Abstract
Effects of ultrasonication, boiling, steaming, microwaving and autoclaving pretreatments on the production of sweet potato protein hydrolysates (SPPH) by single and combined Alcalase (ALC) and Protease (PRO) were investigated, as well as antioxidant activities of SPPH subjected to in vitro gastrointestinal digestion (GID). All pretreatments significantly increased the degree of hydrolysis (DH) and antioxidant activities of SPPH by ALC, PRO and ALC + PRO in the order of autoclaving > steaming, microwaving, boiling > ultrasonication (P < 0.05). GID significantly enhanced antioxidant activities and increased MW <3 kDa peptide fraction contents of all SPPH. Diverse peptides were identified as sporamin A, A precursor and sporamin B before and after GID from LC–QTOF–MS/MS analysis. Peptides with higher antioxidant amino acids of Trp, Tyr, Met, Cys, His and Phe were found after GID. There is a great potential application of SPPH as a novel food ingredient as a natural antioxidant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bioactive peptides with antioxidant activity are one of the most promising natural alternatives to synthetic antioxidants and have been frequently studied by scientists worldwide due to their potential health benefit against oxidative stress [1, 2]. Antioxidant peptides are typically released from protein molecules by enzymatic hydrolysis, microbial fermentation and food processing. Pretreatments on proteins before enzymatic hydrolysis, such as ultrasonication, microwaving and thermal (e.g., boiling, steaming and autoclaving) [3,4,5], are considered helpful in promoting the process of hydrolysis by inducing conformational changes and molecular unfolding of proteins to expose more susceptible peptide bonds to enzymes. These changes can enhance the release of novel bioactive peptides [3, 5], which will exert positive physiological actions in the human body. In vitro gastrointestinal digestion (GID) is considered to be an acceptable approach for determining the bioactive changes of peptides, resulting in more potent peptides [6].
Sweet potato (Ipomoea batatas [L.] Lam) ranks the fifth leading food crop in China, accounts for 90.1 and 67.3% of sweet potato production in Asia and the world, respectively, and contains 1.73–9.14% of protein (dry weight basis) [7, 8]. Sweet potato proteins (SPP) possess good nutritional properties [9], making the development of high-quality proteins from plant sources. SPP hydrolysates (SPPH) produced by enzymatic hydrolysis exhibit certain antioxidant activities [8]. Determining the appropriate pretreatment on SPP before enzymatic hydrolysis may enhance antioxidant activities of SPPH, as well as impact the antioxidant stability of SPPH during GID.
The objective of this study was to investigate the effects of ultrasonication, boiling, steaming, microwaving and autoclaving pretreatments on degree of hydrolysis (DH) and antioxidant activities of SPPH by single and combined enzymatic hydrolysis, thus to improve the production of sweet potato antioxidant peptides. Further, fractionation, peptide distribution and identification of antioxidant peptides in SPPH before and after GID were performed to assess their feasibility as natural antioxidants in foods.
Materials and Methods
Materials
Sweet potatoes (Shang Shu 19 cultivar) were obtained from Shangqiu Academy of Agriculture and Forestry Sciences, Henan province, China. SPP was recovered following our previous report [8]. Alcalase (ALC, 2.4 Au/g) was purchased from Novozymes (Bagsvaerd, Denmark). Protease (PRO) from Aspergillus oryzae and o-phthaladehyde (OPA) were purchased from TCI America Inc. (Portland, OR, USA). Pepsin and pancreatin were purchased from MP Biomedicals LLC (Solon, OH, USA). The 2,2′-Azobis (2-methylpropionamidine) dihydrochloride (AAPH) and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Acros Organics (Morris Plains, NJ, USA).
Pretreatment of SPP
SPP powder was dissolved in deionized water (3%, w/v), and subjected to five different pretreatments described by Uluko et al. [3] and Sun et al. [9] with modifications; ultrasonication at 90 W for 15 min in an ice bath, boiling at 100 °C for 15 min, steaming for 15 min, microwaving at 700 W for 2 min, and autoclaving at 121 °C for 15 min. Untreated sample was the control. After pretreatments, each SPP solution was cooled immediately and subjected to enzymatic hydrolysis.
SPPH by Single and Combined Enzymatic Hydrolysis
SPPH was produced by hydrolyzing SPP (3%, w/v) using ALC and PRO, and the hydrolysis conditions were at pH 8 and 50 °C for ALC; and at pH 7 and 50 °C for PRO, respectively for 2 h. For combined enzymatic hydrolysis, PRO was added to the hydrolysate by ALC for another 2 h (recorded as ALC + PRO) reaction. After hydrolysis, each hydrolysate was boiled for 10 min, centrifuged at 10,000×g for 20 min to collect the supernatant, lyophilized, and stored at −20 °C for subsequent analysis.
