Abstract
In this study, whole and peeled persimmons were dried in the ranges of 55–75 °C of drying temperature in a hot air dryer. The effect of drying temperature and pretreatments on the drying characteristics was determined. Immersing in a solution of 20 % sucrose resulted in an increase in the drying rate of persimmons. A non-linear regression procedure was used to fit five thin-layer drying models available in the literature to the experimental moisture loss data. The Page and Modified Page models have shown a better fit to the experimental drying data as compared to other models. The effective diffusivity was determined to be 9.237 × 10−10–10.395 × 10−10 m2 s−1 for the blanched persimmons and 7.755 × 10−10–9.631 × 10−10 m2 s−1 for immersed sucrose solution persimmons. The activation energies for diffusion were calculated to be 56.09 kJ mol−1 (for blanched) and 10.28 kJ mol−1 (for immersed sucrose solution).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The persimmon (Diospyros kaki L.) is an edible fruit native to East Asia, most likely China, but Italy, Brazil, Israel, Spain, United States, Australia, and New Zealand have significant plantings of this crop. When fully mature the fruit has a tough, glossy, orange-red skin and a yellow-orange flesh, which is very sweet and juicy [1]. A subtropical fruit persimmon, which is rich in bioactive compounds, became a subject for investigation [2, 3]. The bioactivity of persimmon is attributed to its water soluble dietary fibers, minerals, trace elements and phenolics, which determine total antioxidant activity of the fruits [4]. However, fresh persimmon is not available all year around. Therefore, the contents of some important bioactive compounds and the total radical scavenging activities (TRSAs) in fresh and properly dried persimmons were determined and compared [3].
Osmotic dehydration is a method for the partial removal of water from plant tissues by immersion in a hypertonic (osmotic) solution of soluble solute. Osmotic dehydration, which is effective even at ambient temperature and saves the color, flavor and texture of food from heat, is used as a pretreatment to improve the nutritional, sensorial and functional properties of food [5]. The driving force for the diffusion of water from the tissue into the solution is provided by the higher osmotic pressure of the hypertonic solution [6]. Sugar, glucose, fructose, corn syrup and sodium chloride are the common osmotic agents. The influence of the main process variables, such as concentration and composition of osmotic solution, temperature, immersion time, pretreatments, agitation, nature of food and its geometry, and solution to sample ratio on the mass transfer mechanism has been studied extensively [5, 7].
Simultaneous heat and mass transfer takes place during drying process. Convection drying is considered a simultaneous heat and mass transfer process where water is transferred by diffusion from inside the food material to the air–food interface and from the interface to the air stream by convection. Heat is transferred by convection from air to the air–food interface and by conduction to the interior of food. Mathematical models have proved to be very useful design and analysis of these transfer processes during drying. All parameters used by simulation models are directly related to the drying conditions. Furthermore the drying conditions, as directly related to the drying time, are affecting the energy [8, 9].
Thin layer drying equations are used to estimate drying times of several products and also to generalize drying curves [10]. Several investigators have proposed numerous mathematical models for thin layer drying of many fruits. For example, apricot [11], plum [12], grape [13], mulberry [14], apple [15], strawberry [16]. But there are a few studies have been made on thin-layer drying of the persimmon [17, 18].
The objectives of this study are to define drying characteristics and to develop a mathematical model for the thin-layer drying of pretreated persimmons.
2 Materials and methods
2.1 Sample preparation
Persimmons (Diospyros kaki L.) used in this study were purchased from the producers existed at Honaz/DENİZLİ from mid-September. Approximately uniform size persimmons (average diameter and weight 5.50 cm and 100.07 g, respectively) were selected and used. The collected samples were stored at 4 ± 0.5 °C before they were used in experiments. Moisture content of persimmons were determined by the oven drying method at 70 °C in a vacuum oven (Model EV 018, Nuve Laboratory and Sterilization Tech.) for 24 h [19]. The initial moisture content of persimmon samples were 77 ± 1.5 %.
2.2 Drying process
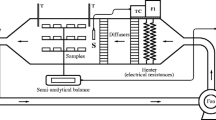
Drying experiments were performed in a cabinet laboratory type dryer existed in the Food Engineering Department of Pamukkale University, Turkey. The dryer consists of a centrifugal fan to supply the air flow, an electric heater, and an electronic proportional controller (ENDA, EUC442, Istanbul, Turkey). The technical properties of cabinet dryer were given in Table 1.
