Abstract
The objective of this research was to investigate the impact of high-intensity ultrasound (HIU) generated by a probe-type sonicator (frequency 20 ± 0.2 kHz and an amplitude of 40%) for 2–20 min on the selected functional and structural properties of egg white proteins (EWPs) and their susceptibility to hydrolysis by alcalase. The protein solubility, foaming, and emulsifying properties were studied as a function of ultrasonication time and related to protein particle and structural properties. The length of ultrasonication exhibited important effect on EWP particle size, uniformity, and charge, affecting also the protein conformation and susceptibility to alcalase hydrolysis and determining functional properties. There was a linear correlation between the particle size decrease and the solubility while a two-step linear correlation between the foam capacity (FC)/foam stability (FS) and particle size was apparent. Specifically, FC and FS sharply increased with decreasing particle size for range from ∼370 to ∼260 nm, and below this range from 260.6 to 68.4 nm, the changes were not that substantial. Besides, the solubility, FC, and FS were directly and linearly related with the absolute value of the particle zeta potential. The overall emulsifying properties were also improved with an increase of sonication time, through both the decrease of the mean particle diameter and the increase of zeta potential, but there was no direct correlation between the emulsion activity/stability index and protein particle size and/or charge. Analysis of EWP structure by Raman spectroscopy revealed that the HIU leads to changes in the secondary structure, while heat and ultrasound generated by the ultrasound bath were not sufficient to exhibit this effect.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to safety and toxicity concerns related to the use of thermal processing technology, the global trend for food industries is to apply new, safe, and environmentally friendly technologies for processing of food components that avoid the quality deterioration and loss in nutritional value (Mirmoghtadaie et al. 2016). The application of power ultrasound based on the interaction of sound waves with frequencies ranging from 16 to 100 kHz with food matrix offers the opportunity to modify and improve some technologically important compounds which are often used in food products, such as proteins (Tian et al. 2015; Ozuna et al. 2015). Unlike nondestructive ultrasound (low-intensity ultrasound, high frequency, typically in the range of 100 kHz to 1 MHz), high-intensity ultrasound (HIU) has been used to provoke changes in some chemical, functional, and physical properties of food ingredients that may be of interest as technological benefit.
The beneficial use of ultrasound is realized through its chemical, mechanical, or physical effects on proteins induced predominantly through cavitation (Chen 2012). This phenomenon involves the rapid formation, growth, and subsequent implosive collapse of nano/microbubbles of gas in a liquid subjected to ultrasound; releasing large magnitude of energy; and inducing localized extreme conditions such as a hot spot with a temperature greater than 5000 K and a pressure greater than 100 MPa. The released energy appears to be strong enough to break the chemical bonds of water to yield H· and OH· radicals, which are very reactive and produce hydrogen peroxide, the most common chemical effect of cavitation (Mason 2015). Physical effects include microstreaming, microstreamers, microjet formation, and shockwaves, as a consequence of the vacuum microbubble implosion. All these effects induced by ultrasound energy could cause important modifications in protein molecules like breaking of covalent bonds, generation of low molecular weight peptides, and/or fragmentation of large aggregates into smaller particles, modifying the functional properties of the proteins (Tavano 2013).
In the food industry, the HIU treatment offers a new approach to modify and tailor the functional properties of food proteins (Barukčić et al. 2015). It has also shown promise for improving enzymatic hydrolysis of natural protein substrates, possibly by increasing protease activity or enhancing protein accessibility, with its effectiveness depending on the type of protease and substrate (Stefanović et al. 2014). The effects of HIU on proteins are increasingly being studied showing that it could induce macroscopic changes like reduced viscosity and turbidity or improve some functional properties such as gelling, solubility, foaming, and emulsifying properties (Arzeni et al. 2012a; O’Sullivan et al. 2016; Zhou et al. 2016). However, the HIU treatment has to be controlled because extended and prolonged treatments often cause aggregation with diminishing functional properties when applied to complex biological systems.
There are many contradictory reports on the influence of sonication on the structural–technological functionalities of various proteins and their peptides. For example, the high-intensity ultrasonication of bovine serum albumin (BSA) has been shown to disrupt the protein secondary structure, increase the surface hydrophobicity and surface charge of BSA particle, but decrease number of free sulfhydryl groups, which was explained by the formation of protein aggregates (Gulseren et al. 2007). Other researchers have also observed the aggregate formation following sonication of proteins, which was thought to be associated with the structural changes in secondary structure of individual proteins such as an increase in β-structure with a concomitant decrease in α-helical structure (Scholz et al. 2004). On the contrary, other studies reported also structural changes but a significant rise in sulfhydryl content as a result of ultrasonication (Huang et al. 2008). For example, the work with ovotransferrin as a model egg white protein system has shown that the content of reactive sulfhydryl (SH) groups increased over 30 and 80% for the samples sonicated for 240 and 480 s at 60 kHz, respectively. This was attributed to the protein structural changes associated with chemical effects of ultrasonic cavitation, generating high-energy intermediates such as hydroxyl and hydrogen free radicals, which caused breakdown of disulfide bonds (Lei et al. 2011).
It has also been reported that the ultrasound treatment of pure β-lactoglobulin resulted in both increase in reactive SH group content and increase in surface hydrophobicity. Although results have suggested that the dissociation of the native dimer into monomers occurred, no change in secondary structure was detected by circular dichroism. Ultrasound has been reported to disrupt the native fold but leave secondary structural elements intact, and similar results were obtained for whey protein isolates (Frydenberg et al. 2016). Studies on effect of ultrasound on protein susceptibility to enzymatic hydrolysis have given particularly contradictory results showing both strong and weak correlations between the hydrolysis rate and intensity of the treatment (Lei et al. 2011; Stefanović et al. 2014). Thus, the effects of ultrasonic waves on proteins appear to be very complex and it is difficult to determine the exact physical and chemical effects of ultrasound on protein structure/functionality due to variability of the measured parameters predominantly resulting from individual differences of natural proteins and their mixtures.
Among a wide range of different protein sources, egg white (albumen) is extensively utilized as a functional ingredient in the food industry, due to its high nutritional quality and unique functional characteristics such as foaming, gelling, and emulsification (Mine 1995). Egg white proteins (EWPs) are essential components of many foods such as bakery products, meringues, meat products, and cookies providing various functions in food quality and stability; however, functional properties of EWPs remain a challenge for industries and need to be considered. Although HIU treatment has shown promise for improving functional properties of some egg white constituents in model systems using the purified protein such as ovalbumin, ovomucoid, ovotransfferin, and lysozyme, works focusing on the potential effects of HIU application on native EWPs are scarce (Arzeni et al. 2012a, b; Stefanović et al. 2014; Tan et al. 2015).
Egg white contains about 88% of water, 11% of proteins, 0.2% of fat, and 0.8% of ash while its protein component is a composite mixture of a number of protein fractions including ovalbumin (∼54%), ovotransferrin (∼12–13.6%), ovomucoid (∼11%), ovomucin (∼3.5%), lysozyme (3.4–3.5%), and over 30 other protein fractions, which all have a role in determining functional properties of albumen. It is known that the effects of sonication on structural and functional changes of proteins are different when they are present as individuals or as mixtures (Chandrapala et al. 2012). For example, ovotransferrin contains 15 disulfide bonds, compared to one disulfide bond in ovalbumin and four disulfide bonds in lysozyme (Huang et al. 2008). Furthermore, the mechanism of ultrasound-induced denaturation and aggregation of proteins in the presence of reducing sugars, sucrose, or salts might show some extraordinary characteristics. So, a dissimilar behavior of the model protein system and natural egg white in relation to ultrasound is expected due to difference in the number of disulfide bonds within the proteins or presence of other additives. Because industrial production handles the complex egg white matrix, all proteins should be considered. Few studies have been carried out on the effect of ultrasound on functional properties of EWPs including surface hydrophobicity, bulk viscosity, foaming, and emulsifying properties (Arzeni et al. 2012a, b), but the structure-function relationship of the EWPs related to their denaturation during the ultrasonication for various periods of the treatment time is not clear.
