Abstract
The aim of this work was to assess the effects of temperature (T), time (t) and pH treatments and an in vitro digestion on the stability of the angiotensin I-converting-enzyme-inhibitory activity (ACEIA) and antithrombotic activity (ATA; assessed as inhibition of platelet aggregation) of selected protein hydrolysates of amaranth named Alb1H103 and GloH88 and GluH24 with dipeptidyl peptidase IV inhibitory activity (DPPIVIA). Heat treatment (40–100 °C) for 1 h showed no significant differences among ACEIA, DPPIVIA and ATA of the heated hydrolysates at pH 4 and 7. There was no statistically significant loss of any bioactivity under heat treatment for 3 h at pH 4.0. Alb1H103 and GluH24 maintained the inhibitory activity of ACE and ATA at pH 7.0 for 3 h, whereas GloH88 maintained ACEIA and ATA for 2.0 h at pH 7.0. The pH effect on hydrolysates bioactivity was assessed in the range of 2.0–12.0. This was negligible on ACEIA, ATA and DPPIVIA. The in vitro digestion was performed using pepsin, trypsin (T) and α-chymotrypsin (C). A previous treatment of hydrolysates with pepsin improved the proteolytic activities of T and C. The hydrolysates kept at 100 °C for 1 h at pH 4.0, showed a significant increase in bioactivity. Conversely, a treatment at pH 7.0 showed no significant difference (p < 0.05) in the hydrolysates bioactivities after their digestion. Thus, biological activity of hydrolysates may be preserved or enhanced, depending on their processing conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, in order to manufacture third generation nutraceuticals (N) and functional foods (FF), bioactive compounds must be added. It is essential that these compounds maintain their biological activity (bio-functionality) under the processing conditions used during their manufacturing. Techno-functional properties, such as foaming and emulsification, of proteins and their hydrolysates are associated with bio-functionality. The loss of stability of bioactivity (bio-functionality) must also be assessed, and all the more so when severe treatment conditions, such as sterilization, grilling and frying, are performed in order to maintain the expected physiological benefit of a food.
The effect of the gastrointestinal digestion on those compounds must also be taken into account so that their bioavailability and bioactivity can occur in the different target organs. The stability of the bioactive compounds in the gastrointestinal tract may impact the final outcome. For example, the antihypertensive and antithrombotic activities (ATA) of peptide fractions of a commercial fermented milk product, made with Lactobacillus casei Shirota and Streptococcus thermophilus, were maintained during storage. However, the same milk product, after an in vitro partial digestion, lost most of its bioactivity and thus its effectiveness as FF (Domínguez-González et al. 2014). On the other hand, amaranth protein hydrolysates with angiotensin I-converting-enzyme-inhibitory activity (ACEIA) and dipeptidyl peptidase IV inhibitory activity (DPPIVIA) remained constant after in vitro or in vivo digestion (Fritz et al. 2011; Soriano-Santos et al. 2015). As a result, the stability of bioactive compounds in foods and foodstuffs must be evaluated throughout the different steps of production and consumption. Such studies, however, are difficult to execute and should initially be carried out using an in vitro model system. These would have to consider not only the interactions of the biological compounds with the molecular structure of the food matrix as there may be other compounds with which they can react, but also other factors such as temperature (T), time (t) of treatment, pH, ionic strength and so forth. Unfortunately, in vitro and in vivo research on the stability of bioactivity of biological compounds, under several processing conditions, is still scarce. Lately there has been a great deal of research on the bioactivity of peptides present in protein hydrolysates as the former offer various physiological benefits such as antioxidant, immunomodulating, ACEIA, ATA, DPPIVIA, opioid, anticancer, etc. (Dexter and Middelberg 2008). Particularly, storage proteins of amaranth grain, in their primary structure, contain peptides with ACEIA (Fritz et al. 2011; Soriano-Santos and Escalona-Buendía 2015), DPPIVIA (Montoya-Rodriguez et al. 2015; Soriano-Santos et al. 2015; Velarde-Salcedo et al. 2013) and antithrombotic activity (Sabione et al. 2015), among others. These bioactivities would be highly relevant in the making of N and FF.
Considerable further research has been conducted by Shevkani and Singh (2015) and Shevkani et al. (2014a, b) on several novel commodities derived from amaranth grain and their use in the design of N and FF. Chemical composition, physicochemical and techno-functional features of proteins, lipids and carbohydrates occurring in amaranth grain flour have been assessed. Even though there has been some work on the physicochemical and surfactant properties of these foodstuffs, their bio-functionality still needs to be studied. Moreover, the chemical composition of foodstuffs derived of amaranth and its influence on techno-functional properties can also affect the bio-functionality. Therefore, this issue should prompt researchers to assess the relationship between bioactivity and the process used to obtain commodities (Soriano-Santos and Escalona-Buendía 2015).
Hypertension, type 2 diabetes mellitus and thrombosis are common diseases for a considerable number of the elderly throughout the world and they also increase the risk of having coronary diseases which could eventually be prevented with the intake of N and FF. Therefore, consumers may be interested in the development of N and FF as these may reduce the undesirable side effects caused by synthetic drugs (Rao et al. 2012). Thus, in this study there was an assessment of the effect of different treatment conditions of T, t and pH, and an in vitro digestion on the stability of the bioactivity of hydrolysates of storage proteins as obtained of A. hypochondriacus L. grain, prior to an animal model assessment.
