Abstract
Plants, via physiological and molecular processes, respond to unsuitable environmental conditions, resulting in stress tolerance. Most previous studies have focused on plant responses to a single abiotic stress, but the effects of combined water deficit and high temperature stresses are more severe and complex than those due to a single stress. Therefore, our study aimed to explore the differences in the damage caused by combined vs. single stresses. Grapevines were subjected to water deficit, high temperature, and water deficit plus high temperature treatments. The transcript levels of heat- and drought-stress genes, activities of photosystem II (PS II) and antioxidant enzymes (superoxide dismutase, catalase, and peroxidase), and changes in abscisic acid (ABA) biosynthesis were evaluated. The activities of PS II and antioxidant enzymes were lower under the water deficit plus high temperature treatment than under the heat treatment alone. The concentration of ABA and the transcript levels of ABA biosynthesis-related genes increased under both types of stress. The enhanced thermo-tolerance observed under drought stress could be attributed to increased PS II efficiency, as well as to changes in antioxidant pathways, mediated by a common regulatory system or including a substantial cross talk between heat- and drought-stress signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, the study of abiotic stresses in plants has advanced considerably. However, most experiments focus on a single environmental stress, whereas several different stresses may occur simultaneously in nature, including high irradiance, extreme temperature, and low water availability, which may affect plant metabolism in a complex manner. For instance, drought and heat stresses often limit plant growth and productivity (Suzuki et al. 2014). Although drought and heat stresses have been widely studied (Queitsch et al. 2000; Wang et al. 2012), their combined impact on plants is poorly understood, and the effect of these stresses on plants is definitely accompanied by physiological and biochemical changes, as well as the transcript levels of resistance genes and proteins.
Plants respond and adapt to biotic or abiotic stresses via biochemical and physiological processes, thereby resulting in stress tolerance mechanisms (Bruce et al. 2007). Chlorophyll a fluorescence was reported as a method to analyze heat-induced changes in the photosystem (PS) II of plant leaves (Camejo et al. 2005; Wen and Ding 2005). Photosynthesis, especially the activity of PS II, is highly sensitive to abiotic stress (Reddy et al. 2004), as it inhibits photosynthetic electron transfer and reduces the photochemical efficiency of leaves. This results in the photosynthetic center mechanism being destroyed, because the leaves cannot effectively absorb light energy (Camejo et al. 2005). Abiotic stresses can also disrupt the equilibrium between the production and clearance of reactive oxygen species (ROS) in plants, and produce harmful substances such as malondialdehyde (MDA). However, plants can limit the ROS-induced harm to their cells by increasing the antioxidant enzyme activities (Meloni et al. 2003; Shah and Nahakpam 2012).
Several studies have suggested that plants are simultaneously or successively subjected to multiple environmental stresses, which enhance their ability to resist environmental stress, a process known as plant cross adaptation. Zhang et al. (2016) found that, in winter wheat, drought improved the resistance to drought stress, and induced heat-tolerance through abscisic acid (ABA), which triggers responses against drought stress (Grossmann et al. 1996; Cho et al. 2012). It is well known that PS II is sensitive to abiotic stresses, and ABA can protect the PS II in leaves from photoinhibition (Ivanov et al. 1995; Kurepin et al. 2015). Furthermore, 9-cis-epoxycarotenoid dioxygenase gene (NCED) and cytochrome protein 707A1 (CYP707A1) are the known ABA synthesis genes. NCED is a key enzyme involved in the cleavage of the 11, 12 double bonds of C40 carotenoids during in ABA biosynthesis (Qin and Zeevaart 1999), while CYP707A1 encodes for ABA 8′-hydroxylase (Wang et al. 2015).
It has been observed that, under field conditions, grapevines are able to respond effectively to different biotic or abiotic stresses in water-depleted soils. This led us to hypothesize that drought priming could ameliorate the physiological and biochemical reactions occurring due to heat stress. Therefore, we conducted an experiment under controlled laboratory conditions to investigate the effects of drought on grapevines under heat stress. Biochemical and physiological processes were analyzed to elucidate the underlying mechanisms of drought-induced heat stress responses.