This work continues a research on the effects of HIU on EWP functional properties. The objectives of this study were to study the effect of length of HIU treatment on functional and structural properties of EWPs, and to determine if there was a correlation between particle and/or structural properties and functional properties. Insight into the link between structural and functional properties of EWPs during the ultrasonication can increase their potential as functional agents in food systems.
Materials and Methods
Materials
Freshly laid chicken eggs (pH 7.6–8.6), kindly provided from PKB “Inshra,” Padinska Skela, were stored at 4 °C and used within 24 h after collection. Egg white was separated manually from the yolk (chalaza was removed) and gently stirred without foam formation to provide homogeneous mixture. The enzyme used in this research for determination the susceptibility of different treated samples to proteolysis was alcalase 2.4 L (EC 3.4.21.14) from Bacillus licheniformis, which was purchased from Sigma-Aldrich (St. Louis, USA). The claimed enzyme activity was ≥2.4 U Anson units/g solid. Ovalbumin (>90% pure by agarose electrophoresis with a molecular weight of 45 kDa) was also from Sigma-Aldrich (St. Louis, USA). The deionized water (18.2 MΩ) used for the experiments was produced using a Thermo Scientific Barnstead Smart2Pure water purification system. All other chemicals were of analytical reagent grade, and they were used without any further purification.
High-Intensity Ultrasound Treatment of EWPs
The series of 10 wt% egg white aqueous solutions were exposed to ultrasound waves using a probe-type sonicator at a frequency of 20 ± 0.2 kHz and an amplitude of 40% (maximum amplitude 40%, 50 ± 2 μm) (Dojcinovic and Volkov-Husovic 2008). A 13-mm high-grade titanium tip was immersed in the liquid, and the liquid is irradiated with an ultrasonic wave directly from the horn tip. The samples were prepared in a custom-made glass reaction water-jacketed vessel of 300-mL capacity where the working volume (∼180 mL) was kept constant for all experiment sets. In all experiments, the horn tip was submerged to a depth of 53 ± 1 mm below the water surface and the overall water depth in the vessel was 160 mm. The constant temperature (25 ± 1 °C) was maintained by circulating thermostated water through a water jacket (ordinary water flow: from 5 to 10 mL/s).
In order to determine the power of ultrasound probe, the initial temperature rise (T) was recorded against time (t) at 30-s intervals with a thermocouple with digital display and then calculated average increase in temperature after several treatments. The power of ultrasound probe was designated calorimetrically according to the following equation (Mason et al. 1992; Tiwari and Mason 2012):
where m is the mass of ultrasound-treated solution (g), C p is the specific heat capacity of the water (4.18 kJ/(g K), and (dT/dt) is the initial rate of change of temperature during ultrasonication, starting at t = 0 (°C/s). The temperature rise was estimated from the slope of the straight portion of the line that was obtained during the first seconds of the experiments in all cases (R 2 ≥ 0.98). The repeatability of power output values estimated using this method in this investigation was satisfactory (STD ˂5%). This showed that in the investigated system, the heat exchange between the treatment chamber and the main vessel was negligible. Similar results were obtained by Kimura et al. (1996) who concluded that the thermal exchange between the coolant and the reaction solution was negligible during the measurement of the initial temperature rise in their reactor.
The value of actual ultrasonic power dissipated in the liquid under adiabatic conditions has been calculated to be 34.11 ± 1.46 W. The pH values of protein model solutions were also checked before and after ultrasound treatment at 20 ± 0.2 kHz for 5–20 min by pH meter Eutech Instrument, Netherlands. Values of pH did not change significantly (P > 0.05) upon the treatment.
For comparison purpose, two different treatments were also carried out. The first treatment was the ultrasound one performed in an ultrasonic water bath with a frequency of 40 kHz (EI-NIS-RO-VEP, Serbia). The acoustic power dissipated in the liquid, determined by a calorimetric method according to a previous work, was 21.3 W (Stefanović et al. 2014). The second was the thermal treatment carried out by heating a EWP solution at 75 °C for 30 min. Upon completion of treatments, samples were used immediately for further analysis or freeze-dried. The control was carried out with a stirring instead of sonication at 25 °C and a speed of 240 rpm.
Functional Properties of Sonicated EWPs
Protein Solubility
Protein solubility of ultrasound-treated EWP solutions was determined at pH 8.0. Samples were suspended in water (10% w/w), and pH was adjusted to 8 using 0.5 M NaOH while stirring at room temperature for 1 h. The samples were then centrifuged at 18,000×g and 4 °C for 15 min. The protein contents in the supernatant were determined using the Sigma Procedure no. TRPO-562 using BSA as the standard (Lai et al. 2010). Solubility was expressed as the percentage of protein remaining in the supernatant as compared to the untreated samples.
Determination of Emulsion Activity and Stability
Emulsion activity index (EAI) and emulsion stability index (ESI) were determined according to the method by Pearce and Kinsella (1978) except for the noted changes in each section. Emulsions of the each EWP dispersion (3% w/w) were prepared with sunflower oil in molar ratio 1:2 and mixing for 90 s using a homogenizer (Yellowline, DI 25 basic, Ica Works Inc., Wilmington, 600 W, 50 V) at 8000 rpm at 25 °C. The 50 mL emulsions were taken from the bottom of the containers and diluted with 10 mL of 0.10% sodium dodecyl sulfate solutions. The absorbance of the diluted emulsions was measured at 500 nm immediately after emulsion formation by UV/vis spectrophotometer (Amersham Bioscience, Ultrospec 3300 pro). The turbidity was calculated by the following formula:
where T is the turbidity, A is the absorbance at 500 nm, and l is the path length (m).
The emulsion activity index (EAI) was then calculated as follows:
where θ is the volume fraction (mL), c is the weight of protein per unit volume of aqueous phase before emulsion is formed (g), and r is the dilution factor.
For determining the emulsion stability, the EWP dispersions were kept at 4 °C for 24 h and analyzed for emulsion activity as described previously. The emulsion stability index (ESI) was calculated by the following formula:
where T is the turbidity value at 0 h, ΔT is the change in turbidity during 24-h period, and Δt is the time interval (24 h).
Determination of Foaming Properties
For assessing foaming properties, foam capacity (FC) and foam stability (FS) were determined.
Analysis was according to the method as described previously (Stefanović et al. 2014). An aliquot (50 mL) of EWP solution (8 mg protein/mL solvent) was placed in a graduated glass cylinder (internal diameter 72.0 mm) in a water bath at 20 °C and whipped for 4 min with a laboratory homogenizer at a speed of 9.500 rpm (Yellowline, DI 25 basic, Ica Works Inc., Wilmington, 600 W, 50 V, 8000–24,000 rpm). After whipping, the propeller was immediately removed and the glass cylinder sealed with parafilm to avoid the foam disruption. The FC was determined by measuring the foam volume after whipping (mL) at 0 min and expressed as percentage of the initial volume before the whipping (mL). The FS was measured by determining foam volume after 30 min of standing (mL) and expressing it as percentage of the FC (Stefanović et al. 2014).
