Abstract
The aim of this study is to report the yield of extraction, as well as the physicochemical and antioxidant properties of extracted chitosan from mud crabs (S.olivacea) as compared to commercial chitosan. The yield obtained for extracted chitosan was 44.57 ± 3.44 % with a moisture and ash content of 9.48 ± 0.59 % and 5.97 ± 0.90 %, respectively. Commercial chitosan demonstrated a higher degree of deacetylation (58.42 ± 2.67 %), water (250 ± 9.90 %) and fat (329 ± 7.07 %) binding capacity, solubility (73.85 %), viscosity (463.25 ± 13.10 %) and also the whiteness value (77.8 ± 0.47) compared to the extracted chitosan, which were only 53.42 ± 0.88 %, 180 ± 0.00 %, 260 ± 0.00 %, 53.38 %, 383.9 ± 28.43 % and 62.1 ± 7.52 %, respectively. The structure of extracted and commercial chitosan was also investigated using Fourier Transform Infrared Spectroscopy (FTIR). In conclusion, the extracted chitosan possessed potential properties similar to the commercial chitosan with high reducing power but low in the scavenging activity on the DPPH and hydroxyl radicals compared to the commercial chitosan.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chitosan, which is a natural and linear polysaccharide made from chitin by a chemical process involving deproteinization, demineralisation, decolouration and deacetylation, has received considerable attention because of its properties. Due to its fungicidal effects and elicitation of defence mechanisms in plant tissue, chitosan has become a useful and highly appreciated as a natural biodegradable high molecular polymer compound which is a nontoxic and bioactive agent. Chitin and chitosan are both polysaccharides, chemically similar to cellulose from which they only differ by the presence or absence of nitrogen in that nitrogen is not present in cellulose (Bautista-Baňos et al. 2006).
There are various usages of crab meat in the food industry including as an ingredient in food products. However, the crab shell is also of benefit to us in that it is effective in fighting cancer and as a natural weight loss supplement. Crab shell is also a source of chitosan, which is a polysaccharide. In Malaysia, mud crab fisheries are limited to estuaries and coastal areas that contain mangroves. Traditionally, mud crabs were exploited by local individuals for immediate consumption, however, the first commercial mud crabs gradually entered local markets and have become a main component of the local crab fishery (Ikhwanuddin et al. 2011).
Chitosan offers a wide range of unique applications in the food industry, including the preservation of foods from microbial deterioration, formation of biodegradable films, and recovery of material from food processing discards. Moreover, it can act as a dietary fibre and as a functional food ingredient. The problem that is associated with the usage of chitosan is its degree of deacetylation. The characterization of chitosan is determined by the degree of N-acetylation (DA), which not only influences the physicochemical characteristics but also its immunological activities (Mahlous et al. 2007).
Physicochemical properties influence the functional properties of chitin and chitosan, which differ according to the crustacean species and method of preparation. Different preparations will result in different physicochemical properties, especially the degree of deacetylation, solubility, viscosity and molecular weight. The functional properties of chitin and chitosan products should be carefully monitored to effectively utilize chitinous products for particular usages (Cho et al. 1998). Chitosan solubility is affected by the presence of free amine groups along the chitosan chain, which allow chitosan to dissolve in diluted aqueous acidic solvents.
Antioxidants are the chemical substances that reduce or prevent oxidation. Natural antioxidants are effective in protecting against oxidative damage and disease (Hayati 2011). It has been found that chitosan has antioxidant activity and many chitosan derivatives have been synthesized and their antioxidant activity assessed accordingly (Li et al. 1992). The antioxidant properties of chitosan and its derivatives have been proposed for application in the biomedical, food, agriculture, biotechnology and pharmaceutical fields (Yen et al. 2008).
The present study aims were to produce value added product from mud crab waste in chitosan production, to investigate its physicochemical and antioxidant properties besides provides a comparison with the available commercial chitosan.
Material and methods
Materials
Mud crab shells (S.olivacea) were obtained from Batu Rakit, Terengganu, Malaysia. Samples were kept chilled in ice during transportation to the laboratory. The crab shells were completely separated from the crab waste in the laboratory, cleaned, placed in polyethylene bags and stored at −20 °C until used. Commercial chitosan (medium molecular weight, catalog number 448877) was obtained from the Sigma-Aldrich Company Ltd, Poole, Dorset, UK. All chemicals used were analytical grade.
