Abstract
Plant fungal relationships should vary with abiotic and biotic factors to minimise plant stress and are likely to vary seasonally and with age. We investigated how fungal colonisation, specifically arbuscular mycorrhizal fungi and dark septate fungal endophytes, would vary with species identity and season, and how these interactions change with ontogeny. Plant roots of adults and seedlings of 9 species were collected from heathland and coastal dune habitats along the Australian east coast in New South Wales. Roots were stained and investigated for arbuscular mycorrhizal fungi and dark septate endophyte structures to determine colonisation strength. Species identity was the most important factor driving colonisation strength, while low rainfall and heatwaves were associated with declining arbuscular mycorrhizal fungi colonisation in the warmest sampling period. AMF colonisation may be supressed by plants under heat and water stress as a way of avoiding loss of limited photosynthates. Dark septate endophyte colonisation was more common in this time period and may assist with the stress of the warmer, drier conditions. Colonisation by arbuscular mycorrhizal fungi differed with age but in unpredictable ways and, along with dark septate endophytes, was evident even in plants that are considered non-mycorrhizal, although more extensive in known mycorrhizal species. The lack of arbuscular mycorrhizal fungi colonisation and the increase in dark septate endophyte colonisation during the most stressful period suggest an uncoupling mechanism in the symbiotic relationship which needs further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plants interact with soil microbes such as bacteria and fungi in a range of facilitatory interactions (Van der Heijden et al. 1998). Root-associated fungi include symbiotic mycorrhizal species, such as arbuscular mycorrhizal fungi (AMF), but also a range of pathogenic fungi and dark septate endophytes (DSE) (Zobel et al. 1997; Jumpponen and Trappe 1998). Symbiotic relationships with fungi assist plants by providing improved tolerance to stress and drought, protection from pathogens and disease, and increased soil stability (Rodriguez et al. 2004; Van Der Heijden and Horton 2009; Singh et al. 2011; Hodge and Fitter 2013). They are crucial to the structure, function, feedback and health of plant communities (Redman et al. 2001; Bücking et al. 2012). Endophytic AMF from the subphylum Glomeromycota commonly colonise herbaceous and woody plant families (Mandyam and Jumpponen 2005; Bücking et al. 2012; Spatafora et al. 2016) and show little selectivity (Klironomos and Hart 2002; Brundrett 2004). Ericoid mycorrhizae (EM) have been found in the roots of the Family Ericaceae (Cairney and Ashford 2002) and are considered central to the success of the Ericaceae family in stressful environments such as heathland (Midgley et al. 2004).
The relationship between plant and fungal endophytes may vary from parasitic through to facilitatory and varies with species (Logan et al. 1989), season (Meney et al. 1993; Mandyam and Jumpponen 2008), and through a plant’s life (termed the mutualist-parasitic continuum) (Moora and Zobel 1996; Lu et al. 2007). The relationship is influenced by a plant’s dependency on fungi as well as abiotic variables such as pH, temperature and nutrients (Brundrett and Kendrick 1990; Mandyam and Jumpponen 2008; Dumbrell et al. 2011; Hazard et al. 2013). Such a dynamic and diverse endophyte-host plant relationship has meant that predictable patterns of variation are still elusive and poorly understood.
Dark septate endophytes (DSE) are considered to be as abundant as AMF and known to occur in the roots of at least 600 plants (Kivlin et al. 2013). Despite their abundance, their presence is often ignored in studies and little is known about their ecological role (Mandyam et al. 2012; Muthukumar et al. 2014). The co-occurrence of AMF and DSE has led to the suggestion that DSE may play a similar role to AMF, although without the saprophytic capabilities of AMF (Fracchia et al. 2011; Kivlin et al. 2013; Huusko et al. 2017). Associations with DSE differ from those with mycorrhizae as they lack a localised interface to transfer nutrients with their host plant (Brundrett 2004). The direct flow of carbohydrates between DSE and a host has not been observed, however, DSE can produce extracellular enzymes to process nutrients that accumulate in organic pools, which are available for uptake by plants (Caldwell et al. 2000; Huusko et al. 2017). Unlike some cases of AMF association, DSE do not form pathogenic associations with the host and have been found to positively influence root morphology and structure (Newsham 2011; Li et al. 2019; Liu and Wei 2019). Plants can benefit indirectly from associations with endophytic fungi such as DSE through increased resistance to herbivores, pathogens and stress (Brundrett 2004). DSE may have various functions and their lack of nutritional exchange does not necessarily correlate to a lack of importance in natural systems (Mandyam and Jumpponen 2005).
Mycorrhizal fungi are usually most active just behind the growing tip of the root. As roots are constantly growing, endophytic fungi must constantly recolonise newly growing tips to establish a continual relationship. This gives the potential for a varying strength in colonisation rate through time and is likely to be affected by the abiotic conditions influencing growth rates of both the fungi and the plant. Thus, there is an expectation that seasonal patterns in colonisation associated with the speed of root growth and soil temperature are likely to occur.
Changes in abiotic variables as a result of seasonality can also influence colonisation of AMF (Muthukumar and Udaiyan 2002; Lingfei et al. 2005; Mandyam and Jumpponen 2008). Changes in light levels can increase photosynthetic capacity with more carbon being available for nutrient exchange (Kothamasi et al. 2001; Redman et al. 2001). Changing seasons can impact soil moisture content and temperature, which in turn influences the composition and colonisation strength of mycorrhizal fungi (Dumbrell et al. 2011; Montero Sommerfeld et al. 2013). Dumbrell et al. (2011) and Montero Sommerfeld et al. (2013) found lower AMF growth in winter in the UK and Chile respectively, while Mandyam and Jumpponen (2008) found lower colonisation in spring in Kansas, USA. Colonisation levels may also be influenced by wider landscape differences. In Australia, soils are characterised by low nutrients, especially nitrogen and phosphorous, which are essential to plant growth (Thomson and Leishman 2004). Many Australian habitats such as heathland and coastal dunes are stressful environments with high light-intensity, poor soil structure, low nutrients, harsh winds, drought and salinity (Hesp 1991; Kothamasi et al. 2001; Keith 2004). In order to survive in these conditions many plant species have specialised belowground adaptations including clustered or hairy roots, dauciform roots, sand-binding roots or associations with nitrogen fixing organisms and mycorrhizal fungi (Thomson and Leishman 2004). Previous studies have shown that AMF colonise plants in these environments (Logan et al. 1989; Mclean et al. 1999; Cairney and Ashford 2002; Gooden et al. 2019). The effect of seasonality can change among plant families; for example, two members of the Cyperaceae family, a family commonly considered non-mycorrhizal, were found to form associations with AMF, with the highest colonisation in winter (Logan et al. 1989; Meney et al. 1993; Brundrett 2009).
