Abstract
Key message
Oxidative stress and the antioxidant enzymes’ activity are higher in damaged than in healthy Juniperus procera trees, in summer than in winter, and in dry than in wet condition.
Abstract
Many of the small stands of Juniperus procera in Saudi Arabia, confined mainly to Aseer Mountains in the southern part of the country, are suffering from branch dieback. As a part of the project on the structural and functional responses of healthy and dieback-affected trees to local environmental conditions of Al-Ghalab, Al-Yazeed, and Saodah locations, this study quantifies the oxidative stress generated and the consequent modulation of proline accumulation and antioxidant enzymes’ activity, as determined by chemical analysis of needle tissues from samples collected in summer and winter seasons. The level of TBARS, which indicated the extent of oxidative stress, was minimum (10.1 nM g−1 f w) at Al-Ghalab and maximum (28.1 nM g−1 f w) at Al-Yazeed, being relatively higher in summer than in winter. Healthy trees had a lower level of TBARS than those suffering from dieback. Proline content showed 147–54 µg g−1 in healthy trees and 460–99 µg g−1 f w in affected ones. Variation in the activity of superoxide dismutase, ascorbate peroxidase, glutathione reductase, and catalase was around 0.7–3.6, 0.01–0.09, 0.02–0.08, and 0.6–3.0 U mg−1 min−1, respectively, in healthy trees, whereas 2.3–6.1, 0.04–0.3, 0.04–0.3, and 2–5.8 U mg−1 min−1, respectively, in the dieback-affected trees of the different locations. Thus, the oxidative stress and the enzymatic stimulation were higher in damaged than in healthy trees and in summer than in winter season. Water-harvesting efforts at the collection sites showed ameliorative effects. Our observations suggest that J. procera tree can be made more tolerant toward stressful condition, and even the risk of dieback can be avoided or minimized by improving soil–water availability through adequate water-harvesting strategies in the drought-affected areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The green cover of the Kingdom of Saudi Arabia (KSA) includes a few tree stands extending over an area of 27,000 km2, mostly in the southwestern region spread over the Sarawat Mountains range. At heights of more than 1800 m, there are stands of Juniperus phoenicea L. (in Hijaz Mountains of the north) and Juniperus procera Hochst ex Endl. (in Asir Mountains of the south), apart from some mixed populations around Taif in the central region (NCWCD and JICA 2006). Juniper forests are likely to emerge as important hill resorts and biodiversity centers of the kingdom in the coming decades. Tree density of these forests ranges from 20 to 150 trees ha−1 depending mainly on topographic conditions. Trees at sloping sites are only a few meters tall, whereas those in plains grow up to 10–15 m (Al-Ghamdi and Jais 2012). The high plateau of Asir Mountains receives 300-to-500-mm rainfall and provides a suitable environment for vegetation. Altitudes between 2000 and 3000 m are often characterized with J. procera communities (NCWCD and JICA 2006; El-Atta and Aref 2010).
These Juniperus stands not only have a narrow genetic diversity, small population size, and low population density, but also face environmental stresses, anthropogenic pressures and, more importantly, incidence of dieback of top branches often leading to death of the tree, despite the fact that junipers are normally drought resistant (Allen et al. 2010). Since they decrease their water consumption during drought periods by withering their branches to reduce the bulk of canopy and protect the main stem axis, pruning has been suggested as a measure to prevent dieback (NCWCD and JICA 2006). Juniper stands located near farmlands, where lower branches of trees are regularly pruned, normally do not experience dieback. While the sudden collective die-off could be an outcome of drought stress caused by global warming, the gradual dieback of branches is seemingly a physiological ailment. In Saudi Arabia, populations of J. procera in Asir Mountains are more severely affected by the dieback incidence than those of J. phoenicea in Hijaz Mountains (NCWCD and JICA 2006).
Atmospheric oxygen, paramagnetic in its ground state, is very unlikely to participate in reactions with organic molecules due to its biradical nature. However, some oxygen species, collectively termed as reactive oxygen species (ROS), are toxic and ready to take part in unlimited chemical oxidation reactions (Das and Roychoudhury 2014). ROS is produced in both stressed and unstressed living cells. Under harsh environmental conditions, they often create ‘oxidative stress’, i.e., an imbalance between production and elimination of ROS, which leads to physiological disturbance in cellular compartments. Each of the ROS has some specific properties with reference to its potential to diffuse the membranes and react at distant sites, cross the membranes as a neutral molecule, and propagate lipid oxidation by extracting protons from polyunsaturated fatty acids (Gill and Tuteja 2010). The various ROS can interact among themselves, forming a third group of ROS, more toxic than the primordial ones. They have diverse properties and may be arranged in their order of reactivity as OH· > O ·−2 > H2O2 (Sweetlove and Moller 2009).
In normal conditions, ROS is produced largely in chloroplasts, mitochondria and peroxisomes as unavoidable by-products of aerobic metabolic processes, such as photosynthesis, photorespiration, and respiration (Ashraf and Harris 2013). Chloroplasts and peroxisomes are the main producers of ROS in green plant parts under light, whereas mitochondria take over this responsibility in the dark or in non-green plant parts (Foyer and Noctor 2003). The level of ROS production in mitochondria is lower (up to 20 times less) than in chloroplasts, at least in C3 plants, but this too is enough to influence numerous cellular processes such as adaptation to stress and programmed cell death (Gill and Tuteja 2010).