In Vitro Gastrointestinal Digestion (GID)
GID of SPPH was performed following the protocol described by Zhang and Mu [10] with modifications. SPPH (3%, w/v) was mixed with pepsin (4%, w/w of protein), and incubated at 37 °C, pH 2 for 2 h (gastric digestion). The samples after gastric digestion were continuously incubated with pancreatin (4%, w/w of protein) at 40 °C, pH 7.5 for another 2 h (intestinal digestion). Afterward, each digested sample was adjusted to pH 7 and heated at 100 °C for 10 min in a water bath. An aliquot of 10 mL was taken at 0, 1 and 2, 3 and 4 h for undigested, during and after gastric and intestinal digestion, respectively.
Degree of Hydrolysis (DH)
DH presenting the percentage of cleaved peptide bonds was determined through monitoring the number of amino groups from hydrolyzed peptide bonds using OPA method reported by Nielsen et al. [11].
Antioxidant Activity
Hydroxyl Radical (·OH) Scavenging Activity
Hydroxyl radical (·OH) scavenging activity was determined according to the method published by Smirnoff and Cumbes [12].
Fe2+-Chelating Ability
Fe2+-chelating ability was determined following the method described by O’Loughlin et al. [13].
Oxygen Radical Absorbance Capacity (ORAC)
Oxygen radical absorbance capacity (ORAC) was determined according to the method described by Zhang and Mu [8]. An aliquot of 20 μL sample solution (1 mg/mL), 20 μL phosphate buffer (75 mM, pH 7.4) and 20 μL 63 nmol/L sodium fluorescein solution were mixed and incubated at 37 °C for 10 min. After adding AAPH (140 μL, 18.28 mM), fluorescence was measured immediately at OD485nm (excitation) and OD535nm (emission) at 37 °C with a microplate reader (Tecan, Gröedig, Austria). ORAC values were presented as μg trolox equivalent per mL sample (μg TE/mL).
Peptides Fractionation and Molecular Weight (MW) Distribution
Peptide fractionation was determined by ultrafiltration with MWCO 10 and 3 kDa of Macrosep® Advance Centrifugal Filter (Port Washington, NY, USA). Peptide fractions with MW >10, 3–10 and < 3 kDa were obtained and MW distribution was calculated as the percentage of protein content in each peptide fraction to total protein content in respective hydrolysate.
Identification of Antioxidant Peptides
Antioxidant peptide identification was analyzed on an ACQUITY ultra-performance LC system (Waters Corp., Milford, USA) coupled with a quadrupole time-of-flight mass spectrometer (LC–QTOF–MS/MS). Injection of 1 μL of 1.6 μg/mL sample solution was made onto an ACQUITY UPLC® BEH C18 column (2.1 × 50 mm, 1.7 μm) with flow rate at 200 μL/min of mobile phase solution A (0.1% formic acid in water) and solution B (95% acetonitrile, 5% H2O and 0.1% formic acid) starting at 1% B for 2 min, then ramped up to 32% B linearly in 60 min, followed by ramping up to 100% B in 20 min. The parameters of data dependent acquisition for MS/MS were set to: capillary voltage 3 kV, sample cone voltage 30 V, extraction cone 4.3 V, scan range 300 to 1300 m/z. The MS/MS spectra were searched by Mascot Matrix Science (MA, USA) and Protein Prospector (SC, USA) with UniProt/Swiss-Prot database for peptide identification.
Statistical Analyses
All experiments were carried out in triplicate measurements, and data were presented as the mean ± SD. Statistical analyses were performed using SAS8.1 (SAS Institute Inc., Cary, NC, USA). Differences were considered statistically significant when P < 0.05.
Results and Discussion
Effects of Pretreatment on DH
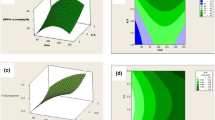
All pretreatments improved DH of SPPH prepared by single and combined enzymatic hydrolysis as shown in Fig. 1a and Online Resource Table S1. Compared with the untreated sample, ultrasonication increased DH of SPPH slightly, while autoclaving, microwaving, boiling and steaming significantly enhanced enzymatic hydrolysis with higher DH (P < 0.05). Among them, autoclaving resulted in the best improvement on DH of SPPH, which were 28.52, 39.05 and 46.34% by ALC, PRO and ALC + PRO, respectively (P < 0.05). For the remaining pretreatments, improvement of DH was seen in the order of microwaving, boiling, steaming and ultrasonication (Fig. 1a, Table S1). These results are similar to enzymatic hydrolysis of fish protein that was also promoted by pretreatments, which was attributed to the exposure of new cleavage sites due to structural changes of protein molecules [5].
