Abstract
The degumming of crude soybean oil with phospholipase A1 (PLA1) and phospholipase C (PLC) was studied, and optimal conditions were obtained for each enzyme. During degumming with PLA1, more fatty acid was found in the oil than would be expected by hydrolysis of only the terminal fatty acid chains, and glycerophosphophorylcholine and glycerophosphoethanolamine were detected in the gums. These observations indicate that acyl-migration of phospholipid fatty acids occurred during PLA1 degumming. In addition, results showed that PLA1 degumming was capable of reducing the phosphorus content in the oil to levels acceptable for physical refining (<10 mg/kg). During degumming with PLC, an increase of 1,2-diacylglycerol was found, as most phosphatidylcholine and phosphatidylethanolamine were hydrolyzed by this enzyme. Treatment with either enzyme slightly decreased the oxidative stability of the oil and most metals were separated with the gums fraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soybean oil is the most abundant vegetable oil in the world. Its high content of unsaturated fatty acids, stability during cooking, and low price make it a valuable part of the food chain and the human diet [1]. To obtain edible oil from most oil-bearing seeds, e.g., soybean, rapeseed, peanut and sunflower seeds, a series of refining operations is needed. These processes can be classified as either chemical refining or physical refining. The former involves degumming, acid neutralization, bleaching and deodorization, while the latter consists of degumming, bleaching and steam distillation [2]. Physical refining offers the advantages of high oil yields and reduced chemical and water use. The latter reduces the environmental impact of the process [3]. Degumming is an important initial step that separates most of the phospholipids and mucilaginous gums from the oil. Failure to remove these impurities can cause oil discoloration and accelerate the formation of off-flavors. Therefore, nearly complete removal of the phospholipids is essential for the production of high-quality oil [4].
Traditional degumming processes, e.g., the water, super, total, and acid degumming processes, cannot guarantee the low phosphorus levels that are required for physical refining (<10 mg/kg), and these techniques are not suitable for oils with high levels of non-hydratable phospholipids (NHP) [5, 6]. Additionally, the alkali refining that follows traditional degumming may decrease the content of micro-nutrients such as tocopherols and sterols. Enzymatic degumming, in contrast, has advantages of reduced acid use, wastewater generation and operating cost, as well as improved product yield [7]. In recent years, researchers have studied enzymatic degumming with different phospholipases [8–10]. In the case of Lecitase Novo, the phosphorus content was reduced to 5 mg/kg in rapeseed oil degumming [9]. In the case of Lecitase Ultra, the phosphorus contents of rice bran and soybean oils were enzymatically degummed to less than 5 and 6 mg/kg after 6.5 and 5 h, respectively [11, 12]. It should be noted that phospholipase A1 (PLA1), phospholipase A2 (PLA2) and phospholipase B all catalyze the production of free fatty acids (FFA) [8, 9], and thus may affect the quality of degummed oil. On the other hand, phospholipase C (PLC) does not cause the formation of FFA because it hydrolyzes the bond between the acylglycerol and the phosphate group. Accordingly, it liberates diacylglycerol (DAG), which will not be removed from the oil and, therefore, will contribute to the refined oil value [13]. However, PLC cannot achieve a complete phosphorus removal of vegetable oils as it only acts on phosphatidylcholine (PC) and phosphatidylethanolamine (PE) [14].
Until now, limited research has focused on the mechanisms of PLA1 and PLC degumming. In this study, we report difference in the degumming efficiency of PLA1 (Lecitase Ultra) and PLC (from Bacillus cereus) when applied to crude soybean oil. We also study the quality changes of the oil after treatment by the two enzymes, and we try to discuss the enzymatic degumming mechanisms by analyzing the phospholipid composition of the gum.
Materials and Methods
Materials
Crude soybean oil was kindly provided by Qingdao Bohai Ltd. (Qingdao, China). PLA1 (Lecitase Ultra) was purchased from A/S Novozymes (Bagsvaerd, Denmark). PLC was prepared by our own laboratory using submerged fermentation of a genetically modified B. cereus. Econa oil purchased from Kao Co. (Japan) was used as the DAG standard. Phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidic acid (PA), lyso-phosphatidylcholine (LPC), lyso-phosphatidylethanolamine (LPE), lyso-phosphatidylinositol (LPI), lyso-phosphatidic acid (LPA), glycerophosphorylcholine (GPC) and p-nitrophenylphosphorylcholine (NPPC) with purities greater than 98 % were purchased from Sigma Chemical Ltd. (St. Louis, MO, USA). Glycerophosphorylethanolamine (GPE) of purity greater than 98 % was kindly provided by Changshu Fushilai Medicine & Chemical Co., Ltd. (Changshu, China). Methanol, isopropanol and n-hexane were purchased from J&K Scientific Ltd. (Beijing, China) and were HPLC grade. All other analytical grade regents were from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China).