Drought, salinity and excessive heat are some major abiotic stresses that can potentially disrupt metabolism and cell structure in plants, eventually ceasing the enzyme-catalyzed reactions (Impa et al. 2012; Golldack et al. 2014). The stress-induced changes often lead to a decline in green leaf area, pigment concentration, photosynthetic rate and other growth parameters, resulting ultimately in a reduced plant growth and yield (Aref et al. 2013a, b, c). Drought-caused inhibition of photosynthesis involves changes in photosynthetic electron-transport capacity, leading to increased production of ROS (Ashraf and Harris 2013). These ROS/free radicals react with lipids, proteins, pigments and nucleic acids, and cause lipid peroxidation, membrane damage and enzyme inactivation, thus affecting the viability of cells (Impa et al. 2012; Anjum et al. 2015). This triggers activation of antioxidant defense system in plants (Lee et al. 2009).
To mitigate and repair the damage caused by the ROS, plants have evolved complex antioxidant systems. Within the cell, superoxide dismutases (SODs) constitute the first line of defence against the ROS, especially in the chloroplasts. Upon exposure to stress, SOD upregulates an array of antioxidant enzymes. Ascorbate peroxidase (APX) is important for detoxification of H2O2 in the chloroplast and the cytosol. Glutathione reductase (GR) normally operates in combination with APX. Under oxidative stress, increased GR activity is required for the ascorbate–glutathione cycle (Anjum et al. 2012). Catalase (CAT) decomposes H2O2 generated at sites other than the chloroplast to water and oxygen. Despite its low affinity toward H2O2, it acts as the principal H2O2 scavenger due to high processing rate, especially in species with high photorespiratory activity under stressful condition (Sahu et al. 2010).
This study was undertaken to determine whether J. procera plants, capable to grow in some drought-affected locations in the Kingdom of Saudi Arabia, experience oxidative stress and, if so, how they manage to withstand it. To understand the phenomenon, accumulation of TBARS and proline and the modulation of some antioxidant enzymes in needle tissues were studied in samples collected from different locations varying in altitude and availability of water in the soil. This appears to be the first attempt to determine the antioxidant enzymes activity in relation to the combination of season, location, and plant health, and a maiden study of the antioxidant defence potential of Saudi Arabian woodlands exposed to a harsh environment.
Materials and methods
Collection sites
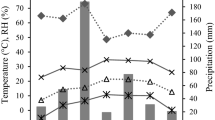
To determine the level of the expected intracellular oxidative stress and the consequent modulation of the ROS-scavenging enzymes within the cell, mature needles of J. procera Hochst ex Endl. were collected in winter (January 3, 2013) and summer (August 15, 2013) from healthy and/or damaged trees growing at Al-Yazeed (N18°05′ and E42°40′; 2225 m a.s.l.), Al-Ghalab (N18°00′ and E42°44′; 2460 m a.s.l.), and four sites (A1, A2, B1, B2) of Saodah (N18°17′ and 42 21–22′; 2900 m a.s.l.) in the Aseer region of Saudi Arabia. Environmental conditions of Al-Yazeed and Al-Ghalab are almost similar, as these places are only a few kilometer distant from each other, the latter being at a slightly higher altitude. The range and the variation pattern of temperature were comparable, but the mean temperature was lower, and the levels of rainfall and relative humidity (RH) were higher at Al-Ghalab (Figs. 1, 2). Since Saodah received a high rainfall during April–August, the RH showed an unusual pattern of being high in summer and low in winter. At Saodah, collection of plant material was made from site A1: an almost plain land area fenced with wire mesh under an FAO project and supported with water-harvesting practice; site A2: an unfenced area at the same location without any water-harvesting arrangement; site B1: a sloping land where water-harvesting was managed (1) by constructing semicircular bunds around tree trunks at the lower side of slope to check the flow of rainwater and (2) by developing terraces to improve the rainwater-harvesting; and site B2: an area with steeper slope at the same location, without fencing or water-harvesting arrangement. The soil was sandy loam or sandy clay loam in general. It was relatively dry, with a pH normal to slightly alkaline, and generally not contaminated with heavy metals. Physicochemical characters of the soil were analyzed in the Central Laboratory of the Department of Soil Science, KSU, Riyadh, following the methods described by Subbaiah and Asija (1956) for available soil N, Watanbe and Olsen (1965) for available P, Jackson (1967) for available K and organic matter, and Anonymous (2011) for other parameters, including available Cu, Fe, Mn, and Zn. Values obtained are given in Tables 1 and 2. The needles detached from four healthy and/or damaged trees at each site were immediately preserved in liquid nitrogen and taken to laboratory for further processing aimed at estimating the lipid peroxidation rate and proline content and determining the level of enzyme activity of SOD, APX, GR, and CAT.
Determination of water deficit and oxidative stress
Water saturation deficit
To assess the level of water stress experienced by the selected stands of juniper trees, small segments of branches (stem + needles) were collected from different sides of the crown around 10.00 a.m. during summer (August), when temperature was high, and in winter (January), when temperature was markedly low. The samples collected in plastic bags were taken immediately for refrigeration, and their fresh weight, turgid weight, and oven-dry weight were obtained. Full turgid weight was obtained by weighing the samples that had been brought to full turgor by enclosing them in a moist chamber for 12 h. For obtaining the dry weight, leaves were dried in an oven (MOV-112, Sanyo, Japan) at 80 °C for 48 h. Water saturation deficit (WSD) was calculated following Morgan (1984), with the help of the following equation:
Lipid peroxidation rate
Oxidative damage to lipids was estimated by determining the content of total 2-thiobarbituric acid reactive substances (TBARS) expressed as equivalents of malondialdehyde (MDA). TBARS content was estimated by the method of Cakmak and Horst (1991) with slight modification. TBARS was extracted from 0.5-g chopped fresh needles, ground in 5 ml of 0.1 % (w/v) trichloroacetic acid (TCA), at 4 °C. Following the centrifugation at 12000×g for 5 min, an aliquot of 1 ml from the supernatant was added to 4 ml of 0.5 % (w/v) TBA in 20 % (w/v) TCA. Samples were incubated at 90 °C for 30 min. Thereafter, the reaction was stopped in ice bath. Centrifugation was performed at 10,000×g for 5 min, and absorbance of the supernatant was read at 532 nm on a spectrophotometer (Model DU-640B, Beckman, USA) and corrected for non-specific turbidity by subtracting from it the absorbance read at 600 nm. The value obtained was used for calculating the TBARS content, using the following formula:
where ε is the specific extinction coefficient (=155 mM cm−1), V is the volume of crushing medium, W is the fresh weight of needles, A 600 is the absorbance at 600-nm wavelength, and A 532 is the absorbance at 532-nm wavelength.