Seasonal changes have also been investigated in DSE (Ruotsalainen et al. 2002; Lingfei et al. 2005; Mandyam and Jumpponen 2008). The melanin in DSE is thought to be useful in stressful situations such as high temperatures or drought (Muthukumar et al. 2014). Ruotsalainen et al. (2002) found no seasonal variation while Mandyam and Jumpponen (2008) found DSE colonisation was highest in spring. Although rarely investigated, root colonisation by DSE appears to be influenced by changes in environmental conditions (Mandyam and Jumpponen 2008; Huusko et al. 2017). Mandyam and Jumpponen (2008) found AMF and DSE colonisation followed dissimilar seasonal trends which may suggest complementary functioning between these two endophytes.
AMF have been suggested to greatly benefit the establishment phase of seedlings – a time of high stress for plants (Abbaspour et al. 2012). This benefit has been found to be a result of AMF making stressful conditions more tolerable through improved soil structure, accumulation of osmotic adjustment compounds, and nutrient acquisition which promotes plant growth and survival (Veenendaal et al. 1992; Moora and Zobel 1998; Jones and Smith 2004; Lu et al. 2007; Abbaspour et al. 2012). Colonisation of seedlings by AMF may also assist in balancing competition with already established adults (Moora and Zobel 1996; Van Der Heijden 2004). As plants age, their resource requirements and ability to obtain these also change and, as such, dependency on mycorrhizae may vary with age (Van Der Heijden 2004; Pietikäinen and Kytöviita 2007; Miller et al. 2014).
Less is known about how relationships between DSE and plants differs across life stages (Andrade-Linares et al. 2011). DSE has been suggested to be of importance to young seedlings more so than to adults (Mandyam and Jumpponen 2005; Andrade-Linares et al. 2011; Muthukumar et al. 2014). Muthukumar et al. (2014) suggested seedlings may preferentially interact with DSE when competition for AMF colonisation is high. In fact, in some habitats DSE colonisation has been found to be higher than AMF or ectomycorrhizae in seedlings (Mandyam and Jumpponen 2005).
This study aimed to investigate the intricacy of plant/ fungal relationships across species and time in landscapes where plant species are known to interact with mycorrhizal fungi. Specifically, three variables known to impact colonisation levels were investigated; species identity, plant age, and seasonality. Three questions were posed:
- 1)
Does the level of fungal colonisation (AMF, EM and DSE) vary between adults and seedlings?
- 2)
Does fungal colonisation vary through seasonal changes?
- 3)
How does fungal colonisation vary with species?
2 Materials and methods
2.1 Study region and habitat
Plant roots were collected from various locations of heathland and coastal dune habitats along the New South Wales (NSW) coast from the Sydney and the South East Coast bioregions. Sites spanned a distance of 307 km from North Durras on the southern NSW coast (35°38′31.48”S 150°18′11.11″E) to Ku-ring-gai Chase National park in Northern Sydney (33°35′55.71”S 151°17′33.62″E). Heathland plants were chosen as plants have adapted to withstand harsh conditions (Keith 2004) including skeletal soils low in nutrients with fire occurring periodically and often at high intensity (NSW Office of Environment and Heritage n.d.). Coastal dunes form an important ecological transition zone between marine and terrestrial environments where plants must overcome unstable sand, harsh winds, high stress from salt and drought, and limited nutrients (Hesp 1991). Species choice was first determined through the encounter of seedlings, followed by collection of adults from a different site. Families with specialised root structures such as proteoid roots were avoided as these are unlikely to have mycorrhizae (Brundrett 2008). Two species of Ericaceae were also collected to investigate EM. Table 1 contains the list of plant species collected and their current known mycorrhizal associations.
2.2 Seasonal collection
Samples were collected over three time periods with different climatic variables; May – June 2017 was the coldest sample with temperature increasing from the second samples collected in the warmer months of August 2017 and the last samples collected between the hot months of December 2017 and January 2018. Average maximum and minimum temperature, and average rainfall were collected for the three time periods from Bureau of Meteorology (BOM) weather stations within the study range and compared to the expected averages for these time periods (Table 2). This study was interested in investigating broad patterns across time rather than site specific microclimates. Köppen Climate Classifications across the study region are Cfa (humid Subtropical) and Cfb (Oceanic) (Climate-data.org n.d.), as a result the temperature and rainfall was similar at each site within a sample period.
2.3 Experimental design and field techniques
Nine species were collected; each with three replicate plant samples collected for each life stage and for each season for a total of 162 samples. Adults and seedlings of the same species were collected from different locations each season, with at least one kilometre between sites to maintain independence of samples. Individual plants were separated by at least 50 m to increase spatial variability. Maximising distance between samples minimises the likelihood of sampling similar AMF communities and as this study was focused on larger landscape processes it is important to avoid local patterns on occurrence of fungi to be sure colonisation of a species was not confounded by local rhizosphere communities (Brundrett 2009). As soil microbe communities can vary over short distances, 50 m was considered far enough apart to maintain independence (Hazard et al. 2013; Huusko et al. 2017). Samples were collected by removing debris around the base of the specimen, digging soil away from the top 10–15 cm of the plant and following the taproot to finer root hairs which were collected. Seedlings were characterised by being less than 10 cm tall and lacking mature reproductive organs, while adults were characterised by being reproductively mature and at least 20 cm tall or long.
