Abstract
Drying properties of syrup prepared from Parinari curatellifolia fruit and cereal based product, zvambwa prepared from the syrup and finger millet (Eleusine coracana) meal were studied using a convective tray drier at temperatures ranging from 30 to 80 °C and air velocity of 0.72 m/s. Nine mathematical models namely Henderson and Pabis, Lewis, Midilli et al., Modified Page, Page, Two Term, Weibull, Modified Page Equation (II) and Wang and Singh were fitted to data for thin layer drying of the products using non-linear regression analysis. Thin layer drying processes for the syrup and zvambwa were best described by the Modified Page model. Effective moisture diffusivities for drying of syrup were higher than those for drying of zvambwa. The activation energies for drying of syrup and zvambwa were 21.0 ± 2.0 kJ/mol and19.0 ± 2.0 kJ/mol respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parinari curatellifolia fruit is obtained from an indigenous tree found mainly in Southern Africa (Kalaba et al. 2009). In Zimbabwe, the fruit ripens during the dry season from the month of July to November in different parts of the country. Parinari curatellifolia fruit has been found to contain soluble carbohydrates, fibre, protein and ash (Benhura et al. 2012). It is a source of nutrients for consumers and is eaten fresh or may be processed into products, for example beverages and porridge (Saka et al. 2004). Processing of fresh fruit is necessary, as they can easily perish, due to lack of cold storage facilities in rural areas (Kalaba et al. 2009).
Communities in Mrewa and Mutoko areas of Mashonaland East in Zimbabwe prepare products called zvambwa, using the syrup of Parinari curatellifolia fruit and finger millet (Eleusine coracana) meal. Zvambwa are biscuit like products moulded from a gel that forms on heating a mixture of the syrup and finger millet meal. The products have a sweet taste and are used as food by the local residents. The syrup and zvambwa have high moisture content which may make them perishable. It is important to apply preservation measures in order to lengthen the shelf stability of the products and this may be achieved by drying them.
Drying is one of the ancient technologies used for preservation of food known to man (Sobukola et al. 2007; Koua et al. 2013). The basic objective of drying food products is removal of water from solid material in order to prevent microbial spoilage (Abano et al. 2011). Several drying methods are used to remove moisture from a variety of food products including fruits and vegetables (Doymaz and Ismail 2011). Sun drying is the most frequently used method to preserve agricultural products for example Uryani plum (Sacilik et al. 2006) and yam (Koua et al. 2013). It is an affordable method of preservation because it uses sunlight, which is a natural source of heat (Doymaz & Ismail 2011). The major limitations of sun drying are that the process takes long and products are exposed to unstable weather conditions, dust, rodents, birds and insects (Doymaz & Ismail 2011; Sacilik et al. 2006; Goyal et al. 2007).
The most widely used commercial drying plants for industrial agrifood byproducts are conventional hot air driers (Abano et al. 2011). Here drying of moist materials is a process involving simultaneous, coupled heat and mass transfer phenomena, which occur inside the material being, dried (Sobukola et al. 2007). Most food materials are dried by thin layer drying (Koua et al. 2013). Thin layer drying (TLD) means to dry one layer of sample particle or slice (Akpinar 2006) and it forms the basis on which drying properties of foods are studied since each food has unique characteristics (Mwithiga & Olwal 2005).
Data from drying kinetics of biological materials is useful in design, optimization and control of drying processes (Sacilik et al. 2006). The drying properties of orange slices (Rafiee et al. 2010), Vitex Donian syrup (Abu & Arogba 2001) and cashew apple (Azoubel et al. 2009) among others have been investigated. However there are no published results on the drying behaviour of syrup of Parinari curatellifolia fruit and zvambwa. Hence the objectives in the current study were to investigate the rates of drying, identify the best mathematical models and determine the effective moisture diffusivities and activation energies for drying of syrup of Parinari curatellifolia fruit and zvambwa. Results of the research will be useful when advising the communities on the best methods for drying the syrup and zvambwa. Drying of the products which preserve them helps in solving food security and nutrition problems in Southern Africa and other regions where Parinari curatellifolia fruit trees and finger millet grow.