Materials and methods
Plant material and growth conditions
This study was conducted in 2016 at the experimental station of Shanghai Academy of Agricultural Science (30°89 N, 121°39 E), Shanghai, China. Grapevines of the widely grown local cultivars, Shenfeng and Shenhua (Vitis vinifera × V. labrusca), were grown in plastic pots of 40 cm height × 15 cm diameter. A mixture of peat moss and perlite (v:v = 1:1) filled each pot. Grapevines (15–20 leaves) were allowed to acclimatize for approximately 2 weeks at 25 °C, 500 μmol m−2 s−1 light intensity, and 65–70% relative humidity. The plants were divided into four groups. A completely randomized experimental design with three biological replicates and three technical replicates was used. One group was kept under normal conditions (N treatment; 25 °C and 0 kPa), another group was subjected to a water deficit treatment until the soil water potential reached − 20 kPa, with the temperature maintained at 25 °C (D treatment; 25 °C and − 20 kPa), the third group was subjected to high temperature (H treatment; 45 °C and 0 kPa), and the fourth group was subjected to double stress (DH treatment; 45 °C and − 20 kPa). Chlorophyll a fluorescence and relative conductivity were measured in Shenfeng and Shenhua and in Shenhua alone, at 3 and 6 h, respectively, after the high temperature treatment, for all treatments. Simultaneously, functional healthy leaves of Shenfeng and Shenhua were immediately preserved in liquid nitrogen and stored at − 80 °C for further analyses.
Leaf chlorophyll a fluorescence
The polyphasic chlorophyll a fluorescence transient was measured using a Plant Efficiency Analyzer (Hansatech Instruments Ltd., King’s Lynn, Norfolk, UK; Strasser et al. 2000). The formulae presented in Table S1 illustrate the biophysical parameters.
Measurement of relative conductivity
Leaf discs (10-mm diameter) were punched from five fresh, whole leaves, added to 10 mL ddH2O, and shaken continuously for 3 h. The initial conductivity value (CV1) and the value measured after boiling the sample for 10 min (CV2) were determined using a conductivity meter (DDS-6110; Chendu Ruizi Analysis and Control Instrument Co., LTD, Chendu, China). The conductivity of ddH2O blank samples (CV0) was determined simultaneously. The relative conductivity (L) for each sample was determined as:
Measurement of ABA concentrations
The samples were extracted with 100 mM phosphate buffer (pH 7.4), and the ABA concentration in the supernatant was then measured using the Phytodetek ABA enzyme immunoassay test kit (Agdia, Elkhart, IN, USA; Zhang et al. 2014).
Quantitative real-time (RT)-PCR
Total RNA was extracted using the E.Z.N.A. Plant RNA Kit (Omega, Bio-Tek, Doraville, GA, USA). First-strand cDNA was obtained using PrimeScript™ RT reagent Kit with gDNA Erase (Perfect Real Time) (Takara, Tokyo, Japan). Gene transcript levels were quantified using the cDNA samples and SYBR Green (Takara), in a LightCycler 480 System (Roche Diagnostics GmbH, Mannheim, Germany). The results were normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and elongation factor 1r (EF1r) following Guillaumie et al. (2013). The stability values (M) of the reference genes were 0.261 (< 1.5) and 0.587 (< 1.5) in Shenfeng and Shenhua, respectively, which indicated that the reference genes were suitable for our experiment. The normalized transcript levels of GLOS1 (Pillet et al. 2012), HSFA2 (Pillet et al. 2012), HSP70 (Hren et al. 2009), MBF1 (Yan et al. 2014), NCED1 (Sun et al. 2010), CYP707A1 (Kondo et al. 2014), and MnSOD (Carvalho et al. 2006) were calculated using the geNorm software (https://genorm.cmgg.be/; Vandesompele et al. 2002). The qRT-PCR primer sequences are listed in Table S2. Three technical replicates were performed for each qRT-PCR assay.
Measurement of MDA content
The samples were extracted with 100 mM phosphate buffer (pH 7.0) and the supernatant was isolated. The MDA content in each sample homogenate was determined using a commercially available kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on thiobarbituric acid (TBA) reactivity, following the manufacturer’s protocol. Briefly, after mixing trichloroacetic acid with the homogenate and centrifuging, TBA was added to the resulting supernatant. The red color developed after the reaction was measured in a DU-800 UV/Vis spectrophotometer (Beckman Coulter, Fullerton, CA, USA) at 532 nm. The MDA content was determined as follows:
where ODs is the OD value for the sample, ODck is the value for the control, ODss is the value for the standard, ODb is the value for the blank, Css is the concentration of the standard substance, and Cs is the concentration of the sample.
