Abstract
This study focused on the purification and characterization of an extracellular β-d-fructofuranosidase or invertase from Aspergillus sojae JU12. The protein was purified by size exclusion chromatography with 5.41 fold and 10.87% recovery. The apparent molecular mass of the enzyme was estimated to be ~ 35 kDa using SDS-PAGE and confirmed by deconvoluted mass spectrometry. The fungal β-d-fructofuranosidase was suggested to be a monomer by native PAGE and zymography, and was found to be a glycoprotein possessing 68.92% carbohydrate content. The products of enzyme hydrolysis were detected by thin layer chromatography and revealed the monosaccharide units, d-glucose and d-fructose. β-d-fructofuranosidase showed enhanced activity at broad pH 4.0–9.0 and activity at a temperature range from 30 to 70 °C, while the enzyme was stable at pH 8.0 and 40 °C, respectively. The β-d-fructofuranosidase activity was lowered by metal ion inhibitors Ag2+ and Hg2+ whereas elevated by SDS and β-ME. The fungal β-d-fructofuranosidase was capable of hydrolyzing d-sucrose and the kinetics were determined by Lineweaver–Burk plot with Km of 10.17 mM and Vmax of 0.7801 µmol min−1. Additionally, the extracellular β-d-fructofuranosidase demonstrated tolerance to high ethanol concentrations indicating its applicability in the production of alcoholic fermentation processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
β-d-fructofuranosidase (EC 3.2.1.26) is also known as invertase and catalyzes the hydrolysis of the disaccharide d-sucrose producing d-glucose and d-fructose. The hydrolytic enzyme produces the invert sugar mixture (1:1) of dextrorotatory and levorotatory monosaccharides, which possesses lower crystallinity than d-sucrose (Alberto et al. 2004). β-d-fructofuranosidase is required in numerous applications in the food industries. The breweries and baking industrial sectors demand β-d-fructofuranosidases due to the property of non-crystallization and hygroscopicity (Bayramoglu et al. 2003). The enzyme is capable to maintain moisture, freshness and softness in food products for longer hours, also for the production of artificial honey soluble β-d-fructofuranosidases are preferred. The sugar mixture obtained from the enzymatic hydrolysis by β-d-fructofuranosidase does not alter the colour, flavour, texture of the food stuffs when compared to acidic hydrolysis treatments (Arica et al. 2000; Shaheen et al. 2008).
β-d-fructofuranosidase are reported in plants (Roitsch and Gonza ́lez 2004; Chaira et al. 2010), microbial diversity such as bacteria (Yoon et al. 2007; Awad et al. 2013), fungi (Kurakake et al. 2010; Rustiguel et al. 2011; Gracida-Rodríguez et al. 2014) and yeasts (Plascencia-Espinosa et al. 2014; Andjelković et al. 2015). β-d-fructofuranosidases are mostly studied in Saccharomyces cerevisiae strains (Rashad and Nooman 2009; Andjelković et al. 2010; Veneshkumar et al. 2011; Shankar et al. 2013). Comparatively, there are lesser findings on β-d-fructofuranosidases from molds which deserves attention (Alves et al. 2013). However, majority of the fungal β-d-fructofuranosidases reported so far are largely filamentous fungi especially from Aspergillus sp. (Lucca et al. 2013; Rustiguel et al. 2015), Penicillium sp. (Flores-Gallegoss et al. Flores-Gallegos et al. 2012), Rhizopus sp. (Goulart et al. 2003) and Fusarium sp. (Wolska-Mitaszko et al. 2007). There is a huge demand for β-d-fructofuranosidases from filamentous fungi with potential characteristic features due to their biotechnological applications for the production of invert sugar syrup, food and beverages. The production of β-d-fructofuranosidases by submerged fermentation (SmF) and solid-state fermentation (SSF) systems have been earlier reported (Alves et al. 2013; Oyedeji et al. 2017).
Extracellular β-d-fructofuranosidases are industrially desired for the ease in down-streaming processes. As per Andjelković et al. (2010), the search for stable extracellular β-d-fructofuranosidases for d-sucrose hydrolysis is ongoing. Thus, new microbial strains producing potential β-d-fructofuranosidases with biotechnological significance are to be identified from the largely unexplored fungal biodiversity. The purification and characterization of β-d-fructofuranosidase is crucial to understand the hydrolytic action and nature of the enzyme. Thus, the aim of the present study was, therefore, to purify and characterize an external β-d-fructofuranosidase from Aspergillus sojae JU12 to unravel the enzymic properties.
Materials and methods
Materials
Acrylamide, N,N′-bis-acrylamide, β-mercaptoethanol (β-ME), coomassie brilliant blue (CBB) R-250, dithiothreitol (DTT), ethylenediaminetetraacetic acid (EDTA), iodoacetamide (IAA), N-acetyl imidazole (NAI), N-bromosuccinimide (NBS), N-ethylmaleimide (NEM), phenylmethylsulphonyl fluoride (PMSF), sodium dodecyl sulphate (SDS) and tosyl-l-phenylalanine chlormethyl ketone (TLCK) were purchased from Sigma-Aldrich, Co, St Louis, MO (USA). Bovine serum albumin (BSA), 3,5-dinitrosalicylic acid (DNS), potato dextrose agar (PDA), Schiff’s reagent, periodic acid, d-sucrose, 2,3,5-triphenyl tetrazolium chloride (TTC) were procured from HiMedia Laboratories (Mumbai, India). Molecular weight marker (medium range) and silica gel 60 F254 plates (20 × 20 cm) were purchased from Merck Pvt. Ltd. (Mumbai, India). All the other chemicals used were of analytical grade.