Proline content
The proline content in the needle samples was estimated by the method of Bates et al. (1973). A 0.05 g of fresh needle material was homogenated in 3 ml of 3 % (w/v) sulphosalicylic acid, 6 N-orthophosphoric acid, and 1 % (w/v) ninhydrin solution. The homogenate was centrifuged at 10,000×g for 10 min. Half ml of the supernatant was taken in test tube to which were added 2 ml of acid ninhydrin and 2 ml of glacial acetic acid. The resultant mixture was boiled at 100 °C in a water bath. The reaction was then terminated by putting the test tubes in ice bath. Then, 6 ml of toluene was added to each tube and mixed vigorously on a cyclomixer for 10–15 s to facilitate quick diffusion/movement of chromophores from the aqueous phase to non-aqueous phase. The toluene layer (upper) was separated from the mixture and the absorbance read at 520 nm on a UV–vis spectrophotometer (Modal Lambda Bio 20, Perkin Elmer, USA), using toluene as the blank. The corresponding concentration of proline was determined against the standard curve processed in the same manner using l-proline (Sigma). The amount of proline has been expressed in μg g−1 f w.
Estimation of antioxidant enzyme activity
Superoxide dismutase (EC 1.15.1.1)
In vitro assay of superoxide dismutase (SOD) activity was done by the method of Dhindsa et al. (1981). A fresh needle material (0.05 g) was homogenized in 2.0 ml of extraction mixture containing 0.5-M phosphate buffer (pH 7.3), 1 % PVP (w/v), 1 % Triton × 100 (w/v) and 0.3-mM EDTA under cold condition (4 °C), with the mortar and pestle kept in ice during the course of homogenization. The homogenate, transferred to centrifuge tubes, was centrifuged at 10,000×g at 4 °C for 10 min.
SOD activity in the supernatant was assayed by its ability to inhibit the photochemical reduction. The assay mixture, consisting of 1.5-ml reaction buffer, 0.2 ml of methionine, 0.1-ml enzyme extract with equal amount of 1-M NaCO3, 2.25-mM NBT solution, 3-mM EDTA, riboflavin, and 1.0 ml of DDW, was taken in test tubes, which were incubated under the light of 15-W inflorescent lamp for 10 min at 25/28 °C. Blank A, containing the above substances of the reaction mixture along with the enzyme extract, was placed in the dark. Blank B, containing the above substances of reaction mixture except enzyme, was placed in light along with the sample. The reaction was terminated by switching off the light, and the tubes were covered with a black cloth. The non-irradiated reaction mixture, containing the enzyme extract, did not develop light blue color. Absorbance of samples along with blank B was read at 560 nm, using the UV–vis spectrophotometer, against the blank A. The difference of % reduction in color between blank B and the sample was then calculated. Fifty percent reduction in color was considered as one enzyme unit (EU), and the activity was expressed in EU mg−1 protein min−1.
Ascorbate peroxidase (EC 1.11.1.11)
The in vitro assay of ascorbate peroxidase (APX) activity was done by the method used by Nakano and Asada (1981). A 0.05 g of the fresh needle material was ground in 4 ml of extraction buffer containing 50-mM phosphate buffer (pH 7.2), 1 % PVP (w/v), 1 % Triton × 100 (w/v), 0.3 mM EDTA. The homogenate was centrifuged at 10,000×g for 10 min at 4 °C. The supernatant was collected and used for the assay. The activity of APX was determined by the decrease in absorbance of ascorbate at 290 nm using the UV–vis spectrophotometer. One ml of the reaction buffer contained 0.5-mM ascorbate, 0.1-mM H2O2, 0.1-mM EDTA, and enzyme extract. The reaction was allowed to run for 3 min at 25 °C. APX activity was calculated using the extinction coefficient (ε) 2.8 mM−1 cm−1 and expressed in enzyme units (EU) mg−1 protein min−1. One unit of enzyme determines the amount necessary to decompose 1 μmol of substrate consumed per minute at 25 °C.
Glutathione reductase (EC 1.6.4.2)
GR activity was determined following the method adopted by Rao (1992). A fresh needle material (0.5 g), ground in 2 cm3 of extraction buffer (0.1 M Na-phosphate, pH 7.0, 3 mM EDTA, 1 % PVP, 1 % Triton × 100), was centrifuged at 7826g for 10 min. The supernatant was immediately assayed for GR activity through glutathione-dependent oxidation of NADPH at 340 nm. One cm3 reaction mixture containing 0.2-mM NADPH, 0.5-mM GSSG, and 0.05 cm3 of enzyme extract was kept for 5 min at 25 °C. Corrections were made for any GSSG oxidation in the absence of NADPH. The activity was calculated using the coefficient of absorbance 6.2 mM−1 cm−1 and expressed in enzyme unit.