Methods
Extraction of carotenoids
Carotenoids were extracted by the method of Shahidi and Synowiecki (1991). The frozen shells were thawed at ambient temperature and washed under running tap water to remove soluble organics, adherent proteins and other impurities. The crab shells were dried in the oven at 70 °C for 24 h or longer until they were completely dried. The dried shells were ground using a grinder and sieved into the size of 500 μm before mixing with cod liver oil in the vortex mixer. The mixture was heated in a water bath at 60 °C for 30 mins. The mixture was then filtered and the filtrates were subjected to vacuum drying at 60 °C for 24 h or longer until completely dried.
Extraction of chitosan
Deproteinization
The mud crab shell waste from the carotenoid extraction process was treated with 2.0 % of potassium hydroxide (KOH) solution with a ratio of ground shell to the solution of 1:20 (w/v), with constant stirring for 2 h at 90 °C to remove the protein. The samples were then filtered under vacuum and the filtrates were washed with tap water for 30 mins until pH neutral (pH, 7). The deproteinized shells were dried in the oven at 60 °C for 24 h (Shahidi and Synowiecki 1991).
Demineralization
The deproteinized mud crab shells were demineralized with 2.5 % (w/v) of hydrochloric acid (HCl) at room temperature (20 °C) for 6 h to remove the mineral content with a ratio of ground shell to the solution of 1:20 (w/v). The samples were then filtered under vacuum and washed for 30 mins with tap water until pH neutral (pH, 7). The demineralized shells were dried in the oven at 60 °C for 24 h (Shahidi and Synowiecki 1991).
Decolouration and dewatering
Decolourizing was achieved by treating the samples with acetone for 10 mins and dried for 2 h at ambient temperature and the resulting residues were then removed. The decolourized shells were then washed in running tap water, rinsed, filtered and dried at 60 °C for 24 h in the oven to obtain crab chitin (Shahidi and Synowiecki 1991).
Deacetylation of chitin
The deacetylation of chitin was then conducted according to the method by Yen et al. (2009). The chitin obtained was treated with 40 % (w/w) aqueous sodium hydroxide (NaOH) with a ratio of chitin to the solution of 1:15 (w/v) at 105 °C for 2 h. Then, the chitin was filtered using a filter pump and washed with deionized water until pH neutral (pH, 7) to obtain the chitosan. The chitosan obtained was then dried at 60 °C for 24 h in the oven.
Characterization of chitosan
Yield, moisture and ash contents
The yield of chitosan was obtained by comparing the weight of the raw material to the weight of chitosan, which was obtained after the treatment, while the moisture and ash contents were determined according to the AOAC (1990) methods.
Determination of degree of deacetylation (DD)
The direct titration method was used to determine the degree of deacetylation of chitosan extracted from mud crabs, which was conducted according to the method by Kjartansson (2008) with some modification. Chitosan samples (0.1 g) were dissolved in 25 ml of 0.06 M HCl for 1 h at room temperature. The solutions were diluted to 50 ml before being titrated with a 0.1 N NaOH to pH 3.75 under constant stirring. The volume of NaOH at pH 3.75 was acquired and recorded. Titration was continued to pH 8 and the total volume of NaOH (0.1 M) was recorded. The degree of deacetylation was then calculated using the following equation:
where, 161.16 is the mass of chitosan monomer, V1 and V2 are the volumes of NaOH solution used, N is the strength of the NaOH solution (0.1 M) and W1 is the mass of sample after correction for moisture. The degree of deacetylation (DD) of the samples was determined in triplicate.
Colour measurements
The colour of extracted and commercial chitosan was measured using a Hunter lab (Hunter Associates Laboratory Inc., Reston, Va.), which was standardized and calibrated using a white plate (X = 78.83, Y = 83.79, Z = 88.60, L* = 3.47, a* = −0.12, b* = −0.32). The chitosan sample was placed in a transparent petri dish. The results were recorded as L*, a*, b* value. The whiteness value of the mud crab and commercial chitosan samples were calculated based on the following equation (Seo et al. 2007):
Water binding capacity
The water binding capacity of extracted and commercial chitosan was measured as referred to Ocloo et al. (2011). Chitosan of 0.5 g was weighed in a centrifuge tube and 10 ml of distilled water was added. The mixture was then vortexed for 1 min in order to dissolve the chitosan before left at an ambient temperature for 30 mins and the tube was shaken for 5 s every 10 mins before being centrifuged at 3,200 rpm for 25 mins. The tube was weighed again after the supernatant was decanted. The water binding capacity was calculated as the following equation:
Fat binding capacity
The fat binding capacity of extracted and commercial chitosan was measured as referred to Ocloo et al. (2011). Chitosan of 0.5 g was weighed in a centrifuge tube and 10 ml of distilled water was added. The mixture was then vortexed for 1 min in order to dissolve the chitosan before left at an ambient temperature for 30 mins and the tube was shaken for 5 s every 10 mins before being centrifuged at 3,200 rpm for 25 mins. The tube was weighed again after the supernatant was decanted. The water binding capacity was calculated as the following equation:
Solubility
The solubility of the extracted and commercial chitosan was determined according to the method by Fernandez-kim (2004). Chitosan powder (0.1 g in triplicate) was placed into a centrifuge tube (known weight) then dissolved with 10 ml of 1 % acetic acid for 30 mins using an incubator shaker operating at 240 rpm and 25 °C. The solution was then immersed in a boiling water bath for 10 mins, cooled to room temperature (25 °C) and centrifuged at 10,000 rpm for 10 mins. The supernatant was decanted. The undissolved particles were washed in distilled water (25 ml) and then centrifuged at 10,000 rpm. The supernatant was removed and undissolved pellets dried at 60 °C for 24 h. Finally, the particles were weighed and the percentage of the chitosan solubility was determined. The solubility of chitosan was calculated using the following equation:
Viscosity
The viscosity of extracted and commercial chitosan was determined according to the method by Ocloo et al. (2011). The extracted chitosan was diluted in 1 % of acetic acid at 1 % concentration on a dry basis. The viscosity of the extracted chitosan was determined using a Brookfield viscometer (Spindle no. 2 at 50 rpm at 25 °C) with values being reported in centipoise units (cP).