Determination of PLA1 and PLC Activity
The PLA1 assay was performed with deoiled soy lecithin emulsion by using the method of Yang et al. [6]. One U/mL of PLA1 is the amount of enzyme solution releasing 1 μmol of titratable FFA per minute under the described conditions. The substrate solution, 25 % deoiled soy lecithin, was emulsified with four volumes of a 4 % polyvinyl alcohol solution (v/v). Four milliliters of the deoiled soy lecithin emulsion, 5 mL of 0.01 M citric acid buffer (pH 5.0), and 1 mL of enzyme solution were mixed and incubated at 37 °C for 10 min. Then 15 mL of 95 % ethanol was added to end the reaction, and the liberated FFA were titrated with 0.05 M NaOH. Blanks were measured with heat-inactivated PLA1 samples (95 °C, 10 min).
PLC activity was determined using NPPC as a substrate [15]. We defined 1 U/mL of PLC as the amount of enzyme solution needed to produce 1 nM nitrophenol per minute by the hydrolysis of NPPC under appropriate conditions. One hundred microliters of the PLC enzyme solution was added to 2 mL of NPPC solution [10 mM NPPC, 250 mM Tris-HCl (pH 7.2), 60 % sorbitol and 1 mM ZnCl2] in a test tube. The tube was incubated at 37 °C for 30 min. Substrate hydrolysis was then quantified by measuring absorbance at 410 nm. The activity of PLC was calculated as follows:
where A is the absorption value at 410 nm and t is the time (in minutes) used in the substrate hydrolysis reaction.
pH Determination and Phosphorus Content Assay
pH determination was carried out according to the procedure from Jahani et al. [11]. The oil emulsion and distilled water were mixed (2 mL of each). After centrifugation, the top layer was pipetted off. The pH value in the aqueous phase was measured with a pH meter. The values obtained were corrected (pHcorrected = pHmeasured − 0.26) to compensate for the dilution effect. The phosphorus content of the sample was determined according to AOCS method Ca 12–55.
Water Degumming Process
The water degumming process of soybean oil was modified based on the method of Yang et al. [16]: oil samples (100 g) were stirred (500 rpm) and heated to 70 °C, and 5 mL of hot distilled water was added dropwise. The mixture was then stirred at 70 °C for 30 min. The hydrated gum from the hot oil was separated by centrifugation (10,000 rpm, 10 min), and the top layer of oil was collected and dried in a rotary evaporator operated at 0.09 MPa and 80 °C. The residual phosphorus content of the water-degummed oil was determined according to AOCS method Ca 12–55.
Citric Acid Degumming Process
The citric acid degumming was modified according to Smiles et al. [17]. Crude soybean oil (100 g) was placed in a 250-mL conical flask fitted with a laboratory stirrer. The oil was heated to 70 °C in a water bath and 0.13 mL of 45 % citric acid was added. After homogenization at 10,000 rpm for 1 min, the mixture was allowed to mix at 70 °C for 30 min with stirring at 500 rpm. Afterwards, 5 mL distilled water was added, and the oil mixture was homogenized at a high shear rate (10,000 rpm) for 1 min. Then the mixture was placed in a water bath (70 °C) with mechanical stirring (500 rpm) for another 20 min, followed by centrifugation at 10,000 rpm for 10 min (HITACHI, model CR21G). The supernatant was collected and dried by rotary evaporation at 0.09 MPa, 80 °C, and the gums were saved for further analyses.