Catalase (EC 1.11.1.6)
The catalase (CAT) activity in the needle was determined by the method of Aebi (1984). Half gram of the fresh needle material was homogenized in 5 ml of extraction buffer containing 0.5-M phosphate buffer (pH 7.3), 0.3-mM H2O2, and 0.3-mM EDTA The homogenate was centrifuged at 10,000×g for 20 min at 4 °C. Catalase activity was determined by monitoring the disappearance of H2O2 by measuring a decrease in absorbance at 240 nm using the UV–vis spectrophotometer. Reaction was carried in a final volume of 2 ml of reaction mixture containing reaction buffer with 0.1-ml 3-mM EDTA, 0.1 ml of enzyme extract, and 0.1 ml of 3-mM H2O2, and allowed to run for 5 min. The activity was calculated using the extinction coefficient (Σ) 0.036 mM−1 cm−1 and expressed in EU (mg−1 protein min−1). One enzyme unit determines the amount necessary to decompose 1 μmol of H2O2 per min at 2 °C.
Statistical analysis
The data obtained on various parameters were subjected to GLM-ANOVA to work out the interactive impact of the factors involved, and the means were compared using least square mean (LSM) at significance level P < 0.0001. The same letters on mean values indicate that these means within a row or column are not significantly different from each other. SAS 9.1.3 package (revised in 2008) was used for processing the data.
Results
Environmental analysis of the locations of tree stands (Al-Ghalab, Al-Yazeed and Saodah) indicates that monthly mean temperature varied from 10 to 21 °C at Al-Ghalab, 14 to 24 °C at Al-Yazeed, and 10 to 18 °C at Saodah. Thus, Al-Yazeed was warmer than the other two locations, which had an almost comparable range of temperature (Figs. 1, 2, 3). The amount of monthly rainfall was 18–35 mm (annual 290 mm) at Al-Ghalab, 7–30 mm (with annual quantum of 220 mm) at Al-Yazeed, and 0–60 mm (annual 240 mm) at Saodah, with the maximum showers in summer months. Consequently, the monthly average of RH varied from 45 to 72 % at Al-Ghalab, 34 to 68 % at Al-Yazeed, and 20 to 60 % at Saodah (Figs. 1, 2, 3), thus Al-Ghalab being the wettest location.
The soils of the locations of study (loam to sandy clay loam) were generally deficient in organic matter (OM), the condition being relatively better at Al-Ghalab and worst at Al-Yazeed. The OM showed correlation with water-harvesting, as is evident from the sites of Saodah. Saturation percentage (SP) and electrical conductivity (EC) also exhibited an almost similar pattern. In general, OM, SP, and EC were higher at Al-Ghalab and at water-harvesting sites (A1 and B1) of Saodah, and relatively low at Al-Yazeed and the unmanaged sites (A2 and B2) of Saodah. The pH was 7 at Al-Ghalab and slightly higher at other sites (Table 1). The proportions of certain macro- and micro-nutrients were also distinctly higher at Al-Ghalab than at other sites; Saodah site B2 was especially poor in most of the nutrients (Table 2).
In general, all trees of J. procera were healthy at Al-Ghalab (Fig. 4a), and damaged due to branch dieback at Al-Yazeed (Fig. 4b). At Saodah, there were mixed populations of healthy and damaged (often partially damaged) trees at sites A1 and A2 (Fig. 4c). On the other hand, all trees were healthy at site B1, while all were damaged at site B2. Collections from healthy as well as damaged trees from all these sites exhibited a marked variation of water saturation deficit (WSD), which was the lowest (26 % in summer; 15 % in winter) in the healthy trees of Al-Ghalab and at site B1 of Saodah managed by water-harvesting through semi-circular bund (25 % in summer; 17 % in winter) or terraces (27 % in summer; 18 % in winter). The WSD was the highest in the damaged trees at Al-Yazeed during both summer (45 %) and winter (38 %), followed by the damaged trees at site B2 of Saodah (38 % in summer; 31 % in winter). At the FAO sites (A1, A2) of Saodah, WSD of the mixed population was 30–37 % in summer and 19–30 % in winter (Fig. 5).
Water saturation deficit of healthy and damaged juniper trees collected from Al-Ghalab, Al-Yazeed, and Saodah sites in summer and winter seasons. A healthy population of Al-Ghalab, B damaged population of Al-Yazeed, C healthy trees of site A1 at Saodah, D damaged trees of site A1 at Saodah, E healthy trees of site A2 at Saodah, F damaged trees of site A2 at Saodah, G healthy population of site B1 (i) at Saodah, H healthy population of site B1 (ii) at Saodah, I damaged population of site B2 at Saodah
Juniper trees suffered badly from dieback at Al-Yazeed, which was a relatively low, dry and warm location. The level of TBARS content, taken as an indicator of the level of oxidative stress in needle tissues, was the highest (up to 28.1 nM g−1 f w) at this location, being slightly greater in summer than in winter. On the other hand, juniper population was healthy at Al-Ghalab, a little higher, colder and wetter location. Hence, TBARS content of the needles was markedly low (10.1–22.5 nM g−1 f w), compared with the Al-Yazeed population. In general, the TBARS value was considerably higher in summer than in winter (Table 3).
At Saodah (the highest location under study), health status of juniper trees varied with site topography. Juniper stands located at a relatively plain land possessed both healthy as well as declining trees, the former having a lower TBARS level than the latter. The TBARS level was invariably higher in summer than in winter, although the oxidative stress in healthy as well as declining trees was higher in both the fenced and unfenced populations of site A, mostly with a non-significant difference. At site B1, where water-harvesting was arranged by making (1) semi-circular bunds or (2) successive terraces, all the trees were healthy. By contrast, all trees were on way to decline through dieback at site B2, which was sloping and without any water-harvesting arrangement (Table 3). The level of TBARS was comparable at sites having semi-circular bunds and/or terraces (site B1), but markedly high in the unmanaged natural population (B2). It was always greater in summer collections than in winter collections (Table 3).
