Abstract
Phospholipase A1 (PLA1), or Lecitase® Ultra, was immobilized on three different supports, calcium alginate (CA), calcium alginate-chitosan (CAC), and calcium alginate-gelatin (CAG), and crosslinked with glutaraldehyde. The results indicated that PLA1–CA retained 56.2% of the enzyme’s initial activity, whereas PLA1–CAC and PLA1–CAG retained 65.5 and 60.2%, respectively. Compared with free PLA1, the optimal pH of immobilized PLA1 shifted to the basic side by 0.5–1.0 pH units and the pH/activity profile range was considerably broadened. Similarly, the temperature-optima of PLA1–CAC and PLA1–CAG increased from 50 to 60 °C, and their thermal stability increased with relative activities of more than 90% that covered a wider temperature range spanning 50–65 °C. In a batch oil degumming process, the final residual phosphorus content was reduced to less than 10 mg/kg with free PLA1, PLA1–CAC and PLA1–CA in less than 5, 6 and 8 h respectively while PLA1–CAG was only able to reduce it to 15 mg/kg in 10 h. When the PLA1–CAC was applied in a plant degumming trial, the final residual phosphorus content was reduced to 9.7 mg/kg with 99.1% recovery of soybean oil. The recoveries of immobilized PLA1–CAC and activity of PLA1 were 80.2 and 78.2% respectively. Therefore, it was concluded that PLA1–CAC was the best immobilized enzyme complex for the continuous hydrolysis of phospholipids in crude vegetable oils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The refining of crude oil consists of degumming, neutralization, bleaching and deodorization. Degumming is the first stage and serves to remove phospholipids and mucilaginous gums from the crude oil. Some common methods are water degumming, acid degumming, deep degumming and membrane processing [1]. Water degumming removes most of the hydratable phospholipids and reduces the phosphorus levels to about 150–-250 mg/kg, while acid degumming reduces the phosphorus levels to below 10 mg/kg. The degummed oil can be further neutralized, bleached and deodorized or bleached and steam refined depending on whether the chemical or physical refining method is used.
Enzymatic degumming, a new degumming method recently employed, has some advantages over the traditional methods such as higher oil yields, consistent degumming results and less water consumption. Furthermore, it is safer, more environmentally friendly and has excellent potential for large scale industrial applications [1–3]. The enzyme, phospholipase A1 (PLA1), has been successfully employed in vegetable oil degumming [1, 3–6]. It hydrolyzes the ester bonds of phospholipids at the sn-1 position and produces 2-acyl-lysophospholipids and free fatty acids. Lysophospholipids are water hydratable and easier to remove, and makes it possible to reduce phosphorus levels to less than 5 mg/kg in rice brain oil [3]. Furthermore, the lysophospholipids retain less oil which results in greater oil yields. The commercial Lecitase® Ultra is a single protein with a molecular mass of 35 kDa, and a high specificity for phospholipids in oil [1, 3–5]. However, the free enzyme can be used only once and can result in higher operating costs. Immobilized enzymes, on the other hand, can be reused many times, which are more stable during storage and use and have a better environment for catalytic activity [7]. Many techniques have been used for enzyme immobilization, such as entrapment in calcium alginate [7], covalent attachment on chitosan and gelatin [8], crosslinking with glutaraldehyde, or a combination of these methods [9, 10]. Calcium-alginate, chitosan, and gelatin are by far the most widely used polymers for immobilization [11]. Sheelu et al. used immobilized Lecitase (Phospholipase A1 in gelatin crosslinked with glutaraldehyde), to efficiently degum rice bran oil without loss of enzyme activity even after 6 recycles [3].
This research investigated the immobilization of PLA1 on calcium-alginate, calcium alginate-chitosan, and calcium alginate-gelatin using glutaraldehyde as a cross-linking reagent, the pH and temperature optima of the immobilized PLA1 and the thermal stability and reusability of the immobilized PLA1. The objective of this research was to provide evidence on the efficacy of the methods used to immobilize PLA1 and on the usefulness of the immobilized enzyme in industrial applications.
