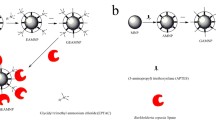
Abstract
In this paper, the free Phospholipase A1 (PLA1) was immobilized on a magnetic carrier. The average particle diameter of the magnetic carrier was 97 ± 1.3 nm, and the average particle diameter of the magnetically immobilized PLA1 was 105 nm ± 1.3 nm. The enzyme activity was 1940.5 U/g. The magnetic enzyme was chemically modified with formaldehyde, dextran-aldehyde, and dextran-aldehyde-glycine. The proportions of primary amino groups in the modified magnetic immobilized enzyme PLA1 were 0, 53.5% and 47.3%, respectively. The optimum pH of the enzyme after chemical modification was 6.5. When the system temperature was 60 °C, the magnetically immobilized PLA1 modified with dextran-aldehyde-glycine had the optimal activity and stability. This chemically modified magnetic immobilized PLA1 was applied to soybean oil degumming at 60 °C, 6.5 h (reaction time), and 0.10 mg/kg (enzyme dosage). The phosphorus content in the degummed oil was 9.2 mg/kg. The relative enzyme activity was 77.6% after 7 reuses which would be potentially advantageous for industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Free enzymes are widely used in food processing, but they are difficult to recycle and reuse. The efficiency of an enzyme can be improved by immobilization (Sheldon and van Pelt 2013). Enzyme immobilization techniques include adsorption, embedding, and covalent cross-linking. Mainly, covalent cross-linking is widely used (De Simone et al. 2018; Yang et al. 2019). The high molecular organic carrier can improve the efficiency of the enzyme, but it is difficult to separate the substrate. The magnetic immobilized enzyme prepared by using ferroferric oxide as a carrier can be rapidly separated from the substrate and can be reused for a long time (Wang et al. 2017). Especially, the nanomagnetic immobilized enzymes have many functional properties and they are a research hotspot of existing immobilized enzymes (Lei et al. 2011). It has been demonstrated that enzymes immobilized on magnetic nanoparticles can be easily separated from the reaction medium, stored and reused (Hu et al. 2008). Nevertheless, after immobilization, the stability is relatively weak compared to the chemically modified enzyme.
Degumming is an important part of the refining process. In this process, different phospholipases hydrolyze ester bonds at different positions of phospholipids to obtain lysophospholipids and fatty acids (Xiang et al. 2020; Sun et al. 2019). The main purpose is to reduce the phosphorus content in the oil. Compared with other methods, enzymatic degumming has many advantages. Degummed oil can meet the need of physical refining, reduce water consumption and wastewater generation (Sampaio et al. 2019). Furthermore, enzymatic degumming can reduce energy consumption and achieve a higher refined oil yield (Fang et al. 2018). With the development of enzymatic degumming technology, many phospholipases have been used for the degumming of vegetable oils, including phospholipase A1 (PLA1), phospholipase A2 (PLA2), phospholipase B (PLB) and phospholipase C (PLC). Particularly, PLA1 is widely used in the degumming of various vegetable oils, has wide applicability and good prospects (Manjula et al. 2011). PLA1 is an enzyme that hydrolyzes phospholipid ester bonds at the sn-1 position to produce 2-acyl-lysophospholipids and fatty acids (Aoki et al. 2007). PLA1 can reduce the phosphorus content in soybean oil and reach an acceptable level of physical refining (Jiang et al. 2014). Presently, degumming process using immobilized phospholipase was widely reported in the literature, however there are few studies on the stability effect of chemically modified PLA1 (Fang et al. 2018).
Chemical modification of enzymes is widely used as a tool to improve enzyme stability (Fang et al. 2018). Aldehydes are commonly used in enzyme modification (Hu et al. 2014) including dextran-aldehyde-glycine (Rueda et al. 2016), dextran-aldehyde (Bi et al. 2016), formaldehyde (Zi-Jian et al. 2016) and so on. The content of the primary amino group is reduced after chemical modification, and the stability of the enzyme is improved (Rodrigues et al. 2009). The modification process introduces a highly hydrophilic polymer into the enzyme molecule. This modification improves the stability of the enzyme without altering its activity. At present, chemical modification can not only change the stability of the enzyme, but also the catalytic activity and specificity of the enzyme (Bhatti et al. 2007; Siar et al. 2018). Thereby, it makes excellent characteristics compared to natural enzyme, and broadens its biological and medicinal applications (Fang et al. 2018).
In this paper, the free PLA1 was immobilized on the carrier to prepare the magnetic immobilized enzyme Fe3O4/SiOx-gP(GMA)-PLA1. Then, the effects of different chemical modification agents on magnetic immobilized PLA1 were investigated. The properties of the chemically modified enzyme and its application for soybean oil degumming were studied. This comparative study on the immobilization technology of PLA1 could have several industrial applications, and in particular vegetable oils degumming process.