2.4 Root staining and investigation
Fungal root colonisation was investigated using a mycorrhizal staining technique adapted from McGonigle et al. (1990). For each specimen, at least 15 thin root segments (<2 mm diameter) were cut into 1 cm pieces and placed in 10% KOH in a 90o C water bath for 60 mins, after which they were rinsed with distilled water. More lignified root segments, such as the Ericaceae, required longer clearing and were placed in the bath for 10 min increments until sufficiently cleared or for a maximum time of 90 mins. Subsequently, the roots were covered with 1% HCl overnight and rinsed the following morning. Specimen jars were filled with 2% Quink in 1% HCl and placed in a 60o C bath for 30 mins to stain the roots. This solution was again rinsed from the roots and they were covered with a de-staining solution for three nights. This process acts to clear excess stain from the root allowing fungal tissue to be seen. At this point, 10 root segments of each specimen were mounted on a slide for investigation.
For each slide the presence of fungal structures, both mycorrhizal and non-mycorrhizal, were calculated using a modification of the magnified intersections method outlined by McGonigle et al. (1990). Using a compound microscope at 40 x magnification, 100 root intersections on each slide were investigated. At each intersection, if the microscope crosshair touched a feature of AMF, EM or DSE its presence was recorded. AMF are characterised by aseptate hyphae, vesicles and arbuscules, while DSE are characterised by septate hyphae and microsclerotia (Likar et al. 2008; Bücking et al. 2012). EM are fungal endophytes characterised by fungal coils that form in fine root hairs (Chambers et al. 2008). For each AMF, DSE and EM structure, a sum was taken of each sample to provide a percentage (%) root colonisation of a given species.
2.5 Data analysis
Three-factor ANOVAs investigated differences amongst seasonality, age and species for aseptate hyphae, arbuscules, vesicles (collectively AMF), septate hyphae and microsclerotia (collectively DSE) (JMP ® Pro; Version 11, SAS Institute Inc., Cary, NC). As only two EM structures were observed, no analyses were undertaken. For all variables, assumptions of normality of residuals were tested graphically and Cochran’s test was performed to test for homogeneity of variances. Both aseptate hyphae and septate hyphae analyses met assumptions and were not transformed. Arbuscules and microsclerotia were transformed using a log(x + 1) transformation and vesicle data was transformed using a log10 transformation. Where ANOVA produced a significant output, post hoc Tukey’s tests were performed to investigate differences among means. For each species, an analysis of covariance investigated whether colonisation of aseptate hyphae was associated with levels of colonisation of septate hyphae with season and ontogeny included in a factorial design.
3 Results
3.1 Arbuscular Mycorrhizal Fungi
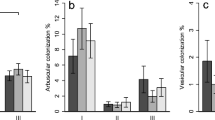
AMF colonisation was found frequently in many species. The percentage of aseptate hyphae colonisation varied among species (F8,53 = 3.002, p < 0.0001), ranging from 26 to 80.2% (Table 3, Fig. 1). Colonisation in A. megalocarpa was four times greater than that of C. glaucescens. All other species showed intermediate levels of colonisation. The two ericaceous species were colonised by aseptate hyphae at equivalent rates to other species. The effect of season on the percentage of aseptate hyphae colonisation differed with ontogeny (F2,53 = 3.002, p = 0.0025) (Table 3). In cool weather both adults and seedlings had similar levels of colonisation while for both the warm and hot seasons colonisation varied significantly but in opposite ways (Fig. 2). In the warm season, aseptate hyphae colonisation was greater in seedlings by 10 + 7.8% while in the hot season the inverse was true, with adults having higher colonisation than seedlings by 14 + 9.9%.
Percentage (%) of aseptate hyphae colonisation of roots of the nine species sampled (+SD). Species are listed in order of AMF association according to Table 1. Species not connected by the same letter are significantly different
The effect of season on the presence of arbuscules varied between ontogeny and species (three-way interaction; F16,53 = 3.91, p = 0.019) (Table 3). The abundance of arbuscules in roots was highly variable among species with abundance during any season ranging from 0 to 71%, but the nine species examined contained arbuscules in a minimum of 2/3 seasons for both adults and seedlings suggesting all may benefit from mycorrhizal associations. For each species, the presence of arbuscules in adults and seedlings were compared in each season (Fig. 3). For most species, differences in the abundance of arbuscules were not evident and did not show a consistent pattern either across season or between adult and juvenile plants. Only one species, P. australe, had higher colonisation in seedlings compared to adults; a result of no adult plants having arbuscules in the warm season. For C. glaucescens, there was a tendency for higher arbuscule colonisation in adults across all three seasons.
Percentage (%) arbuscule colonisation of adults and seedlings of the nine species over three sampling periods; Cool season, warm season, and hot season (+-SD). Comparison among adults and seedlings were undertaken for each seasonal sampling period for each species and statistic differences are indicated by different letters. Species are listed in order of AMF association according to Table 1. NB: For figures a-c the y axis is 100 to reflect their mycorrhizal status while d-f and h-i have maximum of 30% to reflect their lower dependency. As g has an outlier for colonisation % its y differs from all others at 60% maximum. NB: Tukey’s undertaken on transformed data
It was noted that most of the higher abundances of arbuscules in plants occurred in the cool season samples. To facilitate interpretation of this 3-way interaction we also investigated changes in percent colonisation through seasons for both adults and seedlings of each species. For both adults and seedlings 4 species A. megalocarpa, E. longiflora, C. glaucescens and F. nodosa, showed a decrease in colonisation with the change to hotter seasons. E. longiflora was the only species to have a complete absence of arbuscules for both adults and seedlings in the hot season.
Vesicles were found in all species, although at much lower abundances compared with the other AMF structures and differed among species (F8,53 = 2.807, p < 0.0001, Table 3). A. megalocarpa had levels of colonisation three times higher than the second most colonised species F. nodosa. For most species, vesicle colonisation was extremely low with 4 species averaging vesicle colonisation levels less than 1%. Seasonality influenced the number of vesicles among adults and seedlings (F2,53 = 2.807, p = 0.046, Table 3, Fig. 4). In both the cool season and the warm season, vesicle colonisation appeared higher in seedlings however this variation was not significant (Fig. 5). The presence of vesicles varied significantly in the hot season with adults containing more vesicles than seedlings.