Fourier transform infrared (FTIR) spectroscopy analysis
The FTIR analysis spectroscopy of the extracted chitosan was determined according to the method by Mohammed et al. (2013). The completely dried extracted chitosan and commercial chitosan were ground to a very fine powder with potassium bromide (KBr). The dried mixture was pressed under vacuum in a mould to form a KBr disc containing the sample. The FTIR spectra were recorded using a Perkin Elmer FTIR Spectrometer over the frequency range 400–4,000 cm−1 and 16 scans were accumulated. The data were generated using OMNIC series software.
Antioxidant activities of chitosan
Reducing power
Approximately 2.5 ml of the chitosan sample at different concentrations (2, 4, 6, 8 and 10 mg/ml) in 0.2 % acetic acid solution were mixed with 2.5 ml of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 ml of 1 % potassium ferricyanide. The mixture was incubated at 50 °C for 20 mins. Then, 2.5 ml of 10 % trichloroacetic acid was added and the mixture was centrifuged at 3,500 rpm for 20 mins. After being centrifuged, the upper layer (5 ml) was mixed with 5 ml of deionized water and 1 ml of 0.1 % ferric chloride. The absorbances were determined at 700 nm against a blank. A higher absorbance indicates a higher reducing power. The EC50 value (mg/ml) is an effective concentration at which the absorbance is 50 % for reducing power was also determined (Yen et al. 2008).
Scavenging ability on 1, 1-diphenyl-2-picrylhydrazyl radicals
Approximately 4 ml of the chitosan sample at different concentrations (2, 4, 6, 8 and 10 mg/ml) in 0.2 % acetic acid solution was mixed with 1 ml of 10 mM methanolic acid solution containing DPPH radicals. The mixture was shaken vigorously and left to stand for 30 mins in the dark. The absorbance was measured at 517 nm against a blank. The EC50 value (mg/ml) is an effective concentration at which DPPH radicals are scavenged by 50 % was measured (Yen, et al. 2009). The scavenging ability was calculated as follows:
Scavenging ability on hydroxyl radicals
The reaction mixture were contained of 1 ml FeSO4 (9 mM) and 1 ml of salicylic acid (9 mM), chitosan at different concentrations (2, 4, 6, 8 and 10 mg/ml) and 1 mL of H2O2 (10 mM). The mixture was shaken thoroughly and incubated for 1 h at 37 °C. The absorbance of the mixture was measured at 510 nm against a blank. The EC50 value (mg/ml) measured was an effective concentration at which hydroxyl radical is scavenged by 50 %. The scavenging ability was calculated as follows (Zhao et al. 2011):
Statistical analysis
Data were evaluated based on an independent t-test for the carotenoid content of extracted and commercial chitosan. The other characteristics of chitosan samples were also determined in triplicate. The mean of the data obtained was subjected to one-way analysis of variance (ANOVA); a Fisher’s individual error rate test was used to determine the difference among means at the level of 0.05.
Results and discussion
Chitosan characterization
Yield, moisture and ash content
The yield of extracted chitosan, moisture content and ash content extracted from mud crabs was compared to the commercial chitosan, as presented in Table 1. The yield was calculated as the dry weight of chitosan obtained from the extraction of dried mud crab shell powder, which was 44.57 ± 3.44 %. The high yield obtained justifies the potential of mud crab chitosan usage as an economic source for the production of chitosan on an industrial scale due to the availability of mud crabs and the low cost of the source. However, the yield obtained is also affected by the loss of sample mass/weight from excessive removal of acetyl groups from the polymer during deacetylation (i.e. the conversion of chitin to chitosan) (Fernandez-Kim 2004).
