Abstract
In this study, potential Angiotensin Converting Enzyme (ACE) inhibitory activity of common dry beans, dry pinto beans and green lentils were determined and the effects of different heat treatment periods were investigated. In vitro gastrointestinal digestion was applied after heat treatment, and stability of the ACE inhibitory activity was determined. Crude protein extracts of legume samples showed 4.08–28.54% ACE inhibition activities. 30-min heat treatment caused a decrease in ACE inhibitory activity; however, 50-min heat treatment was observed to be beneficial for the release of ACE inhibitory peptides from the three legume species (p < 0.05). In vitro digestion process increased ACE inhibitory activities of the samples (p < 0.05), mainly green lentil digests by a factor of 10.91–23.65 against ACE in comparison with raw sample. It was observed that IC50 values of common beans, pinto beans and green lentils after digestion were 0.78–0.83, 0.15–0.69 and 0.008–0.89 mg protein/mL, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioactive peptides from food sources are defined as peptides from animal or vegetable origin providing regulatory effects in human physiology in addition to supplying adequate and balanced nutrition to human beings [1]. Researches in the field of bioactive peptides have been increased and extensively reviewed for almost 20 years [2, 3]. Several health effects were attributed to bioactive peptides including antimicrobial properties, blood pressure-lowering (ACE inhibitory) effects, cholesterol-lowering ability, antithrombotic and antioxidant activities, enhancement of mineral absorption/bioavailability, cyto- or immunomodulatory effects and opioid activities [1]. Among these bioactive peptides, ACE inhibitory peptides have been well reviewed [4, 5].
ACE (Angiotensin I-converting enzyme; EC 3.4.15.1) is a dipeptidyl carboxypeptidase and plays an important role in human Renin–Angiotensin–Aldosteron System (RAAS). RAAS is a major regulator in human physiology. It controls blood pressure, volume and electrolytes and affects the heart, vasculature and kidney. ACE converts the inactive decapeptide Angiotensin I by cleaving dipeptide from the C-terminus into the potent vasoconstricting octapeptide Angiotensin II. This potent vasoconstrictor is also involved in the release of a sodium-retaining steroid, aldosterone, from the adrenal cortex, which has a tendency to increase blood pressure. ACE also catalyses the degradation of bradykinin, a blood pressure lowering nonapeptide [4].
Inhibition of ACE is considered as a useful therapeutic approach in the treatment of hypertension. Therefore, in the development of drugs to control high blood pressure, ACE inhibition has become an important target. A large number of highly potent and specific ACE inhibitors have been developed as orally active drugs that are used in the treatment of hypertension and congestive heart failure [4]. However, these synthetic ACE inhibitors are known to have irritating side effects such as dry cough, significant drop in energy, angio-oedema, etc. Therefore, ACE inhibitory peptides from food sources are commonly accepted as more advantageous over synthetic drugs [4, 6].
ACE inhibitory peptides can be present in intact food protein, but generally they are not active in that case. These inactive peptides can be released after enzymatic proteolysis, such as during gastrointestinal digestion or during fermentation process [1–3, 7]. Several ACE inhibitory peptides were isolated from food sources. Fish and meat [8–10], milk and dairy products [11–13], egg [14], corn [15–17], wheat [9, 18] were described as precursors of ACE inhibitory peptides.
Legumes are the second food crops that are widely grown in the world following cereals, and they are known to be good sources of dietary protein. Health benefits of legumes were investigated in experimental, epidemiological and clinical studies. Lipid homeostasis control and hypocholesterolemic effects of soybean proteins, glycemic control of a lupin protein, anticarcinogenic effects of protease inhibitors and lectins, therapeutic effects of α-amylase and protein inhibitors on obesity and diabetes were determined in these studies [19]. Adebamowo et al. [20] reported that there was a relationship between bean and lentil consumption and a lower incidence of breast cancer. In a multiethnic case control study, protective effect of legumes except soybeans on prostate cancer was observed [21]. Besides these health-promoting effects, ACE inhibitory peptides were found in soy bean [22], mung bean [23], chickpea [7, 24] and pea [6, 25, 26].