Enzymatic Degumming Process
Enzymatic degumming of soybean oil was modified according to Yang et al. [18]. Crude soybean oil (100 g) was heated to 70 °C in a water bath, and 0.13 mL of a 45 % citric acid solution was added under high shear rate (10,000 rpm) for 1 min. The oil mixture was then allowed to mix at 70 °C for 25 min with stirring at 500 rpm. The temperature was then decreased to 45–65 °C, and a 4 % (w/w) NaOH solution was added to adjust the mixture pH (4.4–6.0). After 5 min of stirring (500 rpm), a 2–4 % volume of distilled water was added, and the enzyme was mixed into the oil under high shear rate (10,000 rpm) for 1 min. The mixture was then stirred (500 rpm) in a water bath at temperature (45–65 °C) for a various times (0–5 h), before being heated to 95 °C for 10 min to inactivate the enzyme. After the reaction, the oil mixture was quickly centrifuged at 10,000 rpm for 10 min (HITACHI, model CR21G). The supernatant was collected and dried by rotary evaporation at 0.09 MPa and 80 °C and the gums were saved for additional analyses. Control runs were conducted maintaining all the conditions as described above but without the addition of enzyme.
FFA Content and Fatty Acid Composition
The FFA content of the sample was determined in accordance with GB/T 5530-2005 (National Standard of the People’s Republic of China, 2005). The fatty acid composition (FA) of the sample was analyzed by GC equipped with a PEG-20000 capillary column (30 m × 0.25 mm × 0.25 µm), a flame ionization detector (FID) and nitrogen as carrier gas. The injection was performed in split mode with a split ratio of 1:50. The fatty acid methyl ester (FAME) solution was prepared with BF3-CH3OH (GB/T 17376-2008). One microliter of FAME solution was injected at an injector temperature of 250 °C, with a column temperature program as follows: 100 °C (3 min), 100–180 °C (20 °C/min), 180 °C (4 min), 180–235 °C (12 °C/min), 235 °C (15 min). The FID temperature was 250 °C, and the carrier gas was nitrogen with a total volumetric flow of 60 mL/min. The FA composition reported was based on the relative FID response areas.
Oxidative Stability and Trace Metal Ion Assay
Oil oxidative stability was analyzed with a Metrohm 743 Rancimat (Herisau, Switzerland) instrument. Samples of 3.0 g were analyzed in a heating block at 110 °C and with a constant air flow of 10 L/h. The elements Fe, Ca, and Mg were analyzed by inductively coupled plasma optical emission spectroscopy according to AOCS method Ca 20–99.
Analysis of Phospholipids in the Gums
Analysis of phospholipids in the gums was modified according to the procedure from Avalli et al. [19]. After degumming, the gum was dried by rotary evaporation, and a residue was obtained. The residue (0.5 g) was dissolved in 5 mL of chloroform–methanol (2:1, v/v), and 0.5 mL of the solution was applied to a silica gel bonded column (Supelclean LC-SI, 6 mL volume, 1 g sorbents, Supelco Bellefonte, USA). After conditioning with hexane, the non-polar lipids were eluted with 3 mL of hexane/diethyl ether (8:2, v/v), followed by 3 mL of hexane/diethyl ether (1:1, v/v). Thin layer chromatography (TLC) was used to test for the removal of non-polar lipids. The recovery of phospholipids was performed by eluting with 4 mL methanol four times, followed by eluting with 4 mL methanol plus 2 mL chloroform/methanol/water (3:5:2, v/v/v) four times. The recovered fraction was collected and dried by rotary evaporation and then re-dissolved in 2 mL of chloroform/methanol (2:1, v/v) before HPLC analysis.
The composition of PC, PE, PI, PA, LPC, LPE, LPI and LPA in the gums was analyzed with an Agilent 1100 series liquid chromatograph equipped with a Thermo Scientific Dionex Corona Ultra RS detector. The column (4.6 × 250 mm, 5 μm particles, Agilent ZORBAX RX-SIL) was performed at 35 °C. Three solvents were used to elute the phospholipids. Solvent A was 10 mM ammonium acetate/isopropanol (1/2, v/v); solvent B was n-hexane; and solvent C was isopropanol. The elution gradient was as follows: 0–22 min (6–18 % A, 40 % B, 54–42 % C), 22–27 min (18 % A, 40 % B, 42 % C), 27–30 min (18–27 % A, 40 %B, 42–33 % C), 27–46 min (27–30 % A, 40 % B, 33–30 % C), 46–50 min (30–6 % A, 40 %B, 30–54 % C), and 50–55 min (6 % A, 40 % B, 54 % C). Samples were applied as 15-μL injections and the flow rate was 1 mL/min.