Prior to drying, persimmon samples were peeled with a sharp peeler and divided into two lots before use. One of the lots was blanched by deeping in hot water at 70 °C for 15 min. These samples were then immediately cooled down in tap water at room temperature to avoid excess heat, and placed on tissue paper to absorb the excess surface water prior to drying. The other lot was immersed in 20 % sucrose solution at 20 °C for 15 min.
The dryer was turned on about 1 h before the drying process to reach steady-state conditions before each drying run. After the steady state conditions for the operation temperatures, both lots (each of which is about 800 g) were hanged by the help of hooks placed on the ceiling of the dryer and dried simultaneously. The drying experiments were performed at 55, 65, and 75 °C air temperatures. The air velocity was kept constant at 0.2 m s−1 in all drying experiments. The relative humidity of ambient air was controlled between 19 and 21 %. During drying, the samples were taken at intervals and weighed, before being returned to the dryer.
At the end of each drying experiment, the final moisture content of the sample was determined. Samples were dried to approximately 35 % final moisture content. Moisture contents were given in wet-basis. The amount of dry matter was calculated by using the mean final moisture content and weight of the dried persimmons. The moisture contents were also expressed in dry basis.
All the experiments were replicated three times at each air temperature and the average values were used.
2.3 Mathematical modelling of drying curves
Mathematical models that describe drying mechanisms of grain and food provide the required temperature and moisture information [20, 21]. The best model describing the drying behaviour of persimmon is selected by testing the five commonly used thin layer drying models given in Table 2. However, the moisture ratio (MR) was simplified to M/M0 instead of the (M − Me)/(M0 − Me) [22, 23].
The coefficient of determination (R2), root mean square error (RMSE) and reduced Chi square (χ2) obtained for these equations were used to compare the relative goodness of fit of experimental data. These parameters can be calculated as follows
where MRexp,i is the ith experimentally observed moisture ratio, MRpre,i is the ith predicted moisture ratio, N is the number of observation and z is the number constants [24].
The best model describing the drying behaviour of persimmons were chosen as the one with the highest coefficient of determination and the least mean relative percent error and the least root mean square error. In addition, reduced Chi square was used to determine the goodness of the fit. The lower values of reduced Chi square, the better goodness of the fit.
2.4 Calculation of effective moisture diffusivity and activation energy
The experimental drying data for the determination of moisture diffusivity was interpreted by Fick’s Second law of diffusion [25]. The solution of Fick’s second law in spherical coordinates is given below (Eq. 3), with the assumption of moisture migration being by diffusion, negligible shrinkage, constant diffusion coefficients and temperature [26, 27]:
The effective moisture diffusivity is calculated using the method of slopes. Diffusion coefficients are typically determined by plotting experimental drying data in terms of ln (MR) versus time [as given in Eq. (4)] [28]. From Eq. (4), a plot of ln (MR) versus time gives a straight line with a slope of
The activation energy (Ea) is calculated by using an Arrhenius type equation [27];
where, D 0 is the pre-exponential factor of the Arrhenius equation (m2 s−1), E a is the activation energy (kJ mol−1), R is the universal gas constant (kJ mol−1 K−1), and T is the absolute air temperature (K). The activation energy is determined from the slope of the Arrhenius plot, ln (D eff) versus T −1.
3 Results and discussion
3.1 Effect of drying temperature on drying time
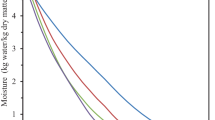
The effect of drying temperature on the drying time of blanched in water (B) and immersed in 20 % sucrose solution (I) persimmons is shown in Fig. 1. It is clear that the moisture content decreases continuously with drying time. The drying times of B samples were 34, 31 and 27 h at 55, 65 and 75 °C, respectively. However, drying times of I samples were 29, 25 and 22 h at the same temperatures, respectively. In conclusion, experimental results showed that the drying air temperature has a significant effect on the evolution of moisture content. Drying rate increased with increasing drying temperatures. This can be attributed to high rate of evaporation from the surface of the sample at higher drying temperatures which leads to higher mass transfer rate in the solid. This is in agreement with results of the study on grape [29], apple [30] and strawberry [31].