Percentage (%) colonisation by vesicles in the nine species sampled (+SD). Species not connected by the same letter indicate statistical difference. Species are listed in order of AMF association according to Table 1. NB: Tukey’s undertaken on transformed data
For most species colonisation of aseptate hyphae was not correlated with levels of colonisation of septate hyphae (p > 0.1). For the two Ericaceae species, there was a significant interaction between ontogeny and septate hyphae but the direction of the trend line in seedlings and adults did not give similar patterns of colonisation for each species (W. pungens, F1,11 = 2.420, p = 0.022; E. longiflora, F1,11 = 4.527, p = 0.0227). We conclude that the relationship was not in a predictable direction.
3.2 Dark Septate Endophytes
As with AMF, DSE colonisation was found in all species. Septate hyphae colonisation was lower than aseptate hyphae colonisation for all species (Fig. 1, Fig. 6). Septate hyphae colonisation differed among species (F8,53 = 2.658, p = 0.0001). The highest colonisation occurred in C. recurvata which was twice the level of colonisation of P. australe, the least colonised species. Septate hyphae colonisation was higher in the hot season than the other seasons (F2,53 = 2.658, p < 0.0001, Fig. 7) but did not vary with age of plant (Table 3). DSE formed intracellular microsclerotia in all species sampled but varied in colonisation with few consistent patterns (F16,53 = 2.718, p = 0.0071, Table 3). Levels were greatest in the cool season for seven species while for A. megalocarpa and C. alba, colonisation was highest in the hot season (Fig. 8).
Percentage (%) of septate hyphae colonisation of roots of the nine species sampled (+-SD). Species are listed in order of AMF association according to Table 1. Species not connected by the same letter are significantly different
4 Discussion
4.1 Arbuscular Mycorrhizal Fungi
Variable patterns of AMF colonisation were able to identify some responses to changes in climate where the effect of climate on colonisation differed with age. In this climate, summer is the most stressful season in heathland and coastal dune environments (Hesp 1991; Kothamasi et al. 2001; Keith 2004) and plants would be predicted to maximise positive biotic interactions during this season, however our results for hyphal and arbuscule colonisation do not support this hypothesis. For most species, aseptate hyphae and arbuscules were not significantly higher in the warmer sampling times. If arbuscules are an indication of a positive interaction between plant and fungi (Zobel et al. 1997; Brundrett 2004), then these appeared to be haphazard in colonisation through time and with age of plant and were not clearly associated with age of plant or season.
The best predictor for levels of colonisation was species identity suggesting species-specific factors are important in determining rates of colonisation. Levels of colonisation should correlate to dependency on the plant-fungal relationship for growth and nutrient acquisition (Meney et al. 1993; Allen et al. 2003; Treseder 2013; Soudzilovskaia et al. 2015). In a meta-analysis Treseder (2013) found a consistent correlation between the percent root length colonised by AMF and plant benefit through improved growth and phosphorus uptake, the latter of which varied with plant functional group. Species were chosen from a wide range of recorded preferences for AMF (Table 1) to assess the validity of these recorded preferences. While there was variation through time, species that have been recorded as obligate mycotrophs tended to have higher colonisation levels of both hyphae and arbuscules (Fig. 1, Fig. 3). P. australe, C. alba and A. megalocarpa all showed consistently high levels of colonisation for all characteristics. However, all species showed colonisation of AMF, including arbuscules, even those recorded as being non-mycorrhizal, suggesting that the terminology of non-mycorrhizal is not particularly useful. Colonisation of roots varies in two ways; through their relationship with AMF and through changes to standing root length where root colonisation can vary as roots grow and may be unrelated to AMF abundance (Treseder 2013). As arbuscules are points of nutrient transfer, all species likely gained some benefits from colonisation. What is yet to be assessed is the level of transfer of nutrients at all levels of arbuscule colonisation and how this is mediated by species-specific root growth rates and overall root availability below ground as habitat available for colonisation.
Interactions are predicted to vary in accordance with the stress-gradient hypothesis where facilitation is more likely in stressful situations while competition will be higher when conditions are favourable (Bertness and Callaway 1994; Bertness et al. 2003). The presence of AMF in all coastal species in our study reflects this broad pattern and mirrors the study by Logan et al. (1989) which found 87% of species in NSW coastal habitats were mycorrhizal. At the scale of habitat, therefore, the stress-gradient hypothesis may explain why all species showed evidence of mycorrhizal associations, even those suggested to be non-mycorrhizal. However, under the stress-gradient hypothesis, AMF would also be predicted to be higher during more stressful seasons, such as summer, which was not found.
A number of explanations could be considered for the lack of an increase in summer colonisation in our species. While soils are well buffered from ambient temperatures and are less likely to heat to lethal levels over summer, high moisture evaporation associated with heatwaves and hot days may yield lower spore availability for colonisation. Secondly, if DSE are more capable of coping with these warmer and potentially drier conditions, then their higher colonisation in plants during warmer times may reduce AMF colonisation, suggesting a level of competition, which needs further investigation. Finally plants may preferably lower their association in response to high stress; an alternative opposing model to the stress-gradient hypothesis. Rainfall in December was average but fell in quick bursts in association with thunderstorms. Coupled with above average temperatures and heatwaves this precipitation was likely drawn away from the skeletal sandy soil quickly by plants and through evaporation, creating a high stress environment. Stress was likely exacerbated by below average rainfall and warmer than average temperatures in the previous two sampling periods (Bureau of Meterology 2017a, 2017b, 2017c). Under plentiful water, there is little cost to plants in providing photosynthates to AMF, however, under water stress, plants will reduce photosynthesis, reducing photosynthates that may be allocated to AMF (Gao et al. 2016). The decline in colonisation suggests there is an uncoupling of the relationships amongst fungi and plants that is predicted under the stress-gradient hypothesis, whereby there is no increase in colonisation when plants are under drought stress as mycorrhizae are suppressed and receiving fewer photosynthate benefits. Under progressive drought stress, arbuscule colonisation in roots can be supressed (Gehring et al. 2017; Sun et al. 2017). Our results, in part, reflect a positive association to water stress with four plant species showing highest arbuscule colonisation in May, when rainfall was highest, and a decline over the sampling period correlated with increasing drought conditions. The short-term variation in this symbiosis over summer may be offset by long-term gains thereby maintaining the importance of the symbiosis (Johnson et al. 1997; Pietikäinen and Kytöviita 2007).
