Abstract
Effects of the pretreatments namely heating, microwave (MW), and ultrasound (US), followed by the enzymatic hydrolysis using different proteases (flavourzyme, neutrase, and protamex) on the protein recovery, umami taste compound content, and antioxidant activities of the Labeo rohita head (LRH) protein hydrolysate was investigated. US and MW pretreatments increased the protein recovery, MSG-like amino acid and flavour 5′-nucleotide contents, equivalent umami concentration (EUC) and antioxidant activities of LRH protein hydrolysates significantly (p < 0.05). The type of enzyme influenced the protein recovery and EUC significantly but did not influence the flavour 5′-nucleotide content of LRH protein hydrolysate (p > 0.05). The highest recovery yield of LRH protein hydrolysate (69.75%) was obtained with the MW pretreatment followed by the protamex hydrolysis, while the highest EUC (41.82 g monosodium glutamate (MSG)/kg) was yielded with the combination of the US pretreatment and the flavourzyme hydrolysis. These results indicate that US and MW pretreatments can help to enhance the recovery yield, umami taste compound content and antioxidant activities of the LRH protein hydrolysate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing demand for fish consumption around the world has led to the development of aquaculture production at an exponential rate (FAO 2016). However, fish processing is associated with the generation of huge amounts of the by-products in the form of heads, bones, skins, scales, fins, and blood, which can account for 50–70% of total body weight of fish (Olsen et al. 2014). At present, some of these by-products are converted to low market value products such as animal feed, fish silages, and fertilisers. However, the main bulk is dumped in the environment, resulting in the loss of valuable components and also environmental pollution (Olsen et al. 2014). It has been observed that most of the fish by-products, especially the fish head contains high protein and are rich in umami taste compounds such as 5′-nucleotides (inosine 5′-monophosphate and guanosine 5′-monophosphate), amino acids (glutamate and aspartate) and some low molecular weight peptides (Cheung and Li-Chan 2014). Thus, the production of new value-added ingredients such as protein hydrolysates, with high umami taste as well as potential biological activity, can pave the way for the complete valorisation of these by-products.
Enzymatic hydrolysis is a specific, simple, safe, eco-friendly method currently used to convert the fish by-products into a more marketable and functional form, namely fish protein hydrolysate (Neves et al. 2017). Proteases such as alcalase, flavourzyme, papain, neutrase, and protamex have been employed for the preparation of the protein hydrolysates from the fish byproducts with different bioactivities (Liaset et al. 2002; Yang et al. 2016; Neves et al. 2017). However, the enzyme–substrate specificity is an important factor that determines the biofunctional properties of protein hydrolysates because it strongly influences its molecular size and the hydrophobic/hydrophilic balance and sequence of peptides. Several studies have been reported that the enzymatically prepared protein hydrolysates from the fish protein possess antioxidant potential (Yang et al. 2016; Neves et al. 2017). In addition to antioxidant potential, previous studies have indicated that fish protein hydrolysates were good sources of umami amino acids (aspartic acid and glutamic acid) and flavour 5′-nucleotides, which can enhance the umami taste of foods (Phat et al. 2016). However, conventional enzymatic extraction facing some limitations in practical applications such as the poor conversion rate of the substrate, low hydrolysis rate of the enzyme and low reaction efficiency due to the compact structural conformation of the protein, insufficient contact frequency between the substrate and enzyme (Qu et al. 2013). Therefore, new techniques are needed to improve the rate of enzymatic hydrolysis and in turn the release more umami amino acids and increase the extraction yield of protein.
Recently, some pretreatments including the US, MW and HT have been used to modify the physical structure of proteins and make them more sensitive to enzymolysis (Uluko et al. 2015; Nguyen et al. 2016; Chen et al. 2017; Ketnawa and Liceaga 2017; Stefanović et al. 2017). Ultrasound could modify the protein conformation by the cavitation effect through affecting hydrogen bonds and hydrophobic interactions and disrupting of the proteins structures (Cheng et al. 2017). MW irradiation could modify protein structure by accelerating organic reactions by modifying chemo-, region-, or stereo-selectivity (Pramanik et al. 2002). On the other hand, heating could bring the structural changes in protein by destroying the inter protein bonds which leads to unfold the peptide chains and expose more number of enzymes cleavage sites (Uluko et al. 2015). Enzyme binding sites might be exposed at greater extent due to the unfolding of protein molecules, which markedly speeds up the enzymatic hydrolysis process of the proteins and promotes the release of large amount, peptides or free amino acids.
India is the second largest producer of freshwater fish in the world after China (FAO 2016). LRH is also one of the most sold and processed fish species in the Indian fish market, which leads to the generation of huge quantities of by-products. The total waste production from LRH fish in India was estimated at approximately 0.9 million tons annually (Kudre et al. 2017). It was noticed that most of the by-products generated, especially the head mass are rich in protein (Ruthu et al. 2014). However, the extraction of protein from the head part of fish is tedious due to the higher content of indigestible fish bones. Therefore, the use of pretreatments, followed by enzymatic hydrolysis of LRH head proteins, could increase the rate of hydrolysis of fish proteins and, consequently, increase the extraction yield, antioxidant potential and taste content of umami. However, the studies on the influence of the pretreatments on enzymatic hydrolysis of the fish head protein are very dearth. Paradoxically, there is no report of the effect of pretreatments (MW US and HT) and the enzymatic hydrolysis on umami taste compound content and antioxidant activities of protein hydrolysates of the LRH head. Therefore, the present study aimed to investigate the effects of HT, MW and US pretreatments, followed by enzymatic hydrolysis using different proteases (flavourzyme, neutrase, and protamex) on extraction yield and umami taste compound content and antioxidant activities of LRH protein hydrolysates.