Although common beans and lentils are widely consumed all over the world, to the best of our knowledge, there are not many studies about their ACE inhibitory activity. It is important to know the effect of heat treatment since cooking is a crucial process to consume dry legumes. Bioavailability is another important aspect for evaluating the possible health effects of the food components. The present study was undertaken to investigate the effects of heat treatment and in vitro gastrointestinal digestion on ACE inhibitory activity of common dry beans, pinto beans and green lentils.
Materials and methods
Different brands of common dry beans (Phaseolus vulgaris L. cv. Dermason), dry pinto beans (Phaseolus vulgaris L.) and green lentils (Lens culinaris L. cv. Sultani) were purchased from a local market.
Pepsin (P7000), PIPES [piperazin-NN′-bis(2-ethan-sulphonic acid)] disodium salt (P3768), pancreatin (P1750), bile extract (B8631); ACE (Angiotensin converting enzyme from rabbit lung, A6778); N-Hippuryl-His-Leu hydrate (H1635); haemoglobin (H2625); phosphate buffered saline (P4417); and Coomassie blue G (B0770) were purchased from Sigma-Aldrich, and sodium borate buffer (82633) and sodium chloride (71378) were purchased from Fluka. All other chemicals were of analytical grade.
Preparation of crude protein extracts
In order to prepare crude protein extracts, an alkaline-assisted process as described by Theodore and Kristinsson [10] and salting out procedure [27] were applied together. Samples were grounded into 60-mesh size with a laboratory mill (Brook Crompton Series 2000). Grounded samples were homogenised in nine volumes of water, and proteins were solubilised by adjusting the pH to 11 with 1 M NaOH. The homogenates were then centrifuged at 10000 × g for 20 min at 4 °C (Thermo Scientific IEC CL31R Multispeed Centrifuge), and the supernatants were collected. The pH values of the supernatants were adjusted to the samples’ isoelectric point (4.5 for common beans and pinto beans; 4.8 for green lentils). Solid ammonium sulphate was added up to 90% saturation and mixtures were slowly stirred for 1.5 h at 4 °C. Precipitates were obtained by centrifugation at 10000 × g for 20 min at 4 °C. Precipitates were dissolved in 50 mM phosphate buffer (pH 7.0) and were dialysed for 24 h at 4 °C against distilled water. The dialysates are called crude protein extracts. If turbidity occurred, they were clarified by centrifugation at 10000 × g for 20 min at 4 °C. The extracts were immediately analysed to determine ACE inhibitory activity, otherwise were stored at −40 °C.
Heat treatment
Whole seeds of dry legume samples were autoclaved at 15 psi (121.1 °C) for 15, 30 and 50 min in a sample:water ratio of 1:3 (w/v).
Preparation of heat-treated protein extracts
Heat-treated samples were homogenised with a hand blender. Protein extraction was practised according to the method described above, which is briefly explained in Fig. 1. The extracts were immediately analysed to determine ACE inhibitory activity, otherwise were stored at −40 °C.
In vitro gastrointestinal digestion
In order to simulate human gastrointestinal digestion, procedure described by Gil-Izquierdo et al. [28] were applied with slight modifications. 10 g of homogenate from heat-treated sample were mixed with 20 mL distilled water in a polystyrene tube and pH was adjusted to 2 with 1 M HCl. 1 mL pepsin solution (4 g pepsin in 100 mL 0.1 N HCl) was added and samples were incubated at 37 °C for 2 h in a shaking water bath (Memmert, Type SV 1422, Germany). At the end of the incubation period, 5 mL of 1 M NaOH was added to stop the reaction in the simulated stomach phase. Mixture was centrifuged to separate particles. Digestive enzymes were separated by ultrafiltration through 5000 MWCO membrane (Vivaspin 6, Sartorius Stedim Biotech GmbH). Filtrate corresponding to stomach digest was collected.
