Abstract
Enzymatic deproteinization of lobster shells is an important step in developing a novel biorefinery process for the recovery of both protein and chitin. This study aimed to develop an efficient enzymatic deproteinization of lobster shells for protein recovery while providing the residual fraction suitable for further chitin recovery. In comparison with conventional incubation, the microwave-intensified enzymatic deproteinization (MIED) of Australian rock lobster shells significantly improved the deproteinization degree from 58 to 85.8 % and reduced the residual protein content from 96.4 to 65.4 mg/g, respectively. The protein hydrolysate produced by MIED had excellent functionality (solubility 91.7 %, water absorption 32 %, oil absorption 2.3 mL/g, foaming 51.3 %, emulsification 91.3 %) and high nutritional quality (34 % essential amino acids, 45.4 mg/g arginine, lysine/arginine ratio 0.69) with potential applications for food industry. With the considerably low residual protein, the MIEDs are suitable for further chitin recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Protein and chitin are the two potentially valuable components of Australian lobster processing by-products. Protein and chitin have been reported to make up about 29 and 23 % on dry weight basis of typical lobster processing by-products, respectively (Tu et al. 1991). The potential economic value and relatively high volume of these by-products offer significant value-added opportunity for the Australian lobster processing industry if chitin and protein in the lobster processing by-products can be economically recovered. However, the recovery and utilization of these two components from Australian lobster processing by-products for food application have not yet been realized. Lobster by-products are currently used for the production of aqua-feed and biofertilizer with low commercial value, or directly discarded with a disposal cost to the industry around $150 per ton. Therefore, the utilization of Australian lobster processing by-products for the recovery of protein and chitin for food applications will reduce operational costs and improve the sustainability of the Australian lobster industry.
Chitin and its derivatives such as chitosan and chito-oligosaccharides (COS) have high economic value because of their desirable physical properties and versatile biological activities with many applications including biomedicine, pharmaceuticals, food, and agriculture (Hayes 2012). Although chitin can be found in various species, crustacean shells are the sole major resource used for the production of industrial chitin (Xu et al. 2008). Shells generated from the lobster processing industry have the potential to offer an inexpensive commercial chitin source because of high chitin content and relatively large quantities generated. The potential value of lobster by-products has already been demonstrated by the use of chitin and its derivatives extracted from lobster shells for biocide and biostimulant applications (Sharp 2013).
Lobster shells have also been identified as an important animal protein source that contains 29 % protein with carotenoprotein making up as much as 16 % of dry lobster shell (Tu 1991). Carotenoprotein is a complex of protein and carotenoid pigments, mainly astaxanthin (Cremades et al. 2001) that possesses high antioxidant activity and may assist in several health benefits (Higuera-Ciapara et al. 2006). Moreover, peptidic fractions generated from the enzymatic hydrolysis of carotenoprotein have been reported to possess good functionalities and high antioxidant activity (Sila et al. 2014). In spite of these developments, it has still lacked a simple but efficient process which could fully recover protein and chitin for value-added products.
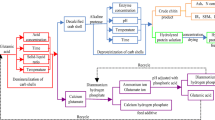
To fill this gap, a biorefinery process has been proposed to recover the protein as the first product by enzymatic deproteinization before extracting chitin as the second product. Previously, the protein generated from chemical deproteinization of lobster shells was not usually recovered due to its incompatibility for food application. The use of proteolytic enzymes for deproteinization of lobster shells could allow the recovery of functionally and nutritionally valuable protein hydrolysates suitable for food application (Gagné and Simpson 1993). Protein hydrolysates derived from crustacean shells have been reported to contain bioactive peptides used as pharmacological tools or as growth-stimulating agents in animal feed (Cudennec et al. 2008). Furthermore, a growing body of scientific evidence suggests that crustacean protein hydrolysates and peptides possess several biological functions promoting human health and prevent some chronic diseases (Lordan et al. 2011). Although a variety of enzymatic processes have been developed for the deproteinization of crustacean shells, the industrial use is still limited because of the inefficient removal of proteins and long processing times (Juan et al. 2014). Improving the enzymatic deproteinization of crustacean shells such as lobster shells by enhancing protein removal and reducing reaction time could make this process more industrially feasible.