Extraction of antioxidant enzymes and measurement of their activities
The samples were extracted with 100 mM phosphate buffer (pH 7.0) and the supernatant was isolated (Li et al. 2013). The activities of catalase (CAT), superoxide dismutase (SOD), peroxidase (POD), and total antioxidant capacity (T-AOC) were determined using commercial assay kits (Nanjing Jiancheng Bioengineering Institute) and a DU-800 UV/Vis spectrophotometer (Beckman Coulter).
One unit of SOD activity was defined as the quantity of SOD required to produce 50% inhibition of nitrite reduction in a 1 mL reaction solution, as measured by the change in absorbance at 550 nm. The activity of SOD was calculated as:
where ODs is the OD value for the sample, ODck is the value for the control, Vm is the total reaction volume, and Vs is the sampling amount.
The activity of the CAT enzyme was calculated as the decrease in absorbance at 405 nm due to the degradation of H2O2. The CAT activity was calculated as:
where ODs is the OD value for the sample, ODck is the value for the control, Vs is the sampling amount, and 271 is the reciprocal of the slope.
The activity of POD was measured as the change in absorbance at 420 nm. The formula used to calculate POD activity was:
where ODs is the OD value for the sample, ODck is the value for the control, Vm is the total reaction volume, Vs is the sampling amount, and t is the reaction duration.
The T-AOC was measured as the change in absorbance at 593 nm. The ability to reduce Fe3+-tripyridine triazine (Fe3+-TPTZ) to produce blue Fe2+-TPTZ in an acidic environment reflects the T-AOC. The standard curve was y = 0.3154x + 0.065; R2 = 0.9989, and the T-AOC was calculated as:
where ODs is the OD value for the sample, ODck is the value for the control, Vm is the total volume of the reaction, Vs is the sampling amount, Vt is the total sampling amount, and W is the fresh weight.
Statistical analysis
Data are presented as mean ± standard deviation. Each experiment was carried out in tri-replicates, and the differences among treatments were assessed using one-way analysis of variance (ANOVA) in SPSS 22.0 software (SPSS Inc., Chicago, IL, USA).
Results
Shenfeng and Shenhua, which are commercially important table-grape cultivars in China, were selected to study the responses to drought and heat stresses. Although drought and high temperature damaged both cultivars, they showed different degrees of damage under the stress treatment at 45 °C. Shenfeng showed heat injury symptoms after 3 h at high temperature and died after 6 h under this treatment, whereas Shenhua showed heat injury symptoms after 6 h at high temperature and eventually died by the end of the treatment duration. Therefore, leaves of Shenfeng and Shenhua after 3 h, and leaves of Shenhua after 6 h at high temperature, were used for the subsequent experiments.
Abiotic stresses might induce the transcript levels of several stress-related genes, including the heat-related genes GLOS1, HSFA2, and HSP70, and drought-related gene MBF1. In Shenfeng (Fig. 1), GLOS1, HSFA2, and HSP70 showed similar expression patterns under all treatments, with a significant induction under treatments H3 and DH3, while the transcript levels of MBF1 were significantly induced under treatments D and DH3. Therefore, in Shenfeng, the transcript level of heat-related genes was specifically enhanced by high temperature, whereas that of MBF1 was specifically induced by water deficit. In Shenhua, the transcript levels of the GLOS1, HSFA2, and HSP70 were induced under treatments H3, DH3, H6, and DH6 (Fig. 2), whereas the drought-related gene MBF1 was enhanced under treatments D, DH3, and DH6 (Fig. 2). These results indicate that the gene responses characteristic of heat and water deficit stresses were typically found in both cultivars.