Materials and Methods
Materials
The phospholipase A1 (Lecitase® Ultra) was obtained from Novozymes A/S (Bagsvaerd, Denmark). The water-degummed soybean oil with a phosphorus content of 142.4 mg/kg was supplied by the Fukang Oil and Fat Co. (Harbin, China). The glutaraldehyde (50%) was from Kemio Chem. Co., (Tianjin, China), and the sodium alginate, gelatin (B type) and chitosan were from Tianjun Chemical Co., (Tianjin, China). All other chemicals were analytical grade.
Immobilization of Phospholipase A1
The phospholipase A1 (PLA1) was immobilized in calcium alginate (PLA1–CA) using the method of Ates and Mehmetoglu with some modification [12]. The mixture of 50 mL of sodium alginate (2.0%)/acetic acid (5.0%) was mixed with 5 mL diluted PLA1 solution (700 U/g) at a speed of 180 rpm. The homogeneous mixture was extruded drop by drop at 5 drops/s using a 10 mL sterile hypodermic syringe needle into a 0.2 M CaCl2 solution to form 2 mm diameter beads.
In a second procedure, the PLA1 was immobilized in a calcium alginate–chitosan (PLA1–CAC) complex using the method in Sun et al. [13] with some modification. A mixture of 25 mL of chitosan (4%)/acetic acid (5%)/CaCl2 (0.2 M) was mixed with 5 mL of PLA1 (700 U/g) at a speed of 180 rpm. Then 25 mL of sodium alginate (4%)/acetic acid (5%) were extruded drop by drop into the mixture at 5 drop/s to form beads.
In a third procedure, the PLA1 was immobilized in a calcium alginate–gelatin (PLA1–CAG) complex using the method in Zhu et al. with some modification [14]. 25 mL of sodium alginate (4%)/acetic acid (5%) were mixed with 25 mL gelatin (8%). Then 5 mL of PLA1 (700 U/g) were added to the 50 mL alginate–gelatin solution at a stirring rate of 180 rpm. CaCl2 (0.2 M) solution was dropped into the mixture at 5 drop/s and stirred at 180 rpm to form beads.
The beads from all three procedures were allowed to harden in their respective solutions for 10 h at 4 °C, then washed three times with sterile distilled water to removal the residual CaCl2. The beads were hardened again in 5 mL of 0.4% glutaraldehyde solution and washed with 10 mL NaHCO3 (0.1 M), 10 Ml NaCl (0.1 M), 10 mL sodium acetate buffer (0.1 M, pH5.5), and 10 mL Tris–HCl buffer (pH 8.0), until free of all cross-linking reagents. The immobilized enzymes were finally freeze-dried.
The activities of PLA1–CA, PLA1–CAC and PLA1–CAG were 1207, 1380 and 1346 U/g respectively. These beads were used in the optimization studies and plant trial.
Enzyme Activity Assay
The activity of PLA1 was determined using the method described by Sheelu et al. [3] with minor modifications. A soybean lecithin emulsion was prepared by dispersing 4 g of soybean lecithin in 100 mL of disodium hydrogen phosphate–citric acid buffer (0.01 M, pH 5.0) and shaking for 5 min in a 50 °C reciprocating water bath (180 rpm). The enzyme solution was prepared by diluting 1 mL of commercial PLA1 in 10 mL of phosphate buffer. 4 mL of the diluted enzyme solution was added to the warmed lecithin emulsion and incubated for 15 min. The hydrolysis reaction was stopped by adding 60 mL of alcohol.
The long chain fatty acids released by PLA1 were neutralized with 0.05 M NaOH addition to maintain a fixed pH of 5.0. The enzyme activity was expressed as micromoles of NaOH consumed per minute which is equivalent to 1 µmol of fatty acid released per min under the assay conditions. The enzyme activity was 6,900 ± 200 U/g.
Effect of pH and Temperature on the Activities of Free and Immobilized Enzymes
A 4% soybean lecithin emulsion in 0.1 M acetate buffer with varying pH values (4.0, 4.5, 5.0, 5.5, 6.0, 6.5, and 7.0) was used to determine optimum pH. The optimum temperature of the free and immobilized PLA1 enzymes was determined in a 4% lecithin emulsion (0.1 M acetate buffer, pH 5.5) using 7 different temperatures (40, 45, 50, 55, 60, 65, and 70 °C). The highest activity under optimum pH or temperature conditions was designated as 100% and the relative activity was defined as the value proportional to that highest activity.