Recently, microwave-assisted extraction (MAE) has been reported as an efficient technique for the extraction of bioactive compounds with high yield, low usage of solvents, and reduced extraction time (Xiao et al. 2008). Microwave has also been used for the pretreatment of biological materials to improve the enzymatic reaction rate (Roy et al. 2003). The pretreatment of materials with microwave has been reported to generate final products with high bioactivities and good functionalities (Lin et al. 2010). Microwave has also been used as an effective method to intensify enzymatic reactions such as the enzymatic hydrolysis of starch and protein (Horikoshi et al. 2015; Lukasiewicz et al. 2009). However, there have been no reports on the microwave-intensified enzymatic deproteinization (MIED) of lobster shells during chitin extraction and recovery of lobster shell protein hydrolysate (LSPH) for food applications. The aim of this work is, therefore, to develop an efficient microwave-intensified enzymatic process for the deproteinization of lobster shells to recover protein while providing the suitable residue materials for subsequent chitin recovery by comparing with the conventional enzymatic process. The LSPHs generated from this new process were further evaluated for their physicochemical functions relevant to food applications.
Materials and Methods
Materials
The shells of Australian rock lobster (Jasus edwardsii) were supplied by Ferguson Australia Pty Ltd as cooked and ground lobster shells (CGLS) with 49.9 % of particle size passing through a 250-μm laboratory sieve.
Alcalase 2.4 L FG, food grade proteolytic enzyme, was supplied by Novozymes Australia Pty Ltd. All the other chemicals used are of analytical grade.
Chemical Composition of Australian Rock Lobster Shells
Moisture was determined by oven drying of Australian lobster shells at 105 °C (AOAC 950.46) until a constant weight was obtained, while ash was quantified by incineration in a muffle furnace at 600 °C (AOAC 920.153). Lipid content was measured using supercritical CO2 extraction reported by Dionisi et al. (1999). Protein content was determined using the method of Synowiecki and Al-Khateeb (2000). Chitin was determined using the method described by Simpson and Haard (1985).
Enzymatic Deproteinization of Lobster Shells by Microwave-Intensified Process
The CGLS (5 g) were mixed with MilliQ water (4 mL/g) in a 250-mL round bottom flask. The pH was adjusted to 8.0 using lactic acid (40 %) and preheated at 55 °C (input energy 70 W, stirring 95 %) for 5 min by a microwave extraction system (Milestone Microwave Laboratory Systems, Model START SYNTH, serial number 131154) to satisfy the optimal pH and temperature for the enzyme alcalase. This microwave system was installed with two built-in sensors including an ATC-FO temperature sensor and infrared sensor to control the temperature with accuracy 99 ± 0.5 %. The alcalase was added with the enzyme/shell ratio 1 % (v/w). The CGLS was then enzymatically deproteinized by setting up firstly 55 °C (input energy 40 W, stirring 95 %) for a desired time (30, 60, and 90 min) and then at 95 °C (input energy 200 W, stirring 95 %) for 5 min to inactive alcalase. After vacuum filtration with sintered metal filter, the residual solid was washed with MilliQ water (10 mL) and then oven-dried to constant weight (referred to as enzymatically deproteinized shells (EDPS)). The dried EDPS was weighed to calculate the weight loss and determine residual protein content while the protein concentration of the filtrate (referred as LSPH) was determined to calculate the degree of deproteinization using the following formula.
Enzymatic Deproteinization of Lobster Shells by Conventional Incubation
The CGLS (5 g) were mixed with MilliQ water (4 mL/g) in a 250-mL conical flask followed by adjusting pH to 8.0 using lactic acid (40 %). The mixture was preheated in a water bath at 55 °C for 15 min. The enzymatic deproteinization of CGLS was then carried out at 55 °C for the desired time (30, 60, and 90 min) by placing the flask in the water bath (Ratek, Model ET22, serial number 908023262). The reaction mixture was manually shaken for 10 s every 30 min during this process. After deproteinization, the flask was boiled for 5 min to inactivate alcalase. Subsequent steps were carried out as per the microwave-intensified enzymatic deproteinization process described above to obtain the LSPH.