Materials and methods
Materials
Chemicals and enzymes
Ophthaldialdehyde (OPA), 9-Fluorenylmethoxycarbonylchloride (FMOC), (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), dithiodipropionic acid, 2,4,6-tripyridyl-s-triazine (TPTZ), 2,2-Diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl (DPPH), Inosine 5′-monophosphate (5′-IMP), Guanosine 5′-monophosphate (5′-GMP), Cytidine 5′-monophosphate (5′-CMP), Adenosine 5′-monophosphate (5′-AMP); Uracil 5′-monophosphate (5′-UMP), Sodium fluorescein, 2,2′-Azobis (2-methylpropionamidine) dihydrochloride, Flavourzyme® 1000 L (mixed endoprotease and exopeptidase from Aspergillus oryzae), Protamex® (endopeptidase from Bacillus sp.), and Neutrase® 0.8 L (endoprotease from Bacillus amyloliquefaciens) and standard of amino acid were procured from Sigma (St. Louis, MO, USA).
Labeo rohita head (LRH)
LRHs were collected from the local fish market and placed in ice at a heads/ice ratio of 1:2 (w/w), then transported to Department of Meat and Marine Sciences, CSIR-Central Food Technological Research Institute, Mysuru. Upon arrival to the laboratory, LRHs were immediately rinsed twice with the cold distilled water to remove the contaminants. Washed LRHs were ground to uniformity by a grinder and then stored in sealed plastic bags at − 80 °C until use but not longer than 2 months.
Methods
Preparation of LRH head slurry
Before pretreatment, frozen samples (50 g) were thawed at 4 °C for 12 h and dispersed in deionised water at a ratio of 1:3 (minced head: water). Then, the mixture was stirred for 15 min using a magnetic stirrer (Corning Magnetic Stirrer PC 210, Thermo Scientific, Marietta, Ohio, USA) to ensure complete dissolution. The mixed head slurry was subjected to following pretreatments.
Pretreatments
Microwave pretreatment
MW pretreatment of LRH slurry (200 mL) was performed using the microwave (Start, Milestone, USA) at a power of 200 W at 50 °C for 5, 10 and 15 min. The sample was agitated at 150 rpm/min throughout the pretreatment. After MW pretreatment, the sample was cooled at room temperature (28–30 °C).
Ultrasound pretreatment
US pretreatment was carried out using a Sonics ultrasound system (VCX-750, Sonics, Newtown, USA) equipped with a flat tip probe set at 20 kHz frequency. Sample (200 mL) was transferred to 500 mL flat bottom conical flask, and the suspension was sonicated with an ultrasonic intensity of 75 W/cm2 for 5, 10 and 15 min. The pulse duration of on-time 3 s and off-time 2 s was used. The flat bottom conical flasks containing sample was placed in an ice bucket during sonication to reduce heat gain.
Heat pretreatment
For heat pretreatment, 200 mL of the sample was transferred to 500 mL of a flat bottom conical flask and closed tightly with the cap. The sample was heated at 90 °C for 5, 10 and 15 min with agitation (150 rpm) in a temperature-controlled water bath (C415A, Rivotek, Mumbai, India). Then, the samples were cooled to room temperature (28–30 °C). An untreated slurry (200 mL) of a head slurry of LRH was considered as a control.
Enzymatic hydrolysis and nitrogen recovery
Enzymatic hydrolysis was performed according to the method described by Bhaskar et al. (2008) with slight modification. The pH of pretreated slurries and the temperature activity values of each enzyme, including Neutrase, Protamex, and Flavourzyme, was adjusted to the optimum condition as followed: (a) flavourzyme (pH 7.0 and 50 °C); (b) neutrase (pH 7.0 and 50 °C) and (c) protamex (pH 8.0 and 55 °C). The initiation of hydrolysis was performed by adding each enzyme at the same activity levels of 1.5 U/100 g LRH. The hydrolysis reaction was run for 2 h with continuous shaking (150 rpm) in a temperature-controlled orbital shaking water bath. After hydrolysis, enzymes were inactivated by heating the suspension at 90 °C for 15 min in a water bath. After that, all hydrolysates were cooled immediately to room temperature (28–30 °C) and centrifuged at 8,000g for 15 min at 15 °C using Legend centrifuge (Sorvall Legend XF, Thermo Fisher Scientific, Langenselbold, Germany). The supernatants were collected and lyophilised at − 55 °C and 0.05 Pa pressure for 24 h using a lyophiliser (CoolSafe 55, ScanLaf A/S, Lynge, Denmark). Dried hydrolysate powders were placed in polyethylene bags, sealed and stored at − 80 °C until analysis.