The relationship between plants under water stress and fungal colonisation is not clear. There are conflicting reports on the relationship between precipitation and AMF with some studies noting a negative correlation (Rabatin 1979; Muthukumar and Udaiyan 2002; Abbaspour et al. 2012) and others finding a positive correlation (Sigüenza et al. 1996; Allen et al. 1998; Apple et al. 2004). Gao et al. (2016) examined mycorrhizal community responses to precipitation and warming over six years and found fungi responded most strongly to increased precipitation regardless of warming. Similarly, Trent et al. (1994) found higher mycorrhizal colonisation at sites associated with higher soil moisture and lower nutrient availability. While there is evidence of an uncoupling as environmental variables shift, more work is needed to distinguish the role of precipitation and stress in plant-fungal relationships through seasons.
4.2 Dark Septate Endophytes
Septate hyphae colonisation occurred in all species, although was less abundant than AMF. Evidence from a range of studies suggests that although DSE are not mycorrhizal endophytes, their presence may have a beneficial effect on plants. Furthermore as no disease symptoms are evident in plants there is no evidence of a negative impact. DSE differ from AMF in the absence of a localised interface of specialised hyphae where nutrient exchange would occur, but they may interact with the host plant and provide benefits. A meta-analysis measuring plant performance influenced by DSE found no negative effects and suggested improved plant performance under controlled conditions (Newsham 2011). While this study was unable to determine if DSE were providing positive impacts to the plant species DSE may play an important role during water-limitation and could sustain plant cells during extended drought through enhanced nutrient and water transport (Barrow 2003; Knapp et al. 2012). Li et al. (2019) found shoot biomass was increased when DSE were present, with both well-watered and water stressed conditions having increased root and total biomass of seedlings. Li et al. (2019) determined plants infected with DSE fungi had increased C and N absorption. While DSE cannot improve uptake of C and N through direct transfer they have been found to process detrital C N and P polymers, in environments where these accumulate in organic pools, which can allow increased access to a host plant (Caldwell et al. 2000).
In the current study, opposite seasonal trends were observed for septate hyphae compared to AMF, with an increase in septate hyphae over summer while microsclerotia had higher colonisation in winter. The melanin content in DSE is known to be related to stress, particularly, drought stress (Knapp et al. 2012). DSE may have been involved in providing stress relief for host plants. Liu and Wei (2019) inoculated seedlings with a strain of DSE and found inoculated seedlings had improved ability to overcome drought stress, maintaining organelle structure and influencing root morphology through regulation of hormone content levels. At the time of this study, 2017/18 was the warmest on record for NSW and the driest year since 2006 (Bureau of Meterology 2018). This may have influenced the soil rhizosphere and the community of mycorrhizal fungi and other fungal endophytes present. During an extended drought in South West United States DSE was found to exclusively colonise the roots (Barrow and Aaltonen 2001). Newsham (2011) found DSE infection was able to improve plant growth by up to 140% and was particularly helpful where nutrient availability was low. While we cannot make a direct link between DSE and benefits to our study species, our pattern of colonisation suggests the increased colonisation of DSE may provide drought tolerance. Future research to examine direct links between DSE colonisation and benefits to plants in an Australian context is needed.
The higher presence of microsclerotia in winter compared to septate hyphae may be indicative of microsclerotia as a vegetative propagule. Increased moisture and lower temperatures may promote the production of DSE storage organs while higher temperatures promoted the production of septate hyphae (Mandyam and Jumpponen 2008).
4.3 Co-colonisation
Colonisation levels of AMF were not related to colonisation levels of DSE for most species, confirming the low correlation found in other habitats (Lingfei et al. 2005; Mandyam and Jumpponen 2008; John et al. 2014), suggesting the factors influencing plant-fungal associations vary between the two endophyte types. The lack of relationship between AMF and DSE colonisation suggests they do not influence each other’s ability to colonise roots.
Plants growing in low-nutrient, high stress environments develop unique mechanisms to cope with and alleviate stress, including symbiotic association which may enhance nutrient uptake and survival (Barrow and Aaltonen 2001). This study suggests that AMF and DSE colonisation are highly variable in colonisation levels with both age and season and only partially conform to broad general patterns. We could not confirm any age-related patterns and while colonisation varied through the seasons, it was not in a particularly predictable way. There was evidence of an uncoupling between AMF and plants as environmental variables became more stressful while DSE may have provided relief to the host plants as drought stress intensified. More work is needed to understand the relationship of mycorrhizal fungi and DSE with host plants in the context of environmental shifts.