The transcript level of stress-related gene in Shenhua under different treatments. N: 0 kPa and 25 °C; D: − 20 kPa and 25 °C; H3: 0 kPa and 45 °C at 3 h; DH3: − 20 kPa and 45 °C at 3 h; H6: 0 kPa and 45 °C at 6 h; DH6: − 20 kPa and 45 °C at 6 h. Significant differences (P ≤ 0.05) were performed by different lower case letters
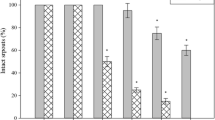
Chlorophyll a fluorescence is an important index that allows the most effective identification of plants under stress. In Shenfeng (Fig. 3, Table 1), the initial fluorescence value of OJIP (FO) increased under different stresses, and the FO of H3 was the highest among all treatments. This trend was also observed in FJ and FI. However, the highest FP value was found in N, and FP was the lowest in H3. One of the most sensitive indicators of chlorophyll a fluorescence, PIABS, decreased under all stress treatments relative to the N treatment and, in Shenfeng, its expression under H3 and DH3 treatments differed significantly from those under N and D treatments (Fig. 3). The relative electrical conductivity of Shenfeng leaves increased under the different stress treatments; it was highest in H3, followed by DH3, and D (Fig. 3). In Shenhua (Fig. 4, Table 2), the initial fluorescence value of OJIP under treatments D, H3, DH3, H6, and DH6 was 491, 935, 626, 1365, and 900, respectively. Thus, the FO value increased under heat stress compared with the N treatment, but was lower under the water deficit plus high temperature treatment. This trend was also observed in FJ and FI values, but FP was lower under high temperature (H3 and H6) than under the remaining treatments (N, D, DH3, and DH6). The PIABS values in D, H3, and DH3 were not different from that in the N treatment, and the PIABS values in H6 and DH6 were significantly lower than those in other treatments. In fact, the PIABS of H6 was the lowest (Fig. 4). The relative electrical conductivity of Shenhua leaves increased significantly under H6 and DH6, showing the highest value under H6. The relative electrical conductivity under D, H3, and DH3 treatments did not differ from that under the N treatment (Fig. 4).
The OJIP curve, PIABS, and relative conductivity in Shenhua under different treatments. N: 0 kPa and 25 °C; D: − 20 kPa and 25 °C; H3: 0 kPa and 45 °C at 3 h; DH3: − 20 kPa and 45 °C at 3 h; H6: 0 kPa and 45 °C at 6 h; DH6: − 20 kPa and 45 °C at 6 h. Significant differences (P ≤ 0.05) were performed by different lower case letters
Stress induces a rapid rise in the OJIP parameters. Therefore, the amplitude of phase K (Wk) can be used as an indicator of the damage caused to the PS II donor site. In Shenfeng, Wk significantly increased under H3 and DH3 treatments (Fig. 5), but no difference was found between treatments D and N. In Shenhua, Wk increased significantly under H3, H6, and DH6 treatments, but no difference was observed between D and DH3 treatments compared with the N treatment (Fig. 6). In Shenfeng, the values of φPo, φEo, and ΨEo displayed no significant changes between N and D treatments, but decreased under H3 and DH3 treatments (Fig. 5). In Shenhua, the values of φPo, φEo, and ΨEo significantly decreased under H6 and DH6 treatments and were lower in H6 than in DH6. However, no differences in φPo, φEo, and ΨEo were observed in the D, H3, and DH3 treatments compared with the N treatment. Changes in the approximate initial slope of the fluorescence transient (Mo) were induced under H3 and DH3 treatments in Shenfeng (Fig. 5) and under H6 and DH6 treatments in Shenhua (Fig. 6). The redox state of photosystem I (δRo) did not display significant changes under any of the treatments in Shenfeng (Fig. 5), but increased under the DH6 treatment in Shenhua (Fig. 6).
The chlorophyll fluorescence parameters in Shenhua under different treatments. N: 0 kPa and 25 °C; D: − 20 kPa and 25 °C; H3: 0 kPa and 45 °C at 3 h; DH3: − 20 kPa and 45 °C at 3 h; H6: 0 kPa and 45 °C at 6 h; DH6: − 20 kPa and 45 °C at 6 h. Significant differences (P ≤ 0.05) were performed by different lower case letters
Water deficit and heat stress treatments significantly increased leaf ABA compared with the N treatment. In Shenfeng, the ABA concentrations were significantly higher in D and DH3 than in N and H3 treatments (Fig. 7). In Shenhua, both stresses enhanced the ABA concentration (Fig. 8). In Shenfeng, the transcript level of NCED1 was induced under D and DH3 treatments and the transcript level of CYP707A1 increased under the H3 treatment (Fig. 7). The transcript levels of NCED1 and CYP707A1 increased under the different stress treatments and showed the highest value under the H6 treatment in Shenhua (Fig. 8).
The ABA concentrations and the transcript level of related genes in Shenhua under different treatments. N: 0 kPa and 25 °C; D: − 20 kPa and 25 °C; H3: 0 kPa and 45 °C at 3 h; DH3: − 20 kPa and 45 °C at 3 h; H6: 0 kPa and 45 °C at 6 h; DH6: − 20 kPa and 45 °C at 6 h. Significant differences (P ≤ 0.05) were performed by different lower case letters
In Shenfeng, the activity of antioxidant enzymes varied under the different treatments (Fig. 9). The activities of SOD, POD, CAT, T-AOC, and MDA content, and the transcript level of did not differ between the D and N treatments, but the activities of SOD, CAT, and MDA content, and the MnSOD transcript level increased under the H3 treatment. The DH3 treatment had no effect on the activities of SOD, POD, and CAT, or the MDA content. In Shenhua, SOD activity showed no significant difference across treatments, whereas POD activity increased under all stress treatments. The CAT activity increased under H3 and DH6 treatments and the MDA content increased under D, H3, H6, and DH6 treatments. The T-AOC increased under H6 and DH6 treatments, while the MnSOD expression increased under H3, H6, and DH6 treatments (Fig. 10).