Determination of extraction yield
The following equation calculated the protein extraction yield (%, w/w):
Determination of degree of hydrolysis (DH)
The DH of the LRH hydrolysates was determined using an ophthaldialdehyde (OPA) assay as described by Nielsen et al. (2001).
Protein and moisture content
Moisture and total nitrogen contents of LRH hydrolysates were determined as per AOAC method (1998). Crude protein was measured by multiplying total nitrogen content by the factor of 6.25.
Determination of free amino acid (FAA) content
The analysis in FAA content of LRH hydrolysate samples was carried out as per the method of Bidlingmeyer et al. (1987). Hydrolysate samples (100 mg) were suspended in 20 mL of 5% trichloroacetic acid and stirred for 1 h at room temperature (28 ± 1 °C), then centrifuged at 10,000 g for 15 min at 4 °C. The supernatants were collected and filtered through a 0.22 µm PTFE membrane filters. FAAs derivatised with the OPA/FMOC reagent were analysed on HPLC (Elite Lachrom 2000, Hitachi, Minato-ku, Tokyo, Japan) equipped with a Zorbax Eclipse AAA column (C18, 4.6 × 150 mm, 3.5 µm, Agilent Technologies, California, USA). The column heater was set at 35 °C, and the mobile phase flow rate was maintained at 1.5 mL·min−1. Eluent A was 40 mM Phosphate buffer pH 7.8, and eluent B was methanol/Acetonitrile/Water (45/45/10). The nonlinear separation gradient was 0–18.1 min (57% B), 18.60 min (100% B), 22.30 min (100% B); 23.20 min (0% B) and 26.00 min (0% B). The amino acids were monitored at 334 nm except for proline, which was detected at 262 nm. Amino acids were identified and quantified by comparing with the authentic standard amino acid mixture. The amino acid content was expressed as g/kg protein.
Determination of 5′-nucleotide content
The 5′-nucleotide analysis was performed according to the method of Pei et al. (2014) with slight modifications. Hydrolysate samples (100 mg) were suspended in 20 mL of ultrapure water and stirred for 2 h at room temperature then heat at 90 °C for 1 min. Mixtures were centrifuged at 6,000 g for 30 min at 4 °C. Supernatants were collected and filtered through a 0.45 µm PTFE membrane. Filtrates were used for determination of 5′-nucleotides using HPLC (Elite Lachrom 2000, Hitachi, Minato-ku, Tokyo, Japan) equipped with a 250 × 4.60 mm, 5 µm C18 column (Gemini, Phenomenex, USA), and a diode array detector set at 254 nm. The separation of 5′-nucleotides was carried out using methanol and 0.05% phosphoric acid at the ratio of 5:95 (v/v) as an isocratic mobile phase. The separation of 5′-nucleotides was carried out at a flow rate of 1 mL min−1 for 30 min. Authentic 5′-nucleotide standards were used for identification and quantification of individual 5′-nucleotide. 5′-nucleotide content was expressed as g/kg protein.
Equivalent umami concentration (EUC)
The EUC value (g MSG/100 g) represents MSG (monosodium glutamate) and 5′-nucleotide concentrations that express the umami intensity in food. The EUC value (g MSG/100 g) was determined following the equation described by Yamaguchi (1991).
where Y is the EUC of the mixture in g MSG/100 g; ai is the concentration (g/100 g LRH hydrolysates) of each umami amino acid [Aspartic acid (Asp) or glutamic acid (Glu)]; aj is the concentration (g/100 g LRH hydrolysates) of each flavour 5′-nucleotide [5′-inosine monophosphate (5′-IMP), 5′-guanosine monophosphate (5′-GMP), or 5′-adenosine monophosphate (5′-AMP)]; bi is the relative umami concentration (g/100 g LRH hydrolysates) for each umami amino acid to MSG (Glu, 1; Asp, 0.077); bj is the relative umami concentration (g/100 g LRH hydrolysates) for flavour 5′-nucleotide to 5′-IMP (5′-IMP, 1; 5′-GMP, 2.3; 5′-AMP, 0.18); and 1218 is a synergistic constant based on the concentration (g/100 g LRH hydrolysate) used. EUC was expressed as g/kg protein.
Determination of antioxidant activities
DPPH radical scavenging activity
The DPPH radical scavenging activity of LRH hydrolysates was carried out as described by Shimada et al. (1992).
Ferric reducing antioxidant power (FRAP)
The FRAP test was carried out according to the method of Benzie and Strain (1999).
Oxygen radical absorbance capacity (ORAC) assay
ORAC assay was performed as per the method of Dávalos et al. (2004).
Statistical analysis
Statistical analysis of data was performed using the Statistica software Version 10 (StatSoft, Tulsa, USA). All experiments were run in triplicate, and a completely randomised design (CRD) was used. Data were subjected to analysis of variance (ANOVA). Comparison of the means was carried out by Duncan’s multiple range test at a significance level of p < 0.05.