Fungal strain and fermentation medium
β-d-fructofuranosidase producing fungus was isolated from black soil of sugarcane growing regions in Maddur (Karnataka, India). The microbial colony was identified by 18S rRNA molecular sequencing as Aspergillus sojae JU12 (GenBank accession number MG051335.1), was used in the present study. The strain was preserved in 40% (v/v) glycerol stocks and revived on PDA medium. The SSF medium consisted of orange peel substrate (20 g) moistened with 50% diluted molasses medium (50% total sugars), fortified with beef extract (1.5%, w/v) as the nitrogen source accompanied with salts and trace elements (w/v) KH2PO4 0.35%, MgSO4·7H20 0.075% and FeSO4·7H20 0001%. The solid-substrate medium was inoculated with 9% (v/w) fungal inocula (1 × 108 spores/ml) and incubated at 37 °C for 120 h for maximum productivity. The enzyme was obtained by mechanical agitation for 1 h at 3 g with 40 ml of extraction buffer and the contents were centrifuged for 10 min, 11, 200 g at 4 °C. The enzyme activity and protein content were assayed in the cell-free supernatant which served as the extracellular crude enzyme.
Determination of β-d-fructofuranosidase activity and protein content
β-d-fructofuranosidase activity was estimated in the reaction assay mixture consisting of 0.1 ml of appropriately diluted enzyme (about 150 U) added to 1% (w/v) d-sucrose in 0.5 ml Tris–HCl (0.1 mol l−1, pH 8.0), and incubated at room temperature (28 ± 2 °C) for 30 min. The reducing sugars were measured by the addition of 1.0 ml DNS and incubated in a boiling water bath for colour development (Miller 1959). The enzyme activity was measured at 540 nm using d-glucose as the standard. One unit of β-d-fructofuranosidase activity was defined as amount of enzyme which released 1 µmol of reducing sugars per min under the assay conditions. The protein content (about 0.09 mg of total proteins) was determined by the method of Lowry et al. (1951) with BSA as the standard. The absorbance was recorded using UV–Visible Spectrophotometer (Systronics Double Beam 2202, India).
Purification of extracellular β-d-fructofuranosidase
The crude β-d-fructofuranosidase extract was precipitated with ammonium sulphate at 80% saturation and incubated at 4 °C overnight. The protein pellet was harvested by centrifugation at 11,200 g for 10 min at 4 °C, and resuspended in minimum aliquots of Tris–HCl buffer (0.1 mol l−1, pH 8.0). The protein was dialysed against the same buffer (0.01 mol l−1) and lyophilized (Freeze dryer, Model LY3TTE, Snijders Scientific, Tilburg Holland) for 7 h. The lyophilized powder served as the partially purified β-D-fructofuranosidase. The enzyme was appropriately diluted and loaded on to the automated size exclusion chromatography column using the fast protein liquid chromatography (FPLC) system (ÄKTAprime plus, GE Healthcare, UK). The Sephadex G-100 column (1.4 cm × 21 cm) equilibration was performed with Tris–HCl (0.05 mol l−1, pH 8.0), and the protein was eluted at a flow rate of 1.2 ml min−1. The eluate of 3.0 ml was collected as a single fraction and the active fractions were pooled, lyophilized based on the protein content and enzyme activity.
Electrophoresis and spectrometry
PAGE and zymography
Native PAGE and zymography for β-d-fructofuranosidase was performed under non-reducing conditions at 4 °C and the samples were separated on a 15% gel. The electrophoresis was carried out at 50 V in the stacking gel (4%) and 100 V in the separating gel (15%) for a duration of about 3–4 h. The protein bands in native PAGE were stained with 0.25% (w/v) CBB R-250 and destained overnight with 5% (v/v) methanol and 7.5% (v/v) glacial acetic acid. The corresponding zymogram was recovered, washed twice in Tris–HCl buffer (0.1 mol l−1, pH 8.0) and incubated at 40 °C for 1 h in the presence of 8% (w/v) d-sucrose solution. The gel was submerged in a developing solution containing 0.2% (w/v) TTC in 0.1 mol l−1 NaOH, and heated in a boiling water bath until appearance of bands (Chaira et al. 2010).
SDS-PAGE
The SDS-PAGE (15%) was performed under reducing conditions at room temperature and was set up similar to the native PAGE (Laemmli 1970). The standard molecular weight medium range markers were used to compare the protein migration. Likewise, post electrophoretic run the reducing gel was stained and destained as that of the native gel (Laemmli 1970; Warchol et al. 2002).
Mass spectrometry
The purified β-d-fructofuranosidase was passed through C18 column (Agilent Poroshell 120, 4.6 × 7.5 mm, 2.7 µm) of the Agilent 1290 Infinity LC–MS system coupled to the quadrupole time of flight (Q-TOF, Agilent 6530). The solvents used were solvent A (0.1% formic acid in water) and solvent B (90% acetonitrile, 0.1% formic acid in water). The chromatography gradient initiated at 3% of solvent B was peaking at 95% within 15 min and lowered at 3% of solvent A. The spectrum was visualized with the Agilent Mass Hunter Qualitative Analysis software.
Thin layer chromatography
The chromatographic plate was spotted with 10 µl of d-sucrose, d-glucose, d-fructose as the standards, and β-d-fructofuranosidase hydrolysate. The sample hydrolysate was prepared in a mixture of 1:1, enzyme and substrate (1%, w/v) in buffer and incubated overnight at 37 °C. The samples were loaded on the TLC plate and placed inside the pre-saturated chamber which consisted of butanol–ethanol–water (5:3:2). The plate was air dried, dipped in the developing solution [0.2% (w/v) orcinol in a mixture of 1:9 (v/v) of H2SO4 and methanol] and heated at 100 °C (Rustiguel et al. 2011).