Materials and methods
Amaranth grain flour and protein hydrolysates
Amaranthus hypochondriacus grain cultivar Revancha obtained from INIFAP-Campus Montecillo, México was used in this research. The whole grain was milled using a Udy mill (Udy Corporation Fort Collins, CO, USA) until a 60-mesh screen defatted flour was obtained. Then albumin 1 (Alb1) and globulin (Glo) were extracted from defatted flour according to the method described by Tovar-Pérez et al. (2009). Briefly, defatted flour (50 g) was mixed with 300 ml 0.04 Na2SO4 containing 20 mM β-mercaptoethanol and stirred for 30 min, centrifuged for 20 min at 13,000g; the supernatant was mixed with 50, 70 and 100% (sat.) (NH4)2SO4. Alb 1 was separated from Glob when supernatant was dialysed for 24 h against distilled water. These crude proteins were lyophilised and stored at 5 °C.
The residue, after Alb1 and Glo were obtained, was treated with 70% ethanol to discard prolamins. Thus glutelins (Glu) were extracted following the method reported by Barba de la Rosa et al. (2010), using 0.1 M Tris buffer at pH 8.0, in a residue/buffer ratio 1:10. Protein content was assessed by the Kjeldahl method (AOAC 2010). Protein extracts were lyophilized and stored at 5 °C until alcalase hydrolysis.
Alcalase hydrolysis of proteins was carried out following the method reported by Tovar-Pérez et al. (2009) with some modifications: 0.5 M phosphate buffer (pH 7.4) was added to a 5 mg/ml of protein solution. The solution was incubated for 5 min at 50 °C; then 2.4 UA/ml of alcalase solution in 0.5 M phosphate buffer was added to each test tube to reach a final ratio E/S = 0.8 UA/g protein. The reaction, at the appropriate time was stopped by adding 100 μl phenylmethylsufonyl fluoride in ethanol (2 mg/ml). Then several amaranth grain protein hydrolysates were obtained at different conditions.
The degree of hydrolysis (DH) was conducted according to the method reported by Condés et al. (2009). Free amino groups, released by alcalase hydrolysis, were assessed by their reaction with 2,4,6-trinitrobenzenesulfonic acid (TNBS). l-Leucine was used as a standard. The DH was calculated as reported by the previous authors.
Molecular weight characterization of amaranth grain storage protein hydrolysates was carried out in agreement with the method used by Tovar-Pérez et al. (2009), using a molecular exclusion column Sephadex G-15 (1.4 × 29 cm; Pharmacia, Uppsala, Sweden) and a Pharmacia LKB FPLC System (Uppsala, Sweden) for peptides separation. 200 μl of hydrolyzed proteins (15 mg/ml) disolved in 32.5 mMK2HPO4–2.6 mM KH2PO4, pH 7.5, which contained 0.4 M NaCl and 20 mM 2-mercaptoethanol, were injected and eluted with the same buffer at 0.2 ml/min. Absorbance at 214 nm was monitored and 0.5 ml fractions were collected. An ultra-low range molecular weight marker (Sigma-Aldrich, St. Louis, MO, USA), containing triose phosphate isomerase 26.6 kDa; myoglobin 17 kDa; α-lactalbumin 14.2 kDa; aprotinin 6.5 kDa; insulin 3.5 kDa; bradykinin 1.06 kDa, was used. All experiments throughout this study were performed in triplicate.
Measurement of biological activity of hydrolysates
The ACEIA was measured by the method of Hayakari et al. (1978), adapted by Tovar-Pérez et al. (2009). The determination of DPPIVIA was performed as reported by Kojima et al. (1980), adapted by Soriano-Santos et al. (2015). The % ACE or % DPPIV inhibition was defined as the percentage of ACE or DPPIV activity inhibited by a given concentration of peptide fraction of hydrolysate obtained from amaranth grain storage proteins (Alb1, Glo or Glu). Captopril and the peptide Diprotin A, which inhibit ACE and DPPIV activities, were used as control in order to compare the protein hydrolysates IC50 values.
The antithrombotic activity was measured as the inhibition of platelet aggregation following the method described by González et al. (2010) with some modifications. Briefly: blood was collected from healthy donors, by venipuncture employing 0.129 mmol/l trisodium citrate as anticoagulant at the blood bank of the National Institute of Cardiology “Ignacio Chávez”. Platelet-rich (PRP) and platelet-poor plasma (PPP) were obtained as described by de la Peña et al. (1993). The samples were centrifuged at 1400×g for 4.5 min at 20–24 °C. PRP was carefully withdrawn and pooled. The platelet count was adjusted to 2 × 103 cells/ml. The PPP was obtained by a second centrifugation at 400×g for 15 min.