The present study also showed that the moisture content of the chitosan extracted from mud crab was 9.48 ± 0.59 %, which was significantly different (p < 0.05) from the commercial chitosan, which was 14.15 ± 0.75 %. A lower moisture content of chitosan indicates better shelf stability and enhances the quality (Ocloo et al. 2011). As chitosan is very hygroscopic in nature, the extracted samples were affected by moisture absorption during storage. The standard moisture content of chitosan for used in application ranged from 5.0 %–15.0 % changing with humidity and form of chitosan whether it in the form of flake or powder (Strusczyk 2006). In addition there was a significant difference (p < 0.05) in the ash content between mud crab and commercial chitosan. The ash content in chitosan is an important parameter as some ash residual of chitosan may affect its solubility and consequently, contribute to lower viscosity, or can affect other more important characteristics of the final product. Ash measurement is an indicator of the effectiveness of the demineralization step for the removal of calcium carbonate (Fernandez-Kim 2004). Chitosan extracted from mud crab shells presented an ash content of 5.97 ± 0.90 %, which was lower than commercial chitosan (7.55 ± 0.05 %). In this study, the mineral content was high due to the concentration of HCl used in the demineralization process for the mud crab, which was low and was not able to demineralise all the minerals that were contained in the chitin samples. Study by Kucukgulmez et al. (2011), showed that the snow crab possessed lower ash content (0.59–0.61 %) than the chitosan extracted from mud crabs. However, No and Meyers (1995) reported that a high quality grade of chitosan should have an ash content of less than 1 %.
Degree of deacetylation (DD)
The process of deacetylation involves the removal of acetyl groups from the molecular chain of chitin, leaving behind a compound (chitosan) with a high degree of chemical reactive amino group (−NH2) (Fernandez-Kim 2004). The degree of deacetylation (DD) is an important parameter that affects the properties, such as solubility, chemical reactivity, and biodegradability, of the chitosan obtained (Lamarque et al. 2005). In this study, the degree of deacetylation of commercial chitosan was found to be significantly higher (58.4 %) (p < 0.05) than the degree of deacetylation of mud crab chitosan (53.4 %) (Table 1). The deacetylation process in this study was only carried out for two hours, which has a great influence on the degree of deacetylation (DD) of the chitosan extracted from mud crabs. The degree of deacetylation (DD) may range from 30 to 95 % depending on the source and preparation procedure (Martino et al. 2005). The degree of deacetylation values are highly dependent on the source and method of purification (No et al. 1989), as well as the type of analytical methods employed, sample preparation and type of instrument used, and various other conditions that may influence the degree of deacetylation analysis. The degree of deacetylation of the extracted chitosan from snow crabs which determined by potentiometric titration was reported as being about 92.19 % (Kucukgulmez et al. 2011).
Colour measurement
Colour measurements were considered according to the whiteness of the extracted chitosan. The commercial chitosan (77.82 ± 0.47) showed the highest degree of whiteness (p < 0.05) as compared to the chitosan extracted from mud crabs (62.10 ± 7.02) (Table 1). The whiteness of the extracted chitosan obtained presented a higher value than the study of Fernandez-Kim (2004) in which the whiteness obtained from different processing protocols ranged from 24.0 to 47.3. This variation might be due to the processing methods employed in the commercial chitosan. Whiteness can be improved by adding bleaching agents. Chitosan powder is quite flabby in nature and its colour varies from pale yellow to white (No and Meyers 1995). According to Seo et al. (2007), the colour of chitosan is also reported to be associated with the content of the carotenoid pigment astaxanthin. In addition, the tan colour of chitosan produced may be caused by the degradation of the pigments that present in the chitin during the deacetylation step.
Water binding capacity (WBC)
There was a significant difference (p < 0.05) in the water binding capacity (WBC) of the mud crab (180 ± 0.00 %) and the commercial chitosan (327 ± 9.99 %), respectively. The extracted chitosan from the mud crab shells showed a lower water binding capacity compared to the commercial chitosan. This is because the isolation process of chitin starts with deproteinization followed by demineralization, which produced lower water binding capacity in the final product of the chitosan. This is also due to the particles size of the chitosan in which better water absorption is achieved with a smaller or finer particle size as is seen in the commercial chitosan. The water binding capacity increased when the demineralization process was conducted first before the deproteinization process. The decolouration step with potassium permanganate and oxalic acid also led to a decrease in the water binding capacity. In addition, chitosan extracted from M. sstebbingi shells had a water binding capacity of 712.99 % (Kucukgulmez et al. 2011). In comparison, the water binding capacity of shrimp chitosan, was reported to be about 582.40 % in the study by Ocloo et al. (2011), while chitosan extracted from crawfish showed a water binding capacity of 660.6 % (Fernandez-kim 2004).