Carbohydrate composition and glycoprotein analysis
To determine the carbohydrate content of the enzyme, the total phenol sulphuric acid method was conducted with d-glucose as standard (Dubois et al. 1956). A primary glycoprotein test was performed at room temperature (28 ± 2 °C), wherein 0.5 ml of β-d-fructofuranosidase (about 150 U) was added to 1.0 ml of concentrated sulfuric acid and absolute ethanol (1:1) and the control was devoid of the enzyme. Furthermore, the enzyme was electrophoresed on SDS-PAGE for glycoprotein detection by Periodic acid-Schiff (PAS) staining (Møller and Poulsen 2002).
Biochemical characterization of purified β-d-fructofuranosidase
Effects of pH and stability
To determine the effect of pH, β-d-fructofuranosidase activity (about 150 U) was measured in various buffers (0.1 mol l−1) of glycine–HCl (pH 2.0–3.0), sodium acetate (pH 4.0–5.0), phosphate (pH 6.0–7.0), Tris–HCl (pH 8.0–9.0), carbonate (pH 10.0) and glycine–NaOH (pH 11.0–12.0) buffers. The enzyme stability with optimal pH buffers (pH 6.0–8.0) was performed from 0 to 6 h at room temperature (28 ± 2 °C) and the enzyme activity was estimated as mentioned previously. The control was considered with maximum activity as 100% (Warchol et al. 2002).
Effects of temperature and stability
The effect of temperature on β-d-fructofuranosidase activity (about 150 U) was investigated from 0 to 80 °C, by pre-incubation with the optimally active buffer (0.1 mol l−1 Tris–HCl, pH 8.0) at various temperatures. The enzyme thermostability was checked at the optimal temperatures (40–60 °C) with the same buffer (pH 8.0), and the activity was assayed as mentioned earlier. The reaction mixture without heat treatment served as the control (100%).
Effects of metal ions
The various metal ions of the concentrations 1 and 10 mM (Ag2+, Ba2+, Ca2+, Co2+, Cu2+, Fe2+, Fe3+, Hg2+, K+, Mg2+, Mn2+, Zn2+) were tested. The enzyme (about 150 U) was pre-incubated with the metal ions for 15 min at optimal pH (0.1 mol l−1 Tris–HCl, pH 8.0) and optimal temperature (40 °C), and the assay was performed as previously. The reaction mixture without the addition of metal ions was considered as control with 100% activity.
Effects of group specific reagents
The effect of group specific reagents was investigated with the concentrations 1 and 10 mM (NEM, NBS, DTT, PMSF, NAI, SDS, EDTA, TLCK, β-ME, IAA). The enzyme (about 150 U) was pre-incubated with the reagents for 15 min, at optimal conditions pH 8.0 and 40 °C and β-d-fructofuranosidase activity was estimated as before. The reaction mixture without any group specific reagents was observed as control (100%).
Determination of kinetics
Michaelis–Menten constant (Km) and maximum velocity (Vmax) was evaluated by measuring the rate of D-sucrose hydrolysis with the optimum buffer and temperature conditions. The kinetic parameters were determined using d-sucrose at 0–50 mM and was calculated using the Lineweaver–Burk plot (Hyper32 software).
Effect of ethanol on β-fructofuranosidase
The effect of ethanol (0–20%, v/v) was tested on enzyme, and the activity was assayed as earlier. The reaction mixture devoid of ethanol was observed as control (Gargel et al. 2014).
Statistical analysis
All the experiments were conducted in triplicates and the results were expressed as mean ± standard deviation (SD) using Microsoft Excel (2016). One way analysis of variance (ANOVA) and means for groups in homogeneous subsets were determined by Duncan’s multiple range test (DMRT) at P0.05, using the statistical software (IBM SPSS Statistics V20.0.0). The values indicated with different letters on the error bars were considered significant.
Results and discussion
Purification studies of β-d-fructofuranosidase
There are various reported species of the genus Aspergillus such as A. niger (L’Hocine et al. 2000; Nguyen et al. 2005; Nadeem et al. 2009), A. nidulans (Alves et al. 2013), A. ochraceus (Guimaraes et al. 2007), A. parasiticus (Lucca et al. 2013), A. phoenicis (Rustiguel et al. 2011), known to produce β-D-fructofuranosidases. The isolated microorganism was identified morphologically by lactophenol cotton blue staining as Aspergillus sp. Further, the isolate was molecular sequenced by 18S rRNA and confirmed to be sojae species, the strain was deposited in the GenBank with the accession number MG051335.1. The gene sequences were subjected to perform Basic Local Alignment Search Tool (BLAST), a tool of the National Center for Biotechnology Information (NCBI) Genbank database. The selected sequences were chosen to be aligned using Clustal Omega, a software which was used to construct the phylogenetic tree. β-d-fructofuranosidases occurs in both intracellular and extracellular forms. The process of purification of enzymes is necessary, to understand enzymic nature or behaviour (Lincoln and More 2017). The purification of secreted β-d-fructofuranosidases from the culture filtrate has low contaminating proteins and is less complex than cellular enzymes. Purification of extracellular β-d-fructofuranosidases from Aspergillus species yields important products crucial for various applications in fermentation industries (Chen et al. 1996). The results of the purification steps of A. sojae β-d-fructofuranosidase is summarized in the Table 1. Majority of the fungal β-d-fructofuranosidases have been partially purified by ammonium sulphate precipitation as the first step of purification (L’Hocine et al. 2000), however, only a few studies have demonstrated acetone or ethanol precipitation (Hayashi et al. 1991; Chávez et al. 1997; Ghosh et al. 2001). A. sojae JU12 β-d-fructofuranosidase (about 90 mg L−1 of activity) was eluted from the Sephadex G-100 column with 5.41 fold and recovery of 10.87%. A. niger β-D-fructofuranosidase was purified by gel filtration on Sephadex G-150 followed by DEAE Sephadex A-50 column and comparatively, a low purification fold of 8.65 and 0.84% yield was observed. Similarly, a purification fold of 3.11 and 9.33% of external β-D-fructofuranosidase was recovered from Fusarium solani (Bhatti et al. 2006). On the other hand, β-D-fructofuranosidase from A. phoenicis (Rustiguel et al. 2011) and A. ochraceus (Guimaraes et al. 2007) were produced by SSF and purified by two chromatographic steps, DEAE cellulose and Sephacryl S-200 to obtain 14.46% yield and purification fold of 18.77; 24% yield and 7.1 fold, respectively. Purification of fungal β-d-fructofuranosidases in multiple steps such as three-stages (Nguyen et al. 2002), four-stages (Ghosh et al. 2001) or as many as eight steps of purification (L’Hocine et al. 2000), reveled lesser than 30% yields and the numerous purifying stages are costly and time consuming.