Physicochemical Properties of LSPH
Protein Solubility
The solubility of LSPH was determined by dispersing 50 mg of sample in 5 mL MilliQ water with the solution pH adjusted to 2, 4, 6, 8, and 10 using HCl 1 M or NaOH 1 M. The solution was magnetically stirred for 30 min at room temperature (20 ± 3 °C) followed by centrifugation at 990×g for 10 min (Beckman Coulter, Allegra X-12R, SN: ALX08813). The protein contents in the supernatants were measured by the method of Lowry et al. (1951) while the total protein of LSPH was determined by Kjeldahl method (AOAC 2000).
Water Absorption Capacity
The ability of LSPH to impact the water absorption capacity of meat products was determined by the method described by Geirsdottir et al. (2011) with some modifications. LSPH (0.1 g) was added to 4.9 g beef mince to produce the beef mince with LSPH while the blank sample was prepared solely using beef mince (5 g). Both the beef mince with and without LSPH were placed in 50-mL centrifuge tubes and mixed with 1 g MilliQ water (WA) by vortex for 1 min. After the mixture was left standing on ice for 30 min, free water was separated by centrifugation at 3000×g for 10 min (Beckman Coulter, Allegra X-12R, SN: ALX08813). The weight of the tubes before (WTB) and after separation of free water (WTA) were recorded to calculate the water absorption capacity of the beef mince.
Oil Absorption Capacity
The oil absorption capacity of LSPH was measured using the procedures described by Beuchat (1977) with slight modifications. One gram of sample (Ws) was mixed with 10 mL canola oil (OA) in a 50-mL centrifuge tube by vortex for 30 s. The sample was then allowed to stand at room temperature (20 ± 3 °C) for 30 min before it was centrifuged at 5000×g for another 30 min (Beckman Coulter, Allegra X-12R, SN: ALX08813). Free oil (OF) was separated by decantation at a 45° angle and the volume of free oil was measured in a 10-mL graduated cylinder.
Emulsifying Capacity and Stability
Emulsifying capacity and stability were measured using the method described by Lawal (2005) with a slight modification. Five milliliters of LSPH solutions (dissolved in MilliQ water) at 10 mg/mL and 5 mL of canola oil were homogenized at 13,500 rpm for 60 s using a homogenizer (Ika, Ultra-Turrax T25). The emulsions were centrifuged at 1100×g for 5 min (Beckman Coulter, Allegra X-12R, SN: ALX08813). The height of the emulsified layer and that of the total contents in the tube was recorded. The emulsifying capacity (EC) was calculated as follows.
Emulsifying stability (ES) was measured by heating the emulsions in a water bath at 80 °C for 30 min before centrifuging at 1100×g for 5 min.
Foaming Capacity
The foaming capacity was determined using the method of Coffman and Garcia (1977) with slight modifications. Ten milliliters of LSPH solution (dissolved in MilliQ water) at 10 mg/mL were foamed by a homogenizer (Ika, Ultra-Turrax T25) at 8000 rpm for 60 s. The volumes of the solutions before (V 1) and after homogenization (V 2) were recorded. The percentage volume increase referred to as index of foam capacity was calculated based on the following equation.
The foaming samples were left to stand at room condition for 60 min, and the volume of samples after standing (V’2) was determined. The foaming stability was determined by the following equation.
Amino Acid Analysis
The amino acid composition of LSPH was analyzed at the Australian Proteome Analysis Facility (APAF), Macquarie University, Sydney.
Statistical Analysis
Except for amino acid profile, all other experiments were carried out in three replicates. The obtained data was subjected to an analysis of variance using a Statistical Analysis System (SAS) 9.1 (SAS and Guide 2003). Duncan’s multiple range test was performed to determine the significant (p < 0.05) difference among means. The Levene test was also used to verify whether the data satisfy the ANOVA condition, and the Kruskall-Wallis test has been applied for the data not satisfying the ANOVA condition.
Results and Discussion
Australian Rock Lobster Shells Rich in Protein and Chitin
Australian rock lobster (J. edwardsii) contains a large proportion of minerals (36 %) while its lipid content is very low (0.6 %). The two significant components of Australian rock lobster shells with high potential economic value are protein and chitin with respective contents of 29 and 25 %. These contents are close to 29 % protein and 23 % chitin, reported in the American cooked lobster waste (Hamarus Americanus) (Tu et al. 1991).