Thermal Stabilities of free and Immobilized Enzymes
The thermal stabilities of the free and immobilized enzymes were determined by incubating the enzymes in 0.1 M acetate buffer (pH 5.5) at various temperatures (50, 55, 60, 65 and 70 °C) for 60 min. After 60 min, the enzymes were immediately cooled to ambient temperature and the residual activities were measured. The relative activity was defined as the value proportional to the initial activity (100%).
Reusability of Immobilized Enzymes
To measure reusability, the immobilized enzymes were placed into oil samples at 50 °C and the hydrolysis of the phospholipids was monitored over 7 cycles. The standard assay procedure was used to measure enzyme activity. After each cycle, the immobilized enzyme was washed with 0.1 M acetate buffer (pH5.5) and reused. The residual activity was defined as the value proportional to the original activity (100%).
Batch Degumming of Soybean Oil
Batch degumming was performed with free and immobilized enzymes using the optimum conditions determined in the previous sections. 200 mL of soybean oil and 0.13 mL citric acid (45%) were added to a 500 mL Erlenmeyer flask, heated to 80 °C and stirred at 900 rpm for 5 min (HYJ30 mixer, Huilong Mixers Co., Ltd. China). The oil was cooled to 60 °C, and then adjusted to pH (5.5–6.0) with 1 M NaOH while stirring at 900 rpm. Water (3 mL) was added to the oil and stirred at 30 rpm for 20 min. The free or immobilized enzyme solution was added to the oil at a dose rate of 250 U/kg (oil mass). The mixture was incubated (60 °C) and stirred (30 rpm) for 10 h. The samples for phosphorus analysis were taken at 1 h intervals.
After 10 h, the reaction mixture was centrifuged at 6,000 g to remove the immobilized PLA1 and mucilaginous gums. The residual phosphorus content in the oil phase was assayed by the AOCS method ca 12–55 [15].
Plant Degumming Trials on Soybean Oil
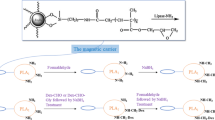
A plant degumming trial on soybean oil was conducted at the Fukang Oil and Fat Co (Harbin, China). The phosphorus content of the water-degummed oil was 142.4 mg/kg. The flow diagram of the oil-degumming process is shown in Fig. 1. Ten tonnes of degummed soybean oil (80 °C) and 6.5 kg citric acid solution (45%) were transported to a multi-mixer (MX-60, Alfa Laval, Sweden) at 10t/h and 6.5 kg/h by a dosing pump (YBND, Jiangyin Fuxin Machinery Co., Ltd. China) respectively. The oil and citric acid were mixed at 960 rpm. The mixture was cooled to 60 °C and adjusted to pH 6.0 with 4% NaOH and mixed again. Water (150 kg/h) was added and mixed in a static mixer (SK-100, Qidong Metallurgy Petrochemical Machinery Co., Ltd. China) then transported to a 15 t stainless steel reaction tank. The PLA1–CAC (250 U/kg oil) was added to the tank and stirred at 30 rpm for 6.5 h at 60 °C.
The final reaction mixture was filtered with a double basket filter (~2 mm) (PSL-S, Jiangsu Juneng Machinery Co., LTD. China) to remove the immobilized PLA1–CAC. After filtering, the reaction mixture was centrifuged at 12,000g to remove mucilaginous gums and produce degummed oil. The phosphorus content and recovery rate of oil was determined as well as the recovery rate of the immobilized PLA1–CAC and activity of the PLA1.
Statistical Analysis
All experiments were performed in triplicate. The data are reported as means ± standard deviations. One-way ANOVA was performed using SPSS 14 Statistical software (SPSS Inc., Chicago, IL). Differences were considered to be significant at p ≤ 0.05, according to Duncan’s Multiple Range Test.