In order to obtain intestine digest, simulated stomach digestion was applied as the same and after incubation period at 37 °C, a dialysis bag (Spectra/Por Dialysis Membrane MWCO:6-8000, Spectrum Laboratories, Inc.) containing 20 mL of 0.15 N PIPES buffer was placed in the tube. Incubation was continued for half an hour and 5 mL of pancreatin/bile extract mixture (0.5 g pacreatin + 3.0 g bile extract in 250 mL 1 N NaHCO3) was added. Incubation lasted for 2 h more and tube was immersed in boiling water for 10 min to stop the reaction. After cooling to room temperature, dialysis bag was rinsed with water and dialysate was filtered through 5000 MWCO ultrafiltration membrane to separate enzymes.
Stomach and intestine digests that were not to be analysed immediately were stored at −40 °C.
Determination of ACE inhibitory activity
ACE inhibition activities of the samples were measured with modifications in the methods described by del Castillo et al. [18] and Theodore and Kristinsson [10].
ACE reactive: 25 mU/mL
Substrate: 5 mM Hip-His-Leu and 0.3 M NaCl were dissolved in 50 mM sodium borate buffer, and pH was adjusted to 8.3.
Analysis was carried out according to 4 tube analysis method.
-
Tube 1: 100 μL ACE + 40 μL deionised water
-
Tube 2: 140 μL deionised water
-
Tube 3: 100 μL ACE + 40 μL sample extract (or digest)
-
Tube 4: 40 μL sample extract (or digest) + 100 μL deionised water
The tubes were incubated at 37 °C for 5 min, and 100 μL substrate was added into each tube. Incubation was continued for 30 min at the same temperature. 150 μL of 1 M HCl was added into tubes to stop the reaction. 1,000 μL ethyl acetate was added into tubes, and tubes were mixed vigorously on vortex mixer. After centrifugation at 1,500 × g for 15 min, 750 μL of the supernatant were collected and put into clean glass beakers. Beakers were placed in boiling water bath, and ethyl acetate was vaporised (approximately within 15 min). Solid hippuric acid in each tube was dissolved in 2-mL deionised water, and absorbance was measured at 228 nm. ACE inhibition activity (%) was determined according to the following formula:
A is the absorbance of ACE + deionised water; B the absorbance of deionised water; C the absorbance of ACE + sample extract (or digest); D the absorbance of deionised water + sample extract (or digest).
A dilution series of samples showing ACE inhibition activity greater than 50% were prepared, and corresponding inhibition activity was determined according to each protein concentration. IC50 values (protein concentration needed to inhibit 50% of the activity of ACE) were determined using GraphPad Prism Version 5.01 (GraphPad Software, Inc., San Diego, CA). Per cent ACE inhibition was graphed versus logarithm of sample concentration. IC50 value was calculated by solving the four-parameter equation below:
where y is the % ACE inhibition activity, x the logarithm of sample concentration, max the plateau value corresponding to 100% inhibition activity, min the plateau value corresponding to 0% inhibition activity.
Determination of protein content
Protein content of crude protein extracts and heat-treated protein extracts were determined according to Bradford procedure [29]. A calibration curve was prepared by using haemoglobin as standard. Protein concentration of digests was determined by absorbance readings taken at 280 and 260 nm [29]. Protein content was calculated according to the formula below:
where A corresponds to absorbance value.
Statistical analysis
All measurements were carried out as triplicates and in parallels.
Differences between ACE inhibitory activity of the samples after heat treatment and in vitro gastrointestinal digestion were tested by ANOVA. Tukey’s test was used to determine significant differences (p < 0.05) by using SPSS for Windows (Version 10.0).
Results and discussion
Protein extraction
In general, isoelectric precipitation has been used to obtain protein extracts or isolates from legumes [24, 30–32]. However, ammonium sulphate precipitation was used in some cases [33]. Also, it was reported that combining a few methods could enhance protein recovery [34]. In this study, two precipitation techniques, isoelectric precipitation alone and isoelectric precipitation combined with ammonium sulphate saturation were used. It was observed that combining the two methods resulted in better protein recovery (0.146 and 0.175 mg protein/mL were measured in extracts of pinto beans obtained by isoelectric precipitation and isoelectric precipitation combined with ammonium sulphate saturation methods, respectively).