3.2 Effect of immersing in sucrose solution on drying time
Moisture content versus drying time is presented in Fig. 1, which shows the effects of immersing in sucrose solution on drying time of whole persimmons. Both moisture content curves for B/I samples had similar moisture content values at starting time of drying. It was clear from Fig. 1 that the whole persimmon samples I before drying were found to have a shorter drying time compared to B ones. However, it can be noted that this sucrose solution application is more pronounced in increasing the drying rate during the early period of drying. Due to the high velocity of penetration of the sugar molecules, a solid enrichment instead of dehydration occurred as the main effect of the process. However, as the sucrose solution concentration increased, the osmotic pressure in the persimmon also increased. According to literature data [32, 33] the water loss and sugar gain increase with sugar solute concentration and immersion time.
3.3 Fitting of the drying curves
The moisture content data from the drying experiment were converted into the moisture ratio (MR) and then fitted to the selected thin-layer drying models listed in Table 2. The statistical results of the different models, including the comparison criteria used to evaluate goodness of the fit, the values of the coefficient of determination (R2), root mean square error (RMSE), and the reduced Chi square (χ2) are presented in Table 3. Based on the criteria of the highest R2 and the lowest RMSE, and χ2, the best model describing the thin-layer drying characteristics of whole persimmons was selected. For all experiments, the R2, RMSE, and χ2 values for models changed between 0.7151 and 0.9988, 0.001008 and 0.482837, 0.000001 and 0.254987, respectively. From Table 3, the highest R2 values and the lowest values of RMSE, and χ2 values were obtained from the Page and Modified Page model. Figures 2 and 3 show the moisture ratios determined by the Page and the Modified Page equations for drying experiments at 65 °C. The Page and Modified Page models have also been suggested by others to describe hot-air drying of tomatoes [25], grapes [29], sweet potato [34] and red bell-pepper [35].
3.4 Effective diffusivity
The values of effective diffusivity (Deff) at different drying temperatures obtained by using Eq. (4) and the estimated values are presented in Table 4. The values of Deff increased progressively as the drying air temperature increased. This was expected. These results closely agreed with those for pear slices [36], chillies [37], banana slices [38]. The values for Deff obtained from this study lie within the general range 10−12 × 10−8 m2 s−1 for drying of food materials [39, 40] and comparable with other reported values of 0.48–1.19 × 10−10 m2 s−1 for sun drying of treated grapes [41]. Doymaz [12] found that Deff was to 4.69 × 10−10 m2 s−1 for treated and 4.26 × 10−10 m2 s−1 for untreated mulberry samples during sun drying [42].
3.5 Activation energy
The temperature dependence of Deff can be described by Arrhenius-type of relationship as given in Eq. (6). The activation energy (Ea) was calculated from the slope of the plot on ln (Deff) versus 1/T in Fig. 4. The plot was found to be a straight line in the range of temperatures investigated, indicating Arrhenius dependence. The activation energies for diffusion calculated from the slope of the straight line of Fig. 4 was 10.28 kJ mol−1 for immersed in 20 % sucrose solution persimmon, 56.09 kJ mol−1 for blanched persimmon. For example, blanched persimmons activation values were higher, but immersed in 20 % sucrose solution persimmon activation values were lower than the (30.64–43.26 kJ mol−1) for persimmon slices drying [18] and apple slices drying (19.96–22.62 kJ mol−1) [43].
4 Conclusions
The effect of pretreatment solution on drying of persimmon using hot air was studied. Whole persimmons treated with 20 % (w/w) sucrose solution and blanched in hot water at 70 °C. Persimmons, which were immersed in sucrose solution, resulted in shorter drying times (thus higher drying rates) than blanched persimmons because of osmotic dehydration. Osmotic dehydration, which is effective even at ambient temperature and saves the color, flavor and texture of food from heat, is used as a pretreatment to improve the nutritional, sensorial and functional properties of food. Sucrose solution was the most significant factors affecting water loss and solid gain during the drying of whole persimmons at 55, 65, and 75 °C air temperatures.