Results and discussion
Effect of pretreatments and proteases on the degree of hydrolysis (DH)
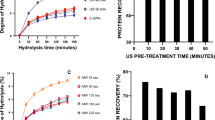
DH is a parameter used to evaluate the degree of protein breakdown. The effects of different pretreatments (US, MW, and HT), pretreatment time (5, 10 and 15 min) and proteases (protamex, neutrase and flavourzyme) on DH of LRH protein are shown in Fig. 1a–c. It has been noticed that the pretreatment time and the type of protease have influenced the DH significantly and ranged from 6.16 to 23.53%. US, MW and HT pretreatments showed the different effects on DH value. Indeed, application of MW pretreatment for 5 min caused a sharp increase in DH compared to the control, regardless of the enzyme tested. The increase of the pretreatment time from 5 to 10 min have no significant effect on DH value (p > 0.05). However, an extension of the MW pretreatment time to 15 min resulted in a decrease in the DH (p < 0.05). The decrease in DH after 10 min could be due to the aggregation of the LRH proteins via the formation of covalent bonds. Aggregation of LRH protein leads to the masking of enzyme binding sites and hence decreased in the DH of LRH protein. It has been reported that application of microwave treatment for a long time caused the aggregation of serum albumin (de Pomerai et al. 2003). In the case of the US pretreatment, the increase of the pretreatment time from 0 to 15 min caused a gradual increase in the DH value. In contrast, the HT pretreatment brought out a significant decrease in DH throughout the pretreatment time compared to the control sample, irrespective of the type of protease. The increase in the DH indicates that the US and MW pretreatments might have caused the destroying of the interactions between the native sequence stretches of the amino acids resulting in the unfolding of the LRH proteins and exposing the enzyme binding sites at greater extents. The exposure of a large number of enzyme binding sites resulted in more hydrolysis of peptide bonds and, thus an increase in the DH. Uluko et al. (2013) also reported that the US and MW pretreatments increased the DH of milk protein. The decrease in the DH of the heat pretreated sample is probably related to the masking of enzyme binding sites caused by denaturation of the proteins via covalent bond formation, making the LRH proteins less sensitive to hydrolysis. A similar result was observed by Guérard et al. (2001), when tuna wastes were preheated, the decrease in the enzymatic hydrolysis rate was noticed. Overall, both US and MW render the protein more sensitive to the enzyme hydrolysis while the heating pretreatment declined the DH of LRH protein. On the other hand, the DH of LRH hydrolysates was highly influenced by the type of enzyme. Hydrolysates prepared by flavourzyme possessed the highest DH as compared to neutrase and protamex for all samples (p < 0.05). Nevertheless, for the heat pretreated samples, neutrase showed slightly higher DH than protamex. The results indicate that flavourzyme has a high affinity towards the LRH proteins compared to neutrase and protamex, resulting in the cleavage of a large number of the peptide bonds. Indeed, the enzyme preparations used herein contain several proteinases with different affinities towards LRH protein. Indeed flavourzyme is a mixture of endopeptidase and exopeptidase that gives it the ability to hydrolyse a large number of peptide bonds and, hence possessed a higher DH. Previous studies have revealed that flavourzyme is active in the hydrolysis of the animal as well as plant based-proteins (Merz et al. 2015; Neves et al. 2017). The difference in the DH of LRH protein hydrolysates might have an impact on the recovery yield, umami amino acid content, equivalent umami concentration and antioxidant activities of LRH hydrolysates.
Degree of hydrolysis of LRH with protamex (a), Degree of hydrolysis of LRH with neutrase (b); Degree of hydrolysis of LRH with flavourzyme (c); Nitrogen recovery yield (NRY) of LRH with protamex (d) Nitrogen recovery yield of LRH with neutrase (e) and Nitrogen recovery yield of LRH with flavourzyme (f). LRH: Labeo rohita head. Results are expressed as the mean ± standard deviation (n = 3). Values followed by different superscript lowercase letters on the bars mean statistically significant differences (p < 0.05). CT: control; US: ultrasound pretreated sample; MW: microwave pretreated sample; and HT: heat pretreated sample
Effect of pretreatments and proteases on nitrogen recovery yield (NRY)
The effects of the pretreatments and protease type on the NRY of hydrolysates from LRH is presented in Fig. 1d–f. The NRY of the hydrolysates was also influenced by the pretreatment time, the type of pretreatment and protease investigated. The NRY of the LRH protein hydrolysate range from 32.24 to 69.75%. Application of the pretreatments (the US, MW, and HT) has significantly affected the NRY of LRH protein hydrolysate (p < 0.05). Overall, the increase in US pretreatment time from 0 to 15 min resulted in a gradual rise in the NRY, regardless of the type of enzyme used. Concerning the MW pretreatment, the NRY sharply increased with the increase of the pretreatment time from 0 and 10 min, regardless of the type of the enzyme used. However, a significant decrease in the NRY was observed when the MW pretreatment time was extended to 15 min (p < 0.05). In contrast, the increase of the HT pretreatment time from 0 to 15 min caused a gradual decline in the NRY. The increase in the NRY of the MW and US hydrolysates is probably due to the substantial disintegration of proteins by the US and MW irradiations and thereby increased in the hydrolysis of the peptide bonds. Meanwhile, the drop in the NRY of the HT hydrolysates could be attributed to the resistance of the heat-denatured aggregated proteins to the enzymatic hydrolysis. This result is in agreement with the lower DH of the HT hydrolysates. Batista et al. (2009) reported that cooking of the sardines prior to enzymatic hydrolysis reduced the NRY. Among the proteases tested, the hydrolysate produced by protamex showed the highest NRY followed by neutrase and flavourzyme, respectively for all pretreated and control LRH samples. Although flavourzyme yielded the highest DH, the NRY was low. This could be explained by the fact that flavourzyme is partially composed of exopeptidase, which is mainly involved in the release of free amino acids while protamex and neutrase are consists of endoenzymes which can release a broad number of large molecular weight peptides, as evidenced by the result of the molecular distribution of the LRH protein hydrolysates (Table S1). Liaset et al. (2002) also found that the enzymatic hydrolysis of Atlantic salmon (Salmo salar, L.) frames by protamex has given a high NRY (76%). Therefore, in terms of NRY, the US or MW pretreatment followed by the protamex hydrolysis of the LRH could be an effective combination. Based on the results of the NRY, the protein hydrolysate extracted from 5 min MW pretreated LRH, 15 min US pretreated LRH, 5 min heat pretreated LRH and untreated LRH was used for the subsequent study.
