Abstract
Chemical modification of lysine residues in Candida rugosa lipase (CRL) was carried out using five different functional ionic liquids, and about 15.4–25.0 % of the primary amino groups of lysine were modified. Enzymatic properties of the native and modified CRLs were investigated in olive oil hydrolysis reaction. Improved thermal stability, catalytic activity in organic solvents, and adaptability to temperature and pH changes were achieved compared with the native enzyme. CRL modified by [choline][H2PO4] showed the best results, bearing a maximum improvement of 16.7 % in terms of relative activity, 5.2-fold increase in thermostability (after incubation at 45 °C for 5 h), and 2.3-fold increase in activity in strong polar organic solvent (80 % dimethyl sulfoxide) compared with the native enzyme. The results of ultraviolet, circular dichroism and fluorescence spectroscopy suggested that the change of the secondary and tertiary structures of CRL caused by the chemical modification resulted in the enhancement of enzymatic performance. The modification of CRL with functional ionic liquids was proved to be a novel and efficient method for improving the enzymatic properties of CRL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipases are a series of versatile biocatalyst with excellent selectivity and activity under mild reaction conditions and play an important role in the development of chemical industries, such as pharmaceuticals, food, energy and fine chemicals [1–4]. However, the industrial application of lipase is often hampered by its high cost and easy inactivation in organic solvent, high temperature and other extreme conditions. Thus, most of the strategies for enzyme engineering are focused on microbiology [5], protein engineering [6], medium engineering [7], immobilization on novel supports, etc. [8]. The traditional technique of chemical modification is a very powerful tool for improving the enzymatic properties [9], leading to the introduction of functional and specificity-determining groups that are inaccessible by conventional mutagenesis techniques, and improvements in enzyme activity and/or stability, which can be achieved at a low cost using a relatively straightforward method. The modifiers usually used for chemical modification of enzymes are aldehydes, anhydrides, amines, fatty acids, halohydrocarbons, polyethylene glycol, dextrans, and so on [9–11].
Room temperature ionic liquids have been widely used as solvents or co-solvents in biocatalytic reactions and have been processed over a decade [12, 13]. In our previous work, we synthesized and grafted different functionalized ionic liquids onto the surface of mesoporous silica SBA-15 (IL-SBA). Lipase was successfully incorporated into IL-SBA by various methods, and the enzymatic properties were improved remarkably [14–17]. Recently, we also showed that various functional ILs with different cations and anions could chemically modify porcine pancreatic lipase (PPL) to increase enzyme activity and thermostability in aqueous solution [18].
Herein, Candida rugosa lipase (CRL), a typical and widely used lipase, was modified with different functionalized ionic liquids (Scheme 1). The structures of native and modified CRLs were studied by ultraviolet, circular dichroism (CD), and fluorescence spectra. The effects of temperature and pH on the enzymatic activity were studied. Moreover, the thermal stability, catalytic activity in organic solvent and kinetic parameters were investigated. This work aims to develop an efficient modification system for improving the enzymatic performance of lipase.
Materials and methods
Materials
CRL (Type VII) and trinitrobenzenesulfonic acid solution (5 % w/v in H2O) were purchased from Sigma-Aldrich China Inc. Carbonyldiimidazole (97 %) was purchased from Aladdin Chemistry Co., Ltd. Ionic liquids (99 %, HPLC) used in this work were purchased from Shanghai Chengjie Chemical Co. Ltd. Hydrochloric acid, acetone, ethanol, methanol, isooctane, anhydrous dimethyl sulfoxide and other reagents were of analytical grade and purchased from SCRC, China. All the solutions were prepared with distilled water.