Proline accumulation was high during summer and low during winter in the healthy population of Al-Ghalab. On the other hand, damaged juniper population of Al-Yazeed contained much higher amounts of proline during both winter and summer seasons (Table 3). In juniper stands at different locations of Saodah, proline accumulation varied from about 460.1 to 735.7 µg g−1 f w in damaged trees and from 330 to 539.6 µg g−1 f w in healthy trees of the fenced (A1) population. In the unfenced (A2) population of this site, proline content was relatively higher in damaged trees but much lower in healthy trees. At site B1, proline content of needle tissues rose up to 374.5 and 506.5 µg g−1 f w at sites with semi-circular bunds and terraces, respectively. In the damaged population of site B2, it was relatively higher. In addition, it was always higher in summer samples than in winter samples (Table 3).
To combat oxidative stress generated in plant tissues, modulation of antioxidant enzymes started, as evident from their enhanced activity. For instance, the activity of superoxide dismutase (SOD) increased to detoxify the superoxide radicals generated seemingly by drought stress. The level of SOD activity was relatively low in collections from Al-Ghalab (healthy trees), being greater in summer than in winter. On the contrary, maximally enhanced activity was recorded in plants growing at Al-Yazeed (badly damaged population). At Saodah, with both healthy and damaged trees present in the fenced (A1) as well as unfenced (A2) tree stands, SOD activity was invariably greater in damaged trees than in healthy trees. Similar reaction was shown by the healthy population of sites B and C, where water-harvesting was done with semi-circular bunds or terraces, and the damaged population of the unmanaged sloping site D. Summer collections showed relatively higher activity than winter collections at the sites studied (Table 3).
Activity of ascorbate peroxidase (APX) also exhibited similar pattern. The healthy population of Al-Ghalab had a relatively low enzyme activity, which increased slightly during the summer months. However, trees at the Al-Yazeed, which were badly damaged, had the maximum enzyme activity (Table 3). In the fenced and unfenced populations of Saodah (site A), both having a mixture of healthy and damaged juniper trees, APX activity was higher in damaged trees than in healthy trees. At site B1, with water-harvesting arrangements in place, all trees were healthy, whereas all trees were damaged at site B2, which has no provision of water-harvesting. Enzyme activity was higher in damaged trees and summer collection than in healthy trees and winter collection at each of the study sites (Table 3).
Similarly, GR activity was considerably low (0.03 EU mg−1 protein min−1) in the healthy population of Al-Ghalab (Table 3), and high in the damaged tree stands at Al-Yazeed, being relatively greater (0.28 EU) in summer collection than in winter collection (0.11 EU). At the different sites of Saodah, the enzyme activity exhibited similar trend of variation with reference to healthy and damaged trees both in the fenced and unfenced populations, the values being relatively higher in summer than in winter (Table 3).
Likewise, catalase activity was the maximum (3.2–5.8 EU mg−1 protein min−1) in the declined tree population of Al-Yazeed, while it varied from 2.0 to 2.7 EU in the healthy population of Al-Ghalab (Table 3). At Saodah, the level of catalase activity differed in the damaged and healthy trees at both managed (A1) and unmanaged (A2) sites. At site B1, the activity was around 2.9EU and 2.7–2.8 EU in the healthy stands benefiting from water-harvesting done by (1) semi-circular bunds and (2) terraces, respectively. The activity in the unmanaged population at site B2 was a bit higher; about 3 EU during winter and 3.4 EU during summer (Table 3).
If the natural, healthy population of juniper trees at Al-Ghalab is taken as control, and the performance of other populations (at Al-Yazeed and Saodah) is compared with it, the increase in the TBARS and proline contents and in the activity of antioxidant enzymes comes out to be greater in terms of percent variation in the damaged tree stand of Al-Yazeed than in the similar stand at Saodah (site B2) both in the summer and winter seasons (Tables 4, 5). Furthermore, a comparison of healthy populations of site B1 at Saodah with the control population at Al-Ghalab reveals that TBARS and proline contents were normally lower in Saodah populations, with the exception for proline content (in summer collection) and TBARS content (in winter collection) in the population on terraces of site B1 (Table 4, 5). The activity of enzymes (except for SOD) was higher in Saodah populations of site B1 during summer (Table 4). However, during winters, it was higher in general in the population supported by semi-circular bunds, but only for SOD and CAT in the population grown on terraces, compared with the control (Table 5).
Discussion
Environmental stress and ROS in plants
Plants are often exposed to many harsh and unavoidable environmental stresses, such as drought, salinity, UV radiation, extreme temperatures (heat shock and chilling), heavy metals, air pollutants, mechanical stress and nutrient deprivation, which affect the development, morphology, physiology, biochemistry and molecular integrity of plants (Iqbal et al. 2005; Mantry et al. 2012). Under normal physiological conditions, reactive oxygen species (ROS) generally remain at stable basal levels in the cells, but the levels of ROS and ROS-induced damage increase under environmental stress (Das and Roychoudhury 2014).
Plant response to environmental stress is dependent on several such factors as plant species, organ and tissue type, intracellular compartments, stage of plant development, and the duration and intensity of the stress (Iqbal et al. 2005, 2010; Aref et al. 2013a). The stress-caused events generally include (1) enhanced ROS generation, (2) a higher expression of genes with antioxidant functions, (3) the consequent increase in concentration of antioxidants, and (4) an improved level of plant tolerance against stress. The various sites of oxygen activation in the plant cell are precisely controlled and tightly coupled to prevent the release of intermediate products. Under stressful situations, this control or coupling is likely to break down, and the consequent dysfunction may result in leakage of activated oxygen. The ROS or free radicals produced under stress react with lipids, proteins, pigments, and nucleic acids and cause lipid peroxidation, membrane damage, and inactivation of enzymes, thus affecting the viability of cells (Gill and Tuteja 2010; Anjum et al. 2015).