Effect of pretreatments on free amino acid content (FAA)
The influence of the pretreatments and protease type on the release of MSG-like and total free amino acids (TFAAs) from LRH is illustrated in Table 1. TFAAs and MSG-like amino acids were ranged from 11.20 to 32.12 g/kg protein and from 0.73 to 3.31 g/kg protein, respectively. The contents of TFAAs in LRH hydrolysates were affected by both pretreatments and type of protease investigated. It was postulated that the MSG-like taste arises from the free aspartic acid and glutamic acid (Phat et al. 2016). US and MW hydrolysates showed a higher content in TFAAs and MSG-like amino acid than CT hydrolysate and HT hydrolysate, respectively, irrespective of the protease used (p < 0.05). This result suggests that US and MW pretreatments have made favourable modifications in the confirmation of LRH proteins, which has led to the pronounced proteolysis and thus to the release of a large amount of the FAAs. The result is in agreement with the higher DHs of the US and MW hydrolysates, compared to the control sample (Fig. 2a). Furthermore, US hydrolysate presented a higher in TFAAs and MSG-like amino acid contents compared to MW hydrolysates, regardless of the type of enzyme (p < 0.05). This result indicates that the US pretreatment promotes the hydrolysis of a larger number of peptide bonds than MW pretreatment, and consequently release of a larger amount of FAAs. Conversely, application of the HT pretreatment resulted in the decrease in TFAAs content of the LRH protein hydrolysate as well as the MSG-like amino acid content over the control, regardless of the protease type used. This observation could be due to the formation of heat-induced aggregations of proteins, resulting in the poor accessibility of peptide bonds. Of the proteases tested, flavourzyme produced the highest TFAAs and MSG-like amino acid contents in LRH hydrolysates compared to other proteases, irrespective of pretreatment tested (p < 0.05). As mentioned previously, flavourzyme contains both endopeptidase and exopeptidase activities. Exopeptidase contained in flavourzyme cleaves the terminal amino acid residues of the polypeptide chains, thereby releasing a large amount of FAAs compared to neutrase and protamex, which are consisted solely of endopeptidases. Generally, flavourzyme is used to produce the flavour amino acids and peptides from proteins (Berends et al. 2014; Merz et al. 2015). The highest MSG-like amino acid content (3.31 g/kg protein) and TFAAs (31.12 g/kg protein) were obtained by the USFL hydrolysate (p < 0.05). Therefore, these results suggested that US or MW pretreatment followed by the flavourzyme hydrolysis promotes the hydrolysis of LRH proteins and the release of a large amount of free MSG-like amino acids.
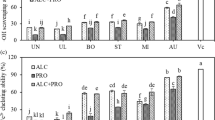
Radical scavenging activity (DPPH), ferric reducing antioxidant power activity (FRAP) and oxygen radical absorbance capacity activity (ORAC) activities of the untreated and pre-treated Labeo rohita head protein hydrolysates. a DPPH, b ORAC and c FRAP. Results are expressed as the mean ± standard deviation (n = 3). Values followed by different superscript lowercase letters on the bars within the same pre-treatment mean statistically significant differences (p < 0.05) among different enzymes, values followed by different superscript capital letters on the bars for the same enzyme mean statistically significant differences (p <0.05) among different pretreatments. CT: control; US: ultrasound pretreated sample; MW: microwave pretreated sample; and HT: heat pretreated sample
Effect of pretreatments on 5′-nucleotide content
The effects of pretreatments and protease type on 5′-nucleotides content of LRH protein are displayed in Table 2. In general, fishes are rich in 5′-nucleotides (5′-AMP, 5′-GMP, 5′-CMP, 5′-UMP and 5′-IMP) which contributes to umami taste sensation (Phat et al. 2016). 5′-AMP, 5′-IMP and 5′-GMP are considered as flavour 5′-nucleotides owing to their contribution to umami taste. In all samples, the main 5′-nucleotide was 5′-AMP (3.07–3.96 g/kg), followed by 5′-GMP (0.40–0.55 g/kg), 5′-IMP (0.32–0.48 g/kg), 5′-CMP (0.21–0.47 g/kg) and 5′-UMP (0.07–0.10 g/kg), respectively. The total 5′-nucleotide and flavour 5′-nucleotide contents of LRH hydrolysates ranged from 4.06 to 5.30 g/kg protein and 3.75 to 4.81 g/kg protein, respectively. Overall, the pretreated hydrolysates showed a higher content in both total 5′-nucleotide and flavour 5′-nucleotide as compared to the control, irrespective of the protease type tested. Moreover, the HT and US hydrolysates showed higher contents in flavour and total 5′-nucleotides compared to MW pretreatment (p < 0.05). The result suggested that all pretreatments enhanced the extraction of the flavour 5′-nucleotides and total 5′-nucleotides. Yue et al. (2016) observed an increase in flavour 5′-nucleotide contents of the crabs subjected to high hydrostatic pressure. Conversely, Poojary et al. (2017) have reported that