Materials and methods
Material
Phospholipase A1, was purchased from Novozymes China Biotechnology Co., Ltd, (free enzyme activity is 8200 U/g). Formaldehyde was obtained from Wokai Chemical Technology Co., Ltd. Glucan (30 mg/mL) was obtained from Sinopharm Chemical Reagent Co., Ltd. Sodium borohydride was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. The dioxane (95%) was obtained from Sinopharm Chemical Reagent Co., Ltd. Soybean oil with free fatty acid content of 0.95 g/100 g, oil phosphorus content of 142.4 mg/kg, soybean variety Beidou No. 41, was obtained from Jiu San Group Harbin Wellcome Food Co., Ltd.
Immobilization of PLA1 and its modification mechanism
The magnetic carrier, enzyme and its chemical modification process are shown in Fig. 1. The modification of magnetically immobilized PLA1 with formaldehyde lead to Fe3O4/SiOx-gP(GMA)-PLA1-CH2O. The modification of the magnetically immobilized PLA1 with dextran-aldehyde lead to Fe3O4/SiOx-g-P(GMA)-PLA1-Dx. The modification of the magnetically immobilized PLA1 with glucan-aldehyde-glycine lead to Fe3O4/SiOx-g-P(GMA)-PLA1-Dx-Gly.
Development, characterization, and modification of magnetically immobilized PLA1
Development of magnetic carrier and magnetically immobilized PLA1
The magnetic carrier and magnetically immobilized PLA1 were prepared by the method of (Yu et al. 2018). The magnetic carrier Fe3O4/SiOx-g-P(GMA) was prepared with reference to atom transfer radical polymerization (ATRP) method. The Fe3O4 nanoparticles and SiOx are combined to obtain Fe3O4/SiOx composite particles. The Fe3O4/SiOx composite particles were modified by APTS method. Multifunctional groups were introduced and Fe3O4/SiOx-g-P (GMA) carrier was obtained after the modification.
Fe3O4/SiOx-g-P (GMA) (1 g) was placed in a beaker and 550 U/ml PLA1 phosphate buffer (pH of 6.5) was added and stirred for 5 h. After filtration, it was washed repeatedly with phosphate buffer solution, and finally filtered to obtain magnetic immobilized PLA1, named Fe3O4/SiOx-g-P(GMA)-PLA1, dried and stored in a refrigerator at 4 °C.
Characterization of magnetic carrier and magnetically immobilized PLA1
Fe3O4/SiOx-g-P(GMA) and Fe3O4/SiOx-g-P(GMA)-PLA1 (3 g) were freeze-dried and dissolved in absolute ethanol for 10 min, and the samples were evenly dispersed (Xie et al. 2020; Yu et al. 2018). The particle size distribution was measured under the following operating conditions; the temperature of 25 °C, counting rate of 87 kCps, measurement angle at 90°, and wavelength at 633 nm. The samples were measured by using a Mastersizer 3000 laser particle sizer (Malvern Panalytical Ltd., Malvern, UK).
Fe3O4/SiOx-g-P(GMA)-PLA1 sample (3 g), was dried in a vacuum drying box, grinded into powder, and pressed into tablets. The Kα ray of Cu was selected, the operating conditions were 1.5406 wavelength, voltage 40 kV, current 40 mA, scanning speed 4°/min, the samples were analyzed by using a D2 Phaser benchtop X-ray diffractometer from Bruker AXS Co. (Karlsruhe, Germany).
Chemical modification of Fe3O4/SiOx-g-P(GMA)-PLA1 with formaldehyde
Modification with formaldehyde was performed according to a previously described method (Real-Guerra et al. 2013). One gram of Fe3O4/SiOx-g-P(GMA)-PLA1 was suspended in 10 mL of 25 mM sodium phosphates at pH 7.0. Then 0.1% (v/v) of formaldehyde was added to the suspension and gently stirred for 3 h at 25 °C. Filtration of the suspension allows to obtain a solid product. This solid was resuspended in 100 mmol/L sodium bicarbonate solutions at pH = 10 and further reduced by using sodium borohydride at a concentration of 1 mg/mL. The modified and reduced solid product was filtered, washed with a phosphate buffer of pH = 7.0, and then thoroughly washed with distilled water to obtain Fe3O4/SiOx-g-P(GMA)-PLA1-CH2O. The formaldehyde modified enzyme was stored at 4 °C for further use.