The sandy loam or sandy clay loam soils of our study sites seem to have a low water-holding capacity, as is evident from their saturation percentage also. The rainfall and RH level were relatively low at sites with damaged trees. The WSD of trees (lowest at Al-Ghalab and highest at Al-Yazeed) showed a negative correlation with tree health. The nutrient status of soils of the different collection sites shows correlation with tree health, suggesting that the level of soil nutrients also has a role in determining the overall performance of juniper stands and that an improved nutrient supply may help in reducing the negative impact of oxidative stress. Reductions in leaf water potential and relative water content, as caused by water stress, display association with stomatal conductance and photosynthetic activity in plant foliage, as observed in Picea abies (Bloedner et al. 2007). In a recent study by Aref et al. (2013b), J. procera growing under water stress was able to photosynthesize at a water potential as low as −5.77 MPa, suggesting that the photosynthetic apparatus of this species remains relatively stable under limited water supply. Our results indicate that a lethal level of stress damages the plant tissue, as observed for the juniper population at Al-Yazeed, whereas a moderate level enhances acclimation, as is evident from stands at Al-Ghalab. The ROS possibly act as abiotic elicitors of antioxidant defense up to a threshold limit, which differs with the degree of sensitivity of plants. Upregulation of antioxidant enzymes under stressful conditions helps in combating the oxidative stress, and often correlates to the type and magnitude of the stress (Gill and Tuteja 2010). Water stress enhances ROS production, inhibits photosynthesis, and alters carbon partitioning (Aref et al. 2013c, 2014). Several ROS-dependent changes such as lipid peroxidation and antioxidative response, often correlate with the degree of the stress, as we observed in this study. Furthermore, plants living in exposed full-sun habitat receiving high irradiance, as in the case of juniper stands studied by us, may be predisposed to suffer photoinhibition (a slow and reversible decline in photosynthetic efficiency due to light absorbance in excess to the requirement for carbon assimilation) and, hence, exhibit poor growth. However, pioneer species may show increased tolerance to high irradiance and enhanced activity of some antioxidant enzymes such as SOD (Favaretto et al. 2011).
Indicators of oxidative stress
Lipid peroxidation, due to increased production of toxic radicals, may cause membrane destabilization and has been extensively used as a marker of oxidative stress (Anjum et al. 2015; Iqbal et al. 2015). TBARS, the cytotoxic product of lipid peroxidation, are normally considered as the major TBA-reacting compounds that indicate the magnitude of the oxidative stress (Qureshi et al. 2007). Increase in lipid peroxidation rate is regarded to be a general response to environmental stress (Jabeen et al. 2009). In this study, increased concentration of TBARS in trees growing in water-deficient areas indicates that water stress is a causal factor for lipid peroxidation.
Increased proline accumulation under water-deficit conditions suggests that water stress also causes situations comparable with those caused by heavy metals, salinity or other similar soil factors (Arshi et al. 2010; Gupta and Huang 2014). Proline, which acts in plant cells as an osmoregulator, a soluble nitrogen sink, a signal of senescence and an indicator of plant resistance to stress, may affect the solubility of various proteins, thus protecting them against denaturation under stressful conditions (Hayat et al. 2012). An increase in proline content, as observed in this study, may be linked to stimulated oxidation or impaired protein synthesis (Hayat et al. 2012; Anjum et al. 2014). Proline is capable to coordinate with other components of antioxidant defence system, such as glutathione, to make the plant withstand a joint attack of abiotic stressors, such as salinity and heavy metals (Anjum et al. 2014). Excessive metabolic disruption may result in death of the plant. It seems that proline accumulates under low water potential up to a certain threshold limit, beyond which the excessive ROS formation might limit its accumulation, as also proposed earlier by Qureshi et al. (2005).
Defense strategies
Upregulation of SODs is essential for combating the oxidative stress and catalyzing the dismutation of O2 − to O2 and H2O2. The increase in SOD activity under stress, as noted in this study, may be due to a de novo synthesis of enzymatic protein (Miller et al. 2010). Juniperus procera has shown enough capacity to upregulate SOD activities. However, very high SOD activities may be harmful for plants due to high H2O2 production, which inhibits other enzymes such as APX (Anjum et al. 2008). Our observations on SOD activity are in line with some earlier findings on effects of water deficit (Anjum et al. 2008) and salinity (Qureshi et al. 2007, 2013; Arshi et al. 2012) on the antioxidant defence system in plants.
APX activity increases in response to enhanced ROS production, but excessive H2O2 might inhibit APX activity under high oxidative stress. In our study, APX activity was especially high in the declining juniper trees, thus being suggestive of its role in detoxification of H2O2. Likewise, the observed increase in GR activity in declining trees could be because (1) the ascorbate–glutathione cycle might be operating at a high rate to detoxify the ROS in these plants, or (2) the reduced glutathione pool might be maintained at high levels, so that it does not become limiting for the synthesis of phytochelatins, the small peptides involved in the sequestration of metal ions in the vacuoles (Anjum et al. 2012; Mendrey 2013).
Our study indicates a role of CAT in defence against oxidative stress through upregulation of its activity in J. procera trees thriving under water stress. In a comparison of the dune- and laboratory-grown plants of Calystegia soldanella, Spano et al. (2013) have shown that despite a higher H2O2 content and lipid peroxidation in dune plants, membrane damage was not significantly different from one in lab plants due to a significantly higher GR and CAT activities in the former.
