, Kang H, Andrabi KI, John R (2017) Abiotic stress: interplay between ROS, hormones and MAPKs. Environ Exp Bot 137:142–157. https://doi.org/10.1016/j.envexpbot.2017.02.010
Rampino P et al (2012) Novel durum wheat genes up-regulated in response to a combination of heat and drought stress. Plant Physiol Biochem 56:72–78
Rivero RM, Mestre TC, Mittler R, Rubio F, Garcia-Sanchez F, Martinez V (2014) The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ 37:1059–1073
Rizhsky L (2002) The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130:1143–1151. https://doi.org/10.1104/pp.006858
Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide the response of arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1683–1696. https://doi.org/10.1104/pp.103.033431
Rollins JA, Habte E, Templer SE, Colby T, Schmidt J, von Korff M (2013) Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J Exp Bot 64:3201–3212. https://doi.org/10.1093/jxb/ert158
Sainz M, Díaz P, Monza J, Borsani O (2010) Heat stress results in loss of chloroplast Cu/Zn superoxide dismutase and increased damage to Photosystem II in combined drought-heat stressed Lotus japonicus. Physiol Plant 140:46–56. https://doi.org/10.1111/j.1399-3054.2010.01383.x
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290:998–1009. https://doi.org/10.1006/bbrc.2001.6299
Salvucci ME, Crafts-Brandner SJ (2004a) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120:179–186. https://doi.org/10.1111/j.0031-9317.2004.0173.x
Salvucci ME, Crafts-Brandner SJ (2004b) Mechanism for deactivation of Rubisco under moderate heat stress. Physiol Plant 122:513–519. https://doi.org/10.1111/j.1399-3054.2004.00419.x
Salvucci ME, Crafts-Brandner SJ (2004c) Relationship between the Heat Tolerance of Photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol 134:1460–1470. https://doi.org/10.1104/pp.103.038323
Seki M, Umezawa T, Urano K, Shinozaki K (2007) Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol 10:296–302. https://doi.org/10.1016/j.pbi.2007.04.014
Sharma AP, Tripathi BD (2008) Biochemical responses in tree foliage exposed to coal-fired power plant emission in seasonally dry tropical environment. Environ Monit Assess 158:197–212. https://doi.org/10.1007/s10661-008-0573-2
Sharma DK, Andersen SB, Ottosen C-O, Rosenqvist E (2012) Phenotyping of wheat cultivars for heat tolerance using chlorophyll a fluorescence. Funct Plant Biol 39:936. https://doi.org/10.1071/fp12100
Sharma DK, Andersen SB, Ottosen C-O, Rosenqvist E (2015) Wheat cultivars selected for high Fv/Fmunder heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol Plant 153:284–298. https://doi.org/10.1111/ppl.12245
Siddiqui MH, Al-Khaishany MY, Al-Qutami MA, Al-Whaibi MH, Grover A, Ali HM, Al-Wahibi MS (2015) Morphological and physiological characterization of different genotypes of faba bean under heat stress. Saudi J Biol Sci 22:656–663. https://doi.org/10.1016/j.sjbs.2015.06.002
Šprtová M, Nedbal L, Marek MV (2000) Effect of enhanced UVB radiation on chlorophyll a fluorescence parameters in Norway spruce needles. J Plant Physiol 156:234–241
Stirbet A, Lazár D, Kromdijk J (2018) Chlorophyll a fluorescence induction: can just a one-second measurement be used to quantify abiotic stress responses. Photosynthetica 56:86–104
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014) Abiotic and biotic stress combinations. New Phytol 203:32–43. https://doi.org/10.1111/nph.12797
Teixeira FK, Menezes-Benavente L, Galvão VC, Margis R, Margis-Pinheiro M (2006) Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular compartments. Planta 224:300–314. https://doi.org/10.1007/s00425-005-0214-8
Upreti K, Murti G, Bhatt R (2000) Response of pea cultivars to water stress: changes in morphophysiological characters, endogenous hormones and yield. Veg Sci 27:57–61
Vadez V et al (2011) Adaptation of grain legumes to climate change: a review. Agron Sustain Dev 32:31–44. https://doi.org/10.1007/s13593-011-0020-6
Verma A, Singh SN (2006) Biochemical and ultrastructural changes in plant foliage exposed to auto-pollution. Environ Monit Assess 120:585–602. https://doi.org/10.1007/s10661-005-9105-5
Way DA, Oren R (2010) Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30:669–688. https://doi.org/10.1093/treephys/tpq015
Yang X et al (2006) Genetic engineering of the biosynthesis of glycinebetaine enhances thermotolerance of photosystem II in tobacco plants. Planta 225:719–733. https://doi.org/10.1007/s00425-006-0380-3
Yuan L-Y, Du J, Yuan Y-H, Shu S, Sun J, Guo S-R (2013) Effects of 24-epibrassinolide on ascorbate–glutathione cycle and polyamine levels in cucumber roots under Ca(NO3)2 stress. Acta Physiol Plant 35:253–262. https://doi.org/10.1007/s11738-012-1071-2
Zandalinas SI, Mittler R, Balfagón D, Arbona V, Gómez-Cadenas A (2017) Plant adaptations to the combination of drought and high temperatures. Physiol Plant 162:2–12
Zhou R, Yu X, Kjær KH, Rosenqvist E, Ottosen C-O, Wu Z (2015) Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Environ Exp Bot 118:1–11. https://doi.org/10.1016/j.envexpbot.2015.05.006
Zhou R et al (2017) Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. https://doi.org/10.1186/s12870-017-0974-x
Zivcak M et al (2013) Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth Res 117(1–3):529–546
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2019/116), King Saud University, Riyadh, Saudi Arabia.
Funding
Researchers Supporting Project Number (RSP-2019/116), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
VR, SUQ, and PA conceived the experimental design and VR, SUQ performed the experiments. MNA performed the statistical analysis. VR and SUQ wrote the first draft of the manuscript. MNA and PA revised the manuscript to present form. All authors read and approved the same for publication.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that no conflict of interest exists.
Informed consent
All authors consent to participate in this manuscript.
Rights and permissions
About this article
Cite this article
Raja, V., Qadir, S.U., Alyemeni, M.N. et al. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 10, 208 (2020). https://doi.org/10.1007/s13205-020-02206-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02206-4