Chemical modification of Fe3O4/SiOx-g-P(GMA)-PLA1 with dextran-aldehyde
Modification of Fe3O4/SiOx-g-P(GMA)-PLA1 was performed using dextran (MW = 70, 000 Da) with 50% oxidation (Orrego et al. 2018). At 25 °C, one gram of Gx-TLL-A was mixed with 10 mL of dextran (30 mg/mL) in 0.2 mol/L sodium phosphate buffer at pH 7.0 and incubated for 12 h. After incubation, the suspension was filtered, resuspended in a 100 mmol/L sodium bicarbonate solution at pH = 10, and further reduced by using a sodium borohydride (1 mg/mL). The reduced solid product was modified with formaldehyde as described above in 2.3.1, and Fe3O4/SiOx-g-P(GMA)-PLA1-Dx was prepared and stored at 4 °C.
Chemical modification of Fe3O4/SiOx-g-P(GMA)-PLA1 with glucan-aldehyde-glycine
Modification of Fe3O4/SiOx-g-P(GMA)-PLA1 with dextran-aldehyde-glycine was performed according to a previously described method (Fuentes et al. 2004). One gram of Fe3O4/SiOx-g-P(GMA)-PLA1 was incubated with 10 mL of a solution containing dextran–aldehyde–glycine (30 mg/mL) in 0.2 mol/L sodium phosphate at pH 7.0 and 25 °C for 12 h. As an end point to the cross-linking reaction, the samples were resuspended in 100 mmol/L sodium bicarbonate solution at pH = 10 and further reduced by using sodium borohydride (1 mg/mL). The obtained Fe3O4/SiOx-g-P(GMA)-PLA1-Dx-Gly was prepared and stored at 4 °C.
Stability of magnetic immobilized PLA1
The magnetically immobilized PLA1 was incubated in phosphate buffers for 4 h at 60 °C to study the effect of different pH on the relative enzymatic activity of magnetically immobilized PLA1. Equally, the immobilized enzyme was incubated at 50 °C in 50 mmol/L phosphate buffer at pH 6.5 to study the effect of incubation time on the relative enzymatic activity. The effect of dioxane (95%) on the relative enzymatic activity of the magnetically immobilized PLA1 was also investigated. The suspension was vigorously stirred and each time a sample was taken using a pipette to obtain a more uniform magnetically immobilized PLA1 suspension (Kajiwara et al. 2017; Ariaeenejad et al. 2020).
Application of magnetically immobilized PLA1
The effect of magnetically immobilized PLA1 on removing soybean oil
The magnetic immobilized PLA1 with the finest stability after chemical modification was applied to the degumming of soybean oil. Soybean oil (300 g) was placed in 500 mL flask, heated to 80 °C in a water bath, citric acid 45% was added and mixed rigorously. The soybean oil mixed with citric acid was cooled, and the pH of the whole system was adjusted to 6.5 by adding a 4% NaOH aqueous solution. One mg of magnetically immobilized PLA1 was soaked with deionized water and added to soybean oil (0.10 mg/kg). The remaining mixture was stirred and lead to react at 65 °C (Yu et al. 2012). The effect of degumming was assessed by measuring the amount of residual phosphorus in soybean oil.
Reuse times of magnetic immobilized PLA1
Magnetically immobilized PLA1 was applied for soybean oil degumming. When the amount of residual phosphorus is below 10 mg/kg, the relative enzyme activity was measured. The enzyme is reused for a new degumming cycle if the enzyme activity is higher than 80%. The number of reuses and changes in relative enzyme activity was recorded until the enzyme activity is less than 80% (Yu et al. 2013b).
Indicator determination of enzyme activity, primary amino and phosphorus content
PLA1 enzyme activity assay
PLA1 activity was determined using the method described previously (Yu et al. 2013b). The enzyme activity was expressed as µmol of NaOH consumed per min which is equivalent to 1 mmol/L of fatty acid released per min under the assay conditions. Phospholipase activity (1 unit) is defined as the amount of phospholipase required to hydrolyze phospholipids to produce 1 µmol of free fatty acid within 1 min under certain conditions. The phospholipase activity of a solid enzyme preparation is expressed as a unit of phospholipase activity measured per gram (g) of immobilized enzyme, ie U/g. The equation for the relative activity of phospholipase is as follows:
Relative activity (%) = A/B × 100%
A: Enzyme activity under different treatment conditions.
B: Enzyme activity in the control group (Control group is the enzyme with the highest activity in the single factor test group).