ACE inhibitory activity of protein extracts
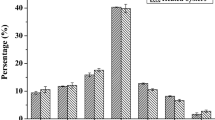
Bioactive peptides can be found in intact food molecule, but they are generally inactive within the sequence of protein molecule. As the first experiment, crude protein extracts were analysed to get information whether legume proteins could exert antihypertensive action in vitro. Figure 2 represents the results.
As can be seen from the figure, samples showed low inhibition activities against ACE ranging from 4.08 to 28.54%. By taking the hypothesis ‘bioactive peptides can be released during food processing’ into consideration, effect of heat treatment on potential ACE inhibitory activity was investigated. The fact that legumes cannot be consumed without cooking was the second reason of this investigation.
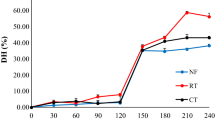
Effects of cooking on the ACE inhibitory activity of dry legumes at 121.1 °C for 15, 30 and 50 min are shown in Fig. 3. Contrary to pinto beans and green lentils, ACE inhibition activity of raw common beans was not statistically different from those of 50-min heat-treated common beans (p > 0.05). Heat treatment for 15 min caused a decrease in ACE inhibitory activity of common beans, but this activity was not different from those of raw sample (p > 0.05), whereas heat treatment for 30 min resulted in a significant decrease in the activity.
It was reported that heat treatment applied to Phaseolus species can lead to formation of disulphide bridges in the protein molecule [32]. It can be concluded that the decrease in ACE inhibition capacity could have been resulted because of a possible change of the protein molecule due to cross bonding (formation of new S–S bonds). Cui et al. [35] reported that while SH group level was decreasing, level of S–S group was increasing. In general, it is considered that disulphide bridges stabilise protein conformation and enhance thermodynamic stability. On that account, formation of new disulphide bridges by heat treatment may have disabled breaking of protein molecule, hence the release of ACE inhibitory peptides. The fact that common beans are rich sources of sulphur-containing amino acids supports this idea. The increase in ACE inhibition activity following 50-min heat treatment can be due to the possible release of bioactive peptides which are still inactive within the protein molecule during 15 and 30-min heat treatments. However, to the best of our knowledge, there were no other studies related to ACE inhibitory activity of Phaseolus species in literature.
ACE inhibitory activity of pinto bean protein extracts obtained after 15- and 50-min heat treatment were found to be similar (p < 0.05). Protein extract of 30-min heat-treated pinto beans showed the lowest ACE inhibitory activity. However, this activity was higher than that of raw sample. Similarly, heat treatment yielded increase in the ACE inhibition capacity of protein extracts of green lentils. A 15-min heat treatment caused an important increase, but when heat treatment lasted for 30 min, it was observed that ACE inhibition activity was decreased. After 50-min heat treatment, an increase in the activity was observed again (from 15.63 to 51.92%) such as observed in both common beans (from 1.32 to 36.99%) and pinto beans (from 24.91 to 47.40%), and the inhibition level reached to the value of inhibition obtained after 15-min cooking time (Fig. 3).
Common beans were found out to be the legume having the lowest ACE inhibition activity following the 15- min and 30-min heat treatments (p < 0.05). However, after the 50-min heat treatment, the activity reached to the level similar to that of pinto beans. The 15-min heat treatment resulted in the same way for pinto beans and green lentils (p > 0.05).
ACE inhibitory activity of legume digests
Digestion is one of the most important processes to release bioactive peptides which are inactive within intact food protein. It is well known that bioactive peptides can be generated by the activity of proteolytic enzymes during digestion [1–3, 7]. Vermeirssen et al. [26] emphasised that ACE inhibitory peptides exert an antihypertensive effect in vivo if they reach the cardiovascular system in an active form. Therefore, upon oral administration, they need to resist complete degradation by gastrointestinal proteases and brush border peptidases, and they have to be absorbed through the intestinal wall with preservation of their physiological activity. During this oral delivery route, degradation of peptides can take place, resulting in an activation or inactivation of their biological activity.