The activities of SOD, POD, CAT, and MDA content in Shenhua under different treatments. N: 0 kPa and 25 °C; D: − 20 kPa and 25 °C; H3: 0 kPa and 45 °C at 3 h; DH3: − 20 kPa and 45 °C at 3 h; H6: 0 kPa and 45 °C at 6 h; DH6: − 20 kPa and 45 °C at 6 h. Significant differences (P ≤ 0.05) were performed by different lower case letters
Discussion
Water deficit and heat stress damage plants
Plants undergo various biochemical, physiological, and molecular damages due to heat or drought stresses. The transcript levels of heat stress-related genes HSFA2, HSP70, and GLOS, and that of drought stress-related gene MBF1, significantly increased under heat or water deficit stress, respectively. Previous studies conducted using Shenfeng and Shenhua had also revealed that HSFA2, HSP70, and GLOS1 expression increased under high- temperature treatments (Zha et al. 2016a, b) and that MBF1 expression was induced by water deficit stress (Yan et al. 2014; Zandalinas et al. 2016). This indicated that specific molecular damage is caused by each of these stresses. Relative electrolyte conductivity was enhanced under water deficit, heat, and water deficit plus heat stress in Shenfeng and Shenhua, but stress injuries and their degrees differed among the different treatments. The relative electrolyte conductivity is greatly influenced by both of these stresses (Bajji et al. 2002; Yin et al. 2008), owing to their effects on the permeability of plant cell membranes. The OJIP curve, some PS II parameters, and ABA concentrations changed under high temperature and water deficit treatments, revealing that PS II and its signaling factors respond positively to stresses, in agreement with previously reported results (Yoshida et al. 2014; Wang et al. 2010).
The difference in the response to high temperature (45 °C) between the two grape cultivars, Shenfeng and Shenhua, was most likely due to their genetic differences. Our results showed that some indicators (PIABS, relative conductivity, Wk, Mo, δRo, φPo, φEo, and ΨEo) under H and DH treatments did not show significant changes in Shenhua after 3 h, but were obviously altered in Shenfeng; after a 6-h period under these treatments, Shenhua also showed differences in these parameters. In addition, unlike the Shenhua, Shenfeng was not able to resist the high temperature stress.
High temperature stress specifically damages PS II and the activity of antioxidant enzymes
In Shenfeng and Shenhua, changes between FO and FI under the DH and D treatments were similar, but significantly lower than those under the H treatment. The greatest increase in FO was recorded under heat stress, indicating that heat stress caused the maximal damage to the leaf photosynthetic system. The initial chlorophyll a fluorescence level at stage P of OJIP corresponds to the state where all QA molecules are in the reduced state. As FP was found to be significantly lower in H and DH treatments than in the N treatment, fast and slow reducing PQ centers might be present in Shenfeng and Shenhua under these stresses. Changes in the OJIP curve under the DH treatment showed a smoother trend than under the H treatment, suggesting that heat stress sharply alters OJIP curves.
Changes in chlorophyll a fluorescence might be used to measure changes in PS II activity, caused directly or indirectly by stress (Su et al. 2014; Sun et al. 2016). Regarding the chlorophyll a energy flow, the photosynthetic mechanism includes the capture of light energy, energy distribution, and electron transport, with a competitive relationship among them (Cendrero Mateo et al. 2012). In our study, the PS II parameters were changed under heat stress, but not under water deficit stress, indicating that the tested water deficit level was not fatal to the plants. In previous studies, moderate and severe drought stress often inhibited the activity of enzymes related to PS II (Dias and Brüggemann 2007; Wang et al. 2011; Zhou et al. 2017), but photosynthetic capacity showed little or no change under low drought stress (Yordanov et al. 2000).