Determination of primary amino group content
Primary amines residues were titration using the picrylsulfonic acid method (Liang et al. 2018). The magnetically immobilized PLA1 (100 mg) was suspended in 0.4 mL of 100 mM sodium bicarbonate at pH 9. The suspension was incubated at 25 °C, and after 10 min 0.1 mL of picrylsulfonic acid (5%, w/w solution) was added. The colored derivatives were filtered and washed consecutively with a saturated NaCl solution, distilled water, and with 100 mM sodium bicarbonate at pH 9. A total of 50 mg of the colored preparations were then re-suspended in 2 mL of sodium bicarbonate (100 mM, pH 9) and their spectra were determined.
Determination of phosphorus content in oils and fats
The phosphorus content in the oil is determined according to the AOCS method of 12–55 (AOCS. 1997).
Data processing method
All experiments were conducted in triplicate. The data were plotted using the Origin 8.5 statistical analysis software (Northampton, MA, USA) and Design Expert 8.0 software (Stat-Ease Inc., Minneapolis, MN, USA).
Results and discussions
Particle size distribution of the magnetic carrier and magnetically immobilized PLA1
The particle size distribution of Fe3O4/SiOx-g-P(GMA) and Fe3O4/SiOx-g-P(GMA)-PLA1 is shown in Fig. 2. The particle size distribution of Fe3O4/SiOx-g-P(GMA) particles is between 59 and 225 nm, and the average particle size is 97 ± 1.4 nm. The particle size distribution of Fe3O4/SiOx-g-P(GMA)-PLA1 particles are in the range of 68–220 nm, and the average particle size is 105 ± 1.4 nm.
X-ray diffraction analysis of magnetically immobilized PLA1
The X-ray diffraction pattern of Fe3O4/SiOx-g-P(GMA)-PLA1 is shown in Fig. 3. The diffraction peaks of the magnetic carrier were 18.30°, 30.20°, 35.60°, 43.20°, 53.40°, 57.10° and 62.60° at 2θ. The diffraction peaks of the magnetically immobilized PLA1 were 18.30°, 30.10°, 35.50°, 43.10°, 53.50°, 57.00° and 62.60° at 2θ. Magnetic carrier and magnetically immobilized PLA1 were consistent with the characteristic peaks of the standard Fe3O4 particles. Fe3O4/SiOx-gP(GMA)-PLA1 has a diffraction peak at the same position as Fe3O4. This result showed that the crystal phase structure of Fe3O4 particles was not modified by the reaction of immobilization (Wang et al. 2014). The crystal phase peak of PLA1 did not appear in the XRD pattern, indicating that the free phospholipase A1 was uniformly distributed on the magnetic carrier.
Effect of chemical modification of magnetically immobilized PLA1
Effect of chemical modification on the primary amino group content of the enzyme
The content and enzyme activity of the remaining primary amino groups of the chemically modified magnetically immobilized PLA1 is shown in the supplementary Table 1. Compared with the unmodified enzyme, Fe3O4/SiOx-g-P(GMA)-PLA1 showed exposed primary amino group. The magnetically immobilized PLA1 was modified with formaldehyde, and the exposed primary amino groups were completely transformed, and modified with dextran-aldehyde and dextran-aldehyde-glycine. Thus, the primary amino group content was reduced by about 50% (Garcia-Galan et al. 2013). The modification of magnetically immobilized PLA1 with dextran-aldehyde-glycine exhibited higher enzymatic activity.
Effect of pH on relative enzyme activity of magnetic immobilized PLA1
Magnetically immobilized PLA1 was incubated in phosphate buffer for 4 h at a temperature of 60 °C. According to Fig. 4) the pH of the reaction system affected the relative enzymatic activity of the magnetically immobilized PLA1. The optimum pH values of the four magnetically immobilized PLA1 were 6.5, the range of pH withstand of the modified magnetically immobilized PLA1 was significantly broadened. At pH 5, the relative enzyme activity was about 84.9%, at pH 8.5, the relative enzyme activity was 83.5%. The relative enzyme activities of the enzyme modified with formaldehyde at lower pH (5) and higher pH (8.5) were 75.8% and 68.3%, respectively. The relative enzyme activities of enzymes modified with dextran aldehyde at low and high pH were 79.7% and 80.9%, respectively. The relative enzyme activities of the unmodified enzyme at low and high pH were 67.3% and 62.8% respectively. The chemically modified pH range of the enzyme is broadened, the chemical modification possibly reduces the unstable primary amino group and changes the environment around the enzyme molecule. In addition, the modified enzyme has the same optimal pH value as the original enzyme, and the modified enzyme can maintain higher activity, indicating that the modifier does not bind to the active center of the enzyme, but forms a protective film around the enzyme. Chahardahcherik et al. (2020) used carboxymethyl dextran to chemically modify L-asparaginase and concluded that chemical modification had no effect on the optimal pH. The changes in pH stability and optimal pH are attributed to the protection of the active site by the modification reagent. Rodrigues et al. (2011) also showed that the barrier function of the modified hydrophilic polymer on the enzyme surface protects the enzyme in harsh conditions.