Results and Discussion
Enzyme Immobilization
The PLA1 enzyme was immobilized on three different supports and the ratio of immobilized enzyme activity to free enzyme activity was designated as the remaining activity. The PLA1–CAC bead had the highest remaining activity (65.5%); the PLA1–CAG bead had the second highest (60.2%) while the PLA1–CA bead was the lowest with 56.2%.
Sodium alginate has been shown to be an effective substrate for immobilizing enzymes, cell organelles, microorganisms, and plant and animal cells [5]. However, due to the high porosity of alginate beads, the entrapped enzymes with molecular weights of 300,000 or less can leach out through the polymer matrix. This would explain the leakage of PLA1 (MW = 35,000) from the structure of calcium alginate gels [7]. To reduce this problem, PLA1 was first entrapped in calcium alginate and then treated with glutaraldehyde to form a crosslinked matrix.
Chitosan has many features favorable for immobilization, such as high protein affinity availability of reactive functional groups; hydrophilicity; mechanical stability and rigidity; regenerative ability; and ease of preparation in different geometrical configurations. These properties provide the system with permeability and a surface area suitable for a chosen biotransformation [16].
Gelatin along with glutaraldehyde as a cross-linking agent can be used for the immobilization of cells and enzymes. In this study, the incorporation of gelatin into sodium alginate increased the remaining activity of PLA1–CAG beads compared to PLA1–CA beads. It was also reported that more than 80% retention of phospholipase A1 activity was observed in hydrogel containing 43.5% gelatin crosslinked with glutaraldehyde [3].
It appears that when calcium alginate is used alone as the substrate, the immobilization of PLA1 does not produce an ideal product, however, when it is combined with chitosan or gelatin, a better immobilized product is obtained.
Effect of pH on the Activities of Free and Immobilized Enzymes
Figure 2 shows the pH-activity profiles of the free and immobilized enzymes from pH 4.0 to 7.0 and 50 °C. The maximum activity for free PLA1 occurs at pH 5.0 which indicates the free enzyme hydrolyzes phospholipids under acid conditions. The optimum pH for PLA1–CA is 5.5, and the optimum pH for PLA1–CAC and PLA1–CAG is 6.0. The activity of the free PLA1 dramatically decreased at pHs greater than 5.0. These plots indicate that the immobilized enzymes are active over a wide pH range compared to the free PLA1. The results also show a shift in the optimum pH (to higher pH values) for the immobilized PLA1. This was attributed to the partitioning effects of the supports. Since the reaction medium and the insoluble support are two different phases, the depletion in one of the phases is possible. When this occurs, the immediate microenvironment of the enzyme on the surface of the support material will be different from that of the bulk solution. This partitioning effect can cause changes to the pH-optimum and these changes becomes more complex when the substrate and/or the products are charged [11, 17]. Compared to free PLA1, the pH-activity profile of the immobilized enzymes showed an upward shift in pH with considerable broadening, due to greater pH stability (Fig. 2). Similar results were found by other researchers on immobilized invertase [18, 19]. When the pH was within the range of 5.5–6.5, the immobilized PLA1 had relative activities greater than 90%. The optimum pH to hydrolyze phospholipids was 4.8–5.1 when free PLA1 (Lecitase® Ultra) was used to degum soybean oil [1], while the optimum pH for partial hydrolysis of soybean oil by PLA1 was 6.8 [20]. The broadened pH/activity profile of immobilized PLA1 may have important benefits when developing industrial applications.
Effect of Temperature on the Activities of Free and Immobilized Enzymes
Figure 3 shows the temperature optima for free PLA1 and PLA1–CA (50 and 55 °C, respectively) and for PLA1–CAC and PLA1–CAG (both 60 °C). Further increases in temperature, above their optima, resulted in lower activities due to thermal denaturation. The shift in optimal temperature toward higher values could be due to the immobilization of the enzyme, which increased its stability and resulted in formation of the enzyme–substrate complex which hindered the access of substrates to the active site [11]. All immobilized enzymes retained more than 90% of their relative activity over a broader range of temperatures from 50 to 65 °C compared to the free enzyme. Immobilization increased the optimum temperature by about 10 °C and thermal stability by 15 °C. Factors that could cause an increase in optimum temperate are diffusion effects, since the immobilized enzyme may be more easily contacted by the substrate due to enhanced mass transfer with increased bulk temperatures [11]. Sheelu immobilized Lecitase in a gelatin hydrogel matrix crosslinked with glutaraldehyde, and found that the optimum temperature increased to 50 °C compared to 37–40 °C for the free enzyme [3]. These results show that the immobilized enzyme can tolerate higher temperatures.