The water binding capacity was found to have a high negative correlation with the degree of deacetylation, molecular weight, viscosity and moisture content. For instant, reversing the steps of chitosan production sequence, such as demineralization and deproteinization, had a pronounced effect on the water binding capacity of chitosan. Study by Fernandez-kim (2004), was stated that deproteinization of demineralized shell showed a higher water binding capacity compared to the process of demineralization of the deproteinized shell. Moreover, the decolouration step also led to a decrease in the water binding capacity of chitosan when it followed deacetylation (Fernandez-kim 2004).
Fat binding capacity (FBC)
The fat binding capacity analysis conducted showed that the chitosan can easily bind or absorb fat. The change in sequence steps in which demineralization is conducted prior to deproteinization and finally deacetylation, results in an increase in fat binding capacity compared to when deproteinization is conducted prior to demineralization and finally deacetylation. Olive oil demonstrated a greater fat binding capacity with crab and crawfish chitosan as compared to the soybean oil, canola oil, corn oil and sunflower oil (Fernandez-kim 2004).
Chitosan extracted from mud crabs presented a lower fat binding capacity (260 ± 0.00 %) compared to the commercial chitosan (329 ± 7.07 %). The result obtained may due to the different sources and the preparation method of the chitin and chitosan. In addition, another factor that determines the absorption of the fat by the chitosan is the size of the particles. This study found that the commercial chitosan had a finer size than the chitosan extracted from the mud crabs. A study by Cho et al. (1998) presented that the FBC of different commercial chitosans were reported to range from 314 to 535 % which is comparable with the mud crab chitosan extracted in this study. However, when the sequence of steps was changed so that demineralization was conducted prior to deproteinization and finally deacetylation, it resulted in an increase in FBC compared to when deproteinization is conducted prior to demineralization and finally deacetylation (Moorjani et al. 1975).
Solubility
There was a significant difference (p < 0.05) in the solubility between the chitosan extracted from mud crabs (53.38 %) and commercial chitosan (73.85 %). The extracted chitosan from mud crabs had low solubility compared to the solubility of the commercial chitosan, which may be due to the incomplete removal of protein. Since the chemical basis of this method is based on the reaction with the amino group, the presence of protein contaminants remaining in the sample during the analysis process could adversely interfere with the results (Fernandez-Kim 2004). The study by Fernandez-Kim (2004) also found that the solubility of crawfish chitosan was in the range of 93.3–94.3 %.
In addition, many attempts have been made to enhance the solubility of chitosan in water. Chitosan has a polymer backbone consisting of hydrophilic functional groups and is hydrophilic in nature; however, chitosan is normally insoluble in water and most common organic solvents. The insolubility of chitosan in aqueous and organic solvents is a result of its crystalline structure, which is attributed to extensive intramolecular and intermolecular hydrogen bonding between the chains and sheets, respectively.
Viscosity
The viscosity of the extracted chitosan obtained from mud crab shells and commercial chitosan was 383.9 cP and 463.25 cP, respectively. There is a significant difference (p < 0.05) in the viscosity value between the chitosan extracted from mud crab shells and commercial chitosan. The extracted chitosan from mud crab shells showed a lower viscosity in the 1 % acetic acid solution compared to the commercial chitosan. This is because the demineralization process in the chitin isolation was conducted for a long time (6 h) and also may due to the high temperature used during deacetylation (105 °C). In addition, the study by Moorjani et al. (1975) showed that an increase in demineralization time resulted in a decrease in the viscosity of chitosan.
Chitin, following the complete removal of protein during the deproteinization process, results in higher chitosan viscosity values, depending on the reagent and shell ratio. Furthermore, the viscosity of chitosan is highly dependent on the degree of deacetylation, molecular weight, concentration of solution, ionic strength, pH and temperature. The process involved in the extraction of chitosan also affects the viscosity of chitosan produced.