Effect of magnetically immobilized PLA1 on thermal stability
Magnetically immobilized PLA1 at pH = 6.5, 60 °C, the effect of incubation time on the relative enzymatic activity of magnetically immobilized PLA1 is shown in Fig. 4b. The chemical modification of the magnetically immobilized PLA1 with formaldehyde induced a slight increase in the thermal stability of the enzyme. After the incubation for 24 h, the relative enzyme activity was 55.8%. The chemical modification with dextran-aldehyde also increased the thermal stability of the immobilized enzyme. After the incubation for 24 h, the relative enzyme activity was 46.8%. The Fe3O4/SiOx-g-P(GMA)-PLA1-Dx-Gly obtained by the modification of dextran-aldehyde-glycine exhibited the highest thermal stability of the enzyme. The relative enzyme activity was close to 70% and the enzyme activity of the unmodified magnetic immobilized enzyme was 42.2% after 24 h of incubation. The chemical modification improved the thermal stability of the enzyme. This result is consistent with those reported by Hassani and Nourozi (2014). Different modification reagents were applied to the modification process of Horseradish Peroxidase, and the thermal stability of the enzyme was improved. Several modification reagents introduce hydrophilic groups into the enzyme, and generally higher degree of hydrophilicity can lead to higher stability. The thermal stability of the modified enzyme is enhanced by the protection of the hydrophilic group from contact with water, thereby improving the stability of the enzyme after chemical modification (Hassani and Nourozi 2014).
Stability of magnetically immobilized PLA1 in organic solvent
Magnetically immobilized PLA1 was assessed at pH = 6.5, 60 °C. The effect of incubation time on the relative enzymatic activity of magnetically immobilized PLA1 was incubated in 95% dioxane solvent, as showed in Fig. 4c. Enzyme stability after chemical modification has been improved. The stability of the enzyme modified with dextran-aldehyde-glycine is the finest, after 70 h of incubation in an organic solvent, the relative enzyme activity is still 66.8%. The relative enzyme activity of magnetically immobilized enzymes modified with formaldehyde and dextran-aldehyde were 53.1% and 47.5%, respectively. The relative activity of unmodified magnetically immobilized enzymes was 43.8%. It shows that the stability of enzymes in organic solvents is improved after chemical modification. Previous study showed that the stability of lipase was improved in an organic solvent system. The hydrophilic polymer surface-modified by the immobilized enzyme can prevent organic solvents to reach the active center of the enzyme, thus improving its stability (Jia et al. 2013). Kajiwara et al. (2019) chemically modified the lipase with dextran. After modification, lipase showed higher stability in the presence of different organic solvents, which is consistent with our results. In the presence of organic solvents, the hydrophilicity around the enzyme molecule is linked to the stability of the enzyme. If the hydrophilicity around the enzyme is low, the enzyme is easily inactivated in the presence of an organic solvent. However, if the hydrophilicity around the enzyme is very high, the tightly bound water molecules will form a good aqueous microenvironment around the enzyme, and the frequency of direct contact between organic solvent molecules and enzyme molecules will be reduced. As a result, the enzyme can maintain its activity in the presence of organic solvents (Kajiwara et al. 2019).
Application effect of magnetic immobilization PLA1
Effect of magnetic immobilized PLA1 on soybean oil degumming
When assessing the pH, the thermal tolerance, and the stability of the magnetic immobilized enzyme in organic solvent, it was found that Fe3O4/SiOx-gP(GMA)-PLA1-Dx-Gly has a wide range of tolerance. Therefore, Fe3O4/SiOx-gP(GMA)-PLA1-Dx-Gly was used for the subsequent study.
At a temperature of 60 °C, pH = 6.5, an enzyme dosage of 0.10 mg/kg, magnetically immobilized PLA1 was applied to soybean oil degumming. The amount of residual phosphorus in the treated oil changed with the degumming time (Fig. 5). The phosphorus content in the two enzyme degumming oils decreased dramatically and then slowly, this is consistent with the changing trend reported by Yu et al. (Yu et al. 2013a, b). After 6.5 h, the phosphorus content in the degummed oil decreased to meet the production requirements (< 10 mg/kg). When the reaction time continues to increase, there is no significant change in the amount of residual phosphorus. Adequate reaction time ensures that the degumming reaction is carried out completely. While an extremely long reaction time reduces the enzyme activity since the temperature of the degummed oil system affects the phospholipase activity center. By comparing the degumming result, it was found that the chemically modified magnetically immobilized PLA1 slightly inhibited the degumming process. Nevertheless, after a few hours, the amount of residual phosphorus in the oil could also meet the production requirements.