Materials and methods
Collection of fruit
Parinari curatellifolia fruit was collected from Mahusekwa, a rural area which is about 50 km South East of Harare in Zimbabwe. Both unripe and ripe fruit were collected in separate polythene bags for convenience of processing, transported to the laboratory and frozen stored at −20 °C. Unripe and ripe fruit of Parinari curatellifolia are illustrated in Figs. 1 (a) and (b) below:
Preparation of syrup
The frozen fruit were left at room temperature to thaw overnight before processing. They were thoroughly washed using running tap water, placed in ten litre buckets and weighed. The unripe and ripe fruit were placed in separate containers. Washed unripe fruit were placed into a washed wooden mortar, filling it to about half of its volume and gently pound with the aid of a wooden pestle to remove pulp and skins from the stones. Initially, vigorous pounding was avoided as some of the fruit escaped from the mortar on colliding with the pestle. Pounding was continued until all skins, pulp and stones separated. Ripe fruit were added to fill the mortar followed by gentle pounding. Pounding was done more vigorously as unripe and ripe fruit mixed. The process was continued until the slurry texture of the ripe fruit pulp was significantly reduced. This state was achieved when unripe and ripe fruit pulps were thoroughly mixed. Increase in proportion of unripe fruit to ripe fruit reduced slurry texture of pulp obtained. A low proportion of unripe fruit to ripe fruit increased slurry texture of pulp making it difficult to squeeze liquid extract containing sugars from such pulp. Five hundred millilitres (500 ml) of water were added to the pulp which was mixed using the pestle and transferred to a clean 50 litre plastic container. The pulping process was repeated with fresh portions of ripe and unripe fruit transferring pulped fruit to the 50 litre container. The process was continued until 17.6 kg of ripe fruit and 23.3 kg of unripe fruit were pulped and the pulp transferred to the 50 litre container. Up to 17.6 litres of water were added to the bulk pulped fruit to allow for extraction of sugars from the pulp and water mixture. The mixture was left to settle for two hours to allow for extraction of the sugars. A liquid extract was squeezed from the mixture using a cheese cloth sterilized in boiling water and collected into a clean handling dish. The work was done while putting on plastic gloves to minimize direct handling of fruit and equipment that was used. On filtration of the pulp and water mixture, 16 litres of extract was obtained. The colour of liquid extract squeezed from the fruit was light yellow. Squeezed pulp residues produced on filtration of the mixture weighed 40.6 kg and were placed into a separate container for use as livestock feed or disposal.
The collected liquid extract was placed into eight litre stainless steel pots and heated to boiling temperature of 91 °C for 11 hours and five minutes on an electric hot plate until the liquid turned into a brownish and viscous syrup with the brown colour darkening further as more water was evaporated. On further heating, the liquid became sweeter as more water was evaporated and a distinct odour of the syrup was smelled. Heating of the extract was continued until the volume of syrup was reduced to 1.5litres. The syrup was transferred to glass bottles that had been cleaned and sterilized in an autoclave. The first batch of fruit processed was designated as F1. The process was repeated with new fruit resulting in batches F2 and F3. Measurements made during the processes for the fruit are recorded in table 1.
The syrup from the three batches was thoroughly mixed to form a composite sample used for preparation of food products. The syrup was stored in a refrigerator at 4 °C until required for use.
Preparation of zvambwa
Four hundred millilitres (400 ml) of Parinari curatellifolia fruit syrup were placed into a 1.5 l stainless steel pot and heated to boiling temperature using a hot plate. To a 1 litre plastic jug containing 200 ml of water, 48.15 g of finger millet meal was added while stirring with a cooking stick to mix the contents. The mixture of water and finger millet meal was added to the boiling syrup of Parinari curatellifolia fruit while stirring to homogenize the contents. Heating of the mixture was continued at a temperature of 95 °C. After 14 minutes, 70.94 g of finger millet meal was stirred into the mixture. The mixture thickened into a gel, as heating continued. More finger millet meal (116.86 g) was added while stirring to allow the thickening gel to homogenize. When the gel had thickened, the hot plate was set to simmering temperature which was maintained for 21 minutes.