In Table 1, ACE inhibitory capacity of legume digests were summarised. Common bean digests showed 1.34–2.53 times inhibitory activity against ACE in comparison with raw sample. Pinto beans showed 2.20–4.73 times inhibitory activity after digestion when compared with crude pinto bean extracts. However, the increase in the inhibitory activity of green lentils was really sharp. Green lentil digests showed 10.91–23.65 times inhibitory activity against ACE in comparison with raw sample. ACE inhibitory activities of stomach and intestine digests of common beans obtained after 15-min heat treatment were similar (p > 0.05) as those of 50-min heat-treated common beans. However, ACE inhibitory activities of 50-min heat-treated digests were higher than those of 15-min heat-treated digests (p < 0.05). Intestine digest of 30-min heat-treated pinto beans showed lower inhibitory activity than intestine digest of 50-min heat-treated pinto beans (p < 0.05). This result is consistent with the finding that 30-min heat-treated pinto beans showed less activity than 50-min heat-treated sample. Determination of intestine digest of 30-min heat-treated pinto beans had lower inhibition activity (24.57% relatively) than stomach digest of 30-min heat-treated pinto beans can be explained by these bioactive peptides were much more susceptible to degradation by digestive enzymes in intestines. Intestine digest of 50-min heat-treated pinto beans was also less effective against ACE when compared with its stomach digest; however, the decrease was less in comparison with 30-min heat-treated one. Hence, it can be concluded that 50-min heat treatment resulted in formation of more resistant bioactive peptides against the activity of digestive enzymes. For stomach digests of green lentils, it was observed that 15-min heat treatment caused the highest inhibitory activity (p < 0.05). However, all the three heat-treatment parameters yielded similar inhibitory activities for intestine digests (p > 0.05).
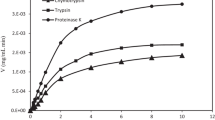
IC50 value is a more reliable parameter to present inhibition activity of a compound since it is based on concentration. However, it is not possible to predict IC50 values of the samples showing less activity than 50%. Figure 4 represents IC50 values of legume digests that show grater inhibitory activity than 50%.
It was observed that IC50 values of common beans, pinto beans and green lentils were 0.78–0.83, 0.15–0.69 and 0.008–0.89 mg protein/mL, respectively. Vermeirssen et al. [6, 25] reported that pea hydrolysate had 0.117 and 0.076 mg/mL IC50 values following pepsin and trypsin + chymotrypsin digestion. In the study of Quist et al. [36] raw peanut digests (digested with pepsin–pancreatin system) and roasted peanut digests were found out to have IC50 values of 0.007–0.19 mg/mL and 0.011–0.10 mg/mL, respectively. Marczak et al. [37] reported that pepsin digest of rapeseed had IC50 value of 0.16 mg/mL and pancreatin digest had IC50 value of 1.30 mg/mL, whereas pepsin–pancreatin digestion resulted in IC50 value of 0.70 mg/mL. Our results are in accordance with the published data related to legume digests.
Among the samples studied, green lentil was observed to be the precursor of ACE inhibitory peptides having the lowest IC50 value. The 50-min heat treatment before digestion process was found to be the most effective for obtaining the lowest IC50 value. Stomach digest of green lentils had lower IC50 value than those of intestine digest following the 50-min heat treatment. As far as our literature survey could ascertain, IC50 values for green lentils digest have not been reported.
Conclusion
The 30-min heat treatment caused a decrease in ACE inhibitory activity of legume samples studied. However, the 50-min heat treatment resulted in an increase in ACE inhibitory activity of the samples. This trend could be attributed to the possible formation of disulphide bridges in the molecule during 30-min heat treatment. The 50-min heat treatment seems to have enhanced release of bioactive peptides, which could not have resulted within 30-min time duration. This hypothesis should be elucidated in more detailed studies.
ACE inhibitory activity of the three legume samples increased following in vitro gastrointestinal digestion. Green lentils showed the highest increase in ACE inhibitory activity. The 15-min heat treatment was the most effective for increasing ACE inhibitory activity of stomach digest; however, for intestine digest of green lentils, all the three heat treatment times resulted in similar effects. It can be concluded that the 50-min heat treatment is more favourable for common beans and pinto beans to increase ACE inhibitory activity.