The effective diffusivity ranged from 7.755 to 9.631 × 10−10 m2 s−1 for the immersed persimmon, and from 9.237 to 10.395 × 10−10 m2 s−1 for blanched persimmons in the temperature range of 55–75 °C. When drying conditions were observed the effective diffusivity was more affected by pretreatments. It is the meaning that pretreatments had more effective than drying temperatures on effective diffusivity. The activation energies for moisture diffusion varied from 10.28 kJ mol−1 (for immersed) to 56.09 kJ mol−1 (for blanched). Page’s and Modified Page’s empirical models showed a good fit for all conditions than the simple exponential model.
Abbreviations
- a, b, c, n :
-
Constants of models
- D eff :
-
Effective diffusivity (m2 s−1)
- D 0 :
-
Pre-exponential factor of the Arrhenius equation (m2 s−1)
- E a :
-
Activation energy (kJ mol−1)
- k :
-
Rate constants in models (h−1)
- M:
-
Moisture content (g water/g dry matter)
- M e :
-
Equilibrium moisture content (g water/g dry matter)
- M 0 :
-
Initial moisture content (g water/g dry matter)
- MR:
-
Moisture ratio
- N :
-
Number of observations
- R :
-
Radius of the persimmon (m)
- R 2 :
-
Determination coefficient
- RMSE:
-
Root mean square error
- T :
-
Temperature (°C)
- t :
-
Drying time (h)
- χ 2 :
-
Reduced Chi square
- z :
-
Number of constants in models
References
Nicoleti JF et al (2007) Influence of drying conditions on ascorbic acid during convective drying of whole persimmons. Drying Technol 25(5):891–899
Uchida S et al (1990) Prolongation of life span of stroke-prone spontaneously hypertensive rats (SHRSP) ingesting persimmon tannin. Chem Pharm Bull 38(4):1049–1052
Park YS et al (2006) Drying of persimmons (Diospyros kaki L.) and the following changes in the studied bioactive compounds and the total radical scavenging activities. LWT-Food Sci Technol 39(7):748–755
Hertog MG et al (1995) Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med 155(4):381–386
Singh B et al (2007) Study of mass transfer kinetics and effective diffusivity during osmotic dehydration of carrot cubes. J Food Eng 79(2):471–480
Rastogi N, Raghavarao K (2004) Mass transfer during osmotic dehydration of pineapple: considering Fickian diffusion in cubical configuration. LWT-Food Sci Technol 37(1):43–47
Kaymak-Ertekin F, Sultanoğlu M (2000) Modelling of mass transfer during osmotic dehydration of apples. J Food Eng 46(4):243–250
Babalis SJ, Belessiotis VG (2004) Influence of the drying conditions on the drying constants and moisture diffusivity during the thin-layer drying of figs. J Food Eng 65(3):449–458
Kaleta A, Górnicki K (2010) Evaluation of drying models of apple (var. McIntosh) dried in a convective dryer. Int J Food Sci Technol 45(5):891–898
Menges HO, Ertekin C (2006) Mathematical modeling of thin layer drying of Golden apples. J Food Eng 77(1):119–125
Toğrul IT, Pehlivan D (2002) Mathematical modelling of solar drying of apricots in thin layers. J Food Eng 55(3):209–216
Doymaz I (2004) Effect of dipping treatment on air drying of plums. J Food Eng 64(4):465–470
Yaldiz O et al (2001) Mathematical modeling of thin layer solar drying of sultana grapes. Energy 26(5):457–465
Maskan M, Göğüş F (1998) Sorption isotherms and drying characteristics of mulberry (Morus alba). J Food Eng 37(4):437–449
Akpinar EK et al (2003) Modeling and experimental study on drying of apple slices in a convective cyclone dryer. J Food Process Eng 26(6):515–541
Beltagy EA et al (2007) Solar drying characteristics of strawberry. J Food Eng 78(2):456–464
Cárcel J et al (2007) Influence of high-intensity ultrasound on drying kinetics of persimmon. Drying Technol 25(1):185–193
Doymaz I (2012) Evaluation of some thin-layer drying models of persimmon slices (Diospyros kaki L.). Energy Convers Manag 56:199–205