Reuse effect of magnetically immobilized PLA1
The Reuse effect of magnetically immobilized PLA1 was assessed at a temperature of 60 °C, pH = 6.5, an enzyme dose of 0.10 mg/kg. The enzyme activity of Fe3O4/SiOx-g-P(GMA)-PLA1-Dx-Gly changed as the number of reuses increases (Fig. 6). The relative activity of the magnetically immobilized PLA1 decreased. Probably the molecules such as phosphorous acid in soybean oil were esterified with certain amino acid residues to cover the surface of the PLA1 which increases the steric hindrance of the magnetically immobilized PLA1 active center. This leads to a decrease in the relative enzyme activity of magnetically immobilized PLA1 (Qu et al. 2016). After 7 cycles, the relative enzyme activity was reduced to 77.6%, which no longer met the need for the degumming process. The Fe3O4/SiOx-g-P(GMA)-PLA1 without chemical modification was reduced to less than 80% after 5 cycles. Therefore, by comparison with the chemical modified PLA1 the number of repeated uses of the magnetically immobilized PLA1 was significantly increased.
Conclusion
In this paper, magnetic carrier and magnetic immobilized enzyme Fe3O4/SiOx-g-P(GMA)-PLA1 were prepared, and the average particle size was nanometer-scale with good dispersion. Three reagents were used to chemically modify the magnetically immobilized PLA1, and it was found that the chemical modification can reduce the content of primary amino groups in the enzyme, and the range of pH tolerance of the magnetically immobilized PLA1 is broadened, thereby increasing the tolerance temperature of the enzyme and its stability in an organic solvent. Chemical modification of magnetically immobilized PLA1 by using dextran-aldehyde-glycine, showed a moderate primary amino group content. The content of primary amino groups is 47.3%, and the higher enzyme activity was 1520.2 U/g. It showed a wide range of pH tolerance, a broadened temperature tolerance and higher stability in an organic solvent. It was applied to soybean oil degumming and compared with the magnetically immobilized PLA1 without chemical modification. The amount of residual phosphorus in the oil was lower than 10 mg/kg which meets the production requirements. The number of repeated uses was increased by 2 times. Therefore, the chemical modification improved the stability of Fe3O4/SiOx-g-P(GMA)-PLA1-Dx-Gly. This finding confirms the enhanced stability of phospholipase and its repeated uses in soybean oil degumming which reduced the degumming cost and showed a promising application for fats and oils industry.
Code availability
Not Applicable.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
AOCS, 1997. Official methods and recommended practices of the American Oil Chemist’s Society. Ca 12–55 Campaign, IL, USA
Aoki J, Inoue A, Makide K, Saiki N, Arai H (2007) Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie 89:197–204. https://doi.org/10.1016/j.biochi.2006.09.021
Ariaeenejad S, Motamedi E, Salekdeh GH (2020) Application of the immobilized enzyme on magnetic graphene oxide nano-carrier as a versatile bi-functional tool for efficient removal of dye from water. Bioresour Technol 319:124228
Bhatti HN, Rashid MH, Asgher M, Nawaz R, Khalid AM, Perveen R (2007) Chemical modification results in hyperactivation and thermostabilization of Fusarium solani glucoamylase. Can J Microbiol 53(2):177–185. https://doi.org/10.1016/S1095-6433(98)00034-8
Bi Y, Yu M, Zhou H, Zhou H, Wei P (2016) Biosynthesis of oleyl oleate in solvent-free system by Candida rugosa Lipase (CRL) immobilized in macroporous resin with cross-linking of aldehyde-dextran. J Mol Catal B Enzym 133:1–5. https://doi.org/10.1016/j.molcatb.2016.05.002
Chahardahcherik M, Ashrafi M, Ghasemi Y, Aminlari M (2020) Effect of chemical modification with carboxymethyl dextran on kinetic and structural properties of L-asparaginase. Anal Biochem 591:113537. https://doi.org/10.1016/j.ab.2019.113537
De Simone A, Naldi M, Bartolini M, Davani L, Andrisano V (2018) Immobilized enzyme reactors: an overview of applications in drug discovery from 2008 to 2018. Chromatographia. https://doi.org/10.1007/s10337-018-3663-5