Degree of hydrolysis (DH) (a),·OH scavenging activity (b), Fe2+-chelating ability (c) and oxygen radical absorbance capacity (ORAC) (d) of sweet potato protein hydrolysates (SPPH) by ALC, PRO and ALC + PRO affected by different pretreatments. ALC, Alcalase; PRO, Protease; ALC + PRO, Alcalase and Protease. UN, untreated; UL, ultrasonication; BO, boiling; ST, steaming; MI, microwaving; AU, autoclaving. Means with different letters on the bar charts are significantly different (P < 0.05)
Effects of Pretreatment on Antioxidant Activity
A significant improvement of pretreatment before hydrolysis on antioxidant activity of SPPH was observed, as well as the enzymatic treatment (Table S1 and Figs. 1b-d). Compared with the untreated sample, ultrasonication increased antioxidant activity of SPPH slightly, while autoclaving pretreatment presented the best enhancement on antioxidant activity of SPPH, followed by steaming, and then by microwaving or boiling (P < 0.05, Table S1 and Figs. 1b-d). Autoclaving pretreatment could also significantly enhance antioxidant activities of hydrolysates from soy protein isolate by enzymatic hydrolysis, resulting from the increase of susceptibility to protein hydrolysis due to the unfolding of protein molecules [14]. In addition, SPPH by ALC + PRO presented higher antioxidant activities, followed by ALC and by PRO (Table S1 and Fig. 1b-d). Among all hydrolysates, SPPH by ALC + PRO with autoclaving pretreatment showed higher·OH scavenging activity, Fe2+-chelating ability and ORAC value, which were 64.37, 87.36 and 106.90 μg TE/mL, respectively.
Changes in Antioxidant Activity during In Vitro Gastrointestinal Digestion (GID)
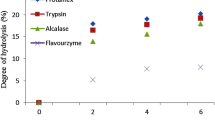
Digested SPPH by ALC slightly decreased in ·OH scavenging activity, that by PRO increased significantly first and then slightly decreased, while that by ALC + PRO presented a significant increase up to 68.92% (4 h) (P < 0.05, Fig. 2a). A general enhancement in antioxidant activities of soybean hydrolysate by ALC was presented after GID [15] suggesting that the hydrolysates after GID might be more active electron or hydrogen donors.
Changes in ·OH scavenging activity (a), Fe2+-chelating ability (b) and Oxygen radical absorbance capacity (ORAC) (c) of sweet potato protein hydrolysates (SPPH) by ALC, PRO and ALC + PRO during in vitro gastrointestinal digestion (GID). ALC, Alcalase; PRO, Protease; ALC + PRO, Alcalase and Protease. Means with different letters on the bar charts are significantly different (P < 0.05)
All SPPH obtained by ALC, PRO and ALC + PRO had higher Fe2+-chelating ability after GID (Fig. 2b). Following gastric digestion by pepsin for 2 h, Fe2+-chelating ability of SPPH significantly increased from 85.20 (ALC), 65.70 (PRO) and 87.36% (ALC + PRO) to 94.74 (ALC), 89.13 (PRO) and 93.82% (ALC + PRO), respectively (P < 0.05) compared to undigested SPPH. It was also reported that GID increased Fe2+-chelating ability of fish protein hydrolysates [6].
ORAC values of all SPPH were highly retained after both gastric and intestinal digestion (Fig. 2c). Following gastric digestion by pepsin (1 and 2 h), ORAC value of SPPH by ALC slightly decreased (2 h); that by PRO was no significant change (2 h); while that by ALC + PRO significantly increased from 106.90 (0 h) to 114.66 μg TE/mL (2 h). ORAC values of all SPPH showed no significant changes with longer (3 and 4 h) GID. Wang et al. [16] also found that ORAC values of different peptide fractions from casein hydrolysate increased after GID.
Changes in Peptides Distribution
SPPH by ALC, PRO and ALC + PRO with autoclaving pretreatment before and after GID (4 h) were selected for peptides distribution analysis due to their high antioxidant activities (Fig. 3). Compared with SPPH before GID, all digested SPPH presented lower content of MW >10 kDa peptide fractions and higher contents of MW 3–10 and < 3 kDa peptide fractions (P < 0.05, Fig. 3). For MW 3–10 kDa peptide fractions, digested SPPH prepared by ALC + PRO (24.32%) exhibited significantly lower content than those by ALC (40.94%) and PRO (48.10%). While for MW <3 kDa peptides, digested SPPH by ALC + PRO showed the highest content of 66.49%, followed by ALC (45.77%) and by PRO (34.88%) (P < 0.05, Fig. 3). Zhang et al. [15] indicated that GID increased MW 1–5 and < 1 kDa peptide fraction contents in soybean protein hydrolysates. In this study, SPPH by ALC + PRO after GID presented the highest amount of MW <3 kDa peptides (Fig. 3), which might be associated with its higher antioxidant activity (Fig. 2).