AOAC (1984) Official methods of analysis of the association of offical analytical chemists, 14th edn. AOAC, Arlington
Parti M (1993) Selection of mathematical models for drying grain in thin-layers. J Agric Eng Res 54:339–352
Doymaz I (2006) Drying kinetics of black grapes treated with different solutions. J Food Eng 76(2):212–217
Diamante LM, Munro PA (1993) Mathematical modelling of the thin layer solar drying of sweet potato slices. Sol Energy 51(4):271–276
Ertekin C, Yaldiz O (2004) Drying of eggplant and selection of a suitable thin layer drying model. J Food Eng 63(3):349–359
Madamba PS et al (1996) The thin-layer drying characteristics of garlic slices. J Food Eng 29(1):75–97
Demiray E, Tulek Y (2012) Thin-layer drying of tomato (Lycopersicum esculentum Mill. cv. Rio Grande) slices in a convective hot air dryer. Heat Mass Transf 48(5):841–847
El-Aouar AA et al (2003) Drying kinetics of fresh and osmotically pre-treated papaya (Carica papaya L.). J Food Eng 59(1):85–91
Özdemir M, Devres YO (1999) The thin layer drying characteristics of hazelnuts during roasting. J Food Eng 42(4):225–233
Tütüncü MA, Labuza T (1996) Effect of geometry on the effective moisture transfer diffusion coefficient. J Food Eng 30(3):433–447
Toğrul IT (2010) Modelling of heat and moisture transport during drying black grapes. Int J Food Sci Technol 45(6):1146–1152
Sacilik K, Elicin AK (2006) The thin layer drying characteristics of organic apple slices. J Food Eng 73(3):281–289
Doymaz I (2008) Convective drying kinetics of strawberry. Chem Eng Process Process Intensif 47(5):914–919
Nahimana H et al (2011) Mass transfer modeling and shrinkage consideration during osmotic dehydration of fruits and vegetables. Food Rev Int 27:331–356
Marcotte M, Maguer M (1991) Repartition of water in plant tissues subjected to osmotic processes. J Food Process Eng 13(4):297–320
Falade KO, Solademi OJ (2010) Modelling of air drying of fresh and blanched sweet potato slices. Int J Food Sci Technol 45(2):278–288
Arslan D, Özcan M (2011) Dehydration of red bell-pepper (Capsicum annuum L.): change in drying behavior, colour and antioxidant content. Food and Bioprod Process 89(4):504–513
Doymaz I (2013) Experimental study on drying of pear slices in a convective dryer. Int J Food Sci Technol 48(9):1909–1915
Zhao D et al (2013) The effect of osmosis pretreatment on hot-air drying and microwave drying characteristics of chili (Capsicum annuum L.) flesh. Int J Food Sci Technol 48(8):1589–1595
Kadam DM, Dhingra D (2011) Mass transfer kinetics of banana slices during osmo-convective drying. J Food Process Eng 34(2):511–532
Zogzas N et al (1996) Moisture diffusivity data compilation in foodstuffs. Drying Technol 14(10):2225–2253
Doymaz I (2011) Thin-layer drying characteristics of sweet potato slices and mathematical modelling. Heat Mass Transf 47(3):277–285
Mahmutoglu T, Emir F, Saygi YB (1996) Sun/solar drying of differently treated grapes and storage stability of dried grapes. J Food Eng 29:289–300
Doymaz I (2004) Pretreatment effect on sun drying of mullberry fruits (Morus alba L.). J Food Eng 65:205–209
Kaya A et al (2007) Drying kinetics of red delicious apple. Biosyst Eng 96(4):517–524
Erbay Z, Icier F (2010) Thin-layer drying behaviors of olive leaves (Olea Europaea L.). J Food Process Eng 33(2):287–308
Roberts JS et al (2008) Drying kinetics of grape seeds. J Food Eng 89(4):460–465
Toğrul H (2006) Suitable drying model for infrared drying of carrot. J Food Eng 77(3):610–619
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demiray, E., Tulek, Y. The effect of pretreatments on air drying characteristics of persimmons. Heat Mass Transfer 53, 99–106 (2017). https://doi.org/10.1007/s00231-016-1797-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-016-1797-2