Chemical modification of CRL
The CRL powder was dissolved in distilled water and magnetic stirred for 30 min at 4 °C. The obtained CRL solution was then concentrated with ammonium sulfate precipitation and transferred into a 10 kDa dialysis membrane to remove excess salt. The ionic liquid and carbonyldiimidazole were dissolved in anhydrous dimethyl sulfoxide (DMSO) for a final concentration of 1.36 M, respectively. The mixture was reacted for 4 h at room temperature to activate the ionic liquid. The activated ionic liquid (0.4 ml) was added dropwise into 15 ml of CRL solution (15 mg/ml) under vigorous stirring. The reaction was allowed to proceed for 24 h at 0 °C. The modified CRL was dialyzed exhaustively against distilled water at 4 °C for 48 h to remove the unreacted modifier molecules. The resulting lipases were denoted as [HOOCMMIm]-CRL-[PF6], [HOOCMMIm]-CRL-[Cl], [HOOCBMIm]-CRL-[Cl], [Choline]-CRL-[H2PO4] and [Choline]-CRL-[NO3], respectively. Protein concentration was determined via the BCA method using bovine serum albumin (BSA) as standard [19].
Determination of the degree of modification
The number of free amino groups presented in CRL before and after chemical modification was estimated using trinitrobenzenesulfonic acid (TNBS) assay procedure [20]. CRL solution (0.5 ml, 100 μg/ml) and 0.01 % TNBS (0.5 ml) were first incubated in 0.25 ml phosphate buffer (0.025 M, pH 8.2) at 37 °C for 2 h. Sodium dodecyl sulfate solution (0.5 ml, 10 % w/v) and hydrochloric acid solution (0.25 ml, 1 M) were added, absorbance was measured at 335 nm in a UV-1200. The degree of modification was calculated by the following equation:
absorbance A and absorbance B mean the absorbance of modified and native enzyme, respectively.
Activity assay
The enzymatic activity of CRL was assayed by the olive oil emulsion method according to the process proposed by Monier et al. [21]. The emulsification solution was prepared by mixing 50 ml of olive oil with 50 ml of gum arabic solution (7 %, w/v). Olive oil emulsification solution (5 ml) and phosphate buffer (5 ml, 0.025 M, pH 7.0) were mixed and incubated in a water bath at 30 °C for 5 min. CRL solution (1 ml, 100 μg/mL) was then added to initiate the reaction under a moderate stirring speed for 3 min. The reaction was stopped by an addition of 15 ml acetone/ethanol (1/1, v/v). The activity of CRL was determined by titration with 0.05 M sodium hydroxide solution. One unit of CRL activity was defined as the amount of enzyme required to release 1 μmol of acid per minute.
Enzymatic properties
Effect of temperature on activity
The activity of CRL was assayed at a temperature ranging from 20 to 50 °C at pH 7 through the activity assay procedure described above.
Effect of pH on activity
The effect of pH (phosphate buffer) on the activity of CRL was determined by conducting the CRL activity assay within the pH ranging from 6.0 to 8.0 at suitable temperatures.
Thermal stability
Thermal stability of CRL was assayed by incubating it in a water bath at 45 °C for 1, 2, 3, 4 and 5 h, respectively. A certain amount of CRL solution was periodically withdrawn for activity assay.
Catalytic activity in organic solvents
Activity of CRL sample in organic solvent was carried out at suitable temperature and pH. The organic solvents used were DMSO, methanol and isooctane. Reaction mixture was set up with increasing percent volume of organic solvents in phosphate buffer (0.025 M) with 10 or 20 % increment.
Determination of kinetic parameters
Experiments for the determination of kinetic parameters, the maximum rate (V max) and the Michaelis constant (K M) were performed at suitable temperature and pH using different concentrations of oil emulsification solution from 40 to 240 mg/ml. The values of K M and V max were calculated from a double reciprocal plot. In all cases, the activity of CRL was determined at 3 min to avoid the possible inhibition that may take place because of the appearance of reaction products.
Characterization of native and modified CRLs
Ultraviolet spectroscopy
Ultraviolet spectrum of CRL was recorded at 25 °C on PerkinElmer-Lambda 25 from 200 to 500 nm. The concentration of enzyme was 25 μg/ml.
Circular dichroism (CD) spectroscopy
The measurement was carried out using a circular cell with 1 mm light path length at 25 °C on JASCO-J810 spectropolarimeter (Jasco Co., Japan) with dilute enzyme solution (14.25 μg/ml). All the CD spectra were averaged by three scans taken under the identical condition and corrected for the solvent background.