the protease hydrolysis of mushrooms had no impact on the extraction of the flavour 5′-nucleotides. In contrast, the heating of the mushrooms at a temperature higher than 70 °C was required for the effective extraction of the flavour 5′-nucleotides (Poojary et al. 2017). The highest flavour 5′-nucleotide content (4.81 g/kg protein) was obtained by USFL hydrolysate (p < 0.05). According to Yang et al. (2016), flavour 5′-nucleotides content can be classified as high (> 5 g/kg), medium (5–1 g/kg), and low (< 1 g/kg). Among LRH protein hydrolysates produced, all US and HT hydrolysates were classified in the high range while all CT and MW hydrolysates were classified in the medium range. Therefore, US and HT pretreatments can be used to enhance the flavour 5′-nucleotide and total 5′-nucleotide contents of LRH hydrolysates.
Effect of pretreatments on equivalent umami concentration (EUC)
EUC value is used to determine the characteristic umami taste of food. It has been reported a high correlation between the EUC value and the human sensory evaluation score (Phat et al. 2016). The association of the flavour 5′-nucleotides and MSG-like amino acids, enhance the umami taste of the food by a synergistic effect. The effects of the pretreatments and the type of protease on the EUC values of the LRH hydrolysates is shown in Table 2. It was observed that EUC values of LRH hydrolysates were influenced by both pretreatments and protease type used and ranged from 10.15 to 41.82 g MSG/kg protein. In comparison with the control, the MW and US pretreatments increased the EUC values of the LRH hydrolysates, while the HT pretreatment decreased its value (p < 0.05), regardless of the protease type used. This result indicates that the US and MW pretreatments enhanced the umami taste compound content of the LRH hydrolysates. Furthermore, EUC of US hydrolysates presented was higher than that of MW hydrolysates for the same protease used. Regardless of the pretreatments, the flavourzyme showed the highest EUC values of hydrolysates than neutrase and protamex enzymes (p < 0.05). The result suggested that the flavourzyme is the effective protease for improving the umami taste content of the LRH hydrolysates. The USFL hydrolysates yielded the highest EUC value (41.82 g MSG/kg protein) while the lower EUC value was obtained by HTNT hydrolysate (p < 0.05). Based on their EUC values, samples can be classified into four levels according to Mau et al. (1997), level 1 (> 100 g MSG/kg dry weight), level 2 (10–100 g MSG/kg dry weight), level 3 (1–10 g MSG/kg dry weight) and level 4 (< 1 g MSG/kg dry weight). Therefore, all LRH hydrolysates are classified in the second level. Thus, US pretreatment followed by flavourzyme hydrolysis of LRH is the promising method for enhancement of the umami taste compound content in LRH hydrolysate.
Effect of pretreatments on antioxidant activities
The effects of different pretreatments and protease type on DPPH, ORAC and FRAP activities of LRH hydrolysates are displayed in Fig. 2. It has been noted that antioxidant properties of produced LRH hydrolysates have been influenced by both pretreatments as well as the type of protease investigated. The antioxidant activities of protein hydrolysate are not reliant on only a single mechanism. Protein hydrolysates contain various antioxidant peptide sequences with different mechanisms of action. Some antioxidant peptides are more effective as radical scavengers or sequester, and other are metal chelating or reducing. Therefore, various antioxidant assays are needed to assess the antioxidant properties of protein hydrolysates. In the present study, variation in the DPPH, ORAC and FRAP activities has been observed with the respective pretreatment and type of protease used. US Hydrolysates exhibited the highest DPPH activities than other hydrolysates at all proteases used, indicating that the US pretreatment was effective to generate a large number of hydrogen donors peptides, which could react with free DPPH radicals and convert them to more stable compounds. When compared the proteases, hydrolysates prepared by neutrase showed the highest DPPH activity. The highest DPPH scavenging activity (12.31 TE/g hydrolysate) was obtained by USNT hydrolysate. Similarly, the US hydrolysates evinced the higher ORAC values, while HT hydrolysates presented the lowest ORAC values, regardless of the protease type used. Nonetheless, no significant difference was observed between the MW hydrolysates and the CT hydrolysate, irrespective of the enzyme used (p > 0.05). Further, neutrase showed higher ORAC values for the US hydrolysates. The USNT hydrolysate obtained the highest ORAC value (818.2 µmol T E/g protein). In the case of FRAP values, protamex had a higher value for US, MW and CT hydrolysates, compared to other proteases. The highest FRAP value was noticeable for MWPT hydrolysate (132.33 TE/g protein), followed by USPT hydrolysate (119.61 TE/g hydrolysate) and CTPT hydrolysate LRH (91.24 TE/g