Antioxidant Activities of Various Peptide Fractions
Antioxidant activities of different MW peptide fractions from SPPH before and after GID were analyzed (Table 1). The ·OH scavenging activity of MW <3 kDa peptides in all SPPH before and after GID were in the range of 75.60% to 82.42%, which were significantly higher than those of MW 3–10 and > 10 kDa peptide fractions (P < 0.05). After GID, a slight decrease in ·OH scavenging activity of MW <3 kDa peptides occurred in SPPH by ALC, while no significant changes were observed in those by PRO and ALC + PRO. Fe2+-chelating ability of MW <3 kDa peptides in all SPPH before GID were higher than those of MW >10 and 3–10 kDa peptide fractions. After GID, Fe2+-chelating ability of MW <3 kDa peptides in all SPPH significantly increased (P < 0.05, Table 1). For ORAC assay, MW <3 kDa peptides in all SPPH before GID showed slightly higher ORAC values than those of MW >10 and 3–10 kDa peptide fractions. After GID, ORAC values of MW <3 kDa peptides in SPPH by ALC presented no significant change, while those in SPPH by PRO and ALC + PRO significantly increased to 130.65 and 126.82 μg TE/mL, respectively. Low MW peptide fractions were reported to eliminate free radicals more effectively, thus exhibited higher antioxidant activity than those of high MW peptides [17].
Identification and Characterization of Peptides
The MW <3 kDa peptides in SPPH by ALC + PRO with autoclaving pretreatment before and after GID presented high contents and strong antioxidant activities, thus it would be important to be selected for identification of peptide sequences (Online Resource Table S2). Peptide sequences of MW <3 kDa peptides in SPPH by ALC + PRO before and after GID were analyzed by LC-QTOF-MS/MS and identified from sporamins A, A precursor or sporamin B (Table S2). Before GID, 29 diverse peptides were identified with MW in the range of 312.15 to 1690.95 Da with 2–15 amino acids; whereas after GID, 35 diverse peptides were found with MW in the range of 312.15–1882.99 Da with 2–18 amino acids. Respective 5 to 18 peptides were found in six fractions from amaranth protein hydrolysates after GID, which showed MW were in the range of 802.31–1665.75 Da with 7–15 amino acids [18].
Amino acids Tyr, Trp and Phe have been reported to show high antioxidant activities due to their hydrogen donation capability; His, with an imidazole group, is considered to have a proton donation ability; Met can be changed to the sulfoxide form by oxidization; Cys contains a sulfur hydrogen [19]. In this study, 13 and 21 identified peptides with one or more antioxidant amino acids were found before and after GID, respectively (underlined in Table S2). The numbers of peptides with Trp, Tyr, Met, Cys, His and Phe residues were increased from 3, 4, 3, 0, 0 and 5 before GID to 4, 7, 9, 3, 6 and 7 after GID, respectively (Table S2). In addition, the percentages of identified peptides with Trp, Tyr, Met, Cys, His and Phe residues were changed from 6.14, 16.55, 4.99, 0, 0 and 10.12% before GID to 5.51, 27.65, 19.18, 3.69, 13.48 and 11.45% after GID, respectively (Fig. S1). All data above demonstrated that SPPH could be a potential source of powerful natural antioxidants, and GID treatment can enhance its activities.
Conclusions
Pretreatments enhanced the DH and antioxidant activities of SPPH treated by ALC, PRO and ALC + PRO, with autoclaving pretreatment exhibiting the greatest improvement, followed by steaming, microwaving, boiling, and ultrasonication. GID significantly increased all determined antioxidant activities of SPPH by ALC + PRO, and high ·OH scavenging activity of that by ALC, whereas there was no significant change in ORAC values of those by ALC and PRO. More MW <3 kDa peptides were found in all SPPH after GID compared to those before GID, and the increase of antioxidant activity of MW <3 kDa peptides in SPPH by ALC + PRO after GID was noticed. Diverse peptides were identified as sporamin A, A precursor and sporamin B before and after GID, and more peptides with antioxidant amino acids were found after GID. These results suggested that SPP could be converted into valuable bioactive peptides through appropriate pretreatment and enzymatic hydrolysis for an application in food system.