Abiotic stresses-related decreases in photosynthesis are usually consistent with a burst of ROS production under abiotic stresses (Kurepin et al. 2015). Over-accumulated electrons with O2 block the photosynthetic electron transport in the reaction centers of PS I and PS II resulting in the production of ROS (Wang et al. 2011; Oukarroum et al. 2015). The antioxidant pathways of plant prevent ROS overproduction via non-enzymatic and enzymatic antioxidants among others mechanisms (Noctor and Foyer 1998). Here, the activities of SOD, POD, and CAT, and the level of MDA significantly increased under the H treatment in Shenfeng and Shenhua, in agreement with the strong stimulation of oxidative capacity by heat stress, as reported in previous studies (Gomes Silva et al. 2010). Thus, water deficit effectively enhanced the antioxidant capacity.
Water deficit triggers cross tolerance to heat stress
Water deficit priming in plants could induce thermo-tolerance. In our study, PS II and the activities of antioxidant enzymes were severely affected by high temperature treatments, but only slightly affected by the combined water deficit plus high temperature treatments. Zhang et al. (2016) reported that drought priming conferred heat tolerance in wheat, as exemplified by increased grain yield, photosynthesis, and anti-oxidation and proteomic activities in leaves subject to both drought and heat stress, relative to those subjected to heat stress only. At the cellular level, the response to adversity is oxidative stress, which can stimulate or induce the improvement of ROS scavenging ability in plants (Bowler et al. 1992). Thus, cross resistance to drought and heat stresses seems to be closely related to the ability of plants to remove ROS.
The cross talk between water deficit and heat stresses involves ABA
In addition to playing an important role in plants’ signal regulation in response to stress (Finkelstein et al. 2002), ABA has been correlated with drought and heat resistance (Reddy et al. 2004; Zhang et al. 2008). Drought and heat stresses induced ABA biosynthesis in Shenfeng and Shenhua. Tolerance to heat stress could be achieved either via protection of PS II activity from photoinhibition (Ivanov et al. 1995), or through the induction of the antioxidant defense system related to ABA accumulation under water deficit stress (Liu et al. 2003). Thus, ABA is likely to play an important role in the cross talk between water deficit and heat stress.
Genes related to ABA biosynthesis are known to be induced by environmental stresses. The transcript level of NCED in Arabidopsis thaliana, cowpea, and avocado was stimulated by drought stress (Chernys and Zeevaart 2000; Iuchi et al. 2000; 2001). Here, water deficit also induced the expression of NCED1, whereas CYP707A1 expression was induced by high temperature. Heat stress has also been shown to increase the ABA concentrations in a previous study (Larkindale et al. 2005). Therefore, there is likely a cross talk between heat- and drought-stress responses, including changes in the expressions of NCED1 and CYP707A1. In addition, ROS are known to mediate ABA signaling in plants through MAP kinases (Jammes et al. 2009) and several ROS-related enzyme activities and products were found to be altered by the abiotic stresses examined in our study. However, how the ROS interact with ABA remains unclear and requires further research.
Author contribution statement
QZ and ALJ conceived and designed the experiment. QZ and YNH procured the materials and methods for the experiment. QZ and XXJ worked carried out data curation and formal analysis. QZ, ALJ, and XPF prepared the manuscript.
Abbreviations
- ABA:
-
Abscisic acid
- CV:
-
Conductivity value
- D:
-
Water deficit treatment
- DH:
-
Water deficit plus high temperature treatments
- GOLS:
-
Galactinol synthesis
- H:
-
High temperature treatment
- HSF:
-
Heat shock transcription factors
- HSP:
-
Heat shock protein
- MBF:
-
Multiprotein bridging factor
- MDA:
-
Malondialdehyde
- OD:
-
Optical density
- PS II:
-
Photosystem II
- TBA:
-
Thiobarbituric acid
References
Bajji M, Kinet JM, Lutts S (2002) The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul 36:61–70
Bowler C, Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Biol 43:83–116
Bruce TJA, Matthes MC, Napier JA, Pickett JA (2007) Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci 173:603–608
Camejo D, Rodríguez P, Morales MA, Dell’Amicoa JM, Torrecillas A, Alarcón JJ (2005) High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol 162:281–289