MIED of Lobster Shells
Weight Loss
The weight losses of shells during enzymatic deproteinization by the microwave and conventional incubation are shown in Fig. 1. In both cases, lobster shells were clearly susceptible to weight loss due to enzymatic reaction. Proteins constituted in the lobster shells could be hydrolyzed during the process and quickly released into the solution leading to the weight loss (Valdez-Pena et al. 2010). As shown in Figure 1, the weight loss increased with the treatment time from 30 to 90 min for both processes, but the weight loss of the 60-min treatment was not significant compared with those of the 30- and 90-min treatment. The highest value of weight loss was found at 90 min of deproteinization with values of 30.3 % for the microwave-intensified process and 24.6 % for the conventional incubation. The weight losses of the lobster shells in the microwave-intensified process were considerably (p < 0.05) higher than those in the conventional incubation at all the investigated times (26.1, 28.2, and 30.3 % for the microwave-intensified process vs 22.5, 23.7, and 24.6 % for the conventional incubation, respectively). The significant differences in the weight loss by the two deproteinization methods could closely relate to the enhanced protein removal ability as a result of proteolytic hydrolysis of shells intensified by microwave (Horikoshi et al. 2015).
Deproteinization Degree
The percentage of protein removed from the lobster shells by proteolysis during enzymatic deproteinization was defined as the deproteinization degree. As shown in Fig. 2, there are significant differences in the deproteinization degree between the samples treated for 30 and 90 min but insignificant for the 60-min treatment with others. The highest deproteinization degrees in both processes were obtained at treatment of 90 min (85.8 % for the microwave-intensified process and 58 % for the conventional incubation). The deproteinization degrees of the microwave-intensified process were significantly (p < 0.05) higher than those of the conventional incubation at all the treatment times. In fact, these deproteinization degrees increased by 18–27.8 % in the microwave-intensified process compared with those in the conventional incubation. A considerable increase (27.8 %) was found at 90 min of deproteinization. The higher deproteinization degree indicates that more proteins were released under the microwave heating. This result is in accordance with the previous studies of Horikoshi et al. (2015) and De La Hoz et al. (2005) where enzymatic reaction rates were reported to increase several times under microwave field relative to conventional heating.
The higher values of both the weight loss and the deproteinization degree indicate that the enzymatic deproteinization of the lobster shells by the microwave-intensified process is far more effective compared with the conventional incubation. However, to further confirm this result as well as to demonstrate the ease for further chitin recovery from the EDPS, the residual proteins in these materials were also investigated.
Residual Proteins in the EDPS
Figure 3 shows the residual proteins in the EDPS generated from the microwave intensification and conventional incubation. The residual proteins in the EDPS reduced significantly with the increase in deproteinization time, and these values together with the removed proteins could make a protein mass balance in the range of 92–107 %. At all the investigated times, the microwave-intensified process had the residual protein values significantly (p < 0.05) lower than those of the conventional incubation by 17–32.2 %. The lowest residual proteins (65.4 mg/g) occurred at 90 min of deproteinization by the microwave-intensified process. With the low residual proteins, the EDPS could be directly demineralized to obtain chitin or further mild deproteinization followed by demineralization to recover purified chitin.
Residual protein content (mg/g) in the EDPS obtained during enzymatic deproteinization by microwave intensification and conventional incubation (results are the means of three replicates; plots with different letters are statistically significant different with p < 0.05; the error bars are the standard deviations)
Functional Property of LSPH
Solubility of LSPH
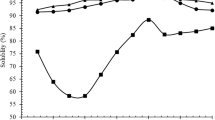
Solubility values for the LSPH and reference protein (egg white protein (EWP) prepared in house by spray-drying of chicken egg purchased from a local supermarket after removing the yolk) in a wide range of pH 2–10 were investigated. As shown in Table 1, LSPH has very high solubility (91.7 %). This result is similar to that of Vieira et al. (1995) where protein hydrolysate derived from lobster by-products was reported to have good solubility. In contrast to EWP, LSPH solubility was not significantly affected by pH change. Its solubility was over 85.5 % in a wide range of pH (2–10) while this value for EWP was only 77.7 % in the same pH range. The solubility of LSPH was higher than that of EWP with an exception at pH 2 and 10 (beyond effective food applications). The high solubility of LSPH over a wide range of pH could be explained by smaller peptides (molecular weight less than 10 KDa) produced by the enzymatic hydrolysis of lobster shell protein, increasing polar and ionizable groups of protein hydrolysates for better interaction with water (Mutilangi et al. 1996). The excellent solubility of the LSPH indicates good potential for food applications since solubility does not only influence emulsification and foaming capacities but also has significant effects on sensory properties (Wilding et al. 1984).