The assays were carried out within 2 h after the blood had been drawn, in a two channel lumi-aggregometer (Model 560 CA and accompanying software Model 810 AGGRO/LINK Chrono-log, Havertown, PA, USA). A 450 μl quantity of PRP was used for each assay. Then 50 μl of the peptide fraction of Mr = 12.6 kDa from Alb1H24 or the peptide fraction Mr = 5.44 kDa from GlobH24 dissolved in dimethyl sulfoxide (DMSO) was added. The platelet aggregation was induced with 5 μM ADP, and the response was recorded over 3 min and estimated in arbitrary units. Light absorption was corrected with PPP and DMSO. The control conditions for platelet aggregation experiments were just platelets induced with 5 μM ADP and the average control aggregation was taken as 100%.
Stability of bioactive peptides as influenced by physical and chemical factors
Peptide fractions of hydrolysates (80 g/l) were separately adjusted to pH 4.0 and 7.0 with buffer solution and heated (40, 60, 80, 100 and 120 °C) for 1 h. The pH effect on bioactivity of hydrolysates was assessed using various buffer solutions at different pH values (1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0, and 12.0), which were heated at 100 °C for 1 h. To investigate the effect of the duration of heat treatment, peptide solutions were adjusted to pH 4.0 or 7.0 and incubated at 100 °C for 1.0, 2.0, 3.0, or 4.0 h. Unheated peptide fractions were used as control (Wu et al. 2014).
Biostability of peptide fractions on an in vitro gastrointestinal digestion
A simulation of human gastrointestinal digestion of peptide fractions was performed in vitro by sequential digestion using pepsin, trypsin and α-chymotrypsin, according to the method reported by Rui (2012). Briefly: Freeze dried peptide fractions were resolubilised in glycine buffer (0.01 M, pH 2.0) at 1.5% (w/v). Pepsin digestion was carried out with E/S = 1/100 (w/w) at 37 °C for 1.5 h. Then the digestion was followed adding trypsin and α-chymotrypsin with E/S 1/150 (w/w) at 37 °C for 2.5 h, maintaining the pH at 6.5 with 1 M NaOH by means of a pH-stat apparatus (MeterLab PHM290, Radiometer-Copenhagen, Denmark). The digestion of the sample was stopped by heating it in boiling water for 10 min.
Statistical analysis
Data were analyzed by means by the analysis of variance (ANOVA) and Tukey multiple comparison test was used to determine the significant differences between means (p < 0.05).
Results and discussion
Bioactivity of amaranth protein hydrolysates
Hydrolysis conditions such as T, t, E/S and % of bioactivity inhibition have previously been reported and evaluated elsewhere by the authors. Table 1 shows the experimental conditions that were used in order to obtain the different selected peptide fractions from hydrolysates of storage proteins of amaranth grain which exhibited ACE inhibitory activity: Alb1H103, GloH88 and DPPIV (GluH24). The peptide fraction Alb1H103 was a hydrolysate that has peptides made up of 4–5 aminoacids (0.55 kDa) with foaming capacity and inhibitory activity of platelet aggregation. Conversely, GloH88 has peptides made up of 3–4 aminoacids (0.40 kDa) with emulsifying capacity (Soriano-Santos and Escalona-Buendía 2015) and also inhibitory activity of platelet aggregation. In this work, ATA of former peptide fractions was observed assessing the inhibitory activity of platelet aggregation triggered by ADP. Blood coagulation is a complex process that involves the confluence of numerous factors and phenomena and consists of three overlapping phases that maintain the integrity of the circulatory system: vascular phase, platelet phase, and coagulate phase. The platelet phase, also called primary hemostasis, implies the adhesion, activation and aggregation of the platelets. The coagulation phase, or secondary hemostasis, involved the participation of the coagulation factors, many of which were proteases synthesised in the liver as zymogens that, in sequential order, lead to the clot formation. This coagulation cascade consists of two triggering pathways, the intrinsic and the extrinsic one, both converging in the common pathway. In order to evaluate the effect of processing conditions on the stability of the potential antithrombotic activity of Alb1H103 and GloH88, a simple method was performed, one that focuses specifically on primary hemostasis. Buame (N-(3-hydroxy-1,3,5(10)-estratrien-17β-yl)butylamine), a synthetic estrogen which is a dose-dependant inhibitor on platelet aggregation, induced by ADP, was used as a control. The method used to assess antithrombotic activity was different from the one reported by Sabione et al. (2015), which was done using clotting tests such as activated partial thromboplastin time test, prothrombin time test and thrombine time test. They found that albumin (3.8 mg/ml) and globulin (2.5 mg/ml) hydrolysates improved the antithrombotic activity whereas glutelin hydrolysate exhibited the highest antithrombotic activity. Nevertheless it was difficult to compare the concentrations of peptide fractions of amaranth protein hydrolysates with antithrombotic activity in the study by Sabione et al. (2015) and present because the trials evaluated different coagulation factors. In spite of this, Alb1H103 (431.1 ± 0.01 mg/ml) and GloH88 (12.8 ± 0.06 mg/ml) had similar antiplatelet activity of the estrogen Buame (400 µM; Flores-Garcia et al. 2012). However, unlike the study by Sabione et al. (2015), no inhibition of platelet aggregation with GluH24 was observed. The peptide fraction GluH24 is a hydrolysate that has peptides made up of 3–4 aminoacids (0.45 kDa). Such fraction displayed DPPIVIA and can control postprandial glycemia in streptozotocin-induced diabetic mice (Soriano-Santos et al. 2015). Table 1 also shows the IC50 value of all hydrolysates. These values were larger than those observed in the controls because the bioactivities were assessed in the corresponding peptide fraction. Moreover, there was neither isolation nor purification of any single peptide in this work. It is known that the more purified a peptide is, the more bioactivity this will exhibit (Li et al. 2004).