protein), respectively. In general, the US and MW pretreatments improved the antioxidant activities of LRH hydrolysates, whereas the HT pretreatment waned the antioxidant activities of LRH hydrolysates when compared to the control samples, regardless of the protease type used. The improvement of antioxidant activities by the US and MW pretreatments was more likely associated with the exposure of hydrophobic interaction sites of the LRH protein to proteases at a greater extent due to unfolding or rearrangement of the LRH protein, as a consequence speed up the hydrolysis rate and release a large number of peptides with high antioxidant activity (Jiménez-Ruiz et al. 2013). US pretreatment has been reported to improve the antioxidant activity of milk protein better than MW and HT pretreatments (Uluko et al. 2013). On the other hand, the decrease in antioxidant activities of the HT hydrolysates was probably due to the low proteolysis of the heat-denatured LRH proteins, resulted in a small number of antioxidant peptides released. Similarly, Uluko et al. (2015) reported that heating had decreased the antioxidant activity of milk protein hydrolysates. Also, LRH hydrolysates produced by neutrase and protamex exhibited better antioxidant activities, followed by flavourzyme. Overall, it was observed that each protease showed their potency towards the generation of specific antioxidant potential peptides from LRH. Jun et al. (2004), proposed that the antioxidant property of protein hydrolysates depends largely on the type of protease used for hydrolysis of proteins. Further, it was postulated that the antioxidant activity of protein hydrolysates is dependent not only on the peptide size but also to a greater extent on their sequences, evidenced the importance of the specificity of the enzyme used.
Conclusion
The results of the present investigation showed that the US or MW pretreatment could be an effective pretreatment to enhance the protein recovery, MSG-like amino acids and flavour 5′-nucleotide contents, equivalent umami concentration (EUC) and the antioxidant activities of LRH protein hydrolysates. On the other hand, HT pretreatment had a negative impact on the enzymatic hydrolysis, protein recovery and antioxidant potential of LRH hydrolysate. US hydrolysates displayed the higher antioxidant potential than MW, HT and CT hydrolysates at all tested proteases. Furthermore, a combination of US pretreatment and flavourzyme produced the hydrolysate with the highest content of free amino acids, MSG-like amino acids, and total 5′-nucleotide. However, protamex showed the highest nitrogen recovery regardless of the pretreatment used. US pretreatment combined with flavourzyme hydrolysis could be a most effective method for the production of LRH hydrolysate rich in the umami taste compounds and antioxidant potential. Therefore, LRH hydrolysates can be used as umami flavour enhancer or nutraceutical in food formulation.
References
AOAC (1998) Official methods of analysis of AOAC international, 16th edn. AOAC International, Gaithersburg
Batista I, Ramos C, Mendonça R, Nunes ML (2009) Enzymatic hydrolysis of sardine (Sardina pilchardus) by-products and lipid recovery. J Aquat Food Prod Technol 18:120–134
Benzie IFF, Strain JJ (1999) [2] Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27
Berends P, Appel D, Eisele T et al (2014) Performance of enzymatic wheat gluten hydrolysis in batch and continuous processes using Flavourzyme. LWT Food Sci Technol 58:534–540
Bhaskar N, Benila T, Radha C, Lalitha RG (2008) Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresour Technol 99:335–343
Bidlingmeyer BA, Cohen SA, Tarvin TL, Frost B (1987) A new, rapid, high-sensitivity analysis of amino acids in food type samples. J Assoc Off Anal Chem 70:241–247
Chen X, Luo Y, Qi B et al (2017) Improving the hydrolysis efficiency of soy sauce residue using ultrasonic probe-assisted enzymolysis technology. Ultrason Sonochem 35:351–358
Cheng Y, Liu Y, Wu J et al (2017) Improving the enzymolysis efficiency of potato protein by simultaneous dual-frequency energy-gathered ultrasound pretreatment: thermodynamics and kinetics. Ultrason Sonochem 37:351–359
Cheung IWY, Li-Chan ECY (2014) Application of taste sensing system for characterisation of enzymatic hydrolysates from shrimp processing by-products. Food Chem 145:1076–1085
Dávalos A, Gómez-Cordovés C, Bartolomé B (2004) Extending applicability of the oxygen radical absorbance capacity (ORAC–Fluorescein) assay. J Agric Food Chem 52:48–54
de Pomerai DI, Smith B, Dawe A et al (2003) Microwave radiation can alter protein conformation without bulk heating. FEBS Lett 543:93–97
FAO (2016) The state of world fisheries and aquaculture 2016. Contributing to food security and nutrition for all. Rome. FAOSTAT Database, FAO. www.fao.org/faostat. 200 pp
Guérard F, Dufossé L, De La Broise D, Binet A (2001) Enzymatic hydrolysis of proteins from yellowfin tuna (Thunnus albacares) wastes using alcalase. J Mol Catal B Enzym 11:1051–1059