In this study, it was shown that common beans, pinto beans and green lentils showed high ACE inhibitory activities in vitro. This result should also be supported by in vivo studies.
References
Hartmann R, Meisel H (2007) Food-derived peptides with biological activity: from research to food applications. Curr Opin Biotech 18:163–169
Meisel H (1997) Biochemical properties of bioactive peptides derived from milk proteins: potential nutraceuticals for food and pharmaceutical applications. Livest Prod Sci 50:125–138
Korhonen H, Pihlanto A (2006) Bioactive peptides: production and functionality. Int Dairy J 16:945–960
Li G-H, Le G-W, Shi Y-H, Shrestha S (2004) Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr Res 24:469–486
Erdmann K, Cheung BWY, Schröder H (2008) The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J Nutr Biochem 19:643–654
Vermeirssen V, van der Bent A, Van Camp J, van Amerongen A, Verstraete W (2004) A quantitative in silico analysis calculates the angiotensin I converting enzyme (ACE) inhibitory activity in pea and whey protein digests. Biochimie 86:231–239
Yust MM, Pedroche J, Girόn-Calle J, Alaiz M, Millάn F, Vioque J (2003) Production of ace inhibitory peptides by digestion of chickpea legumin with alcalase. Food Chem 81:363–369
Byun H-G, Kim S-K (2001) Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process Biochem 36:1155–1162
Saiga A, Kanda K, Weı Z, Okumura T, Kaneko T, Nishimura T (2002) Hypotensive activity of muscle protein and gluten hydrolysates obtained by protease treatment. J Food Biochem 26:391–401
Theodore AE, Kristinsson HG (2007) Angiotensin converting enzyme inhibition of fish protein hydrolysates prepared from alkaline-aided channel catfish protein isolate. J Sci Food Agric 87:2353–2357
Mullally MM, Meisel H, FitzGerald RJ (1997) Angiotensin-I-converting enzyme inhibitory activities of gastric and pancreatic proteinase digests of whey proteins. Int Dairy J 7:299–303
Bütikofer U, Meyer J, Sieber R, Wechsler D (2007) Quantification of the angiotensin-converting enzyme-inhibiting tripeptides Val-Pro-Pro and Ile-Pro-Pro in hard, semi-hard and soft cheeses. Int Dairy J 17:968–975
Quan S, Tsuda H, Miyamoto T (2008) Angiotensin I-converting enzyme inhibitory peptides in skim milk fermented with Lactobacillus helveticus 130B4 from camel milk in Inner Mongolia, China. J Sci Food Agric 88:2688–2692
Miguel M, Alonso MJ, Salaices M, Aleixandre A, Lόpez-Fandiño R (2007) Antihypertensive, ACE-inhibitory and vasodilator properties of an egg white hydrolysate: effect of a simulated intestinal digestion. Food Chem 104:163–168
Suh HJ, Whang JH, Kim YS, Bae SH, Noh DO (2003) Preparation of angiotensin I converting enzyme inhibitor from corn gluten. Process Biochem 38:1239–1244
Kim JM, Whang JH, Suh HJ (2004) Enhancement of angiotensin I converting enzyme inhibitory activity and improvement of the emulsifying and foaming properties of corn gluten hydrolysate using ultrafiltration membranes. Eur Food Res Technol 218:133–138
Kim JM, Whang JH, Kim KM, Koh JH, Suh HJ (2004) Preparation of corn gluten hydrolysate with angiotensin I converting enzyme inhibitory activity and its solubility and moisture sorption. Process Biochem 39:989–994
del Castillo MD, Ferrigno A, Acampa I, Borrelli RC, Olano A, Martίnez-Rodrίguez A, Fogliano V (2007) In vitro release of angiotensin-converting enzyme inhibitors, peroxyl-radical scavengers and antibacterial compounds by enzymatic hydrolysis of glycated gluten. J Cereal Sci 45:327–334
Duranti M (2006) Grain legume proteins and nutraceutical properties. Fitoterapia 77:67–82