Measurement of the Denaturation Degree
The Enzymatic Hydrolysis of EWPs at an Enzyme to Substrate Ratio of 3500 U/g of Protein
The susceptibility of EWP samples to enzymatic hydrolysis was detected by incubation of 180 mL of treated samples (containing 10.7 ± 1.8 mg/mL, protein determined according to the standard Kjeldahl method, N × 6.25, pH 8.0) with a proper amount of alcalase (4.8 AU) at 50 °C (E/S ratio was 2.12 AU/g of protein). The hydrolysis was studied at one E/S ratio. The enzymatic hydrolyses were performed in a mechanically stirred batch reactor at a speed of 240 rpm with temperature and pH control. The pH was maintained by continuous addition of 0.2 M NaOH during the enzyme reaction. The reaction was terminated by heating the mixtures for 15 min at 95 °C and then centrifuged at 12,000×g for 10 min after cooling at room temperature. The supernatant was collected and stored at 4 °C for further analysis.
The progress of the reaction was followed by monitoring the degree of hydrolysis (DH, %) using the pH-stat method. The DH was calculated according to the following equation (Adler-Nissen 1986):
where h is the number of equivalents of peptide bonds hydrolyzed at the time per weight unit, h tot is the total amount of peptide bonds per weight unit of a protein and can be calculated from its amino acid composition (for EWPs h tot is 7.67 mmol/g protein), N b is the normality of the base, B is the consumption of the base in milliliter, α is the degree of dissociation of the α-amino groups (1/α = 1.13 at 50 °C and pH 8.0), and m p is the mass of protein in grams.
Determination of SH Groups
The content of SH groups of EWPs upon treatment with the ultrasound probe was determined spectrophotometrically using 5,5′-(dithiobis-2-nitrobenzoate) (DTNB), which reacted with free SH groups to yield a product with a maximum absorbance at 412 nm. Analysis was performed according to Ellman’s procedure with slight modifications (Shimada and Cheftel 1988). The reactive (surface) SH groups were measured as follows. A solution of HIU-treated or native egg white was diluted to a concentration of 0.05% (w/w) with a standard buffer, pH 8.0, composed of 86 mM Tris, 90 mM glycine, and 4 mM EDTA and centrifuged during 20 min at 12,300×g to remove precipitated proteins. A 0.025-mL Ellman’s reagent (4 mg/mL) was added to 2.5 mL of supernatants and mixed rapidly, and after 15 min at ambient temperature, the absorbance was measured at 412 nm against a reagent blank. The total SH group content was also determined following the same technique but using a denaturing buffer consisting of 86 mM Tris, 8 M urea, and 0.5% (w/v) sodium dodecyl sulfate. The standard and the denaturant buffers were used as reagent blanks instead of protein solutions for both measurements.
Measurement of Particle Properties
Particle Size Distribution and Measurement of Zeta Potential
The particle size, polydispersity indexes (PDIs), and zeta potential of untreated (control), ultrasound-treated, and heat-treated EWPs were performed by dynamic light scattering measurements using a Zetasizer Nano ZS (Malvern Instruments, UK) provided with an apparatus operating at λ = 633 nm produced by an He–Ne laser at a scattering angle 173° at 25 ± 0.1 °C. To determine the effect of ultrasonication treatment time on average particle size, prepared EWP solutions were ultrasonicated with the ultrasound probe for 2, 5, 10, 15, and 20 min (labeled as UPEW-2, UPEW-5, UPEW-10, UPEW-15, and UPEW-20) and compared to samples treated in the ultrasound bath for 15 min (UBEW-15), heat treated for 30 min (TEW-30), and untreated (control, EW). All measurements were carried out immediately after ultrasound or thermal treatment, when the particles were still dispersed in the dispersant. Protein size values were reported as Z-average size. The same apparatus was used for the calculation of zeta potential value from the electrophoretic mobility using the Smoluchowski equation (Shaw 1992):
where ξ is the zeta potential, μ is the mobility, η is the viscosity of the solution, and ε is the dielectric constant of the solvent. The average value and standard deviation of ten measurements per sample were reported.
Spray-Drying Conditions and Field Emission Scanning Electron Microscopy
The samples (pretreated and untreated EWPs) were spray-dried using a pilot-scale spray dryer (BÜCHI Dryer B-290, Büchi, Switzerland). The EWP solutions were fed by a peristaltic pump at a fixed rate of 0.3 L/h. Inlet and outlet air temperatures were set to 120 and 90 °C, respectively. The obtained powder was collected at the cyclone. The products were stored in glass bottles at room temperature, in a dry place, in the absence of light.
The surface morphologies of the obtained pretreated and untreated EWP spray-dried powders were studied by field emission scanning electronic microscopy (FESEM) Mira 3 XMU (Czech Republic) at an accelerating voltage of 5 kV. Prior to the FESEM analysis, the powder samples were coated with gold in an argon atmosphere using a spatter coater.
Molecular Structure Characterization Measured by Raman Spectroscopy
The molecular structures of untreated and ultrasound-treated EWPs were tested by the Raman analysis. Raman spectra were recorded at room temperature (∼20 °C) using an XploRA Raman spectrometer from Horiba Jobin Yvon. The laser excitation wavelength was 532 nm (maximum output power 20–25 mW). Spectra were collected using an acquisition time of 15 s and boxcar smoothing set to 2. All measurements were realized using the spectrometer equipped with 1800 g/mm grating. The averaged spectral data from the scans of samples in the Raman spectrophotometer were baseline corrected and normalized against the phenylalanine band at 1004/cm. The Raman spectra (200–3500/cm) of each sample were performed in triplicate, and the results were reported as the averages of these replicates (relative standard deviation <5%).
Statistical Analysis
In this research, all experiments were carried out at least in triplicate and expressed as means with standard deviation. Analysis of variance (ANOVA), followed by the Tukey test, was performed to compare the effects of ultrasound probe under the significance level of P < 0.05. All statistical analyses including calculations were conducted using OriginPro 9.0 (OriginLab Corporation, MA, USA).
Results and Discussion
Ultrasound Probe Treatment-Induced Changes in Functional Properties of EWPs
EWP solution (10 wt%) was treated for 2, 5, 10, 15, and 20 min using an ultrasonic probe at a frequency of 20 kHz and with an amplitude of 40%. The protein solubility and foaming and emulsifying properties were studied as a function of time, and the results are presented in Fig. 1.
Functional properties of the ultrasound probe-treated EWP solutions as a function of time: a solubility, b foam properties, and c emulsifying properties. The labels indicate samples treated for different times: UPEW-2 ultrasound probe-pretreated EWPs for 2 min, UPEW-5 for 5 min, UPEW-10 for 10 min, UPEW-15 for 15 min, and UPEW-20 for 20 min; EW untreated egg white. Error bars indicate standard deviations
The solubility is an important aspect that needs to be considered due to its considerable effect on other techno-functional properties and quality of the end product. For example, to obtain optimum functionality in foods that requires gelation, emulsifying, and foaming properties, a highly soluble protein is desirable (Mine 1997). The impact of HIU treatment on solubility of EWPs as a function of treatment time is presented in Fig. 1a.