The composition of GPC and GPE in the gums was determined in accordance with Zhang et al. [20]. Samples were analyzed by HPLC–ELSD on a Waters 1525 liquid chromatograph (Waters Corp., Milford, MA, USA). The column (4.6 × 250 mm, 5-μm particles, LiChrospher Si, Sigma–Aldrich Corp.) was performed at 35 °C. The solvents used for elution were methanol (D) and methanol/water (8/1, v/v) (E). The elution gradient was as follows: 0–10 min (60–40 % D), 10–15 min (40 % D), 15–18 min (40–60 % D), and 18–23 min (60 % D). Samples were applied as 15-μL injections and the flow rate was 0.97 mL/min.
The components of the samples were identified and quantified by comparison with peak retention times and calibration curves of standard compounds.
Analysis of DAG in Oil
Degummed oil samples were diluted with n-hexane (10 mg/mL) and analyzed by HPLC–ELSD on a Waters 1525 liquid chromatograph (Waters Corp., Milford, MA, USA) equipped with a LiChrospher Si column (2.0 × 250 mm, 5-μm particles, Hanbon Screrce & Technology Corp., China) at 35 °C. A binary solvent system was used to effect elution. Solvent F was n-hexane/isopropanol (99/1, v/v), and solvent G was n-hexane/isopropanol/glacial acetic acid (1/1/0.01, v/v/v). The elution gradient was as follows: 0–14 min (100–70 % F), 14–15 min (70–100 % F), and 15–20 min (100 % F). Samples were applied as 5-μL injections and the flow rate was 0.3 mL/min. Components of the samples were identified by comparison with peak retention times of standard compounds.
Experimental Design and Statistical Analysis
All experiments were performed in triplicate with all data expressed as mean values ± standard deviations of independent triplicate experiments. Statistical analysis was performed with Origin 8.0 software (OriginLab Ltd., USA). One-way ANOVA was carried out and Tukey adjustment was used to determine the significant difference between treatments. Significant differences were declared at P ≤ 0.05.
Results and Discussion
PLA1 and PLC Activity
The activity of PLA1 and PLC were assayed to be 8,670 and 150 U/mL, respectively. Substrates of PC and PE can be hydrolyzed to form DAG by the PLC used in this study, while substrates of PI or PA cannot be hydrolyzed by this kind of PLC.
Water and Citric Acid Degumming Processes
The phosphorus content of crude soybean oil was originally 697.9 ± 13.6 mg/kg, and after water and citric acid degumming the phosphorus content was reduced to 128.4 ± 2.0 and 28.7 ± 2.1 mg/kg, respectively. The treatment with citric acid could allow for the dissociation of the salts of some NHP to make them slightly more hydratable, facilitating the separation of NHP from oils, and the result obtained was consistent with those of Pan et al. [21].
Enzymatic Degumming Process
Figure 1a shows the effect of reaction time on the residual phosphorus content of degummed soybean oil. Compared with the control sample without enzyme, both PLA1 and PLC reduced the phosphorus content of the degummed oil with an increase in reaction time. The residual phosphorus could be decreased from about 25 mg/kg (Fig. 1a, control) to less than 10 mg/kg by PLA1 treatment, which indicated that PLA1 was capable of hydrolyzing almost all the phospholipids present in the oil when given enough reaction time [22]. However, the treatment of PLC only provided a small decrease from about 25 to 18.2 ± 0.8 mg/kg within 2 h and no further phosphorus reduction was observed at later times. This appears to occur because PLC only catalyzed some phospholipids, such as PC and PE but not PI or PA. Therefore, the PLC degummed oil would need further treatment to be suitable for physical refining.