Carvalho LC, Vilela BJ, Vidigal P, Mullineaux PM, Amâncio S (2006) Activation of the ascorbate-glutathione cycle is an early response of micropropagated Vitis vinifera L. explants transferred to ex vitro. Int J Plant Sci 167(4):759–770
Cendrero Mateo M, Carmo-Silva A, Salvucci M, Moran SM, Hernandez M (2012) Steady-state chlorophyll fluorescence (F s) as a tool to monitor plant heat and drought stress. In: AGU fall meeting abstracts, vol 12, p 421
Chernys JT, Zeevaart JAD (2000) Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol 124:343–354
Cho SM, Kang BR, Kim JJ, Kim YC (2012) Induced systemic drought and salt tolerance by Pseudomonas chlororaphis O6 root colonization is mediated by ABA-independent stomatal closure. Plant Pathol J 28:202–206
Dias MC, Brüggemann W (2007) Differential inhibition of photosynthesis under drought stress in Flaveria species with different degrees of development of the C4 syndrome. Photosynthesis 45:75–84
Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14:15–45
Gomes Silva C, Juárez R, Marino T, Molinari R, García H (2010) Influence of excitation wavelength (UV or visible light) on the photocatalytic activity of titania containing gold nanoparticles for the generation of hydrogen or oxygen from water. J Am Chem Soc 133:595–602
Grossmann K, Scheltrup F, Kwiatkowski J, Caspar G (1996) Induction of abscisic acid is a common effect of auxin herbicides in susceptible plants. J Plant Physiol 149:475–478
Guillaumie S, Ilg A, Réty S, Brette M, Trossat-Magnin C, Decroocq S, Léon C, Keime C, Ye T, Baltenweck-Guyot R, Claudel P, Bordenave L, Vanbrabant S, Duchêne E, Delrot S, Darriet P, Hugueney P, Gomès E (2013) Genetic analysis of the biosynthesis of 2-methoxy-3-isobutylpyrazine, a major grape-derived aroma compound impacting wine quality. Plant Physiol 162:604–615
Hren M, Ravnikar M, Brzin J, Ermacora P (2009) Induced expression of sucrose synthase and alcohol dehydrogenase I genes in phytoplasma-infected grapevine plants grown in the field. Plant Pathol 58:170–180
Iuchi S, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2000) A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiol 123:553–562
Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27:325–333
Ivanov AG, Krol M, Maxwell D, Hüner NPA (1995) Abscisic acid induced protection against photoinhibition of PS II correlates with enhanced activity of the xanthophyll cycle. FEBS Lett 371:61–64
Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, Cho D, Lee S, Giordo R, Strtubtim S, Leonhardt N, Elis BE, Murata Y, Kwak JM (2009) MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc Natl Acad Sci 106(48):20520–20525
Kondo S, Tomiyama H, Rodyoung A, Okawa K, Ohara H, Sugaya S (2014) Abscisic acid metabolism and anthocyanin synthesis in grape skin are affected by light emitting diode (LED) irradiation at night. J Plant Physiol 171:823–929
Kurepin LV, Ivanov AG, Zaman M, Pharis RP, Allakhverdiev SI, Vaughan H, Hüner NPA (2015) Stress-related hormones and glycinebetaine interplay in protection of photosynthesis under abiotic stress conditions. Photosynth Res 126:221–235
Larkindale J, Hall JD, Knight MR, Vierling E (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermo-tolerance. Plant Physiol 138:882–897
Li HX, Xiao Y, Cao LL, Yan X, Li C, Shi HY, Wang JW, Ye YH (2013) Cerebroside C increases tolerance to chilling injury and alters lipid composition in wheat roots. PLoS One 8(9):e73380
Liu F, Andersen MN, Jensen CR (2003) Loss of pod set caused by drought stress is associated with water status and ABA content of reproductive structures in soybean. Funct Plant Biol 30:271–280
Maroco JP, Rodrigues ML, Lopes C, Chaves MM (2002) Limitations to leaf photosynthesis in field-grown grapevine under drought-metabolic and modeling approaches. Funct Plant Biol 29:451–459
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49:249–279
Oukarroum A, Bussotti F, Goltsev V, Kalaji HM (2015) Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ Exp Bot 109:80–88
Pillet J, Egert A, Pieri P, Lecourieux F, Kappel C, Charon J, Gomès E, Keller F, Delrot S, Lecourieux D (2012) VvGOLS1 and VvHsfA2 are involved in the heat stress responses in grapevine berries. Plant Cell Physiol 53:1776–1792
Qin X, Zeevaart JAD (1999) The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci 96:15354–15361
Queitsch C, Hong SW, Vierling E, Lindquist S (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12:479–492