Effect of heat treatment on peptide bioactivity
In a previous work, it was reported that the peptide fractions Alb1H103 and GloH88 of amaranth protein hydrolysates have foaming and emulsifying capacities, whereas GluH24 only displays DPPIVIA (Soriano-Santos and Escalona-Buendía 2015; Soriano-Santos et al. 2015). Alb1H103 and GloH88 might be considered as functional ingredients because of their techno- and bio-functional properties (surfactant properties as well as their ACEIA and ATA). Heat treatment is the most important method in food processing. Consequently, bioactivity assurance of these ingredients should be maintained during thermal processing for a certain time. Figure 1a, a′, a″, b, b′ and b″ shows the effect of heat treatment (40–120 °C) for 1 h, on the bioactivities of hydrolysates, at the two more frequent pH of foods, roughly 4 and 7. There were no significant differences among ACEIA, DPPIVIA and ATA of the heated hydrolysates at pH 4 and 7 and those of unheated hydrolysates (p < 0.05). This indicates that peptides present in acid or weak alkaline food environment could resist thermal processing from 40 to 100 °C. However, at 120 °C treatment, the correspondent bioactivity begins to decrease when compared with that of the unheated hydrolysate (p < 0.05). Furthermore, heating time is also a major factor that may affect the peptide bioactivity present in the hydrolysate. Once it was confirmed that the hydrolysate treatment at 100 °C kept the bio-functional activity, the effect of time on heat treatment was evaluated. With regard to the unheated hydrolysate, there was no statistically significant loss of any bioactivity under heat treatment for 3 h at pH 4.0 (Fig. 2a, a′, a″). Figure 2b, b′ and b″ on the other hand, exhibits that Alb1H103 and GluH24 maintained the inhibitory activity of ACE and ATA at pH 7.0 for 3 h. When compared to the unheated hydrolysates, there was no statistically significant loss of bioactivities in GloH88 under heat treatments just for 2.0 h (p < 0.05) at pH 7.0. Temperature and time may be crucial factors that may lead to loss of bioactivity. Wu et al. (2014) found that the ACEIA of bovine casein-derived peptides showed no significant change after heating at different temperatures (40–100 °C) at pH 7.0 after 2 h of treatment, while heat treatment for 3.0 h or 4.0 h slightly decreased ACEIA (p < 0.05). Those authors claimed that the relationship between peptide structure-bioactivity, was unclear. Heat causes protein denaturation and aggregation during temperature changes from 60 to 90 °C, which may cause that high molecular mass peptides form clusters and, as a result, may hinder binding ability of the enzyme. Thus, bioactivity may diminish or be lost (Bloom et al. 2015). In agreement with research by Wu et al. (2014), this work suggested that hydrolysates with ACEIA could be stable in the heat and time treatments of food processes carried out in temperatures ranging from 60 to 100 °C and also for short periods of time. In a study by Hwang (2010) peptides derived from tuna cooking juice proteins retained ACEIA. They exhibited acceptable stability after various temperatures, pH levels and pressure treatments. In fact, they found that severe thermal processing at 100 °C for 30 min produced minor changes in peptides. Similarly, Wu and Ding (2002) observed that soy-protein-derived peptides with ACEIA also displayed stability at 100 °C for 30 min. Fu et al. (2015) studied the effect of different temperatures on the ACEIA of collagen and found that this kept its inhibitory capacity at relatively low temperatures (20–60 °C). Conversely, after heating for 2 h at 100 °C, they noted a slight reduction of the inhibitory activity. Based on the previous data, it would seem that peptides with ACEIA are more stable in a range of temperature (20–100 °C), at least for 1 h. However, the loss of stability of bioactivity would have to be also assessed under severe treatment conditions, such as sterilization, grilling and frying, in order to assure that the expected physiological benefit of a food is received.