Abbreviations
- AMF:
-
Arbuscular Mycorrhizal Fungi
- DSE:
-
Dark Septate Endophyte
- EM:
-
Ericoid Mycorrhizae
References
Abbaspour H, Saeidi-Sar S, Afshari H, Abdel-Wahhab MA (2012) Tolerance of Mycorrhiza infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J Plant Physiol. Elsevier 169(7):704–709. https://doi.org/10.1016/j.jplph.2012.01.014
Allen EB, Rincón E, Allen MF, Pérez-Jimenez A, Huante P (1998) Disturbance and seasonal dynamics of mycorrhizae in a tropical deciduous forest in Mexico. Biotropica 30(2):261–274. https://doi.org/10.1111/j.1744-7429.1998.tb00060.x
Allen MF, Swenson W, Querejeta JI, Egerton-Warburton LM, Treseder KK (2003) Conceptual framework for complex interactions among plants and Fungi. Annu Rev Phytopathol 41(1):271–303. https://doi.org/10.1146/annurev.phyto.41.052002.095518
>Andrade-Linares DR, Grosch R, Restrepo S, Krumbein A, Franken P (2011) Effects of dark septate endophytes on tomato plant performance. Mycorrhiza 21(5):413–422. https://doi.org/10.1007/s00572-010-0351-1
Apple ME, Thee CI, Smith-Longozo VL, Cogar CR, Wells CE, Nowak RS (2004) Arbuscular mycorrhizal colonization of Larrea tridentata and Ambrosia dumosa roots varies with precipitation and season in the Mojave Desert. Symbiosis, 39
Barrow JR (2003) Atypical morphology of dark septate fungal root endophytes of Bouteloua in arid southwestern USA rangelands. Mycorrhiza Springer 13(5):239–247
Barrow J, Aaltonen R (2001) Evaluation of the internal colonization of Atriplex canescens (Pursh) Nutt. Roots by dark septate fungi and the influence of host physiological activity. Mycorrhiza 11(4):199–205. https://doi.org/10.1007/s005720100111
Bellgard SE (1991) Mycorrhizal Associations of Plant-Species in Hawkesbury Sandstone Vegetation. Aust. J. Bot. 39(4):357–364
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol Elsevier 9(5):191–193
Bertness MD, Bruno JF, Stachowicz JJ (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18(3):119–125
Brundrett MC (2004) Diversity and classification of mycorrhizal associations. Biol Rev Camb Philos Soc 79(3):473–495 Available at: http://www.ncbi.nlm.nih.gov/pubmed/15366760
Brundrett M C (2008) Mycorrhizal associations: the web resource. Digit. Pacific. Available at: https://mycorrhizas.info/. Accessed: 2 March 2017
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320(1–2):37–77
Brundrett M, Kendrick B (1990) The roots and mycorrhizas of herbaceous woodland plants. New Phytol 114:457–468. https://doi.org/10.1111/j.1469-8137.1990.tb00414.x/abstract
Brundrett MC and Abbott LK (1991) Roots of Jarrah Forest Plants .I. Mycorrhizal ssociations of Shrubs and Herbaceous Plants. Aust. J. Bot. 39(5):445–457
Bücking H, Liepold E, Ambilwade P (2012) The role of the Mycorrhizal Symbiosis in nutrient uptake of plants and the regulatory mechanisms underlying these transport processes. Plant Sci 4:108–132. https://doi.org/10.5772/52570
Bureau of Meterology (2017a) June in New South Wales: a dry month with cold nights in the west. Aust. Gov. Available at: http://www.bom.gov.au/climate/current/month/nsw/archive/201706.summary.shtml. Accessed 6 October 2017
Bureau of Meterology (2017b) New South Wales in august 2017: widespread cold nights, but warm days and dry up north. Aust. Gov. Available at: http://www.bom.gov.au/climate/current/month/nsw/archive/201708.summary.shtml. Accessed: 6 October 2017
Bureau of Meterology (2017c) New south wales in may 2017: a dry may with warm days. Aust. Gov. Available at: http://www.bom.gov.au/climate/current/month/nsw/archive/201705.summary.shtml. Accessed: 6 October 2017
Bureau of Meterology (2018) New South Wales in 2017: warmest on record. Available at: http://www.bom.gov.au/climate/current/annual/nsw/archive/2017.summary.shtml
Cairney JW, Ashford AE (2002) Tansley review biology of Mycorrhizal associations of Epacrids (Ericaceae). New Phytol 154(2):305–326
Caldwell BA, Jumpponen A, Trappe JM (2000) Utilization of major detrital substrates by dark-septate, root endophytes. Mycologia 92(2):230–232
Chambers SM, Curlevski NJA, Cairney JWG (2008) Ericoid mycorrhizal fungi are common root inhabitants of non-Ericaceae plants in a south-eastern Australian sclerophyll forest. FEMS Microbiol Ecol 65(2):263–270. https://doi.org/10.1111/j.1574-6941.2008.00481.x
Climate-data.org (n.d.) Climate: New South Wales. Available at: https://en.climate-data.org/oceania/australia/new-south-wales-901/. Accessed 14 November 2019
Dumbrell AJ, Ashton PD, Aziz N, Feng G, Nelson M, Dytham C, Alastair H (2011) Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol 190:794–804. https://doi.org/10.1111/j.1469-8137.2010.03636.x
Fracchia S, Krapovickas L, Aranda-Rickert A, Valentinuzzi VS (2011) Dispersal of arbuscular mycorrhizal fungi and dark septate endophytes by Ctenomys cf. knighti (Rodentia) in the northern Monte Desert of Argentina. J Arid Environ Elsevier 75(11):1016–1023
Gao C, Kim Y-C, Zheng Y, Yang W, Chen L, Ji N-N, Wan S-Q, Guo L-D (2016) Increased precipitation, rather than warming, exerts a strong influence on arbuscular mycorrhizal fungal community in a semiarid steppe ecosystem. Botany 94(6):459–469. https://doi.org/10.1139/cjb-2015-0210
Gehring CA and Connell JH (2006) Arbuscular mycorrhizal fungi in the tree seedlings of two Australian rain forests: occurrence, colonization, and relationships with plant performance. Mycorrhiza 16(2):89–98
Gehring C A, Swaty R L, and Deckert R J (2017) Mycorrhizas, drought, and host-plant mortality. in Mycorrhizal Mediat. Soil Fertil. Struct. Carbon Storage, pp. 279–298. https://doi.org/10.1016/B978-0-12-804312-7.00016-4
Gooden B, Thompson ER, French K (2019) Do native plant associations with arbuscular mycorrhizal fungi and dark septate endophytes differ between reconstructed and remnant coastal dunes? Plant Ecol:1–15. https://doi.org/10.1007/s11258-019-00959-4