Fluorescence spectroscopy
Fluorescence spectrum of CRL (100 μg/ml) was monitored on a spectrofluorometer (PerkinElmer LS55, USA) at 25 °C using a slit width of 5 nm for both excitation and emission. The emission was recorded from 300 to 400 nm, using an excitation wavelength of 270 nm. Three spectra were accumulated and averaged for each sample.
Results and discussion
Determination of modification degree and catalytic activity in aqueous environment
Table 1 summarized the results of the modification degree of CRL. In general, 15.4–25.0 % of the primary amino groups of lysine reacted with the functionalized ionic liquid, and the modification degrees at the same conditions followed the decreasing order [HOOCBMIm]–CRL–[Cl] > [Choline]–CRL–[H2PO4] > [Choline]–CRL–[NO3] > [HOOCMMIm]–CRL–[Cl] > [HOOCMMIm]–CRL–[PF6]. For the same cation (anion), the more kosmotropic the anion (cation), the higher the modification degree was obtained. The kosmotrope was reported to have stronger interactions with water molecules, thus breaking the hydrated shell of the enzyme and allowing the activated cation to graft easily onto the enzyme. The kosmotropicity for the cations was following the order: choline+ < MMIm+ < BMIm+, and for the anion was PF6 − < NO3 − < Cl− < H2PO4 − [22–25].
Generally, chemical modification of CRL will cause a decreased hydrolytic activity. Sánchez-Montero reported that CRL lost more than 80 % hydrolysis activity after modification with polyethylene glycol [26], and over 20 % decrease of activity using dextrans modification [27]. Also, Park showed that copolymer of polyethylene and maleic acid anhydride modification onto CRL caused nearly 20 % decrease of activity in the suitable conditions [28]. By contrast, the modified CRLs obtained in our study maintained a relative higher level of enzyme activity (Table 1). It was found that the activity of the modified CRLs followed the nature of ionic liquids: higher activity was achieved using ionic liquid with chaotrope (cation)–kosmotrope (anion) combination. Compared with the native CRL, the relative activity of CRL was improved by modifying it with a chaotrope–kosmotrope combination of [choline][H2PO4]. Previous study has reported similar activation of the PPL by chaotropic cations and kosmotropic anions in an aqueous environment [18]. It was worth to note that the modification degrees in this study have no association with hydrolysis activities, which was different from the previous research result [18].
Enzymatic properties
Effect of temperature on enzyme activity
Temperature has a profound influence on the enzyme activity. The variations of the relative activities of native and modified CRLs at different temperatures were shown in Fig. 1. As expected, the activities of all the lipases increased gradually with increasing temperature, and the maximum activity was obtained at 30 °C for CRL and 35 °C for modified CRLs. At temperature beyond 35 °C, the relative activity of the modified CRLs exhibited a slow decrease, while it still maintained almost 80 % of its activity from 35 to 50 °C. By contrast, the activity of native CRL declined rapidly, indicating that the modified CRLs had good heat resistance, and the modification possibly altered the conformation of the enzyme.
Effect of pH on enzyme activity
The pH dependence of the CRL hydrolysis reaction was studied within the range of 6.0–8.0, and the maximum activities of the native and modified CRLs were defined as 100 %. As shown in Fig. 2, the suitable pH was 6.5 for all CRLs, the relative activity increased from pH 6.0 to pH 6.5, and then decreased at higher pH levels, especially for native CRL and [HOOCBMIm]-CRL-[Cl]. The broad pH adaptability of the modified CRLs may be attributed to chemical modification. As shown in Fig. 2, the lipases modified by imidazolium-based cations showed better pH adaptability, which may be attributed to the ionic liquid possessing an imidazole ring and a carboxyl functional group. Both have the ability to release H+, resulting in better interaction of the charged group with the lipase molecule, thus reduced the sensitivity of the enzyme to high pH levels [29].