The pot containing the gel was then removed from the heat source and the gel transferred to a wooden plate whose surface had a thin layer of finger millet meal. The finger millet meal prevents the thickened gel from sticking to the surfaces of the plate. The gel was allowed to cool to ambient temperature which was 25 °C and weighed in portions ranging from 120 to 188 g to make a total mass of 615.64 g. The procedure was repeated four more times to produce 3,000 g of gel. The mass of gel was transferred to a polyethene board on which finger millet meal had been spread to form a thin layer. More finger millet meal was added to the gel while mixing and rolling on the board. The gel was levelled to a thin sheet using a wooden rolling pin. More finger millet meal was added to the board to prevent the gel from sticking to the surface of the board and rolling pin. When a sheet of thickness of 3 mm was formed, biscuit like units were moulded using a plastic cup of 57 mm diameter. The biscuit like units called zvambwa were placed on wooden plates on which finger millet meal had been spread to prevent the products from sticking to the surfaces of the plates. The surfaces of zvambwa units were smeared with spared syrup of Parinari curatellifolia fruit using a pastry food brush. Samples of zvambwa are illustrated in Fig. 2 below:
The syrup preserves the product, enhances the appearance and flavour and improves consumer perception of the product. Units of zvambwa moulded for the drying determinations were transferred to polythene lunch boxes each of length, width and height of 25, 16 and 12 cm respectively and refrigerated at 4 °C until required for analysis.
Instrumentation
The drying experiments on the syrup and zvambwa were carried out in a convective tray drier (Armfield Technical Education Company Limited, England). The dimensions of the drier are 193 cm × 28 cm × 28 cm and comprises of a drying chamber, electrical heating element, fan and a temperature regulator.
Temperature of the drying chamber was measured using a digital thermocouple (Comak Electronic, Sussex, UK). Air velocity in the dryer was measured with the aid of an anemometer (Airflow LCA 6,000, Airflow Developments, UK). Relative humidity of the drying chamber was determined using a dry and wet bulb thermometer.
The thickness of syrup layer was determined using a vernier calipers that was vertically dipped into the syrup layer until its lower end touched the bottom of the porcelain plate containing the syrup (Scala Alinox, West Germany). The part of the vernier calipers immersed in the syrup represented the thickness of the syrup layer.
The thickness and diameter of each unit or disc of zvambwa were measured using the vernier calipers.
Masses of the samples recorded during the drying process were measured using a semi-analytical balance (Kerro BL10002A Precision Electronic Balance N. 10123002 KJS Series, Taiwan; Range: 0.01–1,000 g). Time of drying was measured with the aid of a stop watch (Hanhart).
Moisture content of syrup of Parinari curatellifolia fruit and zvambwa
The initial moisture contents of syrup of Parinari curatellifolia fruit and zvambwa were determined by oven drying method (AOAC 2000).
Drying procedure
Experiments were performed at temperatures of 30, 40, 50, 60, 70 and 80 °C. At each temperature the determinations were made in triplicates. One hundred grams of sample were weighed into each of three pre-weighed porcelain plates which were placed into trays in a convective dryer with air velocity set at 0.72 m/s. A sample size of 100 g was used for determination of drying properties of tomatoe slices (Abano et al. 2011). To measure the weight of samples during drying, plates containing samples were withdrawn from the drying unit, weighed on a semi-analytical top loading balance, and replaced in the drying unit. The balance was positioned close to the drying unit for convenience of the weighing process. The dryer was set to a temperature of 30 °C and the conditions were allowed to equilibrate for 30 minutes before introduction of samples and commencement of measurements. Samples were weighed at 15 minutes intervals until constant weight was achieved. Constant weight was considered to be achieved when consecutive weights per sample constantly differed by 0.15–0.2 g. The measurements were repeated with fresh samples at temperatures of 40 up to 80 °C.