Hazard C, Gosling P, Van Der Gast CJ, Mitchell DT, Doohan FM, Bending GD (2013) The role of local environment and geographical distance in determining community composition of arbuscular mycorrhizal fungi at the landscape scale. ISME J Nat Publishing Group 7(3):498–508. https://doi.org/10.1038/ismej.2012.127
Hesp PA (1991) Ecological processes and plant adaptations on coastal dunes. J Arid Environ Elsevier 21(2):165–191
Hodge A, Fitter AH (2013) Microbial mediation of plant competition and community structure. Funct Ecol 27(4):865–875. https://doi.org/10.1111/1365-2435.12002
Huusko K, Ruotsalainen AL, Markkola AM (2017) A shift from arbuscular mycorrhizal to dark septate endophytic colonization in Deschampsia flexuosa roots occurs along primary successional gradient. Mycorrhiza Mycorrhiza 27(2):129–138. https://doi.org/10.1007/s00572-016-0736-x
John J, Lundholm J, Kernaghan G (2014) Colonization of green roof plants by mycorrhizal and root endophytic fungi. Ecol Eng Elsevier B.V 71:651–659. https://doi.org/10.1016/j.ecoleng.2014.08.012
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol Cambridge University Press 135(4):575–585
Jones MD, Smith SE (2004) Exploring functional definitions of mycorrhizas: are mycorrhizas always mutualisms? Can J Bot 82(8):1089–1109. https://doi.org/10.1139/b04-110
Jumpponen ARI, Trappe JM (1998) Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytol. Cambridge University Press 140(2):295–310
Keith DA (2004) Ocean shores to desert dunes: the native vegetation of New South Wales and the ACT. Dept of Environment and Conservation (NSW), Hurstville
Kivlin SN, Emery SM, Rudgers JA (2013) Fungal symbionts alter plant responses to global change. Am J Bot 100(7):1445–1457. https://doi.org/10.3732/ajb.1200558
Klironomos JN, Hart MM (2002) Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 12(4):181–184. https://doi.org/10.1007/s00572-002-0169-6
Knapp DG, Pintye A, Kovács GM (2012) The dark side is not fastidious–dark septate endophytic fungi of native and invasive plants of semiarid sandy areas. PLoS One 7(2):e32570
Kothamasi D, Kuhad RC, Babu CR (2001) Arbuscular Mycorrhizae in plant survival strategies. Trop Ecol 42(1):1–13
Li X, He C, He X, Su F, Hou L, Ren Y, and Hou Y (2019) Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant soil springer, pp. 1–14
Likar M, Bukovnik U, Kreft I, Chrungoo NK, Regvar M (2008) Mycorrhizal status and diversity of fungal endophytes in roots of common buckwheat (Fagopyrum esculentum) and tartary buckwheat (F. tataricum). Mycorrhiza 18(6–7):309–315. https://doi.org/10.1007/s00572-008-0181-6
Lingfei L, Anna Y, Zhiwei Z (2005) Seasonality of arbuscular mycorrhizal symbiosis and dark septate endophytes in a grassland site in Southwest China. FEMS Microbiol Ecol 54(3):367–373
Liu Y, Wei X (2019) Dark Septate Endophyte improves drought tolerance of Ormosia hosiei Hemsley & EH Wilson by modulating root morphology, ultrastructure, and the ratio of root hormones. Forests 10(10):830
Logan VS, Clarke PJ, Allaway WG (1989) Mycorrhizas and root attributes of plants of coastal sand-dunes of New South Wales. Aust J Plant Physiol 16(1):141–146. https://doi.org/10.1071/PP9890141
Lu J, Liu M, Mao Y, Shen L (2007) Effects of vesicular-arbuscular mycorrhizae on the drought resistance of wild jujube (Zizyphs spinosus Hu) seedlings. Front Agric China 1(4):468–471. https://doi.org/10.1007/s11703-007-0077-9
Mandyam K, Jumpponen A (2005) Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud Mycol 53:173–189. https://doi.org/10.3114/sim.53.1.173
Mandyam K, Jumpponen A (2008) Seasonal and temporal dynamics of arbuscular mycorrhizal and dark septate endophytic fungi in a tallgrass prairie ecosystem are minimally affected by nitrogen enrichment. Mycorrhiza 18(3):145–155. https://doi.org/10.1007/s00572-008-0165-6
Mandyam K, Fox C, Jumpponen A (2012) Septate endophyte colonization and host responses of grasses and forbs native to a tallgrass prairie. Mycorrhiza 22(2):109–119. https://doi.org/10.1007/s00572-011-0386-y
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol 115(3):495–501
McLean CB, Cunnington JH, Lawrie AC (1999) Molecular ericoid diversity within from the and between Ericaceae and endophytes Epacridaceae. New Phytol 144(2):351–358
Meney KA, Dixon KW, Scheltema M, Pate JS (1993) Occurrence of vesicular mycorrhizal fungi in dryland species of restionaceae and cyperaceae from south-West Western Australia. Aust J Bot 41(6):733–737. https://doi.org/10.1071/BT9930733
Midgley DJ, Chambers SM, Cairney JWG (2004) Distribution of ericoid mycorrhizal endophytes and root-associated fungi in neighbouring Ericaceae plants in the field. Plant Soil 259(1–2):137–151. https://doi.org/10.1023/B:PLSO.0000020947.13655.9f
Miller RE, Gleadow RM, Cavagnaro TR (2014) Age versus stage: does ontogeny modify the effect of phosphorus and arbuscular mycorrhizas on above- and below-ground defence in forage sorghum? Plant. Cell Environ 37(4):929–942. https://doi.org/10.1111/pce.12209
Montero Sommerfeld H, Díaz LM, Alvarez M, Añazco Villanueva C, Matus F, Boon N, Boeckx P, Huygens D (2013) High winter diversity of arbuscular mycorrhizal fungal communities in shallow and deep grassland soils. Soil Biol Biochem Elsevier Ltd 65:236–244. https://doi.org/10.1016/j.soilbio.2013.06.002
Moora M, Zobel M (1996) Effect of arbuscular mycorrhiza on inter- and intraspecific competition of two grassland species. Oecologia 108(1):79–84. https://doi.org/10.1007/BF00333217
Moora M, Zobel M (1998) Can arbuscular mycorrhiza change the effect of root competition between conspecific plants of different ages? Can J Bot 76(4):613–619. https://doi.org/10.1139/cjb-76-4-613