Thermal stability of CRL
The thermal stability of native and modified CRLs was evaluated by incubating them in a water bath at 45 °C. As presented in Fig. 3, the native lipase lost its initial activity within approximately 2 h (58.0 %), whereas the modified forms retained their initial activity by about 97.8 % for [HOOCMMIm]-CRL-[Cl] and 84.4 % for [choline]-CRL-[H2PO4] under the same conditions. These results indicated that the thermal stability of the modified lipases was much better than that of the native CRL due to the chemical modification with ionic liquids. Our modification method showed better thermal stability than that the modification using citraconic anhydride and maleic anhydride as modifier [30]. In comparison to our previous study for PPL modification [18], the kosmotropic cation modification did not cause a higher stability of CRL, as showed by ionic liquid modification of different lipases which yielded different results.
Catalytic activity in organic solvents
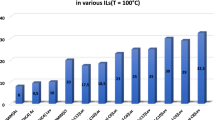
The catalytic activities of the native and modified CRLs in organic solvents were investigated in DMSO, methanol and isooctane at their own suitable temperature and pH. As shown in Figs. 4, 5, 6, the modified CRLs showed better catalytic activity in organic solvents compared with the native one. In strong polar organic solvent (80 % dimethyl sulfoxide), enzymes with choline ionic liquids modification owned more than twofold activity compared with the native enzyme (Fig. 4). And in aqueous methanol, the activities of the lipases decreased, especially the native CRL, whereas the various modified forms showed better catalytic activity (Fig. 5). In aqueous isooctane (Fig. 6), the activity of the lipases all increased to some degree. In 80 % isooctane, the activities of the modified forms ranged from 98.8 to 122.7 %, whereas that of the native CRL retained 96.6 % of the initial activity under the same conditions. The difference in activity may have been a consequence of the altered conformation of the modified enzyme [30, 31].
Kinetic parameters
The kinetic constants (K M and V max) of the native and modified CRLs were determined. The values for all the lipases were calculated by using Lineweaver–Burk plots. The K M value of the native CRL was 150.2 mg/ml, whereas the apparent K M values of the modified CRLs ranged from 142.8 to 208.3 mg/ml, as was shown in Table 2. The V max value of [choline]-CRL-[H2PO4] was 295.0 (μmol/min mg protein), which is noticeably higher than that of the native CRL. These results may be interpreted as follows: the modification changed the conformation of CRL, resulting in a greater probability for substrate-enzyme complex formation or higher accessibility for the substrate to the CRL active site.
Characterization of native and modified CRLs
Ultraviolet spectroscopy
Protein ultraviolet absorption is mainly due to the electronic excitation of aromatic amino acids such as tryptophan and tyrosine. The absorption spectra of these chromophores could be changed with varying conditions. To explore the mechanism of the improvement of the enzymatic properties of CRL upon modification, the ultraviolet spectra of the native and modified CRLs were determined. As shown in Fig. 7, the absorption peak of CRL was approximately 260 nm, with an absorption intensity of 0.45. Compared with the native CRL, the modified forms showed a slight red shift in their ultraviolet peaks and their absorbance values decreased. Changes in the ultraviolet spectra of proteins in the 230 to 270 nm regions are related to transformations in conformation and a decrease in aromatic amino acid exposure [32]. The present results indicated that the ionic liquid modification caused microenvironmental changes in the enzymes.
CD spectroscopy
CD measurements were performed to elucidate the secondary and tertiary structures of the native and modified CRLs in phosphate buffer (pH 7.0). As shown in Fig. 8, the native CRL had negative bands at 208 to 220 nm, which agreed with previous reports [30, 33]. However, a change was observed in the CD spectra of the modified lipases with respect to that of the native CRL, as could be attributed to transformations in both secondary and tertiary structures [34]. The percentages of the secondary structure elements were analyzed using Jwsse32 software (Table 3), the ratios of α-helical, β-sheet, and β-turn structures were altered after modification. The differences in secondary structures of the modified lipases in aqueous buffer may be a result from the changes in the enzymatic properties of the modified CRLs [35].