Adebamowo CA, Cho EY, Sampson L, Katan MB, Spiegelman D, Willett WC, Holmes MD (2005) Dietary flavonols and flavonol-rich foods intake and the risk of breast cancer. Int J Cancer 114:628–633
Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, Paffenbarger RS (2000) Vegetables fruits legumes and prostate cancer: a multiethnic case–control study. Cancer Epidemiol Biomarkers Prev 9:793–804
Wu J, Ding X (2002) Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res Int 35:367–375
Li G-H, Liu H, Shi YH, Le GW (2005) Direct spectrophotometric measurement of angiotensin I-converting enzyme inhibitory activity for screening bioactive peptides. J Pharm Biomed 37:219–224
Pedroche J, Yust MM, Girόn-Calle J, Alaiz M, Millán F, Vioque J (2002) Utilisation of chickpea protein isolates for production of peptides with Angiotensin I-converting enzyme (ACE)-inhibitory activity. J Sci Food Agric 82:960–965
Vermeirssen V, Van Camp J, Devos L, Verstraete W (2003) Release of Angiotensin I Converting Enzyme (ACE) inhibitory activity during in vitro gastrointestinal digestion: from batch experiment to semicontinuous model. J Agric Food Chem 51:5680–5687
Vermeirssen V, Augustijns P, Morel N, Van Camp J, Opsomer A, Verstraete W (2005) In vitro intestinal transport and antihypertensive activity of ACE inhibitory pea and whey digests. Int J Food Sci Nutr 56(6):415–430
Harris ELV (2001) In: Roe SD (ed) Protein purification techniques—a practical approach, 2nd edn. P. Imprenta, Oxford
Gil-Izquierdo A, Zafrilla P, Tomás-Barberán FA (2002) An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Eur Food Res Technol 214:155–159
Dunn MJ (1995) In: Harris ELV, Angal S (eds) Protein purification methods. P. Imprenta, Oxford
Mwasaru MA, Muhammad K, Bakar J, Che Man YB (1999) Effects of isolation technique and conditions on the extractability, physicochemical and functional properties of pigeonpea (Cajanus cajan) and cowpea (Vigna unguiculata) protein isolates. I. Physicochemical properties. Food Chem 67:435–443
Adebowale YA, Adeyemi IA, Oshodi AA, Niranjan K (2007) Isolation, fractionation and characterisation of proteins from Mucuna bean. Food Chem 104:287–299
Tang C-H (2008) Thermal denaturation and gelation of vicilin-rich protein isolates from three Phaseolus legumes: a comparative study. LWT-Food Sci Technol 41:1380–1388
Arcan İ, Yemenicioğlu A (2007) Antioxidant activity of protein extracts from heat-treated or thermally processed chickpeas and white beans. Food Chem 103:301–312
Harris ELV (1995) In: Harris ELV, Angal S (eds) Protein purification methods—a practical approach. P. Imprenta, Oxford
Cui C, Zhou X, Zhao M, Yang B (2009) Effect of thermal treatment on the enzymatic hydrolysis of chicken proteins. Innov Food Sci Emerg 10:37–41
Quist EE, Phillips RD, Saalia FK (2009) Angiotensin converting enzyme inhibitory activity of proteolytic digests of peanut (Arachis hypogaea L.) flour. LWT-Food Sci Technol 42:694–699
Marczak ED, Usui H, Fujita H, Yang Y, Yokoo M, Lipkowski AW, Yoshikawa M (2003) New antihypertensive peptides isolated from rapeseed. Peptides 24:791–798
Acknowledgments
This study has received financial support from the projects TOVAG- 107O797 and 2008 BIL 031 by TUBITAK and EBILTEM, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akıllıoğlu, H.G., Karakaya, S. Effects of heat treatment and in vitro digestion on the Angiotensin converting enzyme inhibitory activity of some legume species. Eur Food Res Technol 229, 915–921 (2009). https://doi.org/10.1007/s00217-009-1133-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-009-1133-x