Jiménez-Ruiz EI, Calderón de la Barca AM, Sotelo-Mundo RR et al (2013) Partial characterization of ultrafiltrated soy protein hydrolysates with antioxidant and free radical scavenging activities: antioxidative ultrafiltrated soy peptides. J Food Sci 78:C1152–C1158
Jun S-Y, Park P-J, Jung W-K, Kim S-K (2004) Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limanda aspera) frame protein. Eur Food Res Technol 219:20–26
Ketnawa S, Liceaga AM (2017) Effect of microwave treatments on antioxidant activity and antigenicity of fish frame protein hydrolysates. Food Bioprocess Technol 10:582–591
Kudre TG, Bhaskar N, Sakhare PZ (2017) Optimization and characterization of biodiesel production from rohu (Labeo rohita) processing waste. Renew Energy 113:1408–1418
Liaset B, Nortvedt R, Lied E, Espe M (2002) Studies on the nitrogen recovery in enzymic hydrolysis of Atlantic salmon (Salmo salar, L.) frames by Protamex™ protease. Process Biochem 37:1263–1269
Mau J-L, Chyau C-C, Li J-Y, Tseng Y-H (1997) Flavor compounds in straw mushrooms volvariella volvacea harvested at different stages of maturity. J Agric Food Chem 45:4726–4729
Merz M, Eisele T, Berends P et al (2015) Flavourzyme, an enzyme preparation with industrial relevance: automated nine-step purification and partial characterization of eight enzymes. J Agric Food Chem 63:5682–5693
Neves AC, Harnedy PA, O’Keeffe MB, FitzGerald RJ (2017) Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chem 218:396–405
Nguyen TT, Zhang W, Barber AR et al (2016) Microwave-Intensified enzymatic deproteinization of australian rock lobster shells (jasus edwardsii) for the efficient recovery of protein hydrolysate as food functional nutrients. Food Bioprocess Technol 9:628–636
Nielsen PM, Petersen D, Dambmann C (2001) Improved Method for Determining Food Protein Degree of Hydrolysis. J Food Sci 66(5):642–646
Olsen RL, Toppe J, Karunasagar I (2014) Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci Technol 36:144–151
Pei F, Shi Y, Gao X et al (2014) Changes in non-volatile taste components of button mushroom (Agaricus bisporus) during different stages of freeze drying and freeze drying combined with microwave vacuum drying. Food Chem 165:547–554
Phat C, Moon B, Lee C (2016) Evaluation of umami taste in mushroom extracts by chemical analysis, sensory evaluation, and an electronic tongue system. Food Chem 192:1068–1077
Poojary MM, Orlien V, Passamonti P, Olsen K (2017) Enzyme-assisted extraction enhancing the umami taste amino acids recovery from several cultivated mushrooms. Food Chem 234:236–244
Pramanik BN, Mirza UA, Ing YH et al (2002) Microwave-enhanced enzyme reaction for protein mapping by mass spectrometry: a new approach to protein digestion in minutes. Protein Sci 11:2676–2687
Qu W, Ma H, Liu B et al (2013) Enzymolysis reaction kinetics and thermodynamics of defatted wheat germ protein with ultrasonic pretreatment. Ultrason Sonochem 20:1408–1413
Ruthu Murthy PS, Rai AK, Bhaskar N (2014) Fermentative recovery of lipids and proteins from freshwater fish head waste with reference to antimicrobial and antioxidant properties of protein hydrolysate. J Food Sci Technol 51:1884–1892
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948
Stefanović AB, Jovanović JR, Dojčinović MB et al (2017) Effect of the controlled high-intensity ultrasound on improving functionality and structural changes of egg white proteins. Food Bioprocess Technol 10:1224–1239
Uluko H, Zhang S, Liu L et al (2013) Effects of microwave and ultrasound pretreatments on enzymolysis of milk protein concentrate with different enzymes. Int J Food Sci Technol 48:2250–2257
Uluko H, Zhang S, Liu L et al (2015) Effects of thermal, microwave, and ultrasound pretreatments on antioxidative capacity of enzymatic milk protein concentrate hydrolysates. J Funct Foods 18:1138–1146
Yamaguchi S (1991) Basic properties of umami and effects on humans. Physiol Behav 49:833–841
Yang F, Hu F, Jiang Q et al (2016) Effect of pretreatments on hydrolysis efficiency and antioxidative activity of hydrolysates produced from bighead carp (Aristichthys nobilis). J Aquat Food Prod Technol 25:916–927
Yue J, Zhang Y, Jin Y et al (2016) Impact of high hydrostatic pressure on non-volatile and volatile compounds of squid muscles. Food Chem 194:12–19
Acknowledgements
This work was financially supported by The World Academy of Sciences (TWAS), Italy and the Department of Biotechnology (DBT), Science and engineering research board (Grant No. ECR/2015/000215) India. We also thank the Director of CSIR-CFTRI, Mysuru, for allowing to access the facilities and permission to publish this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bruno, S.F., Kudre, T.G. & Bhaskar, N. Effects of different pretreatments and proteases on recovery, umami taste compound contents and antioxidant potentials of Labeo rohita head protein hydrolysates. J Food Sci Technol 56, 1966–1977 (2019). https://doi.org/10.1007/s13197-019-03663-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03663-3