Fluorescence spectroscopy
A fluorescence emission from a 270 nm excitation was attributed to tryptophan residues. Thus, tryptophan fluorescence was used to probe structural changes of the modified enzyme compared with the native one. As shown in Fig. 9, the emission maximum at 310 nm of the native CRL did not change upon modification, but an increase was observed in the relative fluorescence intensity as the modification progressed. A previous study reported that the modified CRLs showed a more compact conformation [34]. Combining results from ultraviolet and CD spectroscopy studies, we believe that ionic liquid modification of CRL occurred, and the conformation of CRL was altered to some degree.
Conclusions
In this study, various functional ionic liquids with different cations and anions were grafted onto CRL through lysine coupling, resulting in different degrees of modification. The chemically modified CRLs exhibited improved thermal stability, catalytic activity in organic solvents and adaptability to temperature and pH changes. Modification with ionic liquid [choline][H2PO4] resulted in maximum improvement of CRL in terms of activity, as well as better thermal stability. Furthermore, the ultraviolet, CD, and fluorescence measurements demonstrated that the chemical modification caused change of enzyme conformation to different extent. In addition, in comparison to the previous modification of PPL, the results showed that different kinds of enzymes require different modifiers. Now we are trying to evaluate the mechanism of various catalytic performance improvements caused by different ionic liquids modification using the molecular simulation and new spectroscopy characterization technology.
References
Seelig B, Szostak JW (2007) Selection and evolution of enzymes from a partially randomized non-catalytic scaffold. Nature 448:828–831
Zheng GW, Xu JH (2011) New opportunities for biocatalysis: driving the synthesis of chiral chemicals. Curr Opin Biotechnol 22:784–792
Goswami D, Basu JK, De S (2013) Lipase applications in oil hydrolysis with a case study on castor oil: a review. Crit Rev Biotechnol 33:1–16
Ding Y, Huang H, Hu Y (2013) New progress on lipases catalyzed C–C bond formation reactions. Chin J Org Chem 33:905–914
Deive FJ, Álvarez MS, Sanromán MA, Longo MA (2013) North Western Spain hot springs are a source of lipolytic enzyme-producing thermophilic microorganisms. Bioprocess Biosyst Eng 36:239–250
Hult K, Maurer S, Hamberg A (2012) Rational engineering of Candida antarctica lipase B for selective monoacylation of diols. Chem Commun 48:10013–10015
Durand E, Lecomte J, Barea B, Piombo G, Dubreucq E, Villeneuve P (2012) Evaluation of deep eutectic solvents as new media for Candida antarctica B catalyzed reactions. Process Biochem 47:2081–2089
Forsyth C, Patwardhan SV (2013) Controlling performance of lipase immobilised on bioinspired silica. J Mater Chem 1:1164–1174
Díaz-Rodríguez A, Davis BG (2011) Chemical modification in the creation of novel biocatalysts. Curr Opin Chem Biol 15:211–219
Cowan DA, Fernandez-Lafuente R (2011) Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb Technol 49:326–346
Chalker JM, Bernardes GJ, Lin YA, Davis BG (2009) Chemical modification of proteins at cysteine: opportunities in chemistry and biology. Chem Asian J 4:630–640
Han D, Tang B, Lee YR, Row KH (2012) Application of ionic liquid in liquid phase microextraction technology. J Sep Sci 35:2949–2961
Plechkova NV, Seddon KR (2008) Applications of ionic liquids in the chemical industry. Chem Soc Rev 37:123–150
Zou B, Hu Y, Yu DH, Jiang L, Liu WM, Song P (2011) Functionalized ionic liquid modified mesoporous silica SBA-15: a novel, designable and efficient carrier for porcine pancreas lipase. Colloids Surf B 88:93–99
Zou B, Hu Y, Yu DH, Xia JJ, Tang SS, Liu WM, Huang H (2010) Immobilization of porcine pancreatic lipase onto ionic liquid modified mesoporous silica SBA-15. Biochem Eng J 53:150–153