IC50 value as a result of the effect of heat treatment (40–120 °C). a, a′ and a″ at pH 4.0 and b, b′ and b″ at pH 7.0 for 1 h of selected amaranth peptide fractions on their ACEIA, ATA and DPPIVIA. Values are expressed as mean ± SD. Bars that have the same superscript in a column do not differ significantly (p < 0.05)
IC50 value as a result of the effect of time treatment (1–4 h) of the selected amaranth peptide fractions heated previously at 100 °C a, a′ and a″ at pH 4.0 and b, b′ and b″ at pH 7.0 on their ACEIA, ATA and DPPIVIA. Values are expressed as mean ± SD. Bars that have the same superscript in a column do not differ significantly (p < 0.05)
Effect of pH on the peptide bioactivity
pH effect was assessed on a range of 2.0–12.0 of inhibitory activity of ACE, ATA and DPPIV of Alb1H103, GloH88 and GluH24, respectively. A T = 100 °C was selected for 1 h for this assay, which showed that there was no loss of stability of bioactivity, so any change on it would have to be attributed to the influence of pH treatment. Changes of bioactivity of different peptide fractions of amaranth protein hydrolysates are shown in Fig. 3a–c. Bioactivity depended largely on the solubility of the hydrolysate. In general, as solubility increases, IC50 value of the corresponding bioactivity decreases. Conversely, as solubility decreases, IC50 value of the corresponding bioactivity increases. As for Alb1H103, IC50 values of ACEIA and ATA were roughly constant within the pH range of 5.0–7.0; the lowest inhibitory activity, for both bioactivities, was observed at alkaline pH (8.0–12.0). IC50 value of GloH88, for ACEIA and ATA was kept constant, similar to that of the control, within the pH range of 2.0–8.0. At pH 9.0–12.0, the IC50 values of these hydrolysates increased. GluH24 yielded a very good solubility at acid or alkaline pH. Therefore, within the pH ranges of 2.0–4.0 and 7.0–9.0, the DPPIVIA was constant and similar to that of the control. Within the pH range of 10.0–12.0, the highest inhibitory activity of GluH24 on DPPIV activity was observed. However, at pH 5.0 and 6.0, the DPPIVIA of GluH24 decreased as the IC50 values increased. This may be ascribed to the fact that a heat treatment at those extreme pH ranges can damage some amino acids of the inhibitory peptides. Denaturation and even hydrolysis of some peptides can also affect the inhibitory activity triggered by the pH (Wu et al. 2014). pH modification can also prompt physico-chemical changes of proteins such as denaturation, intermolecular reactions, reaction with sugars, solubility and, thus, cause crosslinking, lysine damage, non specific cleavage of peptides and damaged amino acids prone to pH changes. A further explanation holds that pH changes can affect bioactivity on account of alterations of one or more aminoacids (Hwang 2010). For example, acid treatments damage glutamine and asparagine, and alkaline treatments not only destroy cysteine, serine and threonine, but also produce lysinoalanine and d-amino acids (Kristinsson and Hultin 2003). There are few foodstuffs at acid pH and the treatment at pH over 7.0 is scarce. Therefore, in this work, it was observed that pH did not have a considerable effect on ACEIA, DPPIVIA and ATA. Similarly, a purified hendeca-peptide as obtained of algae protein waste was subjected to incubation at pH 2–10 and temperature 40–100 °C for 1 h and measured for ACEIA. The peptide completely retained its ACEIA, indicating that the purified peptide was both pH and heat-stable (Sheih et al. 2009). The impact of pH (2.0–10.0) on the ACEIA of collagen hydrolysates afforded a significant reduction of ACEIA at strong alkaline conditions (pH 10.0). Similarly, in agreement with our research, collagen peptides exhibited good resistance against the acidic and weak alkaline conditions and heat treatment (Fu et al. 2015).
Stability of bioactivity of hydrolysates on an in vitro gastrointestinal digestion
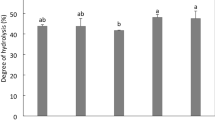
There are more reports about peptides, as isolated from different natural sources, capable of inhibiting ACE than studies about peptides capable of inhibiting DPPIV and antithrombotic activities. By the same token, there are few examples of biopeptides with ACEIA, such as IPP and VPP, derived from milk protein, that have been added to commercial foodstuffs. In addition, conclusive data with respect to their effectiveness on hypertension is scarce not only because there are still insufficient case reports when these tripeptides are incorporated in daily diet plans, but also because functional food regulations are still limited in several countries (Madureira et al. 2010). To the best of our knowledge, no reports on commercial products added with peptides that inhibit DPPIV and antithrombotic activities are known. One of the aims of the present work was to evaluate the bioactivity of the peptide fractions as obtained of amaranth protein hydrolysates once they have undergone gastrointestinal digestion. Therefore a simulation of human gastrointestinal digestion was performed in vitro using pepsin, trypsin (T) and chymotrypsin (C). Because T and C are serin proteases, the activity of the residual peptide, called amaranth trypsin/subtilisin inhibitor (ATSI), present in protein hydrolysates of amaranth grain, was also assessed. ATSI inhibitory activity was evaluated following the protocol by Valdes-Rodriguez et al. (1993). Alb1H103 with a % HD = 42.1 ± 0.4 had a % InhATSI = 35.4 ± 0.6, whereas GloH88 with a % HD = 16.3 ± 1.7 displayed a higher inhibitory activity of serin proteases (%InhATSI = 46.4 ± 0.8). This is relevant in that authors like Hejgaard et al. (1994) observed that a previous treatment of protein extracts of amaranth with pepsin, containing ATSI, improved the proteolytic activities of T and C. Similarly, Fig. 4a, a′, a″ shows that a previous pH treatment at 4.0 or 7.0 (T = 100 °C; t = 1 h) of Alb1H103 and GloH88 and GluH24 influenced the bioactivity after a digestion with T and C. It was found that, in general, when the hydrolysates were kept at 100 °C for 1 h at pH 4.0, the corresponding bioactivity resulted in a significant increase (p < 0.05) as the IC50 (mg/ml) values of ACE decreased from 925 ± 2.2 to 622 ± 0.1 and from 1391 ± 1.4 to 895 ± 0.8 for Alb1H103 and GloH88, respectively. The same effect was obtained when ATA was evaluated as their IC50 (mg/ml) values produced a significant decrease (p < 0.05) from 431.1 ± 0.01 to 226 ± 0.02 and from 12.8 ± 0.06 to 9.4 ± 0.03 for Alb1H103 and GloH88, respectively. DPPIVIA decreased (p < 0.05) their IC50 (mg/ml) from 120 ± 0.6 to 75 ± 0.2 for GluH24 after the enzymatic digestion. This improvement of inhibitory activity of the corresponding bioactivity may be due to the degree of hydrolysis of hydrolysates of Alb1H103 and GloH88 and GluH24 (% DH = 42.1 ± 0.4; 16.3 ± 1.7 and 75.0 ± 0.5, respectively).