Effects of reaction time, reaction temperature, water amount (relative to the weight of oil), enzyme dosage (relative to the weight of oil) and pH on the reduction of phosphorous content of soybean oil: a temperature = 50 °C, water amount = 3 %, PLA dosage = 30 mg/kg, PLC dosage = 300 mg/kg, pH = 5.0, no enzyme for control sample, b reaction time = 4 h for PLA and 2 h for PLC, water amount = 3 %, PLA dosage = 30 mg/kg, PLC dosage = 300 mg/kg, pH = 5.0, no enzyme for control sample, c reaction time = 4 h for PLA and 2 h for PLC, temperature = 50 °C for PLA and 55 °C for PLC, PLA dosage = 30 mg/kg, PLC dosage = 300 mg/kg, pH = 5.0, no enzyme for control sample, d reaction time = 4 h for PLA and 2 h for PLC, temperature = 50 °C for PLA and 55 °C for PLC, water amount = 3.5 %, pH = 5.0, e reaction time = 4 h for PLA and 2 h for PLC, temperature = 50 °C for PLA and 55 °C for PLC, PLA dosage = 40 mg/kg, PLC dosage = 300 mg/kg, water amount = 3.5 %, no enzyme for control sample
Figure 1b shows the effect of reaction temperature on the residual phosphorus content of degummed soybean oil. The PLA1 used in this study has inherent activity towards both phospholipids and triglycerides. At temperatures over 40 °C, the phospholipase activity predominates, and the lipase activity is partly suppressed [6]. In this study, we needed to utilize the phospholipase activity of PLA1, so the reaction temperature was studied between 45 and 65 °C. The residual phosphorus content of the PLA1 or PLC degummed oil showed an initial decreasing trend with temperature but then showed an increasing pattern as the reaction temperature increased further. High temperatures can increases the reaction rate as it reduces the viscosity of the lipid mixture and enhances the contact of the enzyme with substrate at the oil–water surface. However, temperature beyond the enzyme’s optimal value will greatly reduce the stability and half-life of the enzyme [23]. In this study, the optimal reaction temperatures for PLA1 and PLC were 50 and 55 °C, respectively. The residual phosphorus content was reduced to 8.2 ± 0.6 mg/kg at 50 °C by PLA1 within 4 h, and to 15.6 ± 1.2 mg/kg at 55 °C by PLC within 2 h.
Figure 1c shows the effect of the water addition on the residual phosphorus content of the degummed soybean oil. Too little water may not allow the phospholipids particles to flocculate, whereas too much water can promote emulsification making the centrifugal separation of the gums more difficult. In this study, the optimal amount of water was 3.5 % (relative to the weight of oil) for both enzymes, and the residual phosphorus contents were 14.6 ± 0.7 and 7.5 ± 0.6 mg/kg for PLC and PLA1, respectively.
Figure 1d shows the effect of enzyme dosage on the residual phosphorus content of degummed soybean oil. With the other variables fixed, the residual phosphorus content of soybean oil treated by PLA1 and PLC both declined with increasing enzyme dosage first, but then the tendency to decrease became very slow with further addition of enzyme. Phospholipase-catalyzed reactions takes place at the interface between the aqueous phase containing the enzyme and the oil phase containing the phospholipid [22]. When the interfacial area provided by mechanical-stirring is fully accommodated by the substrates (phospholipids) and enzyme, there is no need to add more enzyme. Additionally, any excess enzyme dosage at higher concentrations is more liable to agglomerate, which will reduce the actual effective reaction area on the phases. The optimal enzyme dosages chosen for PLA1 and PLC were 40 and 300 mg/kg, respectively.
Figure 1e shows the effect of pH on the residual phosphorus content of degummed soybean oil. The control sample indicated that the residual phosphorus was continuously decreased by raising the pH. It might be expected if some NHP are decomposed by an acid pretreatment, and the addition of caustic to raise the pH could prohibit the re-forming of the NHP and facilitate their transfer to the aqueous phase [22]. Compared with the control sample, the addition of PLA1 or PLC lowered the residual phosphorus of soybean oil over the range of pH levels studied. The lowest contents of residual phosphorus were obtained at pH 5.0 and pH 5.4 for PLA1 and PLC, respectively.
The optimum reaction parameters for the PLA1 reaction were a 50 °C temperature, 4 h reaction time, 40 mg/kg PLA1 dose, 3.5 % water addition, and a pH of 5.0. The residual phosphorus of PLA1 degummed oil was 4.1 ± 0.3 mg/kg, which is suitable for physical refining. For PLC degumming, the optimum conditions were 55 °C temperature, 2 h of reaction time, 300 mg/kg PLC dose, 3.5 % water addition and a pH of 5.4. These conditions resulted in a residual phosphorus content of 14.1 ± 0.5 mg/kg.