It seemed that the solubility of all samples increased significantly (P < 0.05) after ultrasonication, when compared with the nontreated sample (control), and the highest solubility was observed in a sample which was exposed to ultrasound waves during 15 min. This result was in general agreement with the results of previous studies showing that the ultrasound treatment caused an increase in protein solubility in the case of soy protein isolate and milk protein concentrate (Jambrak et al. 2009; Yanjun et al. 2014). This was attributed to conformational changes of the globular proteins in a manner that hydrophilic parts of amino acids from inside were opened toward water molecules causing the reorganization of water molecules after ultrasound treatment and increased solubility. In addition, the number of charge groups (NH4 +, COO−) appeared to increase permitting greater water–protein interactions due to the higher electrostatic forces. This was also confirmed by an increase in the electrical conductivity (Jambrak et al. 2009). However, the protein solubility can be strongly affected by temperature due to possible thermal denaturation, which caused an increase in protein particle size as a result of ultrasonication. Thus, dissimilar to our result, it has also been reported that EWPs demonstrated a small, but significant, decrease in solubility after HIU treatment conducted at elevated temperatures, which might be associated with the thermal aggregation during the treatment (Arzeni et al. 2012b). In the cited work, authors reported that the highest temperature reached after ultrasound treatment was 49 °C, which was significantly higher than the temperature reached in this work.
The commercial application of EWPs in the food industry is highly dependent on foam properties like FC and FS. For example, the excellent foaming capacity of the egg white and the stability of the resulting foams (even when subjected to heating) are important to the structure and texture of many food protein-based aerated products, including various cakes, confections, meringues, etc. (Van der Plancken et al. 2007). Figure 1b summarizes the foaming properties of the ultrasound probe-pretreated EWPs as a function of the treatment time, and it can be observed that both FC and FS have been increased statistically significant (P < 0.05) after ultrasonication.
However, the HIU treatment resulted in a time-dependent increase in FS, whereas the FC was not significantly affected by the treatment time but was much larger when compared with the nonsonicated sample (control). The short-time HIU treatment (2 min) appeared to be sufficient to provide a rather high effect on FC of EWPs, resulting in a 1.5-fold increase of foam expansion compared to the untreated EWPs. A number of researchers have found that the ultrasound treatment improved FC and FS due to the homogenization effect of ultrasound or increased protein surface hydrophobicity and flexibility allowing a more effective adsorption of the protein molecule onto the air–water interface (Jambrak et al. 2009; Mirmoghtadaie et al. 2016). However, dissimilar to our result, it has been shown that when ovalbumin was ultrasonicated with a standard 20-kHz commercially available horn sonicator, this impacted its foaming ability but not its foaming stability (Xiong et al. 2016). This difference may be a consequence of the complexity of EWPs, which are a composite mixture of a number of protein fractions including ovalbumin and over 30 other protein fractions rather than single component ovalbumin, so egg white is expected to behave in an entirely different manner from ovalbumin. The changes in the foam properties can be associated with the denaturation and unfolding of all protein fractions.
The emulsifying performances of ultrasound-treated EWPs were also tested and compared to their untreated counterpart. As shown in Fig. 1c, the values of EAI and ESI were gradually increased with time of ultrasound treatment and the highest ESI of 205.37 ± 3.24 h was obtained after 20 min of ultrasonication while the highest EAI of 267.60 ± 2.45 m2/g was observed after 15 min of ultrasonication. The results were in agreement with data reported earlier by other authors using globular proteins such as soy glycinin (11S) and soy protein isolate showing increased emulsifying properties with time of sonication (Jambrak et al. 2009; Zhou et al. 2016). The improvement in emulsifying performances of proteins seemed to be associated with the conformational changes resulting in a better interfacial adsorptivity at the interface of oil droplets and was positively correlated with both solubility and surface hydrophobicity. However, the link between surface hydrophobicity and emulsifying properties was not always clear, suggesting that some other factors could contribute to the emulsifying properties of proteins. For example, it appeared that the rate of protein adsorption of ultrasound-treated fish gelatin and soy protein isolate to the oil–water interface remained unchanged regardless of the smaller protein associate sizes and increase in hydrophobicity, when compared with untreated fish gelatin and soy protein isolate (O’Sullivan et al. 2016). Likewise in another study, even though the HIU treatment increased the solubility and surface hydrophobicity of glycinin at low ionic strength (I = 0.06), EAI at I = 0.06 has been reported to decrease, which was attributed to the changes in the molecular flexibility of the protein (Zhou et al. 2016). Thus, HIU affected the functional properties differently depending on the size and nature of the protein as well as environmental conditions like ionic strength and others (Arzeni et al. 2012b; Zhou et al. 2016).
Overall, in this study, the ultrasound treatment using an ultrasonic probe at room temperature and treatment time has been shown to have significant impact on both foaming and emulsifying properties of EWPs and resulted in an enhancement of the surface properties and functionality. The degree of unfolding of the proteins upon ultrasound wave and exposure of functional groups might play a crucial role in the foaming and interfacial property process of the proteins. Furthermore, changes in foaming and emulsifying properties may be due to intramolecular and intermolecular changes related to the size of protein aggregates, as the proteins coalesce and separate simultaneously due to pressure processing. Thus, further studies on the mechanism of physicochemical changes in EWPs during ultrasonication at the molecular level, such as sulfhydryl-disulfide exchange, changes in molecular structure, peptide cleavage, or changes in surface protein particle charge and size, are required for better understanding of the ultrasonication process and link between structural and functional properties of EWPs, increasing their potential as functional ingredients in food systems.
The Effect of HIU on the Denaturation Degree
Several techniques are known in the literature for measuring the degree of denaturation of albumin and other proteins like measuring their susceptibility to enzymatic hydrolysis or the amount of reactive SH groups.
Firstly, the protein structural changes associated with ultrasound application were studied in terms of its susceptibility to hydrolysis by a commercially available food-grade protease, alcalase. Susceptibility to enzymatic hydrolysis has been taken as an indicator of structural integrity for many proteins, including ovalbumin and lysozyme (Van der Plancken et al. 2005). It was apparent from Fig. 2a that the HIU treatment significantly changed the proteolytic pattern of EWPs (P < 0.05) for all examined treatment time. As shown, initial rate and DH increased with the increase of the pretreatment time up to 15 min followed by a gradual decrease with further ultrasonication. The initial increased susceptibility to enzymatic hydrolysis may be attributed to ultrasonically induced conformational changes, which can cause full or partial unfolding of polypeptides and result in exposure of buried peptide bonds, making them more accessible to the enzyme attack. However, HIU treatment for 20 min decreased remarkably the susceptibility of EWPs to enzymatic hydrolysis, suggesting protein aggregation which in turn protected the internal bonds of the proteins.
The time course of hydrolysis by alcalase (a) and content of SH groups (b) as a function of ultrasound treatment time and c comparison of the susceptibility of differently treated EWPs to enzymatic hydrolysis (UBEW-15 ultrasound bath treatment at 40 kHz for 15 min, UPEW-15 ultrasound probe treatment at 20 ± 0.2 kHz for 15 min, TEW-30 thermal treatment at 75 °C for 30 min)
Results of total and reactive (surface) SH group measurements presented in Fig. 2b seemed to confirm further this hypothesis. As shown, the amount of total sulfhydryl groups of EWPs showed a significant increase up to 10 min but then started to decrease with further ultrasonication. The surface sulfhydryl groups showed a similar profile but decreased sharply after 15 min of ultrasonication. These results revealed that the HIU mediated some degree of unfolding, which correlated with improved solubility or emulsifying properties (Fig. 1). Namely, during 10–15 min of ultrasonication, most sulfhydryl residues in EWPs that existed in the interior of protein molecules were exposed with ultrasound denaturation, but they formed S–S bonds by SH oxidation or SH/S–S interchange reaction after prolonged ultrasound treatment. As the EWP susceptibility to hydrolysis and their solubility after HIU treatment for 15 min were rather high as well as foaming and emulsifying properties of EWPs, the decrease in total SH content might be explained by the formation of predominantly intramolecular disulfide bonds due to sulfhydryl oxidation under ultrasonication. On the other side, after HIU for 20 min, the decline in sulfhydryl groups may be attributed to intermolecular disulfide bond formation. Both decreased protein susceptibility to hydrolysis and decreased number of free sulfhydryl groups after prolonged ultrasonication may be attributed to the formation of intermolecular disulfide bond, leading to the protein aggregate formation. However, further research is required to confirm this hypothesis. Data found in bibliography are very dissimilar. Gulseren et al. (2007) and Hu et al. (2013) found that the content of SH groups decreased with the sonication time, while Arzeni et al. (2012b) and Chandrapala et al. (2010) reported that ultrasonication did not change the content of SH group significantly. Similar to our results, Lei et al. (2011) found that the content of total SH group of ovotransferrin solution showed a significant increase as a function of treatment time up to 30 or 60 s at both 20 and 60 kHz, respectively, and then started to decrease. However, the content of reactive sulfhydryl groups, in that case, showed a different trend, increasing considerably at higher ultrasound power with longer sonication time.