Mathematical modeling of drying processes
Drying curves were constructed using data obtained at various temperatures. The moisture content (M) was calculated on a dry weight basis using the formular (Koua et al. 2013).
Where W is the weight of sample and W d is the weight of dry matter in the syrup or zvambwa.
To find suitable mathematical models, moisture content data at different drying temperatures of the samples were converted to moisture ratio (MR) given by the following equation (Azadbakht et al. 2012).
Where M t , M 0 and M e , are moisture content at time of drying, initial moisture content and equilibrium moisture content respectively. Since the values of M e are negligible compared to M t and M 0 , equation (2) may be simplified to equation (3) as given by Berruti et al. (2009).
The drying rates of thin layers of syrup of Parinari curatellifolia fruit and zvambwa were calculated using equation 4 outlined below
Where, Mt is moisture content (g water per g dry matter) at time t, Mt + dt is the moisture content at time t + dt and dt is the change in drying time.
Nine thin layer drying models recorded in Table 2 were fitted to the experimental data of moisture ratio versus drying time. The semi-theoretical models are used to describe the kinetics of drying of foods (Koua et al. 2013).
The correlation coefficient R 2, the root mean square error (RMSE) and reduced chi square (χ 2) were the criteria used to assess the fitness to the models. The highest R 2, lowest χ 2and RMSE were used to determine the goodness of fit (Abano et al. 2011; Koua et al. 2013). The values of χ 2, and RMSE can be calculated as follows:
Where MR exp,i is the ith experimental moisture ratio, MR pred,i is the ith predicted moisture ratio, N and n are the numbers of observations and constants respectively. The best models for describing the drying properties of Parinari curatellifolia fruit syrup and zvambwa were selected on the basis of higher values of R 2 and lower values of χ 2 and RMSE.
Moisture diffusivity and activation energy
Drying processes in agricultural food products have been described using Fick’s second law of diffusion . Fick’s law is mathematically expressed as follows:
Where D eff is the effective moisture diffusivity (m2/s) and t is the drying time (s). Effective moisture diffusivity is an important property used in describing drying processes in foods (Koua et al. 2013). Equation (7) was solved by assuming a one dimensional movement of moisture, no change in volume, constant diffusivity, uniform distribution of initial water content and negligible external resistance. Solution of equation (7) resulted in equation (8), outlined as follows:
For long drying times, equation (8) may be simplified to a form which leaves only the first term of the expansion series as follows:
Equation (9) may be rewritten in logarithmic form as follows:
Where L is half the thickness of layer dried.
Diffusivities were determined by plotting In (MR) against drying time, t giving a straight line with slope expressed as follows:
The dependence of diffusivity, D eff on temperature may be described by the Arrhenius equation as outlined below:
Where E a is the activation energy, D o is Arrhenius factor for the drying process, T is the absolute temperature in degree Kelvins and R is the molar gas constant. Equation (12) was converted to linear form by taking natural logarithms from both sides to yield equation (13) as shown below:
By plotting In (D eff ) against 1/T, a straight line was obtained with slope, −E a /R and y intercept of In (D o ). The activation energy, E a and Arrhenius factor, D o were obtained from slope and y intercept respectively.
Statistical analysis
Drying curves, natural logarithms versus time and Arrhenius plots were constructed using Graph Pad Prism Version 5 software package. Application of mathematical models to data for drying of syrup and zvambwa was done using Data Fit Version 9. Correlation of predicted moisture ratio to experimental moisture ratio was done using Minitab Version16.