Effects of Different Treatments on the Quality of Degummed Soybean Oil
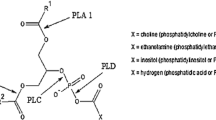
An overview of the effects of different degumming treatments on the quality of soybean oil is given in Table 1. It is well known that PLA1 hydrolyzes sn-1 fatty acid bond of phospholipids to release FFA. Suppose one phospholipid molecule releases only one fatty acid by the treatment of PLA1, there will be about a 0.1 % increase in FFA when the phosphorus content is decreased by 100 mg/kg. In our study, the phosphorus content of soybean oil decreased from 697.9 ± 13.6 to 4.1 ± 0.3 mg/kg by PLA1 degumming, and an increase of about 1.3 % in FFA was observed, which almost doubled the expected value of 0.7 %. The PLA1 used in this study exhibited high phospholipase activity and minor lipase activity at 50 °C [6]. Therefore it does not act as a lipase to hydrolyze the triacylglycerols in the oil. The additional FFA generated during the PLA1 degumming might be attributed to the spontaneous acyl-migration from the sn-2 position to the sn-1 position. So that PLA1 could further catalyze the sn-1 bound fatty acids after acyl-migration. The schematic diagram of this effect is shown in Fig. 2. In contrast, PLC cleaves the phosphorus–oxygen bond between glycerol and phosphate releasing DAG and phosphate esters, thus no significant difference (P > 0.05) was found in the FFA content among crude, control and PLC degummed soybean oils (Table 1).
The FA composition of phospholipids in soybean oil was very close to that of triacylglycerols in the oil. Only a slight decrease was found in stearic acid after PLA1 degumming (P ≤ 0.05). This might be attributed to the difference in fatty acid distribution on the phospholipids.
Compared with the crude soybean oil, the contents of Fe, Ca and Mg were significantly decreased after the degumming treatments (P ≤ 0.05). The pretreatment with citric acid during the degumming process probably facilitates the removal of metal ions by decomposing the metal salts of NHP. Compared with the control sample, the PLA1 treatment significantly reduced the Mg content to about 1 mg/kg (P ≤ 0.05). However, an effect of PLC treatment on the Mg content was not obvious (P > 0.05). As PLA1 decreases the phospholipids more efficiently, almost all the metal ions were separated with the phospholipid fraction.
It is well known that the removal of metal ions could retard the automatic oxidation of oil and improve its oxidative stability. However, in our study, the oxidative stability of soybean oil showed a slight decrease after the different degumming processes (P ≤ 0.05), which might be explained by phospholipids acting as natural antioxidants that postpone the rate of oil oxidation [24, 25].
HPLC–ELSD Analysis of Phospholipids in the Gums
Figure 3 shows the composition of phospholipids in the gum after the different enzyme treatments. The first sample was taken just after enzyme addition to the pretreated oil (t = 0), where phospholipids were not hydrolyzed by the enzyme. In soybean oil, the most abundant phospholipid in the gum detected by HPLC–ELSD is PC (217.8 ± 10.8 mg/g gum), followed by PE (161.4 ± 12.9 mg/g gum), PI (74.6 ± 10.1 mg/g gum) and PA (30.9 ± 4.2 mg/g gum). In PLC degumming (Fig. 3a), most of the PC and PE were hydrolyzed during the first 1.5 h, and only small levels were detected after 2 h. As most PC and PE were hydrolyzed, the relative content of PA and PI in the gum increased steadily along with the reaction time. These results show that the PLC used in this study could only catalyze the phosphate group from PC and PE, and had no activity on PI or PA.
In the PLA1 degumming (Fig. 3b), a fast decrease in PC, PE and PA was observed in the first 2 h, and the tendency to decrease slowed. However, the content of PI decreased faster after 2 h of treatment. Over the same time, the content of LPC, LPE, LPA and LPI showed an initial increasing then a decreasing pattern, and the content of GPC and GPE increased constantly (Fig. 3c). These results indicated that the PLA1 used in this study hydrolyzed all phospholipids in the crude soybean oil, and it was a very efficient catalyst for converting PC and PE to GPC and GPE, respectively. Furthermore, a decrease in LPA and LPI was observed after 1.5 and 3 h, respectively, indicating the existence of further decomposition of these lipids. The reaction trends suggest that the sn-1 fatty acid of phospholipids was hydrolyzed by PLA1 to produce 2-acyl lyso-phospholipids, then spontaneous acyl-migration occurred in which the sn-2 acyl moved to the sn-1 position to form 1-acyl phospholipids, and finally PLA1 hydrolyzed the remaining 1-acyl group to produce the glycerol-phospholipids. These results were consistent with previous reports [12].