Fourier transform infrared (FTIR)
The FTIR spectra of chitosan extracted from mud crab shells and commercial chitosan are presented in Table 2. Extracted chitosan possessed similar functional group as commercial chitosan. In the extracted chitosan, the O-H stretching band was depicted at 3,695.36 cm−1, which showed the alcohol group in the chitosan. According to Pavia et al. (2009), the band identified as the alcohol group (O-H band) was between 3,650 cm-1 to 3,200 cm−1. The stretching band for C-O was at 1,076.79 cm−1, while the commercial chitosan stretching band for C-O was at 1128.21–1129.02 cm−1. In addition, the stretching band of N-H in the extracted chitosan was in the range of 3458.73 cm−1–3,440.48 cm−1, while for the commercial chitosan the stretching band for N-H was in the range of 3,369.11–3,413.07 cm−1. As mentioned by Pavia et al. (2009), the band identified as the amine group (N-H stretching bands) absorbs infrared between 3,500 cm−1 to 3,100 cm−1. Moreover, the bending band of N-H in the extracted chitosan was in the range of 1,622.76–1,623.92 cm−1, while for the commercial chitosan the bending band for N-H was in the range of 1,639.59–1,655.16 cm−1. The band identified as amine group (N-H bending bands) absorbs infrared between 1,640 cm−1 to 1,550 cm−1 (Pavia et al. 2009). The extracted and commercial chitosan showed a different peak on the spectrum because both of chitosans were made from different sources, in which the extracted chitosan was made from mud crab shells while the commercial chitosan was made from shrimp shell. Different sources of chitosan, the method of isolation of chitin and the deacetylation process showed different IR spectra (Duarte et al. 2002). According to Duarte et al. (2002), chitosan that is not completely dry will present the O-H group at different peaks. The band that absorbed between 1,640 cm−1 to 1,550 cm−1 was due to the primary amine groups (N-H), while the absorption between 3,500 cm−1 to 3,100 cm−1 was due to the amide groups which were missing from the deacetylation of chitin. During the deacetylation process, different concentrations and the time taken showed different degrees of deacetylation. A lower concentration of NaOH in the deacetylation process showed a lower degree of deacetylation and the longer the time taken for the deacetylation process will result in a greater degree of deacetylation. Moreover, different degrees of deacetylation showed different peaks in the spectra of FTIR due to the absorption band of the N-H group and O-H group at different IR, which depend on the deacetylation process (Mohammed et al. 2013).
Antioxidant activity
Reducing power
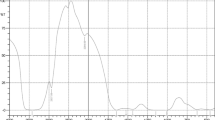
Figure 1 demonstrates that the absorbances of reducing power were gradually increased with the increase in chitosan concentrations. The colour of the solution mixture changed from yellow to green. The highest reading at absorbance of 700 nm, was the sample mixture at a concentration of 10 mg/ml, which was 0.23 nm. A high absorbance indicates a higher reducing power ability of the sample while a lower absorbance shows lower reducing power ability. The comparison of the commercial chitosan with extracted chitosan at a concentration 10 mg/ml showed that there was a significant difference (p < 0.05) between the extracted chitosan (0.23) and the commercial chitosan (0.18) concerning the reducing power value. In the study by Yen et al. (2008) it was found that the reducing power of crab chitosan from snow crab was 0.32 and fungal chitosan from shiitake stipes was 0.42 at 10 mg/ml. The EC50 value for the extracted chitosan for reducing power was 26.11 mg/ml. This result was comparative to the study conducted by Yen et al. (2008), which found that chitosan from snow crab Chionoecetesopilio presented an EC50 value of 20 mg/ml while chitosan from shiitake stipeswas was 14.4 mg/ml (Yen et al. 2008). Hence, in the presence of chitosan as antioxidant, which has a reducing power, it can promote the reduction of Fe3+ to Fe2+, because the amine group (−NH2) in the composition of chitosan can donate hydrogen to the free radical in the reaction. When the hydrogen is donated to the free radical in the initial stage it will break the free radical to become stable and become less readily available for propagation. This shows that chitosan has a potential as a primary antioxidant.
Scavenging ability on 1, 1-diphenyl-2-picrylhydrazyl radicals (DPPH)
The scavenging ability on DPPH of the extracted chitosan were presented in Fig. 2. The commercial chitosan showed lower scavenger ability (28.67 %) at 10 mg/ml than the chitosan extracted from mud crabs as the purple colour of the solution mixture faded. This showed that there was a significant difference between the extracted and commercial chitosan (p < 0.05). The amine group in the chitosan reacted with the DPPH to form stable molecules. The DPPH also reacted with the hydroxyl group in the methanol to reduce the DPPH molecules. Therefore, the purple colour faded with the concentration of chitosan. Research by Yen et al. (2008) showed that the scavenging ability of the DPPH on the fungal chitosan from snow crabs was 46.4 %. However, the chitosan extracted from pink shrimp species Parapenaeuslongirostris and Squilla Mantis possessed scavenging ability on the DPPH of 13 and 5 %, respectively.