Results and discussion
Comparison of drying characteristics between syrup of Parinari curatellifolia fruit and zvambwa
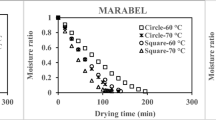
The total drying times of the syrup decreased from 480 to 315 minutes at temperatures that ranged from 30 to 80 °C while drying times for zvambwa decreased from 375 to 255 minutes at the same temperature range (Figs. 3 and 4). The observed differences in the drying times for the syrup and zvambwa may be attributed to differences in the initial moisture content of the two products and differences in molecular arrangements in the matrices of the products. The initial moisture contents of the syrup and zvambwa measured on a dry weight basis were 25.7 ± 0.6 % and 19.8 ± 3.0 % respectively. As shown in Figs. 3 and 4, the moisture ratios of the syrup and zvambwa dried at each temperature decreased with time. The findings are consistent with results obtained for thin layer drying of pumpkin slices (Doymaz 2007), tomato slices (Abano et al. 2011) and Uryani plum (Sacilik et al. 2006). As the temperature increased the drying time decreased. The increasing temperature increased the energy of water molecules allowing for their rapid escape from the matrix of the products. The decrease in the moisture ratio that resulted from loss of moisture from the syrup of Parinari curatellifolia fruit and zvambwa indicates that the internal mass transfer is governed by diffusion similar to the case for thin layer drying in yams (Koua et al. 2013; Falade et al. 2007). As illustrated in Figs. 5 and 6, the drying rates of the syrup and zvambwa decreased with time, until they were almost constant. At all the temperatures considered the drying rates were falling. The drying rates for the products increased with increase in drying temperature with the highest drying rates observed at 80 °C.
Mathematical modeling of drying curves
The Modified Page and Page models had the highest values of R 2 that range from 0.998836 to 0.999573 at the different temperatures and the lowest reduced chi-square and RMSE values (Table 3). At 50 °C, the Modified Page model had lower reduced chi-square and RMSE values than the Page model. At 30 °C, the Modified Page model had a lower RMSE value than the Page model.
The Henderson and Pabis, Lewis, Midilli et al. and Two term models did not fit the data on non-linear regression analysis as they gave R 2 values of zero. The four models yielding R 2 values of zero do not provide a quantitative description of drying of the syrup. The Modified Page model is therefore the best model describing the thin layer drying of the syrup of Parinari curatellifolia fruit. Similarly as for syrup of Parinari curatellifolia fruit, the Modified Page model produced the best non-linear regression results from data for drying of yam slices (Koua et al. 2013).
For zvambwa, the Modified Page and Page models had the highest R 2 values ranging from 0.999461 to 0.999657 (Table 4). Values of R 2 for the Modified Page Equation (II) ranged from 0.999431 to 0.999640. The Wang and Singh model’s R 2 values ranged from 0.968458 to 0.999039 while values for Weibull model ranged from 0.888863 to 0.971997.
The Modified Page model had lower reduced chi-square and RMSE values than the Page model at 70 °C and a lower value of reduced chi-square than the Page model at 60 °C. Hence, the Modified Page model which consistently had the highest R 2 values and the lowest RMSE and χ 2 values provides the best description of the thin layer drying of zvambwa. The similarities in the models describing thin layer drying of the syrup and zvambwa may imply that the mechanism of loss of moisture is the same in the two products.
Comparison of experimental moisture ratio with predicted moisture ratio
Of the models used, Modified Page illustrated the best fit or correlation for experimental and predicted moisture ratios obtained on drying of syrup of Parinari curatellifolia fruit (Fig. 7). Similar results were obtained on correlation of moisture ratios obtained from thin layer drying of yam slices (Koua et al. 2013). For thin layer drying of zvambwa, the Modified Page model also gave the best description (Fig. 8). The results for drying of zvambwa are evident from the good correlation between experimental and predicted moisture ratios for Modified Page as compared with the other models where the correlation was lower. As illustrated in Fig. 7 and 8, MR values are banded along a straight line which shows that the Modified Page model is appropriate for describing drying characteristics of the syrup and zvambwa.
Moisture diffusivity and activation energy
From equation (10), In (MR) was plotted against t for data obtained for thin layer drying of syrup and zvambwa at each temperature. The diffusivities D eff at the different temperatures were calculated from equation (11) and recorded in tables 5 and 6.