Determination of molecular mass, homogeneity and activity of purified β-d-fructofuranosidase
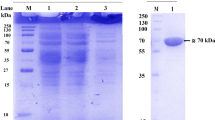
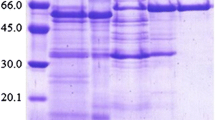
A. sojae β-d-fructofuranosidase represented a single band under native conditions (Fig. 1a), and corresponding bands on β-d-fructofuranosidase zymogram were observed (Fig. 1b). Occurrence of single molecular form of β-d-fructofuranosidases is common in most Aspergillus strains (L’Hocine et al. 2000; Oyedeji et al. 2017) or other fungi (Bhatti et al. 2006). However, this feature is contrasting in few fungal strains depicting oligomeric forms of β-d-fructofuranosidases (Nguyen et al. 2005; Quiroga et al. 1995), as well as yeast β-d-fructofuranosidases that exists as multiple isomers (Andjelković et al. 2010). The purified β-d-fructofuranosidase from A. sojae yielded a single homogenous peak by size exclusion chromatography which corresponded to the apparent molecular mass of 35 kDa on reducing SDS-PAGE (Fig. 1c). Further, the mass of the protein was evaluated by mass spectrometry and found to be 33,251.00 (Fig. 2). The molecular weight of A. sojae β-d-fructofuranosidase was found to be slightly lower than the mass of 37 kDa of the reported fungal strains A. japonicus MU-2 (Hayashi et al. 1992) and Clostridium perfringens (Ishimoto and Nakamura 1997), whereas found to be higher than the filamentous fungus Termitomyces clypeatus which showed a low-size protein of 13.5 kDa, single band in SDS-PAGE (Chowdhury et al. 2009). Molecular masses of some of the reported Aspergillus strains ranged from 66 to 430 kDa (Nadeem et al. 2015). Low molecular weight β-d-fructofuranosidases have been reported in bacterial and yeast β-d-fructofuranosidases, such as Bacillus cereus TA-11 of 26 kDa (Yoon et al. 2007) and Rhodotorula glutinis of 47 kDa, respectively (Rubio et al. 2002). In the present study, homogeneity of the bands in denaturating and non-denaturating electrophoretic gels confirmed the active protein to be a monomer. Comparatively, a low molecular weight monomeric fungal protein from A. sojae is reported, than β-d-fructofuranosidases of other Aspergillus strains. The enzyme hydrolysate revealed a mixture of d-glucose and d-fructose on the thin layer chromatogram (Fig. 3).
Electrophoretic analysis of Aspergillus sojae JU12 β-D-fructofuranosidase. a Native-PAGE: lane 1: crude extract, lane 2: purified enzyme (10 µg). b Zymogram depicting a single pink band of enzyme activity: Purified enzyme (10 µg). c SDS-PAGE: (M) protein molecular weight medium range marker (kDa) phosphorylase b (97.4), bovine serum albumin (66.0), ovalbumin (43.0), carbonic anhydrase (29.0), soyabean trypsin inhibitor (20.1) and lysozyme (14.3); lane 1: crude extract, lane 2: ammonium sulphate fraction (80%) (20 µg), purified enzyme (10 µg). d Glycoprotein staining: lane 1: ammonium sulphate fraction (80%) (20 µg), lane 2: purified enzyme (10 µg)
Glycoprotein analysis of β-fructofuranosidase
β-d-fructofuranosidase from A. sojae was found to be a glycoprotein containing about 68.92% of total sugar content. In the qualitative test, the appearance of brown colour indicated the presence of glycoproteins which was further confirmed by the development of pink coloured PAS stained bands on SDS-PAGE (Fig. 1d), that were coincident to the previously conducted electrophoretic experiments (Fig. 1c). The carbohydrate content of A. sojae β-d-fructofuranosidase was comparatively higher than the reported Aspergillus strains, A. japonicus with 20% (Hayashi et al. 1992) and A. niger with 17% (Nguyen et al. 2005), other fungal strains Aureobasidium sp. possessed 30–53% (Hayashi et al. 1991), Pycnoporus sanguineus with 24% (Quiroga et al. 1995), and bacterial β-d-fructofuranosidase L. reuteri strain showed 48% carbohydrate moiety (De Gines et al. 2000). The internal β-d-fructofuranosidases are found to be non-glycosylated and aggregated, whereas glycosylation in extracellular invertases are found to stabilize the enzyme and prevents thermal denaturation (Kern et al. 1992).