Fang X, Wang X, Li G, Zeng J, Li J, Liu J (2018) SS-mPEG chemical modification of recombinant phospholipase C for enhanced thermal stability and catalytic efficiency. Int J Biol Macromol 111:1032–1039
Fuentes M, Mateo C, García L, Tercero JC, Guisán JM, Fernández-Lafuente R (2004) Directed covalent immobilization of aminated DNA probes on aminated plates. Biomacromol 5:883–888. https://doi.org/10.1021/bm0343949
Garcia-Galan C, Barbosa O, Fernandez-Lafuente R (2013) Stabilization of the hexameric glutamate dehydrogenase from Escherichia coli by cations and polyethyleneimine. Enzyme Microb Technol 52:211–217
Hassani L, Nourozi R (2014) Modification of lysine residues of horseradish peroxidase and its effect on stability and structure of the enzyme. Appl Biochem Biotechnol 172:3558–3569. https://doi.org/10.1007/s12010-014-0756-y
Hu F, Deng C, Zhang X (2008) Development of high-performance liquid chromatography with immobilized enzyme onto magnetic nanospheres for screening enzyme inhibitor. J chromatogr B 871:67–71
Hu Y, Yang J, Jia R, Ding Y, Li S, Huang H (2014) Chemical modification with functionalized ionic liquids: a novel method to improve the enzymatic properties of Candida rugosa lipase. Bioproc Biosyst Eng 37(8):1617–1626. https://doi.org/10.1007/s00449-014-1134-4
Jia R, Hu Y, Liu L, Jiang L, Huang H (2013) Chemical modification for improving activity and stability of lipase B from Candida antarctica with imidazolium-functional ionic liquids. Org Biomol Chem 11:7192–7198. https://doi.org/10.1039/c3ob41076e
Jiang X, Chang M, Wang X, Jin Q, Wang X (2014) A comparative study of phospholipase A1 and phospholipase C on soybean oil degumming. J Am Oil Chem Soc 91:2125–2134
Kajiwara S, Yamada R, Mori H, Hara M, Ogino H (2017) Development of sucrose-complexed lipase to improve its transesterification activity and stability in organic solvents. Biochem Eng J 121:83–87. https://doi.org/10.1016/j.bej.2017.02.002
Kajiwara S, Komatsu K, Yamada R, Matsumoto T, Yasuda M, Ogino H (2019) Modification of lipase from Candida cylindracea with dextran using the borane-pyridine complex to improve organic solvent stability. J Biotechnol 296:1–6
Lei L, Liu X, Li Y, Cui Y, Yang Y, Qin G (2011) Study on synthesis of poly (GMA)-grafted Fe3O4/SiOX magnetic nanoparticles using atom transfer radical polymerization and their application for lipase immobilization. Mater Chem Phys 125:866–871
Liang H, Russell SJ, Wood DJ, Tronci G (2018) A hydroxamic acid–methacrylated collagen conjugate for the modulation of inflammation-related MMP upregulation. J Mater Chem B 6:3703–3715. https://doi.org/10.1039/C7TB03035E
Manjula S, Jose A, Divakar S, Subramanian R (2011) Degumming rice bran oil using phospholipase-A1. Euro J Lipid Sci Technol 113(5):658–664. https://doi.org/10.1002/ejlt.201000376
Orrego AH, Ghobadi R, Moreno-Perez S, Mendoza AJ, Fernandez-Lorente G, Guisan JM, Rocha-Martin J (2018) Stabilization of immobilized lipases by intense intramolecular cross-linking of their surfaces by using Aldehyde-Dextran polymers. Int J Mol Sci 19:553. https://doi.org/10.3390/ijms19020553
Qu Y, Sun L, Li X, Zhou S, Zhang Q, Sun L, Yu D, Jiang L, Tian B (2016) Enzymatic degumming of soybean oil with magnetic immobilized phospholipase A2. LWT - Food Sci Technol 73:290–295. https://doi.org/10.1016/j.lwt.2016.06.026
Real-Guerra R, Carlini CR, Stanisçuaski F (2013) Role of lysine and acidic amino acid residues on the insecticidal activity of Jackbean urease. Toxicon 71:76–83. https://doi.org/10.1016/j.toxicon.2013.05.008
Rodrigues RC, Bolivar JM, Volpato G, Filice M, Godoy C, Fernandez-Lafuente R, Guisan JM (2009) Improved reactivation of immobilized-stabilized lipase from Thermomyces lanuginosus by its coating with highly hydrophilic polymers. J Biotechnol 144:113–119. https://doi.org/10.1016/j.jbiotec.2009.09.002
Rodrigues R, Berenguer-Murcia A, Fernandez-Lafuente R (2011) Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv Synth Catal 353:2216–2238. https://doi.org/10.1002/adsc.201100163
Rueda N, dos Santos JC, Ortiz C, Torres R, Barbosa O, Rodrigues RC, Berenguer-Murcia Á, Fernandez-Lafuente R (2016) Chemical modification in the design of immobilized enzyme biocatalysts: drawbacks and opportunities. Chem rec 16:1436–1455. https://doi.org/10.1002/tcr.201600007