Thermal Stability of Free Enzyme and Immobilized Enzymes
The thermal stability of the free and immobilized enzymes is shown in Fig. 4. The immobilized enzymes retained >74% of their catalytic activity after 1 h incubation at 65 °C, while the free PLA1 retained only 36.3% of its initial activity Moreover, this retained activity was more significant in the case of PLA1–CAC, which exhibited 69.4% relative activity after 1 h at 70 °C, whereas the free PLA1 showed only 20.2% relative activity.
In this study, the enzymes were entrapped in polymer matrices (alginate, gelatin and chitosan) that were crosslinked with glutaraldehyde. The kinetics of the immobilized biocatalysts was affected by diffusion restrictions. That is the increase in thermal stability is due to their attachment to the support material which partly compensates for the strong activity loss suffered by the free enzyme [11]. The increase in the thermal stability implies a reduction in the denaturation reaction. A similar result was reported for PLA1 immobilized on hybrid Gelatin Hydrogel [3].
Reusability of Immobilized Enzymes
The operational stability of the three immobilized PLA1 was tested by recycling. The activity of the first cycle was defined as 100%. The relative activities of the three immobilized enzymes versus number of cycles are shown in Fig. 5. After 4 recycles, PLA1–CAC and PLA1–CAG retained 80% of their initial activity, while PLA1–CA retained only 52% of its initial activity. After seven recycles, the PLA1–CA activity declined to 33%, whereas the PLA1–CAC and PLA1–CAG enzymes still retained 50% and 60% of their initial activities, respectively. However, similar recycle studies using immobilized PLA1 in a rice bran oil medium showed no loss in enzyme activity (±5%) after 10 recycles compared to an aqueous buffer medium where less than 20% of its original activity remained after 10 recycles. This slow loss was attributed to leaching [3].
Other studies have mentioned the loss of soluble enzymes from highly porous alginate beads due to leaching during long storage or repeated use. These leakage problems were circumvented by preparing an insoluble enzyme-concanavalin A complex or by crosslinking the complex with glutaraldehyde before forming the calcium alginate beads [7, 9]. The immobilization of Bacillus kaustophilus leucine aminopeptidase in Ca-alginate/k-carrageenan beads showed no loss in enzyme activity for the first 10 cycles but underwent progressive losses with increase reuse [17]. In the case of PLA1–CAC and PLA1–CAG, the leaching problem appears to be minimal after 4 cycles and may be better when used in an oil medium.
Batch Soybean Oil Degumming by Free and Immobilized PLA1
The free PLA1 and three immobilized PLA1 samples were used to reduce the phospholipid contents in soybean oil. The initial phosphorus content in water-degumming soybean oil was 142.2 mg/kg. The temperature and pH values were maintained at 50–58 °C and pH 5.5–6.0, respectively, to minimize the thermal deactivation of the enzymes, while keeping reasonable reaction rates. The hydrolysis time and residual phosphorus levels are listed in Table 1. Free PLA1 exhibited a steep decline in phosphorus content over 3 h that reached 10 mg/kg after 4 h. The final residual phosphorus content reached ~6 mg/kg after 10 h.
The three immobilized PLA1 exhibited similar profiles. When the phospholipids were hydrolyzed by PLA1–CA and PLA1–CAC, for 7 and 5 h, respectively, the residual phosphorus content was less than 10 mg/kg which is suitable for physical refining of soybean oil. However, hydrolysis by PLA1–CAG only reached ~15 mg/kg after 10 h. The results indicate that the free enzyme was more mobile and more accessible to the phospholipid substrates but levels less than 5 mg/kg were not possible even with prolong incubation.