Biochemical characterization of purified β-fructofuranosidase
Effects of pH and stability
β-d-fructofuranosidase showed high activity from pH 4.0 to 9.0 (Fig. 4a). The stability studies were carried from pH 6.0 to 8.0 and the enzyme was found to be optimally active at pH 8.0 up to 6 h, compared to the lower pH conditions (Fig. 4b). The enzyme activity was significantly influenced by any variation in pH. Mostly, β-d-fructofuranosidases known so far are active in acid ranges, especially from yeasts. There are also many reports on acidic β-d-fructofuranosidases from fungi such as Aspergillus strains which are stable at pH 3.0–6.2 (Nadeem et al. 2009; Lucca et al. 2013). Although the fungal β-D-fructofuranosidase has shown enhanced activity at broad pH ranges, the β-d-fructofuranosidases optimum at alkaline conditions are rarely observed in microorganisms, and this neutral/alkalophilic nature could be beneficial in the development of biosensors or enzyme electrode sensors, digestive aids and biotechnological applications.
Enzymic properties and characteristics of Aspergillus sojae JU12 β-D-fructofuranosidase. a pH-dependent activity profile. b Stability of the enzyme at pH 6.0, 7.0 and 8.0. c Temperature-dependent activity profile. d Thermostability of the enzyme at 40, 50 and 60 °C. Error bars represent the mean ± SD and different letters on the error bars shows the factors as statistically different at P0.05
Effects of temperature and stability
β-d-fructofuranosidase exhibited a thermostable nature and was active at a broad temperature range from 30 to 70 °C, however, a slight decline in activity beyond 60 °C was recorded (Fig. 4c). Thermostability of β-d-fructofuranosidase at 40 °C was found to be more stable than higher temperatures (Fig. 4d). Similar observations were seen in extracellular β-d-fructofuranosidases from T. clypeatus wherein 47 °C was optimally active (Chowdhury et al. 2009), and 38–56 °C was a suitable range for the enzyme from A. parasiticus strain (Lucca et al. 2013). Thermal stability of β-d-fructofuranosidases at physiological temperature ranges are advantageous for applications in production of cough syrups and digestive formulations, or food additives, also required for centre-filled chocolates, cookies, cakes or fondants which necessitate thermostable enzymes.
Effects of metal ions and group specific reagents
The metal ion inhibitors Ag2+ and Hg2+ lowered A. sojae β-d-fructofuranosidase activity (Table 2). Similar observations were recorded in A. japonicas (Hayashi et al. 1992), A. niger (L’Hocine et al. 2000; Nguyen et al. 2005) and A. terreus strains, wherein β-d-fructofuranosidase was completely inhibited by Hg2+ as well as Ag2+ (Giraldo et al. 2014). Hg2+ at 2 mM concentration inhibited β-d-fructofuranosidases from Fusarium oxysporum (Gupta et al. 1989), and also at 5 mM of Hg2+ A. niger β-d-fructofuranosidase was entirely inhibited (Rubio and Maldonado 1995). These metal ions interact with the sulfhydryl groups in β-d-fructofuranosidases which are responsible for enzyme activity, thus leading to conformational changes and protein precipitation. Co2+ showed about 40% inhibition on A. sojae β-d-fructofuranosidases, whereas in case of A. niger β-d-fructofuranosidases 20% inhibition was reported (Nguyen et al. 2005), on the contrary the activity was promoted in S. cerevisiae β-d-fructofuranosidase (Rashad and Nooman 2009). From the Table 2, at both the tested concentrations there was stimulation of β-d-fructofuranosidase activity by Ca2+ and Fe2+, whereas enhancement of activity was found to be higher at 10 mM of Mg2+, Mn2+, K+ and Fe3+ than 1 mM. The effect of other metal ions such as Cu2+, Zn2+, Na+ and few other ions at lower concentrations were associated mostly with enzyme stabilization, which clearly demonstrates the significant action of these ions at the β-d-fructofuranosidase active sites. Likewise, the activity of the purified β-d-fructofuranosidase was elevated by Ca2+ and Mg2+ (Hayashi et al. 1992; Giraldo et al. 2014). The metal ions K+, Na+, Ca2+ (Oyedeji et al. 2017), Mn2+, Cu2+, Fe2+, K+ (Esawy et al. 2014) were found to be mostly β-d-fructofuranosidase activators, while Zn2+ (Nguyen et al. 2005) has also been reported as β-D-fructofuranosidase inhibitors. β-d-fructofuranosidase activity was inhibited in the presence NBS, 15–30% inhibition in case of NAI and PMSF a serine protease inhibitor, 20–50% inhibition by TPTZ. On the contrary, an elevation was observed by SDS and β-ME, while all the other group specific reagents did not exhibit much variation (Table 2). In some reports, the β-d-fructofuranosidase activity of filamentous basidiomycota was not affected by SDS and DTT, whereas completely inhibited by β-ME (Chowdhury et al. 2009). Thus, the influence of metal ions and reagents on enzyme activity is different in fungal β-d-fructofuranosidases and even among the Aspergillus strains.
Kinetics of A. sojae β-d-fructofuranosidases
The kinetics of the enzyme was determined by Lineweaver–Burk plot and Km of 10.17 mM and Vmax of 0.7801 µmol min−1 values were attained (Fig. 5). Aspergillus β-d-fructofuranosidases were found to have highest affinity for d-sucrose substrate (Guimaraes et al. 2007; Alves et al. 2013). A. parasiticus showed similar Km of 10.0 (Lucca et al. 2013), whereas most of the reported β-d-fructofuranosidases demonstrated high Km and Vmax values 117 mM and 12,500 µmol min−1 (Nadeem et al. 2009), 35.67 mM and 3.98 µmol min−1 (L’Hocine et al. 2000) and 35.5 mM and 60 µmol min−1 (Aslam et al. 2013), respectively. From the results, it can be suggested that A. sojae β-d-fructofuranosidase was found to have increased affinity for d-sucrose than the reported fungal and yeast strains.