Furthermore, at pH 4.0 ATSI hydrolysis can be promoted because of pepsin activity. Once ATSI is hydrolysed, no inhibition of serin proteases (T and C) can be observed, so these enzymes continue hydrolysing peptides, thus yielding new peptides with different bioactivities. The resulting biopeptides contained in the hydrolysates can be hydrolysed by T and C because these enzymes can cleave specific peptide bonds, namely, pepsin breaks peptides bonds in Tyr, Phe and Leu residues; trypsin in Arg and Lys residues; chymotrypsin in Trp, Phe, Tyr, Met and Leu residues. Huang et al. (2012) observed that two peptides (PGVGGPLGPIGPCYE and CAYQWQRPVDRIR), derived from tuna cooking juice hydrolysates with DPPIV inhibitory potential, increased significantly upon simulated gastrointestinal digestion. This can be attributed to the fact that new and smaller peptides were formed to increase the content of DPPIV inhibitory peptides. During the simulated gastrointestinal digestion, the peptides were truncated into smaller fragments, some of which increased the DPPIV inhibitory potential. Huang et al. (2012) suggest that the smaller peptides may have greater intestinal permeability than the original ones. They also found that the DPPIV inhibitory potential of other peptides isolated from tuna cooking juice hydrolysates was retained or improved.
In contrast, a treatment at pH 7.0 of Alb1H103, GloH88 and GluH24 (T = 100 °C; t = 1 h) does not facilitate ATSI hydrolysis by pepsin and, consequently, the digestion with T and C can be inhibited by ATSI in such a way that the peptides of each bioactivity do not undergo a further hydrolysis. Therefore no significant difference (p < 0.05) was observed in the bioactivities of the hydrolysates after their digestion. In order to prevent the intestinal digestion from altering the chemical structure of biopeptides, it has been suggested that the hydrolysates be obtained by using only digestive proteolytic enzymes. However, alcalase is one the most sought-after enzymes to hydrolyse proteins from different sources. Orsini et al. (2011) found that biopeptides as obtained of amaranth grain globulin, in an in vitro gastrointestinal digest simulation test, cannot be cleaved by the digestive proteolytic enzymes. To the best of our knowledge, there is not much research on the proteolytic stability of ACEIA peptides with gastrointestinal enzymes. Tavares et al. (2011) found that a modest decrease of ACEIA of peptides as obtained of C. cardunculus, in which IC50 = 336.3 ± 34.9 µg/ml was reduced to a IC50 = 253.6 ± 30.7 µg/ml, when the digestion was over. Conversely, Akilliioglu and Karakaya (2009) noted an increase of ACEIA in pulse hydrolysates after a gastrointestinal digestion. A tripeptide VAP was obtained by hydrolyzing grass carp protein with alcalase and was subjected to separate and combined in vitro digestions by pepsin and chymotrypsin, showing no impact of digestive enzymes on ACEIA of VAP (Chen et al. 2012). Hwang (2010) observed that the ACEIA of oligopeptides, as obtained from tuna cooking juice, showed insignificant changes after an in vitro incubation with gastric enzymes. This suggests that these oligopeptides may be unaffected by the digestion in the gastrointestinal tract. Moreover, previous reports have also shown that small peptides have low susceptibility to hydrolysis by gastric proteases. Sheih et al. (2009) suggested not only that purified peptides are resistant to digestion in the gastrointestinal tract, but also that the active sequence of the peptide would not be destroyed by these enzymes. Collagen peptides were digested by pepsin and pancreatin and afforded an elevated content of free amino acids. The ACEIA of collagen peptides remained stable after an in vitro digestion by gastric proteases. Thus, the collagen peptides may endure digestion considerably through the gastrointestinal tract or they may be partially degraded into smaller peptides whilst retaining their activity. Collagen peptides display potent ACEIA after an in vitro digestion, possibly due to post-translational hydroxylation of proline in collagen (Fu et al. 2015).