HPLC–ELSD Analysis of DAG Content
Figure 4 shows the effect of different treatments on the DAG content of degummed soybean oil. Compared to the crude soybean oil, PLA1 treatment did not change the DAG content in the oil, indicating it was incapable of cleaving the phosphorus–oxygen bond between glycerol and phosphate. In addition, PLA1 used in this study acted as a phospholipase rather than a lipase. Therefore it does not hydrolyze the triacylglycerols in the oil to release DAG. On the other hand, treatment with PLC should increase the DAG content of degummed oil (Fig. 4). Though the DAG content was not quantified in this study, other researchers stressed the importance of the improved oil yield resulting from the use of PLC. For example, an increase of 1 % for every 500 mg/kg phosphorus was claimed in Barton’s recent presentation [26]. The main reasons for this oil yield increase are [1] the DAG formed by hydrolyzing PC and PE is counted as oil and [2] the absolute amount of oil retained in the gum is decreased when the phospholipids are hydrolyzed by PLC.
Effects of different treatments on the DAG content of degummed soybean oil: crude soybean oil; PLA degummed oil: temperature = 50 °C, water amount = 3.5 %, reaction time = 4 h, pH = 5.0, PLA dosage = 40 mg/kg; PLC degummed oil: temperature = 55 °C, water amount = 3.5 %, reaction time = 2 h, pH = 5.4, PLC dosage = 300 mg/kg
Conclusion
The present study showed that PLA1 employed for soybean oil degumming was effective and could reduce the phosphorus content to less than 10 mg/kg under optimal conditions. However, oil treated with PLC would require further treatment to make it suitable for physical refining. After PLA1 degumming, an increase of about 1.3 % FFA was found, approximately double the expected value of 0.7 %, which suggests the existence of acyl-migration during the PLA1 degumming process. Treatment with PLC did not result in increased levels of FFA but it did increase the 1,2-DAG content in the oil by hydrolyzing PC and PE. A kinetic study on the changes of phospholipids composition in the gum was carried, and the results showed that PLA1 degumming could catalyze the conversion of some kinds of phospholipids into glyceryl-compounds under appropriate conditions. The conversion of PC to GPC and PE to GPE were detected, which further supports the existence of acyl-migration during the PLA1 degumming process. A slight decrease in oxidative stability of soybean oil was observed after the degumming treatments, and most of the metal ions contained in the oil were removed together with phospholipids. The FA composition of soybean oil remained almost unchanged after the different treatments.
Abbreviations
- PLC:
-
Phospholipase C
- PLA1 :
-
Phospholipase A1
- PLA2 :
-
Phospholipase A2
- GPC:
-
Glycerophosphophorylcholine
- GPE:
-
Glycerophosphoethanolamine
- DAG:
-
Diacylglycerol(s)
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PI:
-
Phosphatidylinositol
- PA:
-
Phosphatidic acid
- FFA:
-
Free fatty acid(s)
- NHP:
-
Non-hydratable phospholipid(s)
- LPC:
-
Lyso-phosphatidylcholine
- LPE:
-
Lyso-phosphatidylethanolamine
- LPI:
-
Lyso-phosphatidylinositol
- LPA:
-
Lyso-phosphatidic acid
- NPPC:
-
p-Nitrophenylphosphorylcholine
- FA:
-
Fatty acid composition
- FAME:
-
Fatty acid methyl ester(s)
- FID:
-
Flame ionization detector
References
Wang Y, Zhao M, Song K, Wang L, Tang S, Riley WW (2010) Partial hydrolysis of soybean oil by phospholipase A1 (Lecitase Ultra). Food Chem 121:1066–1072
Yu D, Ma Y, Jiang L, Elfalleh W, Shi M, Hu L (2013) Optimization of magnetic immobilized phospholipase A1 degumming process for soybean oil using response surface methodology. Eur Food Res Technol 237:811–817
Dahlke K (1998) An enzymatic process for the physical refining of seed oils. Chem Eng Technol 21:278–281