Unravelling ethanol tolerance of A. sojae β-d-fructofuranosidase
A. sojae β-d-fructofuranosidase was found to be tolerant with maximum activity from 5 to 15% (v/v) of ethanol concentration, whereas at the maximum tested concentration of ethanol (20%, v/v) the activity dropped to 88.04% (Fig. 6). β-d-fructofuranosidase from Candida stellata strain demonstrated enzyme activities of 63 and 58% at 5 and 7.5% of ethanol concentrations, respectively (Gargel et al. 2014). As per Bai et al. (2008), the alcohol fermentation processes in industries usually requires ethanol tolerance up to 10–15% during the completion process. Therefore, the potential extracellular β-d-fructofuranosidase from A. sojae can be effectively utilized in the production of alcohol beverages or alcoholic fermentation as it exhibits good ethanol tolerance.
Conclusion
The present study focuses on the purification and characterization of β-d-fructofuranosidase from Aspergillus sojae JU12 which unravels the enzymic properties, that help in better understanding of its application in industries. A. sojae β-d-fructofuranosidase typically belongs to GH32 family of glycosidases demonstrated characteristics suitable for industrial requirements. The ethanol tolerance level of the fungal β-d-fructofuranosidase indicates its potentiality in the production of alcoholic beverages or fermentation processes. Thus, the extracellular β-d-fructofuranosidase produced from economical agro-wastes was identified to be thermostable at neutral/alkalophilic conditions possessing high affinity for d-sucrose and exhibited efficient ethanol tolerance. The investigation of the physico-chemical properties is crucial so as to further improve enzyme production or design genetic engineering studies. Although β-d-fructofuranosidases have been studied extensively from yeasts, the scope for finding new microbes with better prospects and properties triggers further research in this field.
References
Alberto F, Bignon C, Sulzenbacher G, Henrissat B (2004) The three-dimensional structure of invertase (β-fructosidase) from Thermotoga maritima reveals a bimodular arrangement and an evolutionary relationship between retaining and inverting glycosidases. J Biol Chem 279:18903–18910. https://doi.org/10.1074/jbc.m313911200
Alves JN, Joao A, Luis HSG (2013) Production of invertases by anamorphic (Aspergillus nidulans) and teleomorphic (Emericela nidulans) fungi under submerged fermentation using rye flour as carbon source. Adv Microbiol 3:421–429. https://doi.org/10.4236/aim.2013.35057
Andjelković U, Srdjan P, Zoran V (2010) Purification and characterization of Saccharomyces cerevisiae external invertase isoforms. Food Chem 120:799–804. https://doi.org/10.1016/j.foodchem.2009.11.013
Andjelković U, Milutinović-Nikolić A, Jović-Jovičić N, Banković P, Bajt T, Mojović Z et al (2015) Efficient stabilization of Saccharomyces cerevisiae external invertase by immobilisation on modified beidellite nanoclays. Food Chem 168:262–269. https://doi.org/10.1016/j.foodchem.2014.07.055
Arica MY, Senel S, Alaeddinoglu NG, Patir S, Denizli A (2000) Invertase immobilized on spacer-arm attached poly (hydroxyethyl methacrylate) membrane: preparation and properties. J Appl Polym Sci 75: 1685–1692. https://doi.org/10.1002/(SICI)1097-4628(20000401)75:14/1685/AID-APP1/3.0.CO/2-6
Aslam A, Haq I, Ali S (2013) Purification and characterization of two invertases from mutant strain of Saccharomyces cerevisiae. Pak J Bot 45(1):285–291
Awad GEA, Amer H, Gammal EWE, Helmy WA (2013) Production optimization of invertase by Lactobacillus brevis Mm-6 and its immobilization on alginate beads. Carbohydr Polym 93:740–746. https://doi.org/10.1016/j.carbpol.2012.12.039
Bai FW, Anderson WA, Moo-Young M (2008) Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol Adv 26:89–105. https://doi.org/10.1016/j.biotechadv.2007.09.002
Bayramoglu G, Akol S, Bulut A, Denzili A (2003) Covalent immobilization of invertase onto a reactive film composed of 2-hydroxyethyl methacrylate and glycidyl methacrylate: properties and application in a continuous flow system. Food Chem 84:591–599. https://doi.org/10.1016/s1369-703x(02)00170-5
Bhatti HN, Asgher M, Abbas A, Nawaz R, Sheikh MA (2006) Studies on kinetics and thermostability of a novel acid invertase from Fusarium solani. J Agric FoodChem 54:4617–4623. https://doi.org/10.1021/jf053194g
Chaira N, Smaali I, Besbes S, Mrabet A, Lachiheb B, Ferchichi A (2010) Production of fructose rich syrups using invertase from date palm fruits. J Food Biochem 35:1576–1582. https://doi.org/10.1111/j.1745-4514.2010.00487.x
Chávez FP, Rodriguez L, Díaz J, Delgado JM, Cremataa JA (1997) Purification and characterization of an invertase from Candida utilis: comparison with natural and recombinant yeast invertases. J Biotechnol 53:67–74. https://doi.org/10.1016/s0168-1656(97)01663-5
Chen J, Saxton J, Hemming FW, Peberdy JF (1996) Purification and partial characterization of the high and low molecular weight (S and F-form) of invertase secreted by Aspergillus nidulans. Biochim Biophys Acta 1296:207–218. https://doi.org/10.1016/0167-4838(96)00073-8