Drought stress seems to be the major cause of triggering dieback in the juniper stands studied, as it did in western Europe, leading to decline of several forests (Allen et al. 2010). It is now known that the lack of rapid increase in the level of transcripts of antioxidant enzymes is linked to the role of ROS in signal transduction, which becomes most effective if oxygen radical scavenging systems are not augmented strongly in response to oxidative stress (Fink 2011; Bhattacharjee 2012; Suzuki et al. 2012). Modulation of enzymes, such as SOD, APX, GR, and CAT, prevents plant damage by O2− and H2O2 to a great extent (Sahu et al. 2010). In juniper trees, increased levels of these enzymes provide due resistance against ROS, but in cases where stress exceeds the protective capacity of the defence system, the tree succumbs to the destructive force and falls prey to branch dieback, as we found at Al-Yazeed and a few sites of Saodah, mainly at site B2.
On the whole, oxidative stress, proline accumulation, and antioxidant enzymatic activity were higher in the damaged than in healthy trees and in summer than in winter season. Mutual comparison of the damaged tree populations of Al-Yazeed and Saodah (site B2) revealed that the levels of oxidative stress and its consequent countermeasures were higher in the Al-Yazeed population than in the Saodah population of J. procera. Similar comparison between healthy populations of Al-Ghalab and Saodah (site B1) indicated that water-harvesting arrangement could improve the health of trees, making them even better than in the natural healthy population. The level of oxidative stress was lowest in trees of site B1 at Saodah, where water-harvesting was attempted by developing semi-circular bunds around tree trunks at the lower side of the slope. Thus, J. procera has enough inherent capacity of withstanding arid environment and combating the inner oxidative stress. The level of its tolerance can be enhanced by increasing the soil-water availability possibly by adopting suitable water-harvesting practices.
Author contribution statement
IMR, PRK, HEA and MI proposed, initiated and led the project. Material was collected by PRK and AIA, and laboratory work was done mainly by SK. All the authors contributed equally to analysis of scientific data and preparation of this manuscript.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- DDW:
-
Double distilled water
- EDTA:
-
Ethylene-diamine-tetra-acetic acid
- GR:
-
Gluththione reductase
- GSSG:
-
Glutathione disulfide
- ROS:
-
Reactive oxygen species
- TBARS:
-
Thiobarbituric acid reactive substances
- TCA:
-
Trichloroethanoic acid
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Al-Ghamdi AAM, Jais HM (2012) Study of Juniperus procera and arbuscular mycorhizal fungi (AMF) in Saudi Arabia. Int J Adv Biol Res 2(2):177–183
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Gonzales P, Hogg T, Rigling A, Breshears D et al (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–684
Anjum NA, Umar S, Iqbal M, Khan NA (2008) Growth characteristics and antioxidant metabolism of mungbean genotypes differing in photosynthetic capacity subjected to water deficit stress. J Plant Interact 3:127–136
Anjum NA, Ahmad I, Mohmood I, Pacheco M, Duarte AC, Pereira E, Umar S, Ahmad A, Khan NA, Iqbal M, Prasad MNV (2012) Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—a review. Environ Exp Bot 75:307–324
Anjum NA, Aref IA, Pereira E, Ahmad A, Iqbal M (2014) Glutathione and proline can coordinately make plants withstand the joint attack of osmotic and metal(loid) stresses. Front Plant Sci 5(662):4. doi:10.3389/fpls.2014.00662
Anjum NA, Sofo A, Scopa A, Roychoudhury A, Gill SS, Iqbal M, Lukatkin AS, Pereira E, Duarte AC, Ahmad I (2015) Lipids and proteins—major targets of oxidative modifications in abiotic stressed plants. Environ Sci Pollut Res 22:4099–4121. doi:10.1007/s11356-014-3917-1
Anonymous (2011) Methods manual. Soil testing in India. Ministry of Agriculture, Govt of India, New Delhi
Aref MI, Ahmed AI, Khan PR, El-Atta H, Iqbal M (2013a) Drought-induced adaptive changes in the seedling anatomy of Acacia ehrenbergiana and Acacia tortilis subsp. raddiana. Trees Struct Funct 27(4):959–971. doi:10.1007/s00468-013-0848-2
Aref IM, El-Atta H, Al-Shahrani T, Alazba A, Ahmed AI (2013b) Evaluation of the physiological and growth response of Juniperus procera Hochst. ex Endlicher to some types of microcatchments. Int J Plant Anim Environ Sci 3(1):234–241
Aref MI, El-Atta H, El-Obeid M, Ahmed AI, Khan PR, Iqbal M (2013c) Effect of water stress on relative water and chlorophyll contents of Juniperus procera Hochst. ex Endlicher in Saudi Arabia. Life Sci J 10(4):681–685
Aref MI, Khan PR, Al-Mefarrej H, Al-Shahrani T, Ahmed AI, Iqbal M (2014) Cambial periodicity and wood production in Acacia ehrenbergiana Hayne growing on dry sites of Saudi Arabia. J Environ Biol 35(2):301–310
Arshi A, Ahmad A, Aref IM, Iqbal M (2010) Effect of calcium against salinity-induced inhibition in growth, ion accumulation and proline contents in Cichorium intybus L. J Environ Biol 31:939–944
Arshi A, Ahmad A, Aref IM, Iqbal M (2012) Comparative studies on antioxidant enzyme action and ion accumulation in soybean cultivars under salinity stress. J Environ Biol 33:9–20
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51(2):163–190
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bhattacharjee S (2012) The language of reactive oxygen species signaling in plants. J Bot 2012:22. doi:10.1155/2012/985298
Bloedner C, Majcherczyk A, Kues U, Polle A (2007) Early drought-induced changes to the needle proteome of Norway spruce. Tree Physiol 27:1423–1431