Water Absorption Capacity of Beef Mince With LSPH Addition
Water absorption capacity, the ability of LSPH to imbibe water and retain it against gravitational force within a beef mince matrix, is presented in Fig. 4. With the addition of 2 % LSPH to the beef mince, its water absorption capacity rose significantly by up to 90 %, increased by 32 %. It was 2.5 times higher than that of adding with EWP. This result is consistent with the results of the study of Kristinsson (1998) when salmon fish protein hydrolysate and egg albumin were used in salmon mince patties. The significant improvement in the water absorption of the beef mince with the addition of LSPH could be explained by the presence of polar groups such as –COOH and/or –NH2; these functional groups were likely to have a substantial effect on water absorption capacity (Kristinsson and Rasco 2000a). The water absorption capacity of proteins added to muscle tissue is of great importance to the food industry because retaining water in a food system often improves its texture, reduces its overall energy content per unit mass, and reduces the overall cost of the product. The functional properties of proteins in a food system depend on the water-protein interaction, and the final outcome greatly depends on how well the protein binds and holds water in a food system (Kristinsson and Rasco 2000b). With its excellent water absorption within a protein matrix, the LSPH has potential for use as a water-binding agent in meat products to improve cooking yield, drip losses, and/or as cryo-protective agents.
Oil Absorption Capacity of LSPH
The quantity of oil directly bound to protein is expressed as oil absorption property. As illustrated in Fig. 5, LSPH had oil absorption capacity lower than that of EWP (2.31 mL/g compared with 3.66 mL/g), but it was comparable to that of egg albumin (2.36 mL/g) tested with soybean oil by Kristinsson and Rasco (2000a). The oil absorption capacity of LSPH could be influenced by the bulk density of the protein and peptide sizes (Kinsella and Melachouris 1976). The capacity of LSPH to absorb oil is an important functional property which is useful in some applications required for the meat and confectionary industries Kristinsson and Rasco (2000b).
Emulsification Capacity and Stability of LSPH
In contrast to EWP, the emulsification capacity of LSPH was not affected by pH change, slightly fluctuating around 50 % in a range of pH 2–10 (Table 1). The excellent solubility of LSPH over a wide range of pH would be expected to give the positive results for its emulsification capacity (Klompong et al. 2007). With high solubility, LSPH can quickly diffuse and adsorb at the interface to have positive effects on the emulsification capacity. EWP had very low emulsification capacity at pH 6.0 and 8.0, which coincided with its low solubility at these pH values. Although LSPH had greater initial emulsification capacity than EWP, its emulsification stability was lower than that of EWP; the lower stability is likely to relate to the lower molecular weight of LSPH (less than 10 KDa) as smaller peptides are less effective in stabilizing the emulsions (Klompong et al. 2007).
Foaming Capacity and Stability of LSPH
Similar to EWP, the foaming property of LSPH was significantly influenced by pH change. LSPH had a higher foaming capacity than that of EWP with an exception at pH extremes (pH 2 and 10) (Table 1). The highest foaming capacity of LSPH was found at pH 4 (91.3 %) while the LSPH solubility was low at this pH value. Thus, it seems that the effect of composition and net charge of peptides on the foaming properties outweigh that of its solubility. Foam generated from LSPH was more stable than that from EWP (foaming reduction of LSPH around 6 % compared with 24 % of EWP at pH 4). Foaming is responsible for the desired texture of many food products; thus, LSPH with its ability to form and stabilize foam could find applications in fish-based soufflés and pâté for example.