Chowdhury S, Shakuntala G, Samudra PB, Swagata P, Soumen B, Suman K (2009) Characterization of a novel low molecular weight sucrase from filamentous fungus Termitomyces clypeatus. Process Biochem 44:1075–1082. https://doi.org/10.1016/j.procbio.2009.05.009
De Gines SC, Maldonado MC, De Valdez GF (2000) Purification and characterization of invertase from Lactobacillus reuteri CRL 1100. Curr Microbiol 40:181–184. https://doi.org/10.1007/s002849910036
Dubois M, Gilles KA, Hamilton JK, Rebers PA (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Esawy MA, Kansoh AL, Kheiralla ZH, Ahmed HAE, Kahil TAK, El-Hameed EKA (2014) Production and immobilization of halophilic invertase produced from honey isolate Aspergillus niger EM77 (KF774181). Int J Biotech Well Indus 3: 36–45. http://www.lifescienceglobal.com/pms/index.php/ijbwi/article/view/2195
Flores-Gallegos AC, Castillo-Reyes F, Lafuente CB, Loyola-Licea JC, Reyes-Valdes MH, Aguilar CN et al (2012) Invertase production by Aspergillus and Penicillium and sequencing of an inv gene fragment. Micol Appl Int 24(1): 1–10. http://www.redalyc.org/pdf/685/68522692001.pdf
Gargel CA, Baffi MA, Gomes E, Da Silva R (2014) Invertase from a Candida stellata strain isolated from grape: production and physico-chemical characterization. J Microbiol Biotechnol Food Sci 4:24–28. https://doi.org/10.15414/jmbfs.2014.4.1.24-28
Ghosh K, Dhar A, Samanta TB (2001) Purification and characterization of an invertase produced by Aspergillus ochraceus TS. Ind J Biochem Biophy 38:180-185. http://hdl.handle.net/123456789/15292
Giraldo MA, Gonçalves HB, Furriel RPM, Jorge JA, Guimarães LHS (2014) Characterization of the co-purified invertase and b-glucosidase of a multifunctional extract from Aspergillus terreus. World J Microbiol Biotechnol 30:1501–1510. https://doi.org/10.1007/s11274-013-1570-3
Goulart AJ, Adalberto PR, Monti R (2003) Purificaç ão parcial de invertase a partir de Rhizopus sp. em fermentação semi-sólida. Alim Nutr Araraquara 14(2): 199–203. http://serv-bib.fcfar.unesp.br/seer/index.php/alimentos/article/viewFile/859/738
Gracida-Rodríguez MA, Goncalves HB, Furriel RD, Jorge JA, Guimaraes LHS (2014) Characterization of the co-purified invertase and beta-glucosidase of a multifunctional extract from Aspergillus terreus. World J Microbiol Biotechnol 30:1501–1510. https://doi.org/10.1007/s11274-013-1570-3
Guimaraes LHS, Terenzi HF, Polizeli MLTM, Jorge JA (2007) Production and characterization of a thermostable extracellular β-D-fructofuranosidase produced by Aspergillus ochraceus with agroindustrial residues as carbon sources. Enzyme Microb Technol 42:52–57. https://doi.org/10.1016/j.enzmictec.2007.07.021
Gupta AK, Nagpal B, Kaur N, Rathore P, Singh R (1989) Properties of invertase from Fusarium oxysporum. Proc Indian Natn Sci Acad 55: 505–512. http://www.insa.nic.in/writereaddata/UpLoadedFiles/PINSA/Vol55B_1989_5and6_Art30.pdf
Hayashi S, Nonoguchi M, Takasaki Y, Ueno H, Imada K (1991) Purification and properties of β-fructofuranosidase from Aureobasidium sp. ATCC 20524. J Ind Microbiol 7:251–256. https://doi.org/10.1007/bf01569747
Hayashi S, Matsuzaki K, Takasaki Y, Ueno H, Imada K (1992) Purification and properties of β-fructofuranosidase from Aspergillus japonicus. World J Microbiol Biotechnol 8:276–279. https://doi.org/10.1007/bf01201878
Ishimoto M, Nakamura A (1997) Purification and properties of β-fructofuranosidase from Clostridium perfringens. Biosci Biotechnol Biochem 61:599–603. https://doi.org/10.1271/bbb.61.599
Kern G, Schülke N, Schmid FX, Jaenicke R (1992) Stability, quaternary structure, and folding of internal, external, and core-glycosylated invertase from yeast. Protein Sci 1:120–131. https://doi.org/10.1002/pro.5560010112
Kurakake M, Masumoto R, Maguma K, Kamata A, Saito E, Ukita N et al (2010) Production of fructooligosaccharides by beta-fructofuranosidases from Aspergillus oryzae KB. J Agric Food Chem 58(1):488–492. https://doi.org/10.1021/jf903303w
L’Hocine L, Wang Z, Jiang B, Xu SY (2000) Purification and partial characterization of fructosyltransferase and invertase from Aspergillus niger AS 0023. J Biotechnol 81:73–84
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Lincoln L, More SS (2017) Bacterial invertases: occurrence, production, biochemical characterization, and significance of transfructosylation. J Basic Microbiol. https://doi.org/10.1002/jobm.201700269
Lowry O, Rosebrough N, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Lucca AL, Joao AJ, Luis HSG (2013) Extracellular β-D-fructofuranosidase from Aspergillus parasiticus: optimization of the production under submerged fermentation and biochemical characterization. Afr J Biotechnol 12(38):5678–5687. https://doi.org/10.5897/ajb2013.13029
Miller GL (1959) Use of dinitrosalicyiic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Møller HJ, Poulsen JH (2002) The protein protocols handbook. In: Staining of glycoproteins/proteoglycans in SDS-Gels ed. Walker J.M. pp. 569–574. Humana Press