Conclusion
Alb1H103 and GloH88, as obtained of amaranth grain, exhibited angiotensin I-converting-enzyme-inhibitory activity (ACEIA) and Antithrombotic activity (ATA), and were stable under a heat treatment from 40 to 100 °C at pH 4 and 7 for 3 h, except for GloH88 whose time lapse, at pH 7.0, was shorter (2 h). ATA was evaluated as inhibition of platelet aggregation. GluH24, with dipeptidyl peptidase IV inhibitory activity (DPPIVIA), was also stable in the aforementioned conditions. The pH effect on hydrolysates bioactivity did not have a considerable effect as long as peptides were soluble. With regard to the biological activity of protein hydrolysates, this may be preserved or enhanced depending on their processing conditions. Therefore, the results suggest that these hydrolysates may be considered as biofunctional ingredients to manufacture third generation nutraceuticals and functional foods.
References
Akilliioglu H, Karakaya S (2009) Effects of treatment and in vitro digestion on the angiotensin converting enzyme inhibitory activity of some legume species. Eur Food Res Technol 229:915–921
AOAC (2010) Official methods of analysis. Association of Official Analytical Chemist, Rockville
Barba de la Rosa AP, Barba Montoya A, Martínez-Cuevas P. Hernández-Ledesma B, León-Galván MF, De León-Rodríguez A, González C (2010) Tryptic amaranth glutelin digest induce endothelial nitric oxide production through inhibition of ACE: antihypertensive role of amarant peptides. Nitric Oxide 23:106–111
Bloom KA, Huang FR, Bencharitiwong R, Bardina L, Ross A, Sampson HA, Nowak-Wegrzyn A (2015) Effect of heat treatment on milk and egg proteins allergenicity. Pediatr Allergy Immunol 25:740–746
Chen J, Wanga Y, Zhong Q, Wua Y, Xiaa W (2012) Purification and characterization of a novel angiotensin-I converting enzyme (ACE) inhibitory peptide derived from enzymatic hydrolysate of grass carp Protein. Peptides 33:52–58
Condés MC, Scilingo AA, Añón MC (2009) Characterization of amaranth proteins modified by tripsin proteolysis: structural and functional changes. Food Sci Technol 42:963–970
De la Peña A, Baños G, Izaguirre R, Mandoki JJ, Fernández-G JM (1993) Comparative effect of synthetic amino-estrogens with estradiol on platelet aggregation. Steroids 58:407–409
Dexter AF, Middelberg PJ (2008) Peptides as functional surfactants. Ind Eng Chem Res 47:6391–6398
Domínguez-González K, Cruz-Guerrero A, González-Márquez H, Gómez-Ruiz L, García-Garibay M, Jiménez-Guzmán J, Rodríguez-Serrano G (2014) Antihypertensive and antithrombotic activities of a commercial fermented milk product made with Lactobacillus casei Shirota and Streptococcus thermophillus. Int J Dairy Technol 67:358–362
Flores-Garcia M, Fernández-G JM, León-Martínez M, Hernández-Ortega S (2012) The structures and inhibitory effects of Buame [N-(3-hydroxy-1,3,5(10)-estratrien-17β-yl)-butylamine] and Diebud [N,N 0-bis-(3-hydroxy-1,3,5(10)-estratrien-17β-yl)-1,4-butanediamine] on platelet aggregation. Steroids 77:512–520
Fritz M, Vecchi B, Rinaldi G, Añón MC (2011) Amaranth seed protein hydrolysates have in vivo and in vitro antihypertensive activity. Food Chem 126:878–884
Fu Y, Young JF, Dalsgaard TK, Therkildsen M (2015) Separation of angiotensin I-converting enzyme inhibitory peptides from bovine connective tissue and their stability towards temperature, pH and digestive enzymes. Int J Food Sci Technol 50:1234–1243
González G, Alvarado-Vasquez N, Fernández-G JM, Cruz-Robles D, del Valle L, Pinzón E, Torres I, Rodríguez E, Zapata E, Gómez-Vidales V, Montaño LF, de la Peña A (2010) The antithrombotic effect of the aminoestrogen prolame (N-(3-hydroxy-1,3,5(10)-estratrien-17B-YL)-3-hydroxypropylamine) is linked to an increase in nitric oxide production by platelets and endothelial cells. Atherosclerosis 208:62–68
Hayakari M, Kondo Y, Izumi H (1978) A rapid and simple spectrophotometric assay of angiotensin-converting enzyme. Anal Biochem 84:361–369
Hejgaard J, Dam J, Petersen LC, Bjorn SE (1994) Primary structure and specificity of the major serine proteinase inhibitor of amaranth (Amaranthus caudatus L.) seeds. Biochim Biophys Acta 1204:68–74
Huang SL, Jao CL, Ho KP, Hsu KC (2012) Dipetidyl-peptidase IV activity of peptides derived from tuna cooking juice hydrolysates. Peptides 35:114–121
Hwang JS (2010) Impact of processing on stability of angiotensin I-converting enzyme (ACE) inhibitory peptides obtained from tuna cooking juice. Food Res Int 43:902–906
Kojima K, Ham T, Kato T (1980) Rapid chromatographic purification of dipeptidyl peptidase IV in human submaxillary gland. J Chromatogr A 189:233–240