Cakmak I, Horst J (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2(53):13. doi:10.3389/fenvs.2014.00053
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot 32:93–101
El-Atta H, Aref IM (2010) Effect of terracing on rainwater harvesting and growth of Juniperus procera Hochst ex Endlicher. Int J Environ Sci Technol 7(1):59–66
Favaretto VF, Martinez CA, Soriani HH, Furriel RPM (2011) Differential responses of antioxidant enzymes in pioneer and late-successional tropical tree species grown under sun and shade conditions. Environ Exp Bot 70:20–28
Fink T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194(1):7–15
Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. doi:10.1016/j.plaphy.2010.08.016
Golldack D, Li C, Mohan H, Probst N (2014) Tolerance to drought and salt stress in plants: unraveling the signalling networks. Front Plant Sci 5:art 151:10. doi:10.3389/fpls.2014.00151
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: Physiological, biochemical and molecular characterization. Int J Genom. doi:10.1155/2014/701596 (Art. ID 701596)
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments—a review. Plant Signal Behav 7:11. doi:10.4161/psb.21949
Impa SM, Nadaradjan S, Jagadish SVK (2012) Drought stress induced reactive oxygen species and antioxidants in plants. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants: metabolism, productivity and sustainability. Springer, New York, pp 131–147
Iqbal M, Bano R, Wali B (2005) Plant growth responses to air pollution. In: Chaturvedi SN, Singh KP (eds) Plant biodiversity, microbial interaction and environmental biology. Avishkar Publishers, Jaipur, pp 166–188
Iqbal M, Mahmooduzzafar, Aref IM, Khan PR (2010) Behavioural responses of leaves and vascular cambium of Prosopis cineraria (L.) Druce to different regimes of coal-smoke pollution. J Plant Interact 5(2):117–133
Iqbal M, Ahmad A, Ansari MKA, Qureshi MI, Aref MI, Khan PR, Hegazy SS, El-Atta H, Husen A, Hakeem KR (2015) Improving the phytoextraction capacity of plants to scavenge metal(loid)-contaminated sites. Environ Rev 23(1):44–65. doi:10.1139/er-2014-0043
Jabeen R, Ahmad A, Iqbal M (2009) Phytoremediation of heavy metals: physiological and molecular aspects. Bot Rev 75:339–364
Jackson ML (1967) Soil chemical analysis, 7th edn. Prentice Hall (India) Pvt Ltd, New Delhi, p 498
Lee BR, Li LS, Jung WJ, Jin YLJC, Avice JC, Ourry A, Kim TH (2009) Water deficit-induced oxidative stress and the activation of antioxidant enzymes in white clover leaves. Biol Plant 53:505–510
Mantry N, Patade V, Penna S, Ford R, Pang E (2012) Abiotic stress responses in plants: present and future. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants: metabolism, productivity and sustainability. Springer, New York, pp 1–19
Mendrey KJ (2013) Metal response of Douglas-fir: a comparison of foliar metals and phytochelatin production in trees planted in soils amended with biosolids or metal salts. MS Thesis, University of Washington, USA, p 185
Miller GAD, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Morgan JM (1984) Osmoregulation and water stress in higher plants. Annu Rev Plant Physiol 35:299–319
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbatespecific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
NCWCD, JICA (2006) The management plan for conservation of juniper woodlands. The Final Report of the joint study of National Commission of Wildlife Conservation and Development (NCWCD) and Japan International Cooperation Agency (JICA). pp 142 + 2 appendices
Qureshi MI, Israr M, Abdin MZ, Iqbal M (2005) Responses of Artemisia annua L. to lead and salt-induced oxidative stress. Environ Exp Bot 53:185–193
Qureshi MI, Abdin MZ, Qadir S, Iqbal M (2007) Lead-induced oxidative stress and metabolic alterations in Cassia angustifolia Vahl. Biol Plant 51:121–128
Qureshi MI, Abdin MZ, Ahmad J, Iqbal M (2013) Effect of long-term salinity on cellular antioxidants, compatible solute and fatty-acid profile of Sweet annie (Artemisia annua L.). Phytochemistry 95:215–223
Rao MV (1992) Cellular detoxification mechanisms to determine age dependent injury in tropical plant exposed to SO2. J Plant Physiol 140:737–740
Sahu S, Das P, Ray M, Sabat SC (2010) Osmolyte-modulated enhanced rice leaf catalase activity under salt stress. Adv Biosci Biotechnol 1:39–46
Spano C, Bruno M, Bottega S (2013) Calystegia soldanella: dune versus laboratory plants to highlight key adaptive physiological traits. Acta Physiol Plant 35(4):1329–1336
Subbaiah BV, Asija GL (1956) A rapid procedure for determination of available nitrogen in soils. Curr Sci 25:259–260
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Sweetlove LJ, Moller IM (2009) Oxidation of proteins in plants—mechanisms and consequences. Adv Bot Res 52:1–23
Watanabe FS, Olsen SR (1965) Test of an ascorbic acid method for determining P in water and NaHCO3 extract from soil. Soil Sci Soc Am Proc 29:677–678
Acknowledgments
Financial support provided by the National Plan for Sciences, Technology and Innovation, Saudi Arabia, for sponsoring this study under the research project #10-AGRI 1310-02 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Communicated by W. Bilger.
Rights and permissions
About this article
Cite this article
Aref, I.M., Khan, P.R., Khan, S. et al. Modulation of antioxidant enzymes in Juniperus procera needles in relation to habitat environment and dieback incidence. Trees 30, 1669–1681 (2016). https://doi.org/10.1007/s00468-016-1399-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-016-1399-0