Amino Acid Composition of LSPH
The nutritional value of food depends on the type and amount of amino acids available for body functions (El-Beltagy and El-Sayed 2012). Although biological parameter such as the protein efficiency ratio or net protein utilization are common for assessing protein quality, the protein quality assessment can also be carried out by an analysis of its essential amino acid content or chemical score (Ovissipour et al. 2010). Chemical score provides a nutritive value estimate of a protein by comparing the levels of essential amino acids between the test protein and reference protein. In this study, the determination of chemical score and comparison of essential amino acid content of the LSPH was based on the reference protein recommended by FAO/WHO (1990) for adult humans (FAO/WHO 1990). LSPH produced by microwave-intensified process contains 74 % of protein including 34 % of essential amino acids over total amino acid content (Table 2). The amount of essential amino acids in LSPH was twice the values of those suggested by FAO/WHO with the exception of methionine. Therefore, the essential amino acid composition of LSPH meets or exceeds the essential amino acid recommendation for adult humans. Moreover, LSPH contains very high amount of arginine (45.4 mg/g) that is important for its participation in protein synthesis and other physiological functions such as detoxification and energy conversion (Cao et al. 2008) and plays an important role in cardiovascular disease treatment (Niittynen et al. 1999). Lysine/arginine ratio has also used as an indicator for evaluation of protein nutritional value (Oomah and Mazza 2000). LSPH has a very low lysine/arginine ratio (0.69) compared with meat protein (13.78) (Sidransky 1990). The low lysine/arginine ratio of LSPH suggests that it could be used in meat products to reduce lipidemic and atherogenic effects induced by meat protein on human health. With all these nutritional attributes, LSPH could be potentially used as a supplement to poorly balanced dietary proteins or meat proteins.
Conclusion
Australian rock lobster shells are rich in protein and chitin that could be economically recovered in a novel biorefinery process. The enzymatic deproteinization of lobster shells intensified by microwave was shown highly efficient than that with conventional incubation, achieving higher deproteinization degree and low residual protein content. The lobster shell protein hydrolysate produced by microwave-intensified process has excellent functionalities in terms of its solubility, water absorption, oil absorption, emulsification, foaming, and nutritional values for food applications. Compared with undeproteinized shells and the conventionally deproteinized shells, the microwave-deproteinized shells have significantly lower residual proteins, which could be easily used for further chitin recovery. The microwave-intensified enzymatic deproteinization process is effective and could be considered for protein recovery from lobster shells at large scale.
References
AOAC. (2000). Official methods of analysis. Association of Official Analytical.
Beuchat, L. B. (1977). Functional and electro phonetic characteristics of succinylated peanut flour proteins. Journal of Agricultural and Food Chemistry, 25, 258–261.
Cao, W., Zhang, C., Hong, P., & Ji, H. (2008). Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chemistry, 109(1), 176–183.
Coffman, C. W., & Garcia, V. V. (1977). Functional properties and amino acid content of protein isolate from Mung bean flour. J Food Technology, 12, 473–484.
Cremades, O., Ponce, E., Corpas, R., Gutierrez, J., Jover, M., Alvarez-Ossorio, M., et al. (2001). Processing of crawfish (Procambarus clarkii) for the preparation of carotenoproteins and chitin. Journal of Agricultural and Food Chemistry, 49(11), 5468–5472.
Cudennec, B., Ravallec-Ple, R., Courois, E., & Fouchereau-Peron, M. (2008). Peptides from fish and crustacean by-products hydrolysates stimulate cholecystokinin release in STC-1 cells. Food Chemistry, 111(970–975), 48–55.
De La Hoz, A., Diaz-Ortiz, A., & Moreno, A. (2005). Microwaves in organic synthesis. Thermal and non-thermal microwave effects. [10.1039/B411438H]. Chemical Society Reviews, 34(2), 164–178. doi:10.1039/B411438H.
Dionisi, F., Hug, B., Aeschlimann, J., & Houllemar, A. (1999). Supercritical CO2 extraction for total fat analysis of food products. Journal of Food Science, 64(4), 612–615.