References
Ketnawa S, Wickramathilaka M, Liceaga AM (2018) Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: purification and identification. Food Chem 254:36–46
Delgado MCO, Mónica G, Añón MC, Tironi VA (2015) Amaranth peptides from simulated gastrointestinal digestion: antioxidant activity against reactive species. Plant Food Hum Nutr 70:27–34
Uluko H, Zhang S, Liu L, Tsakama M, Lu J, Lv J (2015) Effects of thermal, microwave, and ultrasound pretreatments on antioxidative capacity of enzymatic milk protein concentrate hydrolysates. J Funct Food 18:1138–1146
Quansah JK, Udenigwe CC, Saalia FK, Yada RY (2013) The effect of thermal and ultrasonic treatment on amino acid composition, radical scavenging and reducing potential of hydrolysates obtained from simulated gastrointestinal digestion of cowpea proteins. Plant Food Hum Nutr 68:31–38
Ketnawa S, Liceaga AM (2017) Effect of microwave treatments on antioxidant activity and antigenicity of fish frame protein hydrolysates. Food Bioprocess Tech 10:582–591
Teixeira B, Pires C, Nunes ML, Batista I (2016) Effect of in vitro gastrointestinal digestion on the antioxidant activity of protein hydrolysates prepared from cape hake by-products. Int J Food Sci Tech 51:2528–2536
FAOSTAT (2016) Production of crops. http://faostat3.fao.org/browse/Q/QC/Ex. Accessed 20 Sept 2018
Zhang M, Mu TH (2017) Identification and characterization of antioxidant peptides from sweet potato protein hydrolysates by Alcalase under high hydrostatic pressure. Innov Food Sci Emerg 43:92–101
Sun M, Mu T, Zhang M, Arogundade LA (2012) Nutritional assessment and effects of heat processing on digestibility of Chinese sweet potato protein. J Food Compos Anal 26:104–110
Zhang M, Mu TH (2016) Optimisation of antioxidant hydrolysate production from sweet potato protein and effect of in vitro gastrointestinal digestion. Int J Food Sci Tech 51:1844–1850
Nielsen P, Petersen D, Dambmann C (2001) Improved method for determining food protein degree of hydrolysis. J Food Sci 66:642–646
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28:1057–1060
O’Loughlin IB, Kelly PM, Murray BA, FitzGerald RJ, Brodkorb A (2015) Molecular characterization of whey protein hydrolysate fractions with ferrous chelating and enhanced iron solubility capabilities. J Agric Food Chem 63:2708–2714
Yoo S-H, Chang YH (2016) Volatile compound, physicochemical, and antioxidant properties of beany flavor-removed soy protein isolate hydrolyzates obtained from combined high temperature pre-treatment and enzymatic hydrolysis. Prev Nutr Food Sci 21:338–347
Zhang Q, Tong X, Qi B, Wang Z, Li Y, Sui X, Jiang L (2018) Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J Func Food 42:298–305
Wang C, Wang B, Li B (2016) Bioavailability of peptides from casein hydrolysate in vitro: amino acid compositions of peptides affect the antioxidant efficacy and resistance to intestinal peptidases. Food Res Int 81:188–196
Phongthai S, D'Amico S, Schoenlechner R, Homthawornchoo W, Rawdkuen S (2018) Fractionation and antioxidant properties of rice bran protein hydrolysates stimulated by in vitro gastrointestinal digestion. Food Chem 240:156–164
Delgado MCO, Nardo A, Pavlovic M, Rogniaux H, Añón MC, Tironi VA (2016) Identification and characterization of antioxidant peptides obtained by gastrointestinal digestion of amaranth proteins. Food Chem 197:1160–1167
Hernández-Ledesma B, Davalos A, Bartolome B, Amigo L (2005) Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulin. Identification of active peptides by HPLC-MS/MS. J Agric Food Chem 53:588–593
Acknowledgements
This work is supported partially by the Alabama Agricultural Experiment Station. The authors also gratefully acknowledge the Beijing Municipal Natural Science Foundation (grant 6182034), the earmarked fund for China Agriculture Research System (CARS-10-B21), National Key R&D Program of China (2016YFE0133600), and financial support from China Scholarship Council.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Authors declare not having any conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 236 kb)
Rights and permissions
About this article
Cite this article
Zhang, M., Huang, TS. & Mu, TH. Production and In Vitro Gastrointestinal Digestion of Antioxidant Peptides from Enzymatic Hydrolysates of Sweet Potato Protein Affected by Pretreatment. Plant Foods Hum Nutr 74, 225–231 (2019). https://doi.org/10.1007/s11130-019-00724-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-019-00724-y