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202
Shah K, Nahakpam S (2012) Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars. Plant Physiol Biochem 57:106–113
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanism, regulation and adaptation. Taylor and Francis, London, pp 443–480
Su X, Wu S, Yang L, Xue R, Wang Y, Zhao H (2014) Exogenous progesterone alleviates heat and high light stress-induced inactivation of photosystem II in wheat by enhancing antioxidant defense and D1 protein stability. Plant Growth Regul 74:311–318
Sun L, Zhang M, Ren J, Qi J, Zhang G, Leng P (2010) Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biol 10:257–268
Sun Y, Liu X, Zhai H, Gao H, Yao Y, Du Y (2016) Responses of photosystem II photochemistry and the alternative oxidase pathway to heat stress in grape leaves. Acta Physiol Plant 38:232
Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014) Abiotic and biotic stress combinations. New Phytol 20:32–43
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:research0034-1
Wang LJ, Fan L, Loescher W, Duan W, Liu GJ, Cheng JS, Luo HB, Li SH (2010) Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol 10:34
Wang X, Cai J, Jiang D, Liu F, Dai T, Cao W (2011) Pre-anthesis high-temperature acclimation alleviates damage to the flag leaf caused by post-anthesis heat stress in wheat. J Plant Physiol 168:585–593
Wang ZX, Chen L, Ai J, Qin HY, Liu YX, Xu PL, Jiao ZQ, Zhao Y, Zhang QT (2012) Photosynthesis and activity of photosystem II in response to drought stress in Amur Grape (Vitis amurensis Rupr.). Photosynthetica 50:189–196
Wang S, Takahashi H, Saito T, Okawa K, Ohara H, Shishido M, Ikeura H, Kondo S (2015) Jasmonate application influences endogenous abscisic acid, jasmonic acid and aroma volatiles in grapes infected by a pathogen (Glomerella cingulata). Sci Hortic 192:166–172
Wen D, Ding Y (2005) Formulation of nanofluids for natural convective heat transfer applications. Int J Heat Fluid Flow 26:855–864
Yan Q, Hou H, Singer SD, Yan XX, Guo RR, Wang XP (2014) The grape VvMBF1 gene improves drought stress tolerance in transgenic Arabidopsis thaliana. Plant Cell Tissue Organ Cult 118:571–582
Yin H, Chen Q, Yi M (2008) Effects of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. Plant Growth Regul 54:45–54
Yordanov I, Velikova V, Tsonev T (2000) Plant responses to drought, acclimation, and stress tolerance. Photosynthesis 38:171–186
Yoshida T, Mogami J, Yamaguchi-Shinozaki K (2014) ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr Opin Plant Biol 21:133–139
Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A, Inupakutika MA, Mittler R (2016) ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J Exp Bot 67:5381–5390
Zha Q, Xi X, Jiang A, Tian YH (2016a) Changes in the protective mechanism of photosystem II and molecular regulation in response to high temperature stress in grapevines. Plant Physiol Biochem 101:43–53
Zha Q, Xi X, Jiang A, Tian YH (2016b) High temperature affects photosynthetic and molecular processes in field-cultivated Vitis vinifera L. × Vitis labrusca L. Photochem Photobiol 92:446–454
Zhang X, Wollenweber B, Jiang D, Liu F, Zhao J (2008) Water deficits and heat shock effects on photosynthesis of a transgenic Arabidopsis thaliana constitutively expressing ABP9, a bZIP transcription factor. J Exp Bot 59:839–848
Zhang H, Zhu H, Pan Y, Yu Y, Luan S, Li L (2014) A DTX/MATE-type transporter facilitates abscisic acid efflux and modulates ABA sensitivity and drought tolerance in Arabidopsis. Mol Plant 7(10):1522–1532
Zhang X, Wang X, Zhong J, Zhou Q, Wang X, Cai J, Dai T, Cao W, Jiang D (2016) Drought priming induces thermo-tolerance to post-anthesis high-temperature in offspring of winter wheat. Environ Exp Bot 127:26–36
Zhou R, Yu X, Ottosen CO, Rosenqvist E, Zhao L, Wang Y, Yu W, Zhao T, Wu Z (2017) Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol 17(1):24
Acknowledgements
This study was partially funded by the Modern Agricultural Industry Technology System (Grape) (CARS-29-10) and Shanghai Sailing Program (Grant number 19YF1443300).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Bavaresco.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zha, Q., Xi, X., He, Y. et al. Water limitation mitigates high-temperature stress injuries in grapevine cultivars through changes in photosystem II efficiency and antioxidant enzyme pathways. Acta Physiol Plant 41, 83 (2019). https://doi.org/10.1007/s11738-019-2875-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2875-0