The ultrasonic device used in the present work was a standard commercially available ultrasonic probe sonicator. In order to verify the impact of the ultrasonic probe treatment (UPT) on the susceptibility of EWPs to enzymatic hydrolysis, these results were compared with our previously results obtained by applying the ultrasound bath and thermal treatment prior hydrolysis (Stefanović et al. 2014). These results are presented in Fig. 2c, and it can be concluded that the UPT accelerated the enzymatic hydrolysis and generated more reproducible results compared to the ultrasound bath treatment under similar conditions, probably by causing the structural changes of EWPs beneficial to promote hydrolysis. This behavior could be explained by the different way of treatments, including a fact that EWPs treated with the ultrasound probe were mostly exposed to high power due to direct contact between tip and sample, whereas in the bath, the sample was inserted together with a flask into the ultrasound bath avoiding a direct contact of sample with the irradiating surface. Ultrasound treatment of EWPs at 20 kHz for 15 min even resulted in higher DH than thermal treatment at 75 °C for 30 min, although the initial rate was lower for UPT.
Particle Properties
Particle Size
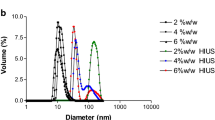
The particle size distribution (PSD) of EWP solution treated with the ultrasound probe during different time at 20 ± 0.2 kHz and the impact of diverse treatments (thermal and ultrasound) on PSD are illustrated in Fig. 3.
Particle size distribution patterns of 10 wt% EWPs treated with an ultrasound probe at 20 ± 0.2 kHz for various times (2, 5, 10, 15, and 20 min). Insert: the influence of several treatments on the particle size distribution of EWPs (UPEW-15 ultrasound probe treatment at 20 ± 0.2 kHz for 15 min (circles); UBEW-15 ultrasound bath treatment at 40 kHz for 15 min (squares); TEW-30 thermal treatment at 75 °C for 30 min (triangles))
The curve pattern of EWP solution prior to ultrasonication (i.e., t = 0) exhibited a peak range from 300 to 600 nm with a maximum at 368.4 ± 10.51 nm and a small peak from 0.5 to 1.1 nm. The width parameter known as the polydispersity index (PDI) was also measured and found to be 0.521 ± 0.011, indicating that the EWP had a broad size distribution. The HIU treatment using the ultrasonic probe at a frequency of 20 kHz caused a shift in the PSD to lower diameters and reduce the PDI value. The average particle size (Z-average) decreased as a function of treatment time up to 15 min followed by a sharp increase with further ultrasonication. The greatest decrease in the particle size was observed when ultrasound was applied for 15 min (from 368.4 to 68.4 nm), which was consistent with the observed increase in the solubility of EWPs and their increased subsequent proteolysis (Figs. 1 and 2). Actually, HIU ruptured the protein granules and facilitated disintegration of particles, which led to an increase of solubility after ultrasonication. Aggregates were broken into smaller particles with narrow size distribution (Table 1) as revealed by the decrease of PDI value with the increase of the treatment time.
These results were in agreement with those of O’Sullivan et al. (2016), who observed a significant reduction in the protein particle size of several animal and vegetable proteins studied by sonication, which was explained by the disruption of noncovalent associative forces like hydrophobic and electrostatic interactions maintaining protein aggregates in solution. Li et al. (2014) also observed a significant reduction in the size of chicken breast meat, with uniform and narrowed distribution, suggesting that the decrease in particle size by sonication could be attributed to the shear effects generated by acoustic cavitation of HIU.
The opposite was observed in our study when the prolonged HIU treatment of EWPs with ultrasonic probe at 20 ± 0.2 kHz for 20 min was applied. This treatment caused a shift to a bimodal size distribution with a marked peak at 260.6 ± 1.67 nm, but a smaller peak at 68.4 nm was still visible. Thus, a population with the higher particle size has become apparent as a result of aggregation between protein molecules, whereas a part of the particles was still intact (∼12%). The prolonged treatment caused an excess denaturation of EWPs enhancing the tendency of molecules toward random aggregation, as also shown by the decrease in the content of SH groups or decreased subsequent proteolysis (Fig. 2). Differently to our results, Arzeni et al. (2012a) also observed an increase in the particle size for sonicated EWPs, but within the whole range studied, which was attributed to the thermal aggregation during the ultrasound treatment. In the cited work, authors reported that the highest temperature reached 49 °C after the ultrasound treatment, which was significantly higher than the temperature reached in our work.
Both treatments of EWPs with ultrasonic bath or heat treatment also caused a significant (P < 0.05) reduction in the particle size, but not to the extent as the ultrasound treatment probe (insert in Fig. 3). As shown, the kind of treatment had a significant impact on the distribution of particle sizes. The EWP sample heated at 75 °C for 30 min showed a major peak at 320.1 nm and broad PSD, whereas for EWP samples treated with both ultrasound probe and bath, the values of these peaks were lower, namely at 68.4 and 164.2 nm, respectively. Besides, the treatment with the ultrasound probe caused a narrow size distribution whose origin might be in cavitational forces of the ultrasound probe. Similarly, researches determined significantly preferable effects of HIU probe on soy protein isolates and concentrate (Jambrak et al. 2009).
The further aim of this study was to determine if there was a correlation between particle properties and functional properties. To verify this, the solubility, foam capacity (FC), and foam stability (FS) were plotted against particle size (Fig. 4). As shown, there was a negative linear correlation between particle size and solubility, suggesting a significant role of the specific free surface on solubility. A decrease in particle size increased the specific free surface and protein–water interactions, which would consecutively result in positive changes in protein solubility. Regarding FC and FS, two clear particle size ranges could be distinguished corresponding to the lower and the higher particle size dependence. Namely, both FC and FS sharply increased with decreasing particle size for a range from ∼370 to ∼260 nm, and below this particle size range throughout the range from 260.6 to 68.4 nm, the changes were not that substantial as they showed almost a straight line behavior. A critical particle size value of 260.6 nm appeared to restrict these two regions.
To verify the obtained relationship between solubility or foaming properties and the particle size, the results obtained for other two treatments (ultrasound bath and heat treatments) are also presented in Fig. 4. It seemed that the values of foaming capacity and stability were well correlated to the particle size predicted from the linear trend obtained for the UPT, suggesting that the kind of energy depending on the particular pretreatment with its specific protein destruction mechanism was not crucial for foaming properties. The values for solubility for both ultrasound bath and heat treatments were, however, significantly lower compared to the predicted value, probably due to the contribution of the specific mechanism of the particular treatment related to changes in the protein secondary structures. However, all data sets suggested that the particle size played an important role in determining these functional properties.