Although immobilized PLA1 was less efficient at hydrolyzing phospholipids compared to free PLA1, the final residual phosphorus content was <10 mg/kg after 8 h for PLA1–CA and PLA1–CAC and also has the advantage of reusable enzymes. Clausen applied free phospholipase A1 (Lecitase® Novo) to reduce the phospholipid content in rapeseed oil to a final phosphorus content of <10 mg/kg (3 h, pH5 and 40–45 °C) [2]. Yang et al. [1] used microbial phospholipase A1 from Thermomyces lanuginosus/Fusarium oxysporum in degumming soybean oil to a final phosphorus content of less than 10 mg/kg (6.3 h, pH 4.8–5.1) on an industrial scale (400 t/day)1. Sheelu used PLA1 to degum rice bran oil and reduced the phosphorus content from 400 mg/kg to 50–70 mg/kg in one cycle [3]. After charcoal treatment and dewaxing, a second enzymatic treatment, brought the phosphorus content to less than 5 mg/kg [3].
Of the three immobilized enzymes, PLA1–CA and PLA1–CAC showed the greatest hydrolytic activity, but PLA1–CAC showed the highest remaining activity, thermal stability, reusability and operational stability.
Plant Trial Degumming of Soybean Oil by PLA1–CAC
Based on the results from the laboratory experiments, PLA1–CAC was selected for soybean oil degumming plant trial in the Fukang Oil and Fat Company (Harbin, China). The immobilized enzyme, PLA1–CAC, was added to 10 t of degummed oil at a rate of 250 U/kg oil. The initial phospholipid content of the oil was 142.4 mg/kg. The hydrolysis conditions were pH 5.8 and 55 °C. The final residual phosphorus content was 9.7 mg/kg after 6.5 h of hydrolysis. The recovery of soybean oil was 99.1%. The recoveries of immobilized PLA1–CAC and activity of PLA1 were 80.2 and 78.2% respectively. The results indicate that the immobilized PLA1 can be used for industrial scale degumming and appears to be robust enough for repeated use in batch reactors
Conclusion
PLA1 samples were successfully immobilized in calcium alginate, calcium alginate–chitosan, and calcium alginate–gelatin matrices, In a batch oil degumming process, the final residual phosphorus content was reduced to less than 10 mg/kg with free PLA1 and immobilized PLA1–CA and PLA1–CAC after 8 h while PLA1–CAG was only able to reduce it to 15 mg/kg after 10 h.
PLA1–CAC was selected for a plant trial using 10 t of soybean oil. The final residual phosphorus content was 9.7 mg/kg after 6.5 h. This residual phosphorus content would be suitable for physical refining in the industry. The recovery of soybean oil was 99.1%. The recoveries of immobilized PLA1–CAC and its activity were 80.2% and 78.2% respectively. The processing costs of the immobilized enzymatic degumming and the conventional acid-degumming technologies are similar. With acid-degumming, the residual phosphorus content in the degummed oil was 25–30 mg/kg. Free fatty acids and the residual phosphorus could be removed from this acid-degummed oil by neutralization and bleaching, which would result in at least 0.8% oil loss. To produce refined soybean oil, the acid-degummed oil would also need to be further deodorized. However, with the immobilized enzymatic degumming process the residual phosphorus content in the degummed oil can be reduced to 5–10 mg/kg, and this amount can be further reduced to 2–3 mg/kg through a decoloring process. Then the degummed oil can be physically refined to produce refined oil [21]. Because the immobilized enzymatic degumming does not involve neutralization and bleaching, the refined soybean oil recovery rate with immobilized enzymatic degumming would be expected to be approximately 0.8% higher than that of acid-degumming, which would generate a profit of 11.5$/ ton of refined soybean oil. Compared with the free enzymatic degumming process, because the immobilized PLA1–CAC can be recycled at least four times, the cost to produce each ton of refined soybean oil through immobilized enzymatic degumming process would be approximately 2.0$ less than that of the free enzymatic process. Therefore, PLA1–CAC is a good candidate for the continuous hydrolysis of phospholipids in crude vegetable oils.