Muthukumar T, Udaiyan K (2002) Seasonality of vesicular-arbuscular mycorrhizae in sedges in a semi-arid tropical grassland. Acta Oecol Elsevier 23(5):337–347
Muthukumar T, Sathiyaraj G, Priyadharsini P, Uma E, Sathiyadash K (2014) Arbuscular mycorrhizal and dark septate endophyte fungal associations in ferns and lycophytes of Palni Hills, Western Ghats, southern India. Rev Bras Bot 37(4):561–581. https://doi.org/10.1007/s40415-014-0085-y
Newsham K K (2011) A meta-analysis of plant responses to dark septate root endophytes. New Phytol. John Wiley & Sons, ltd (10.1111), 190(3), pp. 783–793. https://doi.org/10.1111/j.1469-8137.2010.03611.x
NSW Office of Environment & Heritage (n.d.) Heathlands. NSW Gov. Available at: https://www.environment.nsw.gov.au/threatenedSpeciesApp/VegFormation.aspx?formationName=Heathlands. Accessed: 24 January 2017
Pietikäinen A, Kytöviita MM (2007) Defoliation changes mycorrhizal benefit and competitive interactions between seedlings and adult plants. J Ecol 95(4):639–647. https://doi.org/10.1111/j.1365-2745.2007.01257.x
Rabatin SC (1979) Seasonal and edaphic variation in vesicular-arbuscular mycorrhizal infection of grasses by Glomus tenuis. New Phytol Wiley Online Library 83(1):95–102
Redman RS, Dunigan DD, Rodriguez RJ (2001) Fungal symbiosis from mutualism to parasitism: who controls the outcome, host or invader? New Phytol 151(3):705–716
Rodriguez RJ, Redman RS, Henson JM (2004) The role of fungal symbioses in the adaptation of plants to high stress environments. Mitig Adapt Strateg Glob Chang Springer 9(3):261–272
Ruotsalainen A, Väre H, Vestberg M (2002) Seasonality of root fungal colonization in low-alpine herbs. Mycorrhiza Springer 12(1):29–36
Sigüenza C, Espejel I, Allen EB (1996) Seasonality of mycorrhizae in coastal sand dunes of Baja California. Mycorrhiza Springer 6(2):151–157
Singh LP, Gill SS, Tuteja N (2011) Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal Behav 6(2):175–191. https://doi.org/10.4161/psb.6.2.14146
Soudzilovskaia NA, Douma JC, Akhmetzhanova AA, van Bodegom PM, Cornwell WK, Moens EJ, Treseder KK, Tibbett M, Wang Y-P, Cornelissen JHC (2015) Global patterns of plant root colonization intensity by mycorrhizal fungi explained by climate and soil chemistry. Glob Ecol Biogeogr John Wiley & Sons, ltd (10.1111) 24(3):371–382. https://doi.org/10.1111/geb.12272
Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, Corradi N, Grigoriev I, Gryganskyi A (2016) A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia Taylor & Francis 108(5):1028–1046
Sun X, Shi J, Ding G (2017) Combined effects of arbuscular mycorrhiza and drought stress on plant growth and mortality of forage sorghum. Appl Soil Ecol Elsevier 119:384–391
Sward RJ, Hallam ND, Holland AA (1978) Endogone Spores in a Heathland Area of South-Eastern Australia. Australian Journal of Botany 26(1):29–43
Thomson VP and Leishman MR (2004) Survival of native plants of Hawkesbury Sandstone communities with additional nutrients: effect of plant age and habitat. Aust. J. Bot. 52(2):141–147
Trent JD, Svejcar TJ, Blank RR (1994) Mycorrhizal colonization, hyphal lengths, and soil moisture associated with two Artemisia tridentata subspecies. Gt Basin Nat 54(4):291–300. https://doi.org/10.2307/41712845
Treseder KK (2013) The extent of mycorrhizal colonization of roots and its influence on plant growth and phosphorus content. Plant Soil 371(1–2):1–13. https://doi.org/10.1007/s11104-013-1681-5
van der Heijden MGA (2004) Arbuscular mycorrhizal fungi as support systems for seedling establishment in grassland. Ecol Lett 7(4):293–303. https://doi.org/10.1111/j.1461-0248.2004.00577.x
van der Heijden MGA, Horton TR (2009) Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J Ecol 97(6):1139–1150. https://doi.org/10.1111/j.1365-2745.2009.01570.x
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature Publishing Group 396(6706):69
Veenendaal EM, Monnaapula SC, Gilika T, Magole IL (1992) Vesicular-arbuscular mycorrhizal infection of grass seedlings in a degraded semi-arid savanna in Botswana. New Phytol 121(3):477–485. https://doi.org/10.1111/j.1469-8137.1992.tb02948.x
Zobel M, Moora M, Haukioja E (1997) Plant coexistence in the interactive environment: arbuscular mycorrhiza should not be out of mind. Oikos JSTOR 78(1):202–208
Acknowledgements
We wish to acknowledge the traditional custodians of the land – the Dharawal people, it is upon their land that the research was conducted. We are grateful to Eva Watts, Ben Gooden and Georgia Watson for their assistance with the lab work. The work was supported by the University of Wollongong. Two reviewers and the guest editor provided invaluable advice on how to improve the quality of the manuscript and we thank them for their time and expertise.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s data collection, analysis and manuscript production. Julia Rayment and Kris French collaborated on the conceptualisation, methodology and scope and design of the study. Methodology, collection and analysis of field work, original draft preparation and final edits were completed by the lead scientist, Julia Rayment. Kris French supervised the study and assisted in writing reviews and editing. Shae Jones contributed to the field work, data collection and analysis and comments for manuscript edits.
Corresponding author
Ethics declarations
Conflict of interest
All authors read and approved the final manuscript and declare they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
T Rayment, J., Jones, S. & French, K. Seasonal patterns of fungal colonisation in Australian native plants of different ages. Symbiosis 80, 169–182 (2020). https://doi.org/10.1007/s13199-019-00661-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-019-00661-z