Sampaio KA, Zyaykina N, Uitterhaegen E, De Greyt W, Verhé R, de Almeida Meirelles AJ, Stevens CV (2019) Enzymatic degumming of corn oil using phospholipase C from a selected strain of Pichia pastoris. Lebensm Wiss Technol 107:145–150. https://doi.org/10.1016/j.lwt.2019.03.003
Sheldon RA, van Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc rev 42:6223–6235. https://doi.org/10.1039/c3cs60075k
Siar EH, Arana-Peña S, Barbosa O, Zidoune MN, Fernandez-Lafuente R (2018) Solid phase chemical modification of agarose glyoxyl-ficin: Improving activity and stability properties by amination and modification with glutaraldehyde. Process Biochem 73:109–116. https://doi.org/10.1016/j.procbio.2018.07.013
Sun X, Zhang L, Tian S, Yang K, Xie J (2019) Phospholipid composition and emulsifying properties of rice bran lecithin from enzymatic degumming. LWT - Food Sci Technol 117:108588. https://doi.org/10.1016/j.lwt.2019.108588
Wang X, Liu X, Lei L, Zhu H, Yang Y, Li Y (2014) Preparation and characterization of magnetic microspheres with an epoxy group coating and their applications for lipase immobilization. J Macromol Sci B 53:1348–1363. https://doi.org/10.1080/00222348.2014.928156
Wang H, Lin P, Zhao S, Li S, Lu X, Liang H (2017) Preparation of magnetic microsphere-gold nanoparticle-immobilized enzyme batch reactor and its application to enzyme inhibitor screening in natural extracts by capillary electrophoresis. Chin J Chem 35:943–948
Xiang M, Wang L, Ya Q, Jian Z, Yang S (2020) High-level expression and characterization of a novel phospholipase C from Thielavia terrestris suitable for oil degumming. Int J Biol Macromol 156:740–748. https://doi.org/10.1016/j.ijbiomac.2020.04.104
Xie W, Huang M (2020) Fabrication of immobilized Candida rugosa lipase on magnetic Fe3O4-poly(glycidyl methacrylate-co-methacrylic acid) composite as an efficient and recyclable biocatalyst for enzymatic production of biodiesel. Renew Energ 158:474–486
Yang X, Wang Y, Bai R, Ma H, Wang W, Sun H, Dong Y, Qu F, Tang Q, Guo T, Binks BP (2019) Pickering emulsion-enhanced interfacial biocatalysis: tailored alginate microparticles act as particulate emulsifier and enzyme carrier. Green Chem 21:2229–2233. https://doi.org/10.1039/C8GC03573C
Yu D, Jiang L, Li Z, Shi J, Xue J, Kakuda Y (2012) Immobilization of phospholipase A1 and its application in soybean oil degumming. J Am Oil Chem Soc 89:649–656
Yu D, Ma Y, Jiang L, Elfalleh W, Shi M, Hu L (2013a) Optimization of magnetic immobilized phospholipase A1 degumming process for soybean oil using response surface methodology. Eur Food Res Technol 237:811–817. https://doi.org/10.1007/s00217-013-2057-z
Yu D, Ma Y, Xue SJ, Jiang L, Shi J (2013b) Characterization of immobilized phospholipase A1 on magnetic nanoparticles for oil degumming application. LWT - Food Sci Technol 50:519–525. https://doi.org/10.1016/j.lwt.2012.08.014
Yu D, Zhang X, Zou D, Wang T, Liu T, Wang L, Elfalleh W, Jiang L (2018) Immobilized CALB catalyzed transesterification of soybean oil and phytosterol. Food Biophys 13(2):208–215. https://doi.org/10.1007/s11483-018-9526-7
Zi-Jian WA, Jing-Bo Y, Li GP, Ning-Ning S, Wan-Chun SU, Qi-Sheng PE, Ning LI (2016) Chemical modifications of peptides and proteins with low concentration formaldehyde studied by mass spectrometry. Chin J Anal Chem 44:1193–1199
Acknowledgement
This work was supported by a grant from rice bran high-value steady-state processing technology and intelligent equipment research and demonstration (2018YFD0401101). This work was also supported by a grant from the Province Natural Science Foundation of Heilong Jang: Mechanism of enzymatic degumming process of soybean oil characterized by electrochemical biosensor (LH2020C061).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
Authors have no competing interest to declare.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Ethics approval
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Weining, W., Tang, H., Chen, Y. et al. Chemically modified magnetic immobilized phospholipase A1 and its application for soybean oil degumming. J Food Sci Technol 59, 317–326 (2022). https://doi.org/10.1007/s13197-021-05017-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-021-05017-4