The diffusivities of the syrup and zvambwa increased with increase in temperature (Tables 5 and 6). The increases were expected as there was increase in energy imparted to evaporating water molecules as temperature increased. The resulting increase in vapour pressure further led to high diffusivities. Diffusivity values that ranged from 5.16 × 10−11 to 1.75 × 10−10for drying of syrup and 1.76 × 10−11to 5.30 × 10−11for zvambwa were consistent with published range of 10−12 to 10−8 m2/s applicable to drying of food materials (Abano et al. 2011). The observed increase in diffusivities with increase in temperature was similar to results obtained for drying of yam (Koua et al. 2013), tomato slices (Abano et al. 2011), melon slices (Azadbakht et al. 2012) and Uryani plum (Sacilik et al. 2006).
Values of In (D eff ) and 1/T were calculated from diffusivities and absolute temperatures of the syrup respectively. A linear graph with a negative slope as illustrated in Fig. 9 was obtained on plotting In (D eff ) against 1/T. From the slope of the graph as given in equation (13), the activation energy of the drying process of the syrup of Parinari curatellifolia fruit was 21.0 ± 2.0 kJ/mol.
The activation energy obtained for thin layer drying of the syrup was comparable to 21.32 kJ/mol and 22.8 kJ/mol applicable to Uryani plum (Sacilik et al. 2006) and tomatoe slices (Abano et al. 2011) respectively. The value of the Arrhenius factor D 0 obtained from the y intercept of equation (13) was 2.2 × 10−7 m2/s. Similarly as for the syrup, the activation energy and Arrhenius factor for thin layer drying of zvambwa were deduced from the slope and y intercept of the graph in Fig. 9. The activation energy for drying of zvambwa was 19.0 ± 2.0 kJ/mol. The Arrhenius factor for the product was D 0 = 3.8 × 10−8 m2/s. The activation energy and Arrhenius factor for the syrup were higher than those obtained for zvambwa. Modification of the syrup by finger millet meal used in preparation of zvambwa resulted in a decrease in activation energy and Arrhenius factor or absolute diffusivity. This result is clear evidence of lower energy required to remove water from zvambwa in comparison with energy needed to dry the syrup.
Conclusion
The moisture ratio of syrup and zvambwa at each temperature decreased exponentially with time. The total drying time for each product decreased with increase in drying temperature. Drying rates for the syrup and zvambwa increased with increase in drying temperature. Thin layer drying processes for the syrup and zvambwa were best described by the Modified Page model. Diffusivities for thin layer drying of the syrup were higher than values obtained for drying of zvambwa. Diffusivities for both syrup and zvambwa increased with increase in drying temperature. The activation energy for drying of syrup (21.0 ± 2.0 kJ/mol) was higher than that for drying of zvambwa (19.0 ± 2.0 kJ/mol). Parinari curatellifolia fruit syrup and zvambwa may be dried at temperatures ranging between 30 and 80 °C where electricity and ovens are available. In areas where electricity is not available, zvambwa may be sun dried. Over-drying should be avoided as products become too hard to chew when they excessively lose moisture.