Hu Y, Tang SS, Jiang L, Zou B, Yang J, Huang H (2012) Immobilization of Burkholderia cepacia lipase on functionalized ionic liquids modified mesoporous silica SBA-15. Process Biochem 47:2291–2299
Yang J, Hu Y, Jiang L, Zou B, Jia R, Huang H (2013) Enhancing the catalytic properties of porcine pancreatic lipase by immobilization on SBA-15 modified by functionalized ionic liquid. Biochem Eng J 70:46–54
Jia R, Hu Y, Liu L, Jiang L, Zou B, Huang H (2013) Enhancing catalytic performance of porcine pancreatic lipase by covalent modification using functional ionic liquids. ACS Catal 3:1976–1983
Smith PK, Frohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Habeeb AF (1966) Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem 14:328–336
Monier M, Wei Y, Sarhan A (2010) Evaluation of the potential of polymeric carriers based on photo-crosslinkable chitosan in the formulation of lipase from Candida rugosa immobilization. J Mol Catal B Enzym 63:93–101
Zhao H (2006) Are ionic liquids kosmotropic or chaotropic? An evaluation of available thermodynamic parameters for quantifying the ion kosmotropicity of ionic liquids. J Chem Technol Biotechnol 81:877–891
Zhao H, Olubajo O, Song Z, Sims AL, Person TE, Lawal RA, Holley LA (2006) Effect of kosmotropicity of ionic liquids on the enzyme stability in aqueous solutions. Bioorg Chem 34:15–25
Yang Z (2009) Hofmeister effects: an explanation for the impact of ionic liquids on biocatalysis. J Biotechnol 144:12–22
Zhao H (2010) Methods for stabilizing and activating enzymes in ionic liquids—a review. J Chem Technol Biotechnol 85:891–907
Hernáiz MJ, Sánchez-Montero JM, Sinisterra JV (1999) Modification of purified lipases from Candida rugosa with polyethylene glycol: a systematic study. Enzyme Microb Technol 24:181–190
de la Casa RM, Guisán JM, Sánchez-Montero JM, Sinisterra JV (2002) Modification of the activities of two different lipases from Candida rugosa with dextrans. Enzyme Microb Technol 30:30–40
Park K, Kim H, Maken S, Kim Y, Min B, Park J (2005) Characteristics of the lipase from Candida rugosa modified with copolymers of polyoxyethylene derivative and maleic acid anhydride. Korean J Chem Eng 22:412–417
Bian W, Lou LL, Yan B, Zhang C, Wu S, Liu S (2011) Immobilization of papain by carboxyl-modified SBA-15: rechecking the carboxyl after excluding the contribution of H2SO4 treatment. Micropor Mesopor Mater 143:341–347
Liu JZ, Wang TL, Huang MT, Song HY, Weng LP, Ji LN (2006) Increased thermal and organic solvent tolerance of modified horseradish peroxidase. Protein Eng Des Sel 19:169–173
Szabó A, Kotormán M, Laczkó I, Simon LM (2009) Improved stability and catalytic activity of chemically modified papain in aqueous organic solvents. Process Biochem 44:199–204
Xiong Y, Gao J, Zheng J, Deng N (2011) Effects of succinic anhydride modification on laccase stability and phenolics removal efficiency. Chin J Catal 32:1584–1591
Freitas DD, Abrahão-Neto J (2010) Biochemical and biophysical characterization of lysozyme modified by PEGylation. Int J Pharm 392:111–117
Liu JZ, Wang M (2007) Improvement of activity and stability of chloroperoxidase by chemical modification. BMC Biotechnol 7:23–30
Nordwald EM, Kaar JL (2013) Stabilization of enzymes in ionic liquids via modification of enzyme charge. Biotechnol Bioeng 110:2352–2360
Acknowledgments
This research was supported by the National Science Foundation for Distinguished Young Scholars of China (No. 21225626), the National Natural Science Foundation of China for Young Scholars (Grant No. 20906049), the National Basic Research Program of China (Grant No. 2011CB710800), the Hi-Tech Research and Development Program of China (863 Program, 2011AA02A209).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, Y., Yang, J., Jia, R. et al. Chemical modification with functionalized ionic liquids: a novel method to improve the enzymatic properties of Candida rugosa lipase. Bioprocess Biosyst Eng 37, 1617–1626 (2014). https://doi.org/10.1007/s00449-014-1134-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1134-4