When EAI and ESI were plotted against the average particle size, it was found that they also increased with decreasing particle size within the whole range studied, but the link between EAI and ES with particle size was not clear (data not shown). Specifically, a linear correlation between decreasing particle size and a resulting EAI or ESI increase was not apparent (R 2 was 0.621 and 0.309, respectively). This revealed that the emulsifying properties depended not only on the specific free surface, but also on the other factors like surface charge, hydrophobicity, molecular weight, or changes in the protein secondary structures.
Particle Morphology
The morphologies of spray-dried untreated, HIU-treated, and heat-treated EWP powder samples were examined by FESEM, and the electron micrographs of the spray-dried samples have been shown in Fig. 5. It was found that the powder particles of various treated EWPs showed dramatic changes in surface morphology. The surfaces of ultrasound-treated EWP particles were consistently smooth with some concavities in the surface. The occurrence of concavities in the surface of atomized particles can be associated to the rapid evaporation of the liquid droplets during the spray-drying process, and they could also be observed for the control sample (Fig. 5a) (Tian et al. 2011). However, compared with other samples, more particles from UPT sample were spherically shaped with some dents, but some particles had also a smooth surface without any cracks (Fig. 5b). In addition, the particles from UPT egg white powder were more uniform with a lower size. When the EWPs were subjected to the heat treatment, the network structure was completely changed with the larger particles having various shapes with a sponge effect and rough surface (Fig. 4d). The particle surface was composed of interconnected primary units (particles) and large aggregates of protein can be seen, and it was also composed of discrete entities and showed a porous structure with large and irregular cavities or pores. The particles were much less uniform and homogeneous compared to both ultrasound treatments.
FESEM (magnifications ×8.33 k, bar = 10 μm) images of the untreated EWPs (a), ultrasound probe-treated (UPT) EWPs with frequency 20 ± 0.2 kHz for 15 min (b), ultrasound bath-treated EWPs with frequency 40 kHz for 15 min (c), and thermal pretreated EWPs at 75 °C for 30 min (d). Scale bar is 10 μm in all cases
The mean diameter of the EWP particles obtained for UPT ranged from 1 to 6 μm, which was lower than that for the two other samples, and this difference can add evidence to the hypothesis that ultrasound treatment caused the disruption of protein particle aggregates. These results were consistent with previously obtained results of PSD although PSD was averaged over a large number of particles whilst the SEM images were chosen from a much smaller set of aggregated structures and may not be truly representative of the particle sizes in the sample (Zhang et al. 2016a, b). These values appeared to be highly satisfactory, since particles with a large size changed food texture, having poor dispersibility in finished products. According to the results, both ultrasound treatments seemed to modify the properties of the EWPs in a different way that the heat treatment did and could be a method of improving the functionality of EWPs. This study also can be used as a reference for further studies planned to develop dried egg white products with the desired levels of functional properties.
The Zeta Potential Values of the UPT EWPs
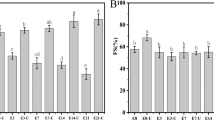
For all EWP samples which were used in this research, the values of zeta potential were negative (Fig. 6), indicating that the EWPs contained more negatively charged amino acid. Prolonging sonication time (5, 10, 15, and 20 min) had apparent effect and led to the significant increase (P < 0.05) of the absolute zeta potential. This suggested that the stability and electrokinetic potential of the EWP solution were maintained well during UPT. The increasing of negative charge of EWP solution which was subjected to the treatment with ultrasound probe has caused the interparticle electrostatic repulsions, disrupting available protein agglomerates and preventing subsequent agglomeration. All these positively affected the stability of the EWP dispersions.
As shown in Fig. 7, there was a linear correlation between the absolute value of the particle zeta potential and the achieved FC and FS increase (R 2 was 0.931 and 0.923, respectively), whereas no direct correlation was found between EAI or ESI and the particle zeta potential (data not shown). Here, the FS dependence had a significantly higher negative slope (−3.002) than did the FC (−1.645), indicating higher impact of HIU on FS. Thus, the foaming properties of EWPs were correlated with both particle size and particle zeta potential. On the other side, the link between particle size/particle charge and emulsifying properties was not clear.
This lack of correlation between particle size/particle charge and emulsifying properties may be a consequence of the complexity of EWPs, which are composed of a mixture of protein fractions rather than single component ovalbumin. To further verify a correlation between particle properties and emulsifying properties, the experiments were conducted in the model system using the purified protein such as ovalbumin, most abundant protein in the egg white. The trend obtained with isolated ovalbumin was quite similar to that after ultrasonication of the native egg white. Briefly, the particle size of ovalbumin decreased as a function of treatment time up to 10 min followed by an increase with further ultrasonication (data not shown). Prolonged treatment caused a shift to a bimodal size distribution with a marked peak at 1033 nm for 15-min HIU treatment and 1090 nm for 20-min HIU treatment, but smaller peaks at 210 and 1450 nm were still visible for both treatment times. The results of the emulsifying properties are consistent with the same results obtained from the mixture of EWP solution with the exception of the optimal length of HIU treatment. Namely, the average EAI and ESI increased as a function of sonication time up to 10 min followed by a sharp decrease with further ultrasonication (Online Resource 1). However, no correlation between EAI or ESI and particle properties was apparent (Online Resource 2), suggesting that some other factors like changes at the protein secondary structure can have a significant impact on egg white emulsifying properties.
EWP Structure Characterization Measured by Raman Spectroscopy
The Raman spectra of pretreated and untreated EWPs were used in order to obtain further information on the protein structural changes after applying various treatments (Fig. 8), and data from selected regions are summarized in Table 2. The structural differences of ultrasonicated EWPs compared to proteins heated in solution have not been clearly elucidated.
The allotments of major Raman bands were accomplished according to the previous researches (Painter and Koenig 1976; Li-Chan 1997; Hu and Du 2000). The amide I band occurred in the 1570–1720/cm region, while the amide II band frequency was fairly constant and occurred at 1555/cm. The S–S stretch vibration region that originated from amino acids with sulfur in the side chain occurred in the 501–511/cm region. Another important region for secondary structure was the C–H bonds from aliphatic residuals occurring at 1420/cm. The amide III band, which showed a strong frequency and width dependence on secondary structure, occurred in the 1226–1338/cm spectral region. The preliminary results of spectrum suggested that the most pronounced bands originated from ovalbumin, the main EW ingredient consisting of the α-helix and β-sheet (Zhang et al. 2016a, b). Additionally, major changes in the position of amide I bands (1570–1720/cm) after thermal and ultrasound treatments should be observed. The mentioned changes were in agreement with literature data which indicated that during EW thermal processing, main changes occurred due to formation of stable β-sheet intermolecular structures [41]. Likewise, the authors introduced that the formation of intermolecular structures was the main consequence of structural changes at molecular level during EW thermal denaturation and aggregation (Painter and Koenig 1976). Mine et al. (1990) emphasized that changes of heat-denatured EW were due to the augment in β-sheet structure as well as an extenuation of helical structure. According to the presented spectra, it was noticed that for all pretreated EWP samples, a reduction in peak intensity was in the amide III region, especially for EW prepared by the UPT.
In order to better report and explain the obtained spectra, the peak intensity values of amide I regions were normalized to the phenylalanine peak (1004/cm) and data from selected regions are summarized in Table 2.
Ultrasound-pretreated EWPs showed a marked increase of peak intensity in the range of 1439–1447/cm, namely 0.21 ± 0.05 and 0.17 ± 0.08 for probe and bath treatment, respectively, compared to 0.09 ± 0.01 for the untreated EW. It is important to point out that the thermal treatment also showed an enhancement in the mentioned peak intensity, even higher compared to the ultrasound bath treatment. Based on the available literature, specified band range can be attributed to the deformation motions of CH2 groups, indicating changes in the environment around the aliphatic side chains after heating or sonication (Ngarize et al. 2004; Zhang et al. 2016a, b). For structure analysis, the formation of disulfide bonds was very significant and those changes in the present study were observed in disulfide region (500–511/cm). For the ultrasound probe-pretreated EWPs, a conformation replacement from 500 to 511/cm was noticed whereas for ultrasound bath and thermal pretreated EWPs, similar but lower conformation shifts were remarked. The conformation shifts in the Raman spectra intimated changes in the disulfide region.