El-Beltagy, A. E., & El-Sayed, S. M. (2012). Functional and nutritional characteristics of protein recovered during isolation of chitin from shrimp waste. Food and Bioproducts Processing, 90, 633–638.
FAO/WHO. (1990). Protein quality evaluation: report of a joint FAO/WHO expert consultation: Organizacion de las Naciones Unidas para la Agricultura y la Alimentacion.
Gagné, N., & Simpson, B. K. (1993). Use of proteolytic enzymes to facilitate the recovery of chitin from shrimp waste. Food Biotechnology, 7, 253–563.
Geirsdottir, M., Sigurgisladottir, S., Hamaguchi, P. Y., Thorkelsson, G., Johannsson, R., Kristinsson, H. G., et al. (2011). Enzymatic hydrolysis of blue whiting (Micromesistius poutassou); functional and bioactive properties. Journal of Food Science, 76(1), C14–C20.
Hayes, M. (2012). Chitin, chitosan and their derivatives from marine rest raw materials: potential food and pharmaceutical applications. In Marine bioactive compounds (pp. 115–128). Springer.
Higuera-Ciapara, I., Felix-Valenzuela, L., & Goycoolea, F. M. (2006). Astaxanthin: a review of its chemistry and applications. Critical Reviews in Food Science and Nutrition, 46, 185–196.
Horikoshi, S., Nakamura, T., Kawaguchi, M., & Serpone, N. (2015). Enzymatic proteolysis of peptide bonds by a metallo-endoproteinase under precise temperature control with 5.8-GHz microwave radiation. Journal of Molecular Catalysis B: Enzymatic, 116, 52–59. doi:10.1016/j.molcatb.2015.03.007.
Juan, C. C.-E., Maria-Josse, V.-M., Adriana, S.-B., Oscar, F. V.-V., Humberto, G.-M., Rosabel, V.-d.-l.-R., et al. (2014). Gluconic acid as a new green solvent for recovery of polysaccharides by clean technologies. In F. Chemat, & M. A. Vian (Eds.), Alternative solvents for natural products extraction. Springer.
Kinsella, J. E., & Melachouris, N. (1976). Functional properties of proteins in foods: a survey. Critical Reviews in Food Science and Nutrition, 7(3), 219–280.
Klompong, V., Benjakul, S., Kantachote, D., & Shahidi, F. (2007). Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chemistry, 102(4), 1317–1327.
Kristinsson, H. G. (1998). Reaction kinetics, biochemical and functional properties of salmon muscle proteins hydrolyzed by different alkaline proteases. University of Washington.
Kristinsson, H. G., & Rasco, B. A. (2000a). Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. Journal of Agricultural and Food Chemistry, 48(3), 657–666.
Kristinsson, H. G., & Rasco, B. A. (2000b). Fish protein hydrolysates: production, biochemical and functional properties. Critical Reviews in Food Science and Nutrition, 40, 43–81.
Lawal, O. S. (2005). Functionality of native and succinylated Lablab bean (Lablab purpureus) protein concentrate. Food Hydrocolloids, 19(1), 63–72.
Lin, Y.-J., Le, G.-W., Wang, J.-Y., Li, Y.-X., Shi, Y.-H., & Sun, J. (2010). Antioxidative peptides derived from enzyme hydrolysis of bone collagen after microwave assisted acid pre-treatment and nitrogen protection. International Journal of Molecular Sciences, 11, 4297–4308.
Lordan, S., Ross, R. P., & Stanton, C. (2011). Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Marine Drugs, 9(6), 1056–1100.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry, 193(1), 265–275.
Lukasiewicz, M., Marciniak, M., & Osowiec, A. (2009). Microwave-assisted enzymatic hydrolysis of starch. Chemistry, ECSOC-13, 1(30).
Mutilangi, W., Panyam, D., & Kilara, A. (1996). Functional properties of hydrolysates from proteolysis of heat‐denatured whey protein isolate. Journal of Food Science, 61(2), 270–275.
Niittynen, L., Nurminen, M.-L., Korpela, R., & Vapaatalo, H. (1999). Role of arginine, taurine 4 and homocysteine in cardiovascular diseases. Annals of Medicine, 31(5), 318–326.