Nadeem H, Rashid MH, Riaz M, Asma B, Javed MR, Perveen R (2009) Invertase from hyper producer strain of Aspergillus niger: physiochemical properties, thermodynamics and active site residues heat of ionization. Protein Peptide Lett 16(9):1098–1105
Nadeem H, Rashid MH, Siddique MH, Azeem F (2015) Microbial invertases: a review on kinetics, thermodynamics, physiochemical properties. Process Biochem 50:1202–1210. https://doi.org/10.1016/j.procbio.2015.04.015
Nguyen QD, Szabó JMR, Claeyssens M, Stals I, Hoschke A (2002) Purification and characterisation of amylolytic enzymes from thermophilic fungus Thermomyces lanuginosus strain ATCC 34626. Enz Microb Technol 31:345–352. https://doi.org/10.1016/s0141-0229(02)00128-x
Nguyen QD, Szabó JMR, Bhat MK, Hoschke A (2005) Purification and some properties of β-fructofuranosidase from Aspergillus niger IMI303386. Process Biochem 40:2461–2466. https://doi.org/10.1016/j.procbio.2004.09.012
Oyedeji O, Bakare MK, Adewale IO, Olutiola PO, Omoboye OO (2017) Optimized production and characterization of thermostable invertase from Aspergillus niger IBK1, using pineapple peel as alternate substrate. Biocatal Agric Biotechnol 16:30232–30238. https://doi.org/10.1016/j.bcab.2017.01.001
Plascencia-Espinosa M, Santiago-Hernández A, Pavón-Orozco P, Vallejo-Becerra V, Trejo-Estrada S, Sosa-Peinado A et al (2014) Effect of deglycosylation on the properties of thermophilic invertase purified from the yeast Candida guilliermondii MpIIIa. Process Biochem 49:1480–1487. https://doi.org/10.1016/j.procbio.2014.05.022
Quiroga EN, Vattuone MA, Sampietro AR (1995) Purification and characterization of the invertase from Pycnoporus sanguineus. BBA-Protein Struct Mater 1251(2):75–80. https://doi.org/10.1016/0167-4838(95)00070-b
Rashad MM, Nooman MU (2009) Production, purification and characterization of extracellular invertase from Saccharomyces cerevisiae NRRL Y-12632 by solid-state fermentation of red carrot residue. Aust J Basic Appl Sci 3(3): 1910–9. http://s3.amazonaws.com/zanran_storage/insipub.com/ContentPages/107091305.pdf
Roitsch T, González M (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9(12):606–613. https://doi.org/10.1016/j.tplants.2004.10.009
Rubio CM, Maldonado CM (1995) Purification and characterization of invertase from Aspergillus niger. Curr Microbiol 31:80–83. https://doi.org/10.1007/bf00294280
Rubio MC, Runco R, Navarro A (2002) Invertase from a strain of Rhodotorula glutinis. Phytochem 61:605–609. https://doi.org/10.1016/s0031-9422(02)00336-9
Rustiguel CB, de Oliveira AHC, Terenzi HF, Jorge JA, Guimaraes LHS (2011) Bio-chemical properties of an extracellular β-d-fructofuranosidase II produced by Aspergillus phoenicis under solid-sate fermentation using soy bran as substrate. Electron J Biotechnol 14(2):1–10. https://doi.org/10.2225/vol14-issue2-fulltext-1
Rustiguel CB, Jorge JA, Guimarães LHS (2015) Characterization of a thermo-tolerant mycelial β-fructofuranosidase from Aspergillus phoenicis under submerged fermentation using wheat bran as carbon source. Biocatal Agric Biotechnol 4(3):362–369. https://doi.org/10.1016/j.bcab.2015.05.004
Shaheen I, Bhatti HN, Ashraf T (2008) Production, purification and thermal characterization of invertase from a newly isolated Fusarium sp. under solid-state fermentation. Int J Food Sci Technol 43:1152–1158. https://doi.org/10.1111/j.1365-2621.2007.01581.x
Shankar T, Thangamathi P, Rama R, Sivakumar T (2013) Optimization of invertase production using Saccharomyces cerevisiae MK under varying cultural conditions. Int J Biochem Biophys 1(3):47–56. https://doi.org/10.13189/ijbb.2013.010301
Veneshkumar R, Vijayakumar R, Jagannathan S, Srinivasan P, Assalam KK, Suganya K (2011) Comparative analysis of the invertase activity by Saccharomyces cerevisiae isolated from cane juice with standard industrial strain. Ann Food Sci Technol 17(4): 7–8. https://www.researchgate.net/publication/216708608_Comparative_analysis_of_the_invertase_activity_by_Saccharomyces_cerevisiae_isolated_from_cane_juice_with_standard_industrial_strain
Warchol M, Perrin S, Grill JP, Schneider F (2002) Characterization of a purified β-fructofuranosidase from Bifidobacterium infantis ATCC 15697. Lett Appl Microbiol 35:462–467
Wolska-Mitaszko B, Jaroszuk-Scisei J, Pszeniczna K (2007) Isoforms of trehalase and invertase of Fusarium oxysporum. Mycol Res 111:456–465. https://doi.org/10.1016/j.mycres.2007.01.018
Yoon MH, Choi WY, Kwon SJ, Yi, SH (2007) Purification and properties of intracellular invertase from alkalophilic and thermophilic Bacillus cereus TA-11. J Appl Biol Chem 50: 196–201. http://agris.fao.org/agris-search/search.do?recordID=KR2008003273
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no known conflicts of interest associated with this publication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lincoln, L., More, S.S. Purification and biochemical characterization of an extracellular β-d-fructofuranosidase from Aspergillus sp.. 3 Biotech 8, 86 (2018). https://doi.org/10.1007/s13205-018-1109-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1109-2