References
Abano EE, Ma H, Qu W (2011) Influence of air temperature on the drying kinetics and quality of tomato slices. J Food Proces Technol 2(5):123
Abu JD, Arogba SS (2001) High temperature drying characteristics and moisture adsorption isotherms of black plum (Vitex Doniana) syrup and syrup-starch. Int J Food Prop 4(2):285–295
Akpinar EK (2006) Determination of suitable thin layer drying curve model for some vegetables and fruits. J Food Eng 73:75–84
AOAC (2000) In W. Horwitz (Edition), Official methods of analysis of the association of official analytical chemists, 17edth edn. AOAC International, Maryland, USA
Azadbakht M, Darvishi H, Rezaeias A, Asghari A (2012) Thin layer drying characteristics and modeling of melon slices (Cucumismelo). J Agric Technol 8(6):1867–1880
Azoubel PM, El-Aouar AA, Tanon RV, Kurozawa LE, Antonio GC, Murr FEX, Park KJ (2009) Effect of osmotic dehydration on the drying kinetics and quality of cashew apple. Int J Food Sci Technol 44:980–986
Benhura C, Benhura MAN, Muchuweti M, Nyagura SF, Gombiro PE (2012) Proximate analysis of Parinari curatellifolia fruit pulp of fruit from parts of Harare and a rural area in Zimbabwe. Pak J Nutr 11(7):541–544
Berruti FM, Klaas M, Briens C, Berruti F (2009) Model for convective drying of carrots for pyrolysis. J Food Eng 92:196–201
Corzo O, BrachoN PA, Väsquez A (2008) Weibull distribution for modelling air drying of coroba slices. LWT Food Sci Technol 41:2023–2028
Doymaz I (2007) The kinetics of forced convective air-drying of pumpkin slices. J Food Eng 79:243–248
Doymaz I, Ismail O (2011) Drying characteristics of sweet cherry. Food and Biopr Proccess 89:31–38
Falade KO, Olurin TO, Ike EA, Aworh OC (2007) Effect of pre-treatment and temperature on air-drying of Dioscorea alata and Dioscorea rotundata slices. J Food Eng 80:1002–1010
Goyal RK, Kingsly ARP, Manikantan MR, Ilyas SM (2007) Mathematical modelling of thin layer drying kinetics of plum in a tunnel dryer. J Food Eng 79:176–180
Hu X, Wang Z, Sun J, Chen F, Liao X (2007) Mathematical modelling on thin layer microwave drying of apple pomace with and without hot air pre-drying. J Food Eng 80:536–544
Kalaba FK, Chirwa PW, Prozesky H (2009) The contribution of indigenous fruit trees in sustaining rural livehoods and concentration of natural resources. J Hort and Fores 1(1):1–6
Koua BK, Koff PME, Fassinou FW, Andoh HY, Gbaha P, Touré S (2013) Evaluation of something layer drying models and effective moisture diffusivity of yam (Dioscorea rotundata) slices. Pak J Food Sci 23(1):1–9
Midilli A, Kucuk H, Yapar Z (2002) A new model for single layer drying. Dry Technol 20:1503–1513
Mwithiga G, Olwal JO (2005) The drying kinetics of kale (Brassica oleracea) in a convective hot air dryer. J Food Eng 71:373–378
Rafiee S, Sharifi M, Keyhani A, Omid M, Jafari A, Mohtasebi SS, Mobli H (2010) Modeling effective moisture diffusivity of orange slice (Thompson Cv.). Int J Food Prop 13(1):32–40
Sacilik K, Elicin AK, Unal G (2006) Drying kinetics of U ryani plum in a convective hot-air dryer. J Food Eng 76:362–368
Saka JDK, Swai R, Mkonda A, Schomburg A, Kwesiga F, Akinnifesi FK (2004) Processing and utilisation of indigenous fruits of the miombo in southern Africa. In: Rao MR, Kwesiga FR (eds) Agroforestry impacts on livelihoods in southern Africa: Putting research into practice. Proceedings of the regional agroforestry conference held in Warmbaths, South Africa 20–24 May, 2002. World Agroforestry centre (ICRAF), Nairobi, Kenya, pp 343–352
Sobukola OP, Dairo OU, Sanni LO, Odunewu AV, Fafiolu BO (2007) Thin layer drying process of some leafy vegetables under open sun. Food Sci and Technol Int 13:35–40
Acknowledgments
The authors acknowledge the support of the University of Zimbabwe for providing funding for the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benhura, C., Kugara, J., Muchuweti, M. et al. Drying kinetics of syrup of Parinari curatellifolia fruit and cereal based product, zvambwa . J Food Sci Technol 52, 4965–4974 (2015). https://doi.org/10.1007/s13197-014-1616-z
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1616-z