Oomah, B., & Mazza, G. (2000). Functional foods. In F. Francis (Ed.), The Wiley Encyclopedia of Science and Technology (2nd ed., Vol. 2, pp. 1176–1182). New York: Wiley.
Ovissipour, M., Benjakul, S., Safari, R., & Motamedzadegan, A. (2010). Fish protein hydrolysates production from yellowfin tuna (Thunnus albacares) head using alcalase and protamex. International Aquatic Research, 2, 87–95.
Roy, I., Mondal, K., & Gupta, M. N. (2003). Accelerating enzymatic hydrolysis of chitin by microwave pretreatment. Biotechnology Progress, 19(6), 1648–1653.
SAS, S., & Guide, S. U. s. (2003). Version 9.1. SAS Institute Inc., Cary, NC.
Sharp, R. G. (2013). A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interaction and improve crop yields. Agronomy, 3, 757–793.
Sidransky, H. (1990). Possible role of dietary proteins and amino acids in Atherosclerosisa. Annals of the New York Academic of Sciences, 598(1), 464–481.
Sila, A., Sayari, N., Balti, R., Martinez-Alvarez, O., Nedjar-Arroume, N., Nasri, M., et al. (2014). Biochemical and antioxidant properties of peptidic fraction of carotenoproteins generated from shrimp by-products by enzymatic hydrolysis. Food Chemistry, 148, 445–452.
Simpson, B., & Haard, N. (1985). The use of proteolytic enzymes to extract carotenoproteins from shrimp wastes. Journal of Applied Biochemistry, 7(3), 212–222.
Synowiecki, J., & Al-Khateeb, N. A. A. Q. (2000). The recovery of protein hydrolysate during enzymatic isolation of chitin from shrimp Crangon crangon processing discards. Food Chemistry, 68(2), 147–152.
Tu, Y. (1991). Recovery, drying and characterization of carotenoproteins from industrial lobster waste. McGill University.
Tu, Y., Simpson, B. K., Ramaswamy, H., Yaylayan, V., Smith, J. P., & Hudon, C. (1991). Carotenoproteins from lobster waste as a potential feed supplement for cultured salmonids. Food Biotechnology, 5(2), 87–93.
Valdez-Pena, A. U., Espinoza-Perez, J. D., Sandoval-Fabian, G. C., Balagurusamy, N., Hernandez-Rivera, A., De-la-Garza-Rodriguez, I. M., et al. (2010). Screening of industrial enzymes for deproteinisation of shrimp head for chitin recovery. Food Science and Biotechnology, 19(2), 553–557.
Vieira, G. H., Martin, A. M., Saker‐Sampaiao, S., Omar, S., & Goncalves, R. C. (1995). Studies on the enzymatic hydrolysis of Brazilian lobster (Panulirus spp) processing wastes. Journal of the Science of Food and Agriculture, 69(1), 61–65.
Wilding, P., Lillford, P. J., & Regenstein, J. M. (1984). Functional properties of proteins in foods. Journal of Chemical Technology and Biotechnology, 34(3), 182–189.
Xiao, W., Han, L., & Shi, B. (2008). Microwave-assisted extraction of flavonoids from Radix Astragali. Separation and Purification Technology, 62(3), 614–618. doi:10.1016/j.seppur.2008.03.025.
Xu, Y., Gallert, C., & Winter, J. (2008). Chitin purification from shrimp waste by microbial deproteinisation and decalsification. Appl Microbiology and Biotechnology, 79, 687–697.
Acknowledgments
The authors wish to acknowledge the Australian government for offering Trung T. Nguyen a PhD scholarship, the South Australian Government and Ferguson Australia Pty Ltd for the IVP funding support from the Premier’s Research and Industry Fund, as well as the Centre for Marine Bio-products Development, Flinders University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, T.T., Zhang, W., Barber, A.R. et al. Microwave-Intensified Enzymatic Deproteinization of Australian Rock Lobster Shells (Jasus edwardsii) for the Efficient Recovery of Protein Hydrolysate as Food Functional Nutrients. Food Bioprocess Technol 9, 628–636 (2016). https://doi.org/10.1007/s11947-015-1657-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1657-y