Kristinsson HG, Hultin HO (2003) Effect of low and high pH treatment on the functional properties of cod muscle proteins. J Agric Food Chem 51:5103–5110
Li GH, Le GW, Shi YH, Sherstha S (2004) Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr Res 24:469–486
Madureira AR, Tavares T, Gomes AMP, Pintado ME, Malcata FX (2010) Physiological properties of bioactive peptides obtained from whey proteins. J Dairy Sci 93:437–455
Montoya-Rodriguez A, Gómez-Favela MA, Reyes-Moreno C, Milán-Carrillo J, González de Mejía E (2015) Identification of bioactive peptide sequences from amaranth (Amaranthus hypochondriacus) seed proteins and their potential role in the prevention of chronic diseases. Compr Rev Food Sci Saf 14:139–156
Orsini DMC, Tironi VA, Añón MC (2011) Antioxidant activity of amaranth protein or their hydrolysates under simulated gastrointestinal digestion. Food Sci Technol 44(1752):1760
Rao S, Sun J, Liu Y, Zeng H, Su Y, Yan Y (2012) ACE inhibitory peptides and antioxidant peptides derived from in vitro digestion hydrolysate of hen egg white lysozyme. Food Chem 135:1245–1252
Rui X (2012) Angiotensin I-converting enzyme inhibitory properties of Phaseolus vulgaris bean hydrolysates: Effects of different thermal and enzymatic digestion treatments. Food Res Int 49:739–746
Sabione AC, Scilingo A, Añon MC (2015) Potential antithrombotic in amaranth proteins and its hydrolysates. Food Sci Technol 60:171–177
Sheih IC, Fang TJ, Wub TK (2009) Isolation and characterization of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem 115:279–284
Shevkani K, Singh N (2015) Relationship between protein characteristics and film-forming properties of kidney bean, field pea and amaranth protein isolates. Intl J Food Sci Technol 50:1033–1043
Shevkani K, Singh N, Kaur A, Rana JC (2014a) Physicochemical, pasting, and functional properties of amaranth seed flours: effects of lipid removal. J Food Sci 79:C1271–C1277
Shevkani K, Singh N, Rana JC, Kaur A (2014b) Relationship between physicochemical and functional properties of amaranth (Amaranthus hypochondriacus) protein isolates. Food Sci Technol 49:541–550
Soriano-Santos J, Escalona-Buendía HB (2015) Angiotensin I-converting enzyme inhibitory and antioxidant activities and surfactant properties of protein hydrolysates as obtained of Amaranthus hypochondriacus L. grain. J Food Sci Technol 52:2073–2082
Soriano-Santos J, Reyes-Bautista R, Guerrero-Legarreta I, Ponce-Alquicira E, Escalona-Buendía HB, Almanza-Pérez JC, Díaz-Godínez G, Román-Ramos R (2015) Dipeptidyl peptidase IV inhibitory activity of protein hydrolyzates from Amaranthus hypochondriacus L. grain and their influence on postprandial glycemia in streptozotocin-induced diabetic mice. Afr J Tradit Complement Altern Med 12:90–98
Tavares T, Contreras M, Amorim M, Pintado M (2011) Novel whey-derived peptides with inhibitory effect against angiotensin-converting enzyme: In vitro effect and stability to gastrointestinal enzymes. Peptides 32:1013–1019
Tovar-Pérez EG, Guerrero-Legarreta I, Farrés-González AN, Soriano-Santos J (2009) Angiotensin I-converting enzyme-inhibitory peptide fractions from albumin 1 and globulin as obtained of amaranth grain. Food Chem 116:437–444
Valdes-Rodriguez S, Segura Nieto M, Chagolla-Lopez A, Verver-y Vargas-Cortina A, Martinez-Gallardo N, Blanco-Labra A (1993) Purification, characterization, and complete amino acid sequence of a trypsin inhibitor from Amaranth (Amaranthus hypochondriacus) seeds. Plant Physiol 103:1407–1412
Velarde-Salcedo AJ, Barrera-Pacheco A, Lara-González S, Montero-Morín G, Díaz-Gois González, de Mejía E, Barba de la Rosa AP (2013) In vitro inhibition of dipeptidyl peptidase IV by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus L.) proteins. Food Chem 136:758–764
Wu J, Ding X (2002) Characterization of inhibition and stability of soy protein derived angiotensin I-converting enzyme inhibitory peptides. Food Res Int 35:367–375
Wu W, Feng-yang Z, Hong-xia CH, Zhan-mei J (2014) Stability and cytotoxicity of angiotensin-I-converting enzyme inhibitory peptides derived from bovine casein. J Zhejiang Univ-Sci B 2:143–152
Acknowledgements
We are grateful to Prof. Abraham Avendaño-Martínez for proofreading and translating the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Sánchez, J., Ponce-Alquicira, E., Pedroza-Islas, R. et al. Effects of heat and pH treatments and in vitro digestion on the biological activity of protein hydrolysates of Amaranthus hypochondriacus L. grain. J Food Sci Technol 53, 4298–4307 (2016). https://doi.org/10.1007/s13197-016-2428-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2428-0