For the purpose of verifying the aforementioned structural changes caused by ultrasound and thermal treatments, the deconvolution of amide I bands has been done (Fig. 9).
Raman spectra of protein secondary structure components contributing to the amide I band were modeled from average spectra of EWPs after ultrasound probe treatment (a), ultrasound bath treatment (b), and after thermal treatment (c). The amide I deconvolved spectra showed contributions from protein secondary structure
Zooming the amide I regions of all investigated EWP samples can indicate the occurrence of minor peaks at 1575/cm after the 15-min UPT, which suggested that amount of histidine residues has become more pronounced (Chi et al. 1999) compared to ultrasound bath and thermal treatments. The review of available literature suggested that the bands that were still perceptible on spectra for ultrasound bath-treated EW at 1604 and 1618/cm can be appointed to the aromatic amino acid chains and their oscillation process (Nawrocka et al. 2015). It can be noticed that the specified bands at 1604/cm from the ultrasound bath-treated EWPs were pronounced and had a higher intensity while for UPT and thermal treated EWPs, the slight shifts of this band at 1600 and 1605/cm, respectively, were observed. Observing the amide I region, it can be emphasized that sonication resulted in decreased peaks at 1670/cm, particularly UPT. This decrease suggested that the part of α-helical structure has decreased (Zhang et al. 2016a, b).
Conclusions
The length of ultrasonication exhibited an important effect on EWP particle size, uniformity, and charge, affecting also the protein conformation and susceptibility to alcalase hydrolysis and determining functional properties. The HIU treatment at 20 kHz for 2–15 min resulted in a decrease in particle size diameter from 368.4 ± 10.51 to 68.4 ± 5.22 nm, reduced the PDI value, and increased the absolute value of zeta potential from −4.68 ± 1.40 to −22.8 ± 1.06 mV (increased negative particle charge). The prolonged treatment for 20 min, however, caused an excess denaturation of EWPs, enhancing the tendency of molecules toward aggregation, as shown by the increase in particle size, decrease in the content of SH groups, or decrease of subsequent EWP proteolysis. During the 15 min of treatment, the greatest increase in solubility by 8.1% with respect to the untreated EWPs was observed, as well as increases in foaming capacity (FC) and foaming stability (FS) by 60.6 and 193.3%, respectively, which have been shown to correlate with protein particle properties.
Therefore, it appeared that the particle properties like particle size and charge and complemented changes in solubility and/or foaming properties could be controlled simply by varying the sonication time. The overall emulsifying properties were also improved with an increase of sonication time, through both decrease of the mean particle diameter and increase of zeta potential, but there was no direct correlation between the emulsion activity/stability index and the surface protein particle size and/or charge. These results suggested that some other factors like changes in protein secondary structure could contribute to the emulsifying properties of EWPs. Analysis of EWP structure by Raman spectroscopy revealed that the ultrasound probe treatment leads to changes in the secondary structure, while heat and ultrasound generated by the ultrasound bath were not sufficient to exhibit this effect.
References
Adler-Nissen, J. (1986). Enzymic hydrolysis of food proteins. London: Elsevier Applied Science Publishers.
Arzeni, C., Martinez, K., Zema, P., Arias, A., Perez, O. E., & Pilosof, A. M. R. (2012b). Comparative study of high intensity ultrasound effects on food proteins functionality. Journal of Food Engineering, 108, 463–472.
Arzeni, C., Pérez, O. E., & Pilosof, A. M. R. (2012a). Functionality of egg white proteins as affected by high intensity ultrasound. Food Hydrocolloids, 29, 308–316.
Barukčić, I., Lisak Jakopović, K., Herceg, Z., Karlović, S., & Božanić, R. (2015). Influence of high intensity ultrasound on microbial reduction, physico-chemical characteristics and fermentation of sweet whey. Innovative Food Science and Emerging Technology, 27, 94–101.
Chandrapala, J., Zisu, B., Palmer, M., Kentish, S., & Ashokkumar, M. (2010). Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrasonics Sonochemistry, 18, 951–957.
Chandrapala, J., Zisu, B., Kentish, A., & Ashokkumar, M. (2012). The effects of high-intensity ultrasound on the structural and functional properties of α-lactalbumin, β-lactoglobulin and their mixtures. Food Research International, 48, 940–943.
Chen, D. (2012). Applications of ultrasound in water and wastewater treatment. In D. Chen, S. K. Sharma, & A. Mudhoo (Eds.), Handbook on application of ultrasound: sonochemistry for sustainability. Florida: CRC Press, Taylor & Francis Group.
Chi, Z., Chen, X. G., Holtz, J. S. W., & Asher, S. A. (1999). UV resonance Raman-selective amide vibrational enhancement: quantitative methodology for determining protein secondary structure. Biochemistry, 37, 2854–2864.
Dojcinovic, M., & Volkov-Husovic, T. (2008). Cavitation damage of the medium carbon steel: implementation of image analysis. Materials Letters, 62, 953–956.
Frydenberg, R. P., Hammershшj, M., Andersen, U., Greve, M. T., & Wiking, L. (2016). Protein denaturation of whey protein isolates (WPIs) induced by high intensity ultrasound during heat gelation. Food Chemistry, 192, 415–423.
Gulseren, I., Guzey, D., Bruce, B. D., & Weiss, J. (2007). Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrasonics Sonochemistry, 14, 173–183.
Hu, H. Y., & Du, H. N. (2000). α-to-β structural transformation of ovalbumin: heat and pH effects. Journal of Protein Chemistry, 19, 177–183.
Hu, H., Fan, X., Zhou, Z., Xu, X., Fan, G., Wang, L., Huang, X., Pan, S., & Zhu, L. (2013). Acid-induced gelation behavior of soybean protein isolate with high intensity ultrasonic pre-treatments. Ultrasonics Sonochemistry, 20, 187–195.
Huang, H., Kwok, K.-C., & Liang, H.-H. (2008). Inhibitory activity and conformation changes of soybean trypsin inhibitors induced by ultrasound. Ultrasonics Sonochemistry, 15, 724–730.
Jambrak, A. R., Lelas, V., Mason, T. J., Krešić, G., & Badanjak, M. (2009). Physical properties of ultrasound treated soy proteins. Journal of Food Engineering, 93, 386–393.
Kimura, T., Sakamoto, T., Leveque, J.-M., Sohmiya, H., Fujita, M., Ikeda, S., & Ando, T. (1996). Standardization of ultrasonic power for sonochemical reaction. Ultrasonics Sonochemistry, 3, S157.
Lai, K. M., Chuang, Y. S., Chou, Y. C., Hsu, Y. C., Cheng, Y. C., Shi, C. Y., Chi, Y., & Hsu, K. C. (2010). Changes in physicochemical properties of egg white and yolk proteins from duck shell eggs due to hydrostatic pressure treatment. Poultry Science, 89, 729–737.
Lei, B., Majumder, K., Shen, S., & Wu, J. (2011). Effect of sonication on thermolysin hydrolysis of ovotransferrin. Food Chemistry, 124, 808–815.