The EC50 value of chitosan extracted mud crab was presented as 11.37 mg/ml, lower than the EC50 of chitosan obtained from squid cartilage which was 4.66 g/ml (Zhao et al. 2011). The effective concentration for scavenging activity on the DPPH increases with a decrease in the concentration because the effectiveness in the antioxidant is inversely correlated with the EC50 value.
DPPH is a stable free radical at room temperature but insoluble in water and the reaction mechanism between the antioxidant and the DPPH radical depends on the structural component of the antioxidant reducing the number of DPPH molecules equal to their number of available hydroxyl groups (Bondet et al. 1997). DPPH in ethanol shows a strong absorption band at 517 nm and the solution appears to be deep violet in colour. The DPPH radicals are scavenged by accepting hydrogen from the antioxidant compound (Wanasundara and Shahidi 2012). The stable DPPH radicals are used to evaluate the antioxidant activities of proton-donating substances according to their hydrogen donating ability. The DPPH radicals accept electrons or hydrogen radicals to form stable molecules. The high scavenging ability showed that it reduces most of the DPPH radical molecules.
Scavenging ability on hydroxyl radicals
Figure 3 depicts that the scavenging ability of the extracted chitosan from mud crabs increased as the concentration increases. The commercial chitosan showed a higher scavenging ability on the hydroxyl radical at a concentration of 10 mg/ml, which was 82.33 ± 0.05 %, while the extracted chitosan was only 59.31 ± 0.29 %. However, there was no significant difference (p > 0.05) between the extracted and commercial chitosan on the scavenging ability on the hydroxyl radical. Based on the results obtained, the increased chitosan concentrations increased the scavenging ability on the hydroxyl radical due to more of the amine group to react with Fe3+ to form the chitosan-Fe2+ complex. Therefore, it will reduce the hydroxyl radical generation and can slow down the rate of oxidation. The scavenging ability on the hydroxyl radical of snow crabs was 88.7 % (Yen et al. 2008), and chitosan from squid cartilage was 38 % (Zhao et al. 2011).
Based on the equation y = 2.755x + 45.057 (R2 = 0.9803), the EC50 of extracted chitosan was 1.79 mg/ml. Chitosan has the ability to scavenge hydroxyl radicals because of the presence of the hydroxyl group in the structure of chitosan. The study by Zhao et al. (2011) showed that polysaccharide is capable of scavenging hydroxyl radicals that have one or more alcohol or phenolic hydroxyl groups and that their scavenging ability is directly related to the number of active hydroxyl groups in the molecule. The free radical scavenging activities are closely related to bond dissociation energy of hydroxyl group (−OH) or amine group (−NH2) and the stability of the formed radicals. The OH and NH2 groups in chitosan are difficult to associate and react with the hydroxyl radical (Rao et al. 2006). Fe3+ was produced when iron sulphate (FeSO4) reacted with hydrogen peroxide (H2O2). Chitosan can prevent the hydroxyl radical complexation of iron. The salicylic acid reduced the Fe3+ into Fe2+.
Conclusion
This study investigated the physicochemical and antioxidant properties of chitosan extracted from mud crab (Scylla sp) shells in which the waste is discarded without being used and causes environmental pollution. Although commercial chitosan showed a high ability in the water binding capacity, fat binding capacity and higher viscosity, the extracted chitosan from mud crabs also has the same potential as the method of extraction and the source of the chitosan are influential factors. Thus, they affected the yield and functional properties of extracted chitosan obtained. Natural chitosan can potentially be obtained from Scylla olivacea with good antioxidant properties. The antioxidant properties of the extracted chitosan from Scylla olivacea increased with the increase in the concentration. The reducing power of the extracted chitosan was high but low in the scavenging activity on the DPPH and hydroxyl radicals compared to the commercial chitosan, which was low in reducing power and high in scavenging ability.
References
Alimuniar A, Zainuddin R (1992) An economical technique for producing chitosan. In: Brine CJ, Sanford PA, Zikakis JP (eds) Advances in Chitin and Chitosan. Elsevier Applied Science, Essex, UK, p 627
AOAC (1990) Official methods of analysis of the Association of Official Analytical Chemistry, 15th edition. The Association of Official Analytical Chemistry: Washington, DC, 1990 Inc.