References
Yang B, Zhou R, Yang J-G, Wang Y-H, Wang W-F (2008) Insight into the enzymatic degumming process of soybean oil. J Am Oil Chem Soc 85:421–425
Clausen K (2001) Enzymatic oil-degumming by a novel microbial phospholipase. Eur J Lipid Sci Technol 103:333–340
Sheelu G, Kavitha G, Fadnavis N (2008) Efficient immobilization of Lecitase in gelatin hydrogel and degumming of rice bran oil using a apinning basket reactor. J Am Oil Chem Soc 85:739–748
Yang B, Wang Y-H, Yang J-G (2006) Optimization of enzymatic degumming process for rapeseed oil. J Am Oil Chem Soc 83:653–658
Yang J-G, Wang Y-H, Yang B, Mainda G, Guo Y (2006) Degumming of vegetable oil by a new microbial lipase. Food Techn Biotechnol 44(1):101–104
Jahani M, Alizadeh M, Pirozifard M, Qudsevali A (2008) Optimization of enzymatic degumming process for rice bran oil using response surface methodology. LWTFood Sci Technol 41:1892–1898
Haider T, Husain Q (2007) Calcium alginate entrapped preparations of Aspergillus oryzae [beta] galactosidase: Its stability and applications in the hydrolysis of lactose. Int J Biol Macromol 41:72–80
Martino A, Pifferi PG, Spagna G (1996) Immobilization of [beta]-glucosidase from a commercial preparation. Part 2. Optimization of the immobilization process on chitosan. Process Biochem 31:287–293
Matto M, Husain Q (2006) Entrapment of porous and stable concanavalin A–peroxidase complex into hybrid calcium alginate–pectin gel. J Chem Technol Biotechnol 81:1316–1323
Zhou Z-d, Li G-y, Li Y-j (2010) Immobilization of Saccharomyces cerevisiae alcohol dehydrogenase on hybrid alginate-chitosan beads. Int J Biol Macromol 47:21–26
Bornscheuer U (2000) Enzymes in lipid modification. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 263–306
Ates S, Mehmetoglu (1997) A new method for immobilization of [beta]-galactosidase and its utilization in a plug flow reactor. Process Biochem 32:433–436
Sun W, Jiang D, Luo Y (2005) Immobilized PLA1 by chitosan/alginate microcapsule and study on Its structure. Food Sci 26:100–103
Zhu M, Ai Z, Zhao Q, Dai Q (2004) Immobilization of α-amylase in calcium alginate-gelatin hydrogels. Food Sci 25:64–68
AOCS (1997) Official methods and recommended practices of the American Oil Chemist’s Society. Ca 12-55 Champaign, IL
Krajewska B (2004) Application of chitin- and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microbiol Technol 35:126–139
Chi M-C, Lyu R-C, Lin L-L, Huang H-B (2008) Characterization of Bacillus kaustophilus leucine aminopeptidase immobilized in Ca-alginate/k-carrageenan beads. Biochem Eng J 39:376–382
Sanjay G, Sugunan S (2006) Enhanced pH and thermal stabilities of invertase immobilized on montmorillonite K-10. Food Chem 94:573–579
Nakane K, Ogihara T, Ogata N, Kurokawa Y (2001) Entrap-immobilization of invertase on composite gel fiber of cellulose acetate and zirconium alkoxide by sol–gel process. J Appl Polym Sci 81:2084–2088
Wang Y, Zhao M, Song K, Wang L, Tang S, Riley WW (2010) Partial hydrolysis of soybean oil by phospholipase A1 (Lecitase Ultra). Food Chem 121:1066–1072
O’Brien RD, Farr WE, Wan PJ (2000) Introduction to fats and oils technology, 2nd edn. AOCS Press, Champaign, pp 154–155
Acknowledgments
This research was founded by the Chinese Higher Technology Research and Development 863 Program (2010AA101503) and the System of Soybean Industry and Technology of State Ministry of Agricultural (nycytx-004).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yu, D., Jiang, L., Li, Z. et al. Immobilization of Phospholipase A1 and its Application in Soybean Oil Degumming. J Am Oil Chem Soc 89, 649–656 (2012). https://doi.org/10.1007/s11746-011-1943-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1943-4