Zamora R, Olmo C, Navarro JL, Hidalgo FJ (2004) Contribution of phospholipid pyrrolization to the color reversion produced during deodorization of poorly degummed vegetable oils. J Agri Food Chem 52:4166–4171
Dijkstra AJ, Van Opstal M (1989) The total degumming process. J Am Oil Chem Soc 66:1002–1009
Yang J-G, Wang Y-H, Yang B, Mainda G, Guo Y (2006) Degumming of vegetable oil by a new microbial lipase. Food Technol Biotechnol 44:101–104
De Maria L, Vind J, Oxenbøll K, Svendsen A, Patkar S (2007) Phospholipases and their industrial applications. Appl Microbiol Biotechnol 74:290–300
Jiang F, Wang J, Kaleem I, Dai D, Zhou X, Li C (2011) Degumming of vegetable oils by a novel phospholipase B from Pseudomonas fluorescens BIT-18. Bioresour Technol 102:8052–8056
Clausen K (2001) Enzymatic oil—degumming by a novel microbial phospholipase. Eur J Lipid Sci Technol 103:333–340
Huang S, Liang M, Xu Y, Rasool A, Li C (2014) Characteristics and vegetable oils degumming of recombinant phospholipase B. Chem Eng J 237:23–28
Jahani M, Alizadeh M, Pirozifard M, Qudsevali A (2008) Optimization of enzymatic degumming process for rice bran oil using response surface methodology. LWT Food Sci Technol 41:1892–1898
Yang B, Zhou R, Yang J-G, Wang Y-H, Wang W-F (2008) Insight into the enzymatic degumming process of soybean oil. J Am Oil Chem Soc 85:421–425
Dayton CL, Galhardo F (2007) Enzymatic degumming utilizing a mixture of PLA and PLC phospholipases. US Patent 2008/0182322 A1
Dijkstra AJ (2009) Recent developments in edible oil processing. Eur J Lipid Sci Technol 111:857–864
Flieger A, Gong S, Faigle M, Neumeister B (2000) Critical evaluation of p-nitrophenylphosphorylcholine (p-NPPC) as artificial substrate for the detection of phospholipase C. Enzym Microb Technol 26:451–458
Yang M, Zhou X, Jin Y (2008) Non-hydrated phosphatide and its quantitative examination. Chinese J Health Lab Technol 18:71–72
Smiles A, Kakuda Y, MacDonald BE (1988) Effect of degumming reagents on the recovery and nature of lecithins from crude canola, soybean and sunflower oils. J Am Oil Chem Soc 65:1151–1155
Yang B, Wang Y-H, Yang J-G (2006) Optimization of enzymatic degumming process for rapeseed oil. J Am Oil Chem Soc 83:653–658
Avalli A, Contarini G (2005) Determination of phospholipids in dairy products by SPE/HPLC/ELSD. J Chromatogr A 1071:185–190
Zhang K, Wang X, Huang J, Liu Y (2012) Purification of l-alpha glycerylphosphorylcholine by column chromatography. J Chromatogr A 1220:108–114
Pan L, Campana A, Toms M (2000) A kinetic study of phospholipid extraction by degumming process in sunflower seed oil. J Am Oil Chem Soc 77:1273–1277
Dijkstra AJ (2010) Enzymatic degumming. Eur J Lipid Sci Technol 112:1178–1189
Xu X, Fomuso LB, Akoh CC (2000) Synthesis of structured triacylglycerols by lipase-catalyzed acidolysis in a packed bed bioreactor. J Agric Food Chem 48:3–10
Kashima M, Cha G-S, Isoda Y, Hirano J, Miyazawa T (1991) The antioxidant effects of phospholipids on perilla oil. J Am Oil Chem Soc 68:119–122
Koga T, Terao J (1995) Phospholipids increase radical-scavenging activity of vitamin E in a bulk oil model system. J Agric Food Chem 43:1450–1454
Barton N (2008) A new process for degumming: the use of phospholipase C to improve yields during refining of high phosphorus vegetable oils. In: 99th AOCS Annual Meeting and Expo, vol 120. Seattle, p 53–658
Acknowledgments
The work was supported by the Public Welfare Research Funds of State Administration of Grain (201313012-03) and the Major State Basic Research Development Program of China (973 Program, 2012CB720802, 2012CB720806).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Jiang, X., Chang, M., Wang, X. et al. A Comparative Study of Phospholipase A1 and Phospholipase C on Soybean Oil Degumming. J Am Oil Chem Soc 91, 2125–2134 (2014). https://doi.org/10.1007/s11746-014-2555-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-014-2555-6