Bautista-Baňos S, Hernandez-Lauzardo AN, Velazquez-del Valle MG, Hernandez-Lopez M, Ait Barka E, Bosquez-Molina E (2006) Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot 25:108–118
Bondet V, Brand-Williams W, Berset C (1997) Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. LWT-Food Sci Technol 30:609–615
Cho YI, No HK, Meyers SP (1998) Physicochemical characteristics and functional properties of various commercial chitin and chitosan products. J Agric Food Chem 46:3839–3843
Duarte ML, Ferreira MC, Marvao MR, Rocha J (2002) An optimised method to determine the degree of acetylation of chitin and chitosan by FTIR spectroscopy. Int J Biol Macromol 31:1–8
Fernandez-kim SO (2004) Physicochemical and functional properties of crawfish chitosan as affected by different processing protocols. Graduate Faculty of Seoul National University, Dissertation of MSc., p 107
Hayati MA (2011) Antioxidant properties of in-vitro goji leaves. Faculty of Applied Science, UniversitiTeknologi Mara, Final year project report, pp 11–20
Ikhwanuddin M, Azmie G, Juariah HM, Zakaria MZ, Ambak MA (2011) Biological information and population features of mud crabs, genus Scylla from mangrove areas of Sarawak Malaysia. Fish Res 108:299–306
Kjartansson GT (2008) Extraction and functional properties of ultrasonicated chitin and chitosan from crustacean by-products. Doctor of philosophy thesis, Graduate School of the University of Massachusetts Amherst, p 158
Kucukgulmez A, Celik M, Yanar Y (2011) Physicochemical characterization of chitosan extracted from Metapenaeus stebbingi shells. J Food Chem 126(3):1144–1148
Lamarque G, Cretenet M, Viton C, Domard A (2005) New route of deacetylation of a-and b-chitins by means of freeze–pump out–thaw cycles. Biomacromolecules 6:1380–1388
Li Q, Dunn ET, Grandmaison EW, Goosen MFA (1992) Applications and properties of chitosan. J. Bioact Compat Polym 7:370–397
Mahlous M, Tahtat D, Benamer S, Nacerkhodja A (2007) Gamma irradiation-aided chitin/chitosan extraction from prawn shells. J Nucl Inst Methods Phys Res Sect B: Beam Interact Mater Atoms 265(1):414–417
Martino AD, Sittinger M, Risbud MV (2005) Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 26:5983–5990
Mohammed MH, Williams PA, Tverezovskaya O (2013) Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll 31:166–171
Moorjani MN, Achutha V, Khasim DI (1975) Parameters Affecting the Viscosity of Chitosan from Prawn Waste. J Food Scie Technol 12:187–191
No HK, Meyers SP (1995) Preparation and Characterization of Chitin and Chitosan-A Review. J Aquat Food Prod Technol 4(2):27–52
No HK, Meyers SP, Lee KS (1989) Isolation and characterization of chitin from crawfish shell waste. J Agric Food Chem 37(3):575–579
Ocloo FCK, Quayson ET, Adu-Gyamfi A, Quarcoo EA, Asare D, Serfor-Armah Y, Woode BK (2011) Physicochemical and functional characteristics of radiation-processed shrimp chitosan. J Radiat Phys Chem 80:837–841
Pavia DL, LampmanG. M Kriz G, S. Vyvyan JR (2009) Introduction to spectroscopy.4th edn. Brooks/Cole, Cengage Learning, USA.
Rao MS, Chander R, Sharma A (2006) Radiation processed chitosan a potent antioxidant. NAARI Annu Conf Radioisotopes Radiat Technol 273:188–194
Seo S, King JM, Prinyawiwatkul W (2007) Simultaneous depolymerisation and decolorization of chitosan by ozone treatment. J Food Sci 72(9):522–526
Shahidi F, Synowiecki J (1991) Isolation and characterization of nutrients and value- added products from snow crab (Chionoecetes Opilio) and shrimp (Pandalus Borealis) processing discards. J Agric Food Chem 39(8):1527–1532
Strusczyk M (2006) Global requirements for medical applications of chitin and its derivatives. Polish Chitin Society. P 98
Wanasundara P, Shahidi F (2012) Antioxidant: Science, technology and applications. Agriculture and Agri-Food Canada Saskatoon Research Center, Memorial University of Newfoundland, St. John’s
Yen MT, Yang JH, Mau JL (2008) Antioxidant properties of chitosan from crab shells. Carbohydr Polym 74:840–844
Yen MT, Yang JH, Mau JL (2009) Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr Polym 75:15–21
Zhao D, Huang J, Hu S, Mao J, Mei L (2011) Biochemical activities of N, O-carboxymethyl chitosan from squid cartilage. Carbohydr Polym 85:832–837
Acknowledgments
The authors acknowledge the financial and technical support provided by the school of food science and Technology, Universiti Malaysia Terengganu for carrying out this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarbon, N., Sandanamsamy, S., Kamaruzaman, S. et al. Chitosan extracted from mud crab (Scylla olivicea) shells: physicochemical and antioxidant properties. J Food Sci Technol 52, 4266–4275 (2015). https://doi.org/10.1007/s13197-014-1522-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1522-4