Li, K., Kang, Z.-L., Zhao, Y.-Y., Xu, X.-L., & Zhou, G.-H. (2014). Use of high-intensity ultrasound to improve functional properties of batter suspension prepared from PSE-like chicken breast meat. Food and Bioprocess Technology, 7, 3466–3477.
Li-Chan, E. C. Y. (1997). The application of Raman spectroscopy in food science. Trends in Food Science and Technology, 7, 361–370.
Mason, T. J. (2015). Some neglected or rejected paths in sonochemistry—a very personal view. Ultrasonics Sonochemistry, 25, 89–93.
Mason, T. J., Lorimer, J. P., & Bates, D. M. (1992). Quantifying sonochemistry: casting some light on a ‘black art’. Ultrasonics, 30, 40–42.
Mine, Y. (1995). Recent advances in the understanding of egg white protein functionality. Trends in Food Science and Technology, 6, 225–232.
Mine, Y. (1997). Effect of dry heat and mild alkaline treatment on functional properties of egg white proteins. Journal of Agricultural and Food Chemistry, 45, 2924–2928.
Mine, Y., Tatsushi, N., & Haga, N. (1990). Thermally induced changes in egg white proteins. Journal of Agricultural and Food Chemistry, 38, 2122–2125.
Mirmoghtadaie, L., Aliabadi, S. S., & Hosseini, S. M. (2016). Recent approaches in physical modification of protein functionality. Food Chemistry, 199, 619–627.
Nawrocka, A., Szymańska-Chargot, M., Miś, A., Ptaszyńska, A. A., Kowalski, R., Waśkoa, P., & Gruszecki, W. I. (2015). Influence of dietary fibre on gluten proteins structure—a study on model flour with application of FT-Raman spectroscopy. Journal of Raman Spectroscopy, 46, 309–316.
Ngarize, S., Adams, A., & Howell, N. K. (2004). Studies on egg albumen and whey protein interactions by FT-Raman spectroscopy and rheology. Food Hydrocolloids, 18, 49–59.
O’Sullivan, J., Murray, B., Flynn, C., & Norton, I. (2016). The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocolloids, 53, 141–154.
Ozuna, C., Paniagua-Martínez, I., Castaño-Tostado, E., Ozimek, L., & Amaya-Llano, S. L. (2015). Innovative applications of high-intensity ultrasound in the development of functional food ingredients: production of protein hydrolysates and bioactive peptides. Food Research International, 77, 685–696.
Painter, P. C., & Koenig, J. L. (1976). Raman spectroscopic study of the proteins of egg white. Biopolymers, 15, 2155–2166.
Pearce, K. N., & Kinsella, J. E. (1978). Emulsifying properties of proteins: evaluation of a turbidimetric technique. Journal of Agricultural and Food Chemistry, 26(3), 716–723.
Shaw, D. L. (1992). Introduction to colloid and surface chemistry (pp. 174–199). London: Butterworth-Heinemann.
Shimada, K., & Cheftel, J. C. (1988). Determination of sulfhydryl groups and disulfide bonds in heat-induced gels of soy protein isolate. Journal of Agricultural and Food Chemistry, 36, 147–153.
Stathopulos, P. B., Scholz, G. A., Hwang, Y.-M., Rumfeldt, J. A. O., Lepock, J. R., & Meiering, E. M. (2004). Sonication of proteins causes formation of aggregates that resemble amyloid. Protein Science, 13, 3017–3027.
Stefanović, A. B., Jovanović, J. R., Grbavčić, S. Ž., Šekuljica, N. Ž., Manojlović, V. B., Bugarski, B. M., & Knežević-Jugović, Z. D. (2014). Impact of ultrasound on egg white proteins as a pretreatment for functional hydrolysates production. European Food Research Technology, 239, 979–993.
Tan, M. C., Chin, N. L., Yasof, Y. A., Taip, F. S., & Abdullah, J. (2015). Improvement of eggless cake structure using ultrasonically treated whey protein. Food and Bioprocess Technology, 8, 605–614.
Tavano, O. L. (2013). Protein hydrolysis using proteases: an important tool for food biotechnology. Journal of Molecular Catalysis B: Enzymatic, 90, 1–11.
Tian, H., Xu, G., Yang, B., & Guo, G. (2011). Microstructure and mechanical properties of soy protein/agar blend films: effect of composition and processing methods. Journal of Food Engineering, 107, 21–26.
Tian, J., Wang, Y., Zhu, Z., Zeng, Q., & Xin, M. (2015). Recovery of tilapia (Oreochromis niloticus) protein isolate by high-intensity ultrasound-aided alkaline isoelectric solubilization/precipitation process. Food and Bioprocess Technology, 8, 758–769.
Tiwari, B. K., & Mason, T. J. (2012). Ultrasound processing of fluid foods. In P. J. Cullen, B. K. Tiwari, & V. P. Valdramidis (Eds.), Novel thermal and non-thermal technologies for fluid foods (pp. 135–157). New York: Elsevier Academic Press.
Van der Plancken, I., Van Loey, A., & Hendrickx, M. E. (2005). Combined effect of high pressure and temperature on selected properties of egg white proteins. Innovative Food Science and Emerging Technologies, 6, 11–20.
Van der Plancken, I., Van Loey, A., & Hendrickx, M. E. (2007). Foaming properties of egg white proteins affected by heat or high pressure treatment. Journal of Food Engineering, 78, 1410–1426.
Xiong, W., Wang, Y., Zhang, C., Wan, J., Shah, B. R., Pei, Y., Zhou, B., Li, J., & Li, B. (2016). High intensity ultrasound modified ovalbumin: structure, interface and gelation properties. Ultrasonics Sonochemistry, 31, 302–309.
Yanjun, S., Jianhang, C., Shuwen, Z., Hongjuan, L., Jing, L., Uluko, H., Yanling, S., Wenming, C., Wupeng, G., & Jiaping, L. (2014). Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. Journal of Food Engineering, 124, 11–18.
Zhang, P., Hua, T., Feng, S., Xua, Q., Zheng, T., Zhou, M., Chu, X., Huang, X., Lu, X., Pan, S., Li-Chan, E. C. Y., & Hua, H. (2016a). Effect of high intensity ultrasound on transglutaminase-catalyzed soy protein isolate cold set gel. Ultrasonics Sonochemistry, 29, 380–387.
Zhang, Z., Arrighi, V., Campbell, L., Lonchamp, J., & Euston, S. R. (2016b). Properties of partially denatured whey protein products: formation and characterization of structure. Food Hydrocolloids, 52, 95–105.
Zhou, M., Liu, J., Zhou, Y., Huang, X., Liu, F., Pan, S., & Hu, H. (2016). Effect of high intensity ultrasound on physicochemical and functional properties of soybean glycinin at different ionic strengths. Innovative Food Science and Emerging Technology, 34, 205–213.
Acknowledgments
The authors wish to extend their appreciation to the Ministry of Education, Science and Technological Development of the Republic of Serbia for their financial support within the EUREKA Project E!6750 and Project III-46010.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Online Resource 1
The emulsion activity/stability index of 2 wt. % ovalbumin treated with high-intensity ultrasound probe for various times (2, 5, 10, 15 and 20 min) at 20±0.2 kHz (GIF 222 kb)
Online Resource 2
Correlations between EAI and ESI and particle size (a) and zeta potential (b) of UPT ovalbumin solution (GIF 29 kb)
Rights and permissions
About this article
Cite this article
Stefanović, A.B., Jovanović, J.R., Dojčinović, M.B. et al. Effect of the Controlled High-Intensity Ultrasound on Improving Functionality and Structural Changes of Egg White Proteins. Food Bioprocess Technol 10, 1224–1239 (2017). https://doi.org/10.1007/s11947-017-1884-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1884-5